Abstract
Background
The main purpose of this study was to compare the effects of various lavage techniques – traditional saline lavage (SL), pulse lavage (PL), closed drainage (CD), and iodine lavage (IL) – on preventing incision-related infection after posterior lumbar interbody fusion.
Material/Methods
Patients with prolapsed lumbar (intervertebral) discs (PLID) undergoing posterior lumbar interbody fusion surgery (PLIF) over the course of 2 years were included and were randomly allocated into 4 groups: the SL group, the PL group, the CD group, and the IL group. Relevant data were recorded, including preoperative conditions, intraoperative lavage time, lavage fluid volume, incision outlook, pain perception, results of routine blood tests, and postoperative infection rate.
Results
The PL, CD, and IL groups showed less intraoperative lavage time, lavage volume fluid, effusion, infection rate, and muscle and lower pain perception compared with the SL group (all P<0.05). Significant differences in white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were observed between preoperative and postoperative data in each group (P<0.01). No significant differences in clinical characteristics, postoperative temperature, suture removal time, incision characteristics, WBC, ESR, and CRP were observed among the PL, CD, IL, and SL groups (P>0.05).
Conclusions
PL, CD, and IL all showed much better postoperative infection prevention in comparison to SL.
MeSH Keywords: Clinical Laboratory Techniques, Clinical Nursing Research, General Surgery
Background
Posterior lumbar interbody fusion (PLIF) is an popular spinal fusion surgery that involves combining bone graft with a certain areas of the lumbar disc to facilitate bone growth between the 2 vertebral elements, thereby preventing the motion of that integral part [1]. This technique has been used as a standard treatment for various spinal pathologies, such as degenerative disk disease, trauma, instability, and pseudarthrosis [1]. Nevertheless, postoperative morbidity caused by wound infection is one of the most common and devastating acute complications of PLIF. A postoperative infection rate ranging from 0.2% to 2.75% has been reported by previous research [2]. Irrigation of the wound surface is performed to remove microbes near the wound, which might thereby decrease the inflammatory reaction rate. Conventional artificial dumping rinse is limited in treatment efficacy and it may trigger secondary infections [3]. Furthermore, frequent symptoms of spinal postoperative infection, including incision pain, fever, and oozing, can rarely be identified at early stages, and prolonged diagnosis of these symptoms may eventually result in surgical failure [4].
Pulsed lavage (PL), which has been widely applied in clinical practice, delivers irrigating solution under pulsed pressure. The pulsed irrigator produces pulsed current under varied pressures to irrigate the wound so that microbes and foreign bodies are removed from the wound and infection can be effectively prevented [5]. In addition, PL promotes granulation tissue growth [6]. Compared with conventional irrigation techniques, PL is also advantageous in enhanced efficiency and economic usage of irrigating fluid. Omnidirectional irrigation is another popular technique that has been employed in irrigating medullary cavities, yet it has rarely been used in postoperative irrigation in spinal surgery.
Closed drainage (CD) has closed area around the wound, which is created by protective materials in conjunction with a biologic semi-permeable membrane that leads irrigating fluid to the wound [7]. In comparison to artificial dumping rinse, CD is more effective in suppressing bacteria growth, thereby preventing postoperative infections. CD not only prevents the corresponding symptoms of infection, but also reduces the pressure required within the lumen [4,8]. CD has been used as a cleansing therapy for open fractures of the extremities and it is also being introduced to clean wounds in surgical operations [9].
Iodine lavage (IL) and traditional saline lavage (SL) rinsing involves simultaneous examination and cleaning of wounds, followed by immersion in 0.1% povidone-iodine solution. SL is used to remove contaminated, dark-colored, or nonviable tissues, whereas IL in conjunction with broad-spectrum bactericide is able to remove necrotic tissues and clean bacteria arising from arthrotomy and laceration [10]. The popularity of IL and SL rinsing is growing in clinical practice, and they both have been introduced to prevent postoperative infection [11].
These 4 irrigation techniques all possess strengths and shortcomings, yet few investigations have compared them in detail. Therefore, this retrospective study was carried out to compare the treatment effectiveness of 4 wound irrigation techniques: SL, PL, CD, and IL.
Material and Methods
Study participants
We recruited 160 patients with prolapsed lumbar intervertebral discs (PLID) who were treated with posterior lumbar interbody fusion surgery (PLIF) from January 1st of 2014 to December 31st of 2015. The included patients were: 1) older than 18 years old without any severe disorders relevant to heart, kidney, lung, or other organs; 2) diagnosed with PLID or lumbar vertebral tube narrow syndrome; 3) met requirements for performance of PLIF; 4) not accompanied by diabetes, lumbar tumor, lumbar tuberculosis, infection, or trauma that could affect inflammation indexes. Patients were excluded if: 1) they suffered from dysfunctions related to the heart, kidney, or other organs, which might prevent surgery; 2) their lumbar disc herniation and lumbar spinal canal stenosis were not clear; 3) they refused surgical interventions. This study was approved by the Ethics Committee of Shanghai Ninth People’s Hospital and the Hospital of Integrated Traditional and Western Medicine of Zhejiang, and all participants signed consent forms. Patients were divided into 4 equal groups (PL, CD, IL, and SL) by random number table method.
Pre-irrigation examination
Venous blood specimens were obtained from fasting patients on the morning before surgery. Preoperative routine examinations were carried out, including routine blood tests, electrolytes, coagulation profile, and electrocardiography (ECG).
Surgery
All patients were put under general anesthesia, and they were placed on the operating table in prone position. Surgery sites were located by X-ray, and then they were disinfected with sterile drapes. Subsequently, intervertebral bone grafting and fusion were conducted from the posterior midline incision. After carefully observing the inflexible soft tissue muscles on both sides of the incision due to pressure and contusion of expanders, tissue scissors were used to cut the surface of necrotic soft tissues until petechial hemorrhages were exposed to the fresh soft tissue. Subsequently, patients underwent different methods of irrigation performed 3 times. Specific operation procedures are as follow.
PL group
Incisions were irrigated with pulsed lavage with application of a pulsatile lavage unit (Apexpulse™ AP-D01). After patients were placed on the operating table in prone position, a sterile basin was put below the incision and the table was covered with waterproof sheeting. Technicians ore protective goggles, waterproof gown, and sterile gloves before performing pulse lavage. Saline was delivered to the surface of the wound through a soft detachable spray nozzle. Finally, the lavage unit was connected to the saline supply (volume: 3000 ml) at 38°C.
CD group
Saline solution was used to flush the incisions in the usual way. Three closed drainage tubes were set up: 2 tubes were placed on the top and ends of incisions under the deep fascia, while the third tube was placed on the end of incisions under subcutaneous tissues. The deep fascia was stitched closely on both sides, while subcutaneous tissues were stitched in the routine way. The irrigation tube was set at 100 ml/h flushing for 24 h, so that the fluid properties and drainage status can be observed closely. According to the clarity of the drainage, liquid adjustments were made to match flow velocity.
IL group
The incision was flushed with 0.1% iodine solution (volume: 200 ml) and soaked for 2 min, then the incisions were flushed with normal saline under adequate hemostasis conditions.
SL group
The incisions were flushed with saline solution in the usual way. Patients were placed in prone position. The sterile basin was placed below the incision and the table was covered by waterproof sheeting. All medical staff wore waterproof gowns and sterile gloves. No postoperative prophylactic antibiotics were used.
After the third irrigation, specimens were obtained via cotton swab from the posterior back muscles and intradiscal space. Bacterial culture was aerobically performed with MacConkey agar medium (MAC) under conditions of 37°C, light 3000~5000 lx, and medium of pH 7.0.
Evaluation of intraoperative index
The corresponding intraoperative lavage time and lavage fluid volume were recorded for each patient to evaluate the surgical effects of different lavage techniques.
Evaluation of postoperative index
General clinical information, including the average body temperature of patients at 1 week after the operation and the corresponding suture removal time, were recorded in each group. Incisions of patients in each group were observed carefully. Signs of red color, swelling, heat at incision site, and effusion were monitored during the course of incision. All signs were classified within 4 grades: none, mild, moderate, and severe. Indicators of reddening, swelling, and heat at the incision site were classified by touch or observation. The wound effusion quantity was used to classify effusion: (1) “none” – the gauze was still dry after 24 h; (2) “mild” – seepage quantity was less than 5 mL in 24 h; (3) “moderate” seepage quantity ranged from 5 mL to 10 mL in 24 h; (4) “severe” – seepage quantity was more than 10 mL in 24 h. Perception of patients with respect to pain caused by debridement was also monitored.
Venous blood was collected from fasting patients on the 7th day after the operation. It was prepared to assess white blood cell count (WBC), erythrocyte sedimentation rate (ESR), and C-reaction protein (CRP) contents. Bacteria at the incision sites were inspected under a microscope and a colony-forming unit was selected for Gram staining. The positive marks showed bacterial growth, whereas negative mark showed no bacterial growth.
Statistical analysis
All data were analyzed by using GraphPad Prism 6.0 (SPSS, Inc., Chicago, IL, USA). Count data were analyzed using the chi-square test. Measurement data, which are displayed in the form of mean ± standard deviation (SD), were compared with the t test or one-way analysis of variance (one-way ANOVA) provided that the assumption of normality was not violated. If the assumption of normality was violated, then the t test was replaced with the Wilcoxon rank-sum test. The significance level for all statistical tests was set at P<0.05.
Results
Preoperative clinical characteristics baseline of participants
A total of 160 participants were equally assigned to SL, PL, CD and IL groups (Table 1). No significant differences were observed in sex distribution (P=0.764), body mass index (P=0.085), smoking (P=0.821), or alcohol consumption (P=0.956).
Table 1.
Clinical baseline characteristics of patients among four groups of SL, PL, CD and IL.
| Items | SL | PL | CD | IL | P |
|---|---|---|---|---|---|
| Sample size | 40 | 40 | 40 | 40 | – |
| Age (years old) | 47.1±13.3 | 44.3±12.7 | 43.6±14.1 | 47.0±12.6 | 0.541# |
| Gender(male/female) | 28/12 | 24/16 | 24/16 | 25/15 | 0.764* |
| BMI (kg/m2) | 26.8±1.5 | 27.1±2.1 | 26.6±1.2 | 27.5±1.7 | 0.085# |
| Smoking (n, %) | 5 (12.5%) | 7 (17.5%) | 6 (15%) | 8 (20%) | 0.821* |
| Alcohol (n, %) | 11 (27.5%) | 13 (32.5%) | 13 (32.5%) | 12 (30%) | 0.956* |
Chi-square test;
single factor analysis of variance (One-Way ANOVA).
BMI – body mass index; SL – traditional saline lavage; PL – pulse lavage, CD – closed drainage, IL – iodine lavage.
Intraoperative index
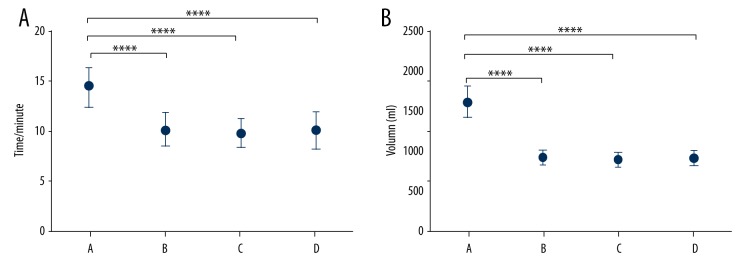
PL, CD, and IL groups showed shorter intraoperative flushing time (Figure 1A) and volume of flushing fluid (Figure 1B) when compared with the SL group (P<0.05), implying that PL, CD, and IL exhibited stronger clinical efficiency in comparison to SL.
Figure 1.
Comparison of the intraoperative lavage time (A) and lavage fluid volume (B) among the 4 irrigation techniques. A – SL group; B – PL group; C – CD group; D – IL group. **** P<0.0001.
Clinical postoperative evaluation
There were no significant differences in average postoperative temperature (P=0.339) or suture removal time (P=0.665) among PL, CD, IL, and SL groups (Table 2). Similarly, there was no significant difference in the adverse events, such as reddening (P=0.219), swelling (P=0.074), or fever (P=0.298), between the SL and the other 3 groups (PL, CD, and IL) (Table 3). However, the other 3 groups exhibited significantly less effusion than the SL group (P<0.001). Furthermore, PL, CD, and IL groups showed significantly less pain perception than in the SL group (P<0.010) (Table 4).
Table 2.
Comparison of average body temperature and suture removal time one week after operation among groups of SL, PL, CD and IL.
| Items | SL | PL | CD | IL | P |
|---|---|---|---|---|---|
| Postoperative temperature (°C) | 36.59±0.30 | 36.49±0.34 | 36.51±0.31 | 36.45±0.37 | 0.339* |
| Suture removal time (d) | 12.6±3.4 | 12.1±1.1 | 12.5±1.5 | 12.5±1.0 | 0.665* |
Single factor analysis of variance (One-Way ANOVA);
SL – saline lavage; PL – pulsed lavage; CD – closed drainage; IL – iodine lavage.
Table 3.
Classification of reddening, swelling, fever and effusion at incision sites after debridement.
| Group | Grading | Reddening (n, %) | Swelling (n) | Fever (n) | Effusion (n) |
|---|---|---|---|---|---|
| SL | None | 21 (52.5%) | 21 (52.5%) | 30 (75.0%) | 13 (32.5%) |
| Mild | 16 (40.0%) | 14 (35.0%) | 8 (20.0%) | 18 (45.0%) | |
| Moderate | 3 (7.5%) | 5 (12.5%) | 2 (5.0%) | 9 (22.5%) | |
| Severe | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Total | 40 (100.0%) | 40 (100.0%) | 40 (100.0%) | 40 (100.0%) | |
| PL | None | 29 (72.5%) | 21 (52.2%) | 35 (87.5%) | 29 (72.5%) |
| Mild | 11 (27.5%) | 19 (47.5%) | 5 (12.5%) | 5 (12.5%) | |
| Moderate | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 6 (15.0%) | |
| Severe | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Total | 40 (100.0%) | 40 (100.0%) | 40 (100.0%) | 40 (100.0%) | |
| CD | None | 30 (75.0%) | 23 (57.5%) | 35 (87.5%) | 31 (77.5%) |
| Mild | 9 (22.5%) | 17 (42.5%) | 4 (10.0%) | 7 (17.5%) | |
| Moderate | 1 (2.5%) | 0 (0.0%) | 1 (2.5%) | 2 (5.0%) | |
| Severe | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Total | 40 (100.0%) | 40 (100.0%) | 40 (100.0%) | 40 (100.0%) | |
| IL | None | 29 (72.5%) | 25 (62.5%) | 37 (92.5%) | 32 (80.0%) |
| Mild | 10 (25.0%) | 13 (32.5%) | 3 (7.5%) | 7 (17.5%) | |
| Moderate | 1 (2.5%) | 2 (5.0%) | 0 (0.0%) | 1 (2.5%) | |
| Severe | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Total | 40 (100.0%) | 40 (100.0%) | 40 (100.0%) | 40 (100.0%) | |
| χ2 | 8.258 | 11.505 | 7.248 | 29.530 | |
| P | 0.219 | 0.074 | 0.298 | <0.001 |
SL – saline lavage; PL – pulsed lavage; CD – closed drainage; IL – iodine lavage.
Table 4.
Classification of incision pain perception before and after debridement among four groups of SL, PL. CD and IL.
| Group | Grading | Pre-debridement (n, %) | Post-debridement (n, %) |
|---|---|---|---|
| SL | None | 0 (0.0%) | 9 (22.5%) |
| Mild | 3 (7.5%) | 21 (52.5%) | |
| Moderate | 17 (42.5%) | 10 (25.0%) | |
| Severe | 20 (50.0%) | 0 (0.0%) | |
| Total | 40 (100.0%) | 40 (100.0%) | |
| PL | None | 0 (0.0%) | 19 (47.5%) |
| Mild | 2 (5.0%) | 21 (52.5%) | |
| Moderate | 19 (47.5%) | 0 (0.0%) | |
| Severe | 19 (47.5%) | 0 (0.0%) | |
| Total | 40 (100.0%) | 40 (100.0%) | |
| CD | None | 0 (0.0%) | 20 (50.0%) |
| Mild | 2 (5.0%) | 19 (47.5%) | |
| Moderate | 17 (42.5%) | 1 (2.5%) | |
| Severe | 21 (52.5%) | 0 (0.0%) | |
| Total | 40 (100.0%) | 40 (100.0%) | |
| IL | None | 0 (0.0%) | 20 (50.0%) |
| Mild | 3 (7.5%) | 18 (45.0%) | |
| Moderate | 19 (47.5%) | 2 (5.0%) | |
| Severe | 18 (45.0%) | 0 (0.0%) | |
| Total | 40 (100.0%) | 40 (100.0%) | |
| χ2 | 0.878 | 24.710 | |
| P | 0.989 | <0.001 |
SL – saline lavage; PL – pulsed lavage; CD – closed drainage; IL – iodine lavage.
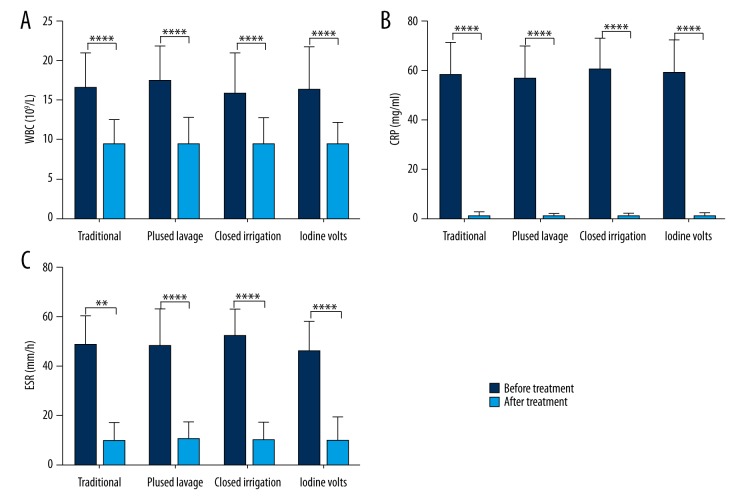
As suggested by one-way ANOVA, no significant difference in WBC, ESR, or CRP were observed in pre-irrigation stages (P=0.555, 0.589, and 0.177, respectively) or in post-irrigation stages (P=0.995, 0.784, and 0.981, respectively) among the 4 groups (all P>0.05) (Table 5). However, there appeared to be significant differences in WBC, CRP, and ESR between the pre-irrigation and post-irrigation stages in all groups (Figure 2, all P<0.001).
Table 5.
Comparison of WBC, ESR and CRP preoperatively and postoperatively among four groups of SL, PL, CD and IL.
| Items | Detection timing | SL | PL | CD | IL | P |
|---|---|---|---|---|---|---|
| WBC (109/L) | Preoperative | 16.38±4.47 | 17.23±4.65 | 15.65±5.17 | 16.28±5.42 | 0.555* |
| Postoperative | 9.40±3.12 | 9.52±3.22 | 9.35±3.32 | 9.43±2.71 | 0.995* | |
| CRP (mg/L) | Preoperative | 58.17±13.06 | 56.3±12.83 | 60.3±13.38 | 58.6±13.25 | 0.589* |
| Postoperative | 1.24±1.60 | 0.97±1.02 | 1.01±1.22 | 1.13±1.27 | 0.784* | |
| ESR (mm/h) | Preoperative | 48.21±11.66 | 47.76±15.14 | 52.01±11.02 | 45.4±12.10 | 0.177* |
| Postoperative | 9.71±7.59 | 10.27±7.39 | 9.85±8.02 | 10.32±8.89 | 0.981* |
Single factor analysis of variance (One-Way ANOVA);
SL – saline lavage; PL – pulsed lavage; CD – closed drainage; IL – iodine lavage.
Figure 2.
Comparison of WBC (A), CRP (B), and ESR (C) between pre-irrigation and post-irrigation stages. **** P<0.0001.
Bacteria in muscle and intervertebral disc
We also inspected bacteria around the incision in muscles and intervertebral discs. The infection rate with respect to muscle in the SL group (20%) was much higher than in the PL (2.5%), CD (0%), and IL (0%) groups (P < 0.001) (Table 6). Nevertheless, there was no significant difference in the infection rate with respect to intervertebral discs among the 4 groups (P=0.104).
Table 6.
Bacteria culture in muscles and intervertebral disc among groups of SL, PL, CD and IL.
| Group | Muscle layer | Infection rate/% | Intervertebral disc | Infection rate/% | ||
|---|---|---|---|---|---|---|
| − | + | − | + | |||
| SL | 32 | 8 | 20 | 37 | 3 | 7.5 |
| PL | 39 | 1 | 2.5 | 40 | 0 | 0 |
| CD | 40 | 0 | 0 | 40 | 0 | 0 |
| IL | 40 | 0 | 0 | 39 | 1 | 2.5 |
| χ2 | 21.070 | 6.154 | ||||
| p | <0.001 | 0.104 | ||||
SL – saline lavage; PL – pulsed lavage; CD – closed drainage; IL – iodine lavage.
Discussion
Exposing spinal lesion areas of PLIF in surgical procedures is challenging, since deep fascia needs to be cut so as to separate the paravertebral muscles, and its implementation is time-consuming. During the surgical process, patients may have an unexpectedly high risk of infection, which may lead to severe chronic diseases [12,13]. To solve this issue, wound irrigation was applied, aimed at cleansing the wounds thoroughly [14]. The current study compared the effectiveness of 3 wound irrigation techniques (PL, CD, and IL) with conventional SL, hoping to determine which method has the best treatment efficacy.
Use of SL requires the largest dose of solution, which makes it time-consuming. In contrast, the irrigation time of PL is shorter because the solution used in PL are vaporific mixtures that are composed of washing solution and compressed air, thereby mitigating local inflammatory responses [15]. In CD, the implementation time and solution dosage are carefully set for an enclosed area that is formed by special materials and biological membranes [9]. Debridement cleans the wound surface of tissues, and provides a relatively clean zone that reduces the corresponding betadine dosage by 0.1% [16]. As was suggested by our study, no significant differences in prevalence of adverse events, including reddening, swelling, or heat at the wound site, were displayed between SL and the other 3 techniques (PL, CD and IL). Also, no significant difference in stitch removal time was observed at 1 week after the operation, indicating that PL, CD, and IL had less influence on patient discomfort than SL. Furthermore, SL cannot be implemented continuously, and its rinse angle and pressure are hard to manage during the cleaning process, which creates increased seepage volume and infection risk. Conversely, PL, CD, and IL were associated with decreased pain and seepage volume when compared with SL, which may expedite the healing of wounds [17,18].
WBC, CRP and ESR have been widely utilized to monitor inflammatory responses in patients undergoing spinal surgery [19,20]. It was shown that post-irrigation hemograms (WBC, CRP, and ESR) were significantly more desirable than pre-irrigation hemograms, suggesting that irrigation can effectively restore the aberrant hemograms to normal levels [21]. There was no significant difference in the post-irrigation hemogram or the bacteria-positive rate among the 4 groups, suggesting that the 4 irrigation techniques might reduce the risk of infection effectively [4,6,22]. Moreover, PL, CD, and IL groups exhibited a lower positive rate of bacteria in muscular tissues than in the SL group, indicating that PL, CD, and IL promote wound healing more than SL [23]. It has been documented that PL is much more effective and efficient in removal of bacteria in complicated musculoskeletal incisions than SL [6,24]. CD effectively prevents infection caused by PLIF incision, which may be correlated with improving microcirculation of the wound, clearance of dead tissues, and generation of new blood vessels [25,26]. Betadine has been verified as a cost-effective antimicrobial with low toxicity, which decreases the corresponding risk of bacterial infection and inflammatory reactions [27,28]. Since the saline in SL is delivered vertically, bacteria may be diffused deeply into the wound, and the risk of infection due to poor bacterial inhibition may be increased. In spite of the above accomplishments, we did not control the solution temperature during implementation of PL, CD, IL, and SL, which may result in biased results. Apart from that, the bleeding amount and extent of soft tissue contusion of subjects were not compared. Hence, we encourage future researchers to address these limitations by conducting studies with rigorous experimental design to control for potential confounding factors.
Conclusions
Overall, the use of PL, CD, and debridement combined with 0.1% betadine irrigation (IL) did not have significant influence on patient discomfort. Compared with SL, we found that PL, CD, and IL exhibited reduced average irrigation time, as well as decreased dosage of solution used, to improve the irrigation efficiency. Other potential strengths of PL, CD, and IL may include decreased seepage volume, which can enhance healing of wounds. Therefore, we conclude that PL, CD, and IL are good solutions for preventing postoperative bacterial infections within muscle layers.
Abbreviations
- PLIF
posterior intervertebral disc fusion surgery
- SL
saline lavage group
- PL
pulse lavage group
- CD
closed drainage group
- IL
iodine lavage group
- WBC
white blood cell
- ESR
erythrocyte sedimentation rate
- CRP
C-reactive protein
Footnotes
Source of support: Departmental sources
Competing interests
The authors declare that they have no competing interests.
References
- 1.DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg. 2008;16:130–39. doi: 10.5435/00124635-200803000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hegde V, Meredith DS, Kepler CK, Huang RC. Management of postoperative spinal infections. World J Orthop. 2012;3:182–89. doi: 10.5312/wjo.v3.i11.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beiner JM, Grauer J, Kwon BK, Vaccaro AR. Postoperative wound infections of the spine. Neurosurg Focus. 2003;15:E14. doi: 10.3171/foc.2003.15.3.14. [DOI] [PubMed] [Google Scholar]
- 4.Rohmiller MT, Akbarnia BA, Raiszadeh K, Canale S. Closed suction irrigation for the treatment of postoperative wound infections following posterior spinal fusion and instrumentation. Spine (Phila Pa 1976) 2010;35:642–46. doi: 10.1097/BRS.0b013e3181b616eb. [DOI] [PubMed] [Google Scholar]
- 5.Scott RG, Loehne HB. 5 questions – and answers – about pulsed lavage. Adv Skin Wound Care. 2000;13:133–34. [PubMed] [Google Scholar]
- 6.Mote GA, Malay DS. Efficacy of power-pulsed lavage in lower extremity wound infections: A prospective observational study. J Foot Ankle Surg. 2010;49:135–42. doi: 10.1053/j.jfas.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Hong HS, Chang MC, Liu CL, Then C. Is aggressive surgery necessary for acute postoperative deep spinal wound infection? Spine. 2008;33:2473–78. doi: 10.1097/BRS.0b013e3181894ff0. [DOI] [PubMed] [Google Scholar]
- 8.Mehbod AA, Ogilvie JW, Pinto MR, et al. Postoperative deep wound infections in adults after spinal fusion: management with vacuum-assisted wound closure. J Spinal Disord Tech. 2005;18:14–17. doi: 10.1097/01.bsd.0000133493.32503.d3. [DOI] [PubMed] [Google Scholar]
- 9.Sampedro MF, Huddleston PM, Piper KE, et al. A biofilm approach to detect bacteria on removed spinal implants. Spine (Phila Pa 1976) 2010;35:1218–24. doi: 10.1097/BRS.0b013e3181c3b2f3. [DOI] [PubMed] [Google Scholar]
- 10.Chang FY, Chang MC, Wang ST, et al. Can povidone-iodine solution be used safely in a spinal surgery? Eur Spine J. 2006;15:1005–14. doi: 10.1007/s00586-005-0975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunisada T, Yamada K, Oda S, Hara O. Investigation on the efficacy of povidone-iodine against antiseptic-resistant species. Dermatology. 1997;195(Suppl 2):14–18. doi: 10.1159/000246025. [DOI] [PubMed] [Google Scholar]
- 12.Archyangelio A, Shakhon A. Using PGD to reduce surgical infection risk. Nurs Times. 2016;112:21–23. [PubMed] [Google Scholar]
- 13.Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35:171–80. doi: 10.1016/j.burns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shetty R, Barreto E, Paul KM. Suction assisted pulse lavage: randomised controlled studies comparing its efficacy with conventional dressings in healing of chronic wounds. Int Wound J. 2014;11:55–63. doi: 10.1111/j.1742-481X.2012.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn DK, Lee S, Moon SH, et al. Bulb syringe and pulsed irrigation: Which is more effective to remove bacteria in spine surgeries? Clin Spine Surg. 2016;29:34–37. doi: 10.1097/BSD.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz JA, Goss SG, Facchin F, Avdagic E, Lantis JC. Surgical debridement alone does not adequately reduce planktonic bioburden in chronic lower extremity wounds. J Wound Care. 2014;23 doi: 10.12968/jowc.2014.23.Sup9.S4. S4, S6, S8 passim. [DOI] [PubMed] [Google Scholar]
- 17.Drugs that delay wound healing. Prescrire Int. 2013;22:94–98. [PubMed] [Google Scholar]
- 18.Corsetti G, D’Antona G, Dioguardi FS, Rezzani R. Topical application of dressing with amino acids improves cutaneous wound healing in aged rats. Acta Histochem. 2010;112:497–507. doi: 10.1016/j.acthis.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6:311–15. doi: 10.1016/j.spinee.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Kraft CN, Kruger T, Westhoff J, et al. CRP and leukocyte-count after lumbar spine surgery: Fusion vs. nucleotomy. Acta Orthop. 2011;82:489–93. doi: 10.3109/17453674.2011.588854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugita S, Hozumi T, Yamakawa K, et al. White blood cell count and C-reactive protein variations following posterior surgery with intraoperative radiotherapy for spinal metastasis. J Spinal Disord Tech. :2015. doi: 10.1097/BSD.0000000000000261. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Lian XF, Xu JG, Zeng BF, et al. Continuous irrigation and drainage for early postoperative deep wound infection after posterior instrumented spinal fusion. J Spinal Disord Tech. 2014;27:E315–17. doi: 10.1097/BSD.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 23.Bowler PG. The 10(5) bacterial growth guideline: Reassessing its clinical relevance in wound healing. Ostomy Wound Manage. 2003;49:44–53. [PubMed] [Google Scholar]
- 24.Svoboda SJ, Bice TG, Gooden HA, et al. Comparison of bulb syringe and pulsed lavage irrigation with use of a bioluminescent musculoskeletal wound model. J Bone Joint Surg Am. 2006;88:2167–74. doi: 10.2106/JBJS.E.00248. [DOI] [PubMed] [Google Scholar]
- 25.Zhang WH, Wu Q, Ma J, Wang JH. [Effects of vacuum drainage combined with heparin irrigation for treatment of scald burns with seawater immersion in rabbits]. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:1481–86. [PubMed] [Google Scholar]
- 26.Owens BD, White DW, Wenke JC. Comparison of irrigation solutions and devices in a contaminated musculoskeletal wound survival model. J Bone Joint Surg Am. 2009;91:92–98. doi: 10.2106/JBJS.G.01566. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Q, Zhang XF, Di DH, et al. Efficacy of different irrigation solutions on the early debridement of open fracture in rats. Exp Ther Med. 2015;9:1589–92. doi: 10.3892/etm.2015.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel KS, Goldenberg B, Schwartz TH. Betadine irrigation and post-craniotomy wound infection. Clin Neurol Neurosurg. 2014;118:49–52. doi: 10.1016/j.clineuro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]