Abstract
Acute kidney injury and fluid overload (FO) are associated with increased mortality in critically ill patients, including the subset supported with extracorporeal membrane oxygenation (ECMO). The indication for and method of application of renal support therapy (RST) during ECMO is largely unknown beyond single-center experiences. The current study uses a survey design to document practice variation regarding RST, including indication, method of interface with the ECMO circuit, and prescribing practices. Sixty-five international ECMO centers (31%) responded to an online electronic survey regarding RST during ECMO. Nearly a quarter of centers (23%) reported using no RST during ECMO. Among those using the therapy, the predominant mode of therapy applied was convection and included slow continuous ultrafiltration and continuous veno-venous hemofiltration. The predominant indication for RST was the treatment (43%) or prevention (16%) of FO. Nephrology rather than critical care medicine is reported as the prescribing service in a majority of centers with a significant difference between US centers and non-US centers. The results of this study identify a wide variation in practice regarding RST during ECMO that will offer multiple important avenues for further research by this group and others regarding the interface of RST and ECMO.
INTRODUCTION
Acute kidney injury (AKI) and the need for renal supportive therapies (RST) are both associated with significant negative effects on outcomes in critically ill adults and children.1–10 Additionally, cumulative fluid overload (FO) during critical illness is associated with mortality and may represent a surrogate of kidney injury.11–22 Among critically ill patients on extracorporeal membrane oxygenation (ECMO) support, AKI and FO are associated with worse outcome.23–33 Despite this known association, remarkably little is known about patients on ECMO receiving concomitant RST for AKI or FO. The majority of published data are from single-center experiences that may not be extrapolated accurately to the larger ECMO population. Of note are the fundamental questions surrounding RST such as the mode employed, the indication for RST, and the method of interfacing with the ECMO circuit.
During ECMO, continuous RST is most often accomplished by including an in-line hemodialysis filter as a shunt off the main ECMO circuit or connecting a conventional continuous RST machine to the circuit.24,31,34 Both convection and diffusion have been studied as modes of RST in the critically ill population, with little clear advantage of one therapy over the other demonstrated to date, despite superior convective “middle molecule” clearance. In the ECMO population, the majority of reported RST has used convective clearance.24,31,35 Little is known about the indication for RST employed by centers durring ECMO beyond single-center experiences.
The Extracorporeal Life Support Organization (ELSO) Registry contains voluntarily reported data on demographics, laboratory and diagnostic information, and clinical course for neonates, children, and adults treated with ECMO around the world. The ELSO data forms do not include data regarding the indication for RST, but simply if it was employed. Because of the lack of data available on RST during ECMO, management is based on local and regional expert opinion, most likely leading to significant variability in practice. This hinders the ability to evaluate the effect of RST on ECMO and other outcomes as well as to evaluate the efficacy of AKI and FO as indications for RST.
The Kidney Intervention During Extracorporeal Membrane Oxygenation study group has been formed to systematically answer questions regarding the use of RST during ECMO. An initial objective of the study group was to understand current RST practice among the international ECMO community. This is a necessary first step toward eventually suggesting a systematic approach toward caring for these patients and ultimately improving outcomes of children receiving RST on ECMO. The goal of this study was to describe RST practice variation during ECMO, specifically regarding the mode employed, indication for RST, and the method of interfacing with the circuit.
METHODS
Study Design and Recruitment
We performed a cross-sectional survey of center practices with regard to RST during ECMO. Individuals subscribing to the ECLSNet ListServe (eclsnet@rufus.origenbio.com), were eligible. ECLSNet ListServe is an electronic forum for information sharing among participating centers. Membership to ECLSNet is voluntary and limited to health care professionals who are members of, or associated with, an institution that is a member of the ELSO. The site is hosted outside of the ELSO administration. An e-mail recruiting participants was posted on the ECLSNet ListServe on March 20, 2010 and included a link to the survey as well as the closure date of the study as April 15, 2010. The e-mail requested only one response per institution to be completed by the program coordinator or medical director. To enhance response rate, a subsequent reminder e-mail was sent out April 1, 2010. No incentive was provided for participation. The questionnaire was anonymous, with no personal data collected except for country location and role of the respondent in ECMO care. Institutional Review Board approval for the study was obtained at the host institution (Vanderbilt University School of Medicine, Nashville, TN) before implementation.
Survey Design
A 29 question web-based survey was created using REDCap electronic data capture tools hosted at Vanderbilt University School of Medicine.36 The survey was created using a modified Delphi technique with the multiple members of the research group as the surveyed experts. Survey questions were designed by one author (G.M.F.) and distributed for comments and modification within the study group. After several similar revisions, the final questionnaire was approved and piloted by all authors. The questionnaire was not tested for reliability or generalizability, but content validity is derived from the method of creation using the modified Delphi method among experts in the field. The survey was designed using branching logic where questions presented are dependent upon answers to the previous question, allowing for an expandable survey dependent upon the responses.
Survey Description
The full survey is available in Appendix A and takes approximately 15 minutes to complete. The survey included center-specific information such as the respondent’s role and the location by continent. Extracorporeal membrane oxygenation related data included age group and indication for ECMO among patients cared for at the institution. Questions specific to RST requested the mode employed, the indication for RST, the prescribing physician group for RST, the method of interface with the ECMO circuit, and equipment used. For questions regarding mode (interface with the circuit and indication for RST), respondents were asked two separate questions, one regarding the range of modes employed and one regarding the mode employed most often.
Statistical Analysis
All variables were categorical and expressed as frequencies and percentages. Comparisons among groups were performed using the chi-square test. Analysis was performed using STATA SE statistical software package (College Station, TX).
RESULTS
Description of ECMO Centers and ECMO Indications
A total of 65 centers subscribing to ECLSnet responded. ECLSnet was unable to provide us with what proportion of the 210 ELSO centers (according to the ELSO website: http://www.elso.med.umich.edu/) currently subscribe to ECLSnet. Therefore, the response rate was estimated as at least 31% (65) of 210 international ELSO centers of which 52 (80%) were US sites (38.2% of published US ELSO centers), 3 (4.6%) were Canadian (30% of published Canadian ELSO centers), 7 (10.8%) were European (13% of published European ELSO centers), and 3 (4.6%) were from Australia or New Zealand (33% of published Australia/New Zealand centers). Of the 65 respondents, 51 (78.5%) were program coordinators, 6 (9.2%) were program directors, and 8 (12.3%) were categorized as “other”. The majority of centers cared for non-adult patients on ECMO; 61 (94%) centers reported caring for neonatal or pediatric patients, 26 (40%) cared for adults on ECMO of which 2 (3%) centers cared exclusively for adults. Thirty (46%) centers reported that their patients had an equal distribution of cardiac and respiratory indications for ECMO, 18 (27.7%) reported the majority indication was cardiac support only, 16 (24.6%) reported the majority indication was respiratory support only, and 1 center did not provide data.
RST Indications and Prescription
Fifteen centers (23%) reported not using any RST during ECMO, and these respondents did not progress through the remainder of the survey as a result of branching logic design. The most frequently reported indications for RST were the treatment of FO (43%), prevention of FO (16%), AKI (35%), electrolyte disturbances (4%), and “other” (2%). As shown in Figure 1, there was a tendency for non-US sites to perform RST more frequently for AKI (46.2% vs. 30.2%), but the difference was not statistically significant (p = 0.5). The predominant indication for RST differed among groups based on the center’s predominant ECMO indication; however, these differences did not reach statistical significance with p > 0.05. Among centers reporting predominant cardiac support, AKI was the most frequent indication for RST, whereas the indication of FO was most frequently reported among centers providing both respiratory and cardiac support (Figure 2).
Figure 1.
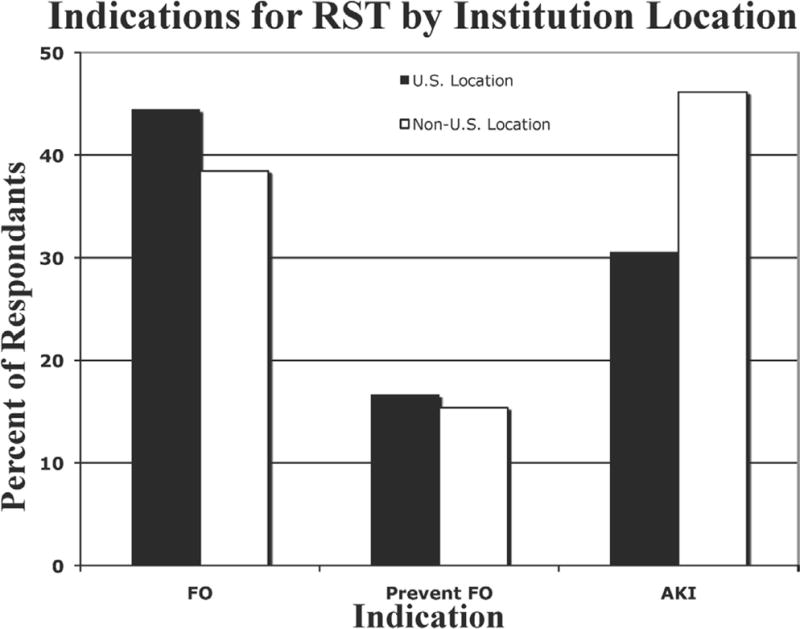
Indications for renal support therapy by location.
Black bars, US center; white bars, non-US center; FO, fluid overload; AKI, acute kidney injury.
Figure 2.
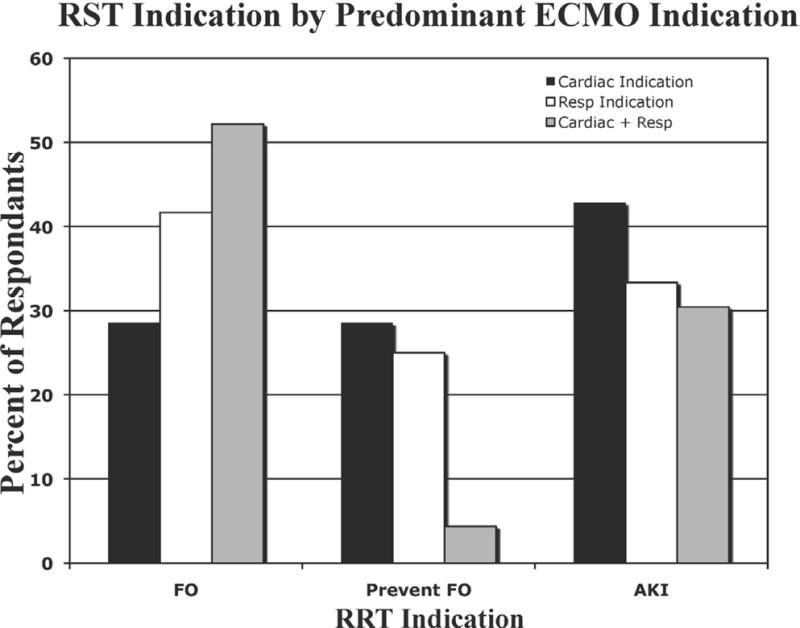
Indications for renal support therapy by predominant indication for ECMO.
Black bars, cardiac; white bars, respiratory; gray bars, cardiac + respiratory; FO, fluid overload; AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation.
Of the centers that reported using RST during ECMO, 63% responded that nephrology authored the RST prescription, 33% of centers reported critical care medicine services wrote the prescription, and 4% described a dual authorship. Centers in the US were much more likely than non-US centers (83.3 vs. 11.1%, p < 0.001) to report that nephrology authored RST prescriptions. Only 74% of centers reported nephrology routinely following patients on RST during ECMO, with US centers more commonly involving the nephrology service (US vs. non-US nephrology consultations were 94.4% and 5.6% respectively, p < 0.001). In centers where the critical care medicine service authored prescriptions, 27.8% also reported nephrology consultation.
RST Modality
The predominant RST modality reported was convection and included continuous veno-venous hemofiltration (CVVH) (43.4%) and slow continuous ultrafiltration (SCUF) (18%). The modality used was affected by method of interface with the ECMO circuit with SCUF predominating in the in-line filter group (47%) and CVVH in the machine interface group (47%) (Figure 3). Of note, 35% of centers using an in-line hemodiafilter reported using CVVH as the primary modality. Among the centers using in-line filters 83% reported using standard intravenous (IV) pumps for ultrafiltrate control, with 17% using scales.
Figure 3.
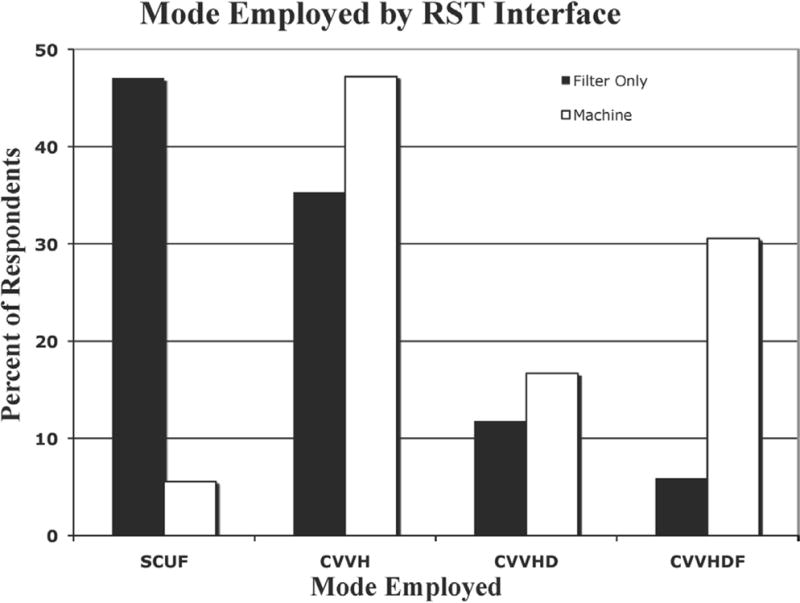
Mode of RST by interface with the ECMO circuit.
Black bars, in-line hemodiafilter; white bars, RST machine connected to circuit; SCUF, slow continuous ultrafiltration; CVVH, continuous veno-venous hemofiltration; CVVHD, continuous venovenous hemodialysis; CVVHDF, continuous veno-venous hemodiafiltration; ECMO, extracorporeal membrane oxygenation; RST, renal support therapy.
RST Interface
Fourteen (21.5%) centers exclusively used an in-line hemodiafilter and 33 (50.8%) centers exclusively used a continuous RRT (CRRT) machine connected to the circuit. For the 17 (26%) centers that routinely included a hemodiafilter in the circuit construction, 53% used a polysulfone membrane. Of the 36 centers that used a machine during ECMO, 30 (83%) used a Gambro (Lakewood, CO) product including the Prisma (25%) and PrismaFlex (58%). These machines were connected to the venous limb only of the ECMO circuit in 42% of centers, to the arterial limb (pre-oxygenator) in 33% of cases, with the remainder representing a blend of connection sites dependent upon ECMO pump type.
DISCUSSION
These survey data summarize several aspects of practice variations that exist in RST use during ECMO, which were previously unreported in the literature. First, nearly one quarter of responding centers report using no RST during ECLS, which is interesting given that AKI incidence may be as high as 72% in certain subgroups and underestimated by current definitions employed in the ELSO registry.37,38 No data were collected during this study to provide further insight into this finding. The authors postulate this may represent multiple factors including lack of recognition of AKI, belief that RST contributes to AKI and is consciously avoided, or lack of support for RST in the intensive care unit. The survey questions used the term “Acute Renal Failure” regarding indications for RST, but did not provide any specific definition that may have led to under-reporting of AKI among respondents. A currently implemented change in the ELSO data registry is the collection of serum-creatinine data on all patients in the registry. This will allow identification of AKI by newer consensus definitions in all patients, thus enabling a comparison of outcomes among those with AKI but who do not receive RST.
For centers using RST during ECMO, use of an in-line hemodiafilter and IV pumps to monitor and manage ultrafiltration rates was common. Although no current published data exist regarding the accuracy of in-line CRRT machines during ECMO, published data show that inaccuracies with IV pumps may be as high as 40% when used to control CVVH.39 Slow continuous ultrafiltration remains a frequent modality for RST during ECMO; however, this therapy modality carries the risk of significant monitoring error and is at risk for producing electrolyte derangements and rapid volume shifts in the ECMO population less than 10 kg. The incidence and prevalence of these complications will be elucidated by the newer data collection tool through the registry that specifically examines patient complications during RST on ECMO.
Indication for RST varied by center location as well as indication for ECMO. It appears that AKI is a problem more predominantly appreciated in the cardiac support group, likely reflecting the documented incidence of renal injury associated with cardiopulmonary bypass. Fluid overload is a more prevalent indication for RST in the respiratory support group, perhaps driven by the group’s desire to control FO and hasten recovery from acute respiratory distress syndrome. Of note, 20% of centers performing RST during ECMO reported “preventing FO” as the indication for RST. This suggests that despite the lack of class Ia evidence that early initiation of RST may prevent complications, there is a prevalent belief that early prevention of FO may prevent significant pulmonary or other complications. This may be driven by several recent studies demonstrating that FO in critically ill patients and in those receiving CRRT is associated with higher mortality even when controlling for severity of illness.11–16,20,29,31,33 Future studies will need to elucidate the definition of FO in the ECMO population and its link to AKI.
Limitations of this study are related to the nature of an anonymous survey posted in a listserv environment. The e-mail was written in English with no translation available, and posted on a listserv with English-language predominance. Additionally, individual centers were not targeted as part of the survey, hence response rates were lower than might have been achieved with targeted recruitment. Finally, survey questions may produce biased results if respondent interpretation differed from the authored intent or if lack of definitions for terms such as “Acute Renal Failure” or “Fluid Overload” led to underreporting because of lack of recognition among respondents.
Despite these limitations, clear themes emerged from this work. First, nearly one quarter of centers report not using any RST during ECMO despite a significant incidence of AKI during ECMO. Second, there is significant practice variation in how clinicians prescribe and perform RST during ECMO with additional differences in the perceived indications for therapy between US and non-US centers. These variations are important to demonstrate before devoting significant effort and resources toward standardizing practice and more detailed evaluation/research. However, the variety in practice is not surprising given the scant amount of data available to guide best practice. No consensus exists regarding optimal mode or dose of RST in the critically ill population. Fluid overload has become a more widely recognized indication for RST in recent years whereas consensus definitions of AKI have increased recognition of organ dysfunction. Yet significant barriers likely still exist among practitioners with regard to the application of RST to all patients with AKI and FO because of under-recognition or disbelief of the data. Another important learning point is that to achieve the eventual goals of RST standardization and performing clinical trials to compare different modes of RST for AKI treatment during ECMO, it will be necessary to arrive at a consensus, ideally at an international level, for knowledge translation and application to be successful. The level of practice variation we have documented greatly supports the recent addition of a renal module to the ELSO registry data forms, which will include data regarding AKI, FO, and details on RST modality used. This information will enable us to better understand the effect of RST and of renal dysfunction during ECMO on outcomes, thereby allowing for goal-directed and targeted future studies to standardize practice and improve ECMO outcomes.
APPENDIX Renal Replacement Therapy during ECLS


References
- 1.Bagshaw SM, George C, Bellomo R. ANZICS Database Management Committee: A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 2.Bailey D, Phan V, Litalien C, et al. Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8:29–35. doi: 10.1097/01.pcc.0000256612.40265.67. [DOI] [PubMed] [Google Scholar]
- 3.Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD. Pediatric acute renal failure: outcome by modality and disease. Pediatr Nephrol. 2001;16:1067–1071. doi: 10.1007/s004670100029. [DOI] [PubMed] [Google Scholar]
- 4.Chang JW, Tsai HL, Wang HH, Yang LY. Outcome and risk factors for mortality in children with acute renal failure. Clin Nephrol. 2008;70:485–489. doi: 10.5414/cnp70485. [DOI] [PubMed] [Google Scholar]
- 5.D’Onofrio A, Cruz D, Bolgan I, et al. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail. 2010;16(Suppl 1):S32–S36. doi: 10.1111/j.1751-7133.2010.00170.x. [DOI] [PubMed] [Google Scholar]
- 6.Lassnigg A, Schmid ER, Hiesmayr M, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36:1129–1137. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 7.Moghal NE, Brocklebank JT, Meadow SR. A review of acute renal failure in children: incidence, etiology and outcome. Clin Nephrol. 1998;49:91–95. [PubMed] [Google Scholar]
- 8.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 9.Uchino S, Kellum JA, Bellomo R, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 10.Symons JM, Chua AN, Somers MJ, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol. 2007;2:732–738. doi: 10.2215/CJN.03200906. [DOI] [PubMed] [Google Scholar]
- 11.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie RS, Seidel K, Symons JM. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;19:1394–1399. doi: 10.1007/s00467-004-1655-1. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein SL, Currier H, Graf CD, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous veno-venous hemofiltration. Pediatrics. 2001;107:1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 15.Michael M, Kuehnle I, Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. 2004;19:91–95. doi: 10.1007/s00467-003-1313-z. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest. 2000;117:1749–1754. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- 18.Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008;12:169. doi: 10.1186/cc6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagshaw SM, Cruz DN. Fluid overload as a biomarker of heart failure and acute kidney injury. Contrib Nephrol. 2010;164:54–68. doi: 10.1159/000313721. [DOI] [PubMed] [Google Scholar]
- 20.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators: A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuller D, Mitchell JP, Calandrino FS, Schuster DP. Fluid balance during pulmonary edema. Is fluid gain a marker or a cause of poor outcome? Chest. 1991;100:1068–1075. doi: 10.1378/chest.100.4.1068. [DOI] [PubMed] [Google Scholar]
- 22.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2011 doi: 10.1097/PCC.0b013e31822882a3. July 14 epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Adolph V, Heaton J, Steiner R, Bonis S, Falterman K, Arensman R. Extracorporeal membrane oxygenation for nonneonatal respiratory failure. J Pediatr Surg. 1991;26:326–30. doi: 10.1016/0022-3468(91)90511-q. discussion 330. [DOI] [PubMed] [Google Scholar]
- 24.Sell LL, Cullen ML, Whittlesey GC, Lerner GR, Klein MD. Experience with renal failure during extracorporeal membrane oxygenation: treatment with continuous hemofiltration. J Pediatr Surg. 1987;22:600–602. doi: 10.1016/s0022-3468(87)80107-0. [DOI] [PubMed] [Google Scholar]
- 25.Weber TR, Connors RH, Tracy TF, Jr, Bailey PV, Stephens C, Keenan W. Prognostic determinants in extracorporeal membrane oxygenation for respiratory failure in newborns. Ann Thorac Surg. 1990;50:720–723. doi: 10.1016/0003-4975(90)90669-w. [DOI] [PubMed] [Google Scholar]
- 26.Zwischenberger JB, Nguyen TT, Upp JR, Jr, et al. Complications of neonatal extracorporeal membrane oxygenation. Collective experience from the Extracorporeal Life Support Organization. J Thorac Cardiovasc Surg. 1994;107:838–48. discussion 848. [PubMed] [Google Scholar]
- 27.Duncan BW, Hraska V, Jonas RA, et al. Mechanical circulatory support in children with cardiac disease. J Thorac Cardiovasc Surg. 1999;117:529–542. doi: 10.1016/s0022-5223(99)70333-8. [DOI] [PubMed] [Google Scholar]
- 28.Zahraa JN, Moler FW, Annich GM, Maxvold NJ, Bartlett RH, Custer JR. Venovenous versus venoarterial extracorporeal life support for pediatric respiratory failure: are there differences in survival and acute complications? Crit Care Med. 2000;28:521–525. doi: 10.1097/00003246-200002000-00039. [DOI] [PubMed] [Google Scholar]
- 29.Meyer RJ, Brophy PD, Bunchman TE, et al. Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med. 2001;2:238–242. doi: 10.1097/00130478-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Shaheen IS, Harvey B, Watson AR, Pandya HC, Mayer A, Thomas D. Continuous venovenous hemofiltration with or without extracorporeal membrane oxygenation in children. Pediatr Crit Care Med. 2007;8:362–365. doi: 10.1097/01.PCC.0000269378.76179.A0. [DOI] [PubMed] [Google Scholar]
- 31.Hoover NG, Heard M, Reid C, et al. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med. 2008;34:2241–2247. doi: 10.1007/s00134-008-1200-y. [DOI] [PubMed] [Google Scholar]
- 32.Askenazi D. Acute Kidney Injury and Renal Replacement Therapy independently predict mortality in neonatal and pediatric non-cardiac patients on extracorporeal membrane oxygenation. Pediatr Criti Care Med. 2011;12:e1–6. doi: 10.1097/PCC.0b013e3181d8e348. [DOI] [PubMed] [Google Scholar]
- 33.Paden ML, Warshaw BL, Heard ML, Fortenberry JD. Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:153–158. doi: 10.1097/PCC.0b013e3181e2a596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santiago MJ, Sánchez A, López-Herce J, et al. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int. 2009;76:1289–1292. doi: 10.1038/ki.2009.383. [DOI] [PubMed] [Google Scholar]
- 35.Heiss KF, Pettit B, Hirschl RB, Cilley RE, Chapman R, Bartlett RH. Renal insufficiency and volume overload in neonatal ECMO managed by continuous ultrafiltration. ASAIO Trans. 1987;33:557–560. [PubMed] [Google Scholar]
- 36.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ELSO. ECLS Registry Report: International Summary. Ann Arbor, MI: Extracorporeal Life Support Organization; 2010. [Google Scholar]
- 38.Smith AH, Hardison DC, Worden CR, Fleming GM, Taylor MB. Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J. 2009;55:412–416. doi: 10.1097/MAT.0b013e31819ca3d0. [DOI] [PubMed] [Google Scholar]
- 39.Sánchez C, López-Herce J, García E, Moreno de Guerra M, Moral R, Carrillo A. Continuous venovenous renal replacement therapy using a conventional infusion pump. ASAIO J. 2001;47:321–324. doi: 10.1097/00002480-200107000-00004. [DOI] [PubMed] [Google Scholar]


