Abstract
Background
The morbidity and mortality burden of the US opioid epidemic falls heavily on reproductive-age women. Information on the patterns of and sources for non-medical use of prescription opioids among reproductive age women, including pregnant women, will inform public health and prevention efforts to mitigate the effects of the opioid epidemic. This study characterized non-medical use of prescription opioids among reproductive-age U.S. women, with a focus on pregnancy status.
Methods
We used nationally-representative data from the National Survey of Drug Use and Health (2005–2014) to examine non-medical use (NMU) of prescription opioids in the past 30 days among females ages 18–44 (N=154,179), distinguishing pregnant women (N=8,069). We used multivariable logistic regression to describe reported sources of opioids, including opioids obtained from a doctor, friend or relative, dealer, or other source.
Results
Nearly 1% of pregnant women and 2.3% of non-pregnant reproductive-age women reported opioid NMU in the past 30 days. Forty-six percent of pregnant women identified a doctor as their source compared with 27.6% of non-pregnant women reporting NMU. Pregnant women reported a friend or relative as their source of opioids less frequently than non-pregnant women (53.8% versus 75.0%), and some pregnant and non-pregnant women acquired opioids from a dealer (14.6% and 10.6%).
Conclusion
Opioid NMU among reproductive-age women is a complex public health challenge affecting a vulnerable population. Pregnant women were more likely than non-pregnant women to list a doctor as their source of opioids for NMU, suggesting the need for targeted policies to address physician prescribing during pregnancy.
Keywords: women’s health, pregnancy, prescription opioids, maternal and child health
1.0. Introduction
The morbidity and mortality burden of the US opioid epidemic falls heavily on reproductive-age women. Between 1999 and 2010, drug overdose deaths related to opioid pain relievers increased fivefold among US women (Mack and Center For Disease Control, 2013). Although on average more men die from drug overdoses than women, including among those treated for opioid use disorders (Evans et al., 2015), the percentage increase in deaths since 1999 is greater among women, and the sex difference in overdose deaths is rapidly disappearing (Mack and Center For Disease Control, 2013). Reproductive-age women are more likely than younger or older women to require emergency care related to opioid misuse and abuse, in part owing to non-medical use of prescription opioid pain relievers (e.g., oxycodone, hydrocodone, fentanyl, morphine) (US Centers for Disease Control and Prevention, 2016). Non-medical use (NMU) is defined as the “intentional use of a medication without a prescription, in a way other than as prescribed, or for the experience or feeling that it causes (Committee on Health Care for Underserved Women and The American College of Obstetricians and Gynecologists, 2012).”
Knowing the source of opioids for NMU is crucial to informing prevention efforts. The majority of persons with recent NMU report obtaining opioids from friends or family, who in turn report obtaining the opioids from medical professionals (Substance Abuse and Mental Health Services Administration, 2014). Indeed, prescribing – practices and policy efforts to address overprescribing – are a focus of broad strategies to combat the opioid epidemic (Dowell et al., 2016). Opioid prescribing has particular relevance as a potential source for NMU among reproductive-age women. A recent study showed that between 2008–2012, nearly 40% of reproductive-age female Medicaid beneficiaries and almost 30% of privately-insured reproductive-age women filled at least one opioid prescription annually (Ailes et al., 2015).
Gender-specific research on opioid NMU is needed, owing to the different use patterns and effects among men and women (Evans et al., 2015; Kerridge et al., 2015). One important aspect of understanding women’s opioid NMU during reproductive years is the potential for pregnancy, given that almost half (45%) of all US pregnancies are unintended (Finer and Zolna, 2016). Further, the percentage of pregnancies that are unintended is substantially higher among women with opioid use disorders (Heil et al., 2011). Over the last decade, NMU of prescription opioids during pregnancy nearly doubled, mirroring national trends in opioid NMU (Pan and Yi, 2013; Patrick et al., 2015a). This increase in prenatal opioid use poses a significant public health concern, with potential risk for both women and infants. Opioid use during pregnancy is associated with increased risk of newborn withdrawal, known as neonatal abstinence syndrome, and preterm birth, which is the largest contributor to infant mortality (Patrick et al., 2015b). Infants diagnosed with neonatal abstinence syndrome have longer, more complicated birth hospitalizations with clinical signs that range from feeding difficulty to seizures (De’Souza, 2015; Creanga et al., 2012; Patrick et al., 2012; Tolia et al., 2015). Women themselves face significant medical and non-medical risks from opioid use during pregnancy, including increased risk of opioid use disorder, which is associated with increased odds of maternal cardiac arrest during delivery and with maternal death (Maeda et al., 2014). Substance use during pregnancy is also associated with broader risks, including intimate partner violence, and parental substance use is associated with involvement with foster care or child protective services (Young et al., 2007).
While pregnant women are an important policy-relevant group because of the risks described above, policy attention must encompass opioid NMU among the broader class of reproductive-age women. In spite of the growing impact of the opioid crisis among women, little of the emergent national attention has focused on addressing opioid use in this group generally, or prior to or during pregnancy specifically. More information on the patterns of and sources for opioid NMU among reproductive age women, including those who are pregnant, will inform public health and prevention efforts to mitigate the effects of the opioid epidemic on women, children and families. The goal of this study was to characterize non-medical prescription opioid use, including sources of opioids, among reproductive-age women in the US, distinguishing women based on pregnancy status.
2.0. Materials and Methods
2.1. Data and study population
We used pooled cross-sectional data from 2005 to 2014 from the National Survey of Drug Use and Health (NSDUH). The NSDUH provides population estimates of substance use and health-related behaviors in the U.S. general population. It utilizes multistage area probability sampling methods to select a representative sample of the U.S. civilian, non-institutionalized population aged 12 years or older for participation in the study. All respondents are ensured privacy when answering survey questions in their home, and sensitive questions are asked confidentially via computer with headphones (Substance Abuse and Mental Health Services Administration, 2013). Weighted annual interview response rates ranged between 71.2% and 76.0% during the study period (Substance Abuse and Mental Health Services Administration, 2015). The current study focused on reproductive age women ages 18–44 (N=154,179; weighted N= 558,385,835). We separately analyzed data for the 8,069 (weighted N= 23,064,218) women who reported that they were pregnant at the time of the survey. The sample of pregnant women is a subset of all reproductive age women, but we analyze them separately because opioid NMU may have different effects and implications during pregnancy.
2.2. Variable measurement
We identified past 30-day opioid NMU as responding yes to the survey question “Have you ever, even once, used any type of prescription pain reliever that was not prescribed for you or that you took only for the experience or feeling it caused?” and indicating that the last use of a prescription pain reliever was in the past 30 days. Additional measures of substance use were also defined within the past 30 days, including alcohol, cigarettes, marijuana, tranquilizer or sedative, and other (cocaine, crack, hallucinogen, inhalant, or stimulant). Alcohol use in the past 30 days was categorized as: heavy use (drinking ≥ 5 drinks on ≥ 5 days in the past 30 days); binge but not heavy (drinking ≥ 5 drinks on at least 1 day in the past 30 days); past 30 day use but not binge or heavy; and no use in the past 30 days (Ko et al., 2015). Cigarette use in the past 30 days was categorized into ≥26 cigarettes per day (≥1.5 packs), 6–25 cigarettes per day (0.5 – 1 packs), ≤5 cigarettes per day (< 0.5 pack), and no smoking within the past 30 days.
Survey respondents with past 30-day opioid NMU were asked 10 questions about the sources of these opioids. Respondents could answer affirmatively to each of the 10 questions, and as such, responses are not mutually-exclusive. We categorized affirmative responses to each question into four non-mutually-exclusive indicators of source of opioids for NMU: doctor (from one or more doctors); friend or relative (from friend or relative for free; bought from friend or relative; took from a friend or relative without asking); dealer (bought from a stranger); and other (wrote fake prescription; stole from a doctor’s office, clinic, hospital, or pharmacy; bought on the internet; got another way).
In order to measure factors that have a known association with opioid NMU, our multivariable analyses controlled for: (1) sociodemographic factors, such as age, race/ethnicity, education, marital status, health insurance, and family characteristics (King et al., 2014; Stine et al., 2009), (2) health and clinical characteristics, including serious psychological distress (Tetrault et al., 2008; Krans and Patrick, 2016), (3) criminal justice system involvement (Saloner et al., 2016), and (4) other substance use (Ko et al., 2015), as described below. Sociodemographics included age categories (ages 12–25, 26–35, and ≥36) and race/ethnicity. Respondents reporting Hispanic ethnicity were categorized as Hispanic and respondents not reporting Hispanic ethnicity were categorized as non-Hispanic Black, non-Hispanic White, and non-Hispanic other. Marital status included married, never married, and widowed, divorced, or separated. Education categories included less than high school, high school graduate, and some post-secondary education or higher. Health insurance categories were private insurance, public insurance (including Medicaid, CHIP, or CHAMPUS), and uninsured. Self-reported health status was reported as excellent, very good, good, and fair or poor. Total family income was categorized as less than $20,000, $20,000-$49,999, $50,000-$74,999, and ≥$75,000. The number of children in the household ages 0–17 was reported as 0, 1, 2, and ≥3. Trimester of pregnancy was included for pregnant respondents. An indicator of serious psychological distress was constructed from the Kessler 6 (K6) scale, a validated scale measuring non-specific psychological distress. We measured serious psychological distress as a dichotomous variable indicated by the highest K6 total score in the past year that exceeded 13 (Kessler et al., 2003). We defined a composite measure of criminal justice system involvement based on arrest at least once in the past year or probation, parole, or supervised release at any point in the past year. Missing values accounted for less than 2% of our sample across all variables.
2.3. Statistical analyses
We described weighted population characteristics and substance use of women in our sample by pregnancy status and by past 30 day opioid NMU. Separately for pregnant and non-pregnant women, we compared those with and without opioid NMU using Pearson chi-square tests.
We investigated associations between the three most frequent sources of opioid NMU – doctor, friend or relative, and dealer – and pregnancy status. We first present univariate results; then models that adjust for: (1) demographic characteristics including age, race, marital status, education, insurance, household income, number of children in household, and year; (2) demographic and health characteristics including self-reported health status and serious psychological distress; (3) demographic and health characteristics and criminal justice system involvement, and (4) fully adjusted models including demographic, health, criminal justice, and substance use variables including past 30 day cigarette, alcohol, marijuana, tranquilizer or sedative, and other drug use. All models were survey-weighted logistic regressions, and separate models were run for each of the three sources.
This study was exempted from review by the University of Minnesota Institutional Review Board. All analyses were conducted using R version 3.3.1.
3.0. Results
Table 1 presents weighted descriptive characteristics of reproductive-age U.S. women by pregnancy status and, for pregnant and non-pregnant women, comparing those with and without NMU of opioids. Four percent of women reported being pregnant at the time of the survey. Among pregnant women, 0.8% reported opioid NMU in the past 30 days. Compared with pregnant women with no opioid NMU, pregnant women with opioid NMU were younger, more likely to be unmarried, less educated, with lower household incomes, less likely to have private health insurance, in poorer self-reported health, more likely to experience serious psychological distress, and to have recent criminal justice system involvement. Also, pregnant women with opioid NMU were most frequently in their first trimester.
Table 1.
Characteristics of U.S. women ages 18–44 by pregnancy status and non-medical use of prescription opioids in the past 30 days
| Characteristic | Pregnant Weighted N=23,064,218 (4.1%) |
Non pregnant Weighted N=535,321,617 (95.9%) |
||||
|---|---|---|---|---|---|---|
| Opioid NMU Weighted N= 191,979 (0.8%) |
No opioid NMU Weighted N=22,872,240 (99.2%) |
P-value | Opioid NMU Weighted N=12,557,402 (2.3%) |
No opioid NMU Weighted N=522,764,215 (97.7%) |
P-value | |
| Age | ||||||
| 18–25 | 63.6% | 37.8% | 0.03 | 44.2% | 29.2% | < 0.001 |
| 26–35 | 26.1% | 48.9% | 30.2% | 31.9% | ||
| ≥36 | 10.3% | 13.3% | 25.6% | 39.0% | ||
| Race | ||||||
| Non-Hispanic Black | 22.4% | 14.0% | 0.39 | 12.4% | 13.9% | < 0.001 |
| Non-Hispanic White | 52.5% | 58.4% | 68.7% | 59.9% | ||
| Non-Hispanic Other | 6.2% | 8.2% | 4.6% | 8.4% | ||
| Hispanic | 18.9% | 19.3% | 14.3% | 17.8% | ||
| Marital Status | ||||||
| Married | 26.0% | 62.0% | < 0.001 | 25.2% | 46.0% | < 0.001 |
| Widowed/Divorced/Separated | 12.2% | 6.3% | 14.9% | 11.8% | ||
| Never Married | 61.8% | 31.6% | 59.9% | 42.3% | ||
| Household Income | ||||||
| <$20,000 | 45.6% | 23.1% | 0.004 | 29.8% | 21.9% | < 0.001 |
| $20,000–$49,999 | 33.1% | 31.5% | 36.2% | 32.8% | ||
| $50,000–$74,99 | 10.8% | 17.3% | 14.9% | 17.1% | ||
| ≥$75,000 | 10.5% | 28.1% | 19.1% | 28.2% | ||
| Education | ||||||
| Less than High School | 29.8% | 16.4% | 0.01 | 18.3% | 12.4% | < 0.001 |
| High School graduate | 31.5% | 26.8% | 32.7% | 27.0% | ||
| Some Post-secondary or more | 38.7% | 56.8% | 49.1% | 60.6% | ||
| Insurance | ||||||
| Private | 23.1% | 54.3% | < 0.001 | 46.8% | 62.9% | < 0.001 |
| Medicaid/CHIP/CH AMPUS | 46.1% | 32.6% | 22.3% | 14.5% | ||
| None | 30.8% | 13.1% | 30.9% | 22.6% | ||
| Number of Children in Household | ||||||
| 0 | 40.7% | 34.0% | 0.81 | 47.7% | 37.1% | < 0.001 |
| 1 | 28.7% | 33.2% | 21.8% | 23.3% | ||
| 2 | 20.4% | 20.6% | 17.9% | 23.8% | ||
| ≥ 3 | 10.2% | 12.2% | 12.5% | 15.8% | ||
| Self-reported Health Status | ||||||
| Excellent | 12.6% | 35.1% | < 0.001 | 13.5% | 27.5% | < 0.001 |
| Very Good | 18.8% | 37.7% | 36.5% | 39.6% | ||
| Good | 45.5% | 22.0% | 34.4% | 25.0% | ||
| Fair/Poor | 23.0% | 5.2% | 15.6% | 8.0% | ||
| Serious Psychological Distress | 52.6% | 12.3% | < 0.001 | 44.6% | 16.9% | < 0.001 |
| Criminal Justice System Involvement | 21.3% | 4.5% | < 0.001 | 11.7% | 3.0% | < 0.001 |
| Trimester | ||||||
| First | 46.5% | 31.0% | 0.05 | --- | --- | --- |
| Second | 33.5% | 35.6% | --- | --- | ||
| Third | 20.0% | 33.4% | --- | --- | ||
| Cigarettes, Past 30 Days | ||||||
| ≤5 cigarettes per day (<1/2 pack) | 30.6% | 7.1% | < 0.001 | 23.4% | 13.0% | < 0.001 |
| 6–25 cigarettes per day (1/2 – 1 pack) | 26.6% | 7.3% | 32.8% | 12.9% | ||
| ≥26 cigarettes per day (≥1 1/2 packs) | 0.1% | 0.5% | 3.0% | 1.1% | ||
| No cigarettes | 42.8% | 85.1% | 40.7% | 73.0% | ||
| Alcohol, Past 30 Days | ||||||
| Heavy use | 12.6% | 0.6% | < 0.001 | 19.9% | 5.3% | < 0.001 |
| Binge, not heavy | 19.5% | 2.3% | 33.5% | 19.8% | ||
| Not binge or heavy | 16.2% | 6.6% | 24.0% | 32.7% | ||
| No use | 51.8% | 90.5% | 22.5% | 42.3% | ||
| Marijuana, Past 30 Days | 41.4% | 2.9% | < 0.001 | 37.2% | 7.0% | < 0.001 |
| Tranquilizer or Sedative, Past 30 Days | 27.4% | 0.2% | < 0.001 | 23.7% | 0.7% | < 0.001 |
| Other (cocaine, crack, hallucinogen, inhalant, stimulant), Past 30 Days | 14.0% | 0.5% | < 0.001 | 19.1% | 1.4% | < 0.001 |
Notes: The unweighted number of pregnant women is 8,069 and the number of nonpregnant women is 146,110. Among pregnant women, the unweighted number of opioid NMU is 96 and the unweighted number of no opioid NMU is 7,973. Among nonpregnant women, the unweighted number of opioid NMU is 4,308 and the unweighted number of no opioid NMU is 141,802.
Among non-pregnant reproductive age women, 2.3% reported opioid NMU in the past 30 days. Compared with non-pregnant reproductive age women with no NMU, non-pregnant women with NMU of opioids were more likely to be non-Hispanic White, to have no children in the household, were younger, less educated, more likely to be unmarried, less likely to have private insurance, and more likely to self-report being in fair/poor health, to have experienced serious psychological distress, and to have recent criminal justice system involvement. Both pregnant and non-pregnant women with opioid NMU had higher rates of past 30 day cigarette smoking, alcohol, marijuana, and other substance use.
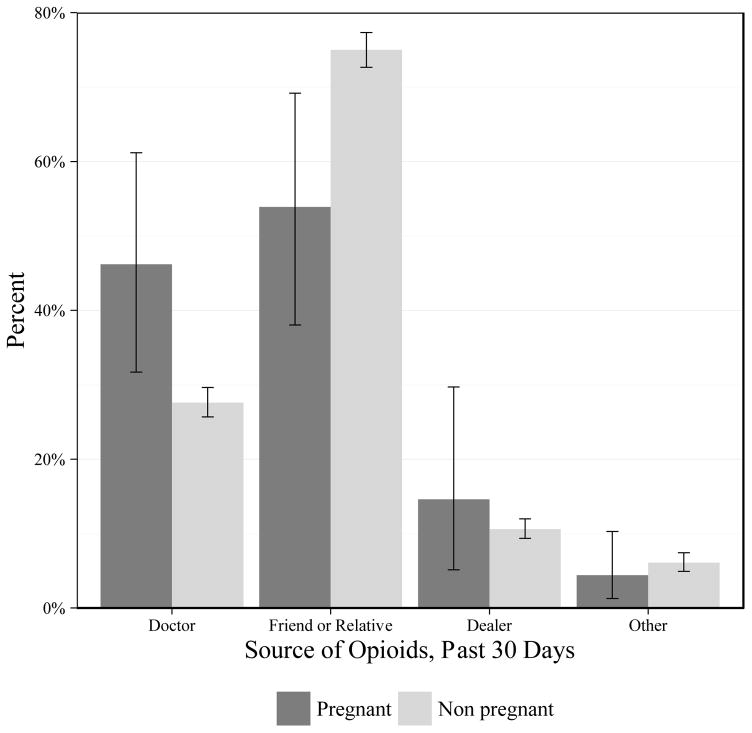
Source of opioids among women with NMU by pregnancy status is shown in Figure 1. A higher share of pregnant women with NMU of opioids reported that their source of opioids was a doctor compared with non-pregnant women with NMU of opioids (46.2% and 27.6%, respectively; p=0.004). Pregnant women reported a friend or relative as a source less frequently compared with non-pregnant reproductive-age women with NMU of opioids (53.9% and 75.0%, respectively; p=0.001). Slightly more pregnant women with opioid NMU reported a dealer as a source relative to non-pregnant women, though the difference was not statistically significant (14.6% and 10.6%, respectively; p=0.44).
Figure 1.
Source of prescription opioids for NMU in the past 30 days among U.S. women age 18–44 by pregnancy status
Notes: Weighted numbers of each source among pregnant women with opioid NMU are: Doctor: 88,720; Friend or Relative: 103,401; Dealer: 28,020, Other: 8,411. Weighted numbers of each source among non-pregnant women with opioid NMU are: Doctor: 3,469,816; Friend or Relative: 9,424,228; Dealer: 1,334,830, Other: 766,478. Unweighted numbers of each source among pregnant women with opioid NMU are: Doctor: 38; Friend or Relative: 53; Dealer: 12; Other: 5. Unweighted numbers of each source among non-pregnant women with opioid NMU are: Doctor: 1,135; Friend or Relative: 3,320; Dealer: 593, Other: 233.
Table 2 shows results from multivariable models examining associations between pregnancy status of women with NMU of opioids and opioid source, separately for those reporting that they obtained opioids from doctors, from friends or relatives, and from dealers (Full model results are shown in Supplemental Tables 1–31). In fully adjusted models, pregnant women had nearly twice the odds of getting opioids from a doctor relative to non-pregnant women (aOR 1.82; 95% CI 0.99, 3.37) but had half of the odds of getting opioids from a friend or relative (aOR 0.51; 95% CI 0.25, 1.04). Pregnant women had higher odds of procuring opioids from a dealer relative to non-pregnant women (aOR 1.36; 95% CI 0.53, 3.49; p=0.53). These differences in source by pregnancy status, which adjust for potential confounders, are measurable, but not statistically significant at p-value<0.05 level (exact p-values are 0.06 and 0.07, respectively), owing in part to small sample sizes in subgroups of pregnant women with opioid NMU sourcing from a doctor (unweighted N=38, weighted N=88,720), from a friend or relative (unweighted N=53, weighted N=103,401), and from a dealer (unweighted N=12, weighted N=28,020).
Table 2.
Associations between sources of opioids and pregnancy status of women ages 18–44 with non-medical use of opioids in the past 30 days
| Unadjusted | Adjusted (1) | Adjusted (2) | Adjusted (3) | Adjusted (4) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | aOR (95% CI) | P-val=ue | aOR (95% CI) | P-value | aOR (95% CI) | P-value | aOR (95% CI) | P-value | |
| Source: Doctor | ||||||||||
| Pregnancy Status | ||||||||||
| Pregnant (vs. not pregnant) | 2.25 (1.28, 3.95) | 0.006 | 1.96 (1.10, 3.49) | 0.03 | 1.94 (1.09, 3.45) | 0.03 | 1.96 (1.09, 3.53) | 0.03 | 1.82 (0.99, 3.37) | 0.06 |
| Source: Friend or Relative | ||||||||||
| Pregnancy Status | ||||||||||
| Pregnant (vs. not pregnant) | 0.39 (0.21, 0.71) | 0.003 | 0.44 (0.23, 0.83) | 0.01 | 0.43 (0.22, 0.82) | 0.01 | 0.44 (0.23, 0.85) | 0.02 | 0.51 (0.25, 1.04) | 0.07 |
| Source: Dealer | ||||||||||
| Pregnancy Status | ||||||||||
| Pregnant (vs. not pregnant) | 1.44 (0.57, 3.62) | 0.44 | 1.34 (0.48, 3.73) | 0.58 | 1.32 (0.48, 3.62) | 0.60 | 1.32 (0.47, 3.73) | 0.60 | 1.36 (0.53, 3.49) | 0.53 |
Notes: Adjusted (1) models include demographic characteristics age, race, marital status, household income, education, insurance, number of children in household, self-reported health status, and year. Adjusted (2) models additionally include serious psychological distress. Adjusted (3) models additionally include criminal justice system involvement. Adjusted (4) models additionally include use of the following substances: cigarettes, alcohol, tranquilizer or sedative, and other illicit drugs. Full multivariable model results are presented in Appendix 13.
A variety of other characteristics of women with NMU of opioids were associated with receiving opioids from a doctor. Figure 2 displays select results for doctor, friend or relative, and dealer models in a forest plot. Full model results are presented in Appendix Tables 1–32. Women who were pregnant, non-White, those having government insurance, and using tranquilizers or sedatives in the past 30 days had greater odds of sourcing opioids from a doctor. Younger women, low income women, women with young family members in the household, and those using alcohol and marijuana in the past 30 days had higher odds of procuring opioids from a friend or relative. Younger women and women using marijuana and tranquilizers or sedatives in the past 30 days had higher odds of sourcing opioids from a dealer. Women experiencing serious psychological distress had greater odds of acquiring opioids from all three sources, though these were not statistically significant.
Figure 2.
Forest plot charting adjusted odds of reporting a doctor, friend or relative, or dealer as the source of opioids by the characteristics of reproductive-age women using opioids non-medically
4.0. Discussion
While the rates of NMU of opioids among reproductive-age women at first glance appear low (at 2.3% and 0.8% among non-pregnant and pregnant women respectively), the public health burden of NMU in this population is quite large. Annually, there are more than 6 million pregnancies each year in the US, occurring among 62 million reproductive-age women (Curtin et al., 2013). Our findings indicate that approximately 1.4 million reproductive-age women (2.3%) and 50,000 pregnant women (0.8%) have recent NMU of opioids. The morbidity and mortality burden of the US opioid epidemic falls heavily on reproductive-age women (Mack et al., 2013). Additionally, NMU of opioids during pregnancy poses substantial health and social risks to women, infants, and families. Policy efforts to address the public health crisis presented by the opioid epidemic must directly confront the particular needs of reproductive-age and pregnant women, who represent a substantial portion of the affected population.
This study’s finding that pregnant women have greater odds than non-pregnant women of listing a doctor as the source of their opioids suggests one possible area for intervention. Nearly half of pregnant women in this sample with opioid NMU reported a doctor as the source of opioids, compared with less than a third of non-pregnant women with opioid NMU. Newly updated prescribing guidelines for opioid pain relievers now include information on pregnant women, but attention to all reproductive-age women is important (Dowell et al., 2016). Pregnant women are a subset of all reproductive-age women. Those who continue opioid NMU during pregnancy may have a more severe form of dependency or may have had health conditions, such as chronic pain, that necessitated prescription opioid medications at point in time prior to the use becoming nonmedical. As such, physician-prescribed opioids as a source for opioid NMU during pregnancy may have originated prior to pregnancy.
Efforts to limit access to opioids for NMU require a multi-faceted approach. Public health detailing, a model using one-on-one educational visits (Kattan et al., 2013), could include tailored information for clinicians that care for reproductive-age and pregnant women. Physician-based systems, such as prescription drug monitoring programs, which have been associated with decreases in opioid-associated adverse outcomes (Patrick et al., 2016), may also hold promise for mitigating the effects of opioid NMU among pregnant women. Given evidence that abrupt discontinuation of opioid use can pose severe risks to the woman and fetus, efforts to curtail prescribing to pregnant women believed to be using opioids non-medically must be combined with concerted efforts to initiate addiction treatment. Opioid maintenance therapy (with methadone or buprenorphine) is the standard of care for pregnant women with opioid use disorder (Hall et al., 2016), and may improve the likelihood that families remain cohesive in cases involving the child welfare system (ACOG Committee on Health Care for Underserved Women and American Society of Addiction Medicine, 2012). Given that rates of opioid prescribing are higher among pregnant Medicaid beneficiaries compared with privately-insured pregnant women (Desai et al., 2014), developing guidelines and targeting physician interventions to community health centers and other settings relying heavily on Medicaid reimbursement are worth considering. More broadly, our findings highlight the relative socioeconomic disadvantage of women with opioid NMU, emphasizing the need for efforts around prescribing to account for the complex interplay of risk factors that affect both the source of opioids and the consequences of opioid NMU.
Fully three-fourths of non-pregnant women reported a friend or relative as source, while over half of pregnant women (almost as many who reported doctor) reported a friend or relative as source. Prior studies describe unused pain medications (including post-surgical medications) as a potential source that may be accessed by friends or relatives for opioid NMU (Bartels et al., 2016; Kennedy-Hendricks et al., 2016a). Disposal of opioids at designated collection sites may hold promise as a policy strategy to address this source (Maughan et al., 2016). Also, a small but substantial portion of reproductive-age women (pregnant and not pregnant) reported that drug dealers were the source of opioids for NMU, emphasizing the importance of ongoing efforts to reduce illegal sales of prescription opioids, but attention should be paid to whether tailored strategies to reduce dealer sourcing for pregnant or reproductive-age women would be warranted.
When neonatal abstinence syndrome is discussed in policy initiatives, the focus is frequently on treatment of infants, not on reproductive-age or pregnant women (De’Souza, 2015; Krans and Patrick, 2016). Further complicating efforts to ensure opioid use disorder treatment for pregnant women are punitive laws that criminalize addiction in the case of pregnancy (Kennedy-Hendricks et al., 2016b; Terplan et al., 2015a; Roberts and Pies, 2011; Guttmacher Institute, 2016). Public health and clinical efforts to promote screening for substance use before and during pregnancy may allow for greater detection, but care must be taken to ensure that such programs take account of the broader policy environment, including the criminalization of substance use during pregnancy. The state of Tennessee, for example, implemented statewide surveillance for neonatal abstinence syndrome, starting in 2013 (Warren et al., 2015). This system has dramatically increased detection rates, but has done so in a context where state law – until 2016 – allowed for criminal prosecution of a pregnant woman for fetal endangerment. Tennessee’s example is instructive and highlights the importance of screening and detection practices that do not discourage prenatal care seeking (Roberts and Pies, 2011).
Opioid NMU among reproductive-age women affects individuals and families at a crucial point in the lifecourse, and efforts to mitigate the health and social impacts of opioid NMU in this population ought to focus on screening and treatment efforts that occur at multiple time points during women’s reproductive lives: during, prior to, or outside the context of pregnancy. The need for woman-centered treatment for substance abuse has greatly outpaced availability (Terplan et al., 2015b), and to be effective, future policy efforts should ensure attention to the particular needs of reproductive-age and pregnant women in prevention and treatment efforts.
4.1. Limitations
While this study presents national estimates of opioid NMU and source of opioids among reproductive-age women in the US, there are several important limitations of the data used in this analysis. First, although self-report is considered a reliable measure for pregnancy status (Overbeek et al., 2013), it is possible that respondents may have misreported or been unaware of their pregnancy status. Opioid NMU and other substance use may be under-reported generally and among pregnant individuals, owing to both recall bias and social desirability bias (McQueen et al., 2015), but NSDUH undertakes considerable efforts to diminish the potential effects of such biases (Substance Abuse and Mental Health Services Administration, 2014). Repeat cross-sections of NSDUH data from 2005–2014 were pooled to increase the analytic sample size, but longitudinal assessment was not possible and there were substantial shifts in awareness of the risks of addiction associated with prescription opioid use among both clinicians and the general public over this time period.
5.0. Conclusions
Opioid NMU has health, social and cost consequences for reproductive-age women, and potential impacts are heightened during pregnancy, when a fetus may also be affected. Opioid NMU among reproductive-age women is a complex public health challenge affecting a vulnerable population. While friends and relatives were the most common source of opioids among reproductive-age women overall, pregnant women were more likely than non-pregnant women to list a doctor as the source of their opioids, suggesting the need for targeted policy efforts to address opioid access and NMU during pregnancy and over the reproductive years. Efforts to address opioid use disorders should build on existing systems for substance use identification and treatment and recognize the myriad needs of women at highest risk of poor outcomes associated with substance use generally and during pregnancy.
Supplementary Material
Highlights.
We studied non-medical use of prescription opioids among reproductive-age women.
1% of pregnant women; 2.3% of non-pregnant women reported past 30 day opioid NMU.
46% of pregnant women reporting NMU identified a doctor as their source of opioids.
Opioid NMU among reproductive-age women affects a vulnerable population.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors:
K. Kozhimannil conceived the study, acquired the data, supervised the analysis, and drafted portions of the manuscript. A. Graves conducted the analysis, contributed to data presentation, and drafted portions of the manuscript. M. Jarlenski, A. Kennedy-Hendricks, S. Gollust, and C. Barry contributed to the conception and design of the study, the analysis and interpretation of the results, data presentation, and public health and policy implications. All authors reviewed the results, revised the article for important intellectual content, and approved the final version.
Conflict of Interest:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katy B. Kozhimannil, Division of Health Policy and Management, University of Minnesota School of Public Health. 420 Delaware St. SE, MMC 729, Minneapolis, MN 55455; Phone: 612-626-3812; Fax: 612-624-2196
Amy J. Graves, Division of Health Policy and Management, University of Minnesota School of Public Health. 420 Delaware St. SE, MMC 729, Minneapolis, MN 55455; Phone: 612-626-3812; Fax: 612-624-2196
Marian Jarlenski, Department of Health Policy and Management, University of Pittsburgh Graduate School of Public Health, 130 DeSoto St, A619, Pittsburgh, PA 15261; Phone: 412-383 5363; Fax: 412-624-3146.
Alene Kennedy-Hendricks, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, 624 N. Broadway, Room 311, Baltimore, MD 21205; Phone: 443-287-5324; Fax: 410-614-4535.
Sarah Gollust, Division of Health Policy and Management, University of Minnesota School of Public Health. 420 Delaware St. SE, MMC 729, Minneapolis, MN 55455; Phone: 612-626-2618; Fax: 612-624-2196.
Colleen L. Barry, Department of Health Policy and Management; Johns Hopkins Bloomberg School of Public Health, 624 N. Broadway, Room 403; Baltimore, MD 21205; Phone: 410-955-3879; Fax: 410-614-4535
References
- ACOG Committee on Health Care for Underserved Women, American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119:1070–1076. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- Ailes EC, Dawson AL, Lind JN, Gilboa SM, Frey MT, Broussard CS, Honein MA Centers for Disease Control and Prevention. Opioid prescription claims among women of reproductive age--United States, 2008–2012. [Accessed August 24, 2016];MMWR. 2015 64:37–41. http://www.ncbi.nlm.nih.gov/pubmed/25611168. [PMC free article] [PubMed] [Google Scholar]
- Bartels K, Mayes LM, Dingmann C, Bullard KJ, Hopfer CJ, Binswanger IA. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One. 2016;11:e0147972. doi: 10.1371/journal.pone.0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Health Care for Underserved Women, The American College of Obstetricians and Gynecologists. Committee Opinion No. 538: Nonmedical use of prescription drugs. Obstet Gynecol. 2012;120:977–982. doi: 10.1097/AOG.0b013e3182723b5a. [DOI] [PubMed] [Google Scholar]
- Creanga A, Sabel JC, Ko JY, Wasserman CR, Shapiro-Mendoza CK, Taylor P, Barfield W, Cawthon L, Paulozzi LJ. Maternal drug use and its effect on neonates: A population-based study in Washington State. Obstet Gynecol. 2012;119:924–933. doi: 10.1097/AOG.0b013e31824ea276. [DOI] [PubMed] [Google Scholar]
- Curtin SC, Abma JC, Ventura SJ, Henshaw SK. Pregnancy rates for U.S. women continue to drop. NCHS Data Brief. 2013;136:1–8. http://www.ncbi.nlm.nih.gov/pubmed/24314113. [PubMed] [Google Scholar]
- De’Souza V. GAO-15-203. Government Accountability Office; Washington, D.C: 2015. Prenatal Drug Use and Newborn Health: Federal Efforts Need Better Planning and Coordination. [Google Scholar]
- Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123:997–1002. doi: 10.1097/AOG.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315:1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Kelleghan A, Li L, Min J, Huang D, Urada D, Hser Y, Nosyk B. Gender differences in mortality among treated opioid dependent patients. Drug Alcohol Depend. 2015;155:228–235. doi: 10.1016/j.drugalcdep.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmacher Institute. State Policies In Brief: Substance Abuse During Pregnancy. Washington, DC: 2016. [Accessed August 24, 2016]. https://www.guttmacher.org/sites/default/files/pdfs/spibs/spib_SADP.pdf. [Google Scholar]
- Hall MT, Wilfong J, Huebner RA, Posze L, Willauer T. Medication-assisted treatment improves child permanency outcomes for opioid-using families in the child welfare system. J Subst Abuse Treat. 2016;71:63–67. doi: 10.1016/j.jsat.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Heil SH, Jones HE, Arria A, Kaltenbach K, Coyle M, Fischer G, Stine S, Selby P, Martin PR. Unintended pregnancy in opioid-abusing women. J Subst Abuse Treat. 2011;40:199–202. doi: 10.1016/j.jsat.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattan JA, Tuazon E, Paone D, Dowell D, Vo L, Starrels JL, Jones CM, Kunins HV. Public health detailing—A successful strategy to promote judicious opioid analgesic prescribing. Am J Public Health. 2016;106:1430–1438. doi: 10.2105/AJPH.2016.303274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Hendricks A, Gielen A, McDonald E, McGinty EE, Shields W, Barry CL. Medication sharing, storage, and disposal practices for opioid medications among us adults. JAMA Intern Med. 2016a;6:1027. doi: 10.1001/jamainternmed.2016.2543. [DOI] [PubMed] [Google Scholar]
- Kennedy-Hendricks A, McGinty EE, Barry CL. Effects of competing narratives on public perceptions of opioid pain reliever addiction during pregnancy. J Health Polit Policy Law. 2016b;41:873–916. doi: 10.1215/03616878-3632230. [DOI] [PubMed] [Google Scholar]
- Kerridge BT, Saha TD, Chou SP, Zhang H, Jung J, Ruan WJ, Smith SM, Huang B, Hasin DS. Gender and nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions - III. Drug Alcohol Depend. 2015;156:47–56. doi: 10.1016/j.drugalcdep.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, Howes MJ, Normand SL, Manderscheid RW, Walters EE, Zaslavsky AM. Screening for serious mental illness in the general population. [Accessed August 24, 2016];Arch Gen Psychiatry. 2003 60:184–189. doi: 10.1001/archpsyc.60.2.184. http://www.ncbi.nlm.nih.gov/pubmed/12578436. [DOI] [PubMed] [Google Scholar]
- King NB, Fraser V, Boikos C, Richardson R, Harper S. Determinants of increased opioid-related mortality in the United States and Canada, 1990-2013: A systematic review. Anesthesiology. 2014;311:367–370. doi: 10.3109/00952990.2015.1047502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JY, Farr SL, Tong VT, Creanga AA, Callaghan WM. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol. 2015;213:201.e1–e201.e10. doi: 10.1016/j.ajog.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krans EE, Patrick SW. Opioid use disorder in pregnancy: Health policy and practice in the midst of an epidemic. Obstet Gynecol. 2016;128:4–10. doi: 10.1097/AOG.0000000000001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack KA Centers for Disease Control and Prevention (CDC) Drug-induced deaths - United States, 1999–2010. [Accessed August 24, 2016];MMWR Suppl. 2013 62:161–163. http://www.ncbi.nlm.nih.gov/pubmed/24264508. [PubMed] [Google Scholar]
- Maeda A, Bateman BT, Clancy CR, Creanga A, Leffert LR. Opioid abuse and dependence during pregnancy: Temporal trends and obstetrical outcomes. Anesthesiology. 2014;121:1158–1165. doi: 10.1097/ALN.0000000000000472. [DOI] [PubMed] [Google Scholar]
- Maughan BC, Hersh EV, Shofer FS, Wanner KJ, Archer E, Carrasco LR, Rhodes KV. Unused opioid analgesics and drug disposal following outpatient dental surgery: A randomized controlled trial. Drug Alcohol Depend. 2016;168:328–334. doi: 10.1016/j.drugalcdep.2016.08.016. [DOI] [PubMed] [Google Scholar]
- McQueen KA, Murphy-Oikonen J, Desaulniers L. Maternal substance use and neonatal abstinence syndrome: A descriptive study. Matern Child Health J. 2015;19:1756–1765. doi: 10.1007/s10995-015-1689-y. [DOI] [PubMed] [Google Scholar]
- Overbeek A, van den Berg MH, Hukkelhoven CW, Kremer LC, van den Heuvel-Eibrink MM, Tissing WJ, Loonen JJ, Versluys AB, Bresters D, Kaspers GJ, Lambalk CB, van Leeuwen FE, van Dulmen-den Broeder E DCOG LATER/VEVO Study Group. Validity of self-reported data on pregnancies for childhood cancer survivors: A comparison with data from a nationwide population-based registry. Hum Reprod. 2013;28:819–827. doi: 10.1093/humrep/des405. [DOI] [PubMed] [Google Scholar]
- Pan IJ, Yi HY. Prevalence of hospitalized live births affected by alcohol and drugs and parturient women diagnosed with substance abuse at liveborn delivery: United States, 1999–2008. Matern Child Health J. 2013;17:667–676. doi: 10.1007/s10995-012-1046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff (Millwood) 2016;35:1324–1332. doi: 10.1377/hlthaff.2015.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015a;35:667. doi: 10.1038/jp.2015.63. [DOI] [PubMed] [Google Scholar]
- Patrick SW, Dudley J, Martin PR, Harrell FE, Warren MD, Hartmann KE, Ely EW, Grijalva CG, Cooper WO. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015b;135:842–850. doi: 10.1542/peds.2014-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures, United States, 2000–2009. JAMA. 2012;307:1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- Roberts SCM, Pies C. Complex calculations: How drug use during pregnancy becomes a barrier to prenatal care. Matern Child Health J. 2011;15:333–341. doi: 10.1007/s10995-010-0594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B, Bandara SN, McGinty EE, Barry CL. Justice-involved adults with substance use disorders: Coverage increased but rates of treatment did not in 2014. Health Aff (Millwood) 2016;35:1058–1066. doi: 10.1377/hlthaff.2016.0005. [DOI] [PubMed] [Google Scholar]
- Stine SM, Heil SH, Kaltenbach K, Martin PR, Coyle MG, Fischer G, Arria AM, Selby P, Jones HE. Characteristics of opioid-using pregnant women who accept or refuse participation in a clinical trial: Screening results from the MOTHER Study. Am J Drug Alcohol Abuse. 2009;35:429–433. doi: 10.3109/00952990903374080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. 2014 National Survey on Drug Use and Health: Methodological Summary and Definitions. Rockville, MD: 2015. http://www.samhsa.gov/data/sites/default/files/NSDUH-MethodSummDefs2014/NSDUH-MethodSummDefs2014.htm#b3-1. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of national findings. 2014 NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. 2014 National Survey on Drug Use and Heath: Field Interviewer Manual. Rockville, MD: 2013. http://www.samhsa.gov/data/sites/default/files/NSDUHmrbFImanual2014_opt.pdf. [Google Scholar]
- Terplan M, Kennedy-Hendricks A, Chisolm MS. Prenatal substance use: Exploring assumptions of maternal unfitness. Subst Abuse. 2015a;9(Suppl 2):1–4. doi: 10.4137/SART.S23328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terplan M, Longinaker N, Appel L. Women-centered drug treatment services and need in the United States, 2002–2009. Am J Public Health. 2015b;105:e50–e54. doi: 10.2105/AJPH.2015.302821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetrault JM, Desai RA, Becker WC, Fiellin DA, Concato J, Sullivan LE. Gender and non-medical use of prescription opioids: Results from a national US survey. Addiction. 2008;103:258–268. doi: 10.1111/j.1360-0443.2007.02056.x. [DOI] [PubMed] [Google Scholar]
- Tolia VN, Patrick SW, Bennett MM, Muthy K, Sousa J, Smith B, Clark RH, Spitzer AR. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372:2118–2126. doi: 10.1056/NEJMsa1500439. [DOI] [PubMed] [Google Scholar]
- US Centers for Disesase Control and Preventon. [Accessed December 20, 2016];Prescription Painkiller Overdoses | VitalSigns. https://www.cdc.gov/vitalsigns/prescriptionpainkilleroverdoses/index.html.
- Warren MD, Miller AM, Traylor J, Bauer A, Patrick SW. Implementation of a statewide surveillance system for neonatal abstinence syndrome - Tennessee, 2013. [Accessed March 21, 2016];MMWR. 2015 64:125–128. http://www.ncbi.nlm.nih.gov/pubmed/25674995. [PMC free article] [PubMed] [Google Scholar]
- Young NK, Boles SM, Otero C. Parental substance use disorders and child maltreatment: Overlap, gaps, and opportunities. Child Maltreat. 2007;12:137–149. doi: 10.1177/1077559507300322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.