Abstract
Summary
Fracture liaison services (FLS) are advocated to improve osteoporosis treatment after fragility fracture, but there are few economic analyses of different models. A population-based 1i [=type C] FLS for non-hip fractures was implemented and it costs $44 per patient and it was very cost-effective ($9200 per QALY gained). Small operational changes would convert it from cost-effective to cost-saving.
Introduction
After fragility fracture, <20% of patients receive osteoporosis treatment. FLS are recommended to address this deficit but there are very few economic analyses of different FLS models. Therefore, we conducted an economic analysis of a 1i (=type C) FLS called “Catch a Break (CaB).”
Methods
CaB is a population-based FLS in Alberta, Canada, that case-finds older outpatients with non-traumatic upper extremity, spine, pelvis, or “other” non-hip fractures and provides telephonic outreach and printed educational materials to patients and their physicians. Cost-effectiveness was assessed using Markov decision-analytic models. Costs were expressed in 2014 Canadian dollars and effectiveness based on model simulations of recurrent fractures and quality-adjusted life years (QALYs). Perspective was healthcare payer; horizon was lifetime; and costs and benefits were discounted 3%.
Results
Over 1 year, CaB enrolled 7323 outpatients (mean age 67 years, 75% female, 69% upper extremity) at average cost of $44 per patient. Compared with usual care, CaB increased rates of bisphosphonate treatment by 4.3 to 17.5% (p < 0.001). Over their lifetime, for every 10,000 patients enrolled in CaB, 4 hip fractures (14 fractures total) would be avoided and 12 QALYs gained. Compared with usual care, incremental cost-effectiveness of CaB was estimated at $9200 per QALY. CaB was cost-effective in 85% of 10,000 probabilistic simulations. Sensitivity analyses showed if “other” fractures were excluded and intervention costs reduced 25% that CaB would become cost-saving.
Conclusions
A relatively inexpensive population-based 1i (=type C) FLS was implemented in Alberta and it was very cost-effective. If CaB excluded “other” fractures and decreased intervention costs by 25%, it would be cost-saving, as would any FLS that was more effective and less expensive.
Keywords: Cost-effectiveness analysis, Fracture liaison services, Fragility fracture, Guidelines, Osteoporosis treatment, Quality improvement
Introduction
Osteoporosis is a chronic and progressive condition associated with low bone mineral density (BMD) and skeletal fragility that causes susceptibility to low-trauma fragility fractures [1–3]. Fractures can cause acute and chronic pain, deformity, diminished quality of life, disability, loss of independence, nursing home admission, and even death [1–3]. Osteoporosis affects at least two million Canadians or about 25% of women and 12% of men older than 50 years [2]. A woman’s lifetime risk of an osteoporosis-related fracture is 40–50% while a man’s is 12–20% [2, 3]. Each year in Canada, there are about 130,000 osteoporosis-attributable fractures at an estimated annual cost of $4.6 billion [4].
At present, many experts have described “a crisis in the treatment of osteoporosis” and have noted that many of the gains made in the last quarter of a century are unraveling [5]. This is particularly true for those who have just suffered an osteoporosis-related fracture; instead of being the group with the highest testing and treatment rates, those who have survived a fracture have rates of treatment of less than 20% [1, 5–9]. To address this care gap, clinicians, experts, and several societies (e.g., American Society of Bone and Mineral Research [ASBMR], International Osteoporosis Foundation [IOF], Osteoporosis Canada [OC]) have strongly endorsed fracture liaison services (FLS) that identify fracture patients and alert their family physicians (1i [= type C]) and arrange investigations such as BMD testing (2i [= type B]) and initiate prescription treatments and longer-term follow-up (3i [= type A]) [5–8]. Although there are a few randomized trials to support FLS, the available evidence suggests that the more intensive (and expensive) the FLS, the more effective it is at managing osteoporosis and thus reducing downstream fractures and improving quality of life [9]. There are, however, even fewer data on whether different FLS models are cost-effective or worthwhile [9, 10].
Thus, we undertook a detailed evaluation of “Catch a Break (CaB),” which is a “1i (= type C)” population-based outpatient FLS [11]. CaB itself was adapted from a randomized controlled trial of another 1i (= type C) intervention that consisted of patient identification with telephonic contacts, educational mail-outs, and patient-specific family physician alerting and outreach that cost about $12 per patient [12–15]. This intervention, tested under the ideal circumstances of a randomized trial, was cost-saving and a budget impact analysis suggested it would “break-even” within 2 years of implementation [15]. In addition, another Canadian province (Manitoba) conducted a randomized trial comparing usual care controls to a similar 1i (= type C) strategy as CaB (but used a different case-finding approach and did not use telephonic outreach for patients) that costs about $8 per patient and that was also cost-saving in formal economic analyses [16, 17]. But it should be acknowledged that both of the aforementioned interventions [12–17] were conducted as randomized trials under ideal circumstances and with adequate resources and infrastructure, and it is well known that when such evidence based on trials is translated into the “real world” that implementation often suffers because of attenuated fidelity, that clinical effectiveness tends to diminish, and that intervention costs may increase [1, 10].
Therefore, we undertook an independent economic evaluation of CaB as it was introduced into the “real world.” Furthermore, although CaB is by strict definition a 1i (= type C) FLS, it is still sufficiently different from other programs in terms of the populations at risk, types of fractures included, methods for determining eligibility, and the manner by which specific intervention components are delivered that it requires a comprehensive and independent evaluation to determine if it should continue and if other jurisdictions should adopt something like CaB. We believe that a formal cost-effectiveness analysis is still needed to better determine value for money and return on investment for and hypothesized that the CaB intervention would be superior to usual care.
Methods
Description of Catch a Break
Health Link is a pre-existing province-wide publicly funded program for patient support and it proffers health advice and counseling by trained staff. Health Link also has the infrastructure to act as a platform for various health-related initiatives such as InformAlberta [18] and CaB. First, administrative and claim data from emergency departments (ED) and ambulatory urgent care centers across Alberta were reviewed to identify patients who may have had a low-trauma (fragility) non-hip fracture. Of note, patients with hip fractures (who are hospitalized, undergo surgical fixation, spend weeks to months in hospital or inpatient rehabilitation facilities, and often transferred to long-term care) were deemed not suitable for CaB because these patients are almost impossible to contact within 6 weeks of fracture and need more intensive osteoporosis management [19]. Indeed, based on high-quality randomized trials, guidelines recommend an inpatient-based nurse case manager or orthogeriatric service (i.e., a 3i [= type A]) to deal with frail and hospitalized hip fracture patients [2]. Thus, CaB was designed to help outpatients with non-traumatic non-hip fractures.
Once patients were identified by administrative data, Health Link staff contacted patients by phone 6 weeks post-fracture and conducted a standardized osteoporosis screening risk assessment using an electronic questionnaire [11, 19] similar to FRAX or CAROC [2, 3]. Participants deemed “high risk” for osteoporosis and recurrent fracture (e.g., a 10-year risk of major osteoporotic fracture = 20% or risk of hip fracture = 3% [2, 3]) were asked to follow up with their family physician for investigations and management of osteoporosis. Participants were also mailed osteoporosis educational materials. Notification of screening results, along with current diagnostic and treatment guidelines, was sent to the family physician of record. High-risk participants who had not yet seen their family physician were contacted at 3 and 6 months with reminders. All participants were contacted at 12 months to complete a final assessment; this cohort represents the CaB intervention group [19].
We stratified fractures into four groups: upper extremity (including rib fractures since rates of low BMD and recurrent fracture are nearly identical to that of upper extremity fractures); spine; pelvis (since rates of low BMD and recurrent fracture are similar to that of hip fracture); and “other” (primarily ankle) fractures [20]. For the mature CaB intervention running for 1 year, we determined that 4633 participants would complete the intervention out of 7323 potentially eligible patients.
Using these same programmatic data, we also simulated a control group (i.e., usual care) based on the outpatients enrolled in CaB that were newly started on bisphosphonate treatment in 6–12 weeks after their non-traumatic non-hip fracture. Because these outpatients had fracture, they were potentially eligible for CaB, but since they were already newly treated, they did not receive any further interventions or follow-up. They were otherwise drawn from exactly the same population and have the same age, sex, incident fracture distributions, and so on, as those who entered CaB. For this control cohort, it was assumed that rates of BMD tests, rates of low bone mass, rates of long-term adherence and persistence with bisphosphonates, and all downstream costs and event rates were the same as for the patients who received the full CaB intervention and were followed for 1 year. For between-group comparisons, chi-squared and t tests as appropriate were used and a two-sided p value of 0.05 was considered statistically significant.
Cost-effectiveness analysis
The cost-effectiveness analysis takes a third party healthcare payor perspective and compares the group of outpatients with fracture receiving the CaB intervention to a simulated usual care control group by using a decision analytic model that incorporates Markov processes. Data regarding population at risk, fracture types, and achieved rates of treatment were drawn directly from CaB. Cost-effectiveness was assessed through a decision analytic model incorporating Markov processes to estimate incremental costs and effectiveness based on quality-adjusted life years (QALYs) gained [21–25]. In general, interventions are considered cost-effective when they either (1) cost less and are more effective or (2) cost more but are more effective than a comparator, but society is willing to pay for this additional cost. In the latter scenario, the incremental cost-effectiveness ratio (ICER), the ratio of incremental cost to incremental health effect, is commonly compared to some threshold level that reflects the amount a society is willing to pay for an additional unit of health [26]. This threshold represents the opportunity cost, relating to health outcomes foregone, by the reallocation of resources needed for the intervention [27]. A QALY-based threshold of $50,000 USD (about $55,000 in 2014 $CDN [28]) is often suggested [29] while a UK study [27] empirically estimated a threshold of £12,936 (about $24,000 in 2014 $CDN); we used these thresholds in our assessments of societal “willingness-to-pay.” We took a lifetime horizon and the perspective of a third party public healthcare payer.
Decision analytic model
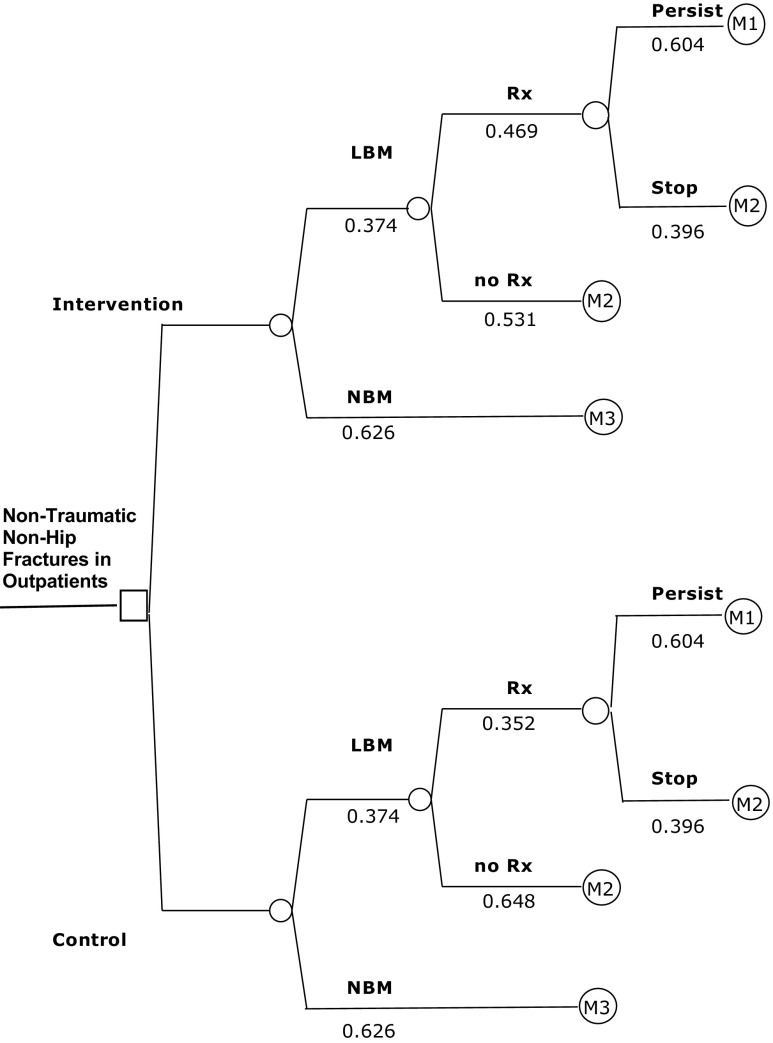
Figure 3 illustrates the model, displaying two study arms and, for each arm, four osteoporosis-related diagnosis and treatment pathways possible in 12 months following fracture. The proportion of patients within each group was derived from CaB data (Table 1). Then, three unique Markov processes were constructed (Fig. 3), and these were distinguished only by their transition probabilities: low bone mass and receiving osteoporosis treatment (M1), low bone mass detected but not receiving osteoporosis treatment (M2), and normal bone mass and appropriately not treated (M3). M1 and M3 represent high-quality guideline-concordant care.
Fig. 3.
Decision tree of the model with Markov processes. Probabilities associated with each chance node are shown under the relevant branch. LBM indicates low bone mass; NBM, normal bone mass; and Rx, treatment with a bisphosphonate. M1, M2, and M3 refer to the individual Markov processes; also, see the “Methods” section and Fig. 2
Table 1.
Distribution of patients by sub-group and study arm
| Sub-group | CaB intervention (%) | Control (%) |
|---|---|---|
| LBM, Rx | 17.5 | 13.2 |
| LBM, no Rx | 19.9 | 24.2 |
| NBM | 62.6 | 62.6 |
| Total | 100.0 | 100.0 |
LBM low bone mass, NBM normal bone mass, Rx treated with a bisphosphonate
The structure of the Markov process was adapted from the IOF cost-effectiveness reference model [23–25] and our prior work [14–16]. The model incorporates 15 health states that simulate the movement of patients from the time of fracture identification at age 50 years to the age of 100 years or death (see Fig. 4). All patients entered the model at one of four initial post-fracture health states (upper extremity, spine, pelvis, other) derived from CaB data. Then, a proportion of the cohort moves to one of the other 11 states once per annual cycle, in accordance with transition probabilities derived from rates specific to the type of subsequent fracture incurred, presence or absence of low bone mass, treatment induced and fracture type-specific reductions in subsequent fractures, and population-based and age-specific death rates.
Fig. 4.
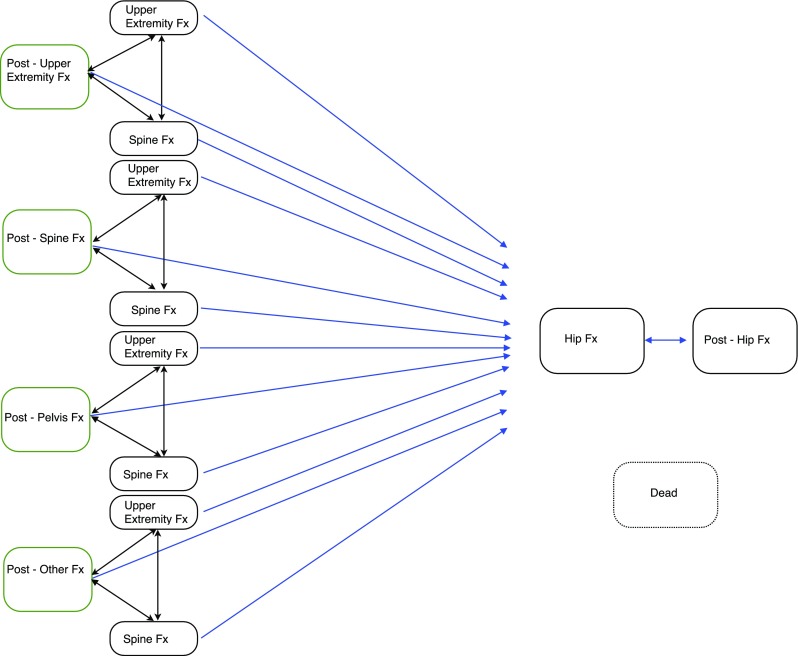
Structure of the Markov process. Adapted from references [23–25]. There are potential transitions from each health state to the dead state that are not shown in the figure, for purposes of clarity. As well, patients can remain in all states for multiple cycles. The Post-Upper Extremity Fx state includes Rib Fx. The Post-Other Fx state includes Ankle Fx
Model assumptions
The model incorporates three simplifying assumptions. First, patients were considered to have normal (T-scores better than or equal to −1.1 at all skeletal sites measured) or treatably low levels of bone mass (T-scores worse than −1.1, encompassing densitometric osteopenia and osteoporosis) based on BMD measurements. Second, we assumed all patients were treated with alendronate or risedronate (hereafter referred to as bisphosphonate treatment), because they are generic and the most widely prescribed bisphosphonates in Canada and have the greatest weight of evidence available for fracture reduction. Last, we assumed that no additional non-hip fractures would occur after a hip fracture, although repeat hip fractures were permitted [23–25].
Model inputs
The mean values and standard errors of all parameters used in the model are presented in Table 5. The key inputs are summarized below:
Repeat fracture rates. We assumed repeat fracture rates for low bone mass patients not treated for osteoporosis were constant with respect to age and fracture site [22–25] and (because there are currently no comparable Alberta-based data) based these fracture rates on recent Manitoba data [18, 30]. Repeat fracture rates for normal bone mass were considered to be the same as for patients with low bone mass treated with bisphosphonates.
Fracture reduction with bisphosphonates. Estimates of fracture reduction were based on meta-analysis and included a pooled 49% reduction in risk of hip and vertebral fractures and a pooled 48% reduction for humeral and distal radial fractures [31]. In the base case analysis, bisphosphonate treatment was for 5-year duration. In the first year of treatment, fracture reduction was assumed to be half the full achievable benefit accrued over 5 years. As well, a residual positive effect of treatment was assumed for 5 years following discontinuation [32]. This residual effect was modeled as linear but with diminishing benefits [32]. One-year persistence with bisphosphonate treatment has been reported as 60% [33], and we assumed this rate of persistence would continue for the next 4 years of treatment. All patients who persisted with bisphosphonate treatment were assumed to have greater than 80% adherence to their medications [15]. We assumed no clinical benefits accrued to the 40% of patients who discontinued treatment in the first year of treatment, and we assumed no clinical benefits associated with over-the-counter calcium and vitamin D supplements. For the latter, we also assumed no costs as these supplements are not covered and patients pay out of pocket.
- Costs. All costs were expressed in constant 2014 CDN dollars and restricted to direct medical costs, i.e., no indirect costs were incorporated into our analyses. In the base case, all costs and outcomes were discounted at 3% per annum [34]. Specific costs included the following (for additional details and information sources, also refer to Table 5):
- Costs of the CaB intervention. Ongoing costs for case-finding and implementing the intervention were based on independently conducted time-motion studies and shown in Table 2. Salary, benefits, and overhead costs related to Health Link callers were included. Ongoing programming (identifying patients and creating mailing lists), administration (mailing, consultants), and other supplies and postage were also included. The cost per patient receiving the intervention was $44. Initial programming costs related to development and testing of the list generating algorithm, database and telephone setup, and one-time training costs were excluded.
- Costs of osteoporosis treatment. We assumed all patients treated with a bisphosphonate received 70 mg per week of generic alendronate or 35 mg per week of generic risedronate. Total annual cost of medication and one annual osteoporosis-related primary care physician visit for re-evaluation and prescription refills plus dispensing fees was $203 per patient [35, 36]. We also assumed bisphosphonates would generate trivial direct medical costs related to side effects [37].
- Costs of subsequent fractures. Estimated annual costs for the health states relate to the management of subsequent clinically symptomatic spine, hip (including pelvis fractures), and upper extremity fractures (including rib). Costs were estimated based on standardized physician fees while other health services and their unit values were obtained from regional and national databases [36, 38]. We assumed hip fractures would require surgical fixation and a 7-day hospital stay [38]. A case-mix method was used for inpatient costs [39]. It was assumed that 80% of patients would be discharged home after hip fracture and 20% to long-term care facilities; costs of long-term care were based on provincial per diems less patient co-payments [40]. Only 10% of patients with clinically symptomatic vertebral fractures were assumed to require inpatient care [41]. Hospital costs were estimated in similar manner as hip fractures [38, 39]. Following discharge, routine care was considered nine follow-up physician visits and one spinal radiograph. The non-hospitalized clinically symptomatic vertebral fracture patients (90%) required four physician visits, two spinal radiographs, and eight visits of outpatient rehabilitation. Patients with subsequent proximal humerus and distal radius fractures were assumed to present to EDs or fracture clinics for treatment and had one closed fracture reduction, two physician follow-up visits, two post-reduction radiographs, eight outpatient rehabilitation visits, and no subsequent surgical repair.
Mortality rates. Patients were assumed to have the same risk of death as the general population, except in the 1 year following a hip fracture [22, 23]. Published life tables were used for age-specific death rates [42], and age-specific excess mortality rates in the 1 year following hip fracture (and spine fracture) were derived from the IOF reference models [25].
Health-related quality of life. We used age-specific utility weights for each health state based on published utilities and their proposed multipliers [25, 43 ].
Table 5.
Model input parameters
| Parameter | Valuesa
Mean (std. error) |
Probability Distribution type for PSA |
Source |
|---|---|---|---|
| Prior probabilities | |||
| Probability of Rx treatment intervention arm | 0.469 (0.021) | Beta | 19 |
| Probability of Rx treatment control arm | 0.352 (0.016) | Beta | 19 |
| Probability of low bone mass | 0.374 (0.012) | Beta | 19 |
| Initial health state proportions | |||
| Post-upper extremity fracture | 0.691 (0.005) | Beta | 19 |
| Post-spine fracture | 0.051 (0.003) | Beta | 19 |
| Post-pelvis fracture | 0.043 (0.002) | Beta | 19 |
| Post-other fracture | 0.215 (0.005) | Beta | 19 |
| Re-fracture probabilities | |||
| Prior upper extremity fracture | |||
| Upper extremity fracture | 0.007 (0.001) | Beta | 17, 30 |
| Spine fracture | 0.006 (0.001) | Beta | 17, 30 |
| Hip fracture | 0.004 (0.001) | Beta | 17, 30 |
| Prior spine fracture | |||
| Upper extremity fracture | 0.011 (0.003) | Beta | 17, 30 |
| Spine fracture | 0.004 (0.001) | Beta | 17, 30 |
| Hip fracture | 0.007 (0.002) | Beta | 17, 30 |
| Prior pelvis/hip fracture | |||
| Upper extremity fracture | 0.017 (0.004) | Beta | 17, 30 |
| Spine fracture | 0.010 (0.003) | Beta | 17, 30 |
| Hip fracture | 0.014 (0.004) | Beta | 17, 30 |
| Prior other fracture | |||
| Upper extremity fracture | 0.002 (0.0002) | Beta | 17, 30 |
| Spine fracture | 0.002 (0.0001) | Beta | 17, 30 |
| Hip fracture | 0.001 (0.0001) | Beta | 17, 30 |
| Relative risk of fracture following treatment | |||
| Spine fracture | 0.520 (0.114) | Beta | 31 |
| Non-spine fracture | 0.510 (0.150) | Beta | 31 |
| Other treatment-related parameters | |||
| Persistence of treatment (probability at 1 year) | 0.604 (0.071) | Beta | 33 |
| Continuation of treatment effect following discontinuation of treatment (years) | Declines over 5 years to zero | Fixed | 32 |
| Mortality | |||
| Probability of all-cause death | Age-specific | ||
| 50 years of age | 0.003 (0.0002) | Beta | 42 |
| 90 | 0.150 (0.005) | Beta | 42 |
| Probability of death in year after hip fracture | Age-specific | ||
| 50 years of age | 0.034 (0.002) | Beta | 25, 42 |
| 90 | 0.258 (0.009) | Beta | 25, 42 |
| Cost ($ per patient per year, except where noted) | |||
| Intervention | 44.34 (2.79) | Gamma | 19 |
| BMD ($ per test) | 119.12 | Fixed | 36 |
| Bisphosphonate treatment | 131.28 | Fixed | 35 |
| Physician visit ($ per visit) | 35.91 | Fixed | 36 |
| Upper extremity fracture | 1397 (70) | Gamma | 36, 38, 39 |
| Upper extremity fracture post-pelvis fracture | 13,854 (693) | Gamma | 36, 38–40 |
| Spine fracture | 1662 (83) | Gamma | 36, 38, 39 |
| Spine fracture post-pelvis fracture | 14,041 (702) | Gamma | 36, 38–40 |
| Hip fracture | 38,332 (1917) | Gamma | 36, 38–40 |
| Late post-hip fracture cost | 12,357 (618) | Gamma | 36, 38–40 |
| Utility | |||
| General population utility score | Age-specific | ||
| 50–59 years | 0.84 (0.013) | Beta | 25 |
| 60–69 years | 0.82 (0.013) | Beta | 25 |
| 70–79 years | 0.80 (0.018) | Beta | 25 |
| 80+ years | 0.74 (0.034) | Beta | 25 |
| Utility weight multipliers | |||
| Hip fracture (1 year post-fracture) | 0.70 (0.035) | Beta | 43 |
| Hip fracture (subsequent years) | 0.80 (0.080) | Beta | 43 |
| Spine fracture | 0.59 (0.065) | Beta | 43 |
| Upper extremity fracture | 0.96 (0.048) | Beta | 43 |
| Discount rate (%) | 3.0 | Fixed | b |
aCosts are expressed in 2014 Canadian dollars (multiply by 0.905 to convert to US dollars [26])
bAssumed
Table 2.
Intervention cost per patient
| Cost item | Cost per patienta |
|---|---|
| Calling—no contact—could not be reached | 1.98 |
| Calling—contacted—declined | 1.39 |
| Calling—incomplete initial survey (patients not eligible for intervention) | 2.55 |
| Calling—patient eligible for intervention—all surveysb | 24.45 |
| Patient and physician packages (mail and email—sent by callers) | 13.45 |
| Programming—lists for callers | 0.34 |
| Consulting osteoporosis specialist | 0.18 |
| Average cost—patients receiving intervention | 44.34 |
aCaller cost per minute = $0.70; 2014 Canadian dollars (multiply by 0.905 to convert to 2014 US dollars [28])
bIncludes cost of unsuccessful call attempts
Deterministic sensitivity analyses
Conventional one-way deterministic sensitivity analyses were conducted to evaluate the robustness of the model. First, we evaluated the impact of excluding “other” fractures from CaB. Collectively, these “other” fractures are not independently associated with an increased risk of subsequent fragility fractures or low bone mass [20]. Second, we assessed the consequences of a 25% decrease (or increase) in the intervention cost. Third, although bisphosphonates are commonly used as the base case analyses in many osteoporosis-related cost effectiveness models [44], other more expensive or “on patent” oral bisphosphonates (e.g., ibandronate) might be used; thus, we conducted sensitivity analyses wherein drug costs were doubled and tripled. Fourth, we re-ran our models assuming only 35% fracture reduction with bisphosphonates; this is the value used by the IOF [25] and approximates the lower bounds of published 95% confidence intervals [31]. Fifth, we included an analysis of the impact of 50% medication adherence rather than the 80% adherence used in the base case and employed recent projections that estimated bisphosphonate effectiveness would thence be only 30% of what could be achieved with optimal adherence [44, 45]. Sixth, we explored the impact of reducing the proportion of patients who persisted with their medication from 60 to 30%. Seventh, because patients may derive a mortality reduction with bisphosphonates, we included a sensitivity analysis to assess an 11% bisphosphonate-related mortality reduction 1 year post-fracture [46]. Eighth, we included the potential for increased mortality following a vertebral fracture and handled it in the same way we incorporated increased mortality after a hip fracture. Ninth, although we used the currently recommended 3% discount rate for both costs and benefits [34], we examined the impact of using higher and lower discount rates. Last, we conducted a two-way sensitivity analysis that simultaneously excluded “other” fractures and reduced intervention costs by 25% as these are the two aspects of the intervention that healthcare administrators could change.
Probabilistic sensitivity analysis
We also conducted a probabilistic sensitivity analysis to assess the impact of parameter uncertainty. Probability distributions were assigned to all variables for which there was uncertainty associated with the base case. We chose a gamma distribution to generate random values for unit costs but otherwise used a beta distribution and then undertook 10,000 simulations. Results were summarized using a cost-effectiveness acceptability curve, which represents the percent of simulations that were cost-effective for each study arm at various potential levels of societal willingness-to-pay. All analyses were conducted using the TreeAge Pro Healthcare Module 2016 (TreeAge Software Inc., Williamstown, MA).
Results
Patient characteristics
At baseline, the average age of eligible patients (n = 7323) was 67 (±11) years and 75% were female. Most (69%) patients had an initial upper extremity fracture (including 14% with rib fractures considered “upper extremity equivalents”), 5% a spine fracture, 4% a pelvis fracture (considered “hip fracture equivalents”), and 22% “other” fractures (17% ankle).
CaB intervention effect
Overall, CaB increased the proportion of patients who appropriately received bisphosphonate treatment to prevent future fracture by 4.3%. Specifically, in the year following fracture, 17.5% (95% CI 15.6–19.4%) of CaB patients were treated with bisphosphonates vs 13.2% (95% CI 12.4–14.0%) of the usual care control group (p < 0.001 for difference).
Base case analysis
The base case model suggests that, over their lifetime, patients exposed to the CaB intervention would be less likely to fracture again compared with usual care. For every 10,000 patients that participated in CaB, an additional 400 patients would be treated with bisphosphonates, resulting in the avoidance of about 4 hip fractures and 14 major osteoporotic fractures in total and a modest gain of 12 QALYs vs usual care (Table 3). Those gains were achieved at an incremental cost of $11 per patient compared to usual care or about $9200 per QALY gained.
Table 3.
Costs and health outcomes per patient by study arm—CaB base case
| Study group | Average cost ($)a | Average hip fracturesb | Average total re-fracturesc | Average QALYsd |
|---|---|---|---|---|
| Intervention | 20,554 | 0.0990 | 0.3661 | 16.6949 |
| Control | 20,543 | 0.0994 | 0.3675 | 16.6937 |
| Incremental Analysis | ||||
| Intervention vs control | 11 | −0.0004 | −0.0014 | 0.0012 |
| ICERe | 9167 | |||
aLifetime average costs per patient, discounted at 3%. These costs are expressed in constant 2014 Canadian dollars (multiply by 0.905 to convert to 2014 US dollars [28])
bRefers to incident hip fractures per patient
cIncludes hip, clinical vertebral, and upper extremity re-fractures per patient
dAverage quality-adjusted life years per patient, discounted at 3%
eICER: Incremental Cost-effectiveness Ratio = Incremental Cost/Incremental QALYs
Deterministic sensitivity analyses
One-way sensitivity analyses indicated that the results of the base case were reasonably robust to most plausible scenarios, i.e., achieving either an ICER of less than $55,000 or dominating usual care (that is, less costly and more effective, see Table 4). The three exceptions were a 500% increase in bisphosphonate price, reduction of treatment persistence to 30% (from the base case of 60%), and assuming only 50% adherence to treatment among those who persisted with treatment for at least 1 year (vs base case of 80%, see Table 4).
Table 4.
Deterministic sensitivity analyses
| Scenario | Incremental Costa | Incremental Effect | ICERa |
|---|---|---|---|
| Base case | 11 | 0.0012 | 9167 |
| Exclude “other” fractures | 0 | 0.0014 | 0 |
| Intervention costs | |||
| 25% decrease | 0 | 0.0012 | 0 |
| 25% increase | 22 | 0.0012 | 18,440 |
| Persistence with treatment | |||
| 30%, rather than 60% | 34 | 0.0006 | 56,667 |
| Adherence with treatment | |||
| 50%, rather than 80% | 57b | 0.0004 | 142,500 |
| Bisphosphonate price | |||
| 100% increase | 31 | 0.0012 | 25,833 |
| 200% increase | 51 | 0.0012 | 42,500 |
| 500% increase | 111 | 0.0012 | 92,500 |
| Effect of bisphosphonate 35% fracture reduction | 31 | 0.0008 | 38,750 |
| Discount rate (rather than 3%) | |||
| 0% discount rate | −30 | 0.0020 | nab |
| 1% discount rate | −13 | 0.0016 | nab |
| 5% discount rate | 26 | 0.0009 | 28,889 |
| Mortality benefit (11% reduction) | 12 | 0.0021 | 5714 |
| Increased mortality 1 year post-spine fracture | 11 | 0.0013 | 8462 |
QALYs quality-adjusted life years, ICER incremental cost-effectiveness ratio
aCosts are expressed in constant 2014 Canadian dollars (multiply by 0.905 to convert to 2014 US dollars [28])
bThe ICER is not applicable since the intervention dominates usual care, i.e., the intervention is less costly and more effective
Of note, there are some scenarios over which CaB administrators might have direct influence and could improve cost-effectiveness. Namely, if “other” fractures were excluded from case-finding, then incremental costs would be reduced to 0, while the incremental effectiveness would improve slightly. Similarly, decreasing intervention costs by 25% by reducing the time spent on detailed risk assessment could also reduce incremental cost to 0. Indeed, the two-way sensitivity analysis showed, by simultaneously excluding “other” fractures and reducing intervention costs by 25%, that incremental cost would decline to −$11 per patient (i.e., CaB would be cost-saving) and the incremental effect could increase to 0.0014 QALYs. Thus, instead of being very cost-effective, the CaB intervention would in fact “dominate” usual care.
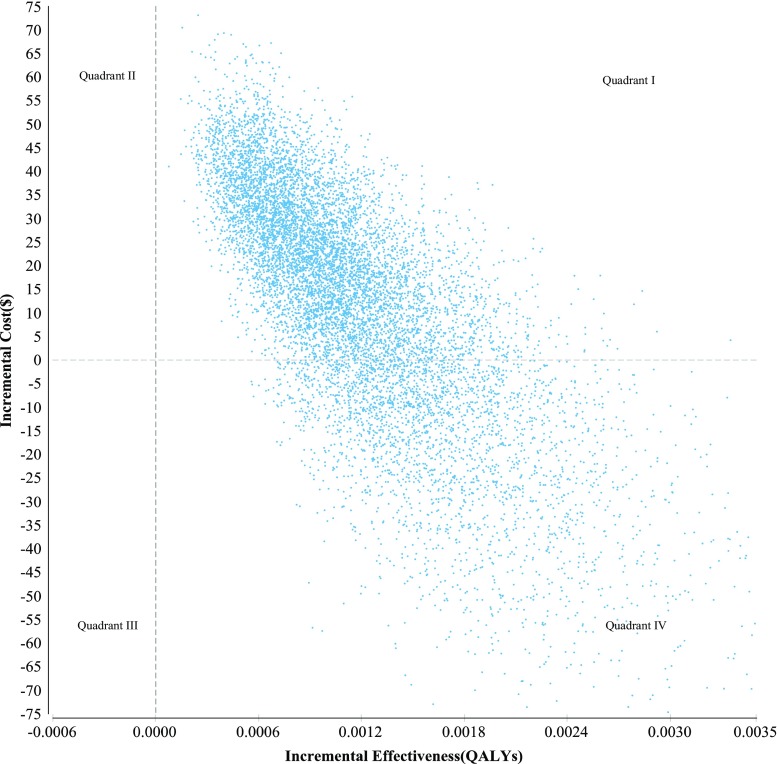
Probabilistic sensitivity analysis
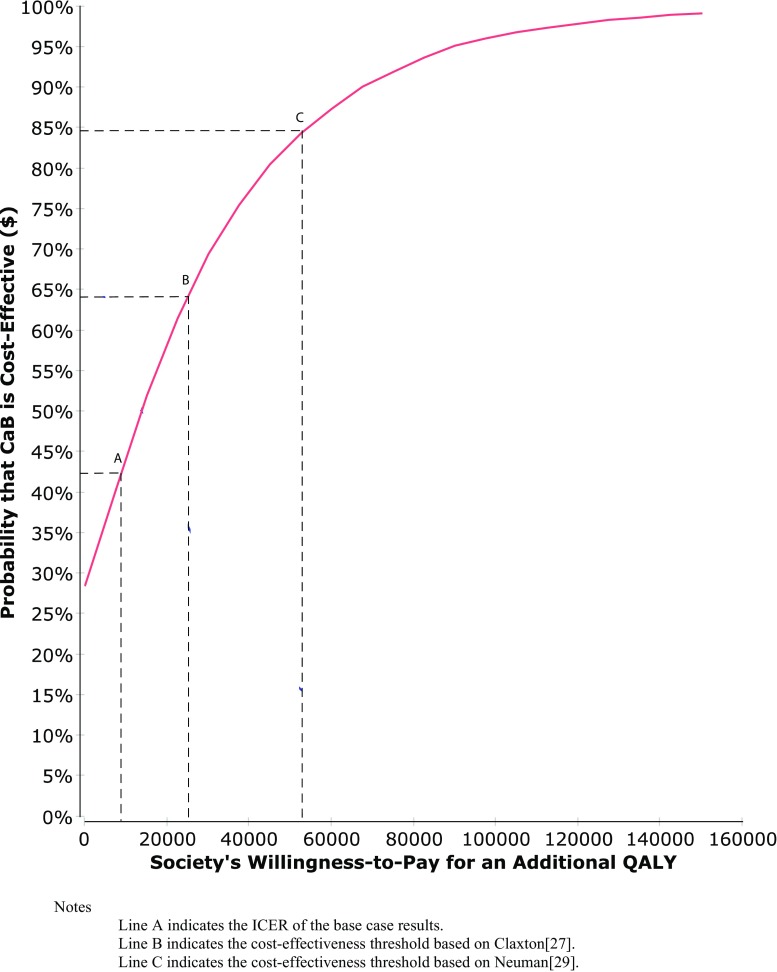
The majority of simulations (71%) fell in Quadrant I (Fig. 1), indicating that the intervention costs more than the usual care but was also more effective. The remainder of the simulations (29%) fell into Quadrant IV, indicating that the intervention was dominant, i.e., less costly and more effective than usual care. The cost-effectiveness acceptability curve for the intervention (red curve Fig. 2) indicates that the intervention would not be cost-effective if society’s willingness-to-pay was set at 0, i.e., the intervention must be cost-neutral or cost-saving to be adopted. At the origin, where the threshold is 0, the probability that the intervention is cost-effective is only 29%. However, the estimated ICER for the intervention is $9167 and lower than either of the thresholds described earlier. Specifically, based on the uncertainty contained in our model, the probability of the intervention being cost-effective is 64% in relation to the $24,000 threshold (B), but 85% if the $55,000 threshold (C) were deemed appropriate.
Fig. 1.
Incremental cost-effectiveness scatter plot
Fig. 2.
Cost-effectiveness acceptability curve. Notes: Line A indicates the ICER of the base case results. Line B indicates the cost-effectiveness threshold based on Claxton [27]. Line C indicates the cost-effectiveness threshold based on Neumann [29]
Discussion
Catch a Break is an ongoing evidence-informed 1i (=type C) FLS intervention designed to identify patients with fragility fracture at high risk of osteoporosis and alert both the patient and their family physician to the need for more urgent management of osteoporosis. Based on earlier randomized trials and recent meta-analysis, CaB would be expected to increase osteoporosis treatment rates by about 16% (95%CI 7–25); when implemented in the real world on a population-wide basis, CaB increased treatment rates by about 4% at a cost of approximately $44 per patient. Though it was both somewhat less effective and somewhat more expensive than prior reports of similar interventions, our economic analysis suggests that the program is still cost-effective. Specifically, for every 10,000 participants who entered CaB, we would expect to prevent 14 major osteoporotic fractures (4 fewer hip fractures) and gain 12 additional QALYs at a cost of $9167 per QALY gained. These findings were robust to numerous deterministic and probabilistic sensitivity analyses and CaB would be considered cost-effective in more than 80% of simulations at conventional societal willingness-to-pay thresholds.
These findings were, analytically, most sensitive to assumptions related to treatment (its costs, adherence, and persistence) and discounting rates. On the other hand, in terms of what those who actually run CaB have influence over, we found that by reducing intervention costs by 25% (perhaps by decreasing efforts on detailed osteoporosis risk assessments) or excluding patients with “other” fractures (consistent with available evidence [20] and other programs [16, 17]) or doing both simultaneously would improve cost-effectiveness substantively; the latter approach would yield cost-savings and CaB would dominate usual care.
Although it is difficult to compare different interventions and programs in terms of their cost-effectiveness, there are at least four similar Canadian programs (or trials) [14–16, 47]. Three of the interventions were similar in structure to CaB, although all three of these studies demonstrated greater effectiveness in terms of increasing treatment rates and (on a per patient basis) cost less than the CaB approach, and all three of these studies were cost-saving and would “break even” within 2–4 years of implementation [14–16]. The other study, the Ontario Fracture Clinic Screening program [47], was more effective at increasing treatment than CaB (increasing treatment rates by about 20% vs usual care) but also costs more than twice per patient of what CaB costs ($97 vs $44) and so it was less cost-effective at $19,132 per QALY gained [47]. But this is still “cost-effective” by conventional standards, and those authors reported that if their program were enabled to order BMD tests and send the results to family physicians (“fast track,” essentially converting their 1i [= type C] model to a 2i [= type B] model), it would be even more cost-effective ($5720 per QALY gained) [47].
Despite some strengths, there are several limitations of our work that ought to be noted. First and foremost, our estimates of effectiveness are not based on randomized trial evidence but rather based on 1 year of follow-up of CaB participants compared with usual care simulations. That said, the estimate of effect is actually smaller than that predicted by meta-analysis (95%CI ranging from 7 to 25%), suggesting the absence of any over-inflation of benefits that might be encountered in an uncontrolled study. Second, although not a limitation of our economic analysis per se, patients hospitalized with hip fractures were excluded altogether and we do not know participant’s BMD or what their long-term rates of recurrent fracture are as we only had 1 year of programmatic data and not lifetime data. Third, all assumptions that we made, by design, were very conservative (e.g., no mortality benefit from bisphosphonates, no increased bone loss over time without treatment, no mortality reduction associated with preventing vertebral fractures) and biased against the intervention, and this is perhaps best exemplified by our choice of discount rate. Although we used the recommended real discount rate of 3% [34], we believe this is quite high in the present low interest rate environment. Since the stream of costs related to the intervention is more weighted toward the present than usual care, a lower discount rate (e.g., 1%) would result in the intervention dominating usual care. Fourth, we did not include up-front setup or training costs for CaB on the one hand nor on the other hand did we include indirect costs such as lost productivity or informal caregiving. Last, even though population-based and consistent with 1i (= type C) FLS endorsed by ASBMR, IOF, and OC, some may be concerned that the results may not be applicable to other nations or jurisdictions.
In conclusion, a population-based implementation of a 1i (=type C) FLS improved treatment of patients with osteoporosis and over the long run would be expected to reduce recurrent fractures, increase quality of life, and be cost-effective under a wide range of assumptions and across different societal thresholds for willingness to pay. More effective and less expensive programs would be expected to be even more cost-effective and perhaps even cost-saving, but this remains to be seen.
Acknowledgements
Supported by Alberta Innovates (STOP-PRIHS grant). SRM holds the Endowed Chair in Patient Health Management supported by the Faculties of Medicine and Dentistry and Pharmacy and Pharmaceutical Sciences at the University of Alberta. LAB is supported by the Canadian Institutes of Health Research (New Investigator) and Alberta Innovates (Population Health Investigator).
Appendix
Compliance with ethical standards
Conflicts of interest
None.
References
- 1.Majumdar SR. A T-2 translational research perspective on interventions to improve post-fracture osteoporosis care. Osteoporos Int. 2011;22(suppl 3):S471–S476. doi: 10.1007/s00198-011-1700-4. [DOI] [PubMed] [Google Scholar]
- 2.Papaioannou A, Leslie WD, Morin S, for the Scientific Advisory Council of Osteoporosis Canada 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2010;182:1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NOF clinicians’ guide to the prevention and treatment of osteoporosis. National Osteoporosis Foundation, 2014 (http://www.nof.org/professionals/cliniciansguide)
- 4.Hopkins RB, Burke N, Keyserlingk CV, Leslie WD, Morin SN, et al. The current economic burden of illness of osteoporosis in Canada. Osteoporos Int. 2016;27:3023–3032. doi: 10.1007/s00198-016-3631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khosla S, Shane E. A crisis in the treatment of osteoporosis. J Bone Miner Res. 2016;31:1485–1487. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 6.International Osteoporosis Foundation Capture the Fracture: best practices framework for fracture liaison services (http://www.capturethefracture.org, last Accessed Feb 1, 2017)
- 7.American Society of Bone and Mineral Research Task Force on Secondary Fracture Prevention: making the first fracture the last fracture (http://www.online library.wiley.com/doi/10.002/jbmr.1698/suppinfo, last Aaccessed Feb 1, 2017) [DOI] [PubMed]
- 8.Osteoporosis Canada: make the first break the last with fracture liaison service (http://www.osteoporosis.ca /fracture-liaison-service /?utm_source=Home+Page& utm_medium=Menu+Button&utm_campaign=FLS, last Accessed Feb 1, 2017)
- 9.Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int. 2013;24:393–406. doi: 10.1007/s00198-012-2090-y. [DOI] [PubMed] [Google Scholar]
- 10.Majumdar SR. Osteoporosis quality improvement interventions: when are the results worth the effort? CMAJ. 2012;184:279–280. doi: 10.1503/cmaj.111999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juby A, Bayne T, Yuksel N, Hanley DA. Using an existing telephone health information service to improve the diagnosis and treatment of osteoporosis post fragility fracture. Age Ageing. 2011;40(suppl 2):115. [Google Scholar]
- 12.Majumdar SR, Rowe BH, Folk D, Johnson JA, Holroyd BH, et al. A controlled trial to increase detection and treatment of osteoporosis in older patients with a wrist fracture. Annals Intern Med. 2004;141:366–373. doi: 10.7326/0003-4819-141-5-200409070-00011. [DOI] [PubMed] [Google Scholar]
- 13.Majumdar SR, Johnson JA, McAlister FA, Bellerose D, Russell AS, et al. Multifaceted intervention to improve osteoporosis diagnosis and treatment in patients with recent wrist fracture: a randomized controlled trial. CMAJ. 2008;178:569–575. doi: 10.1503/cmaj.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majumdar SR, Johnson JA, Lier DA, et al. Persistence, reproducibility, and cost-effectiveness of an intervention to improve the quality of osteoporosis care after a fracture of the wrist. Osteoporos Int. 2007;18:261–270. doi: 10.1007/s00198-006-0248-1. [DOI] [PubMed] [Google Scholar]
- 15.Majumdar SR, Lier DA, Rowe BH, Russell AS, McAlister FA, Maksymowych WP, Hanley DA, Morrish DW, Johnson JA. Cost-effectiveness of a multifaceted intervention to improve quality of osteoporosis care after wrist fracture. Osteoporos Int. 2011;22:1799–1808. doi: 10.1007/s00198-010-1412-1. [DOI] [PubMed] [Google Scholar]
- 16.Majumdar SR, Lier DA, Leslie WD. Cost effectiveness of two inexpensive post-fracture osteoporosis interventions: results of a randomized trial. J Clin Endocrinol Metab. 2013;98:1991–2000. doi: 10.1210/jc.2013-1034. [DOI] [PubMed] [Google Scholar]
- 17.Leslie WD, LaBine L, Klassen P, Dreilich D, Caetano PA. Closing the gap in postfracture care at the population level: a randomized controlled trial. CMAJ. 2012;184:290–296. doi: 10.1503/cmaj.111158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letourneau S. Health Link Alberta: a model for successful health service integration. Healthcare Quarterly. 2009;13(Supp):56–60. doi: 10.12927/hcq.2009.21099. [DOI] [PubMed] [Google Scholar]
- 19.Alberta Bone and Joint Health Institute, Alberta Health Services. Catch a Break: preliminary (1 year) report. Calgary, Alberta, Canada; 2016
- 20.Morin SN, Lix LM, Leslie WD. The importance of previous fracture site on osteoporosis diagnosis and incident fractures in women. J Bone Miner Res. 2014;29(7):1675–1680. doi: 10.1002/jbmr.2204. [DOI] [PubMed] [Google Scholar]
- 21.Sonnenberg F, Beck R. Markov models in medical decision-making: a practical guide. Med Decis Mak. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 22.Tosteson AN, Jonsson B, Grima DT, O'Brien BJ, Black DM, Adachi JD (2001) Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporos Int 12:849–857 [DOI] [PubMed]
- 23.Johnell O, Jönsson B, Jönsson L, Black D. Cost effectiveness of alendronate for the treatment of osteoporosis and prevention of fractures. PharmacoEconomics. 2003;21:305–314. doi: 10.2165/00019053-200321050-00002. [DOI] [PubMed] [Google Scholar]
- 24.Zethraeus N, Borgström F, Ström O, Kanis J, Jönsson B. Cost-effectiveness of the treatment and prevention of osteoporosis—a review of the literature and a reference model. Osteoporos Int. 2007;18:9–23. doi: 10.1007/s00198-006-0257-0. [DOI] [PubMed] [Google Scholar]
- 25.Strom O, Zethraeus N, Borgström F, Johnell O, Jönsson B, Kanis J. IOF cost-effectiveness reference model: background document. International Osteoporosis Foundation (http://www.iofbonehealth.org, last Accessed Feb 1, 2017)
- 26.Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. Third. Oxford: Oxford University Press; 2005. [Google Scholar]
- 27.Claxton K, Martin S, Soares M, Rice N, Spackman, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1–504. doi: 10.3310/hta19140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bank of Canada. Daily noon exchange rates: 10 year lookup. (http://www.bankofcanada.ca /rates/exchange/10-year-lookup , Accessed on Feb 1, 2017)
- 29.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 30.Hodsman AB, Leslie WD, Tsang JF, Gamble GD. 10-year probability of recurrent fractures following wrist and other osteoporotic fractures in a large clinical cohort: an analysis from the Manitoba Bone Density Program. Arch Intern Med. 2008;168:2261–2267. doi: 10.1001/archinte.168.20.2261. [DOI] [PubMed] [Google Scholar]
- 31.Maclean C, Newberry S, Maglione M, McMahon M, Ranganath V. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 32.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 33.Majumdar SR, McAlister FA, Johnson JA, Bellerose D, Siminoski K, et al. Interventions to increase osteoporosis treatment in patients with “incidentally” detected vertebral fractures on chest radiographs. Am J Med. 2012;125:929–936. doi: 10.1016/j.amjmed.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 34.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 35.Government of Alberta. Drug benefits list. Edmonton, Alberta, Canada. 2013
- 36.Alberta Health Care Insurance Plan. Schedule of medical benefits (procedures and price list). Edmonton, AB, Canada; 2012
- 37.Schousboe JT, Nyman JA, Kane RL, Ensrud KE. Cost-effectiveness of alendronate therapy for osteopenic postmenopausal women. Ann Intern Med. 2005;142:734–741. doi: 10.7326/0003-4819-142-9-200505030-00008. [DOI] [PubMed] [Google Scholar]
- 38.Canadian Institute for Health Information. CMG+ Client Tables. 2014. (https://secure.cihi.ca/estore/productFamily.htm?locale=en&pf=PFC2544&lang=fr&media=0 Accessed on Feb 1, 2017)
- 39.Canadian Institute for Health Information. DAD resource intensity weights and expected length of stay. Ottawa, ON, Canada; 2010. (http://www.cihi.ca/cihi-ext-portal/internet/en/document/ standards and data submission/standards/case mix/bul_31may11_d, last Accessed Feb 1, 2017)
- 40.Alberta Health and Wellness. Continuing care—accommodation charges. (http://www.health.alberta.ca/services/continuing-care-accommodation-charges.html, last Accessed Feb 1, 2017)
- 41.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 42.Statistics Canada(2013) Life tables—Canada, provinces and territories, 2009–2011 (84–537- X). Ottawa, Ontario, Canada
- 43.Peasgood T, Herrmann K, Kanis JA, Brazier JE. An updated systematic review of health state utility values for osteoporosis related conditions. Osteoporos Int. 2009;20:853–868. doi: 10.1007/s00198-009-0844-y. [DOI] [PubMed] [Google Scholar]
- 44.Patrick AR, Schousboe JT, Losina E, Solomon DH. The economics of improving medication adherence in osteoporosis: validation and application of a simulation model. J Clin Endocrinol Metab. 2011;96:2762–2770. doi: 10.1210/jc.2011-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanis JA, Cooper C, Hiligsmann M, Rabenda V, Reginster JY, Rizzoli R (2011) Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int 22:2565–2573 [DOI] [PubMed]
- 46.Bolland MJ, Grey AB, Gamble GD, Reid IR (2010) Effect of osteoporosis treatment on mortality: a metaanalysis. J Clin Endocrinol Metab 95:1174-81 [DOI] [PubMed]
- 47.Yong JH, Masucci L, Hoch JS, Sujic R, Beaton D (2016) Cost-effectiveness of a fracture liaison service—a realworld evaluation after 6 years of service provision. Osteoporos Int 27:231–240 [DOI] [PubMed]