Abstract
Background
The performance of estimated glomerular filtration rate (eGFR) have been proved to vary according to the races of the target population. The eGFR equations have not been validated in the Chinese cancer population received chemotherapy. Meanwhile, serum cystatin C (CysC), urea, β2 microglobulin (β2-MG), and creatinine (SCr) were also evaluated in a cohort of Chinese cancer patients.
Material/Methods
A total of 1000 cancer patients undergoing combination chemotherapy and 108 healthy volunteers were included in this study, and their renal function parameters were evaluated. The eGFR values were compared with reference GFR (rGFR) according to correlation, consistency, precision, and accuracy. Receiver operating characteristic (ROC) curves were used to evaluate the discriminating ability of the GFR equations and serological indicators of renal function.
Results
(1) The equations contained CysC had the same varying tendency as rGFR in relation to the chemotherapeutic cycle. (2) eGFRscr+cysc and eGFRChinese scr+cysc worked better than the other equations, as indicated by a stronger correlation, less bias, improved precision, higher accuracy, and greater AUC. (3) CysC was more sensitive than the other serological indicators for identifying early renal injury. (4) Each parameter showed different characteristics in subgroups of Chinese cancer patients.
Conclusions
CysC was the most sensitive marker for early renal injury. Among the 8 most commonly used eGFR equations, the combination equation eGFRscr+cysc and eGFRChinese scr+cysc exhibited the best performance in the assessment of the renal function of Chinese cancer patients.
MeSH Keywords: Cystatin C; Drug Therapy, Combination; Glomerular Filtration Rate; Renal Insufficiency, Chronic
Background
As the average age of a population increases, a corresponding increase in the prevalence of malignant tumors can be seen. The use of chemotherapeutic agents in patients with malignant tumors has led to a major increase in the overall survival time of cancer patients. Despite this survival increase, patients with malignant tumors are at high risk for renal impairment (RI) caused by chemotherapy. Chronic kidney disease (CKD) has been reported in 15–50% of cancer patients [1,2]. Therefore, kidney function should be monitored to recognize RI as early as possible [3]. The evaluation of renal function should be performed as part of the follow-up during and after chemotherapy in cancer patients.
Glomerular filtration rate (GFR) is the most important parameter of kidney function, and inulin clearance remains the criterion standard GFR tracer; however, it is inconvenient and difficult to measure. The clearance of radionuclide (51Cr-EDTA or 99mTc-DTPA) is considered to be closest to that of inulin, but it is radioactive and its use is time-consuming. Therefore, there is growing interest in finding accurate and simple methods to estimate the GFR to access glomerular filtration function. Serum creatinine (SCr) is most commonly used to estimate GFR, but the serum levels of creatinine are often influenced by many non-renal factors, such as sex, muscle mass, protein intake, and metabolism. The low-molecular-weight protein (12.8 kDa) cystatin C (CysC) is being considered as a replacement for SCr for the estimation of GFR. Unlike SCr, it is not affected by dietary protein intake and is independent of changes in GFR [4]. The Cockcroft-Gault (CG) formula, published in 1976, was developed to predict creatinine clearance [5]. The formula derived from the Modification of Diet in Renal Disease (MDRD) study included 6 variables (creatinine, urea, albumin, age, sex, and race) [6]; this equation was simplified in 2000 to include only 4 variables and was subsequently re-expressed for use with standardized SCr in 2007 [7]. Both the creatinine and CysC methods have been standardized, and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations for the estimation of GFR were established in 2012 [8,9]. To exclude the influence of race, the CKD-EPI developed a creatinine-based equation with an ethnicity adjustment in 2011 [10]. The Chinese eGFR Investigation Collaboration developed a modified equation for use in Chinese CKD patients [11,12]. The utility of eGFR equations in evaluating renal function in cancer patients has been reported in many studies [13–16]. However, the aim of the present study was to reappraise the performances of the new CKD-EPI 2012 equations, the Chinese equations, and the MDRD equations in a Chinese oncology cohort who had received chemotherapy. We also evaluated SCr, urea, β2-microglobulin (β2-MG), and CysC performance in the diagnosis of RI in the cohort of Chinese cancer patients.
Material and Methods
Patient selection
A total of 1000 cancer patients (596 males and 404 females) aged 25–83 years (average age 60.3±10.4 years) were recruited from the patients admitted to Tianjin Medical University Cancer Institute and Hospital during the period from October 2012 to December 2014. All patients received combination chemotherapy (with or without platinum), and when they finished chemotherapy, we screened out 545 cancer patients with CKD. The inclusion criteria for patients with CKD according to the KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease included the following: (1) Presence of 1 or more of the following markers of kidney damage: a. albuminuria (AER ≥30 mg/24 h; ACR ≥30 mg/g [≥3 mg/mmol]), b. urine sediment abnormalities, c. electrolyte and other abnormalities due to tubular disorders, d. abnormalities detected by histology, f. structural abnormalities detected by imaging, and g. history of kidney transplantation; and (2) GFR <60 ml/min/1.73 m2 (GFR categories G3a–G5). Either of the above criteria needed to be present for >3 months for inclusion. The exclusion criteria were: (1) acute kidney function deterioration, (2) severe cardiac insufficiency, (3) pleural or abdominal effusion, (4) edema, (5) diabetes, (6) primary kidney disease, and (7) disabled limbs or amputation. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. The members of the control group were selected from a group of volunteers and healthy individuals (n=108).
Ethics statement
All patients signed a written informed consent form prior to inclusion in the study and our study was approved by our local Ethics Committee.
Specimen and data collection
Three days before specimen collection, patients were asked to avoid a high-protein diet, meat, and strenuous exercise. In addition, the specimens were collected after 8 h of fasting, and the optimal blood sampling time was 7: 00 AM to 9: 00 AM. For outpatients, sitting for 15 min was required before specimen collection. Then, we collected serum specimens before chemotherapy and after the first, second, third, fourth, fifth, and last chemotherapy cycles. Prior to chemotherapy and after all chemotherapy cycles, all collected serum specimens were allowed to stand for 2 h, followed by centrifugation at 3000 rpm for 5 min. The 99mTc-diethylenetriamine pentaacetic acid (99mTc-DTPA) dual-plasma clearance was applied to measure GFR on the same day. The procedures used to process the samples were the same for the control and experimental groups. For each enrolled individual, age, sex, race, body surface area (BSA), SCr, CysC, urea, β2-MG, and reference GFR (rGFR) before chemotherapy and after each chemotherapy cycle were recorded.
Laboratory tests
The serum creatinine was measured by the enzymatic method (IDMS calibrated) (Shanghai Rongsheng Biotech Co., Ltd, Shanghai, China.). The GLDH Kinetic Assay (Beckman-Coulter Experiment System (SuZhou.) Co., Ltd, Suzhou, Jiangsu, China.) was used to measure urea levels. In addition, β2-MG and CysC were determined using the latex-enhanced immunoturbidimetric method (Beijing Strong Biotechnologies, Inc, Beijing, China.) and latex immunoturbidimetric method (Beijing Strong Biotechnologies, Inc, Beijing, China), respectively. The levels of all 4 parameters were determined using a BECKMAN-COULTER AU5800 device (Beckman-Coulter Instruments, Brea, CA, USA).
GFR measurement
The reference method for measuring GFR was the 99mTc-DTPA dual-plasma clearance, and the following equation was used to calculate rGFR (ml/min/1.73 m2): [D ln(P1/P2)/(T2–T1)] exp{[(T1 ln P2)–(T2 ln P1)]/(T2–T1)}×0.93×1.73/BSA (D: dosage of drug injected, T1: 120 min, P1: amount of plasma activity at T1, counts per min×ml−1,T2: 240 min, P2: amount of plasma activity at T2, counts per min×ml−1).
Estimated GFR equation
The expression of all equations is summarized in Table 1. GFR was calculated using 8 estimation formulas: MDRD equation [6], IDMS-MDRD equation [7], Chinese equations [11,12], CKD-EPI equations using creatinine alone [9], CysC alone or both [8], and the CKD-EPI 4-level equation [10]. rGFR categories were assigned according to the KDIGO 2012 Clinical Practice Guidelines for the Evaluation and Management of Chronic Kidney Disease as follows: G1 normal or high (>90 ml/min/1.73 m2), G2 mildly decreased (60–89 ml/min/1.73 m2), G3a mildly to moderately decreased (45–59 ml/min/1.73 m2), G3b moderately to severely decreased (30–44 ml/min/1.73 m2), G4 severely decreased (15–29 ml/min/1.73 m2), and G5 kidney failure (<15 ml/min/1.73 m2). In this study, early and moderate RI were defined as a GFR <90 ml/min/1.73 m2 and GFR <60 ml/min/1.73 m2, respectively.
Table 1.
GFR-predicting equations used in this study.
| 2009 CKD-EPI creatinine equation(eGFRscr) =141×min(SCr/κ, 1)α × max(SCr/κ, 1–1.209 × 0.993Age [×1.018 if female] [×1.159 if black], (κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males) |
| 2012 CKD-EPI cystatin C equation(eGFRcysc) =133 × min(SCysC/0.8, 1)−0.499 × max(SCysC/0.8, 1)−1.328 × 0.996Age [×0.932 if female] |
| 2012 CKD-EPI creatinine-cystatin C equation(eGFRscr+cysc) =135 × min(SCr/κ, 1)α × max(SCr/κ, 1)−0.601 × min(SCysC/0.8, 1)−0.375 × max(SCysC/0.8, 1)−0.711 × 0.995Age [× 0.969 if female] [× 1.08 if black](κ is 0.7 for females and 0.9 for males, α is −0.248 for females and −0.207 for males) |
| CKD-EPI 4 level equation(eGFRCKD-EPI Asian) = 141 × min(SCr/κ, 1)α × max(SCr/κ, 1)−1.210 × 0.993Age × 0.993 [if female] × 1.16 [if Black] × 1.05 [if Asian] × 1.01 [if Hispanic and Native American] (κ is 0.7 for females and 0.9 for males, α is −0.412 for females and −0.328 for males) |
| MDRD equation using 4 variable (eGFRMDRD) =186 × SCr−1.154 × Age−0.203[× 0.742 if female] |
| IDMS-MDRD equation(eGFRIDMS-MDRD) =175 × SCr−1.154 × Age−0.203[× 0.742 if female] |
| Modified MDRD equation for Chinese CKD patients(eGFRChinese MDRD) =186 × SCr−1.154 × Age−0.203[× 0.742 if female] × 1.233(if Chinese) |
| Estimated GFR equation combining SCr and cysC by Chinese eGFR Investigation Collaboration(eGFRChinese scr+cysc) =169 × SCr−0.608 × SCysC−0.63 × Age−0.157 × 0.83(if female) |
Statistical analysis
All results are expressed as the mean values ± standard deviations. Results were considered to be significant at p<0.05. The independent samples t test was used to compare the experimental and control groups or 2 subgroups. One-way analysis of variance (ANOVA) was used for the comparison of the 3 subgroups. The paired t test was used to compare the indexes before and after chemotherapy. Correlations of parameters with rGFR were assessed using Pearson’s (r) correlation coefficients. The accuracy refers to the percentage of GFR estimates within 10% and 30% of the rGFR. Accuracy between the formulas was compared using the chi-square test. A Bland-Altman plot, created using GraphPad Prism 6.00, was used to analyze the agreement between the rGFR and the values derived from the 8 eGFR equations. To determine the diagnostic consistency of the equations compared with the rGFR, receiver operating characteristic (ROC) curves were constructed and analyzed. A cutoff value was then proposed for each marker or equation with the best sensitivity and specificity. All statistical analyses were performed in SPSS 17.0 for Windows.
Results
Clinical characteristics of the enrolled population
The characteristics of the cancer population (n=1000) and the control population (n=108) are listed in Table 2. The serum Cr and CysC levels increased significantly in the cancer patients compared with the control group (p<0.01).
Table 2.
Characteristics of the enrolled population.
| Cancer (pre chemotherapy) | Control | p | |
|---|---|---|---|
| Number | 1000 | 108 | |
| Gender (M/F) | 596/404 | 55/53 | 0.099 |
| Age (years) | 60.25±10.42 | 61.82±11.01 | 0.251 |
| BSA (m2) | 1.73±0.12 | 1.73±0.15 | 0.834 |
| Serum Cr (μmol/l) | 72.38±21.12 | 52.02±7.44 | <0.001* |
| Urea (mmol/l) | 5.79±5.02 | 4.55±1.32 | 0.169 |
| β2-MG (mmol/l) | 2.60±1.26 | 1.71±0.44 | 0.065 |
| CysC (mg/l) | 1.03±0.34 | 0.83±0.18 | 0.003* |
| rGFR (ml/min/1.73 m2) | 90.07±23.96 | 106.04±19.69 | 0.057 |
| Operated/unoperated | 457/543 | ||
| Cancer type | n | Chemotherapy regimens | |
| Non-Hodgkin lymphoma | 185 | R-CHOP | |
| Hodgkin lymphoma | 40 | ABVD | |
| Lung cancer | 443 | TP/EC | |
| Esophagus cancer | 168 | FP/DC | |
| Gastric cancer | 164 | FOLFOX4 |
Data were expressed mean ±SD.
Independent samples T test, p<0.01.
R-CHOP − Rituximab + Cyclophosphamide + Adriamycin + Vincristine + Prednisone; ABVD − Adriamycin + Bleomycin + Vinblastine + Dacarbazine; TP − Taxol + Cis-platinum; EC − Epirubicin + Cyclophosphamide; FP − Fluorouracil + Cis-platinum; DC − Docetaxel + Camptosar; FOLFOX4 − Oxaliplatin + Calcium folinatc + Fluorouracil.
Evaluation of renal function parameters in different cycles of chemotherapy
The cancer patients were divided into 2 subgroups according to their chemotherapy regimen. rGFR and the 4 serological indicators of renal function in different cycles of chemotherapy are presented in Table 3. The number of chemotherapy cycles depends on what the physician at the beginning had decided to give to the patient, depending on cancer disease, performance state, and comorbidities. Patients may also stop chemotherapy for different reasons (such as renal failure). To eliminate the influence of these factors, methods are needed to compare the parameters after chemotherapy with those before chemotherapy. When cancer patients finished their chemotherapy, they were no longer involved in the study. rGFR began to decrease after 1 chemotherapeutic cycle in patients who received platinum-containing protocols, while β2-MG and CysC in the serum increased significantly (Table 3). However, for patients who received non-platinum-containing protocols, rGFR instead began to decrease after 4 chemotherapeutic cycles, and only CysC demonstrated an obvious rise at the same time (Table 3).
Table 3.
Comparison of parameters of patients in different cycles of chemotherapy.
| n | rGFR (ml/min/1.73 m2) | Serum Cr (μmol/l) | Urea( mmol/l) | β2-MG (mmol/l) | CysC (mg/l) | |
|---|---|---|---|---|---|---|
| Platinum-containing protocols | ||||||
| Before CT | 440 | 91.48±24.26 | 74.40±25.11 | 6.44± 7.27 | 2.52± 1.10 | 1.01±0.37 |
| After 1 cycle chemotherapy | 440 | 84.45±24.35** | 75.09±26.16 | 5.73 ±2.89 | 2.73 ±1.59* | 1.19 ±0.49** |
| After 2 cycles chemotherapy | 424 | 83.55±25.17** | 74.76 ±33.18 | 5.81 ±3.73 | 2.83 ±1.69** | 1.25 ±0.54** |
| After 3 cycles chemotherapy | 398 | 83.65±26.28** | 73.53±25.69 | 5.92±5.41 | 2.77 ±1.25** | 1.25 ±0.49** |
| After 4 cycles chemotherapy | 365 | 78.68±24.01** | 74.92 ±25.07 | 5.83±1.99 | 2.82 ±1.27** | 1.34±0.45** |
| After 5 cycles chemotherapy | 323 | 70.74±21.52** | 82.13±53.54 | 6.09 ±3.05 | 3.09±1.82** | 1.57±0.59** |
| After 6 or more cycles chemotherapy | 274 | 62.13±19.31** | 81.38 ±32.02** | 7.29 ±3.95 | 3.44±2.29** | 2.09 ±1.68** |
| Non-platinum-containing protocols | ||||||
| Before CT | 560 | 88.97±23.73 | 70.80±17.26 | 5.28±1.73 | 2.67± 1.38 | 1.05±0.32 |
| After 1 cycle chemotherapy | 560 | 90.67±23.90 | 68.20±16.63 | 5.17 ±1.77 | 2.64 ±1.07 | 1.07±0.38 |
| After 2 cycles chemotherapy | 555 | 90.97±25.03 | 67.37±16.16 | 5.16±1.64 | 2.69 ±1.09 | 1.09 ±0.41 |
| After 3 cycles chemotherapy | 528 | 91.90±26.66 | 66.20±16.14 | 5.16±1.83 | 2.61 ±1.10 | 1.09 ±0.37 |
| After 4 cycles chemotherapy | 485 | 87.84±24.81* | 66.29 ±18.27 | 5.50±4.31 | 2.51 ±1.31 | 1.19±0.39** |
| After 5 cycles chemotherapy | 428 | 84.37±22.91* | 67.06±23.02 | 5.37±2.06 | 2.60±1.06 | 1.25±0.41** |
| After 6 or more cycles chemotherapy | 356 | 73.73±20.46** | 71.06 ±31.82 | 5.96 ±3.60* | 3.12±2.08 | 1.52±0.48** |
Data were expressed mean ±SD. CT – chemotherapy.
Paired t-test, make comparison to before CT, p<0.05.
Paired t-test, make comparison to before CT, p<0.01.
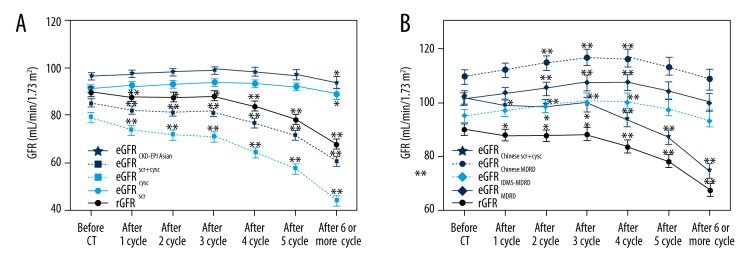
In Figure 1, the mean rGFR and eGFR values from all estimates for all 1000 cancer patients are presented in relation to the chemotherapeutic cycle. The results show that with the increasing number of chemotherapeutic cycles, rGFR gradually decreased and the rGFR began to decrease significantly after 1 cycle of chemotherapy (p<0.05). eGFRcysc, eGFRscr+cysc, and eGFRChinese scr+cysc had the same varying tendency as rGFR. eGFRscr and eGFRCKD-EPI Asian began to decrease after 6 or more cycles of chemotherapy. However, with more cycles of chemotherapy, eGFRMDRD, eGFRIDMS-MDRD, and eGFRChinese MDRD gradually increased, before decreasing after 5 cycles of chemotherapy.
Figure 1.
(A, B) cGFR (thick line) and the various eGFR presented according to chemotherapeutic cycle with 95% confidence intervals. * Paired t test, comparison before and after CT, p<0.05. ** Paired t test, make comparison before and after CT, p<0.01.
Evaluation of renal function parameters in cancer patients with CKD
Of the 1000 cancer patients, 545 patients with CKD were selected as a second experimental group when they finished chemotherapy. As shown in Table 4, CysC and eGFRChinese scr+cysc had strong correlations with rGFR in both the experimental and control groups. We also found that for the control group, the combined SCr and CysC(SCr-CysC) equations had good test-retest reliability, as the ICC calculations were greater than 0.75. However, in the experimental group, all equations had good test-retest reliability except the eGFRMDRD and eGFRChinese MDRD equations. The eGFRscr and eGFRscr+cysc equations yielded the least amount of bias in the control and experimental groups, respectively. However, eGFRChinese scr+cysc and eGFRscr+cysc equations had the least absolute values of bias in the control and experimental groups, respectively. The best precision and accuracy for the 2 groups were obtained with the eGFRChinese scr+cysc and eGFRscr+cysc equations. We considered that the combination equation eGFRscr+cysc performed best for the control and experimental groups, while the eGFRscr and eGFRChinese scr+cysc equations performed better for the control and experimental groups, respectively.
Table 4.
Comparison of the eGFR regarding correlation, bias, precision and accuracy in control group and experimental group.
| Performance | eGFRscr | eGFRcysc | eGFRscr+ cysc | eGFRCKD-EPI Asian | eGFRMDRD | eGFRIDMS-MDRD | eGFRChinese MDRD | eGFRChinese scr+cysc |
|---|---|---|---|---|---|---|---|---|
| Control (n=108) rGFR 106.04±19.69 | ||||||||
| eGFR | 105.84± 11.92 | 92.01± 18.60 | 99.81± 14.93 | 111.45± 12.50 | 118.76± 23.62 | 111.74± 22.22 | 131.26± 27.91 | 119.14± 22.28 |
| Correlation (r) | 0.785** | 0.772** | 0.866** | 0.709** | 0.698** | 0.698** | 0.696** | 0.927** |
| ICC | 0.629** | 0.745** | 0.833** | 0.642** | 0.685** | 0.692** | 0.656** | 0.920** |
| Bias (95%CI) | −0.20 (−25.88~ 25.48) | −14.03 (−37.73~ 9.67) | −6.23 (−21.84~ 9.38) | 5.41 (−20.05~ 30.87) | 12.73 (−18.16~ 43.61) | 5.70 (−23.73~ 35.13) | 25.22 (−11.14~ 61.58) | 13.10 (6.99~ 19.22) |
| Absolute values of bias | 25.48 | 37.73 | 21.84 | 30.87 | 43.61 | 35.13 | 61.58 | 19.22 |
| SD of bias | 13.10 | 12.09 | 7.97 | 12.99 | 15.76 | 15.01 | 18.55 | 3.12 |
| Accuracy P10% | 60.19% | 34.26% | 75% | 52.78% | 36.11% | 50.93% | 21.30% | 50.93% |
| Accuracy P30% | 99.07% | 92.59% | 100% | 96.30% | 87.96% | 93.52% | 65.74% | 100% |
| Patients with CKD (n=545) rGFR 62.36±21.90 | ||||||||
| eGFR | 79.36± 24.45 | 42.63± 17.63 | 56.38± 18.44 | 83.70± 25.82 | 88.18± 35.25 | 82.98± 33.14 | 94.90± 40.05 | 71.14± 25.65 |
| Correlation (r) | 0.785** | 0.772** | 0.898** | 0.785** | 0.784** | 0.784** | 0.721** | 0.911** |
| ICC | 0.780** | 0.754** | 0.885** | 0.775** | 0.738** | 0.758** | 0.691** | 0.900** |
| Bias (95%CI) | 17.00 (−8.81~ 42.82) | −19.72 (−43.66~ 4.22) | −5.97 (−18.30~ 6.36) | 21.34 (−5.80~ 48.48) | 25.82 (−11.07~ 62.72) | 20.62 (−12.87~ 54.12) | 32.54 (−12.80~ 77.89) | 8.78 (−3.02~ 20.58) |
| Absolute values of bias | 42.82 | 43.66 | 18.30 | 48.48 | 62.72 | 54.12 | 77.89 | 20.58 |
| SD of bias | 13.17 | 12.21 | 6.29 | 13.85 | 18.82 | 17.09 | 23.13 | 6.02 |
| Accuracy P10% | 15.78% | 9.17% | 62.94% | 7.89% | 10.09% | 15.60% | 4.22% | 46.24% |
| Accuracy P30% | 55.60% | 40.92% | 99.45% | 40.55% | 34.31% | 51.19% | 24.77% | 97.98% |
rGFR and eGFR were expressed as mean ±SD in mL/min/1.73 m2; r – Pearson’s correlation with the rGFR;
p<0.01.
ICC – Intraclass Correlation Coefficient; P10 – refers to percentage of GFR estimates within 10% of rGFR; P30 – refers to percentage of GFR estimates within 30% of rGFR.
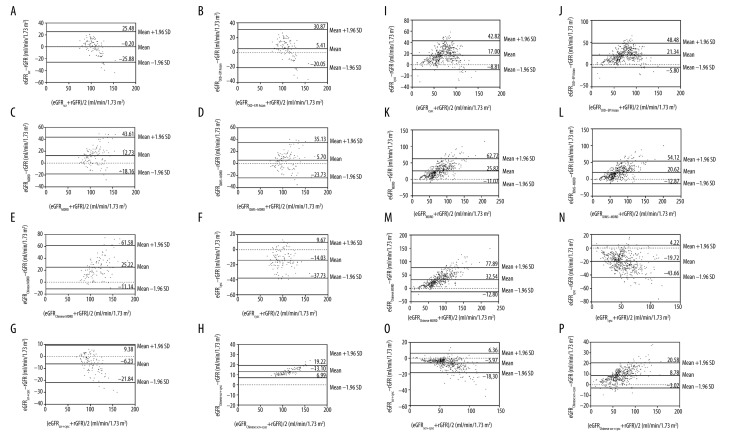
A Bland-Altman plot analysis was then applied to describe agreement between the rGFR and different estimated GFR values in the control and experimental groups (Figure 2). The results showed that compared with the other estimated GFR equations, the eGFRscr and eGFRscr+cysc equations yielded minimum bias for the control and experimental groups, respectively.
Figure 2.
Bland-Altman plot for differences between estimated GFR and cGFR. Solid line represents the mean of difference and 95% limits of agreement of the mean of difference between GFR. The estimated GFR of control and experimental groups are respectively shown in the (A–H) and (I–P) plots, respectively.
Comparison of the diagnostic accuracy of various GFR equations and serological indicators
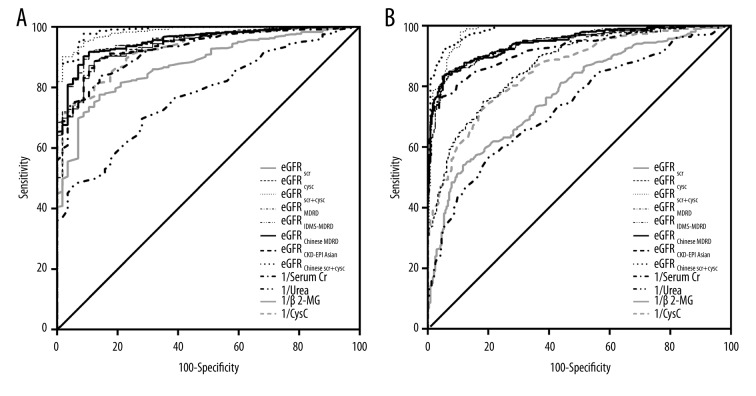
The ROC curve was used to evaluate the discriminating ability of the GFR equations and serological indicators of renal function (Figure 3). In this study, GFR <90 ml/min/1.73 m2 and GFR <60 ml/min/1.73 m2 were set as critical values, which were regarded as markers of early and moderate RI, respectively. In addition, the KDIGO guidelines recommend a decrease in cisplatin dose when GFR is <60 ml/min/1.73 m2, which was the other reason for setting 60 ml/min/1.73 m2 as a critical value. The results indicated that for both early and moderate RI, eGFRChinese scr+cysc and eGFRscr+cysc had the largest and second largest AUCs, respectively, indicating that the combination SCr-CysC equations had improved diagnostic accuracy over the other equations. Among the 4 serological indicators, CysC had the largest AUC (0.926, p<0.001) for early RI, and serum Cr had the largest AUC (0.924, p<0.001) for moderate RI. The coordinates of the ROC curves were used to define cutoff values with acceptable sensitivity and specificity for each formula, and serological indicators, cutoff values, sensitivities, specificities, and AUCs are presented in supplemental data, Table 2.
Figure 3.
Receiver operator characteristic (ROC) curve analysis for measures and various formulas in Chinese cancer patients with CKD. a: ROC curves with cGFR <90 ml/min/1.73 m2; b: ROC curves with cGFR <60 ml/min/1.73 m2.
Results according to subgroup
We further conducted a subgroup analysis for the experimental group according to GFR category, sex, age, and surgical options (Table 5). The results of our investigation were as follows: 1) The rGFR values for the 545 cancer patients with CKD were divided into 6 categories. SCr, urea, β2-MG, and CysC increased with increasing CKD stage, but the increases followed different trends. One-way ANOVA showed that the serum CysC increased significantly in the experimental group compared with the control group in each category (p<0.05), while SCr began to increase significantly in G3a. Urea and β2-MG showed a marked increase in G2 (p<0.05). 2) Male patients had higher SCr and CysC levels compared with female patients. 3) rGFR was significantly decreased in cancer patients over the age of 55 years, and the other parameter values were not significantly different between any of the age groups. 4) None of the renal parameter values were significantly different between the operated and unoperated cancer patients.
Table 5.
Results of renal function parameters in subgroups.
| n | rGFR (ml/min/1.73 m2) | Serum Cr (μmol/l) | Urea (mmol/l) | β2-MG (mmol/l) | CysC (mg/l) | |
|---|---|---|---|---|---|---|
| Stratification by GFR category | ||||||
| G1 | 57 | ≥90 | 52.07±9.96 | 4.63±1.26 | 1.95±0.50 | 1.09±0.20* |
| G2 | 184 | 60–89 | 66.10±10.88 | 5.64±1.68** | 2.81±1.02** | 1.43±0.27** |
| G3a | 204 | 45–59 | 89.09±16.19** | 6.50±2.28** | 3.49±1.57** | 1.76±0.42** |
| G3b | 71 | 30–44 | 117.44±23.81** | 8.57±2.69** | 4.84±1.86** | 2.30±0.50** |
| G4 | 22 | 15–29 | 188.50±41.66** | 14.96±7.83** | 7.49±3.85** | 3.09±0.43** |
| G5 | 7 | <15 | 438.14±225.06** | 25.34±8.83** | 15.14±8.05** | 6.59±4.26** |
| Stratification by gender | ||||||
| Male | 325 | 63.08±23.18 | 94.66±62.20 | 7.09±3.73 | 3.60±2.22 | 1.84±0.98 |
| Female | 220 | 60.71±18.61 | 78.19±40.41** | 6.37±4.42 | 3.55±2.94 | 1.60±0.69** |
| Stratification by age | ||||||
| <55 | 112 | 70.88±23.40#,@ | 85.71±69.46 | 6.33±2.98 | 3.21±2.10 | 1.62±0.55 |
| 55~65 | 206 | 60.04±22.11& | 95.65±67.56 | 7.10±3.98 | 3.84±3.10 | 1.88±1.13 |
| ≥65 | 227 | 60.26±19.92& | 86.14±35.18 | 6.92±4.34 | 3.54±1.90 | 1.73±0.81 |
| Stratification by surgical options | ||||||
| Operated | 259 | 62.25±22.85 | 90.97±59.65 | 6.80±3.60 | 3.38±1.83 | 1.76±0.82 |
| Unoperated | 286 | 62.45±21.05 | 88.45±54.42 | 6.93±4.27 | 3.78±2.91 | 1.77±0.98 |
Data were expressed as mean ±SD.
Independent samples T test, p<0.05;
Independent samples T test, p<0.01;
One way ANOVA compared with patients aged <55, 55~65, ≥65, respectively.
Discussion
Because of the high prevalence of RI, it is necessary to monitor renal function in cancer patients receiving chemotherapy, especially platinum-containing chemotherapy. Notably, several studies have indicated that platinum-based chemotherapy causes acute kidney injury in 25% of patients [17–19]. GFR is the best indicator of renal function and is the main basis for the diagnosis and staging of CKD, as well as for evaluation of the severity of kidney disease and therapeutic outcomes [20]. Many studies, as well as the KDIGO guidelines, indicate the dose of cisplatin should be reduced when GFR is below 60 ml/min/1.73 m2 [13,21,22]. Thus, precise measurement of GFR is needed for renal function monitoring after chemotherapy in these patients to adjust to appropriate medication doses as well as to detect early renal injury.
Inulin clearance remains the criterion standard GFR tracer; however, it is inconvenient and difficult to measure. The clearance of radionuclide (51Cr-EDTA or 99mTc-DTPA) is considered to be closest to that of inulin, but it is radioactive and its use is time-consuming. Therefore, scholars have developed many equations to estimate GFR, which can be divided into 3 categories: creatinine-based, which remain the most prevalent, followed by cystatin C-based and creatinine/cystatin C-based. All the equations were developed in CKD patients without cancer. However, whether these equations can be used to replace the assessment of rGFR in cancer patients whose SCr and CysC production has been changed by the disease is unclear.
In our study, creatinine-based equations (eGFRscr, eGFRCKD-EPI Asian, eGFRMDRD, eGFRIDMS-MDRD, and eGFRChinese MDRD) were studied. Their changes with increased numbers of chemotherapeutic cycles were not obvious compared with those obtained from equations containing CysC. We also found that creatinine-based equations overestimated rGFR during chemotherapy (Figures 1, 2, Table 4). These findings are in accordance with the results of Salek et al. [13]. This is likely because creatinine is not only filtrated through glomeruli, but is also secreted from the proximal tubuli, leading to overestimation [23]. eGFRscr is the current equation recommended for use in clinical practice [24]. Nonetheless, in our study, the eGFRscr equation did not provide the best reflection of the GFR value for Chinese cancer patients with CKD, although it did perform better in the control group. The 4-level race equation eGFRCKD-EPI Asian was considered more accurate than the 2-level race equation eGFRscr in the Chinese dataset [10]. However, in our study, the eGFRscr equation was found to be superior to the eGFRCKD-EPI Asian equation in Chinese cancer patients with CKD, which is consistent with the results of previous studies [25,26]. In addition, Levey considered that when the calibration of SCr methods is traceable to the SCr reference system, the GFR should be estimated using the eGFRIDMS-MDRD equation [7]. Accordingly, our study showed similar results; specifically, eGFRIDMS-MDRD performed the best among the 3 MDRD equations. However, they all seemed to be unsuitable for assessing renal function in Chinese cancer patients with CKD. SCr did not increased until the GFR had already deteriorated to below 60 mL/min/1.73 m2 (Table 5), and GFR at this level indicates the dose of cisplatin should be reduced. From this point of view, the creatinine-based equations to estimate GFR are not safe for patients with injured renal function. Therefore, we do not recommend this method in cancer patients, whose renal function gradually deteriorate with reinforced courses of chemotherapy.
We evaluated whether a cystatin C-based equation provided by CKD-EPI (eGFRcysc) is also applicable in Chinese cancer patients. It is disappointing that CysC alone did not improve the estimation. The comparison of eGFRcysc with rGFR indicates that eGFRcysc was underestimated rGFR during chemotherapy (Figures 1, 2, Table 4). It is easy to establish a deteriorative staging of CKD or erroneous diagnosis as CKD to influence the dose of chemotherapy in cancer patients. In addition, eGFRcysc had lower AUC in both early and moderate RI (supplemental data, Table 2). This finding may be due to an elevation in the CysC value due to cancer (Table 2). Our findings are consistent with those of previous studies [13,27–29], probably due to the role of CysC as a cysteine protease inhibitor. However, several studies have revealed no correlation between CysC concentration and tumor burden [30,31].
It is important to note that the combination of equations using both SCr and CysC performed better in the studied cohort of Chinese cancer patients. eGFRscr+cysc was the most appropriate because it had the least bias, the highest accuracy, and second largest AUCs for early and moderate RI (Table 4, Figures 2, 3, supplemental data, Table 2). In addition, the eGFRChinese scr+cysc equation had the strongest correlation, best precision, and largest AUCs for early and moderate RI (Table 4, supplemental data, Table 2, Figure 3), indicating that the combination creatinine-cystatin C equations had higher diagnostic accuracy than the others. Our results are in accordance with previous studies [32–35]. The GFR of Chinese cancer patients with CKD can be estimated accurately by creatinine-cystatin C equations, which is of great help for the choice of therapeutic strategies and adjustment of drug dosage.
Our results regarding the evaluation of 4 serological indicators for renal function indicated that CysC, which can detect minor changes in renal function at an early stage with the largest AUC (supplemental data, Table 2, Figure 3) and increased significantly when rGFR ≥90 ml/min/1.73 m2 (Table 5), is a superior indicator to other contemporary markers and traditional markers, which is consistent with the results of several previous studies [20,23,36,37]. CysC increased significantly after the early cycle of chemotherapy (Table 3). These results indicate that CysC is an early indicator of injured renal function in all cancer patients. In patients with moderate RI, SCr had the largest AUC, and the cutoff value was 83.33 μmoL/L for moderate RI, indicating that SCr had high diagnostic accuracy and that the dosage of cisplatin should be reduced when the concentration of SCr is >83.33 μmoL/L. Interestingly, CysC and SCr increased significantly in cancer patients, which was in agreement with the results of several studies [38–40]. It is necessary and helpful to refine the equations to estimate GFR for cancer patients, which will be a future focus of further research.
We found that β2-MG also demonstrated good sensitivity for detecting early RI for patients receiving platinum-containing protocols (Table 3). In addition, β2-MG was also a remarkable indicator for monitoring early renal injury in patients receiving chemotherapy with platinum-containing protocols. We found that the operation did not affect kidney function index in our study. As shown in our results, sex and age are important factor for the assessment of kidney function, due to the higher levels of SCr and CysC in male patients and lower rGFR in patients over the age of 55 years (Table 5). Therefore, physicians should use different standards in patients of different sexes or ages to evaluate renal function.
Conclusions
Cancer patients differ from the general population and are a very special group of patients. Our study showed that individual differences exist between rGFR and all eGFR, which has a great impact on the discovery rate of CKD and potential drug dosage adjustment. In summary, our study shows that CysC is the most sensitive marker for early renal injury, and the combination equations using both SCr and CysC performed better in the studied cohort of Chinese cancer patients. It is important to monitor the change in GFR when CysC is abnormal to detect early renal injury in time. One important limitation of this study is that only 7 patients with CKD stage 5 were enrolled. Therefore, a further study is necessary to broaden the research to include a larger patient population.
Acknowledgement
Grateful acknowledgement is made to my supervisor Mrs. Zhou Yunli who gave me considerable help by means of suggestion, comments, and criticism. Her encouragement and unwavering support has sustained me through frustration and depression. Without her pushing me ahead, the completion of this thesis would be impossible.
Footnotes
Conflict of interest
None.
Source of support: This work was funded by grants from the National Natural Science foundation of China (81201653) and the Natural Science Foundation of Tianjin (16JCYBJC26000) to Zhou Y, the National Natural Science foundation of China (81502519) to DongDong, and the National Natural Science foundation of China (31501091) to ShaoJie
References
- 1.Launay-Vacher V. Epidemiology of chronic kidney disease in cancer patients: Lessons from the IRMA study group. Semin Nephrol. 2010;30:548–56. doi: 10.1016/j.semnephrol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Janus N, Launay-Vacher V, Byloos E, et al. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer. 2010;103:1815–21. doi: 10.1038/sj.bjc.6605979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleber M, Cybulla M, Bauchmuller K, et al. Monitoring of renal function in cancer patients: an ongoing challenge for clinical practice. Ann Oncol. 2007;18:950–58. doi: 10.1093/annonc/mdm055. [DOI] [PubMed] [Google Scholar]
- 4.Tangri N, Stevens LA, Schmid CH, et al. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. 2011;79:471–77. doi: 10.1038/ki.2010.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–72. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 8.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. New Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79:555–62. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–44. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 12.Ma YC, Zuo L, Chen JH, et al. Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int. 2007;72:1535–42. doi: 10.1038/sj.ki.5002566. [DOI] [PubMed] [Google Scholar]
- 13.Salek T, Vesely P, Bernatek J. Estimated glomerular filtration rate in oncology patients before cisplatin chemotherapy. Klin Onkol. 2015;28:273–77. [PubMed] [Google Scholar]
- 14.Lauritsen J, Gundgaard MG, Mortensen MS, et al. Reliability of estimated glomerular filtration rate in patients treated with platinum containing therapy. Int J Cancer. 2014;135:1733–39. doi: 10.1002/ijc.28816. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz M, Lahoti A, O’Brien S, et al. Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer. 2015;121:3894–904. doi: 10.1002/cncr.29587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funakoshi Y, Fujiwara Y, Kiyota N, et al. Prediction of glomerular filtration rate in cancer patients by an equation for Japanese estimated glomerular filtration rate. Jpn J Clin Oncol. 2013;43:271–77. doi: 10.1093/jjco/hys235. [DOI] [PubMed] [Google Scholar]
- 17.Solanki MH, Chatterjee PK, Xue X, et al. Magnesium protects against cisplatin-induced acute kidney injury without compromising cisplatin-mediated killing of an ovarian tumor xenograft in mice. Am J Physiol Renal Physiol. 2015;309:F35–47. doi: 10.1152/ajprenal.00096.2015. [DOI] [PubMed] [Google Scholar]
- 18.Solanki MH, Chatterjee PK, Gupta M, et al. Magnesium protects against cisplatin-induced acute kidney injury by regulating platinum accumulation. Am J Physiol Renal Physiol. 2014;307:F369–84. doi: 10.1152/ajprenal.00127.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaeidi A, Rasoulian B, Hajializadeh Z, et al. Cisplatin toxicity reduced in human cultured renal tubular cells by oxygen pretreatment. Ren Fail. 2013;35:1382–86. doi: 10.3109/0886022X.2013.829406. [DOI] [PubMed] [Google Scholar]
- 20.Du Y, Sun TT, Hou L, Guo JJ, et al. Applicability of various estimation formulas to assess renal function in Chinese children. World J Pediatr. 2015;11:346–51. doi: 10.1007/s12519-014-0532-7. [DOI] [PubMed] [Google Scholar]
- 21.Gonzales-Vitale JC, Hayes DM, Cvitkovic E, Sternberg SS. The renal pathology in clinical trials of cis-platinum (II) diamminedichloride. Cancer. 1977;39:1362–71. doi: 10.1002/1097-0142(197704)39:4<1362::aid-cncr2820390403>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Patterson WP, Reams GP. Renal toxicities of chemotherapy. Semin Oncol. 1992;19:521–28. [PubMed] [Google Scholar]
- 23.Bolke E, Schieren G, Gripp S, et al. Cystatin C – a fast and reliable biomarker for glomerular filtration rate in head and neck cancer patients. Strahlenther Onkol. 2011;187:191–201. doi: 10.1007/s00066-010-2203-5. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DW, Jones GR, Mathew TH, et al. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: New developments and revised recommendations. Med J Aust. 2012;197:224–25. doi: 10.5694/mja11.11329. [DOI] [PubMed] [Google Scholar]
- 25.Kong X, Ma Y, Chen J, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating glomerular filtration rate in the Chinese population. Nephrol Dial Transplant. 2013;28:641–51. doi: 10.1093/ndt/gfs491. [DOI] [PubMed] [Google Scholar]
- 26.Lamb EJ, Stevens PE. Estimating and measuring glomerular filtration rate: Methods of measurement and markers for estimation. Curr Opin Nephrol Hypertens. 2014;23:258–66. doi: 10.1097/01.mnh.0000444813.72626.88. [DOI] [PubMed] [Google Scholar]
- 27.Nakai K, Kikuchi M, Fujimoto K, et al. Serum levels of cystatin C in patients with malignancy. Clin Exp Nephrol. 2008;12:132–39. doi: 10.1007/s10157-008-0043-8. [DOI] [PubMed] [Google Scholar]
- 28.Bodnar L, Wcislo GB, Elichowski G, et al. [Cystatin C as a marker of glomerular filtration rate in patients with ovarian cancer]. Pol Merkur Lekarski. 2008;24:307–11. [in Polish] [PubMed] [Google Scholar]
- 29.Shibata K, Yasuda Y, Kobayashi R, et al. Renal function evaluation in patients with cancer who were scheduled to receive carboplatin or S-1. Clin Exp Nephrol. 2015;19:1107–13. doi: 10.1007/s10157-015-1115-1. [DOI] [PubMed] [Google Scholar]
- 30.Lankisch P, Wessalowski R, Maisonneuve P, et al. Serum cystatin C is a suitable marker for routine monitoring of renal function in pediatric cancer patients, especially of very young age. Pediatr Blood Cancer. 2006;46:767–72. doi: 10.1002/pbc.20581. [DOI] [PubMed] [Google Scholar]
- 31.Mojiminiyi OA, Marouf R, Abdella N, et al. Serum concentration of cystatin C is not affected by cellular proliferation in patients with proliferative haematological disorders. Ann Clin Biochem. 2002;39:308–10. doi: 10.1258/0004563021902017. [DOI] [PubMed] [Google Scholar]
- 32.Chew-Harris JS, Florkowski CM, George PM, Endre ZH. Comparative performances of the new chronic kidney disease epidemiology equations incorporating cystatin C for use in cancer patients. Asia-Pac J Clin Oncol. 2015;11:142–51. doi: 10.1111/ajco.12312. [DOI] [PubMed] [Google Scholar]
- 33.Fan L, Levey AS, Gudnason V, et al. Comparing GFR estimating equations using cystatin C and creatinine in elderly individuals. J Am Soc Nephrol. 2015;26:1982–89. doi: 10.1681/ASN.2014060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemoine S, Panaye M, Pelletier C, et al. Cystatin C-creatinine based glomerular filtration rate equation in obese chronic kidney disease patients: impact of deindexation and gender. Am J Nephrol. 2016;44:63–70. doi: 10.1159/000447365. [DOI] [PubMed] [Google Scholar]
- 35.Kukla A, Issa N, Jackson S, et al. Cystatin C enhances glomerular filtration rate estimating equations in kidney transplant recipients. Am J Nephrol. 2014;39:59–65. doi: 10.1159/000357594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasouli HA, Moghadam MM, Tabatabaiefar M, et al. Comparing cystatin C changes as a measure of renal function before and after radiotherapy in patients with stomach cancer. Acta Med Iran. 2012;50:43–46. [PubMed] [Google Scholar]
- 37.Wang M, Zhang L, Yue R, et al. Significance of Cystatin C for early diagnosis of contrast-induced nephropathy in patients undergoing coronary angiography. Med Sci Monit. 2016;22:2956–61. doi: 10.12659/MSM.897241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demirtas S, Akan O, Can M, et al. Cystatin C can be affected by nonrenal factors: A preliminary study on leukemia. Clin Biochem. 2006;39:115–18. doi: 10.1016/j.clinbiochem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Yano M, Hirai K, Naito Z, et al. Expression of cathepsin B and cystatin C in human breast cancer. Surg Today. 2001;31:385–89. doi: 10.1007/s005950170126. [DOI] [PubMed] [Google Scholar]
- 40.Uyeturk U, Sarici H, Kin Tekce B, et al. Serum omentin level in patients with prostate cancer. Med Oncol. 2014;31:923. doi: 10.1007/s12032-014-0923-6. [DOI] [PubMed] [Google Scholar]