Abstract
Background: The older adult population is expanding, living longer, with multiple chronic conditions. Understanding and managing their needs over time is an integral part of defining successful aging. Population health is used to describe the measurement and health outcomes of a population. Objectives: To define population health as applied to older adults, summarize lessons learned from current research, and identify potential interventions designed to promote successful aging and improved health for this population. Method: Online search engines were utilized to identify research on population health and health interventions for older adults. Results: Population health management (PHM) is one strategy to promote the health and well-being of target populations. Interventions promoting health across a continuum tend to be disease, risk, or health behavior specific rather than encompassing a global concept of health. Conclusion: Many existing interventions for older adults are simply research based with limited generalizability; as such, further work in this area is warranted.
Keywords: population health, population health management, successful aging, older adults
Introduction
According to the U.S. Census Current Population Report, the older adult population (65 years and older) will almost double from 43.1 million in 2012 to 83.7 million in 2050 due to the aging Baby Boomers and increases in life expectancy (Ortman, Velkoff, & Hogan, 2014). This number also reflects a larger representation of minorities among older adults increasing from 20.7% in 2012 to 39.1% in 2050. The National Council on Aging (2014) reported that 92% of older adults have at least one chronic disease and 77% have at least two chronic diseases. The most common chronic conditions or diseases include hypertension, coronary heart disease, stroke, diabetes, and cancer (Ward & Schiller, 2013). Although these conditions affect all older adults, they disproportionately affect older African Americans who also have less access to quality health care and lower health care utilization (Miller, Kirk, Kaiser, & Glos, 2014; Rooks et al., 2008).
Health care utilization and support service needs are increasing among older adults. Due to the increasing population size and number of comorbidities among older adults, it is unrealistic to address their health needs solely on either an individual disease or condition-related basis; thus, a population strategy is required. Historically, the term population health has been used broadly in a number of contexts often mirroring public health. Population health is defined as a “concept of health” characterized by both objective and subjective determinants and health outcomes of a population (Kindig & Stoddart, 2003). The determinants and outcomes of population health are a function of three overarching domains of well-being: physical, psychological, and social well-being. Well-being is a subjective term that describes self-reported general life satisfaction and the positive and negative emotions associated with health status (Diener, 2000). Examples of determinants include physical activity, perceived social support, stress, depressive symptoms, cognition, and loneliness, and a sense of purpose. Examples of outcomes include self-rated health, medical services utilization and expenditures, and mortality.
This subjective and psychosocial (i.e., psychological and social) aspect of health goes beyond a medical diagnosis, and reflects a growing need to better understand the population health continuum for older adults and how to integrate successful aging into that model. Successful aging has been studied extensively in gerontology. Rowe and Kahn (1987, 1997) first defined successful aging as the low probability of disease and disease-related disability, high cognitive and physical function capacity, and active engagement with life. Other researchers within the field expanded on successful aging theory to include additional factors such as adaptation, motivation, emotion, internal and external resources, and stress (Baltes & Baltes, 1990; Depp & Jeste, 2006; Kahana & Kahana, 1996). Health is more than just a condition or disease; it includes the psychosocial components as well. Successful aging reflects a combination of both objective and subjective measures of health, emphasizing its role in population health for older adults. Therefore, placing the “concept of health” across a continuum provides a framework in which both the subjective and objective health status indicators can be better understood. Furthermore, a continuum indicates that successful aging and health management can be implemented at multiple health stages (Depp & Jeste, 2006; P. Martin et al., 2015).
Continuum of Population Health
The field of population health has primarily focused on a medical model when placing individuals on a health continuum. Stratification of the population generally relies on a combination of individuals’ chronic disease diagnosis codes and health expenditure levels. As Figure 1, developed for this review, illustrates, the health of older adults is conceptualized on a population health continuum based on the numbers and severity of diagnosed chronic diseases (such as diabetes or heart disease), obtained from administrative claims databases. On one end of the continuum are the healthiest older adults whom we call healthy and active (disease-free, low health care expenditures). These older adults have no chronic health conditions or disease; staying healthy and active is critical to them. In the middle of the continuum are the majority of older adults who are classified into one of two groups: at risk but healthy or coping with disease (few and well-managed diseases, relatively low health care expenditures). Older adults in the at risk but healthy group are managing a chronic condition (such as hypertension) and their focus is to stay healthy, prevent the progression of their condition, and prevent future chronic conditions. Those in the coping with disease (multiple chronic conditions; higher health care expenditures) group may have multiple conditions or diseases and their focus is on slowing the progression of these conditions and/or reversing the damage. Finally, the very ill (high–very high health care expenditures) group consists of older adults who have had severe medical episodes and need constant or regular medical intervention.
Figure 1.
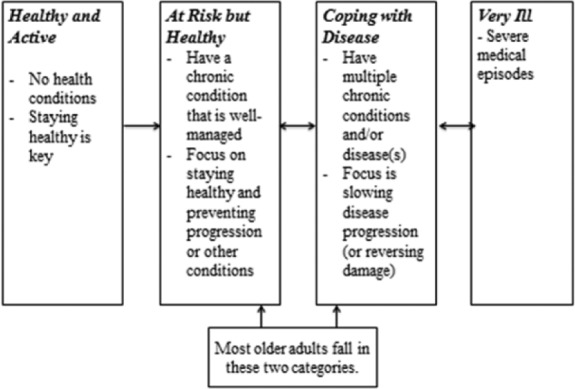
The continuum of population health for older adults.
The majority of older adults will develop health conditions as they age. However, their subjective health and well-being may indicate that they are healthier than the medical model indicates. Therefore, an important component of this population health continuum is to integrate the subjective perspective into the medical model. The population health continuum allows for subjectivity by including self-report data on health perceptions and the psychological aspects of health, broadening the definition of successful aging. Therefore, the purpose of this article is to (a) consider definitions of health for older adults, (b) review research that promotes health management of older adults, and (c) identify lessons learned and future directions to promote successful population health management (PHM) programs/interventions.
The Health of Older Adults
Defining Health for Older Adults
The World Health Organization (WHO; 2006) defines health as “a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity.” (p.1) The specificity of this definition has been criticized as it defines the majority of the population as “unhealthy” and lacks the subjectivity of health (Huber et al., 2011; Jadad & O’Grady, 2008). Health includes aspects defined by the individual and its meaning reflects individuals’ ability to adapt to their environment and manage their well-being (Huber et al., 2011). Therefore, a holistic perspective on health that includes a more objective construct such as physical functioning and the more subjective constructs of psychological well-being, social well-being, and self-rated successful aging can provide a more accurate health description and a comprehensive perspective on the quality of life of older adults.
Physical functioning
Physical functioning relies primarily on objective determinants and outcomes as it relates to issues that result as a function of a combination of age, illness, and mobility. The objective measures of age and chronic illness are particularly relevant as physical decline occurs (Holmes, Powell-Griner, Lethbridge-Cejku, & Heyman, 2009; Hughes et al., 2001). For example, some physical limitations of older adults are characterized by the inability to walk a quarter of a mile, stand for 2 hrs, stoop, bend or kneel, and lift or carry something more than 10 pounds (equal to bags of groceries; Holmes et al., 2009). There are a number of characteristics associated with reduced physical functioning, which also includes activities of daily living (ADLs; for example, bathing, dressing, etc.) and risk of fall. These characteristics include age, minority status, being female, lower education, depressive symptoms, and illnesses such as diabetes, arthritis, and heart failure (Bruce, Seeman, Merrill, & Blazer, 1994; V. C. Chang & Do, 2015; Dunlay et al., 2015; Gregg et al., 2000; Holmes et al., 2009; Lin et al., 2003; Reginster, 2002). Outcomes of poor physical functioning include continued decline, falls, worse mental health, and increased mortality (Ambrose, Paul, & Hausdorff, 2013; Makizako et al., 2015).
Another aspect of physical functioning limitations is sensory impairment. For older adults, the decline of hearing and vision has a significant impact on their well-being (Schneider et al., 2011). Determinants of sensory impairment include age, gender, and race (Lin, Thorpe, Gordon-Salant, & Ferrucci, 2011). Outcomes of sensory impairment include increased risk of falls, depression, cognitive impairment, and loneliness (Lin, 2011; Schneider et al., 2011).
Psychological well-being
Psychological well-being is a multidimensional construct that personifies the human capacity to continue to grow, adapt to our environment, and evolve as we age. For older adults in particular, psychological well-being is characterized as the ability to deal with life situations, to have and maintain positive and close relationships, self-acceptance of both self and others, and autonomy (Ryff, 1995; Ryff & Keyes, 1995). Predictors of high psychological well-being for older adults include social support, physical activity, spirituality, and higher socioeconomic status (Black et al., 2015; Holland & Holahan, 2003; Levin & Chatters, 1998). Conversely, depression, stress, and anxiety are predictors of low psychological well-being (Allerhand, Gale, & Deary, 2014; Ormel et al., 1998; Ryff, 2014). Outcomes of high psychological well-being include lower rates of cognitive decline, higher levels of resilience, and greater life satisfaction (Bowling & Iliffe, 2011; Gerstorf, Lovden, Rocke, Smith, & Lindenberger, 2007; Ryff, 2014). Notably, high psychological well-being is a key factor in successful aging and reduced mortality rates for both physically healthy and unhealthy older adults (Chida & Steptoe, 2008; Rowe & Kahn, 1997; Ryff, 2014; Steptoe, Deaton, & Stone, 2015).
Social well-being
Social well-being has been defined as the relative ability to deal with social challenges and how well one functions in the social world (Keyes, 1998). There are five major components of social well-being: social integration, social contribution, social coherence, social actualization, and social acceptance (Keyes, 1998). Social well-being has a significant impact on the health of older adults, with higher levels of social well-being related to lower levels of cortisol and inflammatory factors that contribute to a number of diseases including cardiovascular disease (CVD; Ryff, Singer, & Love, 2004). As adults age, their social networks and social roles change; some of these changes enhance their social well-being and some detract from it. For example, social networks may decrease in size but satisfaction with social networks may increase (Carstensen, 1992; Carstensen, Fung, & Charles, 2003).
Social relationships are an important indicator of quality of life, with those who have negative social relationships reporting worse quality of life independent of disease status (Liao & Brunner, 2016; Rook, 1984). Close positive family ties have particular relevance for the well-being of older adults (Fuller-Iglesias, Webster, & Antonucci, 2015). Negative social factors that reduce the social well-being of older adults include loneliness and social isolation (Singh & Misra, 2009). Loneliness can result from feeling a loss of meaning in life, lack of independence, and loss of loved ones (Smith, 2012). Social support is also a key influence on the social well-being of older adults. Among older adults, high levels of social support are associated with lower stress and better mental and physical well-being (Fuller-Iglesias, 2015; Thoits, 2011).
There is also an emphasis in the literature on the importance of incorporating more subjective data into the model of successful aging as the objective measures (disease and functioning) severely limit the number of individuals who are considered to age successfully (Cho, Martin, & Poon, 2012, 2015; Pruchno, Wilson-Genderson, & Cartwright, 2010). For example, Pruchno et al. (2010) proposed a two-factor model of successful aging by incorporating objective and subjective data. Objective data included physical functioning, pain (frequency, intensity, and interference), and number of chronic conditions. Subjective questions asked participants how successfully or how well they thought they had aged and life ratings. This two-factor model found that age and gender had strong associations with objective aging but not with the subjective factor. Additional research by Cho et al. (2012) found that only about 15% of octogenarians fit the Rowe and Kahn criteria of successful aging. However, the subjective aspect of successful aging identified 62% as successful agers. These studies highlight successful aging as a fluid and modifiable construct on the continuum of health. On the health continuum, some individuals may be coping with disease and are working to manage multiple chronic conditions and feel they have also aged successfully. As a result of this subjective aspect, they may incorporate better health behaviors that improve their objective health status.
Connecting the subjective and objective aspects of health for older adults within the context of population health and successful aging is a natural fit. The theory of population health acknowledges this subjectivity within the context of objective metrics of health and provides a structure to measure and interpret the holistic health of older adults.
PHM: Programs and Research
PHM programs promote health and well-being across the continuum of population health and have three basic goals: to improve health, improve the health care experience, or reduce health care costs (Berwick, Nolan, & Whittington, 2008). Primarily, PHM programs seek to keep healthy people healthy, reduce the progression of disease, and reduce the risk of future disease for those who have chronic conditions.
The concept of PHM programs, sometimes referred to as wellness health programs (WHP), originated in the employee sector. In this model, the population is stratified on a combination of health risks and diseases. Programs are then targeted to match the health needs of individuals along this continuum from no health risks/no diseases to multiple chronic diseases to catastrophic illness episodes. With the utilization of incentives, these programs have been able to reach large portions of employee populations. The financial impact resulting from risk reduction and/or disease management has been positive but weak as many employers do not include all the components necessary to make these programs successful (Goetzel & Ozminkowski, 2008; Grossmeier, Terry, Anderson, & Wright, 2012).
Interventions to Promote Health for Older Adults
To our knowledge, there is little scientific research that examines or evaluates a comprehensive PHM program for older adults. Those programs labeled as PHM for older adults target populations with serious risks and conditions rather than the general population (LaMantia et al., 2015; Stefanacci, Reich, & Casiano, 2015). Studies on health promotion for older adults tend to be randomized trials that are health behavior (smoking, physical activity), risk (fall), or disease (diabetes or heart disease) specific (Byrne, Barry, & Petry, 2012; Jorgensen, Laessoe, Hendriksen, Nielsen, & Aagaard, 2012; Van Hoecke, Delecluse, Bogaerts, & Boen, 2014; Zbikowski et al., 2011). Some of these studies have demonstrated success with small sample sizes and unknown long-term effects.
Lifestyle behavior interventions
The purpose of a lifestyle behavior intervention is to modify poor lifestyle behaviors to improve health, reduce risk of disease, or slow progression of disease (Glanz, Rimer, & Viswanath, 2008; Montaño & Kasprzyk, 2008). On the continuum of health, the majority of older adults already have chronic conditions and therefore lifestyle modification is of great significance. The three most relevant modifiable lifestyle behaviors are physical inactivity (or sedentary lifestyle), healthy diet, and tobacco usage. These lifestyle behaviors have strong associations with a number of diseases (Mokdad et al., 2003; Warburton, Nicol, & Bredin, 2006). Therefore, lifestyle behavior modifications can prevent older adults from moving further along the continuum of health. Successful interventions require regular and frequent contact with participants and need to include multiple modes of delivery (e.g., in-person, online, etc.; Mouton & Cloes, 2015; Svetkey et al., 2008; Zbikowski, Magnusson, Pockey, Tindle, & Weaver, 2012). In addition, addressing lifestyle behaviors requires multiple levels of interventions (Sallis, Owen, & Fisher, 2008). For example, interventions to improve healthy eating are only successful if there is sustainable access to healthy food.
Utilizing a theoretical approach when designing a lifestyle intervention is of great importance and a lack of integrating theory may partly explain limited successful outcomes (Glanz, Rimer, & Viswanath, 2008; Painter, Borba, Hynes, Mays, & Glanz, 2008). Theories such as the health belief model or the transtheoretical model of health behavior change are the most frequently used (Painter et al., 2008; Prochaska & Velicer, 1997; Rosenstock, 1974). These theories incorporate the individual’s level of willingness to change, assessment of risk, and self-efficacy (Prochaska & Velicer, 1997; Rosenstock, 1974). Self-efficacy, a belief in the ability to achieve success, is of particular importance in health behavior change for older adults (Grembowski et al., 1993; Schwarzer & Renner, 2000). Older adults with high self-efficacy have lower health risks than those with low self-efficacy (Grembowski et al., 1993). Self-efficacy has also been found to be a significant predictor of physical activity, smoking cessation, and healthy diet (Bauman et al., 2012; Grembowski et al., 1993; McAuley et al., 2011).
Physical activity declines as adults age with those more than the age of 60 reporting the lowest levels (Troiano et al., 2008). Interventions designed specifically for older adults to increase physical activity can be successful by improving physical functioning, cognition, disease management, and psychological well-being (Black et al., 2015; Colcombe & Kramer, 2003; Cress, Buchner, Questad, Esselman, & Schwartz, 1999). Healthy food consumption and physical activity are frequently linked together in research because of their relationship to each other and their shared relationship with obesity (Rosenheck, 2008; Villareal, Apovian, Kushner, & Klein, 2005; Wareham, van Sluijs, & Ekelund, 2005). The rising rate of obesity is the number one health concern for Americans (Flegal, Carroll, Ogden, & Curtin, 2010); a number of interventions have aimed to improve older adults’ eating habits to reduce their weight and improve physical functioning. These studies find that combining both diet and physical activity yields the most successful results (Rejeski, Mihalko, Ambrosius, Bearon, & McClelland, 2011; Villareal et al., 2011; Witham & Avenell, 2010).
As noted on the population health continuum, health behavior interventions can be for those at risk of developing a condition or disease or to manage a condition or disease and reduce the risk of other conditions and diseases. One diabetes prevention study randomized non-diabetics to one of three groups: lifestyle modification (diet and exercise), medication, or control group (Knowler et al., 2002). Those randomized to the lifestyle intervention group were the least likely to develop diabetes over a 3-year period, highlighting the importance of modifiable health behaviors.
Fall risk interventions
As the number of severity of chronic conditions or diseases increases across the continuum of health, so does the risk of falls. Falls are the leading cause of fatal and non-fatal injuries and have a significant impact on quality of life (Houry, Florence, Baldwin, Stevens, & McClure, 2016). Although falls are considered a preventable health issue, it is estimated that about 40% of older adults fall every year, increasing health care utilization and costs (Ambrose et al., 2013; Houry et al., 2016). Predictors of falls and recurrent falls include gender (female), visual impairment, cognitive impairment, medications, poor physical functioning, poor balance, reduced gait, the need of a walking assistant (cane, walker), fear of falling, low body weight, and history of falls (Ambrose et al., 2013; Mirelman et al., 2012; Rubenstein, 2006; Shumway-Cook, Baldwin, Polissar, & Gruber, 1997).
The U.S. Preventive Service Task Force (Moyer, 2012) recommends exercise, physical therapy, and vitamin D to reduce the risk of falls. Exercise interventions, in particular, have demonstrated high success in reducing the risk of falls (J. T. Chang et al., 2004; Rubenstein, 2006). Some novel approaches of exercise, such as Tai Chi and videogames (Nintendo Wii), have been tested. These interventions are considered low cost and have demonstrated success in reducing fall risk and improving physical functioning with the added benefit of participant enjoyment (Bieryla & Dold, 2013; Jorgensen et al., 2012; Li et al., 2005). These interventions also demonstrate that across the continuum of health, there are a number of opportunities to reduce risk and improve well-being.
Psychosocial interventions
Several interventions aim to improve psychological or social well-being; however, these studies demonstrate mixed results (Hogan, Linden, & Najarian, 2002; Netz, Wu, Becker, & Tenenbaum, 2005). Physical activity and mindfulness training interventions have significant effects on psychological well-being (Carmody & Baer, 2008; Chiesa & Serretti, 2009; Netz et al., 2005; Penedo & Dahn, 2005). However, many mindfulness interventions are related to chronic pain or high levels of stress (Chiesa & Serretti, 2009; Morone & Greco, 2007). Interventions to improve social well-being for older adults have also shown some benefits (Dickens, Richards, Greaves, & Campbell, 2011). One meta-analysis reviewing social well-being interventions for older adults found that the most successful were those that used a theoretical approach, group support, and had active participation (Dickens et al., 2011). In addition, the use of technology interventions have also been successful in improving the psychological and social well-being of older adults (Jones, Ashurst, Atkey, & Duffy, 2015; Shapira, Barak, & Gal, 2007). One study found that older adults who completed an Internet-use training program reported greater contact with friends and family, less loneliness, and better mental well-being (Jones et al., 2015). As these programs demonstrate, the overlap of clinical and psychosocial determinants and outcomes demonstrates the need for a more comprehensive and integrative model addressing health and health promotion across the continuum of health.
Disease management interventions
Disease management (DM) programs were originally developed for high-cost patients dealing with a specific chronic disease such as diabetes, asthma, or congestive heart failure (CHF). Their purpose was to implement evidence-based practice to reduce cost by reducing emergency room visits and hospitalizations, improving quality of life, and improving clinical outcomes (Shelton, 2002). However, a number of reviews and meta-analyses have found that DM programs generally do not reduce cost and have limited health outcomes (de Bruin, Heijink, Lemmens, Struijs, & Baan, 2011; McCall & Cromwell, 2011; Pimouguet, Le Goff, Thiébaut, Dartigues, & Helmer, 2011; Savard, Thompson, & Clark, 2011). However, literature supporting the benefits of DM programs indicates that they can improve quality of life and reduce cost especially for two of the most expensive and common conditions: CVD and diabetes (Chodosh et al., 2005; Griffo et al., 2013; The Look AHEAD Research Group, 2010; Tricco et al., 2012; Yu et al., 2004).
Cardiac rehabilitation programs target individuals who have experienced or are at risk of a cardiac event (heart attack, angioplasty, stent surgery). The program provides a supervised exercise program, education, and counseling to improve health and prevent further cardiac events (Fletcher et al., 2013). Research has demonstrated the benefits of cardiac rehabilitation to improve clinical outcomes, increase physical activity, improve quality of life, and reduce mortality (Lavie, Berra, & Arena, 2013; Lavie & Milani, 2011; B.-J. Martin et al., 2012; Oldridge, 2012; Shepherd & While, 2012). Cardiac rehabilitation is most effective for patients who adhere to the program (B.-J. Martin et al., 2012), yet interventions to increase adherence have mixed results (Antypas & Wangberg, 2014; Karmali et al., 2014). Factors that improve adherence include self-monitoring, action planning, and individual counseling (Karmali et al., 2014), as well as strong social networks, social support, and self-efficacy (Tkatch et al., 2011; Woodgate, Brawley, & Shields, 2007).
Type 2 diabetes is a serious growing health concern. In 2010, the Centers for Disease Control and Prevention (CDC, 2011) reported that 27% of adults 65 and older have diabetes, which is a significant cause of other serious health conditions including kidney failure, heart disease, and stroke. One of the strongest risk factors for diabetes is obesity; therefore, improving one’s health behaviors and health lifestyle are the most effective ways to reduce risk for and manage diabetes (Knowler et al., 2002). A number of studies have demonstrated that diabetes self-management education (DSME) can be effective in preventing and managing the disease (Haas et al., 2013; Stellefson, Dipnarine, & Stopka, 2013). However, older adults are underrepresented in clinical trials related to diabetes management and treatment, and there are different guidelines for treating diabetes and its comorbidities for older adults (Kirkman et al., 2012). Factors that contribute to the success of DSME programs include high levels of social support and self-efficacy and the use of theory-based or technology-based programs (Byrne et al., 2012; Ceriello et al., 2012; Cotter, Durant, Agne, & Cherrington, 2014; Rosland et al., 2015).
The lack of consistent results for DM programs highlights the need for a more comprehensive approach rather than targeting just one disease or health behavior (Krause et al., 2006; Shelton, 2002). The overlap of both the determinants and outcomes of multiple chronic conditions indicates that health behavior, risk, and DM interventions and programs are not independent and should be addressed with a more integrated approach. Programs attempting to do this are small in nature and the feasibility of implementation on a larger scale is unknown (Krause et al., 2006). Furthermore, some research demonstrates that an increase in conditions may actually decrease the likelihood of completing a DM program such as cardiac rehabilitation (Forhan, Zagorski, Marzonlini, Oh, & Alter, 2013). Therefore, a number of challenges need to be addressed when considering comprehensive PHM programs.
Case management (CM)/care coordination interventions
Chronic DM becomes more complex as individuals move along the continuum of health to the very ill category. Multiple chronic conditions require a multidimensional approach to addressing the health needs of older adults, especially with concurrent medical episodes (Boyd et al., 2005). In CM programs, case managers help organize the health care needs of high-cost individuals by coordinating a multidisciplinary team to provide high-quality care, and thus help improve quality of life and reduce costs prior to and post hospitalization (Hickam et al., 2013). Individuals are identified for these programs through Hierarchal Condition Category (HCC) risk scores, which are derived from medical claims (McCall & Cromwell, 2011). CM programs are endorsed by the Centers for Medicare and Medicaid Services (CMS) and covered by Medicare although their limited impact on reducing cost has been documented (McCall & Cromwell, 2011; Schore, Brown, & Cheh, 1998).
CM program success is frequently determined by reduced hospitalizations (Askren-Gonzalez & Frater, 2012). Key factors, identified through CMS data, that contribute to successful programs include frequent in-person contact, assistance in communication between patients and their providers, education, medication management, and extensive transition care after hospitalizations (Brown, Peikes, Peterson, Schore, & Razafindrakoto, 2012). Additional variables that contribute to the success of CM programs include the length of time engaged in the program and focusing on individuals with the highest risk (Hawkins et al., 2015; Peikes, Peterson, Brown, Graff, & Lynch, 2012). There is also a significant need to include a mental health component in these programs (Bao, Casalino, & Pincus, 2013; Zulman et al., 2014).
Although CM programs can be beneficial, they primarily target those at the end of the health continuum (consequently a relatively small percentage of the population). Importantly, there is little focus on quality of life and lifestyle modifications that could restore quality of life post hospitalization and reduce repeat episodes, ultimately reducing cost. Individuals should not have to wait until they are at the end of this continuum to receive comprehensive quality and effective care. Therefore, when developing a comprehensive PHM program for older adults, all of these components could have the potential to promote successful aging independent of an individual’s objective health status.
Conclusions and Recommendations
The current health trajectory for older adults is to treat a condition or disease as it is diagnosed and to treat it solely from a medical perspective. There is little emphasis on the subjective or psychosocial components that significantly influence health. The integration of both the subjective and objective concepts of health is necessary to consider in health management programs and research for older adults. PHM programs have yet to be strategically incorporated into successful aging initiatives for older adults. Implementation of PHM programs requires (a) successful program designs, (b) distribution mechanisms and proven access for older adults, (c) funding through existing agencies (e.g., Medicare, health plans), and (d) a comprehensive evaluation of the successful elements of a PHM program.
The concept of successful aging is not theoretical; it is a practical theory that can be incorporated into the health and well-being of older adults regardless of their position on the health continuum. Currently, attention and resources often focus on the very ill who are not considered to be successful agers, rather than addressing the health needs of the majority of older adults. Furthermore, successful aging does not consider that very ill older adults may need more than just management of their physical diseases with psychological and social programs to restore quality of life during and post illness. Therefore, perhaps the most cost-effective measures to consider include reducing the risk of disease and slowing disease progression, which can be achieved most effectively through comprehensive lifestyle interventions. In addition, programs that adopt factors shown to promote successful interventions, such as frequent in-person contact, multiple modes of delivery, and care coordinators, could lead to significant changes in the health and well-being of older adults regardless of health status. One issue to consider regarding a successful intervention is the ability to reach the older adult population. Previous methods have used telephone or “snail” mail, which have proven to be time-consuming, expensive, and have low attrition rates (Jancey et al., 2006; Taylor-Davis, Smiciklas-Wright, Davis, Jensen, & Mitchell, 1998). Many of the successful interventions reviewed in this article utilized technology as it has become increasingly more popular and effective. In 2012, a Pew Research Report (Zickuhr & Madden, 2012) found that more than half of older adults are online using smartphones, email, and social networking sites. In particular, smartphone applications have been successful in health promotion (Bert, Giacometti, Gualano, & Siliquini, 2014; Zickuhr & Madden, 2012). These applications provide a low-cost and easily accessible method to reach older adults regardless of mobility or health status. Other technology tools, such as web-based programs or videogames, have also demonstrated success in health promotion (Cotter et al., 2014; Jorgensen et al., 2012)
Another point to consider is the growing needs of minority older adults. As noted earlier, there has historically been a significant racial disparity in access to quality health care (Miller et al., 2014, Rooks et al., 2008). The Affordable Care Act (ACA) provides free personalized preventive care for Medicare beneficiaries, a program designed to provide better access to quality care for minorities. However, analysis of utilization of these services found that African Americans are less likely to use them (Hu, Jensen, Nerenz, & Tarraf, 2015). These data demonstrate that PHM programs will need to be not only comprehensive but also inclusive for minority older adults.
On a practical level, comprehensive PHM programs designed for the health of older adults are expensive. CM programs are funded by Medicare but these programs are geared toward high-cost/high-risk patients, whereas other interventions and programs are funded by grants through academic institutions. This leaves the question of who will administrate and pay for real-world program implementation. However, as noted previously, many of the successful interventions have implemented low-cost practices such as online programs and in-home interventions that are practical and enjoyable. As noted, the use of technology in health promotion has demonstrated its effectiveness in improving health behaviors, adherence to management, and health-related quality of life (Irvine, Gelatt, Seeley, Macfarlane, & Gau, 2013; Joe & Demiris, 2013; Stellefson, Chaney et al., 2013; Street, Gold, & Manning, 1997). Furthermore, just as millions of older adults purchase supplemental health insurance, perhaps an evidence-based, fee-for-service health program may be an option for health care organizations to offer.
A comprehensive PHM program would need to move beyond the clinical model and focus heavily on the psychosocial components of health and how they may influence well-being. Stratification would require more than just claims data, number of chronic conditions, or prescription drugs. Rather, self-reported survey data that measure psychosocial elements such as loneliness, resilience, and self-efficacy, or measuring personality characteristics may provide a clearer picture of what an individual really needs. Tailoring programs that target these population subgroups, along with an evaluation to assess these outcomes, may be most effective for future PHM programs.
Health plan organizations might also consider evidence-based, low-cost PHM to promote health and well-being and as a strategy to help manage health care costs. For example, UnitedHealthcare has developed and implemented a pilot health and wellness program utilizing four modes of delivery: online, telephonic, in-person classes, and discounts for gym memberships. The program, titled At Your Best, is available, at no additional cost, to insured members covered under an AARP® Medicare Supplement Insurance Plan insured by UnitedHealthcare Insurance Company, and is geared toward all older adults regardless of their health status. Although it is too early to determine the success of this program, engagement rates thus far are steadily increasing.
PHM for older adults has not yet been fully recognized or implemented as an area of health promotion. However, the potential to improve health and well-being for older adults across the health continuum is viable. These programs may provide an opportunity to integrate successful aging at all stages of health and improve both the objective and subjective components of health.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Allerhand M., Gale C. R., Deary I. J. (2014). The dynamic relationship between cognitive function and positive well-being in older people: A prospective study using the English Longitudinal Study of Aging. Psychology and Aging, 29, 306-318. doi: 10.1037/a0036551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose A. F., Paul G., Hausdorff J. M. (2013). Risk factors for falls among older adults: A review of the literature. Maturitas, 75, 51-61. [DOI] [PubMed] [Google Scholar]
- Antypas K., Wangberg S. C. (2014). An Internet-and mobile-based tailored intervention to enhance maintenance of physical activity after cardiac rehabilitation: Short-term results of a randomized controlled trial. Journal of Medical Internet Research, 16(3), e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askren-Gonzalez A., Frater J. (2012). Case management programs for hospital readmission prevention. Professional Case Management, 17, 219-226. [DOI] [PubMed] [Google Scholar]
- Baltes P. B., Baltes M. M. (1990). Psychological perspectives on successful aging: The model of selective optimization with compensation. In Baltes P. B., Baltes M. M. (Eds.), Successful aging: Perspectives from the behavioral sciences (pp. 1-34). New York, NY: Cambridge University Press. [Google Scholar]
- Bao Y., Casalino L. P., Pincus H. A. (2013). Behavioral health and health care reform models: Patient-centered medical home, health home, and accountable care organization. The Journal of Behavioral Health Services & Research, 40, 121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman A. E., Reis R. S., Sallis J. F., Wells J. C., Loos R. J., Martin B. W. Lancet Physical Activity Series Working Group. (2012). Correlates of physical activity: Why are some people physically active and others not? The Lancet, 380, 258-271. [DOI] [PubMed] [Google Scholar]
- Bert F., Giacometti M., Gualano M. R., Siliquini R. (2014). Smartphones and health promotion: A review of the evidence. Journal of Medical Systems, 38(1), Article 9995. [DOI] [PubMed] [Google Scholar]
- Berwick D. M., Nolan T. W., Whittington J. (2008). The triple aim: Care, health, and cost. Health Affairs, 27, 759-769. doi: 10.1377/hlthaff.27.3.759 [DOI] [PubMed] [Google Scholar]
- Bieryla K. A., Dold N. M. (2013). Feasibility of Wii Fit training to improve clinical measures of balance in older adults. Clinical Interventions in Aging, 8, 775-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S. V., Cooper R., Martin K. R., Brage S., Kuh D., Stafford M. (2015). Physical activity and mental well-being in a cohort aged 60-64 years. American Journal of Preventive Medicine, 49, 172-180. doi: 10.1016/j.amepre.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A., Iliffe S. (2011). Psychological approach to successful ageing predicts future quality of life in older adults. Health and Quality of Life Outcomes, 9, Article 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C. M., Darer J., Boult C., Fried L. P., Boult L., Wu A. W. (2005). Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. The Journal of the American Medical Association, 294, 716-724. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Peikes D., Peterson G., Schore J., Razafindrakoto C. M. (2012). Six features of Medicare coordinated care demonstration programs that cut hospital admissions of high-risk patients. Health Affairs, 31, 1156-1166. [DOI] [PubMed] [Google Scholar]
- Bruce M. L., Seeman T. E., Merrill S. S., Blazer D. G. (1994). The impact of depressive symptomatology on physical disability: MacArthur studies of successful aging. American Journal of Public Health, 84, 1796-1799. doi: 10.2105/AJPH.84.11.1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne S., Barry D., Petry N. M. (2012). Predictors of weight loss success. Exercise vs. dietary self-efficacy and treatment attendance. Appetite, 58, 695-698. doi: 10.1016/j.appet.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody J., Baer R. A. (2008). Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. Journal of Behavioral Medicine, 31, 23-33. [DOI] [PubMed] [Google Scholar]
- Carstensen L. L. (1992). Social and emotional patterns in adulthood: Support for socioemotional selectivity theory. Psychology and Aging, 7, 331-338. doi: 10.1037/0882-7974.7.3.331 [DOI] [PubMed] [Google Scholar]
- Carstensen L. L., Fung H. H., Charles S. T. (2003). Socioemotional selectivity theory and the regulation of emotion in the second half of life. Motivation and Emotion, 27, 103-123. doi: 10.1023/A:1024569803230 [DOI] [Google Scholar]
- Center for Disease Control and Prevention. (2011). National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. [Google Scholar]
- Ceriello A., Barkai L., Christiansen J. S., Czupryniak L., Gomis R., Harno K., Owens D. (2012). Diabetes as a case study of chronic disease management with a personalized approach: The role of a structured feedback loop. Diabetes Research and Clinical Practice, 98, 5-10. [DOI] [PubMed] [Google Scholar]
- Chang J. T., Morton S. C., Rubenstein L. Z., Mojica W. A., Maglione M., Suttorp M. J., Shekelle P. G. (2004). Interventions for the prevention of falls in older adults: Systematic review and meta-analysis of randomised clinical trials. British Medical Journal, 328, Article 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang V. C., Do M. T. (2015). Risk factors for falls among seniors: Implications of gender. American Journal of Epidemiology, 181, 521-531. doi: 10.1093/aje/kwu268 [DOI] [PubMed] [Google Scholar]
- Chida Y., Steptoe A. (2008). Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic Medicine, 70, 741-756. doi: 10.1097/PSY.0b013e31818105ba [DOI] [PubMed] [Google Scholar]
- Chiesa A., Serretti A. (2009). Mindfulness-based stress reduction for stress management in healthy people: A review and meta-analysis. The Journal of Alternative and Complementary Medicine, 15, 593-600. [DOI] [PubMed] [Google Scholar]
- Cho J., Martin P., Poon L. W. (2012). The older they are, the less successful they become? Findings from the Georgia centenarian study. Journal of Aging Research, 2012, Article 695854. doi: 10.1155/2012/695854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J., Martin P., Poon L. W. (2015). Successful aging and subjective well-being among oldest-old adults. The Gerontologist, 55, 132-143. doi: 10.1093/geront/gnu074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh J., Morton S. C., Mojica W., Maglione M., Suttorp M. J., Hilton L., Shekelle P. (2005). Meta-analysis: Chronic disease self-management programs for older adults. Annals of Internal Medicine, 143, 427-438. [DOI] [PubMed] [Google Scholar]
- Colcombe S., Kramer A. F. (2003). Fitness effects on the cognitive function of older adults a meta-analytic study. Psychological Science, 14, 125-130. [DOI] [PubMed] [Google Scholar]
- Cotter A. P., Durant N., Agne A. A., Cherrington A. L. (2014). Internet interventions to support lifestyle modification for diabetes management: A systematic review of the evidence. Journal of Diabetes and its Complications, 28, 243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress M. E., Buchner D. M., Questad K. A., Esselman P. C., Schwartz R. S. (1999). Exercise: Effects on physical functional performance in independent older adults. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 54, M242-M248. [DOI] [PubMed] [Google Scholar]
- de Bruin S. R., Heijink R., Lemmens L. C., Struijs J. N., Baan C. A. (2011). Impact of disease management programs on healthcare expenditures for patients with diabetes, depression, heart failure or chronic obstructive pulmonary disease: A systematic review of the literature. Health Policy, 101, 105-121. [DOI] [PubMed] [Google Scholar]
- Depp C. A., Jeste D. V. (2006). Definitions and predictors of successful aging: A comprehensive review of larger quantitative studies. American Journal of Geriatric Psychiatry, 14, 6-20. doi: 10.1097/01.JGP.0000192501.03069.bc [DOI] [PubMed] [Google Scholar]
- Dickens A. P., Richards S. H., Greaves C. J., Campbell J. L. (2011). Interventions targeting social isolation in older people: A systematic review. BMC Public Health, 11, Article 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E. (2000). Subjective well-being: The science of happiness and a proposal for a national index. American Psychologist, 55, 34-43. [PubMed] [Google Scholar]
- Dunlay S. M., Manemann S. M., Chamberlain A. M., Cheville A. L., Jiang R., Weston S. A., Roger V. L. (2015). Activities of daily living and outcomes in heart failure. Circulation: Heart Failure, 8, 261-267. doi: 10.1161/circheartfailure.114.001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. (2010). Prevalence and trends in obesity among US adults, 1999-2008. The Journal of the American Medical Association, 303, 235-241. [DOI] [PubMed] [Google Scholar]
- Fletcher G., Ades P., Kligfield P., Arena R., Balady G., Bittner V., Gerber T. (2013). Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation, 128, 873-934. [DOI] [PubMed] [Google Scholar]
- Forhan M., Zagorski B. M., Marzonlini S., Oh P., Alter D. A. (2013). Predicting exercise adherence for patients with obesity and diabetes referred to a cardiac rehabilitation and secondary prevention program. Canadian Journal of Diabetes, 37, 189-194. [DOI] [PubMed] [Google Scholar]
- Fuller-Iglesias H. R. (2015). Social ties and psychological well-being in late life: The mediating role of relationship satisfaction. Aging & Mental Health, 19, 1103-1112. doi: 10.1080/13607863.2014.1003285 [DOI] [PubMed] [Google Scholar]
- Fuller-Iglesias H. R., Webster N. J., Antonucci T. C. (2015). The complex nature of family support across the life span: Implications for psychological well-being. Developmental Psycholology, 51, 277-288. doi: 10.1037/a0038665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D., Lovden M., Rocke C., Smith J., Lindenberger U. (2007). Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology, 43, 705-718. doi: 10.1037/0012-1649.43.3.705 [DOI] [PubMed] [Google Scholar]
- Glanz K., Rimer B. K., Viswanath K. (2008). Health behavior and health education: Theory, research, and practice. In Glanz K., Rimer B. K., Viswanath K. (Eds.), Health behavior and health education: Theory, research, and practice (4th ed.). San Francisco, CA: Jossey-Bass. [Google Scholar]
- Goetzel R. Z., Ozminkowski R. J. (2008). The health and cost benefits of work site health-promotion programs. Annual Review of Public Health, 29, 303-323. doi: 10.1146/annurev.publhealth.29.020907.090930 [DOI] [PubMed] [Google Scholar]
- Gregg E. W., Beckles G. L., Williamson D. F., Leveille S. G., Langlois J. A., Engelgau M. M., Narayan K. M. (2000). Diabetes and physical disability among older U.S. adults. Diabetes Care, 23, 1272-1277. [DOI] [PubMed] [Google Scholar]
- Grembowski D., Patrick D., Diehr P., Durham M., Beresford S., Kay E., Hecht J. (1993). Self-efficacy and health behavior among older adults. Journal of Health and Social Behavior, 34, 89-104. [PubMed] [Google Scholar]
- Griffo R., Ambrosetti M., Tramarin R., Fattirolli F., Temporelli P. L., Vestri A. R., . . . Tavazzi L. (2013). Effective secondary prevention through cardiac rehabilitation after coronary revascularization and predictors of poor adherence to lifestyle modification and medication. Results of the ICAROS Survey. International Journal of Cardiology, 167, 1390-1395. [DOI] [PubMed] [Google Scholar]
- Grossmeier J., Terry P. E., Anderson D. R., Wright S. (2012). Financial impact of population health management programs: Reevaluating the literature. Population Health Management, 15, 129-134. doi: 10.1089/pop.2010.0086 [DOI] [PubMed] [Google Scholar]
- Haas L., Maryniuk M., Beck J., Cox C. E., Duker P., Edwards L., Kolb L. (2013). National standards for diabetes self-management education and support. Diabetes Care, 36(Suppl. 1), S100-S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins K., Parker P. M., Hommer C. E., Bhattarai G. R., Huang J., Wells T. S., Yeh C. S. (2015). Evaluation of a high-risk case management pilot program for Medicare beneficiaries with Medigap coverage. Population Health Management, 18, 93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickam D. H., Weiss J. W., Guise J.-M., Buckley D., Motu’apuaka M., Graham E., Saha S. (2013). Outpatient case management for older adults with medical illness and complex care needs (Report Number 13). Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Hogan B. E., Linden W., Najarian B. (2002). Social support interventions: Do they work? Clinical Psychology Review, 22, 381-440. [DOI] [PubMed] [Google Scholar]
- Holland K. D., Holahan C. K. (2003). The relation of social support and coping to positive adaptation to breast cancer. Psychology & Health, 18, 15-29. doi: 10.1080/0887044031000080656 [DOI] [Google Scholar]
- Holmes J., Powell-Griner E., Lethbridge-Cejku M., Heyman K. (2009, July). Aging differently: Physical limitations among adults aged 50 years and over: United States, 2001-2007 (NCHS Data Brief No. 20). Atlanta, GA: Centers for Disease Control. [PubMed] [Google Scholar]
- Houry D., Florence C., Baldwin G., Stevens J., McClure R. (2016). The CDC injury center’s response to the growing public health problem of falls among older adults. American Journal of Lifestyle Medicine, 10, 74-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Jensen G. A., Nerenz D., Tarraf W. (2015). Medicare’s annual wellness visit in a large health care organization: Who is using it? Annals of Internal Medicine, 163, 567-568. [DOI] [PubMed] [Google Scholar]
- Huber M., Knottnerus J. A., Green L., van der Horst H., Jadad A. R., Kromhout D., Smid H. (2011). How should we define health? British Medical Journal, 343, Article d4163. doi: 10.1136/bmj.d4163 [DOI] [PubMed] [Google Scholar]
- Hughes V. A., Frontera W. R., Wood M., Evans W. J., Dallal G. E., Roubenoff R., Singh M. A. F. (2001). Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 56, B209-B217. doi: 10.1093/gerona/56.5.B209 [DOI] [PubMed] [Google Scholar]
- Irvine A. B., Gelatt V. A., Seeley J. R., Macfarlane P., Gau J. M. (2013). Web-based intervention to promote physical activity by sedentary older adults: Randomized controlled trial. Journal of Medical Internet Research, 15(2), e19. doi: 10.2196/jmir.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad A. R., O’Grady L. (2008). How should health be defined? British Medical Journal, 337, Article a2900. doi: 10.1136/bmj.a2900 [DOI] [PubMed] [Google Scholar]
- Jancey J., Howat P., Lee A., Clarke A., Shilton T., Fisher J., Iredell H. (2006). Effective recruitment and retention of older adults in physical activity research: PALS study. American Journal of Health Behavior, 30, 626-635. [DOI] [PubMed] [Google Scholar]
- Joe J., Demiris G. (2013). Older adults and mobile phones for health: A review. Journal of Biomedical Informatics, 46, 947-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. B., Ashurst E. J., Atkey J., Duffy B. (2015). Older people going online: Its value and before-after evaluation of volunteer support. Journal of Medicine Internet Research, 17(5), e122. doi: 10.2196/jmir.3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen M. G., Laessoe U., Hendriksen C., Nielsen O. B. F., Aagaard P. (2012). Efficacy of Nintendo Wii training on mechanical leg muscle function and postural balance in community-dwelling older adults: A randomized controlled trial. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 68, 845-852. doi: 10.1093/gerona/gls222 [DOI] [PubMed] [Google Scholar]
- Kahana E., Kahana B. (1996). Conceptual and empirical advances in understanding aging well through proactive adaptation. In Bengtson V. L. (Ed.), Adulthood and aging: Research on continuities and discontinuities (pp. 18-40). New York, NY: Springer. [Google Scholar]
- Karmali K. N., Davies P., Taylor F., Beswick A., Martin N., Ebrahim S. (2014). Promoting patient uptake and adherence in cardiac rehabilitation. The Cochrane Database of Systematic Reviews, 6, Article CD007131. doi: 10.1002/14651858.CD007131 [DOI] [PubMed] [Google Scholar]
- Keyes C. L. M. (1998). Social well-being. Social Psychology Quarterly, 61, 121-140. [Google Scholar]
- Kindig D., Stoddart G. (2003). What is population health? American Journal of Public Health, 93, 380-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman M. S., Briscoe V. J., Clark N., Florez H., Haas L. B., Halter J. B., Odegard P. S. (2012). Diabetes in older adults. Diabetes Care, 35, 2650-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler W. C., Barrett-Connor E., Fowler S. E., Hamman R. F., Lachin J. M., Walker E. A., Nathan D. M. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine, 346, 393-403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause C. M., Jones C. S., Joyce S., Kuhn M. E., Curtin K., Murphy L. P., Lucas D. R. (2006). The impact of a multidisciplinary, integrated approach on improving the health and quality of care for individuals dealing with multiple chronic conditions. American Journal of Orthopsychiatry, 76, 109-114. [DOI] [PubMed] [Google Scholar]
- LaMantia M. A., Alder C. A., Callahan C. M., Gao S., French D. D., Austrom M. G., Boustani M. A. (2015). The aging brain care medical home: Preliminary data. Journal of the American Geriatrics Society, 63, 1209-1213. doi: 10.1111/jgs.13447 [DOI] [PubMed] [Google Scholar]
- Lavie C. J., Berra K., Arena R. (2013). Formal cardiac rehabilitation and exercise training programs in heart failure: Evidence for substantial clinical benefits. Journal of Cardiopulmonary Rehabilitation and Prevention, 33, 209-211. doi: 10.1097/HCR.0b013e31829f95c9 [DOI] [PubMed] [Google Scholar]
- Lavie C. J., Milani R. V. (2011). Cardiac rehabilitation and exercise training in secondary coronary heart disease prevention. Progress in cardiovascular diseases, 53, 397-403. [DOI] [PubMed] [Google Scholar]
- Levin J. S., Chatters L. M. (1998). Religion, health, and psychological well-being in older adults findings from three national surveys. Journal of Aging and Health, 10, 504-531. [DOI] [PubMed] [Google Scholar]
- Li F., Harmer P., Fisher K. J., McAuley E., Chaumeton N., Eckstrom E., Wilson N. L. (2005). Tai Chi and fall reductions in older adults: A randomized controlled trial. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 60, 187-194. doi: 10.1093/gerona/60.2.187 [DOI] [PubMed] [Google Scholar]
- Liao J., Brunner E. J. (2016). Structural and functional measures of social relationships and quality of life among older adults: Does chronic disease status matter? Quality of Life Research, 25, 153-164. doi: 10.1007/s11136-015-1052-1 [DOI] [PubMed] [Google Scholar]
- Lin E. H., Katon W., Von Korff M., Tang L., Williams J. W., Jr., Kroenke K., Arean P. (2003). Effect of improving depression care on pain and functional outcomes among older adults with arthritis: A randomized controlled trial. The Journal of the American Medical Association, 290, 2428-2429. [DOI] [PubMed] [Google Scholar]
- Lin F. R. (2011). Hearing loss and cognition among older adults in the United States. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 66, 1131-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Thorpe R., Gordon-Salant S., Ferrucci L. (2011). Hearing loss prevalence and risk factors among older adults in the United States. The Journals of Gerontology, Series A: Biological Sciences & Medical Sciences, 66, 582-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Look AHEAD Research Group. (2010). Long term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes: Four year results of the Look AHEAD trial. Archives of Internal Medicine, 170, 1566-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makizako H., Shimada H., Doi T., Tsutsumimoto K., Lee S., Hotta R., Suzuki T. (2015). Cognitive functioning and walking speed in older adults as predictors of limitations in self-reported instrumental activity of daily living: Prospective findings from the Obu study of health promotion for the elderly. International Journal of Environmental Research and Public Health, 12, 3002-3013. doi: 10.3390/ijerph120303002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B.-J., Hauer T., Arena R., Austford L. D., Galbraith P. D., Lewin A. M., Aggarwal S. (2012). Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation, 126, 677-687. doi: 10.1161/CIRCULATIONAHA.111.066738 [DOI] [PubMed] [Google Scholar]
- Martin P., Kelly N., Kahana B., Kahana E., Willcox B. J., Willcox D. C., Poon L. W. (2015). Defining successful aging: A tangible or elusive concept? The Gerontologist, 55, 14-25. doi: 10.1093/geront/gnu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E., Mullen S. P., Szabo A. N., White S. M., Wójcicki T. R., Mailey E. L., Erickson K. (2011). Self-regulatory processes and exercise adherence in older adults: Executive function and self-efficacy effects. American Journal of Preventive Medicine, 41, 284-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall N., Cromwell J. (2011). Results of the Medicare health support disease-management pilot program. New England Journal of Medicine, 365, 1704-1712. doi: 10.1056/NEJMsa1011785 [DOI] [PubMed] [Google Scholar]
- Miller N. A., Kirk A., Kaiser M. J., Glos L. (2014). Disparities in access to health care among middle-aged and older adults with disabilities. Journal of Aging & Social Policy, 26, 324-346. [DOI] [PubMed] [Google Scholar]
- Mirelman A., Herman T., Brozgol M., Dorfman M., Sprecher E., Schweiger A., . . . Hausdorff J. M. (2012). Executive function and falls in older adults: New findings from a five-year prospective study link fall risk to cognition. PLoS ONE, 7, Article e40297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad A. H., Ford E. S., Bowman B. A., Dietz W. H., Vincor F., Bales V. S., Marks J. S. (2003). Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. The Journal of the American Medical Association, 289, 76-79. doi: 10.1001/jama.289.1.76 [DOI] [PubMed] [Google Scholar]
- Montaño D. E., Kasprzyk D. (2008). Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In Glanz K., Rimer B. K., Viswanath K. (Eds.), Health behavior and health education: Theory, research, and practice (4th ed., pp. 67-96). San Francisco, CA: Jossey-Bass. [Google Scholar]
- Morone N. E., Greco C. M. (2007). Mind–body interventions for chronic pain in older adults: A structured review. Pain Medicine, 8, 359-375. [DOI] [PubMed] [Google Scholar]
- Mouton A., Cloes M. (2015). Efficacy of a web-based, center-based or combined physical activity intervention among older adults. Health Education Research, 30, 422-435. doi: 10.1093/her/cyv012 [DOI] [PubMed] [Google Scholar]
- Moyer V. A. (2012). Prevention of falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine, 157, 197-204. [DOI] [PubMed] [Google Scholar]
- National Council on Aging. (2014). Healthy aging: Fact sheet (p. 2). Washington, DC: Author. [Google Scholar]
- Netz Y., Wu M.-J., Becker B. J., Tenenbaum G. (2005). Physical activity and psychological well-being in advanced age: A meta-analysis of intervention studies. Psychology and Aging, 20, 272-284. [DOI] [PubMed] [Google Scholar]
- Oldridge N. (2012). Exercise-based cardiac rehabilitation in patients with coronary heart disease: Meta-analysis outcomes revisited. Future Cardiology, 8, 729-751. [DOI] [PubMed] [Google Scholar]
- Ormel J., Kempen G. I. J. M., Deeg D. J. H., Brilman E. I., van Sonderen E., Relyveld J. (1998). Functioning, well-being, and health perception in late middle-aged and older people: Comparing the effects of depressive symptoms and chronic medical conditions. Journal of the American Geriatric Society, 46, 39-48. doi: 10.1111/j.1532-5415.1998.tb01011.x [DOI] [PubMed] [Google Scholar]
- Ortman J. M., Velkoff V. A., Hogan H. (2014). An aging nation: The older population in the United States. Retrieved from https://www.census.gov/prod/2014pubs/p25-1140.pdf
- Painter J. E., Borba C. P., Hynes M., Mays D., Glanz K. (2008). The use of theory in health behavior research from 2000 to 2005: A systematic review. Annals of Behavioral Medicine, 35, 358-362. [DOI] [PubMed] [Google Scholar]
- Peikes D., Peterson G., Brown R. S., Graff S., Lynch J. P. (2012). How changes in Washington University’s Medicare coordinated care demonstration pilot ultimately achieved savings. Health Affairs, 31, 1216-1226. [DOI] [PubMed] [Google Scholar]
- Penedo F. J., Dahn J. R. (2005). Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry, 18, 189-193. [DOI] [PubMed] [Google Scholar]
- Pimouguet C., Le Goff M., Thiébaut R., Dartigues J. F., Helmer C. (2011). Effectiveness of disease-management programs for improving diabetes care: A meta-analysis. Canadian Medical Association Journal, 183, E115-E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J. O., Velicer W. F. (1997). The transtheoretical model of health behavior change. American Journal of Health Promotion, 12, 38-48. [DOI] [PubMed] [Google Scholar]
- Pruchno R. A., Wilson-Genderson M., Cartwright F. (2010). A two-factor model of successful aging. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences, 65, 671-679. doi: 10.1093/geronb/gbq051 [DOI] [PubMed] [Google Scholar]
- Reginster J. Y. (2002). The prevalence and burden of arthritis. Rheumatology, 41, 3-6. [PubMed] [Google Scholar]
- Rejeski W. J., Mihalko S. L., Ambrosius W. T., Bearon L. B., McClelland J. W. (2011). Weight loss and self-regulatory eating efficacy in older adults: The cooperative lifestyle intervention program. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences, 66, 279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook K. S. (1984). The negative side of social interaction: Impact on psychological well-being. Journal of Personality and Social Psychology, 46, 1097-1108. [DOI] [PubMed] [Google Scholar]
- Rooks R. N., Simonsick E. M., Klesges L. M., Newman A. B., Ayonayon N. H., Harris T. B. (2008). Racial disparities in health care access and cardiovascular disease indicators in Black and White older adults in the Health ABC Study. Journal of Aging and Health, 20, 599-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheck R. (2008). Fast food consumption and increased caloric intake: A systematic review of a trajectory towards weight gain and obesity risk. Obesity Reviews, 9, 535-547. [DOI] [PubMed] [Google Scholar]
- Rosenstock I. M. (1974). Historical origins of the Health Belief Model. Health Education & Behavior, 2, 328-335. [Google Scholar]
- Rosland A.-M., Kieffer E., Spencer M., Sinco B., Palmisano G., Valerio M., Heisler M. (2015). Do pre-existing diabetes social support or depressive symptoms influence the effectiveness of a diabetes management intervention? Patient Education & Counseling, 98, 1402-1409. doi: 10.1016/j.pec.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J. W., Kahn R. L. (1987). Human aging: Usual and successful. Science, 237, 143-149. [DOI] [PubMed] [Google Scholar]
- Rowe J. W., Kahn R. L. (1997). Successful aging. The Gerontologist, 37, 433-440. [DOI] [PubMed] [Google Scholar]
- Rubenstein L. Z. (2006). Falls in older people: Epidemiology, risk factors and strategies for prevention. Age and Ageing, 35, ii37-ii41. [DOI] [PubMed] [Google Scholar]
- Ryff C. D. (1995). Psychological well-being in adult life. Current Directions in Psychological Science, 4, 99-104. doi: 10.1111/1467-8721.ep10772395 [DOI] [Google Scholar]
- Ryff C. D. (2014). Psychological well-being revisited: Advances in the science and practice of eudaimonia. Psychotherapy and Psychosomatics, 83, 10-28. doi: 10.1159/000353263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff C. D., Keyes C. L. (1995). The structure of psychological well-being revisited. Journal of Personality and Social Psychology, 69, 719-727. doi: 10.1037/0022-3514.69.4.719 [DOI] [PubMed] [Google Scholar]
- Ryff C. D., Singer B. H., Love G. D. (2004). Positive health: Connecting well-being with biology. Philosophical Transactions-Royal Society of London, Series B: Biological Sciences, 359, 1383-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis J. F., Owen N., Fisher E. B. (2008). Ecological models of health behavior. In Glanz K., Rimer B. K., Viswanath K. (Eds.), Health behavior and health education: Theory, research, and practice (4th ed., pp. 465-486). San Francisco, CA: Jossey-Bass. [Google Scholar]
- Savard L. A., Thompson D. R., Clark A. M. (2011). A meta-review of evidence on heart failure disease management programs: The challenges of describing and synthesizing evidence on complex interventions. Trials, 12, Article 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. M., Gopinath B., McMahon C. M., Leeder S. R., Mitchell P., Wang J. J. (2011). Dual sensory impairment in older age. Journal of Aging and Health, 23, 1309-1324. [DOI] [PubMed] [Google Scholar]
- Schore J. L., Brown R. S., Cheh V. A. (1998). Case management for high-cost Medicare beneficiaries. Health Care Financing Review, 20, 87-101. [PMC free article] [PubMed] [Google Scholar]
- Schwarzer R., Renner B. (2000). Social-cognitive predictors of health behavior: Action self-efficacy and coping self-efficacy. Health Psychology, 19, 487-495. [PubMed] [Google Scholar]
- Shapira N., Barak A., Gal I. (2007). Promoting older adults’ well-being through Internet training and use. Aging & Mental Health, 11, 477-484. [DOI] [PubMed] [Google Scholar]
- Shelton P. S. (2002). Disease management programs. Disease Management & Health Outcomes, 10, 461-467. [Google Scholar]
- Shepherd C. W., While A. E. (2012). Cardiac rehabilitation and quality of life: A systematic review. International Journal of Nursing Studies, 49, 755-771. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A., Baldwin M., Polissar N. L., Gruber W. (1997). Predicting the probability for falls in community-dwelling older adults. Physical Therapy, 77, 812-819. [DOI] [PubMed] [Google Scholar]
- Singh A., Misra N. (2009). Loneliness, depression and sociability in old age. Industrial Psychiatry Journal, 18, 51-55. doi: 10.4103/0972-6748.57861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M. (2012). Toward a better understanding of loneliness in community-dwelling older adults. The Journal of Psychology: Interdisciplinary and Applied, 146, 293-311. doi: 10.1080/00223980.2011.602132 [DOI] [PubMed] [Google Scholar]
- Stefanacci R. G., Reich S., Casiano A. (2015). Application of PACE principles for population health management of frail older adults. Population Health Management, 18, 367-372. doi: 10.1089/pop.2014.0096 [DOI] [PubMed] [Google Scholar]
- Stellefson M., Chaney B., Barry A. E., Chavarria E., Tennant B., Walsh-Childers K., Zagora J. (2013). Web 2.0 chronic disease self-management for older adults: A systematic review. Journal of Medical Internet Research, 15(2), e35. doi: 10.2196/jmir.2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellefson M., Dipnarine K., Stopka C. (2013). Peer reviewed: The chronic care model and diabetes management in US primary care settings: A systematic review. Preventing Chronic Disease, 10, Article E26. doi: 10.5888/pcd10.120180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A., Deaton A., Stone A. A. (2015). Subjective wellbeing, health, and ageing. The Lancet, 385, 640-648. doi: 10.1016/s0140-6736(13)61489-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street R. J., Gold W. R., Manning T. R. (1997). Health promotion and interactive technology: Theoretical applications and future directions. Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Svetkey L. P., Stevens V. J., Brantley P. J., Appel L. J., Hollis J. F., Loria C. M., Smith P. (2008). Comparison of strategies for sustaining weight loss: The weight loss maintenance randomized controlled trial. The Journal of the American Medical Association, 299, 1139-1148. [DOI] [PubMed] [Google Scholar]
- Taylor-Davis S., Smiciklas-Wright H., Davis A. C., Jensen G. L., Mitchell D. C. (1998). Time and cost for recruiting older adults. Journal of the American Geriatrics Society, 46, 753-757. [DOI] [PubMed] [Google Scholar]
- Thoits P. A. (2011). Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior, 52, 145-161. doi: 10.1177/0022146510395592 [DOI] [PubMed] [Google Scholar]
- Tkatch R., Artinian N. T., Abrams J., Mahn J. R., Franks M. M., Keteyian S. J., Schwartz S. (2011). Social network and health outcomes among African American cardiac rehabilitation patients. Heart & Lung: The Journal of Acute and Critical Care, 40, 193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco A. C., Ivers N. M., Grimshaw J. M., Moher D., Turner L., Galipeau J., Manns B. (2012). Effectiveness of quality improvement strategies on the management of diabetes: A systematic review and meta-analysis. The Lancet, 379, 2252-2261. [DOI] [PubMed] [Google Scholar]
- Troiano R. P., Berrigan D., Dodd K. W., Masse L. C., Tilert T., McDowell M. (2008). Physical activity in the United States measured by accelerometer. Medicine & Science in Sports & Exercise, 40, 181-188. [DOI] [PubMed] [Google Scholar]
- Van Hoecke A. S., Delecluse C., Bogaerts A., Boen F. (2014). Effect of need-supportive physical activity counseling on well-being: A 2-year follow up among sedentary older adults. Journal of Physical Activity & Health, 11, 1492-1502. [DOI] [PubMed] [Google Scholar]
- Villareal D. T., Apovian C. M., Kushner R. F., Klein S. (2005). Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obesity Research, 13, 1849-1863. [DOI] [PubMed] [Google Scholar]
- Villareal D. T., Chode S., Parimi N., Sinacore D. R., Hilton T., Armamento-Villareal R., Shah K. (2011). Weight loss, exercise, or both and physical function in obese older adults. New England Journal of Medicine, 364, 1218-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D. E., Nicol C. W., Bredin S. S. (2006). Health benefits of physical activity: The evidence. Canadian Medical Association Journal, 174, 801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. W., Schiller J. S. (2013). Prevalence of multiple chronic conditions among US adults: Estimates from the National Health Survey 2010. Preventing Chronic Disease, 10, Article 120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareham N. J., van Sluijs E. M., Ekelund U. (2005). Physical activity and obesity prevention: A review of the current evidence. Proceedings of the Nutrition Society, 64, 229-247. [DOI] [PubMed] [Google Scholar]
- Witham M. D., Avenell A. (2010). Interventions to achieve long-term weight loss in obese older people A systematic review and meta-analysis. Age and Ageing, 39, 176-184. [DOI] [PubMed] [Google Scholar]
- Woodgate J., Brawley L. R., Shields C. A. (2007). Social support in cardiac rehabilitation exercise maintenance: Associations with self-efficacy and health-related quality of life. Journal of Applied Social Psychology, 37, 1041-1059. doi: 10.1111/j.1559-1816.2007.00198.x [DOI] [Google Scholar]
- World Health Organization. (2006). Constitution of the World Health Organization, 45th Edition, 2006. (pp. 1-18). Author; Retrieved from www.who.int/governance/eb/who_constitution_en.pdf [Google Scholar]
- Yu C.-M., Lau C.-P., Chau J., McGhee S., Kong S.-L., Cheung B. M.-Y., Li L. S.-W. (2004). A short course of cardiac rehabilitation program is highly cost effective in improving long-term quality of life in patients with recent myocardial infarction or percutaneous coronary intervention. Archives of Physical Medicine and Rehabilitation, 85, 1915-1922. [DOI] [PubMed] [Google Scholar]
- Zbikowski S. M., Jack L. M., McClure J. B., Deprey M. S., Javitz H. S., McAfee T. A., Swan G. E. (2011). Utilization of services in a randomized trial testing phone and web-based interventions for smoking cessation. Nicotine & Tobacco Research, 13, 319-327. doi: 10.1093/ntr/ntq257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbikowski S. M., Magnusson B., Pockey J. R., Tindle H. A., Weaver K. E. (2012). A review of smoking cessation interventions for smokers aged 50 and older. Maturitas, 71, 131-141. [DOI] [PubMed] [Google Scholar]
- Zickuhr K., Madden M. (2012). Older adults and internet use. Washington, DC: Pew Internet & American Life Project. [Google Scholar]
- Zulman D. M., Ezeji-Okoye S. C., Shaw J. G., Hummel D. L., Holloway K. S., Smither S. F., . . . Asch S. M. (2014). Partnered research in healthcare delivery redesign for high-need, high-cost patients: Development and feasibility of an Intensive Management Patient-Aligned Care Team (ImPACT). Journal of General Internal Medicine, 29, 861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]


