Abstract
The purpose of this study is to assess the effectiveness of mycophenolate mofetil (MMF) in treating Takayasu arteritis (TA) patients. Embase, Cochrane Library, Pubmed, Clinicaltrials. Gov and three Chinese literature databases (VIP, CNKI, WanFang) were searched; randomized-controlled trials and observational studies that compared the efficacy before and after treatment with MMF were included. The efficacy outcomes were disease activity, the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) values and steroid dosage. The results were expressed as mean differences with 95% confidence intervals. Compared with the baseline, there were significant reductions in the ESR (−14.92 [25.35, −4.48]), CRP values (−12.99 [−23.29, −2.68]) and the steroid dosage (−17.64 [−24.89, −10.4]) after the addition of MMF, and the disease tended to stabilize. Therefore, MMF might be an alternative immunosuppressive drug for TA for the control of disease activity and to taper the steroid dosage.
Electronic supplementary material
The online version of this article (doi:10.1007/s00296-017-3704-7) contains supplementary material, which is available to authorized users.
Keywords: Mycophenolate mofetil, Takayasu arteritis, Efficacy, Meta-analysis, Systematic review, Steroid dosage
Introduction
Takayasu arteritis (TA) is a chronic inflammatory disease that mainly affects large arteries such as the aorta and its major branches with an unknown etiopathogenesis [1, 2]. The inflammatory process of Takayasu’s arteritis causes thickening, narrowing or occlusion of the affected vessels and finally results in various symptoms. As well as monetary considerations, patients are concerned about their quality of life. A study demonstrated that the quality of life of TA patients, including both physical and mental components, was worse than that of age-matched, healthy patients [3]. Studies on the management of TA patients are rare, and oral glucocorticoid agents were recommended as first-line therapy [4]. However, many patients require large maintenance steroid doses and are exposed to the risk of chronic toxicity [5]. Nearly, half of all patients will relapse during tapering and, consequently, will require additional immunomodulating drugs, such as azathioprine, cyclophosphamide, and methotrexate [4]. Therefore, we should search for an optimum treatment to improve the therapeutic effect and to reduce disease activity so that the patients’ quality of life is improved.
In recent years, mycophenolate mofetil (MMF) has been widely used as a concomitant drug for TA, and some researchers have studied the application of MMF in TA patients in their countries. MMF may control the disease activity and allow for tapering of the steroid dosage [5, 6]. Nevertheless, to our knowledge, there is no published study assessing the explicit efficacy of treating TA with MMF combined with a steroid systematically. Thus, we performed a systemic review and meta-analysis to evaluate the effectiveness of MMF in patients with TA.
Methods
We conducted a meta-analysis following the methods specified in the Cochrane Handbook for Systematic Reviews of Intervention [7]. Outcome measures included disease activity (including imaging examinations), the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) values and the steroid dosage.
Data sources
Eligible trials were identified through electronic searches (conducted by two independent reviewers, D. D. and Y. W.). Searches were performed in Embase, Cochrane Library, Pubmed, Clinicaltrials. Gov and three Chinese literature databases (VIP, CNKI, WanFang) from their inception until September 2015. The search was limited to English or Chinese articles. The search strategy is shown in Table S1. In addition, the reference lists of eligible studies were also scanned to identify additional relevant studies.
Study selection
The electronic search results were imported to a management software, and the duplicate results were deleted. Two reviewers (D. D. and Y. W.) independently screened all titles and abstracts for eligible studies. Studies were included if they met the following criteria: (1) TA was diagnosed unequivocally; (2) MMF was used for treatment; (3) the study design was a randomized controlled trial (RCT) or observational study; (4) the study included one of the predefined outcome measures; and (5) the study was published in English or Chinese.
Data extraction
Two authors extracted data independently (D. D. and Y. W.). Any dispute was settled by discussion or by a third investigator. Study characteristics were extracted from each study, including first author identification, year of publication, sample size, study location, study design, sex, age, duration of TA, duration of MMF therapy, and other concomitant drugs before MMF.
Laboratory parameters (e.g., the ESR value, CRP value, steroid dose, and disease activity) before the first introduction of MMF were extracted as baseline values (before MMF) and were compared to the same parameters extracted at the end of the study (after MMF).
Any drop in the ESR or/and CRP values was considered as efficacy due to the therapy. If patients did not relapse or the disease was stable during the steroid tapering, we also considered the treatment to be effective.
Quality assessment
The Newcastle–Ottawa Scale (NOS) was adopted to evaluate the quality of the included studies [8].
Data analysis
The meta-analysis was accomplished by RevMan 5.1 (Cochrane IMS). A fixed effects model was selected and Cochrane Q χ² and I² statistics were used to estimate the heterogeneity among studies. I² values of over 25%, 50% and 75% represent low, moderate and high heterogeneity, respectively [9, 10]. p values of 0.05 were used to determine statistical significance. The results were calculated using the Mantel–Haenzsel method and are expressed as mean differences (MD) for continuous outcomes with 95% confidence intervals.
A sensitivity analysis was performed to test the robustness of the main results. We re-analyzed the data using a random effects model. The results for the sensitivity analysis are only reported if the conclusions differed.
Results
Literature search
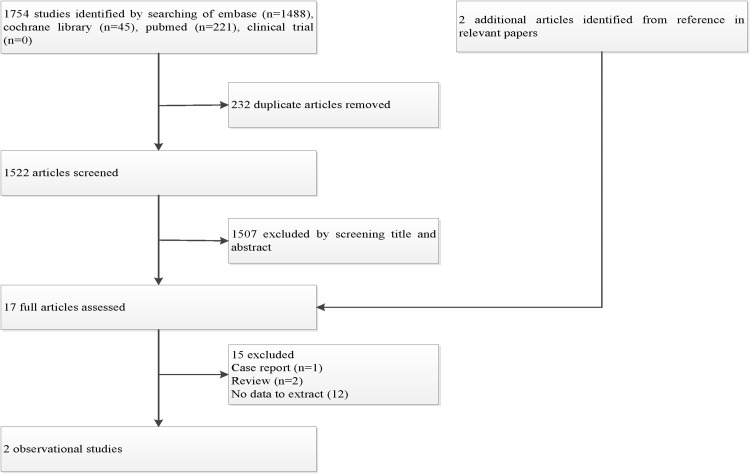
The study selection process for inclusion is shown in Fig. 1. The electronic searches identified 1524 potentially relevant articles. After initially excluding duplicates and the initial screening, 17 relevant articles were selected, and 15 articles were excluded for the reasons shown in Table S2. A total of 2 articles involving 31 patients were included in the meta-analysis. We did not obtain any additional studies by scanning the reference lists of eligible studies.
Fig. 1.
Flow chart of study selection
Study characteristics
The characteristics of the included studies are summarized in Table 1. Thirty-one patients diagnosed with TA according to the American College of Rheumatology (ACR) criteria were involved [11]. Of these, two patients abandoned the studies due to their severe adverse events related to MMF [5, 6]. Fifteen patients had received at least one immunosuppressive drug before the administration of MMF (12 azathioprine, 4 methotrexate, and 1 chlorambucil). Because these two studies used different criteria to assess disease activity, we are unable to pool the data.
Table 1.
Characteristics of the included studies
| References | Country, study design | Sample size (male/female) | Age (years) | TA duration (months) | MMF therapya (months) | Other immunosuppressive drugs before MMF (n) |
|---|---|---|---|---|---|---|
| Goel et al. [5] | India, retrospective | 21b (2/19) | Mean ± SD: 31.9 ± 13.8 | Mean ± SD: 35.5 ± 28.4 Range:1–120 |
Mean ± SD: 9.6 ± 6.4 | Azathioprine (10) |
| Shinjo et al. [6] | Brazil, prospective | 10c (3/7) | Mean ± SD: 29.9 ± 8.9 Range: 18–40 |
Mean ± SD: 57.5 ± 65.8 | Mean ± SD: 23.3 ± 12.1 | Methotrexate (4)d,e
Azathioprine (2)d Chlorambucil (1)e |
TA Takayasu’s arteritis, MMF mycophenolate mofetil
aMycophenolate mofetil as an alternative immunosuppressive drug accompanying steroids in controlling TA disease activity
bOne patient was not used to evaluate efficacy because of a skin rash, and 11 patients were on steroids alone before MMF
cOne patient was not used to evaluate efficacy due to a severe headache, and five patients were on steroids alone before MMF
dOne received methotrexate + azathioprine
eOne received chlorambucil + methotrexate
Evaluation of efficacy
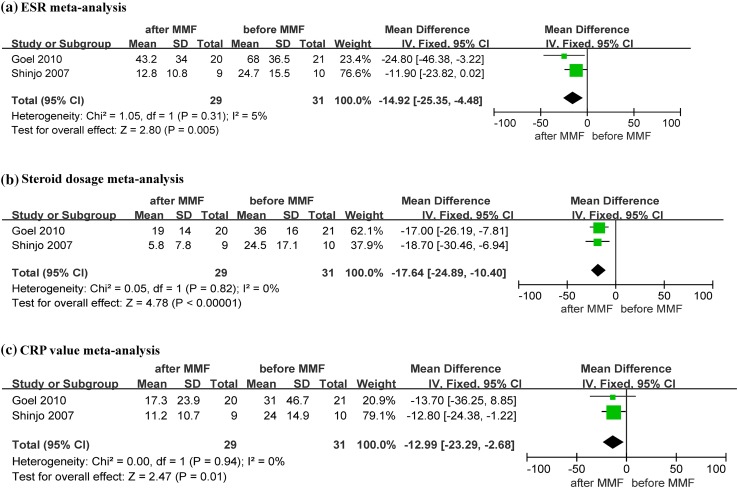
A summary of meta-analysis for efficacy is shown in Table 2; forest plots are shown in Fig. 2. The raw data are shown in Table S3. We performed a fixed effects meta-analysis including 31 patients assigned to the “before” MMF group and 29 patients assigned to the “after” MMF group.
Table 2.
A summary of the meta-analysis for efficacy
| Lab parameter | No. of studies contributing data | MD (95%) | No. of participants of experimental group | No. of participants of control group | I² (%) | p |
|---|---|---|---|---|---|---|
| ESR | 2 | −14.92 [25.35, −4.48] | 29 | 31 | 5 | 0.005 |
| CRP | 2 | −12.99 [−23.29, −2.68] | 29 | 31 | 0 | 0.01 |
| Steroid dosage | 2 | −17.64 [−24.89, −10.4] | 29 | 31 | 0 | <0.00001 |
Fig. 2.
Forest plots
Erythrocyte sedimentation rate
Both studies reported a great reduction in the ESR from baseline to the end of the study period, with a mean difference of −14.92 (95% CI −25.35 to −4.48, p = 0.005). This result is shown in Fig. 2a. No significant heterogeneity was found in the analysis (I² = 5%, p = 0.31).
Steroid dosage
Both studies reported significant decreases in the steroid dosage from baseline to the end of the study. This result is shown in Fig. 2b. The mean difference was −17.64 (95% CI −24.89 to −10.4, p < 0.00001). Insignificant heterogeneity was found among the included studies (I² = 0%, P = 0.82).
C-reactive protein values
The mean difference of the CRP values was −12.99 (95% CI −23.29 to −2.68, p = 0.01). This result is shown in Fig. 2c. There was no significant heterogeneity among the included studies (I² = 0%, p = 0.94).
Disease activity
Goel et al. [5] assessed disease activity with Indian Takayasu’s arteritis activity score (ITAS) [12] scoring system, while Shinjo et al. [6] used the National Institutes of Health (NIH) [11] criteria. Regardless of which evaluation standard was used, both studies reported significant improvement in the disease activity, and all patients were stable at the end of the study, except two patients who dropped out of the studies.
The quality assessment of the included studies
Using the 9-point scoring system, the scores of the included studies were 8 and 9. The results of the specific quality scores from the NOS system are summarized in Table S4.
Discussion
Primary guidelines recommend immunosuppressive agents, such as azathioprine, cyclophosphamide, and methotrexate as second-line agents [4]. Nevertheless, the long-term administration of such drugs can cause serious side effects. For example, cyclophosphamide can cause cystitis, bladder cancer and infertility (especially in TA patients) [13]. Although less toxic than cyclophosphamide, methotrexate can cause severe bone marrow depression, which may lead to life-threatening infections or spontaneous hemorrhage. These drugs are only to be used carefully when withdrawal from steroids is difficult [14]. Therefore, it is necessary to identify alternative immunosuppressants with lower hepatotoxicity, nephrotoxicity and other severe side effects. Recently, MMF was used to treat TA patients who did not tolerate azathioprine, cyclophosphamide, or methotrexate [1, 2, 5, 6, 15–18], and it achieved a favorable response [2, 5, 6].
Although there are no established specific biological markers to estimate disease, patients with TA often present with higher ESRs and CRP values [19]. Therefore, reduced ESRs and CRP values seem to be a good indicator of disease remission. The results of the present meta-analysis demonstrated that MMF is effective in controlling disease activity and tapering the steroid dosage. Moreover, MMF could significantly decrease the ESR and CRP values. Similar results were reported by Erica Daina [2]. A sensitivity analysis further confirmed that our findings were robust (results shown in Table S5).
However, our study has several limitations. First, although we searched widely, there were only two observational studies included, and the sample size was small (only included 31 patients). Second, because of the lack of data, imaging examination results could not be pooled, although this is an important measurement of long-term efficacy. Third, the use of observational studies in a meta-analysis is liable to the biases and confounding factors that are inherent in the original studies.
In conclusion, MMF might be an alternative immunosuppressive drug for TA incontrolling disease activity and tapering the steroid dosage. However, further research with longer follow-up periods and more participants is needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the authors of the included articles for their thoughtful work. And we thank the study team for their cooperation.
Compliance with ethical standards
This article does not contain any studies with animals or human participants performed by any of the authors.
Conflict of interest
Author Danping Dai, Author Haiying Jin, Author Yangyang Wang, Author Yiyang Mao, and Author Hao Sun declare that they have no conflict of interest.
Funding
This study was not supported by any funding.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00296-017-3704-7) contains supplementary material, which is available to authorized users.
References
- 1.Keser G, Direskeneli H, Aksu K. Rheumatology (United Kingdom) 2014;53(5):793–801. doi: 10.1093/rheumatology/ket320. [DOI] [PubMed] [Google Scholar]
- 2.Daina E, Schieppati A, Remuzzi G. Mycophenolate mofetil for the treatment of takayasu arteritis: report of three cases. Ann Intern Med. 1999;130(5):422–426. doi: 10.7326/0003-4819-130-5-199903020-00013. [DOI] [PubMed] [Google Scholar]
- 3.Abularrage CJ, Slidell MB, Sidawy AN, Kreishman P, Amdur RL, Arora S. Quality of life of patients with Takayasu’s arteritis. J Vasc Surg. 2008;47(1):131–137. doi: 10.1016/j.jvs.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 4.Erbel R, Aboyans V, Boileau C, et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 5.Goel R, Danda D, Mathew J, Edwin N. Mycophenolate mofetil in Takayasu’s arteritis. Clin Rheumatol. 2010;29(3):329–332. doi: 10.1007/s10067-009-1333-6. [DOI] [PubMed] [Google Scholar]
- 6.Shinjo SK, Pereira RMR, Tizziani VAP, Radu AS, Levy-Neto M. Mycophenolate mofetil reduces disease activity and steroid dosage in Takayasu arteritis. Clin Rheumatol. 2007;26(11):1871–1875. doi: 10.1007/s10067-007-0596-z. [DOI] [PubMed] [Google Scholar]
- 7.Deeks JJ, Higgins JPT, Altman DG (2011) Chapter 9: analysing data and undertaking meta-analyses. In Higgins J, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. (updated March 2011). The Cochrane Collaboration. http://www.cochrane-handbook.org. Accessed 25 March 2014
- 8.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 9.Evangelou E, Ioannidis J, Patsopoulos NA. Uncertainty in heterogeneity estimates in meta-analysis. Br Med J. 2007;335(7626):914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Educ Debate. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919–929. doi: 10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Misra R, Danda D, Rajappa SM, et al. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010) Rheumatology (Oxford) 2013;52(10):1795–1801. doi: 10.1093/rheumatology/ket128. [DOI] [PubMed] [Google Scholar]
- 13.Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med. 1996;124(5):477–484. doi: 10.7326/0003-4819-124-5-199603010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez-Ureña S, Molina JF, García CO, Cuéllar ML, Espinoza LR. Pancytopenia secondary to methotrexate therapy in rheumatoid arthritis. Arthritis Rheum. 1996;39(2):272–276. doi: 10.1002/art.1780390214. [DOI] [PubMed] [Google Scholar]
- 15.Liang P, Hoffman GS. Advances in the medical and surgical treatment of Takayasu arteritis. Curr Opin Rheumatol. 2005;17:16–24. doi: 10.1097/01.bor.0000146607.65808.37. [DOI] [PubMed] [Google Scholar]
- 16.Cong X-L, Dai S-M, Feng X, et al. Takayasu’s arteritis: clinical features and outcomes of 125 patients in China. Clin Rheumatol. 2010;29(9):973–981. doi: 10.1007/s10067-010-1496-1. [DOI] [PubMed] [Google Scholar]
- 17.Freitas DS, Camargo CZ, Mariz HA, Arraes AED, De Souza AWS. Takayasu arteritis: assessment of response to medical therapy based on clinical activity criteria and imaging techniques. Rheumatol Int. 2012;32(3):703–709. doi: 10.1007/s00296-010-1694-9. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt J, Kermani TA, Bacani AK, et al. Diagnostic features, treatment, and outcomes of Takayasu arteritis in a US cohort of 126 patients. Mayo Clin Proc. 2013;88(8):822–830. doi: 10.1016/j.mayocp.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Terao C, Yoshifuji H, Mimori T. Recent advances in Takayasu arteritis. Int J Rheum Dis. 2014;17(3):238–247. doi: 10.1111/1756-185X.12309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.