Abstract
Two type I DnaJ homologs DjA1 (DNAJA1; dj2, HSDJ/hdj-2, rdj1) and DjA2 (DNAJA2; dj3, rdj2) work similarly as a cochaperone of Hsp70s in protein folding and mitochondrial protein import in vitro. To study the in vivo role of DjA1, we generated DjA1-mutant mice. Surprisingly, loss of DjA1 in mice led to severe defects in spermatogenesis that involve aberrant androgen signaling. Transplantation experiments with green fluorescent protein-labeled spermatogonia into DjA1−/− mice revealed a primary defect of Sertoli cells in maintaining spermiogenesis at steps 8 and 9. In Sertoli cells of DjA1−/− mice, the androgen receptor markedly accumulated with enhanced transcription of several androgen-responsive genes, including Pem and testin. Disruption of Sertoli–germ cell adherens junctions was also evident in DjA1−/− mice. Experiments with DjA1−/− fibroblasts and primary Sertoli cells indicated aberrant androgen receptor signaling. These results revealed a critical role of DjA1 in spermiogenesis and suggest that DjA1 and DjA2 are not functionally equivalent in vivo.
Keywords: androgen receptor, chaperone, DnaJ, Sertoli cell, spermiogenesis
Introduction
The molecular chaperones are a group of proteins that mediate folding of de novo-synthesized proteins, refolding of misfolded proteins, translocation of proteins into organelles, assembly of proteins, and their degradation (Gething and Sambrook, 1992; Bukau and Horwich, 1998; Frydman, 2001; Hartl and Hayer-Hartl, 2002). Among groups of molecular chaperones, members of Hsp70 chaperone proteins are engaged in all these biological processes with the aid of DnaJ homologs. Three major members of the Hsp70 family are present in the mammalian cytosol: constitutive Hsc70, inducible Hsp70, and testis-specific Hsp70-2. Over 40 members of DnaJs present in mammals are classified into three types according to their structural features (Ohtsuka and Hata, 2000). We have focused on cochaperone activities of the most authentic type of DnaJ homologs (type I or subfamily A). Four members of mammalian type I DnaJs are DjA1 (DNAJA1, HomoloGene #20385) and DjA2 (DNAJA2, #21193) in cytosol, DjA3 (DNAJA3, #36170) in mitochondria, and DjA4 (DNAJA4, #23110) in testis cytosol.
All members of DnaJ contain J domain. A conserved sequence motif (HPD) in the J domain accelerates ATPase activity of Hsp70, and ADP-bound state of Hsp70 tightly binds polypeptide substrate (Bukau and Horwich, 1998). This ATPase cycle of Hsp70 with productive substrate binding is a key to the chaperone function of Hsp70. In addition to binding to Hsp70, DnaJ itself associates with peptide substrate by a domain distinct from the J domain. Recently, a structure of a model polypeptide substrate in complex with the substrate-binding domain of a type I DnaJ homolog was reported (Li et al, 2003).
As a biological source of molecular chaperones, rabbit reticulocyte lysate has been used for various in vitro studies including protein translocation, folding, assembly, and degradation. Using this chaperone source, we identified members of the Hsc70-based chaperone system that are necessary for protein import into mitochondria and refolding of denatured proteins (Terada et al, 1997; Terada and Mori, 2000). Chaperone activities required for mitochondrial protein import and protein refolding were abolished by either single depletion of Hsc70 or simultaneous depletion of DjA1 and DjA2, but not by depletion of DjB1 (DNAJB1, HomoloGene #5105). We further analyzed folding activity using purified Hsc70, Hsp70, and Hsp70-2, and found that these three Hsp70s are essentially similar in folding activity, but require specific type I DnaJ homologs. Potent folding activities are found in Hsp70s–DjA1 and Hsp70s–DjA2 pairs, but not in Hsp70s–DjA4 nor Hsp70s–DjB1 pair (Hafizur et al, 2004). These in vitro results demonstrate that DjA1 and DjA2 are functionally equivalent and act as equally potent cochaperones of the Hsp70s.
The biological action of androgens is mediated through the androgen receptor (AR). AR is a member of the steroid receptor family and mediates differential regulation of target genes to elicit a variety of physiological processes, such as male sexual differentiation and maturation, and maintenance of spermatogenesis (Nieschlag and Behre, 1997). For the maintenance of spermatogenesis, dual actions of follicle stimulating hormone (FSH) and intratesticular testosterone are dominant. These hormones bind to their respective receptors, FSH-R and AR in Sertoli cells, and modulate Sertoli cell function in a cyclical manner. FSH binding to Sertoli cells ultimately activates transcription factors, such as cAMP response element-binding protein (CREB) (Don and Stelzer, 2002). CREB is highly active at stages II–V and AR at stages VII and VIII, and these factors promote stage-specific and germ cell-dependent regulation of gene expression in Sertoli cells (Don and Stelzer, 2002; De Gendt et al, 2004).
In the present study, we generated DjA1-mutant mice to study the functional role of DjA1. Although DjA2 was expected to compensate for loss of DjA1, the DjA1-null mice were defective in late stages of spermatogenesis. Transplantation experiments revealed a critical defect of Sertoli cells in maintaining spermiogenesis at steps 8 and 9. In DjA1−/− Sertoli cells, the amount of AR was increased, transcription of several androgen-responsive genes was enhanced, and Sertoli–germ cell contact was disrupted. These results reveal a critical role of DjA1 in spermiogenesis through AR-mediated signaling in Sertoli cells.
Results
Genomic organization and targeting of the mouse DjA1 gene
As shown in Figure 1A, the mouse DjA1 gene is 12 kb with nine exons (accession # AB183426). Proximate to a putative transcription start site, a typical heat shock element (GAAnnTTCnnGAA) is present. As in the case of the human DjA1 mRNA species (Terada and Mori, 2000), species of 1.6 and 2.4 kb were detected in mouse (Figure 1B). We assumed that these two species are derived from two poly(A) signal sequences in exon 9 from EST database search (data not shown). The predicted sizes from a putative transcription start site (accession # AF055664) are 1448 and 2242 nt, respectively. Thus, the two mRNA species differ in length of the 3′-untranslated region.
Figure 1.
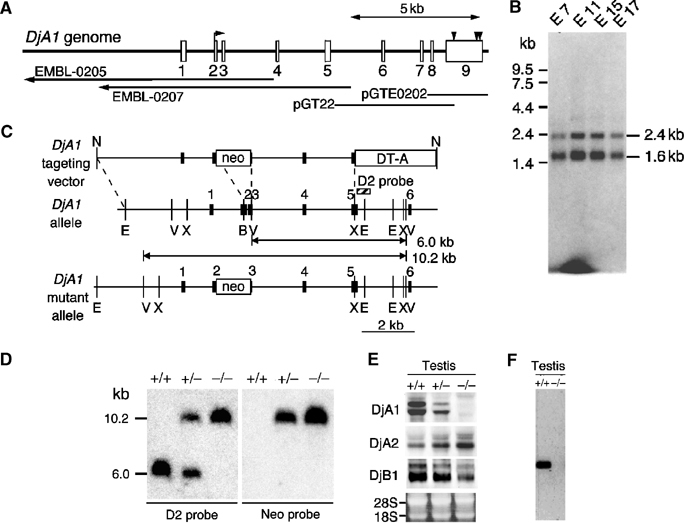
Genomic structure of mouse DjA1 and generation of DjA1-mutant mouse. (A) Schematic diagram of DjA1 gene from C57BL/6 mouse. Exons are indicated by rectangles. An arrow in exon 2 indicates the initial methionine. Arrowheads in exon 9 represent positions of putative poly(A) signal sequences. Relative positions of phage clones (EMBL-0205 and -0207) and plasmid clones (pGT22 and pGTE0202) are indicated by lines (accession # AB183426). (B) RNA blot analysis for DjA1. A filter with mouse embryonic stage poly(A)+ RNAs (Clontech) was hybridized, using as probes 32P-labeled mouse DjA1 cDNA. (C) Targeting vector for DjA1 mutation. Exons 1–6 are indicated by filled rectangles. The predicted sizes of normal and targeted alleles and the location of D2 probe used in Southern blot analysis (D) are shown. B, BsaBI; E, EcoRI; N, NotI; V, EcoRV; X, BstXI. (D) Southern blot analysis by EcoRV digestion of genomic DNAs (10 μg each). (E) Northern blot analysis of DjA1 from testis. Total RNA samples were analyzed using digoxigenin-labeled cRNA probes. Integrity of the RNA samples was verified using ethidium bromide staining. (F) Western blot analysis of DjA1 from testis.
A null allele of the DjA1 gene in an embryonic stem (ES) cell line was generated with a positive/negative-targeting vector (Figure 1C). Insertion of the neomycin phosphotransferase gene (neo) results in deletion of most of the J domain and the G/F-rich domain. The J domain is essential for interaction with members of Hsp70 family proteins (Bukau and Horwich, 1998). The vector was introduced into the TT2 ES cells, and 28 homologous recombinant clones among 96 G418-resistant clones were isolated. Two independent clones were injected into ICR eight-cell-stage embryos to generate chimeric mice. Male chimeras were mated with C57BL/6 females to generate DjA1+/− mice. The DjA1+/− mice had no obvious defect and were interbred to generate DjA1−/− mice. Southern blot analysis confirmed the presence of DjA1−/−, DjA1+/− and wild-type offspring (Figure 1D). DjA1−/− mice were born at a slightly lower Mendelian ratio than expected (data not shown). The DjA1−/− mice lived for over 1 year. The absence of DjA1 mRNA and protein in DjA1−/− mice was confirmed (Figure 1E and F). As a control, we checked expression of DjA2 and found that DjA2 mRNA was increased in DjA1−/− testis by about 2.4-fold, whereas DjB1 mRNA was decreased by about 2.2-fold.
Phenotypic abnormalities of male DjA1−/− mouse
The DjA1−/− mice apparently grew normally as did their DjA1+/− littermates. However, average weights of male DjA1−/− mice were always less than those of male DjA1+/− littermates (Figure 2A and B). In contrast, the growth curves for female DjA1−/− and DjA1+/− mice were virtually identical. Interestingly, the curve for male DjA1−/− mice coincides well with that for female littermates.
Figure 2.
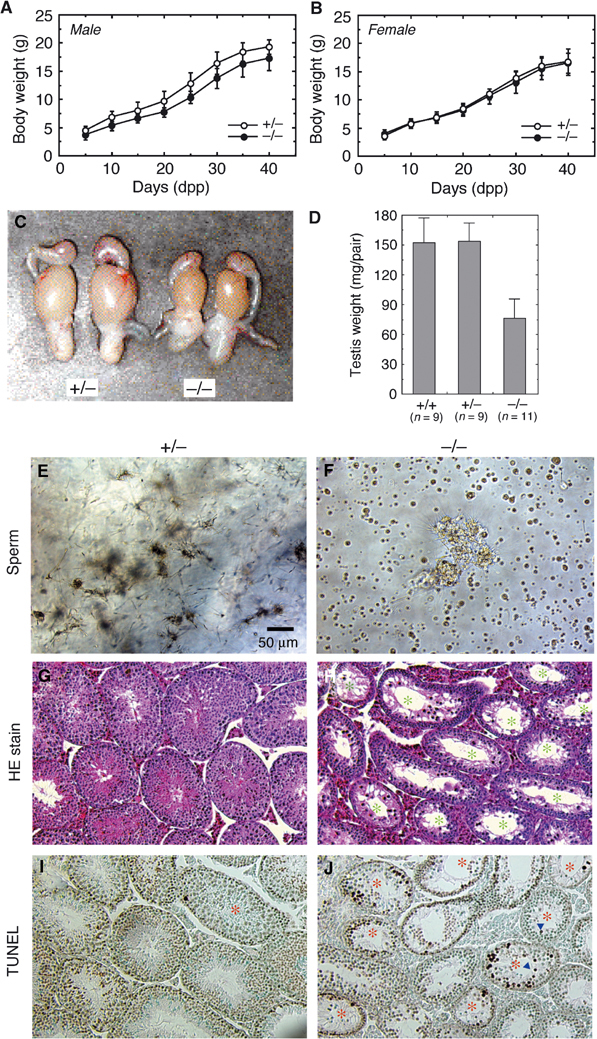
Phenotypic abnormalities of DjA−/− male mice. (A, B) Growth curves for male (A) and female (B) DjA1-mutant mice (n=15 for each genotype of each sex). Littermates of the DjA1+/+ mice showed virtually identical curves to DjA1+/− mice. (C) Gross anatomy of male genital tracts from DjA1+/− and DjA1−/− mice (4 months of age). Adipose tissues were removed from the tracts. (D) Testis weight of each phenotype at 4 months of age. (E, F) Spermatozoa from cauda epididymis of the DjA1+/− (E) and DjA1−/− (F) mice. (G, H) Hematoxylin–eosin-stained sections of DjA1+/− (G) and DjA1−/− (H) testes. Atrophied tubules are marked with asterisks. (I, J) Apoptotic cells in DjA1+/− (I) and DjA1−/− (J) testes. Apoptotic cells were labeled in situ using the TUNEL method and visualized using 3,3′-diaminobenzidine as a substrate. Sections were counterstained with methyl green. Tubules with TUNEL-positive cells are marked with asterisks. Arrowheads indicate apoptotic cells that lost contact with Sertoli cells. Bar=50 μm (E–J).
When male DjA1−/− mice were mated with females, there was a marked reduction in their fertility (Table I). Copulatory behavior in DjA1−/− males was normal, but DjA1−/− mature males showed grossly smaller sizes of testis and seminal vesicle (Figure 2C and D; data not shown). The DjA1−/− males had normal urogenital tracts and descended testes. Serum level of FSH was slightly higher (DjA1−/−, 55.9±8.39 ng/ml; DjA1+/−, 42.5±11.1 ng/ml), and that of testosterone in DjA1−/− was within the range in DjA1+/− (0.3–8.7 ng/ml).
Table 1.
Fertility of male DjA1-mutant mice
| Fertilitya (%) |
||
|---|---|---|
| Genotype | DjA1−/− female | DjA1+/− female |
| DjA1−/− male | 6.9 (2/29) | 8.1 (3/37) |
|
DjA1+/− male |
90 (28/31) |
100 (21/21) |
| aEach genotype of male mice (3 months of age) was mated with the indicated genotype of females for 8 weeks (1–3 females per male). Percentage of fertility and numbers of male mice mated are shown. | ||
In the DjA1−/− mature males, there was a drastic reduction in the number of spermatozoa from cauda epididymis (Figure 2E and F). Number of spermatozoa in DjA1−/− male was decreased to about 7% of that in DjA1+/− male, and >95% of spermatozoa from DjA1−/− male lost their motility (n=3). Much cell debris was also present in epididymis fluid. It should be noted that a small population of DjA1−/− males (<9%) produce considerable numbers of normal spermatozoa, as these males retain fertility (Table I). In contrast, fertility of female DjA1−/− mice showed little difference from that of female DjA1+/− animals in terms of days required for primiparity and numbers of pups per litter (data not shown).
Defects of spermatogenesis in DjA1−/− mice
The DjA1−/− male mice had impairment of spermatogenesis in over 50% of the seminiferous tubules (Figure 2G and H). The degree of damage ranged from tubules with intraepithelial vacuoles of varying sizes to near atrophied tubules consisting of Sertoli cells and spermatogonia. Elongated spermatids were rarely present in DjA1−/− tubules. Apoptotic spermatocytes were present in 58% of DjA1−/− tubules, whereas few were detected in DjA1+/− tubules (Figure 2I and J). ATP level was similar in both DjA1−/− and DjA1+/− testes (about 3.0 μmol/g tissue, n=3). Many apoptotic spermatocytes in the lumen of tubules might be sloughed off and drained away into the epididymis (Figure 2F). The apoptotic cells in the lumen frequently lost contact with Sertoli cells. In juvenile DjA1−/− mice, the peak of spermatocyte apoptosis during the first wave of spermatogenesis was delayed by several days (data not shown).
Expression of type 1 DnaJ proteins in testis
We reported that three kinds of type I DnaJs (DjA1, DjA2, and DjA4) are strongly expressed in testis (Abdul et al, 2002). Immunohistochemistry showed that DjA1 is expressed prominently in primary and secondary spermatocytes (Figure 3A). In contrast, spermatogonia, Sertoli cells, and Leydig cells were less positive. The signals found in the basement membrane of tubules and in restricted portions of interstitial areas are apparently nonspecific, because they were also detected in the DjA1−/− section (Figure 3B). Results of in situ hybridization and Northern blot analyses coincided well with that of immunohistochemical analysis (Figure 3C–E).
Figure 3.
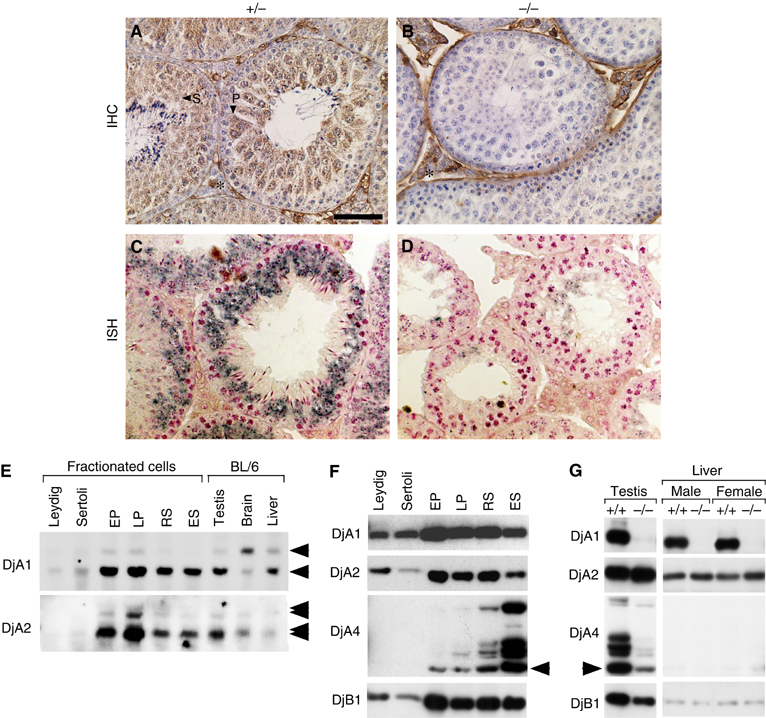
High expression of type I DnaJs in testis and increased expression of DjA2 in DjA1−/− mice. (A, B) Sections of DjA1+/− and DjA1−/− testes were mounted on the same slide glass, and decorated with a mAb against DjA1. Peroxidase activity was visualized using 3,3′-diaminobenzidine as a chromogen. Sections were counterstained with hematoxylin. Signals of DjA1 in primary and secondary spermatocytes are indicated with P and S, respectively (A). Nonspecific signals in the interstitial area are indicated with asterisks (A, B). (C, D) In situ hybridization of DjA1 mRNA from DjA1+/− and DjA1−/− testes was carried out using digoxigenin-labeled DjA1 probe. Alkaline phosphatase activity was visualized using 5-bromo-4-chloro-3-indolyl phosphate-nitro blue tetrazolium as a chromogen. Sections were counterstained with nuclear fast red. (E) Total RNA samples from fractionated testicular cells and mouse tissues (5 μg each) were subjected to Northern blot analysis, using digoxigenin-labeled DjA1 and DjA2 cRNAs as probes. Arrowheads indicate positions of 2.4 and 1.6 kb bands (DjA1) and 2.1, 2.2, 3.1 and 4.0 kb bands (DjA2). Protein samples of testicular cells (F) or tissues (G) were subjected to Western blot analysis using a monoclonal anti-DjA1, or polyclonal anti-DjA2 or anti-DjB1 antibodies (0.4 μg/ml each), or antiserum against DjA4 (1/1000 dilution). An arrowhead indicates position of expected size of DjA4. EP and LP, early and late stage pachytene spermatocytes; RS and ES, round and elongated spermatids. Bar=50 μm (A–D).
In Northern blots, strong expression of DjA1 mRNA was observed in spermatocytes (Figure 3E). The strongest signal was found in the pachytene stage. Expression in testicular somatic cells was much lower than those in spermatocytes, brain, and liver. Expression of DjA2 mRNA was similar to that of DjA1 mRNA.
The results of Western blot analysis accorded well with those of Northern blot. DjA1 and DjA2 proteins are expressed most strongly at the pachytene stage and less strongly in somatic cells (Figure 3F). The level of DjA2 protein was lowest in Sertoli cells. Expression pattern of DjB1 protein was similar to that of DjA2. DjA4 protein of 46 kDa was present in germ cells and was expressed more strongly as the spermatogenesis proceeded. Additional larger species were also increased along with spermiogenesis. Expression pattern of DjA4 protein accorded well with that of immunohistochemical analysis (Hafizur et al, 2004). At least four extra species of DjA4 protein were present (49–66 kDa). We found additional robust expression of 1.8 kb mRNA species in the testis as well as the ordinary 3.3 kb species (Abdul et al, 2002).
Expression of DnaJ proteins was compared in testis and liver (Figure 3G). As expected from mRNA analysis (Figure 1E), expression of DjA2 protein was slightly increased in DjA1−/− mice, whereas that of DjB1 was decreased. Consistent with marked decrease in the number of elongated spermatids, DjA4 protein was decreased in DjA1−/− testis. DjA4 was not detected in the liver.
Defect of spermiogenesis in recipient DjA1−/− mouse
To clarify the capacity of the microenvironment for supporting germ cell differentiation in the DjA1−/− mouse, we transplanted green fluorescent protein (GFP)-expressing spermatogonia from transgenic mice into the seminiferous tubules of DjA1−/− mice, and 10 weeks later, GFP-expressing spermatogonia had settled well in the tubules of both DjA1−/− and DjA1+/− mice (Figure 4A and B).
Figure 4.
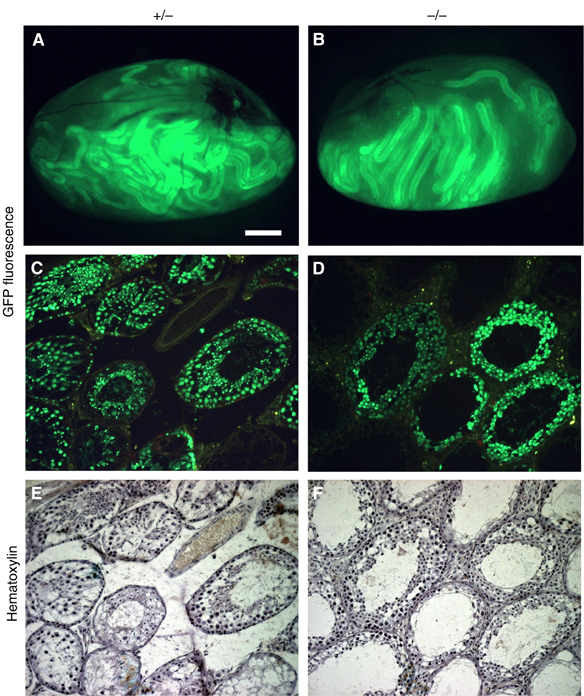
Defect of spermiogenesis in DjA1−/− seminiferous tubules. GFP-labeled spermatogonia from GFP-transgenic mice were transplanted into seminiferous tubules of DjA1+/− and DjA1−/− mice (n=2 and 4, respectively). (A, B) Observation of whole testes under a fluorescent stereomicroscope at 2 months after transplantation. Representative sections under fluorescent microscope (C, D) and the same sections stained with hematoxylin (E, F). Bar=1 mm (A, B) and 75 μm (C–F).
Histological evaluation of the cross-sections of seminiferous tubules revealed spermatogenesis of the transplanted spermatogonia (Figure 4C–F). However, elongated spermatids were absent in DjA1−/− testes, while fully elongated spermatids were present in DjA1+/− testes. The round spermatids present in the transplanted DjA1−/− tubules were at steps 8 and 9, according to the living cell map that permits accurate identification of male germ cells with GFP fluorescence (Ventela et al, 2002). We conclude that DjA1−/− testis has a prominent defect in spermiogenesis, and that DjA1−/− Sertoli cells cannot support round spermatid differentiation.
Morphology of supporting cells in DjA1−/− seminiferous tubules
Testosterone is the critical hormone in spermiogenesis at steps 7 and 8 (Lindsey and Wilkinson, 1996). The action of androgens involves gene regulation in Sertoli cells through AR (De Gendt et al, 2004). In DjA1+/− testis, positive immunostaining of AR was observed in nuclei of somatic cells, such as Leydig cells, smooth muscle cells surrounding blood vessels, peritubular myoid cells, and Sertoli cells (Figure 5A and C). In Sertoli cells, the signal intensity varied depending on stages of spermatogenesis. The strongest signal of Sertoli cell nuclei was observed in tubules at stage VII, as documented (Bremner et al, 1994; Vornberger et al, 1994). In DjA1−/− testis, AR signal was present in nuclei of somatic cells (Figure 5B and D). The signal in nuclei indicates the presence of transcriptionally active AR. However, Sertoli cell nuclei of DjA1−/− were stained more strongly than those of DjA1+/−. Furthermore, most nuclei of DjA1−/− Sertoli cells became round, lost contact with basement membrane, and dislocated toward lumen of the seminiferous tubule. The mean distance between the center of Sertoli cell and the basement membrane in DjA1+/− was 2.8±1.1 μm, whereas that in DjA1−/− mice was 7.2±3.4 μm (n=70). Prominent increase of AR protein and slight increase of glucocorticoid receptor (GR) in DjA1−/− testis was observed (Figure 5E). Similar increase of these two receptors in prostate was observed.
Figure 5.
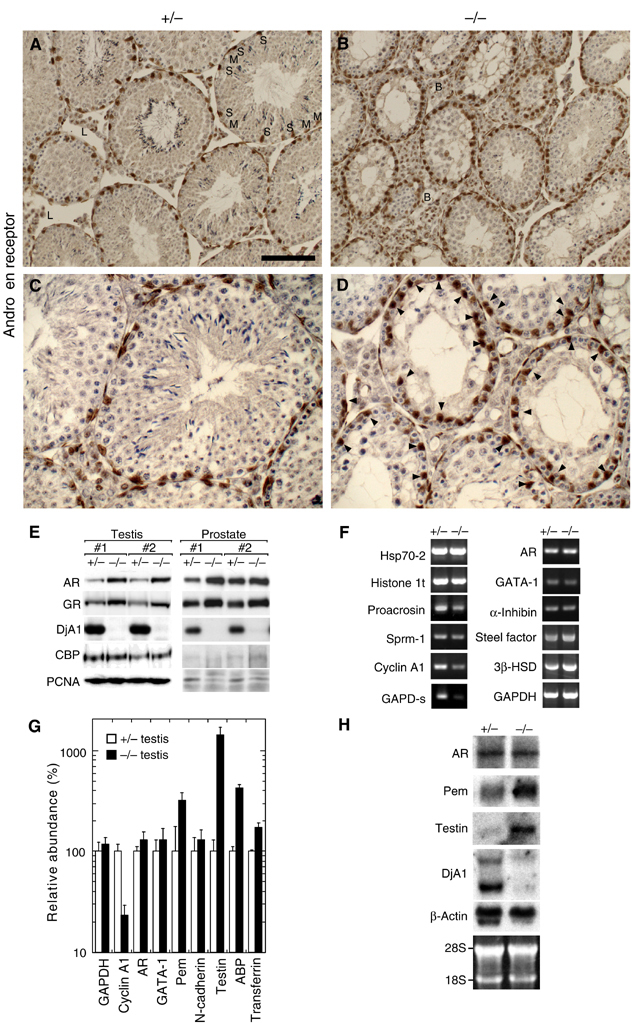
Aberrant functions of Sertoli cell as revealed by dislocated nuclei, accumulation of AR protein without mRNA increase, and induction of testosterone-responsive genes. Sections of DjA1+/− (A, C) and DjA1−/− (B, D) testes on the same slide glass were probed with AR antibody. Signals were visualized using 3,3′-diaminobenzidine as a chromogen. Sections were counterstained with hematoxylin. Some AR-positive cells in (A, B) are marked with capital letters. Strong AR signals in Sertoli cell nuclei are indicated with arrowheads (D). B, smooth muscle cell surrounding blood vessel; M, myoid cell; L, Leydig cell; S, Sertoli cell. (E) Western blot analysis of total protein from testis and prostate. Protein samples were prepared under denaturing conditions from two pairs of littermates (3 months of age). The respective pairs of mice were derived from independent ES strains. (F) Semiquantitative PCR analysis. Total RNAs were isolated from the testes of littermates (4 months of age). (G) Real-time PCR analysis. Values were normalized for levels of β-actin expression (n=5 pairs of littermates), and mean value for respective gene expression for DjA1+/− mice was set as 100%. (H) Northern blot analysis of Pem and testin mRNAs. Total RNA samples were analyzed using 32P-labeled probes. Bar=100 μm (A, B) and 50 μm (C, D).
Increased transcription of androgen-responsive genes in DjA1−/− testis without enhanced AR transcription
To determine the extent of germ cell development in DjA1−/− mice, we next examined the transcription of genes known to be developmentally expressed in spermatogenic cells. Hsp70-2, a member of the Hsp70 family was found to be expressed at the first wave of spermatogenesis and present from leptotene/zygotene spermatocytes (Dix et al, 1997). Semiquantitative RT–PCR analysis demonstrated that the transcript is present in DjA1−/− testis (Figure 5F). Histone H1t and proacrosin mRNAs are known to be expressed in mid-pachytene spermatocytes, and Sprm-1 and cyclin A1 mRNAs at the end of prophase of meiosis I (Dix et al, 1997). Amounts of these marker mRNAs are decreased in DjA1−/− testis. Real-time PCR analysis also confirmed the decreased amount of cyclin A1 mRNA (Figure 5G), the amount in DjA1−/− testis being 25% of that in DjA1+/− testis. Expression of the Gapd-s gene begins in spermatids. In accord with the decreased number of spermatids in DjA1−/− tubules, transcript from the Gapd-s gene was practically absent in DjA1−/− tubules.
We next investigated transcription of genes expressed in Sertoli cells. Strikingly, expression of the AR gene was similar in DjA1+/− and DjA1−/− testis (Figure 5G and H). The discrepancy between mRNA and protein levels suggests post-transcriptional events that lead to accumulation of AR protein. Expression of the genes for GATA-1, α-inhibin, and Steel factor, which are specific to Sertoli cells, and that for 3β-HSD, which is specific to Leydig cells (Walther et al, 1996), was similar in DjA1−/− and DjA1+/− mice.
Since Sertoli cell dysfunction was suspected to be a defect in spermiogenesis and since the amount of AR protein was unexpectedly and markedly increased in DjA1−/− mice, we further analyzed several genes specifically expressed in Sertoli cells. An orphan homeobox gene, Pem, is preferentially expressed in stages VII and VIII (Lindsey and Wilkinson, 1996). Coincident with an increased amount of AR protein in nuclei of DjA1−/− Sertoli cells, Pem expression was increased 3.2-fold in DjA1−/− mice. The androgen-responsive sequence in the Pem promoter region has been identified (Rao et al, 2003), and more recently, Pem expression was found to be severely decreased in mice with a selective knockout of the AR gene in Sertoli cells (De Gendt et al, 2004). These results suggests that the increased amount of AR in DjA1−/− Sertoli cells directly enhances the transcription of Pem. Expression of another androgen-responsive gene, testin, was increased 10-fold in DjA1−/− mice. Testin is a Sertoli cell secretory product, which preferentially localizes on the Sertoli cell side of the ectoplasmic specialization (a modified adherens junction (AJ)) surrounding developing spermatids (Cheng and Mruk, 2002). In contrast, expression of the gene for neural cadherin, a component of cell adhesion molecules of ectoplasmic specialization (Perryman et al, 1996), was similar between DjA1+/− and DjA1−/− mice. Finally, expression of the genes for androgen-binding protein (ABP) and transferrin was increased in DjA1−/− testis by 4.3- and 1.7-fold, respectively. These two genes are classical markers of mature Sertoli cells.
Repression of AR transactivation by DjA1
To assess AR transactivation directly, we did an MMTV-luciferase reporter assay with exogenous human AR in mouse embryo fibroblasts (MEFs) (Figure 6A). In the absence of androgen, basal MMTV-luciferase activity of DjA1−/− cells was lower than that of DjA1+/− cells. In the presence of natural androgens, luciferase activity in DjA1−/− cells was 1.5- to 1.7-fold higher than that in DjA1+/− cells. The increased activity in DjA1−/− cells was repressed by expression of human DjA1 (−/−MEF+hDjA1) to the level of DjA1+/− cells (+/−MEF), indicating that DjA1 is a negative modulator for AR transactivation. Expression of DjA1 in DjA1+/− cells had little effect (+/−MEF+hDjA1). These findings demonstrate that the transcriptional activity of AR is enhanced in DjA1−/− cells.
Figure 6.
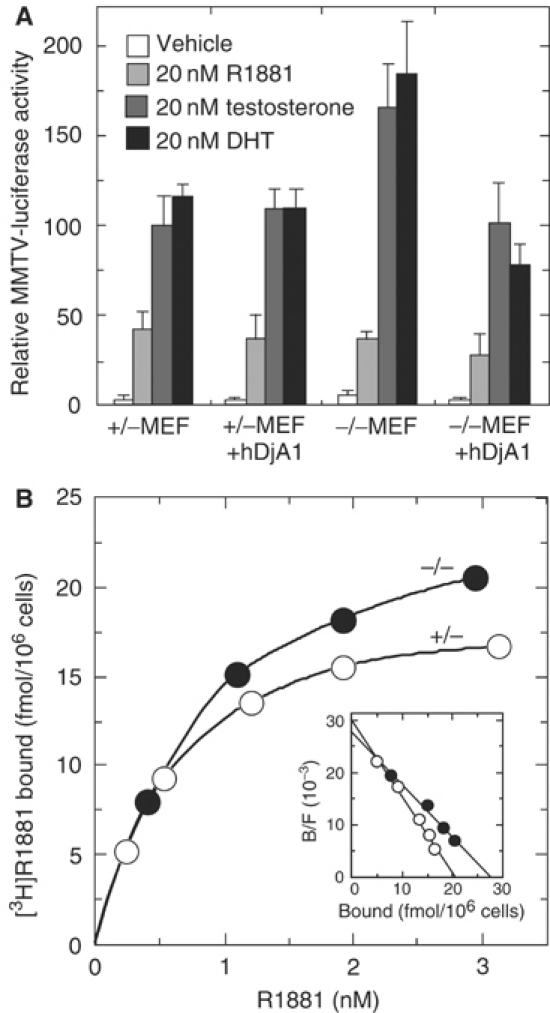
Repression of AR transactivation by DjA1 and decreased androgen binding to the receptor. (A) Luciferase reporter assay in DjA1+/− and DjA1−/− MEFs. The MMTV-luciferase activities were normalized for internal reporter activities and are represented as values relative to the activity of DjA1+/− cells in the presence of testosterone. Internal reporter activities for DjA1+/− and DjA1−/− MEFs were 100±13 and 34±1.9 U, respectively. Values are shown as mean±s.d. of three experiments. DHT, 5α-dihydrotestosterone. (B) Saturation curve and Scatchard plot (inset) of [3H]R1881 binding to primary Sertoli cells.
In contrast, methyltrienolone (R1881), an artificial androgen, activated luciferase activity to similar levels in both MEFs. Coexpression of human DjA1 had little effect.
Binding of androgen in Sertoli cells
The saturation curve and Scatchard plot of [3H]R1881 binding to primary Sertoli cells are shown in Figure 6B. R1881 is a synthetic androgen that is not metabolized and does not significantly bind to ABP (van Roijen et al, 1997). The dissociation constant (Kd) for [3H]R1881 to DjA1−/− Sertoli cells was 1.0 nM, while that for DjA1+/− cells was 0.69 nM. The latter value accords well with data in the literature. The binding sites for DjA1−/− and DjA1+/− Sertoli cells were 28 and 21 fmol/106 cells, respectively.
Integrity of AJs in testis
To explore integrity of AJs of Sertoli–spermatid junction and inter-Sertoli cell junction, we investigated expression of nectin-2 (Figure 7A and B). Nectin-2 was present in a ring-like staining pattern at the basal compartment of DjA1−/− and DjA1+/− tubules. This pattern represents inter-Sertoli cell junctions (Ozaki-Kuroda et al, 2002; Mueller et al, 2003). In the Sertoli–spermatid junction, nectin-2 from Sertoli cells and nectin-3 from elongated spermatids (step 9 and thereafter) form a heterotypic AJ. Nectin-2 and -3 localized this heterotypic AJ in DjA1+/− tubules (Figure 7A and C). In DjA1−/− tubules, staining of nectin-2 was strong in the adluminal compartment and surrounded round spermatids (Figure 7B and D). In contrast, staining of nectin-3 was marginal. Expression of nectin-2 and -3 was confirmed by Northern blot analysis (Figure 7E). Nectin-3 was expressed only in elongated spermatids. Reduced expression of nectin-3 in DjA1−/− testis coincides well with reduced number of elongated spermatids. Reduced expression of smaller nectin-2 mRNA species was also observed. This species appears after the onset of spermiogenesis (Ozaki-Kuroda et al, 2002).
Figure 7.
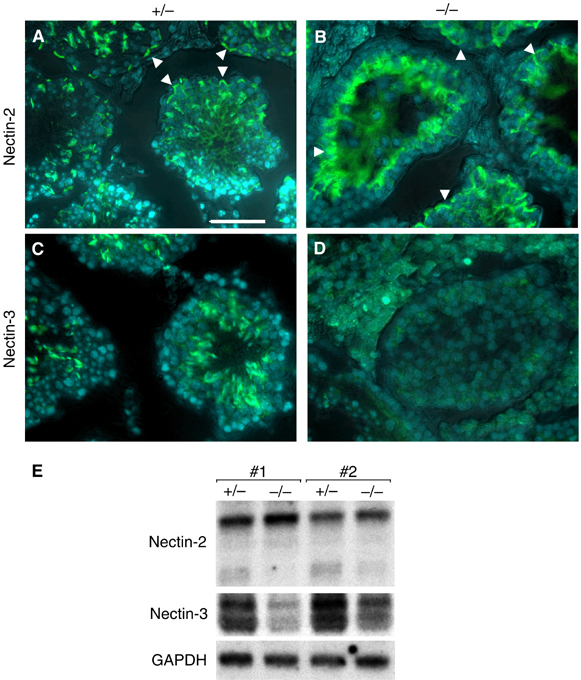
Expression of nectin-2 and -3 in testis of DjA1+/− and DjA1−/− mice. Frozen testis sections (7 μm) of DjA1+/− (A, C) and DjA1−/− (B, D) mice were stained with rat mAb 502-57 (green) against nectin-2 (A, B) and with rat mAb 103-A1 (green) against nectin-3 (C, D). Nuclei were visualized with Hoechst 33258 DNA stain (blue), and co-immunofluorescence and phase contrast images were combined (A–D). Nectin-2-positive signals within basal compartment are indicated by arrowheads (A, B). (E) Northern blot analysis of nectin-2 and -3 mRNAs. Total RNA samples were analyzed using 32P-labeled probes. Bar=50 μm (A–D).
Discussion
Previous in vitro studies have established that a specific member of type I DnaJs, either DjA1 or DjA2, is necessary for Hsp70s to exhibit potent chaperone activities (Terada et al, 1997; Terada and Mori, 2000). Both DnaJs are present in many tissues examined and equally efficient; however, their individual physiological roles remain obscure. To assess whether DjA1 exhibits a redundant or unique function, we prepared DjA1−/− mice. Loss of the DjA1 cochaperone mainly resulted in severe defects in spermatogenesis that involve aberrant androgen signaling with markedly accumulated AR in Sertoli cells. Increased amount of GR may also contribute to the defect in spermiogenesis. We also found disruption of Sertoli–germ cell AJ. There are many syndromes that accompany primary testicular hypogonadism with unknown pathogenesis (Diemer and Desjardins, 1999). Our findings point that loss of DjA1 function leads to an inherited disease with hypogonadism that involves quantitatively and qualitatively aberrant AR protein in Sertoli cells.
AR signaling in DjA1−/− mice
In the current experiments, we found an unexpected accumulation of AR in DjA1−/− Sertoli cells. AR signaling in DjA1−/− cells is hyperactivated in transactivating androgen-responsive genes. The most notable defect in DjA1−/− mice was spermiogenesis at steps 8 and 9, which overlap androgen-dependent stages. We found increased levels of Pem mRNA in DjA1−/− mice. In contrast, expression of Pem mRNA is severely decreased in the absence of AR in Sertoli cells (De Gendt et al, 2004). The absence and also hyperactivation of AR signaling in Sertoli cells are apparently deleterious for spermiogenesis.
The levels of AR mRNA and protein peak in Sertoli cells are at stages VII and VIII (Bremner et al, 1994; Vornberger et al, 1994; Shan et al, 1995), and that of GATA-1 at stages VII–IX (Yomogida et al, 1994). Since mRNA levels of AR, as well as neural cadherin and GATA-1, showed little difference between DjA1+/− and DjA1−/− testes, tubules at spermatid steps 8 and 9 do not constitute significant proportion in DjA1−/− tubules. The highest levels of Pem, testin, androgen-binding protein, and transferrin proteins also overlap these steps (Ritzen et al, 1982; Zong et al, 1994; Lindsey and Wilkinson, 1996; Maguire et al, 1997). Since these four mRNAs are clearly induced in DjA1−/− testis (Figure 5G), stage-specific gene expression in Sertoli cells is obviously disordered.
The accumulation of AR protein is apparently a post-transcriptional event. One possible event that involves DjA1 is the assembly of AR with the Hsp90/Hsp70-based chaperone system in the cytosol (Pratt, 1997). Since Hsp70 is a component of this multicomplex chaperone system, involvement of a DnaJ homolog is expected (Fliss et al, 1999; Pratt and Toft, 2003). Alternatively, proteasome-mediated degradation of AR may be suspected. The role of Hsp70 in facilitation of protein degradation has been increasingly studied, and AR degradation by Hsp70–DjB1–CHIP system was recently reported (Thomas et al, 2004). Other possibility is the assembly and disassembly of coregulators of AR (Gelmann, 2002; Heinlein and Chang, 2002). Coregulators participate in chromatin modification, as well as recruitment of the basal transcriptional machinery. Some coregulators enhance transcription (coactivators), while others inhibit it (corepressors). Defect in assembly and disassembly of these coregulators may well affect transactivation activity of AR protein. Indeed the dynamic assembly and disassembly of large steroid receptor–coactivator complexes within nuclei was recently demonstrated, and the Hsp90 and Hsp70 chaperone systems including yeast type I DnaJ were the necessary factors for mobility of the steroid receptors within nucleus (Elbi et al, 2004).
Disruption of adherens junction in DjA1−/− testis
Sertoli–spermatid junctions are important for maintenance of spermiogenesis (Cheng and Mruk, 2002). Increased frequency of apoptotic spermatocytes with vacuoles, lack of the elongated spermatids, and morphological abnormalities of Sertoli cells in DjA1−/− tubules result in sloughing of premature germ cells by a defect in AJs. Indeed, AJs in the adluminal compartment were disturbed in DjA1−/− tubules. Furthermore, marked increase of testin mRNA in DjA1−/− tubules (Figure 5) is not only due to aberrant AR signaling, but also due to disruption of AJs. Although functions of testin remain to be clarified, a surge in testin expression is an excellent marker for disruption of Sertoli–germ cell AJs (Cheng and Mruk, 2002).
Enhanced apoptotic cell death in DjA1−/− spermatocytes
Although the transplantation experiment revealed Sertoli cell dysfunction, role(s) of DjA1 in spermatocyte cannot be ruled out. In fact, loss of DjA1 frequently involved apoptotic cell death in spermatocytes, where both DjA1 and DjA2 are strongly expressed. Presence of a heat shock element in DjA1 gene corroborates heat induction of DjA1 mRNA in various cultured cells (Terada and Mori, 2000). Indeed, the induction of stress-inducible Hsp70 and DjA1 was noted in heat-shocked mouse testis (Rockett et al, 2001). Simultaneous induction of the Hsp70–DjA1 pair may contribute to an intracellular protective effect(s) against various stress conditions during spermatogenesis. Severe defects in spermatogenesis were found in simultaneous disruption of heat shock factors (Wang et al, 2004). A possible target for DjA1 in spermatocyte is a proapoptotic protein Bax, which promotes germinal cell death during spermatogenesis (Print and Loveland, 2000), and we found that the Hsp70–DjA1 pair directly inhibits apoptosis by preventing mitochondrial translocation of Bax (Gotoh et al, 2004). Loss of DjA1 may partly contribute to this intracellular event in spermatocytes, in addition to an intercellular effect(s) related to aberrant Sertoli cell function.
Materials and methods
Generation of DjA1-deficient mice
To generate a DjA1-deficient mouse, a C57BL/6 mouse liver EMBL3 SP6/T7 phage genomic library (1 × 106 PFU) was screened with DjA1 cDNA (nucleotide 1-287, accession # AA172971). In constructing the targeting vector, the neo cassette was placed between the BsaBI site of exon 2 and EcoRV site of exon 3. Lengths of the homologous regions were 4.6 and 4.9 kb in the targeting vector at the 5′ and 3′ sides of the neo cassette, respectively. The targeting vector was linearized with NotI and introduced into TT2 ES cells as described (Yagi et al, 1993a, 1993b). Homologous recombinant ES clones were identified using PCR and Southern blot analysis. PCR primers used were 5′-GTTAGCCTTGGCAATACAGGAAA-3′ as the 3′-side primer in the DjA1 gene and 5′-ATCGCCTTCTTGACGAGTTCTTCTG-3′ as the 5′-side primer in the neo gene. A 423-bp fragment of the DjA1 gene (nucleotides 9352–9964, accession # AB183426) that was proximal to the 3′-end of the homologous region of the targeting vector and a 614-bp fragment of the neo gene were used as probes for Southern blot analysis. Two mutant mouse strains were generated from two independent homologous recombinant ES clones. The genotype of each mouse was routinely determined using PCR with tail biopsy samples. To detect normal and mutant alleles, primers 5′-GGCAGAAGCAGTTGGATCTTTTC-3′ and 5′-GGCCAAACACTAAACACATTAAG-3′ were used. To detect the mutant allele, primers 5′-ATCGCCTTCTTGACGAGTTCTTCTG-3′ and 5′-GGCCAAACACTAAACACATTAAG-3′ were used. The mice were treated according to the guidelines of Kumamoto University for animal and recombinant DNA experiments with approval from the committee of Kumamoto University.
Northern blot, semiquantitative PCR, and quantitative real-time PCR analyses
The testicular cells were fractionated using an elutriator, as described (Meistrich, 1977; Koga et al, 1998; Tadokoro et al, 2002). Total RNA was electrophoresed in formaldehyde–agarose gels, and blotted onto nylon membranes. Digoxigenin-labeled antisense RNA probes and 32P-labeled DNA probes were prepared from mouse cDNAs. Chemiluminescence signals were analyzed using a LAS1000plus image analyzer (Fuji Photo Film). For PCR analysis, total RNA from testis was prepared using the RNeasy kit (Qiagen). The first strand cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen) and random hexamers, followed by RNase H treatment. The cDNA was amplified for 25 and 30 cycles for semiquantitative PCR. Real-time PCR was performed in triplicate using iQ SYBR green supermix (Bio-Rad Laboratories). The amplification product was verified to give a single band of the expected size and was quantified with standard curves obtained from 102–106 copies of plasmid template. Values were normalized to levels of β-actin. The following oligos were used: GAPDH forward, 5′-CCCCTTCATTGACCTCAACTACA-3′; GAPDH reverse, 5′-TGCTTCACCACCTTCTTGATGTC-3′; β-actin forward, 5′-GAGACCTTCAACACCCCAGCCAT-3′; β-actin reverse, 5′-TACTCCTGCTTGCTGATCCACAT-3′; ABP forward, 5′-AGCCTGGGAAACTGTGATGTGGA-3′; ABP reverse, 5′-CGGGTATCGTTGCTAGGTCTCTG-3′; AR forward, 5′-GGGGACATGCGTTTGGACAGTA-3′; AR reverse, 5′-CAAGGCAGCAAAGGAATCTGGT-3′; GATA-1 forward, 5′-TGTGTGAACTGTGGAGCAACGGC-3′; GATA-1 reverse, 5′-AAATAGAGGCCGCAGGCATTGCA-3′; N-cadherin forward, 5′-TGGGGATATTGGGGACTTCATT-3′; N-cadherin reverse, 5′-AAAGGTACAAAAAGGCACATAAA-3′; Pem forward, 5′-CCAGGTATGGAAGCTGAGGGT-3′; Pem reverse, 5′-TCAAATCAAAATCTCGGTGTCGC-3′; testin forward, 5′-ATGATCGCTGTTCTCTTCCTAGC-3′; testin reverse, 5′-TCAGACGATGGGGTATGTGGC-3′; transferrin forward, 5′-ATCTGGGAGATTCTCAAAGTG-3′; transferrin reverse, 5′-AGTGTGGCAGGACTTCTTGCC-3′. Other oligos used have been described previously (Walther et al, 1996; Dix et al, 1997). All pairs of oligos except GAPDH were designed in different exons.
Histology and immunohistochemistry
The testes were fixed in Bouin's fixative at 4°C for 12–16 h, dehydrated, and embedded in paraffin block. Sections of 5 μm thick were deparaffinized and stained with a hematoxylin–eosin solution. For immunohistochemical analysis, deparaffinized sections were autoclaved in citrate buffer to retrieve antigenicity. A monoclonal anti-DjA1 (NeoMarkers, MS-225, 20 ng/ml) and a polyclonal anti-AR (Santa Cruz Biotechnology, SC-816, 50 ng/ml) antibody were used. The signal was amplified using a tyramide signal amplification biotin system kit (Perkin-Elmer). Rat monoclonal antibodies (mAbs) against nectin-2 and -3 (Medical & Biological Laboratories) were used for immunofluorescence analysis as described (Mueller et al, 2003). Alexa 488-conjugated anti-rat antibody (Molecular Probes, 10 μg/ml) was used as a secondary antibody. Images were captured by Olympus BX50 fluorescence microscope equipped with Penguin 600CL camera system (Pixera). Apoptotic cells were detected using an in situ apoptosis detection kit (Takara Bio).
In situ hybridization
The testes were fixed in modified Davidson's fluid (Latendresse et al, 2002), dehydrated, and embedded in a paraffin block. Sections of 5 μm thickness were deparaffinized, digested for 10 min with 50 μg/ml proteinase K, and then prehybridized in hybridization mixture containing 50% formamide, 5 × SSC, and 250 μg/ml yeast RNA. Hybridization was carried out using 0.4 μg/ml of DIG-labeled antisense probe for 12 h at 42°C. A stringent wash was performed in 0.2 × SSC at 65°C. The probe was detected using alkaline phosphatase-coupled anti-DIG antibody (Roche, 1:5000 dilution).
MEF isolation and transfection
MEFs from the DjA1+/+ and DjA1−/− embryos were prepared, as described (Goldman, 1998). They were cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 25 mM glucose and 10% fetal bovine serum (FBS) in a humidified atmosphere with 5% CO2. The cells, cultured in 24-well (2 × 104 cells/well), were transfected with 0.25 μg/well of pGL3-MMTV (Saitoh et al, 2002) as the reporter, 0.25 ng/well of pRL-CMV (Promega) as the internal control, 5.0 ng/well of pCMV-AR as the expression vector for human AR (Saitoh et al, 2002), and 0.24 μg/ml pCAGGS-HSDJ as the expression vector for DjA1 (Kanazawa et al, 1997), using 2.5 μl of Lipofectamine 2000 reagent (Invitrogen). The total amount of vector added to each well was adjusted to 0.5 μg by adding an empty vector. The medium was replaced after 5 h with DMEM containing 10% charcoal-treated FBS and 20 nM androgen, and cultured for 24 h. Activities of reporter genes were determined using a dual-luciferase reporter assay system (Promega).
Western blot analysis
To detect endogenous AR in testis, the tissue was disrupted in 10% trichloroacetic acid using a glass-Teflon homogenizer. The protein precipitate was collected by centrifugation, and solubilized into solution containing 7.2 M urea, 1.6% Triton X-100, 1% lithium dodecyl sulfate, and 0.1 M Tris base. Protein was separated using 2–15% polyacrylamide–SDS gel electrophoresis and processed for Western blot analysis. Rabbit anti-DjA2 (dj3) and anti-DjB1 (dj1) antibodies, rat anti-Hsc70 (1B5) mAb, and rabbit anti-DjA4 (mmdj4) serum were as described (Terada et al, 1995; Terada and Mori, 2000; Hafizur et al, 2004). Rabbit anti-GR (SC-1004), anti-CBP (SC-369), and anti-PCNA (SC-7909) antibodies were obtained from Santa Cruz Biotechnology.
Analysis of AR in primary Sertoli cells
Primary Sertoli cells were prepared essentially as described (Anway et al, 2003), and cultured (34°C, 5% CO2) in phenol red-free, HEPES-buffered DMEM/F12 medium supplemented with 10% FBS, 1 μM retinyl acetate, 200 nM testosterone, 10 μg/ml insulin, 5 μg/ml transferrin, and 10 ng/ml epidermal growth factor. Cells were used for subsequent experiments betweens 2 and 3 weeks after isolation. For whole-cell-binding assay, media of Sertoli cells (4 × 105 cells/35-mm dish) were replaced with DMEM/F12 supplemented with 2% charcoal-treated FBS. Cells were incubated with 0.2–3 nM [3H]R1881 (Perkin-Elmer) at 34°C for 1 h, then washed with ice-cold PBS, solubilized in RIPA buffer, and subjected to liquid scintillation counting. Nonspecific binding was assessed by competition with 400-fold excess radio-inert R1881.
Transplantation of GFP-labeled spermatogonia
Donor GFP-labeled spermatogonia were prepared as described (Ohta et al, 2000). Busulfan was administered intraperitoneally at a dose of 40 mg/kg to destroy the spermatogenic cells in recipient mice (four DjA1−/− mice and two DjA1+/− mice). After 4 weeks, spermatogonia were transplanted via the efferent ductules (Ventela et al, 2002).
Acknowledgments
We thank Toshihiko Yanase and Hajime Nawata (Kyushu University) for pGL3-MMTV and pCMV-AR plasmids, Hiroyuki Nakanishi (Kumamoto University) for suggestions on analysis of AJ, Kikuko Uchino (Kumamoto University) for menstrual analysis, and Gen Yamada (Kumamoto University) for support in blastocyst injections. We also thank Rieko Shindo and Yasuko Indo for technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (13680789 to KT and 14037257 to MM), a grant from the Sumitomo Foundation, and a grant from the Naito Foundation.
References
- Abdul KM, Terada K, Gotoh T, Hafizur RM, Mori M (2002) Characterization and functional analysis of a heart-enriched DnaJ/ Hsp40 homolog dj4/DjA4. Cell Stress Chaperones 7: 156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Folmer J, Wright WW, Zirkin BR (2003) Isolation of Sertoli cells from adult rat testes: an approach to ex vivo studies of Sertoli cell function. Biol Reprod 68: 996–1002 [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Millar MR, Sharpe RM, Saunders PT (1994) Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology 135: 1227–1234 [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92: 351–366 [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD (2002) Cell junction dynamics in the testis: Sertoli–germ cell interactions and male contraceptive development. Physiol Rev 82: 825–874 [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G (2004) A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101: 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer T, Desjardins C (1999) Developmental and genetic disorders in spermatogenesis. Hum Reprod Update 5: 120–140 [DOI] [PubMed] [Google Scholar]
- Dix DJ, Allen JW, Collins BW, Poorman-Allen P, Mori C, Blizard DR, Brown PR, Goulding EH, Strong BD, Eddy EM (1997) HSP70-2 is required for desynapsis of synaptonemal complexes during meiotic prophase in juvenile and adult mouse spermatocytes. Development 124: 4595–4603 [DOI] [PubMed] [Google Scholar]
- Don J, Stelzer G (2002) The expanding family of CREB/CREM transcription factors that are involved with spermatogenesis. Mol Cell Endocrinol 187: 115–124 [DOI] [PubMed] [Google Scholar]
- Elbi C, Walker DA, Romero G, Sullivan WP, Toft DO, Hager GL, DeFranco DB (2004) Molecular chaperones function as steroid receptor nuclear mobility factors. Proc Natl Acad Sci USA 101: 2876–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss AE, Rao J, Melville MW, Cheetham ME, Caplan AJ (1999) Domain requirements of DnaJ-like (Hsp40) molecular chaperones in the activation of a steroid hormone receptor. J Biol Chem 274: 34045–34052 [DOI] [PubMed] [Google Scholar]
- Frydman J (2001) Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem 70: 603–647 [DOI] [PubMed] [Google Scholar]
- Gelmann EP (2002) Molecular biology of the androgen receptor. J Clin Oncol 20: 3001–3015 [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J (1992) Protein folding in the cell. Nature 355: 33–45 [DOI] [PubMed] [Google Scholar]
- Goldman A (1998) Isolation of fibroblasts. In Cells—A Laboratory Manual, Spector DL, Goldman RD, Leinwand LA (eds) pp 41–47. Plainview, NY: Cold Spring Harbor Press [Google Scholar]
- Gotoh T, Terada K, Oyadomari S, Mori M (2004) hsp70–DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ 11: 390–402 [DOI] [PubMed] [Google Scholar]
- Hafizur RM, Yano M, Gotoh T, Mori M, Terada K (2004) Modulation of chaperone activities of Hsp70 and Hsp70-2 by a mammalian DnaJ/Hsp40 homolog, DjA4. J Biochem 135: 193–200 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295: 1852–1858 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C (2002) Androgen receptor (AR) coregulators: an overview. Endocr Rev 23: 175–200 [DOI] [PubMed] [Google Scholar]
- Kanazawa M, Terada K, Kato S, Mori M (1997) HSDJ, a human homolog of DnaJ, is farnesylated and is involved in protein import into mitochondria. J Biochem 121: 890–895 [DOI] [PubMed] [Google Scholar]
- Koga M, Tanaka H, Yomogida K, Tsuchida J, Uchida K, Kitamura M, Sakoda S, Matsumiya K, Okuyama A, Nishimune Y (1998) Expression of selenoprotein-P messenger ribonucleic acid in the rat testis. Biol Reprod 58: 261–265 [DOI] [PubMed] [Google Scholar]
- Latendresse JR, Warbrittion AR, Jonassen H, Creasy DM (2002) Fixation of testes and eyes using a modified Davidson's fluid: comparison with Bouin's fluid and conventional Davidson's fluid. Toxicol Pathol 30: 524–533 [DOI] [PubMed] [Google Scholar]
- Li J, Qian X, Sha B (2003) The crystal structure of the yeast Hsp40 Ydj1 complexed with its peptide substrate. Structure 11: 1475–1483 [DOI] [PubMed] [Google Scholar]
- Lindsey JS, Wilkinson MF (1996) Pem: a testosterone- and LH-regulated homeobox gene expressed in mouse Sertoli cells and epididymis. Dev Biol 179: 471–484 [DOI] [PubMed] [Google Scholar]
- Maguire SM, Millar MR, Sharpe RM, Gaughan J, Saunders PT (1997) Investigation of the potential role of the germ cell complement in control of the expression of transferrin mRNA in the prepubertal and adult rat testis. J Mol Endocrinol 19: 67–77 [DOI] [PubMed] [Google Scholar]
- Meistrich ML (1977) Separation of spermatogenic cells and nuclei from rodent testis. Methods Cell Biol 15: 15–54 [DOI] [PubMed] [Google Scholar]
- Mueller S, Rosenquist TA, Takai Y, Bronson RA, Wimmer E (2003) Loss of nectin-2 at Sertoli–spermatid junctions leads to male infertility and correlates with severe spermatozoan head and midpiece malformation, impaired binding to the zona pellucida, and oocyte penetration. Biol Reprod 69: 1330–1340 [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Behre HM (1997) Andrology: Male Reproductive Health and Dysfunction. Berlin, New York: Springer [Google Scholar]
- Ohta H, Yomogida K, Dohmae K, Nishimune Y (2000) Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development 127: 2125–2131 [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Hata M (2000) Mammalian HSP40/DNAJ homologs: cloning of novel cDNAs and a proposal for their classification and nomenclature. Cell Stress Chaperones 5: 98–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, Nishimune Y, Takai Y (2002) Nectin couples cell–cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol 12: 1145–1150 [DOI] [PubMed] [Google Scholar]
- Perryman KJ, Stanton PG, Loveland KL, McLachlan RI, Robertson DM (1996) Hormonal dependency of neural cadherin in the binding of round spermatids to Sertoli cells in vitro. Endocrinology 137: 3877–3883 [DOI] [PubMed] [Google Scholar]
- Pratt WB (1997) The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol Toxicol 37: 297–326 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med 228: 111–133 [DOI] [PubMed] [Google Scholar]
- Print CG, Loveland KL (2000) Germ cell suicide: new insights into apoptosis during spermatogenesis. BioEssays 22: 423–430 [DOI] [PubMed] [Google Scholar]
- Rao MK, Wayne CM, Meistrich ML, Wilkinson MF (2003) Pem homeobox gene promoter sequences that direct transcription in a Sertoli cell-specific, stage-specific, and androgen-dependent manner in the testis in vivo. Mol Endocrinol 17: 223–233 [DOI] [PubMed] [Google Scholar]
- Ritzen EM, Boitani C, Parvinen M, French FC, Feldman M (1982) Stage-dependent secretion of ABP by rat seminiferous tubules. Mol Cell Endocrinol 25: 25–33 [DOI] [PubMed] [Google Scholar]
- Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ (2001) Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod 65: 229–239 [DOI] [PubMed] [Google Scholar]
- Saitoh M, Takayanagi R, Goto K, Fukamizu A, Tomura A, Yanase T, Nawata H (2002) The presence of both the amino- and carboxyl-terminal domains in the AR is essential for the completion of a transcriptionally active form with coactivators and intranuclear compartmentalization common to the steroid hormone receptors: a three-dimensional imaging study. Mol Endocrinol 16: 694–706 [DOI] [PubMed] [Google Scholar]
- Shan LX, Zhu LJ, Bardin CW, Hardy MP (1995) Quantitative analysis of androgen receptor messenger ribonucleic acid in developing Leydig cells and Sertoli cells by in situ hybridization. Endocrinology 136: 3856–3862 [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y (2002) Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev 113: 29–39 [DOI] [PubMed] [Google Scholar]
- Terada K, Kanazawa M, Bukau B, Mori M (1997) The human DnaJ homologue dj2 facilitates mitochondrial protein import and luciferase refolding. J Cell Biol 139: 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada K, Mori M (2000) Human DnaJ homologs dj2 and dj3, and bag-1 are positive cochaperones of hsc70. J Biol Chem 275: 24728–24734 [DOI] [PubMed] [Google Scholar]
- Terada K, Ohtsuka K, Imamoto N, Yoneda Y, Mori M (1995) Role of heat shock cognate 70 protein in import of ornithine transcarbamylase precursor into mammalian mitochondria. Mol Cell Biol 15: 3708–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Dadgar N, Aphale A, Harrell JM, Kunkel R, Pratt WB, Lieberman AP (2004) Androgen receptor acetylation site mutations cause trafficking defects, misfolding, and aggregation similar to expanded glutamine tracts. J Biol Chem 279: 8389–8395 [DOI] [PubMed] [Google Scholar]
- van Roijen JH, Ooms MP, Weber RF, Brinkmann AO, Grootegoed JA, Vreeburg JT (1997) Comparison of the response of rat testis and accessory sex organs to treatment with testosterone and the synthetic androgen methyltrienolone (R1881). J Androl 18: 51–61 [PubMed] [Google Scholar]
- Ventela S, Ohta H, Parvinen M, Nishimune Y (2002) Development of the stages of the cycle in mouse seminiferous epithelium after transplantation of green fluorescent protein-labeled spermatogonial stem cells. Biol Reprod 66: 1422–1429 [DOI] [PubMed] [Google Scholar]
- Vornberger W, Prins G, Musto NA, Suarez-Quian CA (1994) Androgen receptor distribution in rat testis: new implications for androgen regulation of spermatogenesis. Endocrinology 134: 2307–2316 [DOI] [PubMed] [Google Scholar]
- Walther N, Jansen M, Ergun S, Kascheike B, Ivell R (1996) Sertoli cell lines established from H-2Kb-tsA58 transgenic mice differentially regulate the expression of cell-specific genes. Exp Cell Res 225: 411–421 [DOI] [PubMed] [Google Scholar]
- Wang G, Ying Z, Jin X, Tu N, Zhang Y, Phillips M, Moskophidis D, Mivechi NF (2004) Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility. Genesis 38: 66–80 [DOI] [PubMed] [Google Scholar]
- Yagi T, Nada S, Watanabe N, Tamemoto H, Kohmura N, Ikawa Y, Aizawa S (1993a) A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal Biochem 214: 77–86 [DOI] [PubMed] [Google Scholar]
- Yagi T, Tokunaga T, Furuta Y, Nada S, Yoshida M, Tsukada T, Saga Y, Takeda N, Ikawa Y, Aizawa S (1993b) A novel ES cell line, TT2, with high germline-differentiating potency. Anal Biochem 214: 70–76 [DOI] [PubMed] [Google Scholar]
- Yomogida K, Ohtani H, Harigae H, Ito E, Nishimune Y, Engel JD, Yamamoto M (1994) Developmental stage- and spermatogenic cycle-specific expression of transcription factor GATA-1 in mouse Sertoli cells. Development 120: 1759–1766 [DOI] [PubMed] [Google Scholar]
- Zong SD, Zhu LJ, Grima J, Aravindan GR, Bardin CW, Cheng CY (1994) Cyclic and postnatal developmental changes of testin in the rat seminiferous epithelium—an immunohistochemical study. Biol Reprod 51: 843–851 [DOI] [PubMed] [Google Scholar]


