Dear Editor
An emerging literature base is examining the association between exercise and prognosis after a diagnosis of early-stage breast, colorectal, or prostate cancer.(Friedenreich et al., 2016) Pooling of data across available studies suggests that higher levels of exercise / physical activity are associated with significant reductions in risk of recurrence or cancer-specific mortality, however findings of individual studies are inconsistent.(Friedenreich et al., 2016; Jones, 2015) In vivo models of UVB-induced skin carcinogenesis and syngeneic models of melanoma consistently demonstrate, however, that exercise inhibits primary tumor growth.(Michna et al., 2006; Pedersen et al., 2016) Here, we examined the relationship between pre-diagnosis exercise and clinical outcomes in 2,465 patients with primary cutaneous invasive melanoma participating in the Gene, Environment, and Melanoma (GEM) study(Begg et al., 2006). We also examined whether this relationship differed on the basis of select tumor features.
The detailed study methods are described in the supplementary materials. In brief, patients with single primary cutaneous melanoma participating in the GEM study,(Begg et al., 2006) an international population-based prospective cohort study, were analyzed (total n=3,579). The institutional review board at the coordinating center (MSK) and each participating institution approved the study protocol. To minimize reverse causality, patients with second or higher order invasive melanoma (n=1,110), patients dying within 6 months of enrollment (n=3), and a single patient with missing data were excluded, bringing the final analytic cohort to 2,465 patients. Vital status was followed through 2007 or 2008.(Begg et al., 2006) Dates and causes of death were obtained from National Death Indexes, cancer registries, and municipal records.(Thomas et al., 2014) At enrollment, participants reported frequency and duration of various exercise activities (e.g., biking, walking, jogging) performed during at least 10 days between 9am and 5pm over the 12 month period prior to diagnosis. Standardized metabolic equivalent (MET) values (Ainsworth et al., 2011) were assigned to each activity; ‘dose’ was calculated by multiplying the frequency of sessions per week by average session duration, weighted by the standardized activity MET. Individual activities were summed together to calculate a total MET-hours per week (MET-hrs·wk−1). Total MET-hrs·wk−1 was categorized via an unbiased quartile split (≤3, >3 to ≤20.2, >20.2 to ≤48.4, and >48.4 MET-hrs·wk−1). Median follow-up was 7.7 (range: 5.6 – 8.7) years.
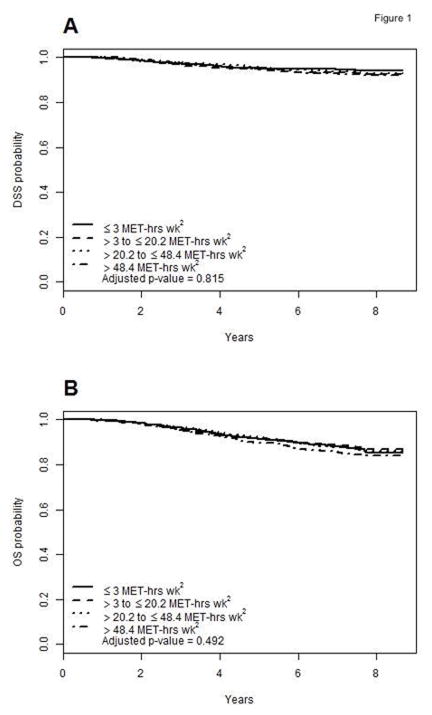
During the follow-up period, a total of 341 deaths were observed, with 163 deaths from melanoma. For the overall cohort (n=2,465), exercise was not significantly associated with the risk of death from melanoma (P=0.815; Fig. 1A). Compared to ≤3 MET·hrs·wk−1, the adjusted hazard ratio was 1.22 (95% CI, 0.78 to 1.89) for >3 to ≤20.2 MET·hrs·wk−1, 1.02 (95% CI, 0.65 to 1.62) for >20.2 to ≤48.4 MET·hrs·wk−1, and 1.09 (95% CI, 0.69 to 1.70) for >48.4 MET-hrs·wk−1. Similarly, exercise was not significantly associated with the risk of death from any cause (P=0.492; Fig. 1B). Compared to ≤3 MET·hrs·wk−1, the adjusted HR was 1.01 (95% CI, 0.74 to 1.36) for >3 to ≤20.2 MET·hrs·wk−1, 0.82 (95% CI, 0.60 to 1.11) for >20.2 to ≤48.4 MET·hrs·wk−1, and 0.89 (95% CI, 0.66 to 1.19) for >48.4 MET-hrs·wk−1. There were no significant interactions between exercise and disease-specific survival (DSS) (Fig. 2) on the basis of any clinicopathologic or molecular features of the tumor (all p-values >0.1).
Figure 1.
Figure 2.
In summary, we found that pre-diagnosis exercise was not associated with a reduction in cancer-related or overall mortality after a diagnosis of invasive melanoma. These findings both support and refute the current evidence examining the relationship between exercise and clinical outcomes in patients with solid tumors. For example, in a review of 26 studies including a total of 38,560 patients with breast, colorectal, or prostate cancer, ‘high’ exercise / physical activity was only associated with a significant reduction in cancer mortality among 11 (42%) studies.(Friedenreich et al., 2016) Differences in study methodology likely contributed to these divergent findings; it is becoming increasingly apparent, however, that the exercise – cancer prognosis relationship may differ as a function of tumor heterogeneity.(Jones, 2015; Jones et al., 2016) The design of the GEM study permitted for the first time a categorization of patients into homogeneous subgroups according to novel molecular predictors such as TILs grade and BRAF/NRAS status. Nevertheless, melanoma responses to exercise were not altered on the basis of any selected tumor features. The strengths of this study include the large, population-based cohort, long-term follow-up, and standardized pathologic and molecular review. Nevertheless, we need to keep in mind that the GEM study was designed to examine germ-line variants in the CDKN2A gene and other common genetic variants and known environmental risk factors, such as sun exposure (Begg et al., 2006), but was not specifically designed to evaluate exercise activity, perhaps resulting in some misclassification. Relatedly, our findings are likely impacted (possibly temporally) by residual confounding parameters such as disease characteristics (tumor aggressiveness) and other important prognostic factors, such as comorbid disease conditions. Indeed, there were significant differences in age, sun exposure and disease characteristics across exercise quartiles at diagnosis. However, we have no data on whether or how exercise behavior may have changed over the post-diagnosis time period, changes which might have contributed to the null findings in this study. Finally, data collection was conducted in 2000 – since this time the treatment paradigm and prognosis of primary melanoma patients,(Postow et al., 2015) as well as adherence to sun protective behaviors(Moyer and Force, 2012) has changed dramatically, thus limiting the generalizability of our findings.
In summary, we found that pre-diagnosis exercise was not associated with prognosis after primary melanoma. The emerging impact of exercise as well as other host-related factors (e.g., body composition, body weight, diet) on prognosis in other solid tumors (Goodwin et al., 2010) creates a solid rationale for prospective studies specifically designed to investigate the role of lifestyle factors on clinical outcomes after primary melanoma.
Supplementary Material
Acknowledgments
Funding / Support: This study was supported by grants R01CA112243, R01CA112524, R01CA112243-05S1, R01CA112524-05S2, U01CA83180, CA098438, R33CA10704339, P30CA016086, P30CA014089, and P30CA008748 from the National Cancer Institute; grant P30ES010126 from the National Institute of Environmental Health Sciences; and a University of Sydney Medical Foundation Program grant (Dr Armstrong). LWJ is supported by research grants from the National Cancer Institute, AKTIV Against Cancer, and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Conflicts of Interest: There are no conflicts of interest.
References
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Medicine and science in sports and exercise. 2011;43:1575–81. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- Begg CB, Hummer AJ, Mujumdar U, Armstrong BK, Kricker A, Marrett LD, Millikan RC, Gruber SB, Culver HA, Zanetti R, et al. A design for cancer case-control studies using only incident cases: experience with the GEM study of melanoma. Int J Epidemiol. 2006;35:756–64. doi: 10.1093/ije/dyl044. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0067. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Meyerhardt JA, Hursting SD. Host factors and cancer outcome. J Clin Oncol. 2010;28:4019–21. doi: 10.1200/JCO.2010.31.5143. [DOI] [PubMed] [Google Scholar]
- Jones LW. Precision Oncology Framework for Investigation of Exercise As Treatment for Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:4134–7. doi: 10.1200/JCO.2015.62.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Kwan ML, Weltzien E, Chandarlapaty S, Sternfeld B, Sweeney C, Bernard PS, Castillo A, Habel LA, Kroenke CH, et al. Exercise and Prognosis on the Basis of Clinicopathologic and Molecular Features in Early-Stage Breast Cancer: The LACE and Pathways Studies. Cancer Res. 2016;76:5415–22. doi: 10.1158/0008-5472.CAN-15-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna L, Wagner GC, Lou YR, Xie JG, Peng QY, Lin Y, Carlson K, Shih WJ, Conney AH, Lu YP. Inhibitory effects of voluntary running wheel exercise on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2006;27:2108–15. doi: 10.1093/carcin/bgl057. [DOI] [PubMed] [Google Scholar]
- Moyer VA, Force USPST. Behavioral counseling to prevent skin cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:59–65. doi: 10.7326/0003-4819-157-1-201207030-00442. [DOI] [PubMed] [Google Scholar]
- Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016;23:554–62. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NE, Kricker A, Waxweiler WT, Dillon PM, Busman KJ, From L, Groben PA, Armstrong BK, Anton-Culver H, Gruber SB, et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol. 2014;150:1306–314. doi: 10.1001/jamadermatol.2014.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.