Abstract
Familial risks of lung cancer are well-established, but whether lung cancer clusters with other discordant cancers is less certain, particularly beyond smoking-related sites, which may provide evidence on genetic contributions to lung cancer aetiology.
We used a novel approach to search for familial associations in the Swedish Family-Cancer Database. This involved assessment of familial relative risk for cancer X in families with increasing numbers of lung cancer patients and, conversely, relative risks for lung cancer in families with increasing numbers of patients with cancers X. However, we lacked information on smoking.
The total number of lung cancers in the database was 125 563. We applied stringent statistical criteria and found that seven discordant cancers were associated with lung cancer among family members, and six of these were known to be connected with smoking: oesophageal, upper aerodigestive tract, liver, cervical, kidney and urinary bladder cancers. A further novel finding was that cancer of unknown primary also associated with lung cancer. We also factored in histological evidence and found that anal and connective tissue cancers could be associated with lung cancer for reasons other than smoking. For endometrial and prostate cancers, suggestive negative associations with lung cancer were found.
Although we lacked information on smoking it is prudent to conclude that practically all observed discordant associations of lung cancer were with cancers for which smoking is a risk factor.
Short abstract
Among family members of lung cancer patients, 7 other cancers were found, all of which were smoking related http://ow.ly/ZLnt30csVfZ
Introduction
A family history of lung cancer has been found among approximately 10% of first-degree relatives (FDRs), and the familial relative risk (RR) is 1.9 between parents and offspring and 2.5 between siblings [1, 2]. Lung cancer has been found to be associated with other discordant (different) cancers such as bladder, kidney and cervical cancers [3, 4]. However, in an international multicentre case–control study, no association was found [5]. In a systematic analysis of familial risks in a cohort study between discordant sites, lung cancer showed 13 significant discordant associations, and most of them were smoking-related, with the exception of endocrine cancers [6]. The highest associations were with upper aerodigestive tract (1.84) and oesophageal cancers (1.73) between siblings, but these were lower (1.14 and 1.45) between parents and offspring [6]. Due to the strong influence of smoking it has been difficult to establish a germline architecture for lung cancer. Compared to many common cancers, only very few high-risk predisposing genes are known: for example, lung cancer may be a manifestation in Li–Fraumeni and retinoblastoma syndromes [7, 8]. Recent genome-wide association studies (GWASs) have reported more than 15 low-risk loci [9]. These include CHRNA3/5 genes, which are associated with nicotine dependence and propensity to smoke, the 5p15.33 locus containing the TERT gene, RAD52, CDKN2A and TP63 [8]. Some genetic loci may be specific to histological types of lung cancer, such as the rare variants BRCA2 p.Lys3326X and CHEK2 p.Ile157Thr that predispose to squamous cell lung cancer [8]. Although some of the low-risk variants of the above genes are known to predispose to other cancers, the familial risks conveyed by low-risk genes are usually so small that they individually could hardly explain any associations between lung cancer and discordant cancers [10].
Data for associated discordant cancers may provide useful information about shared genetic and environmental risk factors. In the case of lung cancer it would be of particular interest to focus on clusters with sites that are not tobacco-related, because familial clustering of smokers influences familial risks. Spouse correlations for lung cancer are among the highest noted (RRs in wives 1.58 and in husbands 1.63) [1]. As we had no individual smoking data for the current and past Swedish population we categorised different cancers based on whether they were considered to be associated with smoking [11]. We applied a novel approach to search for familial associations of lung cancer with other cancers using the most recent updated version of the Swedish Family-Cancer Database. This involved assessment of familial RRs for cancer X in families with increasing numbers of lung cancer patients or, conversely, familial RRs for lung cancer in families with increasing numbers of patients with cancers X. Histology-specific associations were also tested.
Methods
In the Swedish Family-Cancer Database, a total of 15.7 million individuals are organised in families with cancer data from the Swedish Cancer Registry. Since 1958, cancers have been registered, and the latest follow-up of the database includes cancers up to and including 2012. The offspring generation is constituted of all individuals born from 1932 onwards with parental linkage and includes 8.6 million people, among which 428 942 cancers were diagnosed. Their maximal age can be 80 years, whereas the ages of their biological parents (the parental generation) were not limited. Using the 7th revision of the International Classification of Diseases (ICD-7), the 29 most common cancers were used in the analysis. Since the 1960s, histological type has been recorded by pathological–anatomical coding.
We have described in a previous study the methods of calculating familial RRs for individuals whose FDRs (parents and/or siblings) were diagnosed with cancer [2]. In brief, incidence rates for persons with affected relatives were compared to rates for those whose relatives had no cancer. Incidence rates were obtained by counting cases and person-years according to family history. The follow-up for cancer in offspring commenced from the beginning of 1958, the birth year or immigration year, whichever came latest. The follow-up was terminated when a person was diagnosed with cancer, emigrated or died, or at the end of 2012, whichever came first. RRs were adjusted for sex, age group, calendar period, residential area and socioeconomic status to account for potential confounders. These variables were used as covariates in a Poisson regression model to obtain adjusted RRs and corresponding confidence intervals (CIs) for 5%, 1% and 0.1% significance levels. Trend tests were performed by modelling the number of familial cancers as a continuous covariate. Analyses were also conducted in the reverse order, i.e. by calculating RRs for lung cancer when FDRs were diagnosed with other concordant cancers. The two types of analyses are illustrated in figure 1. On the left side, RR is calculated for cancer X; person-years at risk are calculated for all offspring, and probands (family history) are from all FDRs. On the right side, the reverse analysis is illustrated: RR is calculated for lung cancer (LC). For parent–offspring generations these were independent analyses, but for siblings the pairs of cancer were the same and thus not completely independent. Separate analyses were carried out according to sex and lung cancer histology. Sex was considered for the case but not for the proband. Small cell histology included the oat cell type, and large cell histology included undifferentiated lung cancer.
FIGURE 1.
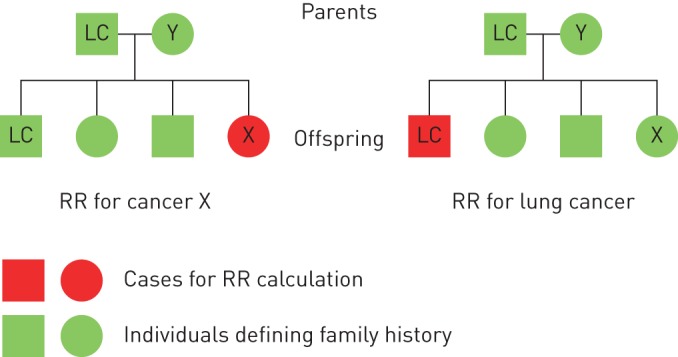
Flowchart for calculating the relative risk (RR) for cancer X and lung cancer (LC). A family had four cancer patients (one LC patient and one cancer Y patient in the parental generation; one LC patient and one cancer X patient in the offspring). When RR for cancer X was calculated (left), the patient with cancer X in the offspring was used as the case for RR calculation and the two LC patients in first-degree relatives (FDRs) were used as probands. When RR for LC was calculated (the reverse analysis, right), the LC patient in the offspring was used as the case and the patient with cancer X in FDRs was used as proband.
For classification of evidence on smoking-related cancers we adopted summary statements from the assessment of the International Agency for Research on Cancer (IARC): lung, oral cavity, naso-, oro- and hypopharynx, nasal cavity and accessory sinuses, larynx, oesophagus, stomach, pancreas, colorectum, liver, kidney (body and pelvis), ureter, urinary bladder, uterine cervix and ovary (mucinous) and myeloid leukaemia [11]. The evaluation also noted that a positive association was observed between tobacco smoking and female breast cancer. For cancers of the endometrium (post-menopausal) and of the thyroid, IARC considered that there is evidence suggesting lack of carcinogenicity; this IARC terminology simply means that smoking is not associated with these cancers. However, it is likely that some smoking-related cancer data may have been published after the IARC evaluation, which was conducted in September/October 2009. Notably, for example, strong evidence on smoking association was published for cancer of unknown primary (CUP), and we included CUP among smoking-related cancers even though IARC did not evaluate this cancer [12, 13].
For calling results to suggest true familial association between lung and a discordant site, we needed to consider the number of tests carried out. A total of 28 discordant sites were included in the two-way analysis (RR for cancer X by lung cancer and RR for lung cancer by cancer X), each in two proband categories (1 affected proband or 2+ affected probands). This added up to 112 comparisons. A Bonferroni corrected p-value would thus be 0.0004. We therefore needed to detect three significant associations at the 5% level (0.05×0.05×0.05=0.00013) to meet the Bonferroni correction of the p-value. Only two significant associations at 1% significance levels reached the same combined significance (0.0001). Of note, the applied significance levels were cut-off limits of minimal significance; for example, the 0.001% limit may include association with far higher significance. We also carried out histology-specific analysis on four types of lung cancer. However, for these analyses, case numbers in many individual categories were small and thus the statistical power was low, which made p-value adjustments redundant because even the nominal p-values were not significant.
Results
The total number of lung cancers was 125 563, and of these, a total of 24 377 were in the 0–80 year offspring generation for which the RRs were calculated. Among offspring with lung cancer, 21 602 (88.6%) had no FDR diagnosed with lung cancer, while 2598 (10.7%) had one and 177 (0.7%) had at least two FDRs with concordant lung cancer (table 1). The total number of patients with familial lung cancer was 4499. The RR for any lung cancer was 2.11 when one FDR was diagnosed with lung cancer and it increased to 3.75 when at least two FDRs were diagnosed (both significant at the 0.1% level). The reference was families with no lung cancer in FDRs. Compared to families with no lung cancer in FDRs, the RRs for all histological types were also highly significant when at least one FDR was diagnosed. Of note, only concordant histology was considered. When one family member was diagnosed with lung cancer, RRs for histological types decreased from large cell (2.80) to small cell (2.56), squamous cell (SCC, 2.40) and adenocarcinoma (2.37). A trend test for increasing RRs by the number of concordant lung cancers yielded a p-value of <0.0001 for all histological types except small cell lung cancer.
TABLE 1.
Risk of concordant histology-specific lung cancer
| Histologic type | Negative family history | One lung cancer case in the family | At least two lung cancer cases in the family | Trend test p-value | ||
| Cases | RR (95% CI) | Cases | RR (95% CI) | |||
| Overall | 21 602 | 2598 | 2.11# (2.00–2.23) | 177 | 3.75# (3.07–4.57) | <0.0001¶ |
| Adenocarcinoma | 9342 | 334 | 2.37# (2.08–2.69) | 11 | 5.83# (2.93–11.61) | <0.0001¶ |
| SCC | 3894 | 147 | 2.40# (1.95–2.94) | 5 | 7.81# (2.63–23.23) | <0.0001¶ |
| Small cell | 2513 | 25 | 2.56# (1.56–4.21) | 0 | 0.0013¶ | |
| Large cell | 2981 | 31 | 2.80# (1.91–4.09) | 0 | <0.0001¶ | |
RR: relative risk; SCC: squamous cell carcinoma. #: significantly increased RR at the two-sided 5% level, 1% level and 0.1% level; ¶: statistically significant.
RRs were increased and the trend test was significant for 10 out of 12 smoking-related discordant cancers when one or two or more family members were diagnosed with lung cancer (table 2, upper part). The RRs ranged from 1.07 (colorectal cancer) to 1.43 (CUP) when one FDR had lung cancer, but for upper aerodigestive tract cancer the RR reached 2.55 when two or more FDRs were diagnosed with lung cancer. For ovarian cancer and leukaemia, no increase was observed, but smoking association with these cancers has been limited to certain subtypes. In the reverse analysis, for RRs for lung cancer when increasing numbers of FDRs were diagnosed with discordant cancers, the trend tests were significant for 7 out of 12 associations (table 2, lower part). However, lung cancer risk was significant only in families with two or more oesophageal cancer patients (6.59).
TABLE 2.
Risk of smoking-related cancer when family members were diagnosed with lung cancer and risk of lung cancer when family members were diagnosed with smoking-related cancer
| Cancer site | Negative family history | One cancer case in the family | At least two cancer cases in the family | Trend test p-value | ||
| Cases | RR (95% CI) | Cases | RR (95% CI) | |||
| Risk for discordant cancer | ||||||
| Upper aerodigestive tract | 8025 | 630 | 1.39# (1.23–1.57) | 42 | 2.55# (1.61–4.04) | <0.0001§ |
| Oesophagus | 2547 | 195 | 1.34# (1.17–1.54) | 11 | 2.02+ (1.14–3.57) | <0.0001§ |
| Stomach | 5048 | 358 | 1.28¶ (1.08–1.52) | 13 | 1.27 (0.53–3.04) | 0.0066§ |
| Colorectum | 33 315 | 1972 | 1.07+ (1.01–1.13) | 73 | 1.09 (0.83–1.43) | 0.0129§ |
| Liver | 5506 | 369 | 1.23¶ (1.09–1.40) | 18 | 1.62 (0.94–2.82) | 0.0004§ |
| Pancreas | 6147 | 424 | 1.25# (1.10–1.41) | 20 | 1.59 (0.91–2.77) | 0.0002§ |
| Cervix | 8427 | 497 | 1.16¶ (1.05–1.28) | 26 | 1.83¶ (1.20–2.80) | 0.0005§ |
| Ovary | 9406 | 546 | 1.07 (0.98–1.17) | 18 | 0.98 (0.61–1.59) | 0.1948 |
| Kidney | 8843 | 583 | 1.25# (1.14–1.38) | 14 | 0.83 (0.46–1.51) | <0.0001§ |
| Urinary bladder | 12 984 | 894 | 1.22# (1.09–1.36) | 47 | 1.74+ (1.08–2.80) | 0.0002§ |
| Leukaemia | 14 028 | 587 | 0.97 (0.89–1.06) | 19 | 0.95 (0.59–1.52) | 0.4591 |
| CUP | 8469 | 663 | 1.43# (1.25–1.65) | 39 | 2.29¶ (1.33–3.96) | <0.0001§ |
| Risk for lung cancer | ||||||
| Upper aerodigestive tract | 23 775 | 597 | 1.40# (1.27–1.55) | 5 | 1.11 (0.38–3.26) | <0.0001§ |
| Oesophagus | 24 130 | 243 | 1.55# (1.31–1.83) | 4 | 6.59¶ (1.81–24.00) | <0.0001§ |
| Stomach | 23 526 | 834 | 1.02 (0.90–1.16) | 17 | 1.27 (0.53–3.06) | 0.6576 |
| Colorectum | 22 113 | 2159 | 1.07 (1.00–1.14) | 105 | 1.01 (0.76–1.34) | 0.0674 |
| Liver | 23 727 | 639 | 1.21# (1.09–1.33) | 11 | 2.06 (0.98–4.35) | 0.0001§ |
| Pancreas | 23 777 | 592 | 1.10 (0.99–1.23) | 8 | 1.24 (0.48–3.22) | 0.0831 |
| Cervix | 23 879 | 494 | 1.38# (1.23–1.55) | 4 | 1.93 (0.55–6.80) | <0.0001§ |
| Ovary | 23 881 | 492 | 1.11 (1.00–1.25) | 4 | 0.89 (0.26–3.02) | 0.0691 |
| Kidney | 23 701 | 666 | 1.17¶ (1.05–1.31) | 10 | 1.33 (0.56–3.19) | 0.0041§ |
| Urinary bladder | 23 290 | 1061 | 1.24# (1.12–1.36) | 26 | 1.47 (0.80–2.70) | <0.0001§ |
| Leukaemia | 23 820 | 543 | 1.03 (0.92–1.15) | 14 | 1.74 (0.88–3.45) | 0.4233 |
| CUP | 23 531 | 835 | 1.32# (1.21–1.45) | 11 | 1.67 (0.77–3.62) | <0.0001§ |
RR: relative risk; CUP: cancer of unknown primary. #: significantly increased RR at the two-sided 0.1% level; ¶: significantly increased RR at the two-sided 1% level; +: significantly increased RR at the two-sided 5% level; §: statistically significant.
Among the 14 cancers without established relation to smoking (according to IARC), a significant trend test was observed for anal, breast and squamous cell skin cancers. However, only the latter showed a suggestive “dose–response” by the number of FDRs diagnosed with lung cancer (table 3, upper part). Prostate and endometrial cancers showed a negative trend. Of the two cancers lacking evidence of carcinogenicity (endometrial and thyroid cancers, according to IARC), endometrial cancer showed a negative trend test. In the reverse analysis, for RRs for lung cancer in families with increasing numbers of patients with connective tissue tumours and non-Hodgkin lymphoma, trend tests were increased to p=0.04 and 0.03, respectively (table 3, lower part).
TABLE 3.
Association analysis of lung cancer and cancer not related to smoking
| Cancer site | Negative family history | One cancer case in the family | At least two cancer cases in the family | Trend test p-value | ||
| Cases | RR (95% CI) | Cases | RR (95% CI) | |||
| Risk of discordant cancer | ||||||
| Small intestine | 1700 | 118 | 1.24¶ (1.01–1.53) | 3 | 0.88 (0.25–3.08) | 0.0600 |
| Anus | 1005 | 81 | 1.41+ (1.09–1.82) | 2 | 0.95 (0.20–4.50) | 0.0147§ |
| Breast | 71 906 | 4258 | 1.05¶ (1.01–1.10) | 168 | 1.17 (0.93–1.47) | 0.0141§ |
| Other female genitals | 1256 | 86 | 1.24 (0.97–1.58) | 4 | 1.56 (0.52–4.72) | 0.0747 |
| Prostate | 58 584 | 3188 | 0.95 (0.91–0.99) | 92 | 0.76 (0.59–0.96) | 0.0031§ƒ |
| Testis | 7162 | 341 | 1.16 (0.99–1.37) | 10 | 1.27 (0.50–3.20) | 0.0637 |
| Melanoma | 27 054 | 1389 | 0.98 (0.91–1.05) | 52 | 1.11 (0.78–1.59) | 0.7434 |
| Skin, squamous cell | 10 893 | 658 | 1.09 (1.00–1.19) | 34 | 1.56¶ (1.07–2.28) | 0.0138§ |
| Nervous system | 21 223 | 1005 | 1.04 (0.98–1.12) | 41 | 1.31 (0.95–1.80) | 0.0829 |
| Endocrine glands | 9132 | 524 | 1.10 (0.99–1.23) | 13 | 0.80 (0.40–1.58) | 0.1614 |
| Connective tissue | 3585 | 178 | 1.08 (0.93–1.26) | 7 | 1.30 (0.61–2.74) | 0.2627 |
| Non-Hodgkin lymphoma | 13 566 | 750 | 1.07 (0.99–1.16) | 25 | 1.02 (0.67–1.57) | 0.1164 |
| Hodgkin lymphoma | 4257 | 169 | 0.97 (0.82–1.16) | 11 | 2.17¶ (1.10–4.29) | 0.6850 |
| Myeloma | 3856 | 222 | 1.02 (0.88–1.18) | 9 | 1.14 (0.56–2.29) | 0.7010 |
| Endometrium# | 10 689 | 542 | 0.89 (0.82–0.97) | 23 | 1.00 (0.66–1.50) | 0.0148§ƒ |
| Thyroid gland# | 5308 | 259 | 1.00 (0.89–1.12) | 15 | 1.78¶ (1.11–2.83) | 0.4398 |
| Risk of lung cancer | ||||||
| Small intestine | 24 274 | 102 | 1.22 (0.94–1.59) | 1 | 2.74 (0.19–39.18) | 0.1375 |
| Anus | 24 321 | 56 | 1.47 (0.92–2.35) | 0 | 0.1303 | |
| Breast | 21 972 | 2260 | 1.03 (0.97–1.09) | 145 | 1.12 (0.91–1.38) | 0.1881 |
| Other female genitals | 24 269 | 108 | 1.17 (0.87–1.59) | 0 | 0.3152 | |
| Prostate | 21 676 | 2533 | 0.97 (0.91–1.03) | 168 | 0.86 (0.68–1.09) | 0.1209 |
| Testis | 24 309 | 67 | 1.25 (0.92–1.71) | 1 | 2.57 (0.20–32.67) | 0.1430 |
| Melanoma | 23 777 | 579 | 1.07 (0.96–1.20) | 21 | 1.62 (0.92–2.86) | 0.1010 |
| Skin, squamous cell | 23 622 | 742 | 1.01 (0.91–1.12) | 13 | 0.98 (0.45–2.11) | 0.8944 |
| Nervous system | 23 775 | 593 | 1.10 (0.99–1.22) | 9 | 1.10 (0.47–2.57) | 0.0844 |
| Endocrine glands | 23 979 | 388 | 1.23 (0.81–1.87) | 10 | 3.06 (0.23–40.86) | 0.4277 |
| Connective tissue | 24 220 | 157 | 1.29¶ (1.02–1.64) | 0 | 0.0448§ | |
| Non-Hodgkin lymphoma | 23 722 | 646 | 1.13¶ (1.01–1.26) | 9 | 1.10 (0.44–2.75) | 0.0295§ |
| Hodgkin lymphoma | 24 283 | 92 | 1.01 (0.76–1.36) | 2 | 3.45 (0.48–24.84) | 0.7743 |
| Myeloma | 24 102 | 273 | 0.95 (0.82–1.11) | 2 | 0.79 (0.14–4.60) | 0.4776 |
| Endometrium | 23 837 | 535 | 1.00 (0.89–1.13) | 5 | 0.69 (0.20–2.36) | 0.9491 |
| Thyroid gland | 24 207 | 170 | 1.10 (0.88–1.38) | 0 | 0.3905 | |
RR: relative risk. #: according to the International Agency for Research on Cancer, the evidence on endometrial and thyroid cancers indicated a lack of carcinogenicity from smoking; ¶: significantly increased RR at the two-sided 5% level; +: significantly increased RR at the two-sided 1% level; §: statistically significant; ƒ: RR decreased with increasing number of lung cancer cases.
Sex-specific analyses are presented in tables S1 and S2, but no significant differences were found.
Analyses by lung cancer histology are shown in tables S3 (RRs for discordant cancer) and S4 (RRs for lung cancer). With the exception of anal and connective tissue cancers, no other cancer showed histology that would be distinct from smoking aetiology (table 4). For families with anal cancer, lung adenocarcinoma was increased in the two-way analyses (RRs of 1.60 and 1.77, both with significant trends). For connective tissue cancer, RRs for lung adenocarcinoma were also increased in the two-way analyses.
TABLE 4.
Risk of cancer when family members were diagnosed with histology-specific lung cancer and risk of histology-specific lung cancer when family members were diagnosed with discordant cancer
| Cancer site and histological type | Negative family history | One lung cancer case in the family | At least two lung cancer cases in the family | Trend test p-value | ||
| Cases | RR (95% CI) | Cases | RR (95% CI) | |||
| Risk of discordant cancer | ||||||
| Anus | ||||||
| Adenocarcinoma | 1062 | 26 | 1.60# (1.08–2.36) | 0 | 0.0284¶ | |
| SCC | 1067 | 21 | 1.27 (0.84–1.94) | 0 | 0.2732 | |
| Small cell | 1083 | 5 | 1.21 (0.51–2.84) | 0 | 0.6734 | |
| Large cell | 1085 | 3 | 0.71 (0.24–2.14) | 0 | 0.5231 | |
| Connective tissue | ||||||
| Adenocarcinoma | 3697 | 71 | 1.48# (1.15–1.91) | 2 | 3.68 (0.82–16.61) | 0.0022¶ |
| SCC | 3730 | 40 | 0.89 (0.62–1.30) | 0 | 0.5455 | |
| Small cell | 3756 | 14 | 1.12 (0.64–1.94) | 0 | 0.6982 | |
| Large cell | 3758 | 12 | 0.93 (0.51–1.73) | 0 | 0.8255 | |
| Risk of lung cancer | ||||||
| Anus | ||||||
| Adenocarcinoma | 9660 | 27 | 1.77# (1.10–2.84) | 0 | 0.0307¶ | |
| SCC | 4040 | 6 | 0.95 (0.35- 2.62) | 0 | 0.9230 | |
| Small cell | 2534 | 4 | 1.00 (0.32–3.11) | 0 | 0.9986 | |
| Large cell | 3005 | 7 | 1.49 (0.45–4.88) | 0 | 0.5399 | |
| Connective tissue | ||||||
| Adenocarcinoma | 9619 | 68 | 1.40# (1.01–1.94) | 0 | 0.0575 | |
| SCC | 4027 | 19 | 0.94 (0.54–1.64) | 0 | 0.8300 | |
| Small cell | 2518 | 20 | 1.58# (1.04–2.40) | 0 | 0.0482¶ | |
| Large cell | 2989 | 23 | 1.53# (1.02–2.29) | 0 | 0.0542 | |
RR: relative risk; SCC: squamous cell carcinoma. #: significantly increased RR at the two-sided 5% level; ¶: statistically significant.
Discussion
Numerous family studies on lung cancer have been published and reviewed, such as the meta-analysis by Lissowska et al. [5]. The summary odds ratios have been 1.66 for case–control studies and 1.96 for cohort studies. Many studies have also assessed the risk among non-smokers for whom the summary case–control odds ratio was 1.37 [5]. According to an international pooling of case–control studies, the odds ratio for familial lung cancer in “ever-smokers” was 1.55, while for non-smokers it was 1.25; odds ratios for non-smokers were not significant (1.09/1.10) for paternal and maternal family histories, respectively [14]. However, there are known concerns about the reliability of case–control data on family history of cancer and of smoking habits and thus there may be large margins of error in the estimates of familial risk of lung cancer among non-smokers [15, 16]. However, even modelling studies suggest that all familial risk of lung cancer cannot be explained by clustering of smoking in families [17].
The focus of the present study was on analyses of discordant familial associations with lung cancer. Although the subject has been addressed in previous studies, none has had the statistical power or rigour of the present study [3–6]. The standout conclusion from the present study is that all compelling discordant associations were with smoking-related cancers. Smoking is likely to bias any observational studies to such an extent that it may be difficult to demonstrate discordant associations unrelated to smoking. Lung cancer studies are overwhelmingly dominated by smokers due to the historic smoking rates in the population; only 14% of the familial cases were classified as non-smokers in the international lung cancer pooling study [14]. Although we lacked information on smoking, the unique advantage of the present study is the cohort design, which uses reliable and essentially complete nationwide family and cancer data. With 4499 familial lung cancers, the present study is by far the largest single study on familial lung cancer.
The association of oesophageal cancer with lung cancer reached the highest (minimal) significance (p=0.001×0.05×0.001×0.01=5×10−10). The corrected significance level (p-value of 0.0004, see Methods) was also reached for upper aerodigestive tract, liver, cervical, kidney and urinary bladder cancers and CUP. For CUP, a cancer that is equally as common as pancreatic or kidney cancer in Sweden, the data are completely novel and have mechanistic implications; the strong association with primary lung cancer suggests that the hidden primary cancer was also located in the lung in the family member diagnosed with CUP [18, 19]. The joint significance for pancreatic cancer was 0.001, for stomach cancer it was 0.01 and for colorectal cancer it was 0.05, each based on single RRs. Lung cancer histology did not essentially differ between concordant lung cancer and discordant familial cancers. As in previous case–control studies, both sexes and all main histological types showed increased familial risks [5, 14].
Among cancers for which a smoking relation has not been established according to IARC (although some individual studies support the role of smoking), only single associations were increased for small intestinal, anal, squamous cell skin and connective tissue cancers, non-Hodgkin lymphoma and Hodgkin lymphoma. Such single associations do not constitute evidence of a true familial risk. However, for two of these cancers (anal and connective tissue cancers), association with lung adenocarcinoma histology was observed in the two-way analyses. Moreover, for anal cancer the associations with lung adenocarcinoma were the only significant results. Of course, there are a large number of known risk factors for anal cancers, such as human papilloma virus infection, and a weak effect of smoking has also been reported [20]. IARC's evaluation for endometrial (and thyroid) cancer considered that the evidence indicated lack of carcinogenicity by smoking, which in the IARC terminology means that smoking is “probably not carcinogenic” to these cancers (in humans). The mechanism in endometrial cancer may be a reduction of oestrogen levels and/or earlier menopause in women that smoke [11]. Accordingly, a negative trend test was observed for endometrial cancer, but also for prostate cancer. However, the evidence for prostate cancer and smoking collected by IARC was considered largely null [11].
In summary, by applying stringent statistical criteria we found seven discordant cancers that were associated with lung cancer among FDRs. Six of these were previously implicated in the context of smoking: oesophageal, upper aerodigestive tract, liver, cervical, kidney and urinary bladder cancers. A novel association is described for CUP. By considering histological evidence, anal and connective tissue cancers could be associated with lung cancer for reasons other than smoking. For endometrial and prostate cancers, suggestive negative associations with lung cancer were found, probably relating to interference of smoking with endogenous hormonal levels [11]. As we lacked smoking data we can only conclude that all significant discordant familial associations observed in this study were with smoking-related cancers. From a public health perspective, this underscores the importance of supporting smoking cessation as a means to reduce cancer incidence and mortality. The role of smoking should be considered in clinical counselling of patients with smoking-related cancers and of their family members.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Tables S1-S4 00006-2017_supplementary_tables (205.4KB, pdf)
Disclosures
A. Hemminki 00006-2017_Hemminki (1.2MB, pdf)
Acknowledgements
A. Hemminki is the Jane and Aatos Erkko Professor of Oncology at the University of Helsinki (Helsinki, Finland). We are grateful to Patrick Reilly (Center for Primary Health Care Research, Lund University, Lund, Sweden) for language editing.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Support statement: This work was funded by German Cancer Aid, EU Transcan funding by the German Federal Ministry of Education and Research, University of Helsinki and Helsinki University Central Hospital, and the Swedish Research Council for Health, Working Life and Welfare (FORTE) (registration numbers 2013-1836 and 2014-0804), and the Swedish Research Council (2012-2378 and 2014-10134), as well as ALF funding from Region Skåne. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: Disclosures can be found alongside this article at openres.ersjournals.com
References
- 1.Frank C, Fallah M, Ji J, et al. The population impact of familial cancer, a major cause of cancer. Int J Cancer 2014; 134: 1899–1906. [DOI] [PubMed] [Google Scholar]
- 2.Frank C, Fallah M, Sundquist J, et al. Population landscape of familial cancer. Sci Rep 2015; 5: 12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amundadottir LT, Thorvaldsson S, Gudbjartsson DF, et al. Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Med 2004; 1: e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Hemminki K. Familial and second lung cancers: a nation-wide epidemiologic study from Sweden. Lung Cancer 2003; 39: 255–263. [DOI] [PubMed] [Google Scholar]
- 5.Lissowska J, Foretova L, Dabek J, et al. Family history and lung cancer risk: international multicentre case–control study in Eastern and Central Europe and meta-analyses. Cancer Causes Control 2010; 21: 1091–1104. [DOI] [PubMed] [Google Scholar]
- 6.Hemminki K, Sundquist J, Brandt A. Do discordant cancers share familial susceptibility? Eur J Cancer 2012; 48: 1200–1207. [DOI] [PubMed] [Google Scholar]
- 7.Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med 2008; 359: 2143–2153. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Cuesta L, McKay JD. Genomic architecture of lung cancers. Curr Opin Oncol 2016; 28: 52–57. [DOI] [PubMed] [Google Scholar]
- 9.Zanetti KA, Wang Z, Aldrich M, et al. Genome-wide association study confirms lung cancer susceptibility loci on chromosomes 5p15 and 15q25 in an African-American population. Lung Cancer 2016; 98: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemminki K, Forsti A, Lorenzo Bermejo J. The ‘common disease–common variant’ hypothesis and familial risk. PLoS ONE 2008; 3: e2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IARC. Personal Habits and Indoor Combustions. Lyon, International Agency for Research on Cancer, 2012. [Google Scholar]
- 12.Hemminki K, Chen B, Melander O, et al. Smoking and body-mass-index as risk factors for subtypes of cancer of unknown primary. Int J Cancer 2015; 136: 246–247. [DOI] [PubMed] [Google Scholar]
- 13.Kaaks R, Sookthai D, Hemminki K, et al. Risk factors for cancers of unknown primary site (CUP) – results from the prospective EPIC cohort. Int J Cancer 2014; 135: 2475–2481. [DOI] [PubMed] [Google Scholar]
- 14.Cote ML, Liu M, Bonassi S, et al. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. Eur J Cancer 2012; 48: 1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang E, Ekström Smedby K, Hjalgrim H, et al. Reliability of self-reported family history of cancer in a large case–control study of lymphoma. J Natl Cancer Inst 2006; 98: 61–68. [DOI] [PubMed] [Google Scholar]
- 16.Fiederling J, Shams AZ, Haug U. Validity of self-reported family history of cancer: a systematic literature review on selected cancers. Int J Cancer 2016; 139: 1449–1460. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo Bermejo J, Hemminki K. Familial lung cancer and aggregation of smoking habits: a simulation of the effect of shared environmental factors on the familial risk of cancer. Cancer Epidemiol Biomarkers Prev 2005; 14: 1738–1740. [DOI] [PubMed] [Google Scholar]
- 18.Hemminki K, Ji J, Sundquist J, et al. Familial risks in cancer of unknown primary: tracking the primary sites. J Clin Oncol 2011; 29: 435–440. [DOI] [PubMed] [Google Scholar]
- 19.Hemminki K, Sundquist K, Sundquist J, et al. Location of metastases in cancer of unknown primary are not random and signal familial clustering. Sci Rep 2016; 6: 22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffey K, Beral V, Green J, et al. Lifestyle and reproductive risk factors associated with anal cancer in women aged over 50 years. Br J Cancer 2016; 114: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Tables S1-S4 00006-2017_supplementary_tables (205.4KB, pdf)
A. Hemminki 00006-2017_Hemminki (1.2MB, pdf)


