Abstract
Summary
Model-based economic evaluation was performed to assess the cost-effectiveness of zoledronic acid. Although zoledronic acid was dominated by alendronate, the incremental quality-adjusted life year (QALY) was quite small in extent. Considering the advantage of once-yearly injection of zoledronic acid in persistence, zoledronic acid might be a cost-effective treatment option compared to once-weekly oral alendronate.
Introduction
The purpose of this study was to estimate the cost-effectiveness of once-yearly injection of zoledronic acid for the treatment of osteoporosis in Japan.
Methods
A patient-level state-transition model was developed to predict the outcome of patients with osteoporosis who have experienced a previous vertebral fracture. The efficacy of zoledronic acid was derived from a published network meta-analysis. Lifetime cost and QALYs were estimated for patients who had received zoledronic acid, alendronate, or basic treatment alone. The incremental cost-effectiveness ratio (ICER) of zoledronic acid was estimated.
Results
For patients 70 years of age, zoledronic acid was dominated by alendronate with incremental QALY of −0.004 to −0.000 and incremental cost of 430 USD to 493 USD. Deterministic sensitivity analysis indicated that the relative risk of hip fracture and drug cost strongly affected the cost-effectiveness of zoledronic acid compared to alendronate. Scenario analysis considering treatment persistence showed that the ICER of zoledronic acid compared to alendronate was estimated to be 47,435 USD, 27,018 USD, and 10,749 USD per QALY gained for patients with a T-score of −2.0, −2.5, or −3.0, respectively.
Conclusion
Although zoledronic acid is dominated by alendronate, the incremental QALY is quite small in extent. Considering the advantage of annual zoledronic acid treatment in compliance and persistence, zoledronic acid may be a cost-effective treatment option compared to alendronate.
Electronic supplementary material
The online version of this article (doi:10.1007/s00198-017-3973-8) contains supplementary material, which is available to authorized users.
Keywords: Cost-effectiveness analysis, Fracture prevention, Health economics, Osteoporosis, Zoledronic acid
Introduction
Among the osteoporotic fractures, hip fracture in particular imposes not only a clinical burden on patients by aggravating their QOL and health outcomes but also a significant socioeconomic burden of medical expenses for the treatment as well as a nursing care. Survival rates reported for patients who have experienced a hip fracture—81, 49, and 26% for 1, 5, and 10 years, respectively—are lower than rates for the general population [1]. Also, a health-related QOL score (utility value) of patients in the year following a hip fracture was reduced by 11.5% compared to the baseline [2]. According to a national medical expenditure survey by the Japanese Ministry of Health, Labor and Welfare, bone density and bone structure as well as fracture-related expenditure in the population aged 65 and over were estimated to be 891.5 billion JPY in 2012 and 943.6 billion JPY in 2013 [3].
As anti-osteoporosis drugs have become widely used, the incidence rate of hip fracture has declined in the USA and Europe; however, it is still on the rise in Japan. A national survey of hip fractures estimated new fractures at 148,100 (male, 31,300; female, 116,800) in 2007 and 175,700 (male, 37,600; female, 138,100) in 2012, an increase of about 27,600 in 5 years [4, 5]. As the Japanese population ages, the mounting burden caused by the increased incidence of osteoporotic fractures is of grave concern.
Drug therapy is considered to be an effective measure against the burden incurred by osteoporotic fractures, and a wide variety of options are available today. Zoledronic acid is a bisphosphonate that showed preventive efficacy on osteoporotic fractures in HORIZON-PFT (Pivotal Fracture Trial), a large population-based randomized trial conducted overseas [6]. One of the major issues with osteoporosis is the low rate of patients receiving treatment. According to the in-country report, the prescription rate for osteoporotic drugs in patients who experienced a hip fracture was 18.7%, while those who had no treatment was 53.3%, suggesting that the number of patients receiving treatment is insufficient [7]. A yearly intravenous injection of zoledronic acid is expected to facilitate long-term treatment for patients and avoid the adverse effects caused by oral bisphosphonates that require daily, weekly, or monthly dosing.
Although the drug therapy for osteoporosis definitely reduces the risk of fracture and is anticipated to reduce the total treatment cost for osteoporotic fracture, there is the possibility of an increase in the total cost of medication. Recently, the cost-effectiveness of various drug therapies for osteoporosis has been studied in advanced countries, and the results are having an effect on decision-making in clinical practice as well as on healthcare policies. A health economic evaluation of zoledronic acid conducted in Finland, Norway, and Holland by Akehurst and colleagues reported that zoledronic acid is cost-effective compared with basic treatment (placebo, calcium, and vitamin D) or other existing bisphosphonates [8].
In Japan, cost-effectiveness of alendronate therapy in osteopenic women has been examined. The results indicated that osteoporosis treatment should be considered only for a high-risk population on the basis of age, bone mineral density (BMD), and number of clinical risk factors [9]. However, to date, no health economic evaluation of the drug therapy including zoledronic acid for postmenopausal women with osteoporosis and with a history of fracture has been reported in Japan. In addition, there are epidemiological characteristics of a lower incidence rate of hip fracture [10, 11] and a higher incidence of vertebral fracture [12, 13] in Japanese compared to Western people. The Japanese healthcare system also differs from that of Europe and USA including the drug prices, treatment fees, and socially acceptable thresholds of the incremental cost-effectiveness ratio (ICER). For these reasons, direct application of the results of studies in Western countries to the Japanese population is problematic. The objective of this study was to estimate the cost-effectiveness of zoledronic acid in Japanese women with osteoporosis who have experienced a previous vertebral fracture from the perspective of the Japanese healthcare system.
Methods
Model structure
A model-based, cost-effectiveness analysis was conducted to evaluate the cost-effectiveness of zoledronic acid plus basic treatment (once-a-year injection of zoledronic acid 5 mg + calcium + vitamin D supplement) compared with alendronate plus basic treatment (once-weekly alendronate 35 mg + calcium + vitamin D supplement) or basic treatment alone (calcium + vitamin D supplement) in Japanese women with osteoporosis having a history of vertebral fracture. We targeted Japanese women having osteoporosis with a vertebral fracture as a hypothetical cohort. The base case was a 70-year-old women with a femoral neck BMD T-score of −2.5 (=0.565 g/cm2). In this model, the T-score is based on the young adult mean (0.79 ± 0.09 g/cm2) used in a study by Soen et al., i.e., T-score − 2.5 = (0.565–0.790) / 0.09 [14].
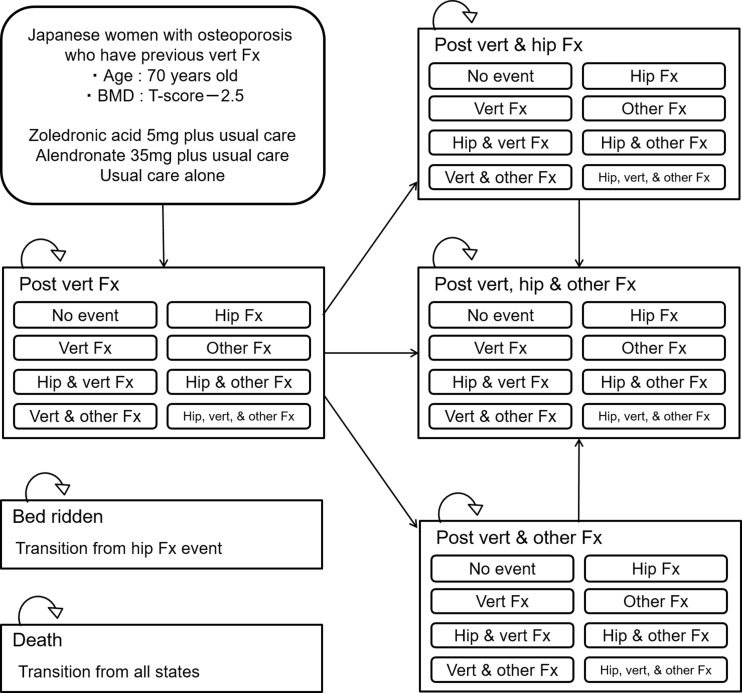
Cohort-based Markov models have been frequently used for economic evaluation of osteoporotic interventions. However, this modeling approach is limited by the “memoryless” feature of the process, which is known as the Markov assumption [15]. This assumption means that once a patient has moved from one state to another, the model will have “no memory” regarding where the patient came from. When transition probabilities depend on prior events (ex: prior fracture event), this “memory” should be reflected in the model. To overcome the “memoryless” feature of the Markov process, we developed a patient-level state-transition model to predict long-term costs and quality-adjusted life years (QALYs) associated with each therapy. The seven medical events (post vertebral fracture; post vertebral and hip fracture; post vertebral and other fracture; post vertebral, hip, and other fracture; bedridden; and death) used in the model are shown in Fig. 1. In this model, “other fracture” is defined as a proximal humeral or distal radius fracture. Also, vertebral fracture in this model refers to clinical vertebral fracture; morphological vertebral fracture was not considered. In this model simulation, subjects started with in the state of having a history of vertebral fracture and faced with various risks of fracture depending on their age, femoral neck BMD, and treatment received. The cycle length of the model was defined as 1 year. During each cycle of the simulation, subjects experienced one of nine clinical events (no event; vertebral fracture; hip fracture; other fracture; vertebral and hip fracture; vertebral and other fracture; hip and other fracture; vertebral, hip, and other fracture; death). In the case of the occurrence of a fracture, the patient health status was changed to post fracture with an additional risk for subsequent fractures. We assumed that a certain number of subjects having a hip fracture would attain “bedridden” status. The model was developed and analyzed using TreeAge Pro 2016 (TreeAge Software, Williamston, MA, USA).
Fig. 1.
Model structure. Vert vertebral, Fx fracture
Transition probabilities
A transition probability on a fracture event and death was calculated using the declining exponential approximation of life expectancy (DEALE) method based on the incidence rate derived from published sources. In the case of using an exponential function for the distribution, probability (p) of an event occurring over a time interval (t) was calculated using incidence rate (r) according to the following formula: p = 1 − exp.(−rt) [15].
We developed equations for age and a femoral neck BMD-specific fracture rate using a series of methods developed by De Laet and colleagues [16] and epidemiological data of Japanese women with osteoporosis or osteopenia. We first developed equations for the age-specific fracture rate for hip and vertebral fractures by using data on fracture rates of Japanese women and curve-fitting techniques [13, 17, 18]. An exponential curve was created using Stata14 (StataCorp LP, College Station, TX, USA), while exponential and sigmoid functions were used for hip and vertebral fractures, and other fractures, respectively, from the perspective of Akaike information criterion (AIC) values of approximate expression and clinical relevance.
Following the method suggested by De Laet and colleagues, we formulated an equation for age- and BMD-specific fracture on the basis of the age-specific fracture rate in Japan, BMD distributions of femoral neck BMD by age group, and relative risk (RR) of fracture per 1 SD reduction in BMD (see supplementary material S-1) [13, 16–19]. The process of formulating the equation is shown in the S-2. Incident rates of subsequent fractures for those with a precious fracture were calculated by multiplying the age- and BMD-specific fracture rate by the RR of subsequent fracture (S-3) [20]. For those who have a number of fractures, risk was calculated by multiplying by the RR more than once (S-3) [20]. The RR of fracture for those who have a risk factor independent of BMD was estimated by combining overseas meta-analysis and national epidemiological data (S-3) [21–24]. Incident rates of fractures when receiving treatment was calculated in the same manner by multiplying the formulated equation for fracture incidence rate by the RR of each fracture (S-3) [6]. In this study, we considered only symptomatic clinical vertebral fractures and did not include morphometric vertebral fractures in the model. The proposition of clinical vertebral fractures among all vertebral fractures was assumed to be 30% (S-3) [25].
The age-specific mortality rate was obtained from a simplified life table reported in 2014 by the Ministry of Health, Labor and Welfare in Japan [26]. The odds ratio for death 10 years after hip fractures was formulated by the curve-fitting derived from the data reported by Tsuboi et al. (S-1) [1]. The mortality rate for subjects who experienced a hip fracture was calculated by multiplying the mortality from the life table by the aforementioned odds ratio equation. The probability of becoming bedridden after hip fracture was derived from a published source in Japan (S-3) [27].
Efficacy of treatment
In the zoledronic acid arm, treatment consisted of 3 years of zoledronic acid intravenous injection at a dosage of 5 mg per year. According to the HORIZON-PFT details, the subjects in both arms received calcium and vitamin D [6]. In the alendronate arm, patients received once-weekly oral alendronate therapy at a dosage of 35 mg per week. The fracture risk reduction effect of zoledronic acid and alendronate was derived from a recently published network meta-analysis [28]. We modeled the residual effects of zoledronic acid and alendronate, assuming a linear decline in efficacy of fracture risk over 3 years, after 3 years of drug treatment (S-3) [29]. In the model for the base case analysis, full compliance with zoledronic acid and alendronate treatment was assumed. Influenza-like symptoms (acute phase reaction) are suggested as an adverse event of zoledronic acid; however, low- to mid-level symptoms are considered transient, disappearing within about 3 days. Given that the adverse events from zoledronic acid on QOL, outcome of patients, and cost are limited, they were not considered in this model. Additionally, adverse events associated with alendronate therapy were not considered because their impact on long-term costs and clinical benefits was relatively small. We assumed that patients either in the zoledronic acid arm or in the alendronate arm who experienced a secondary fracture will continue preventive zoledronic acid therapy or alendronate therapy unless their status is changed to “bedridden.”
Costs
We considered medical costs and nursing care costs from the perspective of the Japanese healthcare and nursing care system. Supplementary material S-4 summarizes input values for cost parameters used in the model. All costs were calculated in Japanese yen (JPY) and converted to US dollars (USD) at the currency rate of 1 USD = 120 JPY. The annual treatment cost of zoledronic acid was calculated using the Japanese national drug pricing standard (39,485 JPY) plus consulting fee (470 JPY) [30, 31]. Costs for alendronate, calcium, and vitamin D were based on the Japanese national price list for drugs [32]. Annual medical cost estimations were based on standard clinical practice in Japan [27, 31]. In each arm, patients were assumed to see a doctor every 2 months and having bone metabolism and bone density measured twice a year (using dual-energy x-ray absorption). Medical costs due to a fracture event were obtained from published sources in Japan, which were then adjusted to be closer in value to the most recent medical fees using a past revision rate [33–35]. For bedridden patients, we used data for patients provided with nursing care level 5 under the Nursing Care Insurance Scheme in Japan [36].
Utilities
Values input for QOL parameters used in the model are summarized in the S-4. The estimating equation to determine the QOL value for event-free subjects by age group was developed using linear regression analysis based on the EQ-5D-3L descriptive system for Japanese elderly people [37]. The QOL value for patients who experienced fracture events was calculated by multiplying the event-free QOL value by the percent reduction in QOL associated with fracture events. Decrease in the rate of QOL between the first year after experiencing a fracture and the subsequent years was calculated based on reported values by the EQ-5D-3L for patients with osteoporotic fractures [2]. Given the lack of data on QOL values for bedridden patients, to obtain the estimated values, we used QOL values for patients with nursing care level 5 based on a national research report [38].
Base case analysis
A Monte Carlo simulation using mean cost and mean QALY of a hypothetical cohort of 1000 people was performed with 1000 iterations to estimate lifetime expected costs and expected QALY for the zoledronic acid arm, for the alendronate arm, and for the basic treatment arm. An annual discount rate of 2% was applied to both cost and benefit [39]. Based on the estimated expected costs and expected QALY, the incremental cost-effectiveness ratio (ICER) was estimated as an indicator of cost-effectiveness using the following formula: ICER = (Costdrug therapy – Costcomparator) / (QALYdrug therapy – QALYcomparator). A willingness to pay (WTP) threshold of 50,000 USD per QALY gained was used as the acceptable level for ICER. We also estimated the incremental net monetary benefit (INMB) as another indicator of cost-effectiveness, assuming a WTP threshold of 50,000 USD per QALY gained, using the following formula: WTP × (QALYdrug therapy – QALYcomparator) − (Costdrug therapy – Costcomparator) [15]. A positive value for INMB indicates that the drug therapy is cost-effective compared to the comparator at a WTP of 50,000 USD per QALY gained. To verify the validity of the simulation, a 10-year probability of hip and major fractures (hip, vertebral, and others) was calculated. The simulation was also performed on these populations with a femoral neck BMD of T-score of −2.0, −2.5, or −3.0 and at 65, 70, or 75 years of age.
Sensitivity analysis
Deterministic sensitivity analyses were performed to examine the influence of key parameter uncertainties in the model on the base case analysis. Assessed parameters and ranges are shown in Supplementary materials S 1, 3, and 4. The plausible ranges for each parameter were determined based on reported values, such as 95% confidence interval (CI) in published sources, or expert opinion.
To estimate a probabilistic distribution of ICER and perform a probabilistic assessment of the cost-effectiveness, probabilistic sensitivity analyses was performed using 1000 iterations of a second-order Monte Carlo simulation. Probabilistic distribution was determined based on the modeled parameters that are available in the published sources, such as 95% CI (S-3). By sampling the value for each parameter from the determined probabilistic distribution, simulations of the expected costs, expected QALY and ICER were performed 1000 times. We constructed cost-effectiveness acceptability curves based on the 1000 iterations of a second-order Monte Carlo simulation and estimated an acceptable probability of zoledronic acid treatment from the cost-effectiveness perspective, assuming a WTP threshold of 50,000 USD per QALY gained.
A scenario analysis was performed to examine the cost-effectiveness of zoledronic acid by T-score in 70-year-old patients having one of the following risk factors: current smoking, high alcohol intake, or a family history of hip fracture. We also performed a scenario analysis considering the advantage of once-yearly injection of zoledronic acid for continuing treatment. In the model for this scenario analysis, we assumed that a certain proportion of patients in zoledronic acid arm or alendronate arm discontinued their prescribed therapy within the initial cycle and they received calcium and vitamin D after treatment discontinuation as in basic treatment arm. Based on a representative database comprising longitudinal prescription data, the proportion of patients who continued drug therapy for over a year in zoledronic acid arm and alendronate arm was estimated to be 65.62 and 44.76%, respectively (S-3) [40]. In addition, we examined the influence of treatment persistence on the cost-effectiveness of zoledronic acid with different combinations of the proportion of patients who continued treatment for over a year in zoledronic acid arm and alendronate arm.
Results
Base case analysis
The results of the base case analysis are shown in Table 1. For 70-year-old osteoporotic patients with a femoral neck BMD T-score of −2.5, the respective 10-year probability of hip fracture and the 10-year probability of major fractures were estimated to be 2.90 and 17.40% for the zoledronic acid arm, 2.80 and 18.50% for the alendronate arm, and 3.60 and 22.9% for the basic treatment alone arm. Three years of zoledronic acid treatment followed by 3 years of residual were simulated to have a 19.4% risk reduction in hip fracture (RR = 0.806) and a 24% risk reduction in major fractures (RR = 0.760) compared to basic treatment alone. Compared to 3 years of alendronate treatment followed by 3 years of residual effects, the RR values for hip fracture and major fractures in the zoledronic acid arm were estimated to be 1.036 and 0.941, respectively. The alendronate treatment group showed incremental costs of 210 USD per person and conferred additional QALYs of 0.067 compared to the basic treatment group, resulting in an ICER of 3143 USD per QALY gained. Zoledronic acid treatment was dominated by alendronate treatment with incremental QALY of −0.002 and incremental cost of 430 USD. INMB of zoledronic acid compared to alendronate was estimated to be −507 USD. The incremental costs and incremental QALYs of zoledronic acid compared to alendronate tented to be small with an increase of T-score. Although the incremental QALYs nearly unchanged by age of patients, the incremental costs showed a decreasing trend with an increase of patient age. The base case results indicated that zoledronic acid treatment was dominated by alendronate and showed an incremental QALY of −0.004 to −0.000 and an incremental cost of 430 USD to 493 USD, resulting in an INMB of −411 USD to −665 USD.
Table 1.
Base case results
| 10-year fracture probability (%) | RR of hip fracture | RR of major fracture | QALYs | Incremental QALYs |
Cost (USD) | Incremental cost (USD) |
ICER (USD per QALY) | INMB (USD) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hip fracture | Vertebral fracture | Other fracture | ||||||||||
| 70 years old, T-score −2.0 | ||||||||||||
| BT | 2.20 | 9.20 | 6.20 | − | − | 11.327 | − | 17,507 | − | − | − | |
| ALN + BT (vs BT) | 1.70 | 7.10 | 5.50 | 0.773 | 0.813 | 11.370 | 0.043 | 18,208 | 701 | 16,460 | 1429 | |
| ZOL + BT (vs ALN + BT) | 1.80 | 6.50 | 5.20 | 1.059 | 0.944 | 11.370 | −0.000 | 18,608 | 400 | Weakly dominated | −411 | |
| 65 years old, T-score −2.5 | ||||||||||||
| BT | 2.50 | 9.50 | 7.00 | − | − | 13.468 | − | 23,991 | − | − | − | |
| ALN + BT (vs BT) | 2.00 | 7.40 | 6.20 | 0.800 | 0.821 | 13.543 | 0.075 | 24,079 | 88 | 1166 | 3669 | |
| ZOL + BT (vs ALN + BT) | 2.00 | 6.70 | 5.90 | 1.000 | 0.936 | 13.541 | −0.002 | 24,572 | 493 | Dominated | −582 | |
| 70 years old, T-score −2.5 | ||||||||||||
| BT | 3.60 | 12.00 | 7.30 | − | − | 11.165 | − | 20,345 | − | − | − | |
| ALN + BT (vs BT) | 2.80 | 9.30 | 6.40 | 0.778 | 0.808 | 11.231 | 0.067 | 20,555 | 210 | 3143 | 3131 | |
| ZOL + BT (vs ALN + BT) | 2.90 | 8.40 | 6.10 | 1.036 | 0.941 | 11.230 | −0.002 | 20,985 | 430 | Dominated | −507 | |
| 75 years old, T-score −2.5 | ||||||||||||
| BT | 5.10 | 14.80 | 7.20 | − | − | 8.907 | − | 16,737 | − | − | − | |
| ALN + BT (vs BT) | 3.90 | 11.30 | 6.40 | 0.765 | 0.797 | 8.968 | 0.061 | 16,993 | 255 | 4192 | 2791 | |
| ZOL + BT (vs ALN + BT) | 4.00 | 10.20 | 6.00 | 1.026 | 0.935 | 8.967 | −0.002 | 17,358 | 366 | Dominated | −455 | |
| 70 years old, T-score −3.0 | ||||||||||||
| ALN + BT | 4.60 | 12.00 | 7.50 | − | − | 11.035 | − | 23,823 | − | − | − | |
| ZOL + BT (vs ALN + BT) | 4.70 | 10.90 | 7.10 | 1.022 | 0.942 | 11.031 | −0.004 | 24,298 | 474 | Dominated | −665 | |
| BT(vs ALN + BT) | 5.90 | 15.70 | 8.60 | 1.283 | 1.253 | 10.934 | −0.101 | 24,469 | 646 | Dominated | −5692 | |
BT: basic treatment (placebo + calcium + vitamin D), ZOL: once-yearly injection of zoledronic acid 5 mg, ALN: once-weekly oral alendronate 35 mg. 1 USD = 120 JPY (February 2016 exchange rate). All strategies were ranked according to costs
RR relative risk, QALYs quality-adjusted life years, ICER incremental cost-effectiveness ratio, INMB incremental net monetary benefit
Sensitivity analysis
The results of our deterministic sensitivity analyses are summarized in Fig. 2. The tornado diagram for the INMB of zoledronic acid compared to alendronate (Fig. 2) showed that the RR of hip fracture with zoledronic acid and the annual cost of zoledronic acid had a relatively strong effect on the estimated INMB. If the RR of hip fracture with zoledronic acid was equal to 0.34 (lower limit of 95% CI), the zoledronic acid incurred an additional cost of 98 USD per person and conferred an additional 0.016 QALY compared to alendronate, which resulted in an INMB of 719 USD and an ICER of 5890 USD per QALY gained. Assuming the lower cost of zoledronic acid (−30%), the alendronate incurred an additional cost of 247 USD per person and conferred an additional 0.002 QALY, which resulted in an ICER of 159,760 USD per QALY gained compared to zoledronic acid (INMB = 170 USD). In this case, zoledronic acid becomes a cost-effective option compared to alendronate applying a WTP of 50,000 USD per QALY gained.
Fig. 2.
Results of the deterministic sensitivity analyses for the base case. Tornado diagram for the INMB of zoledronic acid compared to alendronate
The results of the probabilistic sensitivity analysis were used to construct the cost-effectiveness acceptability curves shown in S-5, and the probability of zoledronic acid of being cost-effective was estimated according to the level of BMD, assuming that the WTP threshold is 50,000 USD per QALY gained. The probability that zoledronic acid treatment is the most cost-effective option for patients with a T-score of −2.0, −2.5, or −3.0 were estimated to be 7.5, 9.7, or 12.9%, respectively. The probability that the alendronate becomes most cost-effective option ranged from 87.1 to 91.2% with a WTP of 50,000 USD per QALY gained.
The results of the scenario analysis considering additional risk factors are shown in S-6. The scenario analyses for the INMB of zoledronic acid compared to alendronate indicated that the INMB of zoledronic acid was below 0 USD in those who have an additional risk factor and became lower in higher-risk group (S-6). The results of the scenario analysis considering treatment persistence are shown in Tables 2 and 3. If we assumed that the proportion of patients who continued treatment for over a year was 65.62% for zoledronic acid arm and 44.76% in alendronate arm, the ICER of zoledronic acid compared to alendronate was estimated from 7576 USD to 48,865 USD per QALY gained (Table 2). If zoledronic acid was expected to improve the proportion of patients who continued drug therapy for over a year by 10% compared with alendronate, the ICER was ranged from 23,225 USD to 44,940 USD per QALY gained (Table 3). In addition, the ICER of zoledronic acid compared to alendronate was less than 24,203 USD per QALY gained, if zoledronic acid was expected to improve the proportion of patients who continued treatment for over a year by 20% compared with alendronate (Table 3).
Table 2.
Results of scenario analysis on treatment persistence
| 10-year fracture probability (%) | RR of hip fracture | RR of major fracture | QALYs | Incremental QALYs |
Cost (USD) | Incremental cost (USD) |
ICER (USD per QALY) | INMB (USD) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hip fracture | Vertebral fracture | Other fracture | ||||||||||
| 70 years old, T-score −2.0 | ||||||||||||
| BT | 2.20 | 9.20 | 6.20 | – | – | 11.331 | – | 17,530 | – | – | – | |
| ALN + BT (vs BT) | 2.00 | 8.30 | 5.90 | 0.909 | 0.920 | 11.346 | 0.015 | 17,830 | 300 | 19,904 | 453 | |
| ZOL + BT (vs ALN + BT) | 1.90 | 7.30 | 5.50 | 0.950 | 0.907 | 11.356 | 0.010 | 18,326 | 497 | 47,435 | 27 | |
| 65 years old, T-score −2.5 | ||||||||||||
| BT | 2.50 | 9.50 | 7.00 | – | – | 13.472 | – | 23,988 | – | – | – | |
| ALN + BT (vs BT) | 2.30 | 8.60 | 6.60 | 0.920 | 0.921 | 13.503 | 0.031 | 24,029 | 41 | 1322 | 1499 | |
| ZOL + BT (vs ALN + BT) | 2.20 | 7.60 | 6.20 | 0.957 | 0.914 | 13.520 | 0.017 | 24,447 | 418 | 25,067 | 416 | |
| 70 years old, T-score −2.5 | ||||||||||||
| BT | 3.60 | 12.00 | 7.30 | – | – | 11.167 | – | 20,364 | – | – | – | |
| ALN + BT (vs BT) | 3.30 | 10.80 | 6.90 | 0.917 | 0.917 | 11.193 | 0.026 | 20,460 | 95 | 3620 | 1222 | |
| ZOL + BT (vs ALN + BT) | 3.10 | 9.50 | 6.40 | 0.939 | 0.905 | 11.209 | 0.015 | 20,868 | 408 | 27,018 | 347 | |
| 75 years old, T-score −2.5 | ||||||||||||
| BT | 5.20 | 14.80 | 7.30 | – | – | 8.905 | – | 16,755 | – | – | – | |
| ALN + BT (vs BT) | 4.60 | 13.30 | 6.80 | 0.885 | 0.905 | 8.934 | 0.029 | 16,885 | 130 | 4523 | 1309 | |
| ZOL + BT (vs ALN + BT) | 4.40 | 11.60 | 6.30 | 0.957 | 0.903 | 8.948 | 0.014 | 17,252 | 367 | 25,747 | 346 | |
| 70 years old, T-score −3.0 | ||||||||||||
| ALN + BT | 5.30 | 14.10 | 8.10 | – | – | 10.979 | – | 24,209 | – | – | – | |
| ZOL + BT (vs ALN + BT) | 5.00 | 12.40 | 7.50 | 0.943 | 0.905 | 11.000 | 0.022 | 24,443 | 235 | 10,749 | 857 | |
| BT (vs ZOL + BT) | 66.00 | 15.70 | 8.60 | 1.200 | 1.217 | 10.935 | −0.065 | 24,534 | 91 | Dominated | −3341 | |
BT: basic treatment (placebo + calcium + vitamin D), ZOL: once-yearly injection of zoledronic acid 5 mg, ALN: once-weekly oral alendronate 35 mg. 1 USD = 120 JPY (February 2016 exchange rate). All strategies were ranked according to costs
RR relative risk, QALYs quality-adjusted life years, ICER incremental cost-effectiveness ratio, INMB incremental net monetary benefit
Table 3.
Results of sensitivity analysis on proportion of 1-year treatment persistence
| Proportion of 1-year persistence for zoledronic acid (%) | |||||||
|---|---|---|---|---|---|---|---|
| 90 | 80 | 70 | 60 | 50 | ZOL + BT (vs ALN + BT) | ||
| Proportion of 1 year persistence for alendronate (%) | 80 | 362 | 344 | 322 | 294 | 244 | Incremental cost (USD) |
| 0.008 | 0.002 | −0.004 | −0.010 | −0.017 | Incremental QALYs | ||
| 44,940 | 225,388 | Dominated | Dominated | Dominated | ICER (USD per QALY) | ||
| 70 | 359 | 340 | 319 | 291 | 240 | Incremental cost (USD) | |
| 0.015 | 0.008 | 0.002 | −0.003 | −0.010 | Incremental QALYs | ||
| 24,203 | 41,005 | 129,970 | Dominated | Dominated | ICER (USD per QALY) | ||
| 60 | 370 | 352 | 331 | 302 | 252 | Incremental cost (USD) | |
| 0.022 | 0.015 | 0.009 | 0.003 | −0.003 | Incremental QALYs | ||
| 17,125 | 23,317 | 35,793 | 86,844 | Dominated | ICER (USD per QALY) | ||
| 50 | 398 | 380 | 359 | 330 | 280 | Incremental cost (USD) | |
| 0.029 | 0.022 | 0.017 | 0.011 | 0.004 | Incremental QALYs | ||
| 13,745 | 16,933 | 21,617 | 30,453 | 63,877 | ICER (USD per QALY) | ||
| 40 | 388 | 370 | 348 | 319 | 269 | Incremental cost (USD) | |
| 0.036 | 0.030 | 0.024 | 0.018 | 0.012 | Incremental QALYs | ||
| 10,740 | 12,481 | 14,660 | 17,685 | 23,225 | ICER (USD per QALY) | ||
BT: basic treatment (placebo + calcium + vitamin D), ZOL: once-yearly injection of zoledronic acid 5 mg, ALN: once-weekly oral alendronate 35 mg. 1 USD = 120 JPY (February 2016 exchange rate)
QALYs quality-adjusted life years, ICER incremental cost-effectiveness ratio
Discussion
In this study, we estimated the cost-effectiveness of zoledronic acid therapy for Japanese women with osteoporosis and a history of vertebral fracture using state-transition model. Our results demonstrate that zoledronic acid therapy for patients aged 65 and over with a T-score of −2.0 is likely to be dominated by alendronate therapy with quite small difference in QALYs. Furthermore, we found that zoledronic acid is less effective and more costly for those patients who have one of the risk factors―current smoking, high alcohol intake, and a family history of hip fracture compared to alendronate. The perspective of our study is novel in terms of examining the cost-effectiveness of zoledronic acid using various combinations of age, BMD, and number of clinical risk factors compared with alendronate. To estimate incidence rates of osteoporotic fracture in Japanese women, the use of a large cohort-based fracture risk assessment tool, such as FRAX developed by WHO, is preferable. However, in building the model, we did not use FRAX because the algorithm and parameter estimates are not available to the public. In the present study, we developed original prediction equations for the incidence rate of fracture based on the epidemiological data published in Japan. Our model considered the risk of not only hip fracture and clinical vertebral fracture but also of other osteoporotic fractures such as proximal humerus and distal radius fractures. The validity of our prediction equation was verified by comparison of the predicted 10-year osteoporotic fracture probabilities in our model with those derived from FRAX [9, 41]. The 10-year index case fracture probabilities in our model were confirmed to be almost identical to those from FRAX; thus, we consider this model to assess the cost-effectiveness of osteoporosis therapy in Japanese women to be appropriate.
The cost-effectiveness of zoledronic acid varies from country to country. A study in Finland reported that zoledronic acid dominated alendronate in 50- to 80-year-old patients who have a history of fracture and a T-score of −2.5 [8]. In a Norwegian study, zoledronic acid has also been shown to be dominant (more effective and less costly) compared to alendronate in patients aged 70 and 80 years; in patients aged 50 and 60 years, the ICER of zoledronic acid compared with alendronate was NOK 76,188 and NOK 83,954 per QALY gained, respectively [8]. In Holland, the ICER of zoledronic acid compared with alendronate ranged from 36,927 EUR per QALY in patients aged 60 years to 48,383 EUR per QALY in patients aged 80 years [8]. However, the results of our base case analyses showed that zoledronic acid was dominated by alendronate. Several factors are suggested for this gap including the differences in the fracture risk of baseline, discount rate of cost and health benefits, fracture treatment costs, and drug costs. One of the possible reasons for the gap is a setting of efficacy on the RR for hip fracture. To date, there have been no head-to-head clinical trials with these drugs, and therefore, no evidence for direct comparison for fracture risk decline has been established. Hence, we indirectly evaluated the cost-effectiveness of zoledronic acid therapy compared to oral alendronate therapy using the evidence from network meta-analysis. The results of our base case analyses showed that zoledronic acid was slightly less effective and costly than alendronate (incremental QALY, −0.004 to −0.000; incremental cost, 366 to 493 USD). Zoledronic acid was more effective in preventing major osteoporotic fractures (RR = 0.935–0.944) but less effective in preventing hip fracture (RR = 1.000–1.059) compared to alendronate. Deterministic sensitivity analyses of our model indicated that parameters associated with hip fracture had a relatively strong effect on the cost-effectiveness of osteoporotic treatments. Based on the published network meta-analysis, the RR of hip fracture compared to basic treatment was estimated to be 0.50 in the zoledronic acid arm and 0.45 in the alendronate arm. Therefore, we consider the main explanation for our results to be the settings related to hip fracture. The results from the study in Finland, Norway, and Holland indicated that zoledronic acid was a cost-effective or cost-saving option compared with alendronate, which were considerably different from our results [8]. We consider these gaps to be mainly derived from the difference in the setting of the RR for hip fracture compared to basic treatment. They estimated the RR for hip fracture by zoledronic acid to be 0.59 from the HORIZON-PFT and the RR by alendronate to be 0.62 from the economic evaluation reported by NICE, assuming that the treatment effect observed in clinical trials was independent of patient’s baseline risk factors. Although various studies have used data of the restraining effects on fractures derived from different sources for model analysis, we believe that caution is necessary when handling such data. For an assessment of cost-effectiveness comparing a variety of treatments, a more appropriate indirect comparison such as network meta-analysis is required. In our study, we estimated the comparative effectiveness among multiple osteoporotic treatments by using a recently published network meta-analysis, which we considered to be the best source of available data [28]. However, network meta-analyses generally require advanced technical skills and thorough understanding. The results vary depending on the trials included in a syntactic analysis as well as the timing of the trials. Therefore, caution needs be taken when using these analyses.
The results of the deterministic sensitivity analysis on patients with a T-score of −2.5 indicated that a parameter such as the efficacy of zoledronic acid has a relatively strong impact on the base case result. If the RR of hip fracture with zoledronic acid was equal to 0.34 (lower limit of 95% CI), the ICER of zoledronic acid compared to alendronate was 5890 USD per QALY gained. In other words, judgment of the cost-effectiveness of zoledronic acid compared to alendronate may vary if the restraining effect of zoledronic acid on hip fracture is higher. Our analysis thus suggests that zoledronic acid treatment might be cost-effective compared to alendronate for a selected population, that is, one in which the treatment has a high therapeutic effect. A subgroup analysis of HORIZON-PET by Eastell et al. indicated that zoledronic acid has preventive effects on vertebral fracture in relatively young women with a normal creatinine clearance with BMI ≥25 kg/m2 [42]. However, they found that background factors had no statistical significance on the hip fracture preventive effects [42]. Therefore, using the results of our analysis requires an understanding that the cost-effectiveness of zoledronic acid is sensitive to the degree of the restraining effect on hip fracture. Our simulation was based on the annual drug cost of zoledronic acid which has only recently been approved in Japan. If the annual drug cost of zoledronic acid is 30% lower than the set value of 39,485 JPY (329 USD), the ICER of alendronate compared to zoledronic acid was 159,760 USD per QALY gained. In this case, zoledronic acid becomes a cost-effective option compared to alendronate applying a WTP of 50,000 USD per QALY gained.
Probabilistic sensitivity analysis showed that the probability of zoledronic acid of being cost-effective in patients with a T-score of −2.0, −2.5, and −3.0 was 7.5, 9.7, and 12.9% compared with alendronate treatment and basic treatment alone when using the WTP of 50,000 USD per QALY, respectively. The probability that alendronate becomes the most cost-effective option ranged from 87.1 to 91.2% in those patients. In terms of medical economics, our results suggest that for this patient group, the use of alendronate is recommended.
The main limitation of our analysis is the assumptions about compliance and persistence of the treatment. In the base case analysis of this study, we assumed that there was no difference in the compliance and persistence between zoledronic acid and alendronate. However, one of the major issues with osteoporosis is poor adherence and persistence with drug therapies in clinical practice, which may affect the cost-effectiveness in real world. Although the real-life compliance and persistence data of zoledronic acid is not yet available in Japan, it has been reported that 65.5% of the patients received a second infusion of zoledronic acid after 1 year, while 44.8% of patients remained on the weekly oral alendronate treatment in Germany [40]. Therefore, we performed scenario analysis considering treatment persistence by using this data. The scenario analysis showed that zoledronic acid might be a cost-effective treatment option compared to weekly oral alendronate (ICER, 10,749 USD to 47,435 USD per QALY gained). Furthermore, we estimated the ICER of zoledronic acid with different combinations of the proportion of patients who could continue drug therapy for over a year in zoledronic acid arm and alendronate arm. As a result, we found that zoledronic acid might be cost-effective compared to alendronate if zoledronic acid was expected to improve the proportion of patients who continued treatment for over a year by 10% compared to alendronate with a few exceptions. Recently, Kishimoto and Maehara reported that the 1 year medication possession ratio (MPR), which was used as an indicator of compliance, was 70.6% for weekly oral bisphosphonates in Japan [43]. If we assumed the proportion of patients who continued therapy for over a year in alendronate arm was equal to 70% based on the previous report, the cost-effective threshold for the proportion of treatment persistence for over a year in zoledronic acid arm was estimated to be about 80% or more. The data obtained from this study will possibly facilitate decision-making to determine medical practice. However, comparable data with zoledronic acid or other bisphosphonates on compliance and persistence are insufficient at present. The advantage of an annual injection of zoledronic acid in compliance and persistence in Japanese setting should be challenges for the future studies.
Another limitation is that we estimated the efficacy of zoledronic acid treatment based on the network meta-analysis using a randomized clinical trial that was conducted in countries other than Japan. A subgroup analysis of HORIZON-PET indicated that background factors such as race (Caucasian, Asian, or other) and geographical region (Europe, Asia, or the Americas) had no statistically significant effect on the preventive effects of the treatment on osteoporotic fractures [42]. Therefore, we concluded that, to some extent, extrapolating the preventive effect of zoledronic acid from the population of HORIZON-PET to Japanese women would be acceptable. The sensitivity analysis showed that the uncertainty about the efficacy of zoledronic acid on the RR for hip fracture had a relatively large impact on the ICER of preventive zoledronic acid therapy, and therefore, the efficacy of zoledronic acid in postmenopausal Japanese women with osteoporosis should be determined to confirm the validity of our results. Although the average age of patients in HORIZON-PET was 73, we assumed the average age of a patient to be 70 and ranged from 65 to 75 in the base case analysis. The subgroup analysis showed that for the preventive effects on vertebral fracture, there was a statistically significant difference between the treatment and the age of patients [42]. Therefore, the RR of vertebral fracture by zoledronic acid should vary depending on the age of the patient. However, our sensitivity analysis showed that the impact of the RR for vertebral fracture from zoledronic acid on the ICER was relatively small. Therefore, we used a fixed value for the point estimate of the RR for vertebral fracture from zoledronic acid.
Finally, it is important to note that the cost-effectiveness of zoledronic acid treatment for Japanese osteoporosis patients varies depending on the social acceptability of the ICER threshold. The WTP threshold of 50,000 USD per QALY in the USA and 20,000–30,000 GBP per QALY in the UK is commonly acceptable [44, 45]. However, as noted above, it varies from country to country, and there is no international consensus. In Japan, a study by Ohkusa and Sugawara has proposed a WTP threshold of 6,350,000–6,700,000 JPY (52,917–55,833 USD) per QALY gained [46]. Although still controversial, the population for which drug treatment is cost-effective may vary depending on Japanese societal WTP for an additional QALY.
Conclusion
Although once yearly zoledronic acid treatment in Japanese osteoporosis patients with a history of vertebral fracture is dominated by weekly oral alendronate, the difference in effectiveness is quite small in extent. Considering the advantage of annual zoledronic acid for continuing treatment, zoledronic acid may be a cost-effective treatment option compared to alendronate.
Electronic supplementary material
(DOCX 341 kb)
Acknowledgements
The authors would like to thank Frances Ford for providing English language editing services on behalf of Springer Healthcare Communications. This assistance was funded by the Asahi Kasei Pharma Corporation.
Compliance with ethical standards
Conflicts of interest
Kensuke Morwaiki has received speaker honoraria, consulting fees, and reimbursement for attending meetings from AbbVie GK; Asahi Kasei Pharma Corp.; Chugai Pharmaceutical Co., Ltd.; Deloitte Tohmatsu Consulting LLC; Eli Lilly Japan K.K.; MSD K.K.; and Medtronic Japan Co., Ltd. Mitsuko Mouri declares no conflict of interest associated with this manuscript. Hiroshi Hagino has received research grants and/or consulting fees from Asahi Kasei Pharma Corp.; Astellas Pharma Inc.; Chugai Pharmaceutical Co., Ltd.; Eisai; Eli Lilly Japan K.K.; Mitsubishi Tanabe Pharma Corporation; MSD K.K.; Ono Pharmaceutical Co., Ltd.; Pfizer Japan Inc.; Taisho Toyama Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; and Teijin Pharma Limited.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00198-017-3973-8) contains supplementary material, which is available to authorized users.
An erratum to this article is available at http://dx.doi.org/10.1007/s00198-017-4006-3.
References
- 1.Tsuboi M, Hasegawa Y, Suzuki S, Wingstrand H, Thorngren KG. Mortality and mobility after hip fracture in Japan: a ten-year follow-up. J Bone Joint Surg Br. 2007;89:461–466. doi: 10.1302/0301-620X.89B4.18552. [DOI] [PubMed] [Google Scholar]
- 2.Hagino H, Nakamura T, Fujiwara S, Oeki M, Okano T, Teshima R. Sequential change in quality of life for patients with incident clinical fractures: a prospective study. Osteoporos Int. 2009;20:695–702. doi: 10.1007/s00198-008-0761-5. [DOI] [PubMed] [Google Scholar]
- 3.Japan Ministry of Health, Labour, and Welfare (2016) [Internet]. Tokyo, Japan: Japan Ministry of Health, Labour, and Welfare; [cited 2016 Nov 30]. Available from: http://www.mhlw.go.jp/toukei/list/37-21.html. Japanese
- 4.Orimo H, Yaegashi Y, Hosoi T, et al. Hip fracture incidence in Japan: estimates of new patients in 2012 and 25-year trends. Osteoporos Int. 2016 doi: 10.1007/s00198-015-3464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orimo H, Yaegashi Y, Onoda T, Fukushima Y, Hosoi T, Sakata K. Hip fracture incidence in Japan: estimates of new patients in 2007 and 20-year trends. Arch Osteoporos. 2009;4(1–2):71–77. doi: 10.1007/s11657-009-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 7.Hagino H, Sawaguchi T, Endo N, Ito Y, Nakano T, Watanabe Y. The risk of a second hip fracture in patients after their first hip fracture. Calcif Tissue Int. 2012;90:14–21. doi: 10.1007/s00223-011-9545-6. [DOI] [PubMed] [Google Scholar]
- 8.Akehurst R, Brereton N, Ariely R, et al. The cost effectiveness of zoledronic acid 5 mg for the management of postmenopausal osteoporosis in women with prior fractures: evidence from Finland, Norway and the Netherlands. J Med Econ. 2011;14:53–64. doi: 10.3111/13696998.2010.545563. [DOI] [PubMed] [Google Scholar]
- 9.Moriwaki K, Komaba H, Noto S, et al. Cost-effectiveness of alendronate for the treatment of osteopenic postmenopausal women in Japan. J Bone Miner Res. 2013;28:395–403. doi: 10.1002/jbmr.1755. [DOI] [PubMed] [Google Scholar]
- 10.Ross PD, Norimatsu H, Davis JW, et al. A comparison of hip fracture incidence among native Japanese, Japanese Americans, and American Caucasians. Am J Epidemiol. 1991;133:801–809. doi: 10.1093/oxfordjournals.aje.a115959. [DOI] [PubMed] [Google Scholar]
- 11.Hagino H, Katagiri H, Okano T, Yamamoto K, Teshima R. Increasing incidence of hip fracture in Tottori prefecture, Japan: trend from 1986 to 2001. Osteoporos Int. 2005;16:1963–1968. doi: 10.1007/s00198-005-1974-5. [DOI] [PubMed] [Google Scholar]
- 12.Ross PD, Fujiwara S, Huang C, et al. Vertebral fracture prevalence in women in Hiroshima compared to Caucasians or Japanese in the US. Int J Epidemiol. 1995;24:1171–1177. doi: 10.1093/ije/24.6.1171. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara S, Kasagi F, Masunari N, Naito K, Suzuki G, Fukunaga M. Fracture prediction from bone mineral density in Japanese men and women. J Bone Miner Res. 2003;18(8):1547–1553. doi: 10.1359/jbmr.2003.18.8.1547. [DOI] [PubMed] [Google Scholar]
- 14.Soen S, Fukunaga M, Sugimoto T, et al. Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab. 2013;31:247–257. doi: 10.1007/s00774-013-0447-8. [DOI] [PubMed] [Google Scholar]
- 15.Briggs A, Sculpher M, Claxton K (2006) Decision modelling for health economic evaluation. Oxford University Press, USA, New York
- 16.De Laet CE, van Hout BA, Burger H, Hofman A, Pols HA. Bone density and risk of hip fracture in men and women: cross sectional analysis. BMJ. 1997;315:221–225. doi: 10.1136/bmj.315.7102.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagino H, Furukawa K, Fujiwara S, et al. Recent trends in the incidence and lifetime risk of hip fracture in Tottori, Japan. Osteoporos Int. 2009;20:543–548. doi: 10.1007/s00198-008-0685-0. [DOI] [PubMed] [Google Scholar]
- 18.Hagino H, Yamamoto K, Ohshiro H, Nakamura T, Kishimoto H, Nose T. Changing incidence of hip, distal radius, and proximal humerus fractures in Tottori prefecture, Japan. Bone. 1999;24:265–270. doi: 10.1016/S8756-3282(98)00175-6. [DOI] [PubMed] [Google Scholar]
- 19.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16(7):737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 22.Kanis JA, Johansson H, Oden A, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35:1029–1037. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 24.Japan Ministry of Health, Labour, and Welfare (2012) National Health and Nutrition Survey [Internet]. Tokyo, Japan: Japan Ministry of Health, Labour, and Welfare; [cited 2016 Mar 18]. Available from: http://www.mhlw.go.jp/stf/houdou/0000032074.html. Japanese
- 25.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 26.Japan Ministry of Health, Labour, and Welfare (2010) Life table for Japanese [Internet]. Tokyo, Japan: Japan Ministry of Health, Labour, and Welfare; [cited 2016 Mar 18]. Available from: http://www.e-stat.go.jp/SG1/estat/GL08020103.do?_toGL08020103_listID=000001111987requestSender=dsearch. Japanese
- 27.Hayashi Y. Economical viewpoint for treatment of osteoporosis. Nihon Rinsho. 2007;65(Suppl 9):609–614. [PubMed] [Google Scholar]
- 28.Murad MH, Drake MT, Mullan RJ, et al. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2012;97:1871–1880. doi: 10.1210/jc.2011-3060. [DOI] [PubMed] [Google Scholar]
- 29.Tosteson AN, Jonsson B, Grima DT, O'Brien BJ, Black DM, Adachi JD. Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporos Int. 2001;12:849–857. doi: 10.1007/s001980170036. [DOI] [PubMed] [Google Scholar]
- 30.Japan Ministry of Health, Labour, and Welfare (2016) [Internet]. Tokyo, Japan: Japan Ministry of Health, Labour, and Welfare; [cited 2016 Nov 30]. Available from: http://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000142226.pdf. Japanese
- 31.Medical Fee Schedule (2016) Igaku-tsushin-sya, Tokyo, Japan, Japanese
- 32.National Health Insurance Price List Japan (2016) Jihou Press, Tokyo, Japan, Japanese
- 33.Hagino H. Cost-effectiveness of the treatment for osteoporosis. Nihon Rinsho. 2002;60(Suppl 3):645–654. [PubMed] [Google Scholar]
- 34.Kondo A, Zierler BK, Isokawa Y, Hagino H, Ito Y. Comparison of outcomes and costs after hip fracture surgery in three hospitals that have different care systems in Japan. Health Policy. 2009;91:204–210. doi: 10.1016/j.healthpol.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Negami S. Comparison of cost for distal radius fractures before and after DPC revision by using EVE. Journal of Sapporo Social Insurance General Hospital. 2011;20:43. [Google Scholar]
- 36.Reward for nursing care (2014) Igaku-tsushin-sya, Tokyo, Japanese
- 37.Nawata S, Yamada Y, Ikeda S, Ikegami N. EuroQol study of the elderly general population: relationship with IADL and other attributes. Journal of Health Care and Society. 2000;10:75–86. doi: 10.4091/iken1991.10.2_75. [DOI] [Google Scholar]
- 38.Imai H, Fujii Y, Fukuda Y, Nakao H, Yahata Y. Health-related quality of life and beneficiaries of long-term care insurance in Japan. Health Policy. 2008;85:349–355. doi: 10.1016/j.healthpol.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda T, Shiroiwa T, Ikeda S, et al. Guideline for economic evaluation of healthcare technologies in Japan. J Natl Inst Public Health. 2013;62:625–640. [Google Scholar]
- 40.Volker Z, Karel K, Ioannis K, et al. Persistence and compliance of medications used in the treatment of osteoporosis analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther. 2012;50(5):315–322. doi: 10.5414/CP201632. [DOI] [PubMed] [Google Scholar]
- 41.Moriwaki K, Noto S. Validation of fracture risk model in Japanese women compared with Frax. Value Health. 2015;18:A698. doi: 10.1016/j.jval.2015.09.2606. [DOI] [Google Scholar]
- 42.Eastell R, Black DM, Boonen S, et al. Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab. 2009;94:3215–3225. doi: 10.1210/jc.2008-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kishimoto H, Maehara M. Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos. 2015;10:231. doi: 10.1007/s11657-015-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gafni A, Birch S. Incremental cost-effectiveness ratios (ICERs): the silence of the lambda. Soc Sci Med. 2006;62(9):2091–2100. doi: 10.1016/j.socscimed.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 45.National Institute for Health and Care Excellence: Guide to the Methods of Technology Appraisal 2013 [cited 2016 Mar 18]. Available from: https://www.nice.org.uk/article/pmg9/chapter/foreword [PubMed]
- 46.Ohkusa Y, Sugawara T. Research for willingness to pay for one QALY gain. Iryo To Shakai. 2006;16:157–165. doi: 10.4091/iken.16.157. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 341 kb)