Abstract
Background and purpose
For the first time, delivered dose to the rectum has been calculated and accumulated throughout the course of prostate radiotherapy using megavoltage computed tomography (MVCT) image guidance scans. Dosimetric parameters were linked with toxicity to test the hypothesis that delivered dose is a stronger predictor of toxicity than planned dose.
Material and methods
Dose–surface maps (DSMs) of the rectal wall were automatically generated from daily MVCT scans for 109 patients within the VoxTox research programme. Accumulated-DSMs, representing total delivered dose, and planned-DSMs, from planning CT data, were parametrised using Equivalent Uniform Dose (EUD) and ‘DSM dose-width’, the lateral dimension of an ellipse fitted to a discrete isodose cluster. Associations with 6 toxicity endpoints were assessed using receiver operator characteristic curve analysis.
Results
For rectal bleeding, the area under the curve (AUC) was greater for accumulated dose than planned dose for DSM dose-widths up to 70 Gy. Accumulated 65 Gy DSM dose-width produced the strongest spatial correlation (AUC 0.664), while accumulated EUD generated the largest AUC overall (0.682). For proctitis, accumulated EUD was the only reportable predictor (AUC 0.673). Accumulated EUD was systematically lower than planned EUD.
Conclusions
Dosimetric parameters extracted from accumulated DSMs have demonstrated stronger correlations with rectal bleeding and proctitis, than planned DSMs.
Keywords: Rectal toxicity, VoxTox, Dose–surface maps, Delivered dose, Prostate radiotherapy
In prostate radiotherapy, the correlation between dose to rectum and toxicity has been the focus of many research studies [1], [2], [3], [4], [5], [6], [7]. The rectum is one of the dose-limiting organs when planning intensity-modulated radiotherapy (IMRT) to the prostate due to the risk of radiation-induced adverse effects. Modern systems for inverse IMRT treatment planning iteratively seek to achieve an optimal plan, delivering maximal dose to the tumour volume and minimal dose to healthy organs. Current normal tissue complication probability (NTCP) models and conventional treatment planning constraints are based upon dose–volume histogram (DVH) data to minimise the risk of toxicity. With ever improving disease control [8], [9] and survival rates [10], post-treatment quality of life becomes an increasingly significant consideration during treatment planning, alongside target coverage.
The DVH-based approach to radiotherapy treatment planning has been criticised for lacking in spatial dose consideration [2]. Consequently, accumulation of DVHs is not dosimetrically representative and results in false overestimations of dose. A review by Landoni et al. [11] emphasises the need to assess associations between spatial dose patterns and late toxicity [12], particularly as results may reveal inhomogeneous intra-organ radiosensitivities.
Several groups have explored alternative approaches for parametrisation of dose distributions in order to establish links with toxicity. Methods have included dose–surface histograms [1], [5], [13], [14], dose–surface maps [1], [5], [15], dose–line histograms [14], principal component-based pattern analysis [16], and voxel-based approaches for identifying rectal subregions [2], [6], [7]. These studies have been limited in their analysis by the availability of planned dose data only, based on a single anatomical snapshot in time.
A common recommendation in the literature has been the need to establish dose-toxicity models based on delivered dose [17]. However, this has proven technically challenging to date due to hardware and software limitations. These challenges have been addressed within the VoxTox Research Programme [18] where contours generated from on-treatment megavoltage computed tomography (MVCT) image guidance scans are used to calculate daily delivered dose. This approach has made it possible to account for the effect of interfractional anatomical variation. Total delivered dose can be estimated by accumulating daily delivered dose throughout the course of radiotherapy. Studies by the VoxTox group have demonstrated that the rectum moves more than previously predicted based on estimates from prostate motion [19], and that planned dose is not equal to delivered dose [20].
The dose–surface map (DSM) approach has been implemented within this study as a solution enabling meaningful accumulation and conservation of geometric information, an advantage over the DVH methodology. The concept of accumulating DSMs to estimate total delivered dose has been applied previously for the bladder [13]. By extracting spatial parameters from DSMs of delivered dose, and linking with the archive of patient follow-up data available within VoxTox, it was hypothesised that stronger correlations could be established with late toxicity than previously achievable using planned dose alone. Ultimately, improved dose-toxicity modelling based on delivered dose could facilitate real-time in silico prediction of NTCP within the clinical pathway.
Material and methods
VoxTox study design & patient information
The VoxTox research programme is an observational study linking radiation dose to toxicity outcomes [18], [20]. It received approval from the National Research Ethics Service (NRES) Committee East of England (13/EE/0008) in February 2013 and is part of the UK Clinical Research Network Study Portfolio (UK CRN ID 13716).
One hundred and nine prostate cancer patients were selected from the discovery cohort of the VoxTox research programme [18]. This cohort (Table 1) comprised patients treated prior to the formal collection of baseline data, but for whom prospective follow-up data of at least 2 years were available (median 4 years). Early VoxTox patients were selected based on expected benefit from IMRT rather than conventional 3D conformal radiotherapy. Patients in this study were included on the basis of availability of pre-existing toxicity status from clinical notes, or no reported toxicity, and was limited to those prescribed IMRT to a dose of 74 Gy in 37 fractions, the standard of care in the UK at the time [21]. VoxTox patients are treated with TomoTherapy® (Accuray, Sunnyvale, CA). Manual contouring of the anatomy on the kilovoltage computed tomography (kVCT) planning scan was performed according to local procedures [19], adapted from clinical trials. Daily MVCT image guidance scans were acquired immediately prior to treatment delivery for the purposes of online target localisation [22]. Following our department protocol, scans were inspected for rectal dilation and if deemed excessive, remedial action was taken prior to delivery of radiation therapy [23].
Table 1.
Baseline characteristics for the 109 VoxTox participants. Prescribed dose to the prostate was 74 Gy in 37 fractions over 7.5 weeks. All patients were treated with androgen deprivation therapy. IBD = inflammatory bowel disease, IQR = interquartile range, PSA = prostate-specific antigen, SD = standard deviation.
| Clinical data for VoxTox patients (n = 109) | |
|---|---|
| Age, years | |
| Median (IQR) | 68 (64–71) |
| Range | 51–80 |
| T stage,n(%) | |
| T1A/T1B/T1C/T1X | 24 (22%) |
| T2A/T2B/T2C/T2X | 34 (31%) |
| T3A/T3B/T3X | 45 (41%) |
| T4 | 0 |
| Not known | 6 (6%) |
| Gleason score,n(%) | |
| 6 | 23 (21%) |
| 7 | 44 (40%) |
| ⩾8 | 39 (36%) |
| Not known | 3 (3%) |
| PSA (ng/ml) | |
| Median (IQR) | 11 (7–20) |
| Mean (SD) | 20 (30) |
| Not known | 3 (3%) |
| Clinical history | |
| Diabetes | 10 (9%) |
| Hypertension | 35 (32%) |
| IBD or diverticular disease | 7 (6%) |
| Previous pelvic surgery | 7 (6%) |
| Haemorrhoids past 12 months | 3 (3%) |
| Any previous TURP | 9 (8%) |
| Not known | 9 (8%) |
Dose–surface map construction & dose accumulation
Within the VoxTox research programme, MVCT scans are multifunctional; primarily for the purpose of routine image guidance, they also provide a platform for calculation of delivered dose. The rectum was identified on each MVCT image series using an in-house autocontouring system based on a customised Chan-Vese segmentation algorithm [24]. Delivered dose was independently calculated using a locally implemented ray-tracing algorithm [25], [26] and the rectal contour-of-the-day, accounting for inter-fraction motion. Automation and integration of dose calculation and contouring systems were essential for large-scale processing of the 4142 scans in this study.
Planned and daily DSMs were generated based on algorithms described by Buettner et al. [1] and Murray et al. [15]. The rectal wall was considered the structure of interest, and was treated as a tubular surface rather than a volume. Contours were virtually ‘cut’ along the superior–inferior axis and ‘unfolded’ to a two-dimensional plane. The ‘cutting point’ was identified as the point on the contour surface directly posterior to the centre of mass of the rectal outline, on each CT slice [20].
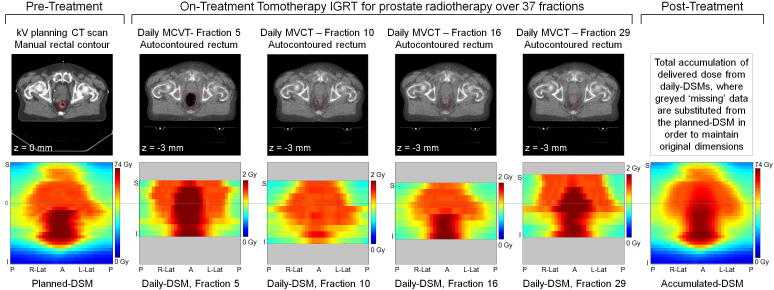
The height of the planned-DSM was defined by the number of slices of the manually contoured rectum on the kV planning scan (slice thickness 3 mm). The circumference of the rectal contour on each slice was normalised such that the unfolded width of the planned-DSM was equal to the height. Daily delivered DSMs calculated from the image-guidance MVCT scans (slice thickness 6 mm) were normalised to the same width as the planned-DSM but were restricted in height by the field of view (FOV), resulting in a shorter DSM, as shown in Fig. 1.
Fig. 1.
Generation of planned, daily and accumulated dose surface maps.
Rectal DSMs were calculated for each treatment fraction, and corrected for daily couch shifts. For the purposes of dose accumulation, any ‘missing’ dose data cropped by the restricted FOV superiorly or inferiorly were substituted from the planned-DSM [20] in order to maintain common dimensions between final accumulated-DSM and planned-DSM. The final accumulated-DSM was resampled to match the 3 mm resolution of the planned-DSM, producing an easily comparable and interpretable spatial representation of total delivered dose to the rectal wall throughout the course of prostate radiotherapy [25] (illustrated in Fig. 1).
The use of planned-DSM data as a surrogate beyond the boundaries of the MVCT FOV was considered an acceptable estimate under the assumption that the relative anatomical motion of the rectum becomes more confined by surrounding musculature as the distance from the prostate increases [20]. However, this could have reduced potential differences between planned and accumulated dose, and was a limitation of the analysis.
Dose parameters & clinical endpoints
Dose was parametrised from DSMs using two methods implemented in MATLAB® (MathWorks®, Natick, MA):
-
1.
Calculation of Equivalent Uniform Dose (EUD)
-
2.
Fitting of DSM ‘dose-widths’ to discrete isodose clusters
EUD reduces the dose information extracted from the DSMs to a single generalised value which allows comparison between inhomogeneous dose distributions [27]. An ‘a’ value of 11.11 was used in the EUD calculation [28]. Spatial dose information was generated by reproducing Buettner’s ellipse-fitting method [1], reporting the most significant dose quantifier, the lateral extent, termed here the ‘DSM dose-width’.
For a given isodose level, a binary image was created from the DSM by assigning a pixel value of 1 to doses greater than or equal to the nominated isodose, with lower doses assigned a value of 0. An ellipse was then fitted to the largest central cluster. The maximum lateral extent of the ellipse was projected onto the DSM axis, accounting for any rotation with respect to the DSM coordinate system. The resulting DSM dose-width, expressed as a the percentage of total normalised DSM width, allowed parametrisation of the geometrical dose distribution which would have been masked using a DVH approach.
For each patient, EUD and DSM dose-widths for isodose levels of 30, 40, 50, 60, 65, and 70 Gy were calculated from planned-DSM and accumulated-DSM. Doses less than 30 Gy were not included as DSM dose-width results became dominated by extrapolated values greater than 100%, indicating that the entire rectal circumference was receiving less than or equal to the selected isodose level. This was identified as a limitation of the ellipse fitting method when seeking to analyse low dose toxicity correlations. Doses greater than 70 Gy were also excluded from toxicity analyses due to the increasing frequency of 0% DSM dose-widths, indicating that doses greater than or equal to the selected isodose level were not received by the rectal wall. Only 49/109 patients recorded a non-zero result from accumulated DSM at 74 Gy, reducing to 10/109 at 75 Gy, compared with 106/109 and 64/109 respectively from planned-DSM. It was identified that a 0% DSM result could conceal information leading to misinterpretation of data when performing AUC calculations so results at these isodose levels were not reported. Despite these restriction, the dose levels included within this study incorporate the 39–61 Gy range at which Buettner [1] determined significant correlations between lateral extent and toxicity.
Study specific clinical reporting forms were developed for robust collection of toxicity data, and raw data were used to populate recognised systems, including: Common Terminology Criteria for Adverse Events (CTCAE) v4.03 [29], Late Effects of Normal Tissues/Subjective, Objective, Management, Analytic (LENT SOMA) scores [30]; Radiation Therapy Oncology Group (RTOG) grading system [31]; University of California, Los Angeles, Prostate Cancer Index (UCLA-PCI) questionnaire [32]. Receiver Operator Characteristic (ROC) curves (Fig. 2) were generated using SPSS® (IBM® 23.0.0.2) to evaluate the link between dosimetric parameters extracted from planned and accumulated DSMs, and the six most prevalent clinical endpoints, listed in Table 2.
Fig. 2.
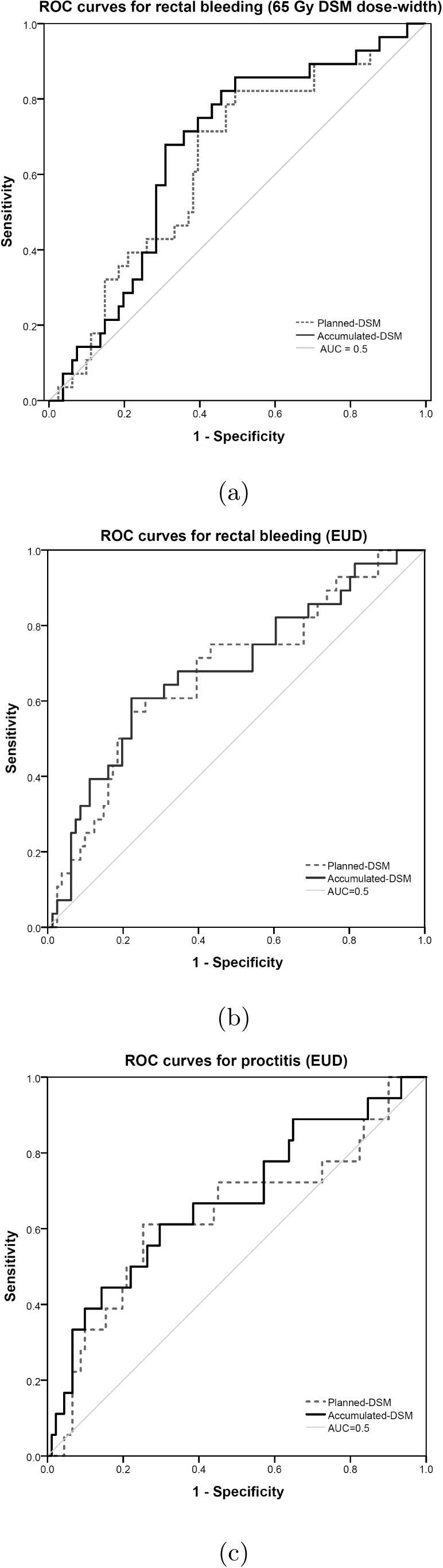
Receiver operator characteristic curves for (a) rectal bleeding with 65 Gy DSM dose-widths, (b) rectal bleeding with EUD, and (c) proctitis with EUD.
Table 2.
Clinical endpoints, scoring systems and incidence rates of the 6 most frequently reported toxicities within the patient sample (*Data were missing for 4 patients so sample size was reduced accordingly).
| Clinical Endpoint | Scoring System | Incidence % (n) |
|---|---|---|
| Rectal Bleeding ⩾Grade 1 (Rectal Bleeding ⩾Grade 2) |
CTCAE [29] (LENT SOMA [30]) |
25.7 (28/109) |
| Proctitis ⩾Grade 2 | RTOG [31]/ Gulliford [35] |
16.5 (18/109) |
| Sphincter Control ⩾Grade 1 | LENT SOMA [30] | 10.1 (11/109) |
| Rectal Pain ⩾Grade 1 | CTCAE [29]/ LENT SOMA [30] |
15.6 (17/109) |
| Bowel bother ⩾Grade 1 | UCLA-PCI [32] | 30.7 (32/105*) |
| Bowel bother ⩾Grade 2 | UCLA-PCI [32] | 11.5 (12/105*) |
The mean area under the curve (AUC), with associated upper and lower 95% confidence intervals (CIs), was calculated for each ROC curve as a measure of the level of association between dosimetric parameter and toxicity. An ideal correlation would have an AUC of 1. Results were reported for dosimetric parameters with AUC ⩾ 0.6 and lower 95% CI ⩾ 0.5, considered statistically significant by Gulliford et al. [33].
Results
Rectal bleeding
Twenty-eight patients reported rectal bleeding CTCAE ⩾Grade 1, which was equivalent to ⩾Grade 2 (LENT SOMA). The AUC was greater for all accumulated DSM dose-widths than planned DSM dose-widths up to 70 Gy (Table 3). At 30, 40 and 60 Gy, the lower 95% CI boundaries for the planned DSM dose-widths extended below 0.5, but remained above this threshold for the corresponding accumulated DSM dose-widths (Fig. 3a). The strongest spatial predictor of rectal bleeding was accumulated 65 Gy DSM dose-width (AUC 0.664), and the largest difference between planned and accumulated DSM dose widths was at 60 Gy (AUC difference 0.035).
Table 3.
Mean Area Under the Curve (AUC) for planned and accumulated DSM dose-widths and EUD corresponding to rectal bleeding ⩾Grade 2 (LENT SOMA) and ⩾Grade 1 (CTCAE), n = 28/109. The greater AUC of each parameter has been presented in bold.
| Dose Level (Gy) | Mean AUC (Planned) | Mean AUC (Accumulated) |
|---|---|---|
| 30 | 0.606 | 0.629 |
| 40 | 0.603 | 0.621 |
| 50 | 0.627 | 0.635 |
| 60 | 0.608 | 0.643 |
| 65 | 0.635 | 0.664 |
| 70 | 0.659 | 0.642 |
| EUD | 0.673 | 0.682 |
Fig. 3.
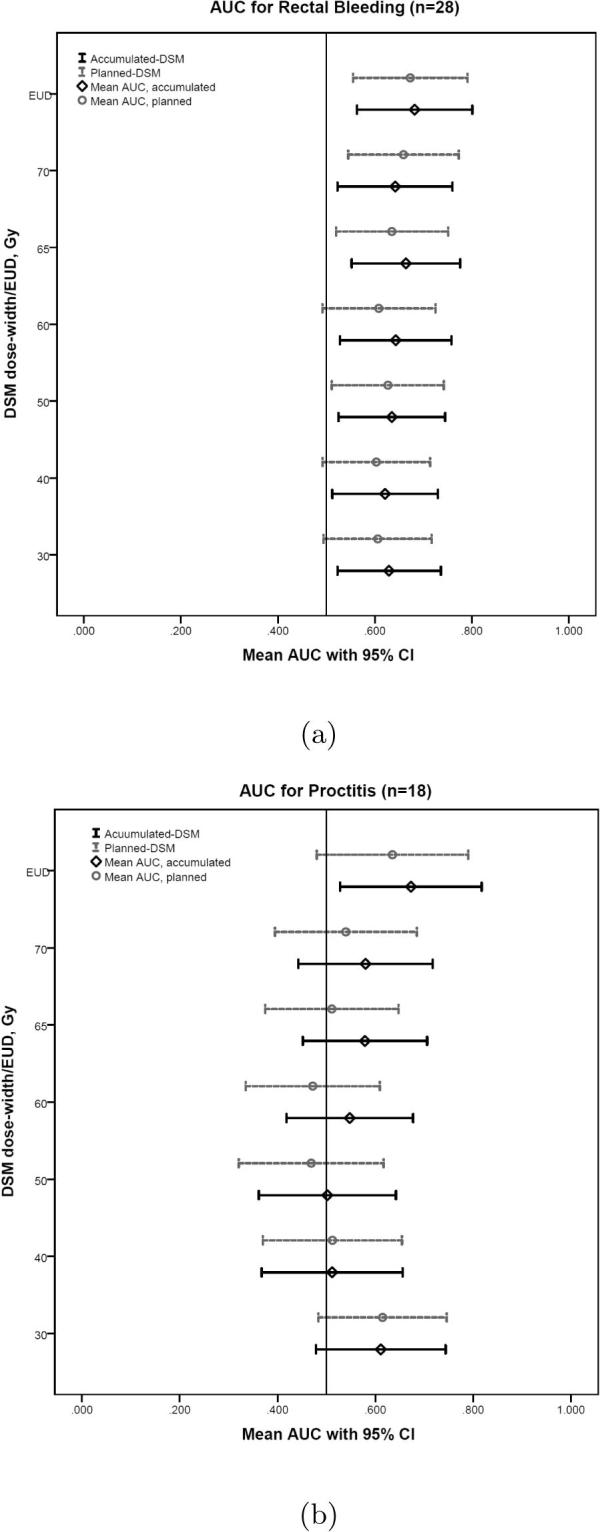
High-low plots of mean AUC and 95% confidence intervals for (a) Rectal Bleeding and (b) Proctitis, where results were considered significant if mean AUC ⩾0.6 and lower 95% CI ⩾0.5.
Overall, for both planned and accumulated DSMs, the AUC was greater for EUD than from respective DSM dose-widths, with the strongest predictor of rectal bleeding being accumulated-EUD (AUC 0.682).
Proctitis
The RTOG definition of proctitis considers urgency and frequency of bowel movements, as well as the presence of rectal mucous/blood. Eighteen patients reported RTOG proctitis ⩾Grade 2. Accumulated-EUD (AUC 0.673) was the only dosimetric parameter with AUC ⩾ 0.6 and lower 95% CI ⩾ 0.5 (Fig. 3b). Accumulated DSM dose-widths had greater AUC than planned DSM dose-widths at 50, 60, 65 and 70 Gy, and were equivalent at 40 Gy. At 30 Gy, the AUC of planned DSM dose-width was slightly higher than the accumulated AUC (0.004 difference).
Remaining clinical endpoints
For the remaining clinical endpoints (LENT SOMA sphincter control ⩾Grade 1; CTCAE/LENT SOMA subjective rectal pain ⩾Grade 1; UCLA-PCI “Overall, how big a problem have your bowel habits been for you during the last 4 weeks?”, bowel bother ⩾Grade 1 and ⩾Grade 2), EUD and DSM dose-widths had little discriminatory power from planned-DSM or accumulated-DSM. No dosimetric parameter was found to have AUC ⩾ 0.6 and lower 95% CI ⩾ 0.5. Results have been included as supplementary data.
Equivalent uniform dose
EUD produced the greatest AUCs for rectal bleeding and proctitis, indicating a stronger association than the spatial parameters investigated. In both cases, accumulated-EUD generated a higher AUC than planned-EUD. For all patients, EUD of accumulated-DSM was lower than that of planned-DSM (mean difference −2.2 Gy, standard error 0.3 Gy, range [−0.3, −7.1] Gy).
Discussion
Radiation dose received by the rectal wall during prostate radiotherapy was calculated and accumulated using DSMs. Geometric aspects of dose distribution - information not distinguishable from DVHs – were parametrised using DSM dose-widths. EUD was calculated to compare planned and accumulated DSMs using a single metric. Extracted dosimetric parameters were evaluated against six clinical endpoints reported by patients within the VoxTox research programme. Previous dose-toxicity investigations in the literature have been limited to planned dose only. This study has demonstrated, for the first time, that delivered dose can be a stronger predictor of toxicity in the case of rectal bleeding and proctitis in prostate radiotherapy.
Toxicity rates reported in the literature have been variable. The rate of bowel toxicity ⩾Grade 2 (CTCAE), 5 year cumulative incidence, amongst VoxTox prostate patients was 17%. This falls within the bowel toxicity ⩾Grade 2 (RTOG) range of 13.7–24.9% for IMRT over the same timeframe, reported by Dearnaley et al. [9] and Wortel et al. [34], respectively. The rates of incidence indicate that toxicity remains an important clinical issue.
Many associations were found between DSM dose-widths with rectal bleeding. Accumulated DSMs generated greater AUCs than planned DSMs for 5 DSM dose-width levels up to 70 Gy. The strongest correlation between rectal bleeding and any spatial parameter was the 65 Gy DSM dose-width from accumulated dose (AUC 0.664). At 30, 40 and 60 Gy, accumulated DSM dose-widths produced AUC ⩾ 0.6 and lower 95% CI ⩾ 0.5, where corresponding planned DSM dose-widths did not. These thresholds were considered indicative of significance following the methods of Gulliford et al. [33]. The greatest difference between planned and accumulated AUCs was observed at the 60 Gy DSM dose-width. Overall, the results compared well with the findings of Buettner et al. [1] who reported the most significant correlation with rectal bleeding to be the 61 Gy lateral extent (AUC 0.66), derived from planned dose data.
Accumulated EUD was found to have the strongest correlation overall with rectal bleeding (AUC 0.682), and was the only predictor of proctitis (AUC 0.673).
For all patients, accumulated-EUD was systematically lower than planned-EUD. A contributory factor was possibly the inherent blurring of high dose regions during accumulation. Upon visual inspection of daily DSMs, the differences in size, shape and position of the high dose region due to anatomical variation was clearly visible (for example, shown in deep red in Fig. 1). During accumulation, high doses were superimposed in overlap regions, but reduced where isodose edges differed, due to averaging over the full course of radiotherapy. This affected the maximum dose of the accumulated-DSM, on which EUD calculation was heavily weighted.
The dose-blurring effect could also have been responsible for the increased frequency of 0% DSM dose-width results at high dose levels from accumulated-DSMs with respect to planned-DSMs. At 70 Gy, 4/109 patients recorded a 0% accumulated DSM dose-width (including 1 patient experiencing toxicity), whereas all corresponding planned DSM dose-widths had non-zero results. Furthermore, dose levels could not be considered independent variables, as a low 70 Gy DSM dose-width was likely to be associated with a low 65 Gy DSM dose-width, and a cooler plan overall. These issues were not accounted for within the scope of this study.
The generally lower reported values for EUD and DSM dose-widths from accumulated dose compared with planned dose should not be interpreted as delivered treatment erring on the ’safe side’ in terms of dose to rectum. Where current NTCP models are based on planned dose, the presented results suggest that the same magnitude of risk would be associated with a systematically lower delivered dose.
The findings show that the difference in dose between patients with and without rectal toxicity is greater from delivered dose than planned dose. This indicates that dosimetric parameters from accumulated-DSMs could provide new information to improve understanding of the relationship between dose and toxicity. The single parameter EUD was a superior predictor of rectal bleeding and proctitis than spatial dose quantifiers. However, DSM-dose widths produced several strong correlations with rectal bleeding, and for 5/6 dose levels, accumulated dose generated AUC values greater than planned dose.
The ability to preserve and accumulate spatial dose information throughout treatment is a novel process requiring careful consideration of data interpretation and parametrisation. Future work may involve exploring alternative methods for geometrical quantification of spatial dose distributions in order to determine stronger correlations with toxicity. Analysis of delivered dose to the rectal wall could facilitate the identification of inhomogeneous intra-organ radiosensitivities, allowing shape-based dose constraints to be derived. Spatial considerations could complement current DVH-based approaches to treatment planning.
Through novel characterisation of delivered dose, beyond the limitations of the static planned DVH, the aim is to determine those parameters strongly associated with rectal toxicity which could be incorporated into multivariate NTCP models. Emerging dose quantifiers could be integrated into planning constraints, as well as being prospectively monitored throughout treatment. Delivered dose can be accumulated in ’real-time’ and analysed with each fraction, allowing on-treatment toxicity risk assessment. Towards the end of the course of treatment, if toxicity prediction was found to be lower than planned, the decision could be made to increase the total delivered dose to the target. The potential scope for further individualisation and adaptation of treatment could ultimately reduce rates of toxicity incidence and improve clinical outcomes.
Conclusion
Parametrisation of delivered dose to the rectal wall during prostate radiotherapy has revealed stronger correlations with rectal bleeding and proctitis than achievable from planned dose. New information from accumulated delivered dose could lead to improved dose-toxicity modelling in the future, with the aim of reducing post-treatment toxicity.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to thank the patients who participated in the study, the referring physicians, and VoxTox Research Facilitator, Michael Simmons. LEAS is supported by the University of Cambridge W D Armstrong Trust Fund; AMB, MRR and KH are supported by the VoxTox Programme Grant, which is funded by Cancer Research UK (CRUK); JES was supported by a CRUK Clinical Research Fellowship; DJN is supported by a CRUK Clinical Research Fellowship; NGB is Principle Investigator of the CRUK VoxTox Programme and is supported by the NIHR Cambridge Biomedical Research Centre.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2017.04.008.
Supplementary data
References
- 1.Buettner F., Gulliford S.L., Webb S., Sydes M.R., Dearnaley D.P., Partridge M. Assessing correlations between the spatial distribution of the dose to the rectal wall and late rectal toxicity after prostate radiotherapy: an analysis of data from the MRC RT01 trial (ISRCTN 47772397) Phys Med Biol. 2009;54:6535–6548. doi: 10.1088/0031-9155/54/21/006. [DOI] [PubMed] [Google Scholar]
- 2.Acosta O., Dréan G., Ospina J.D. Voxel-based population analysis for correlating local dose and rectal toxicity in prostate cancer radiotherapy. Phys Med Biol. 2013;58:2581–2595. doi: 10.1088/0031-9155/58/8/2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wortel R.C., Witte M.G., van der Heide U.A. Dose–surface maps identifying local dose-effects for acute gastrointestinal toxicity after radiotherapy for prostate cancer. Radiother Oncol. 2015;117:515–520. doi: 10.1016/j.radonc.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Coloigner J., Fargeas A., Kachenoura A. A novel classification method for prediction of rectal bleeding in prostate cancer radiotherapy based on a semi-nonnegative ICA of 3D planned dose distributions. IEEE J Biomed Health Informat. 2015;19:1168–1177. doi: 10.1109/JBHI.2014.2328315. [DOI] [PubMed] [Google Scholar]
- 5.Buettner F., Gulliford S.L., Webb S., Partridge M. Modeling late rectal toxicities based on a parameterized representation of the 3D dose distribution. Phys Med Biol. 2011;56:2103–2118. doi: 10.1088/0031-9155/56/7/013. [DOI] [PubMed] [Google Scholar]
- 6.Dréan G., Acosta O., Ospina J.D. Identification of a rectal subregion highly predictive of rectal bleeding in prostate cancer IMRT. Radiother Oncol. 2016;119:388–397. doi: 10.1016/j.radonc.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Gael Dréan G., Acosta O., Lafond C., Simon A., de Crevoisier R., Haigron P. Interindividual registration and dose mapping for voxelwise population analysis of rectal toxicity in prostate cancer radiotherapy. Med Phys. 2016;43:2721–2730. doi: 10.1118/1.4948501. [DOI] [PubMed] [Google Scholar]
- 8.Dearnaley D.P., Jovic G., Syndikus I. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 9.Dearnaley D., Syndikus I., Mossop H. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Research UK. Prostate cancer survival statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/survival#ref-2. Accessed: November 2016
- 11.Landoni V., Fiorino C., Cozzarini C., Sanguineti G., Valdagni R., Rancati T. Predicting toxicity in radiotherapy for prostate cancer. Phys Med. 2016;32:521–532. doi: 10.1016/j.ejmp.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 13.Palorini F., Botti A., Carillo V. Bladder dose–surface maps and urinary toxicity: Robustness with respect to motion in assessing local dose effects. Physica Med. 2016;32:506–511. doi: 10.1016/j.ejmp.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Hamlett L.J., McPartlin A.J., Maile E.J. Parametrized rectal dose and associations with late toxicity in prostate cancer radiotherapy. Br J Radiol. 2015;88:20150110. doi: 10.1259/bjr.20150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray J., McQuaid D., Dunlop A. Su-e-j-14: a novel approach to evaluate the dosimetric effect of rectal variation during image guided prostate radiotherapy. Med Phys. 2014;41:157. [Google Scholar]
- 16.Söhn M., Alber M., Yan D. Principal component analysis-based pattern analysis of dose–volume histograms and influence on rectal toxicity. Int J Radiat Oncol Biol Phys. 2007;69:230–239. doi: 10.1016/j.ijrobp.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 17.Jaffray D.A., Lindsay P.E., Brock K.K., Deasy J.O., Tomé W. Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. Int J Radiat Oncol Biol Phys. 2010;76:S135–S139. doi: 10.1016/j.ijrobp.2009.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CompRT VoxTox. Linking radiation dose at the voxel level with toxicity. http://www.comprt.org/research/voxtox. Accessed: August 2016
- 19.Scaife J.E., Harrison K., Romanchikova M. Random variation in rectal position during radiotherapy for prostate cancer is two to three times greater than that predicted from prostate motion. Br J Radiol. 2014;87:20140343. doi: 10.1259/bjr.20140343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scaife J.E., Thomas S.J., Harrison K. Accumulated dose to the rectum, measured using dose-volume histograms and dose-surface maps, is different from planned dose in all patients treated with radiotherapy for prostate cancer. Br J Radiol. 2015;88:20150243. doi: 10.1259/bjr.20150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence (NICE). Prostate cancer: diagnosis and treatment (CG175). http://www.nice.org.uk/guidance/cg175. Accessed: November 2016 (2014)
- 22.Burnet N.G., Adams E.J., Fairfoul J. Practical aspects of implementation of helical tomotherapy for intensity-modulated and image-guided radiotherapy. Clin Oncol. 2010;22:294–312. doi: 10.1016/j.clon.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Thomas S.J., Ashburner M., Tudor G.S.J. Intra-fraction motion of the prostate during treatment with helical tomotherapy. Radiother Oncol. 2013;109:482–486. doi: 10.1016/j.radonc.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Sutcliffe MP, Harrison K, Scaife JE, Parker MA, Romanchikova M. Auto-contouring of the rectum on megavoltage computed tomography images. Cambridge University Engineering Department Technical Report 2015;CUED/C-MICROMECH/TR. 100.
- 25.Thomas S.J., Romanchikova M., Harrison K. Recalculation of dose for each fraction of treatment on TomoTherapy. Br J Radiol. 2016;89:20150770. doi: 10.1259/bjr.20150770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas S.J., Eyre K.R., Tudor G.S.J., Fairfoul J. Dose calculation software for helical tomotherapy, utilizing patient ct data to calculate an independent three-dimensional dose cube. Med Phys. 2012;39:160–167. doi: 10.1118/1.3668061. [DOI] [PubMed] [Google Scholar]
- 27.Niemierko A. Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys. 1997;24:103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 28.Michalski J.M., Gay H., Jackson A., Tucker S.L., Deasy J.O. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:123–129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. department of health and human services NIoH. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 2010. Accessed: July 2016. URL: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE4.032010-06-14QuickReference5x7.pdf.
- 30.LENT SOMA tables table of contents. Radiother Oncol. 1995;35:17–60. [PubMed] [Google Scholar]
- 31.Pilepich M., Asbell S., Krall J. Correlation of radiotherapeutic parameters and treatment related morbidity—analysis of RTOG study 77–06. Int J Radiat Oncol Biol Phys. 1987;13:1007–1012. doi: 10.1016/0360-3016(87)90038-1. [DOI] [PubMed] [Google Scholar]
- 32.Litwin M.S., Hays R.D., Fink A., Ganz P.A., Leake B., Brook R.H. The UCLA prostate cancer index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Gulliford S.L., Partridge M., Sydes M.R., Andreyev J., Dearnaley D.P. A comparison of dose–volume constraints derived using peak and longitudinal definitions of late rectal toxicity. Radiother Oncol. 2010;94:241–247. doi: 10.1016/j.radonc.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 34.Wortel R.C., Incrocci L., Pos F.J. Late side effects after image guided intensity modulated radiation therapy compared to 3D-conformal radiation therapy for prostate cancer: results from 2 prospective cohorts. Int J Radiat Oncol Biol Phys. 2016;95:680–689. doi: 10.1016/j.ijrobp.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Gulliford S.L., Foo K., Morgan R.C. Dose–volume constraints to reduce rectal side effects from prostate radiotherapy: Evidence from MRC RT01 trial ISRCTN 47772397. Int J Radiat Oncol Biol Phys. 2010;76:747–754. doi: 10.1016/j.ijrobp.2009.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.