Abstract
Declines in cardiovascular deaths have been dramatic for men but occur significantly less in women. Among patients with symptomatic ischemic heart disease (IHD), women experience relatively worse outcomes compared with their male counterparts. Evidence to date has failed to adequately explore unique female imaging targets and their correlative signs and symptoms of IHD as major determinants of IHD risk. We highlight sex-specific anatomic and functional differences in contemporary imaging and introduce imaging approaches that leverage refined targets that may improve IHD risk prediction and identify potential therapeutic strategies for symptomatic women.
Keywords: imaging, prognosis, sex, women
For more than 2 decades, population case fatality rates for cardiovascular (CV) disease have been higher for women compared with men (1). Recent declines in CV deaths in men have been dramatic; yet declines are significantly less for women than men (2,3). The term ischemic heart disease (IHD) now broadly includes higher risk status associated with symptomatic patients with obstructive and nonobstructive coronary artery disease (CAD), including coronary microvascular disease (CMD) (4). Among patients with IHD, women experience relatively worse outcomes ranging from stable angina to acute coronary syndromes (ACS) and heart failure compared with men (5–8). Determining sex-specific causality has been elusive because series often include only women (9), are invasive coronary angiographic series (6,10), or include cohorts of women with attempted case-matching to men, thus limiting identification of a unique female risk profile (11). For example, the National Institutes of Health National Heart, Lung, and Blood Institute–sponsored Women’s Ischemia Syndrome Evaluation (WISE) included only symptomatic women undergoing a variety of ischemia and other physiological testing without comparative assessments of male patients (9). The ensuing selection and other biases represent sizable challenges to uncover sex-specific findings that may explain the higher risk status of women with IHD compared with men. Evidence to date fails to explore unique female imaging targets and their correlative signs and symptoms of IHD as major determinants of IHD risk. This paper highlights sex-specific anatomic and functional differences across imaging targets and introduces contemporary imaging approaches that leverage refined targets that may improve IHD risk prediction and identify potential therapeutic strategies for symptomatic women.
LIMITATIONS OF DEMAND ISCHEMIA TESTING IN WOMEN
Traditional diagnostic approaches for the assessment of risk associated with IHD are derived from the notion that identification of the consequences of flow-limiting stenosis(es) in major epicardial coronary arteries represents the major mechanism for ischemia. Accordingly, this concept is extended to clinical practice guidelines and appropriate use criteria (12,13). Furthermore, these approaches depend on a patient’s ability to exercise and an accurate assessment pre-test probability of obstructive CAD to guide test selection. Most integrated risk scores poorly categorize women as to their pretest CAD likelihood, with variable point values assigned to risk factors resulting in an over- or underestimation of CV risk (14). Moreover, women commonly present with more atypical, less exertional symptoms, which confound candidate selection and accurate assessment of pre-test risk. Importantly, a sizable proportion of women are unable to exercise maximally (those with prevalent obesity, diabetes, and orthopedic limitations), which may contribute to the lower reported sensitivity of the stress electrocardiogram (29 studies, 62% sensitivity) than stress imaging tests such as stress echocardiography (14 studies, 79% sensitivity) and single-photon emission computed tomography (SPECT) (14 studies, 81% sensitivity) from a recent meta-analysis (15). In addition to a reduced diagnostic accuracy of the exercise electrocardiogram alone for epicardial CAD, equivocal results are frequent and lead to physician uncertainty and contribute to further, perhaps unnecessary, testing of women (16).
Moreover, the traditional diagnostic goal for symptomatic women and men in whom IHD is suspected has been the detection of obstructive CAD requiring revascularization. It is now clear that this search for a functionally limiting obstructive stenosis is at a mismatch with the much greater prevalence of nonobstructive CAD in women versus men (17). For many years, this has led to the misperception of a high rate of “false-positive” (i.e., abnormal stress test results with nonobstructive CAD) findings for women. According to a recent systematic review, the range of abnormal test findings in the setting of nonobstructive CAD is 16% to 32% for stress testing using electrocardiography, nuclear, echocardiography, and cardiac magnetic resonance (CMR) (18). Conventional stress imaging also has technical artifact issues related to breast tissue, obesity, and lung disease with poor exercise capacity, further contributing to reduced test accuracy (4). For women, the misperception of a high “false-positive” rate may prompt greater uncertainty and inaction on the part of the treating physician. Documented ischemia on stress testing for women is rarely followed by intensification or alterations in anti-ischemic therapies or referral to coronary angiography (19). Compared with men, women consistently receive less intensive care, including fewer antianginal medications, less frequent coronary angiography or revascularization, and fewer lifestyle or risk factor–modifying treatments (20–23). Even when accounting for sex differences in risk factor prevalence, smaller body size, higher bleeding risk, and other factors, women have decidedly worse outcomes after coronary revascularization, particularly in the near term. The lack of symptom-driven care for women with demonstrable ischemia is a contributor to their worsening IHD outcomes. In addition, at 1 year after the index evaluation, nearly 40% of symptomatic women have persistent or worsening symptoms (24). The extent to which our diagnostic evaluation is not tailored to women may be at the core of suboptimal care. However, there also likely remains an unexplained residual gap in knowledge with regard to treatment effectiveness and strategies of care optimized for women with IHD. Additional imaging markers not in use in our contemporary diagnostic evaluation may hold promise to improve identification of high-risk women.
SEX-SPECIFIC ATHEROSCLEROTIC PLAQUE VULNERABILITY
Decades of data demonstrate that the culprit ACS lesion often occurs in a previously documented nonobstructive stenosis, revealing that there is much to learn regarding ischemia and atherosclerotic plaque as contributors to symptoms and future IHD risk (25). Coronary thrombosis is the most common precursor of ACS (26,27), and evidence supports unique sex-specific mechanisms of ACS, including differences in plaque rupture, erosion, and calcified nodules (26,28,29). Plaque rupture is more common in men with culprit lesions exhibiting atherosclerotic plaque features including thin-cap fibroatheroma (thin fibrous caps with a large thrombogenic lipid-rich necrotic core), positive remodeling, and a high plaque burden (27,28,30–32). More unique to women is the plaque erosion as a precursor of ACS (33–36), which has been variably associated with more fibrous plaque (p < 0.001), less thin-cap fibroatheroma (p < 0.001), a lower plaque burden (p = 0.003), and a reduced remodeling index (p = 0.003) (26,33–37). These data support a sex-specific etiology for ACS, underscoring the importance of varying plaque features unique to women compared with men. Results identifying unique atherosclerotic plaque features as precursors of worsening or unstable symptoms have direct applicability to the pool of female candidates undergoing evaluation for suspected IHD. Importantly, atherosclerotic plaque features associated with ACS are also reported in ~50% of stable IHD patients (38,39). The extent to which other imaging markers may be female specific and whether the totality of risk is uniquely defined in women are unknown.
ADVANCED IMAGING APPROACHES TO UNIQUELY IDENTIFY RISK IN WOMEN
A plethora of novel technology was introduced in the past decade that may improve IHD risk assignment in women. These approaches are reviewed as anatomic visualization of atherosclerotic plaque and the extent and severity of obstructive CAD and provocative imaging to demonstrate myocardial ischemia. Ancillary markers as well as combined anatomic with functional parameters imaged sequentially or using hybrid technology are also discussed.
ANATOMIC IMAGING TO DETECT IHD RISK IN WOMEN
X-RAY ANGIOGRAPHY
Invasive coronary angiography has been the traditional endpoint of a diagnostic evaluation for IHD patients. Nonobstructive CAD (i.e., <50% stenosis) is more common in symptomatic women than men (4,17,40). From the American College of Cardiology CathPCI Registry (N = 375,886 [~50% women] from >600 hospitals), nearly one-half of black, Hispanic, Native American, Asian, and white women with stable IHD had nonobstructive CAD (6). A recent statement from the American College of Cardiology CV Disease in Women Committee focused on redefining the term nonobstructive CAD to incorporate the burden of atherosclerotic plaque and vascular dysfunction, which is increasingly cited as contributory to symptom provocation in women (17).
Our current strategy for women and men with demonstrated ischemia has been to obtain a follow-up invasive angiogram. Yet the high rate of nonobstructive CAD on invasive angiography coupled with infrequent clinical measurement of atherosclerotic plaque provides an opportunity for alternative noninvasive approaches that may be more efficient and effective. Invasive coronary angiography yields greater inefficiency as it is reimbursed at a rate substantially higher than coronary computed tomography angiography (CTA) or magnetic resonance angiography (MRA) and is ineffective because there is a low diagnostic yield with invasive angiography (41) (frequent documentation of <50% stenosis) and a 3% to 4% procedural complication rate (e.g., vascular bleed, heart failure, ACS, or death) (6). Given the low diagnostic yield of current approaches leading to invasive angiography (41) and the limitations of lumenography, particularly for women, the concept of noninvasive angiography examining beyond coronary stenosis to include prevalent atherosclerotic plaque features (38,39,42,43) is worthy of exploration. Coronary CTA and MRA may detail atherosclerotic plaque extent and severity more easily than routine x-ray angiography (Figure 1), an important advantage when recognizing the distinct vascular biology of CAD in women.
FIGURE 1. Invasive Angiogram With Nonobstructive CAD.
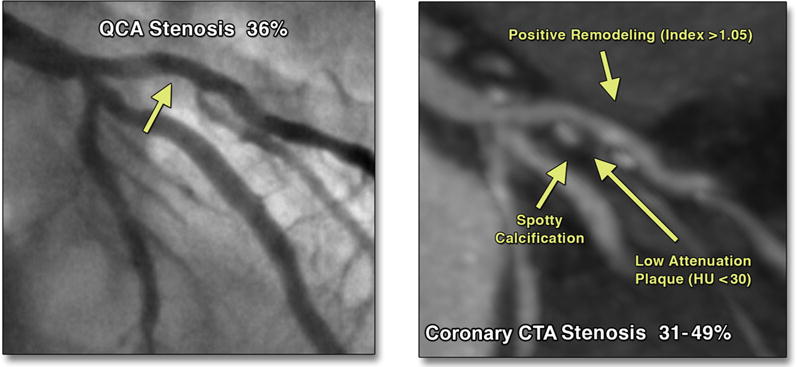
Analysis using QCA. (Left) Coronary computed tomography angiography (CTA) evidence of high-risk plaque including positive remodeling, spotty calcification, and low-attenuation plaque; Hounsfield units (HU) <30. (Right) The invasive angiography reveals mild CAD, whereas coronary CTA identifies high-risk plaque features in the same patient with mild CAD. CAD = coronary artery disease; QCA = quantitative coronary angiography.
CORONARY COMPUTED TOMOGRAPHY ANGIOGRAPHY
Coronary CTA has emerged as a noninvasive option for coronary angiography and atherosclerotic plaque characterization (30,44) including a host of features from luminal narrowing and plaque location, burden, remodeling, and composition. Contemporary coronary CTA technology has improved spatial and temporal resolution and volume coverage, allowing for acquisition of motion-free images of the heart. Coronary CTA has demonstrated excellent accuracy for the detection of obstructive CAD (45–47) and coronary plaque compared with intravascular ultrasound with sensitivity and specificity measures ≥90 (44,48–50).
There is now well-established evidence of the proportional increase in major adverse event risk by the number of arteries with obstructive CAD seen on coronary CTA (51,52), consistent with invasive data (53). From a large series (N = 23,854), coronary CTA nonobstructive CAD was associated with an elevated mortality risk (Figure 2) (51). In several large registries, the number of vessels with nonobstructive plaque had a 2- to 6-fold elevated risk of death (52,54–56). Thus, there is also a graded increase in risk based on the number of vessels or segments with identifiable nonobstructive plaque observed with coronary CTA or invasive coronary angiography (57,58). Among patients with evidence of calcified plaque, major adverse events were reported more often in those with co-occurring noncalcified plaque (59,60). Moreover, in 1,102 patients with nonobstructive CAD, the 6-year death rate was 1.4% for calcified plaque, 3.3% for mixed plaque, and 9.6% for noncalcified plaque (p < 0.0001) (61). Similar to invasive series, women have less obstructive CAD seen on coronary CTA (6,14,62,63) but have a higher mortality risk with multivessel CAD (51). Data are available with regard to sex differences in atherosclerotic plaque (11,51,63,64), with most using visual (nonquantitative) plaque assessment (11,51,64). The number of nonobstructive plaques predicted death in women (p = 0.003), even when adjusting for obstructive CAD, findings not replicated in men (65). From the CONFIRM (COronary CT EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) registry, nonobstructive CAD was associated with an approximately 2-fold increased major adverse event risk in women (11). As well, 5-year outcomes were higher for women than men with 2 to 3 vessels with plaque (51). Coronary artery calcium (CAC) scoring was also recently evaluated in a subset of symptomatic CONFIRM patients with nonobstructive CAD (64). The vast majority (86%) of patients with no luminal stenosis (i.e., 0%) had a CAC score <10. By comparison, among patients with a stenosis >0% but <50%, mortality increased in proportion with the CAC score, ranging from 0.8% to 9.8% for CAC scores of 0 to ≥400 (p < 0.0001). The CAC score in this symptomatic patient subset of 2,820 was independently predictive of all-cause mortality (p < 0.0001) and death or myocardial infarction (MI) (p < 0.0001), even in models controlling for CAD risk factors and presenting symptoms. These pioneering coronary CTA investigations added to intravascular ultrasound data (66–68) by examining patients with no previous CAD and identifying atherosclerotic plaque from coronary CTA akin to that defined invasively. This evidence supports a largely unmeasured plaque burden and a lack of detail on sex-specific patterns of atherosclerosis. However, it is unknown whether high-risk atherosclerotic plaque features (e.g., low-attenuation plaque, noncalcified plaque, arterial remodeling) are uniquely different in women compared with men.
FIGURE 2. Unadjusted 3-Year Survival for Women Versus Men by the Extent of CAD by Coronary CTA.
Comparative all-cause survival estimates from the CONFIRM (COronary CT EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) registry in women and men. In women and men, nonobstructive CAD was associated with worsening survival. Reprinted with permission from Min et al. (51).
MAGNETIC RESONANCE ANGIOGRAPHY
Contemporary MRA techniques for atherosclerotic imaging have advanced considerably (69). In addition to anatomic imaging, Hays et al. (70) optimized phase contrast magnetic resonance (MR)–based coronary artery flow measurement coupled with simple hand grip exercise to demonstrate abnormal coronary endothelial function compared with control subjects in a majority female cohort with nonobstructive CAD. Remarkably little has been published to date on advanced MRA-based plaque characterization to analyze sex differences in CAD, highlighting opportunities to fill important knowledge gaps (71).
ADVANCES IN STRESS IMAGING FOR WOMEN
Evidence is robust regarding the role of inducible perfusion or wall motion abnormalities, impaired flow reserve, and other imaging markers as predictive of major adverse event risk, with more limited evidence of unique risk estimates among women (7,40,72,73). In this section, we highlight recent advances in imaging, including echocardiography, CMR, SPECT, and positron emission tomography (PET) that have incorporated a multiparametric approach to uniquely identify women at elevated IHD risk.
ECHOCARDIOGRAPHY
In women presenting acutely with chest pain, in the absence of diagnostic electrocardiographic or biomarker evidence of ischemia, echocardiography remains a valuable and readily available bedside test, facilitated by progressive miniaturization (74). In addition to ruling out wall motion abnormalities, echocardiography is valuable for screening for disease entities including hypertensive, hypertrophic, and Takotsubo cardiomyopathies. The use of myocardial contrast echocardiography may help to facilitate recognition of the latter, reflecting the contribution of microvascular disease (75).
Most of the work on sex-specific benefits of echocardiography has focused on the ability of stress echocardiography to recognize anatomically defined coronary disease with a higher sensitivity and specificity than nonimaging tests (15). Although the use of myocardial perfusion stress echocardiography increases the accuracy of this test, event rates (driven by revascularization) were significantly higher in men after an abnormal real time myocardial contrast echocardiography (men, 35%; women, 16%, p = 0.02) (76). In a small series, Doppler-derived measures of impaired coronary flow velocity reserve (77) have been shown to be prognostically significant, although not specifically studied in women (78). In 1 report of 369 octogenarians (58% women), the all-cause mortality rates were 9.8% and 3.7% among patients with a coronary flow velocity reserve ≤1.93 and >1.93 (p = 0.001) (79).
Exercise echocardiography as an initial screening test can also be particularly beneficial for the recognition of noncoronary heart disease. The assessment of diastolic dysfunction and especially the recognition of a likely abnormal filling pressure response to exercise may explain nonspecific symptoms, including dyspnea, which may be attributable to coronary as well as noncoronary heart disease (80). The detection of abnormal myocardial mechanics (assessed using tissue Doppler imaging and speckle-tracking echocardiography) may facilitate the recognition of myocardial dysfunction as an explanation of exercise intolerance (81). The extent to which this finding may be due to CMD in women remains undefined.
CARDIAC MAGNETIC RESONANCE
Due to high spatial resolution, a lack of limitation by body habitus and windows, a lack of ionizing radiation, and high diagnostic accuracy, stress MR is well suited to evaluate suspected IHD in women.
Dobutamine CMR remains a useful test for ischemia (82,83), with particular utility for women with poor acoustic windows (84). Increasing availability of rapid high-resolution techniques has made vasodilator perfusion imaging the first-line stress CMR modality. In the CMR and SPECT for diagnosis of coronary heart disease (CE-MARC [Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease]) single center trial (n = 752) (85), CMR demonstrated accuracy superior to that of SPECT. From a predefined secondary analysis of CE-MARC, the CMR examination, which included as MRA, function, perfusion, and scar imaging, had high sensitivity for CAD detection in both women and men (89% vs. 86%, p = 0.57), with similar specificity measurements (>80%) (86). However, the sensitivity of SPECT was significantly lower in women than men in this study (51% vs. 71%, p = 0.007). The higher diagnostic performance of stress CMR compared with SPECT (area under the curve, 0.76 ± 0.04 vs. 0.63 ± 0.05; p = 0.033) was also demonstrated by the MR-IMPACT II (A Study of Gadodiamide Injection in Myocardial Perfusion Magnetic Resonance Imaging) study (87). Specifically in women, stress perfusion CMR has a high accuracy (87%, n = 147) (88) and prognostic value (hazard ratio: ~50, n = 168) (89).
Advanced techniques to evaluate perfusion and metabolism appear promising. Semiquantitative evaluation can generate a myocardial perfusion reserve index whose lower value in 118 symptomatic women and 21 reference subjects from the WISE (Women’s Ischemia Syndrome Evaluation) project predicted ≥1 abnormal invasive coronary reactivity testing variable (90). Abnormal myocardial flow reserve on vasodilator stress CMR in symptomatic women may indicate CMD (91). Dark rim artifact can be problematic when distinguishing from true perfusion defects in microvascular disease, which may be subendocardial. Advances in perfusion imaging may eliminate this artifact (92); until there is widespread clinical availability of such techniques, one must follow interpretive guidelines that emphasize transmurality and persistence of the perfusion defect beyond the initial frames of the first-pass acquisition to distinguish true-positive from false-positive results (93).
Mordini et al. (94) showed that fully quantitative assessment of stress perfusion MR images outperforms both semiquantitative and qualitative methods of interpretation for identification of obstructive CAD in a cohort of patients (n = 67, 33% women) referred for coronary angiography, with an endocardial-to-epicardial ratio <0.50, identifying segments with abnormal perfusion. This may be due to the ability of fully quantitative perfusion to increase linearly over a range of flow rates, enabling this method to yield even better diagnostic accuracy (95). The increase in signal-to-noise ratios and higher spatial resolution with higher field strength 3-T scanners may further increase the sensitivity of stress MR perfusion testing (96).
Blood oxygenation level–dependent (BOLD) imaging can be used to assess regional myocardial oxygenation as an assessment of microvascular dysfunction and ischemia by measuring paramagnetic deoxyhemoglobin, as reflected in the difference in signal intensity of the myocardium at rest and stress when imaged with this sequence. A study of 22 patients with obstructive CAD (≥50% stenosis on quantitative coronary angiography) showed cutoff values of stress myocardial blood flow (MBF) of <2.45 ml/min/g and a BOLD signal intensity change of <3.74% to correlate with ischemic segments on 3-T imaging. There was good correlation of ischemia using BOLD CMR and 15O-water PET (97). Many BOLD-CMR studies have limited sample sizes and lack invasive coronary reactivity testing data (98).
Exercise CMR is on the horizon as a promising modality for women who can perform exercise stress testing. A recent study evaluated 115 subjects (38% women) with treadmill exercise stress CMR and found that the presence of inducible regional wall motion abnormalities identified those at risk of future IHD events (99).
CMR spectroscopy uses MR signals from nuclei, such as phosphorus-31, to provide insights into metabolic activity. In women with chest pain and no epicardial CAD, a subgroup of subjects demonstrated an abnormal decrease in myocardial phosphocreatine-to-adenosine triphosphate ratios with hand-grip exercise, similar to that seen in those with obstructive CAD (100). At 3 years of follow-up, abnormal hand-grip testing on phosphorus-31 MR spectroscopy stress testing was predictive of increased IHD events, the majority of which were chest pain hospitalization (101). Further technological advances may enable cardiac MR spectroscopy to further elucidate metabolic dyscrasias in women with suspected IHD, affording targeted therapy.
Late gadolinium enhancement imaging is routinely included as part of the CMR examination, adding powerful diagnostic and prognostic value. In a cohort of subjects (50% women) with a low prevalence of CAD, unrecognized subendocardial or transmural MI on late gadolinium enhancement CMR was detected in 20% of subjects (102). More women had unrecognized MI (45%) compared with recognized MI (18%). In a prospective study of suspected IHD patients (34% women), CMR-detected unrecognized MI was an independent predictor of CAD mortality with a hazard ratio of 17.4 (103). Importantly, older individuals (52% women) with unrecognized MI by CMR were less likely to use anti-ischemic or risk factor–modifying therapies compared with those with recognized MIs (104). Underdetected MIs with undertreatment and intervention highlight opportunities to improve worse IHD outcomes in female patients.
Advances in CMR-based parametric mapping are rapidly being translated to assess ischemic or at-risk myocardium (105). Myocardial edema and inflammation can be targeted with quantitative T2 mapping (Figure 3) (106) to delineate ischemic and injured myocardium more reliably, overcoming the limitations of traditional T2-weighted imaging (107). T1 mapping has also been proposed as a useful technique to image myocardium at risk (108), with recent reports raising the intriguing possibility of noncontrast stress CMR with native T1 alone (109).
FIGURE 3. Myocardial Inflammation and Impaired Myocardial Perfusion Reserve by CMR.
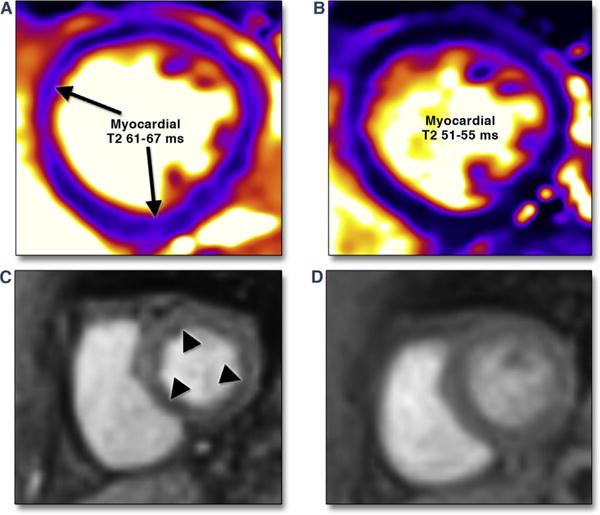
A 37-year-old female was admitted with visual changes. Serologic testing and neuroimaging yielded a diagnosis of lupus cerebritis. Significant resting tachycardia (heart rate 120 to 130 beats/min) raised concern of lupus myocarditis, confirmed by CMR examination that showed significantly elevated myocardial T2 values (A). Immunomodulatory therapy was initiated, with both improvement visual symptoms and normalization of heart rate. She returned to the hospital approximately 5 months later with chest pain and lack of inflammatory markers with a resting heart rate of 75 beats/min. ECG and serial troponin-I measurements were negative for myocardial injury. CMR showed normalized T2 values (B); vasodilator stress adenosine infusion produced severe chest pain, and concomitant first-pass perfusion imaging showed diffuse subendocardial hypoperfusion (C). Minimal residual chest pain was present upon termination of adenosine, with near-complete normalization of perfusion (D). Chest pain attributed to impaired myocardial perfusion reserve has reduced with angiotensin converting enzyme inhibitor therapy.
STRESS MYOCARDIAL PERFUSION SPECT AND PET
There is abundant evidence of the utility of stress myocardial perfusion SPECT imaging in women (4,7,19,110–115). False-positive findings in women who are obese or with large breasts reduce the diagnostic accuracy of myocardial perfusion SPECT (81% sensitivity and 78% specificity in women) (18). Studies have reported improved interpretive accuracy when integrating multiple parameters from the SPECT study, including gated left ventricular ejection fraction and wall motion findings, with the perfusion findings, which can aid in discerning true- from false-positive findings. Moreover, use of validated attenuation correction algorithms or the addition of 2-position supine/prone imaging has been reported to improve diagnostic specificity in numerous studies (116–118). Despite the limitations of SPECT, data support equivalent risk stratification by sex across population cohorts, whereby risk ranges from low for normal findings to high for moderate to severe myocardial perfusion SPECT abnormalities, and this pattern is similar for women and men (4,7,115). Evidence is also available of risk stratification in diverse cohorts of African-American and Hispanic women using myocardial perfusion SPECT (119,120). The newly introduced high-speed SPECT camera technology is capable of measuring blood flow (similar to PET) and reduced radiation exposure, marked improvements in nuclear imaging over conventional SPECT technology, which may disproportionately benefit women.
In women, stress myocardial perfusion PET has several advantages over the more commonly performed SPECT imaging including the following: 1) improved spatial and temporal resolution; 2) high diagnostic and prognostic accuracy (121–124); 3) segmentation of subepicardial and epicardial perfusion; and 4) quantification of absolute MBF and CFR (125). Computed tomography is used to generate an attenuation map of the chest for correction of breast tissue attenuation, the latter of which is a notable limitation of SPECT in women. The safety profile of PET is markedly beneficial with an effective radiation dose of ~2 to 3 mSv using the perfusion tracers rubidum-82 and ammonia-13N versus ~14 mSv for technetium-99 m SPECT (126).
There is a growing evidence base with vasodilator stress myocardial perfusion PET, and evidence supports a high diagnostic and prognostic accuracy for both women and men (122,123). The diagnostic accuracy of PET is decidedly higher than that of SPECT (88% vs. 67%, p = 0.009) (122). The evidence with stress myocardial perfusion PET reveals a directly proportional relationship between the extent and severity of stress abnormalities and major adverse CAD events including CAD mortality (Figure 4) (123,127,128). These data reveal a similar prognostic pattern with PET and SPECT, although no formal comparison has been performed.
FIGURE 4. Cumulative Cardiac Mortality Rates by the Percentage of Abnormal Stress Myocardium With Rubidium-82 PET Imaging.
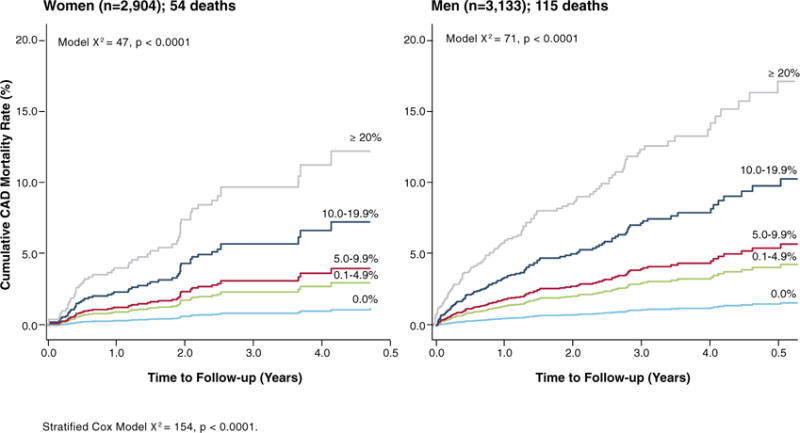
Comparative CAD mortality estimates from the PET Prognosis Registry in women and men. In women and men, the percentage of the myocardium that was abnormal was associated with a graded increase in CAD mortality. CAD = coronary artery disease; PET = positron emission tomography.
PET CFR measures absolute MBF into the perfused myocardium and is affected by the extent and severity of atherosclerotic plaque within the epicardial coronary arteries, arterial remodeling, and CMD. In patients with a reduced CFR, the frequency of nonobstructive CAD is greater in women, whereas obstructive CAD is more common in men (127). A PET CFR of ~2 or lower is a consistent threshold of higher adverse events (129,130). In short-term follow-up of ~1 year, a higher hazard ratio (~5) for a reduced CFR suggests a temporal relationship to CAD events (129). Importantly, there is a synergistic relationship among ischemia severity, CFR, and major CAD events (128,130). In patients with ischemia, a reduced CFR doubles their CAD death rate (128). CMD has been the focus of attention (131) due to the high rate of nonobstructive CAD in women compared with men. CMD is defined as chest pain and reduced CFR occurring without an obstructive epicardial stenosis (often including mild CAD and normal coronaries) and largely occurs in women. From the literature, there is an unclear distinction between atherosclerotic microvascular dysfunction with mild epicardial CAD compared with “pure” microvascular dysfunction with normal epicardial coronary arteries. These are likely 2 different manifestations of CMD but have been incompletely defined heretofore. From the WISE study, ~80% of 200 women with nonobstructive CAD had mild but notable nonstenotic atherosclerosis (132). Similarly, Lee et al. (133) reported detectable atherosclerotic plaque in 139 patients with nonobstructive CAD and evidence of endothelial dysfunction by invasive coronary reactivity testing. Thus, the extent to which epicardial nonobstructive CAD has been excluded from published findings focusing on CMD remains unclear. In 1 recent report, no prognostic difference was identified between women and men without obstructive CAD and a PET CFR <2.0 (134). This latter definition included a fair number of patients with demonstrable atherosclerotic plaque, and in a secondary analysis of patients with a CAC score of 0, no prognostic differences were identified between women and men with a PET CFR <2.0 (134). To date, no sufficiently powered series has clearly delineated flow impairment in the coronary microvasculature in the absence of nonobstructive epicardial CAD and noted sex differences.
The data are unfolding on the added parameters of absolute MBF and incorporation of coronary CTA findings as additive or interactive with myocardial perfusion parameters. Importantly, separation of the influence of nonobstructive atherosclerosis on reduced flow reserve remains an important differentiator of epicardial CAD versus CMD. If the primary determinant of risk from reduced CFR is derived from epicardial atherosclerotic plaque, then guided anti-ischemic and risk factor–modifying management approaches may prove useful for the millions of women presenting for evaluation with suspected IHD with documented nonobstructive CAD. This would allow for more fruitful research toward the use of contemporary anti-ischemic regimens as reducing IHD risk in women. Moreover, the prognostic significance of PET subepicardial ischemia, an early manifestation of ischemia specific to IHD risk in women, has not been adequately explored in the scientific literature.
Myocardial perfusion imaging technology includes a focus on expanded applications for PET CFR but also a substantial role for PET-CT imaging. Minimal data are available on the correlation of coronary CTA findings with stress myocardial perfusion ischemia, reporting that calcified and noncalcified plaque are more often associated with an abnormal myocardial perfusion scan or reduced CFR on PET (135–137). Stress myocardial perfusion abnormalities and reduced CFR occur commonly with obstructive CAD but also occur in 20% to 40% of patients with nonobstructive CAD (135–137). Thus, the burden of atherosclerotic plaque is quite substantial in patients with suspected IHD but is uncommonly measured in tandem. The evidence is robust that there is a directly proportional relationship between stress-induced abnormalities and the major adverse event risk for women and men alike (4,7,114,132). Yet, for women with documented ischemia, major adverse event rates range widely from 3% to 10% per year, supporting our contention that undetected atherosclerotic plaque features may contribute to the varying clinical event rates observed for women (7,138–140). Data suggest that the interplay of calcified, noncalcified, or mixed plaque coupled with reduced CFR may improve IHD risk detection (141). Multimodality image fusion integrating myocardial segmentation of coronary anatomy, myocardial perfusion imaging, and MBF is possible and has tremendous promise in the identification of functionally limiting atherosclerosis.
NOVEL PARADIGMS AND KNOWLEDGE GAPS
Women are differentially affected by IHD through the evolution of their symptoms from initial chest pain to ACS (Central Illustration). Although many of the data on atherosclerotic plaque features focus on its relationship with ACS, sex-specific patterns in stable IHD as links to persistent/worsening symptoms represent sentinel changes, and, perhaps, as intermediate links to ACS. More evidence is needed to support novel paradigms using state-of-the-art imaging strategies to detect and characterize IHD in women. Novel evidence is needed to develop sex-specific profiles of atherosclerotic plaque correlating with perfusion, MBF, fractional flow reserve, CFR, and other physiological measures such as shear stress (142). The emergence of fractional flow reserve computed tomography (143,144) suggests a “game-changing” approach that combines CAD imaging with hemodynamic assessment of plaque; a similar case is being made for coronary CTA with vasodilator perfusion imaging (145,146). Evidence of smaller coronary arteries and higher resting coronary artery flow in women compounds the challenges for CAD imaging and should be addressed. With integrated imaging strategies that deliver anatomic and physiological targets in diagnosis, evidence is needed to direct specific treatment strategies.
Demonstration of improved precision in diagnosis and refined imaging-guided treatment may be insufficient to support the incorporation of advanced techniques into novel evaluation paradigms without comparative effectiveness research that includes the perspectives of all relevant stakeholders including patients, advocacy experts, and clinicians. The ongoing CE-MARC 2 trial will evaluate across multiple centers the comparative effectiveness of multiparametric CMR versus SPECT (147). The randomized MR-INFORM (MR Perfusion Imaging to Guide Management of Patients With Stable CAD) trial will test the hypothesis that CMR perfusion is noninferior to invasive angiography with fractional flow reserve, based on the endpoints of death, MI, or repeat revascularization (148). Moreover, the National Institutes of Health National Heart, Lung, and Blood Institute–sponsored ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial is randomizing patients with moderate to severe ischemia (on CMR, nuclear, and echocardiography) to an initial invasive or optimal medical therapy approach. These trials are imaging-guided therapeutic strategies that target sufficient enrollment of women and will add tremendously to inform the diagnostic evaluation and treatment of women with IHD. Nevertheless, these trials focus on diagnosis and treatment of obstructive CAD and do not address the needs of women and men with nonobstructive CAD. Additional clinical trials focusing on standardized noninvasive approaches and therapeutic strategies of care for IHD women with nonobstructive CAD are warranted. One such example is the ongoing iPOWER (Improving diagnosis and treatment of women with angina pectoris and microvascular disease) registry, which examines transthoracic echocardiography during rest and dipyridamole stress with measurement of CFR by Doppler imaging of the left anterior descending artery in IHD women with nonobstructive CAD (149).
All of these questions must be addressed across the expanse of presentations associated with IHD, from stable IHD to ACS. Establishing temporal relationships among plaque characteristics, myocardial ischemia, and clinical sequelae is needed to inform our understanding of drivers of worse outcomes for women with IHD and to define allocation of societal resources toward improving outcomes for our female patients.
CENTRAL ILLUSTRATION. A Working Model of Imaging Targets Identifying Ischemic Heart Disease Risk in Women.
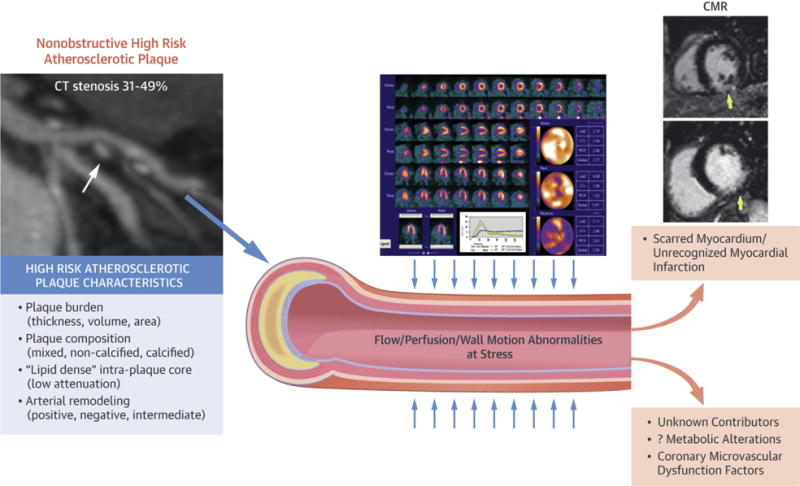
Potential influential factors contributing to high rates of nonobstructive coronary artery disease (CAD) in women. Investigations have implicated altered flow reserve and provocative ischemia as determinants of risk, even in women with nonobstructive CAD. Other factors may further refine risk including scarring and unrecognized myocardial infarction. Each of these proposed parameters may differentially affect short- or long-term outcomes in women.
Accreditation and Designation Statement.
The American College of Cardiology Foundation (ACCF) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
The ACCF designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Method of Participation and Receipt of CME Certificate.
To obtain credit for this CME activity, you must:
Be an ACC member or JACC: Cardiovascular Imaging subscriber.
Carefully read the CME-designated article available online and in this issue of the journal.
Answer the post-test questions. At least 2 out of the 3 questions provided must be answered correctly to obtain CME credit.
Complete a brief evaluation.
Claim your CME credit and receive your certificate electronically by following the instructions given at the conclusion of the activity.
Acknowledgments
Author Disclosure: Dr. Raman has received research support from Siemens Healthcare; and is a co-inventor and founding member of EXCMR. Dr. Min is a consultant for HeartFlow; is on the Scientific Advisory Board of Arineta; has ownership of MDDX and Autoplak; has a research agreement with GE Healthcare; and is the recipient of grants NIH/NIHLBI R01HL111141, NIH/NIHLBI R01HL115150, NIH/NIHLBI R01HL118019, NIH/NIHLBI U01HL105907, and NPRP09-370-3-089. Dr. Bucciarelli-Ducci is a consultant for Circle Cardiovascular Imaging. Dr. Bairey Merz has received grant support from Gilead, Practive Point, and Medscape. Dr. Ferdinand is a consultant for Amgen, Sanofi, Boehringer Ingelheim, and Eli Lilly; and has received research support from Boehringer Ingelheim. Dr. Pepine received grant UL1TR001427 from the National Center for Advancing Translational Sciences. Dr. Shaw has received the Dean’s Distinguished Faculty Award and the Albert E. Levy Scientific Research Award from Emory University; and has received grant support from the Woodruff Foundation and the Antinori Foundation, and grants NIH-NHLBI R01HL118019-02, R01HL111150, and 1U01HL10556-01; and is a past president of the American Society of Nuclear Cardiology and President-Elect of the Society of Cardiovascular Computed Tomography. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Jonathon Leipsic, MD, served as Guest Editor for this paper.
ABBREVIATIONS AND ACRONYMS
- ACS
acute coronary syndrome(s)
- BOLD
blood oxygenation level dependent
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CTA
computed tomography angiography
- CFR
coronary flow reserve
- CMD
coronary microvascular disease
- CMR
cardiac magnetic resonance
- CV
cardiovascular
- IHD
ischemic heart disease
- MBF
myocardial blood flow
- MI
myocardial infarction
- MR
magnetic resonance
- MRA
magnetic resonance angiography
- PET
positron emission tomography
- SPECT
single-photon emission computed tomography
Footnotes
CME Editor: Ragavendra R. Baliga, MD
This article has been selected as this issue’s CME activity, available online at http://www.acc.org/jacc-journals-cme by selecting the CME tab on the top navigation bar.
CME Objective for This Article: After reading this article the reader should be able to provide an updated review on advances in noninvasive stress imaging and noninvasive coronary angiography in the evaluation of women presenting with stable, suspected ischemic heart disease.
CME Editor Disclosure: JACC: Cardiovascular Imaging CME Editor Ragavendra R. Baliga, MD, has reported that he has no relationships to disclose.
Medium of Participation: Print (article only); online (article and quiz).
Go to http://www.acc.org/jacc-journals-cme to take the CME quiz for this article.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Ni H, Coady S, Rosamond W, et al. Trends from 1987 to 2004 in sudden death due to coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;157:46–52. doi: 10.1016/j.ahj.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Wang Y, Spertus JA, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–45. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mieres JH, Gulati M, Bairey Merz N, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation. 2014;130:350–79. doi: 10.1161/CIR.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 5.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–11. doi: 10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LJ, Shaw RE, Merz CN, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–75. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MB, Maddox TM, Langner P, Plomondon ME, Rumsfeld JS, Duvernoy CS. Characteristics and outcomes of women veterans undergoing cardiac catheterization in the Veterans Affairs Healthcare System: insights from the VA CART Program. Circulation Cardiovasc Qual Outcomes. 2015;8:S39–47. doi: 10.1161/CIRCOUTCOMES.114.001613. [DOI] [PubMed] [Google Scholar]
- 9.Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–61. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 10.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. J Am Coll Cardiol Img. 2012;5:S62–72. doi: 10.1016/j.jcmg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Leipsic J, Taylor CM, Gransar H, et al. Sex-based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology. 2014;273:393–400. doi: 10.1148/radiol.14140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM) Circulation. 2011;124:2423–32. doi: 10.1161/CIRCULATIONAHA.111.039255. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolor RJ, Patel MR, Melloni C, et al. Noninvasive Technologies for the Diagnosis of Coronary Artery Disease in Women. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 16.Christman MP, Bittencourt MS, Hulten E, et al. Yield of downstream tests after exercise treadmill testing: a prospective cohort study. J Am Coll Cardiol. 2014;63:1264–74. doi: 10.1016/j.jacc.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepine CJ, Ferdinand KC, Shaw LJ, et al. Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66:1918–33. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. doi: 10.1080/15360280802537332. Available at: https://effectivehealthcare.ahrq.gov/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=1132. Accessed March 4, 2016. [DOI] [PubMed]
- 19.Shaw LJ, Mieres JH, Hendel RH, et al. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation. 2011;124:1239–49. doi: 10.1161/CIRCULATIONAHA.111.029660. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LJ, Miller DD, Romeis JC, Kargl D, Younis LT, Chaitman BR. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med. 1994;120:559–66. doi: 10.7326/0003-4819-120-7-199404010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–62. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 23.Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672–93. doi: 10.1161/01.CIR.0000114834.85476.81. [DOI] [PubMed] [Google Scholar]
- 24.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–15. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 25.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–71. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 26.Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–58. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narula J, Strauss HW. The popcorn plaques. Nat Med. 2007;13:532–4. doi: 10.1038/nm0507-532. [DOI] [PubMed] [Google Scholar]
- 28.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 29.Hong MK, Mintz GS, Lee CW, et al. The site of plaque rupture in native coronary arteries: a three-vessel intravascular ultrasound analysis. J Am Coll Cardiol. 2005;46:261–5. doi: 10.1016/j.jacc.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 30.Osborn EA, Jaffer FA. Imaging atherosclerosis and risk of plaque rupture. Curr Atheroscler Rep. 2013;15:359. doi: 10.1007/s11883-013-0359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolodgie FD, Gold HK, Burke AP, et al. Intra-plaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–25. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 32.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–82. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 33.Lafont A. Basic aspects of plaque vulnerability. Heart. 2003;89:1262–7. doi: 10.1136/heart.89.10.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheifer SE, Canos MR, Weinfurt KP, et al. Sex differences in coronary artery size assessed by intravascular ultrasound. Am Heart J. 2000;139:649–53. doi: 10.1016/s0002-8703(00)90043-7. [DOI] [PubMed] [Google Scholar]
- 35.Han SH, Bae JH, Holmes DR, Jr, et al. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008;29:1359–69. doi: 10.1093/eurheartj/ehn142. [DOI] [PubMed] [Google Scholar]
- 36.Vaccarino V. Ischemic heart disease in women: many questions, few facts. Circ Cardiovasc Qual Outcomes. 2010;3:111–5. doi: 10.1161/CIRCOUTCOMES.109.925313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higuma T, Soeda T, Abe N, et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. J Am Coll Cardiol Intv. 2015;8:1166–76. doi: 10.1016/j.jcin.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Maehara A, Mintz GS, Bui AB, et al. Morphologic and angiographic features of coronary plaque rupture detected by intravascular ultrasound. J Am Coll Cardiol. 2002;40:904–10. doi: 10.1016/s0735-1097(02)02047-8. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Elvira G, Coma-Canella I, Artaiz M, Paramo JA, Barba J, Calabuig J. Characterization of coronary plaques with combined use of intravascular ultrasound, virtual histology and optical coherence tomography. Heart Int. 2010;5:e12. doi: 10.4081/hi.2010.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 41.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–26. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 43.Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66:337–46. doi: 10.1016/j.jacc.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 44.Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. J Am Coll Cardiol Img. 2011;4:537–48. doi: 10.1016/j.jcmg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 46.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 47.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 48.Cheng VY, Nakazato R, Dey D, et al. Reproducibility of coronary artery plaque volume and composition quantification by 64-detector row coronary computed tomographic angiography: an intraobserver, interobserver, and interscan variability study. J Cardiovasc Comput Tomogr. 2009;3:312–20. doi: 10.1016/j.jcct.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Rinehart S, Vazquez G, Qian Z, Murrieta L, Christian K, Voros S. Quantitative measurements of coronary arterial stenosis, plaque geometry, and composition are highly reproducible with a standardized coronary arterial computed tomographic approach in high-quality CT datasets. J Cardiovasc Comput Tomogr. 2011;5:35–43. doi: 10.1016/j.jcct.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Lehman SJ, Schlett CL, Bamberg F, et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. J Am Coll Cardiol Img. 2009;2:1262–70. doi: 10.1016/j.jcmg.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Min JK, Dunning A, Lin FY, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 52.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 53.Mark DB, Nelson CL, Califf RM, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–25. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 54.Lin FY, Shaw LJ, Dunning AM, et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58:510–9. doi: 10.1016/j.jacc.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 55.Ostrom MP, Gopal A, Ahmadi N, et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol. 2008;52:1335–43. doi: 10.1016/j.jacc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 56.Pundziute G, Schuijf JD, Jukema JW, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49:62–70. doi: 10.1016/j.jacc.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 57.Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–91. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 58.Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–63. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoo DH, Chun EJ, Choi SI, et al. Significance of noncalcified coronary plaque in asymptomatic subjects with low coronary artery calcium score: assessment with coronary computed tomography angiography. Int J Cardiovasc Imaging. 2011;27(Suppl 1):27–35. doi: 10.1007/s10554-011-9968-1. [DOI] [PubMed] [Google Scholar]
- 60.Lee MS, Chun EJ, Kim KJ, Kim JA, Yoo JY, Choi SI. Asymptomatic subjects with zero coronary calcium score: coronary CT angiographic features of plaques in event-prone patients. Int J Cardiovasc Imaging. 2013;29(Suppl 1):29–36. doi: 10.1007/s10554-013-0257-z. [DOI] [PubMed] [Google Scholar]
- 61.Ahmadi N, Nabavi V, Hajsadeghi F, et al. Mortality incidence of patients with nonobstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011;107:10–6. doi: 10.1016/j.amjcard.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 62.Grunau GL, Ahmadi A, Rezazadeh S, et al. Assessment of sex differences in plaque morphology by coronary computed tomography angiography–are men and women the same? J Women’s Health. 2014;23:146–50. doi: 10.1089/jwh.2013.4496. [DOI] [PubMed] [Google Scholar]
- 63.Otaki Y, Gransar H, Cheng VY, et al. Gender differences in the prevalence, severity, and composition of coronary artery disease in the young: a study of 1635 individuals undergoing coronary CT angiography from the prospective, multinational confirm registry. Eur Heart J Cardiovasc Imaging. 2015;16:490–9. doi: 10.1093/ehjci/jeu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah S, Bellam N, Leipsic J, et al. Prognostic significance of calcified plaque among symptomatic patients with nonobstructive coronary artery disease. J Nucl Cardiol. 2014;21:453–66. doi: 10.1007/s12350-014-9865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaw LJ, Min JK, Narula J, et al. Sex differences in mortality associated with computed tomographic angiographic measurements of obstructive and nonobstructive coronary artery disease: an exploratory analysis. Circ Cardiovasc Imaging. 2010;3:473–81. doi: 10.1161/CIRCIMAGING.109.860981. [DOI] [PubMed] [Google Scholar]
- 66.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 67.Cheng JM, Garcia-Garcia HM, de Boer SP, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMOIVUS study. Eur Heart J. 2014;35:639–47. doi: 10.1093/eurheartj/eht484. [DOI] [PubMed] [Google Scholar]
- 68.Calvert PA, Obaid DR, O’Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. J Am Coll Cardiol Img. 2011;4:894–901. doi: 10.1016/j.jcmg.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Lin K, Carr JC. MR imaging of the coronary vasculature: imaging the lumen, wall, and beyond. Radiol Clins North Am. 2015;53:345–53. doi: 10.1016/j.rcl.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hays AG, Kelle S, Hirsch GA, et al. Regional coronary endothelial function is closely related to local early coronary atherosclerosis in patients with mild coronary artery disease: pilot study. Circ Cardiovasc Imaging. 2012;5:341–8. doi: 10.1161/CIRCIMAGING.111.969691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Makowski MR, Henningsson M, Spuentrup E, et al. Characterization of coronary atherosclerosis by magnetic resonance imaging. Circulation. 2013;128:1244–55. doi: 10.1161/CIRCULATIONAHA.113.002681. [DOI] [PubMed] [Google Scholar]
- 72.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pepine CJ, Kerensky RA, Lambert CR, et al. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol. 2006;47:S30–5. doi: 10.1016/j.jacc.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 74.Testuz A, Muller H, Keller PF, et al. Diagnostic accuracy of pocket-size handheld echocardiographs used by cardiologists in the acute care setting. Eur Heart J Cardiovasc Imaging. 2013;14:38–42. doi: 10.1093/ehjci/jes085. [DOI] [PubMed] [Google Scholar]
- 75.Abdelmoneim SS, Mankad SV, Bernier M, et al. Microvascular function in Takotsubo cardiomyopathy with contrast echocardiography: prospective evaluation and review of literature. J Am Soc Echocardiogr. 2009;22:1249–55. doi: 10.1016/j.echo.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 76.Laiq Z, Smith LM, Xie F, Chamsi-Pasha M, Porter TR. Differences in patient outcomes after conventional versus real time perfusion stress echocardiography in men versus women: a prospective randomised trial. Heart. 2015;101:559–64. doi: 10.1136/heartjnl-2014-306869. [DOI] [PubMed] [Google Scholar]
- 77.Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48–62. doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- 78.Dikic M, Tesic M, Markovic Z, et al. Prognostic value of calcium score and coronary flow velocity reserve in asymptomatic diabetic patients. Cardiovasc Ultrasound. 2015;13:41. doi: 10.1186/s12947-015-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cortigiani L, Borelli L, Raciti M, et al. Prediction of mortality by stress echocardiography in 2835 diabetic and 11 305 nondiabetic patients. Circ Cardiovasc Imaging. 2015;8:e002757. doi: 10.1161/CIRCIMAGING.114.002757. [DOI] [PubMed] [Google Scholar]
- 80.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891–900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 81.Kraigher-Krainer E, Shah AM, Gupta DK, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–56. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100:1697–702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 83.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99:763–70. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 84.Raman SV, Donnally MR, McCarthy B. Dobutamine stress cardiac magnetic resonance imaging to detect myocardial ischemia in women. Prev Cardiol. 2008;11:135–40. doi: 10.1111/j.1751-7141.2008.08243.x. [DOI] [PubMed] [Google Scholar]
- 85.Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–60. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Greenwood JP, Motwani M, Maredia N, et al. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circulation. 2014;129:1129–38. doi: 10.1161/CIRCULATIONAHA.112.000071. [DOI] [PubMed] [Google Scholar]
- 87.Schwitter J, Wacker CM, Wilke N, et al. Superior diagnostic performance of perfusion-cardiovascular magnetic resonance versus SPECT to detect coronary artery disease: the secondary endpoints of the multicenter multivendor MR-IMPACT II (Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial) J Cardiovasc Magn Reson. 2012;14:61. doi: 10.1186/1532-429X-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klem I, Greulich S, Heitner JF, et al. Value of cardiovascular magnetic resonance stress perfusion testing for the detection of coronary artery disease in women. J Am Coll Cardiol Img. 2008;1:436–45. doi: 10.1016/j.jcmg.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 89.Coelho-Filho OR, Seabra LF, Mongeon FP, et al. Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient’s sex. J Am Coll Cardiol Img. 2011;4:850–61. doi: 10.1016/j.jcmg.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation Circ Cardiovasc Imaging. 2015;8:e002481. doi: 10.1161/CIRCIMAGING.114.002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doyle M, Fuisz A, Kortright E, et al. The impact of myocardial flow reserve on the detection of coronary artery disease by perfusion imaging methods: an NHLBI WISE study. J Cardiovasc Magn Reson. 2003;5:475–85. doi: 10.1081/jcmr-120022263. [DOI] [PubMed] [Google Scholar]
- 92.Salerno M, Sica C, Kramer CM, Meyer CH. Improved first-pass spiral myocardial perfusion imaging with variable density trajectories. Magn Reson Med. 2013;70:1369–79. doi: 10.1002/mrm.24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hundley WG, Bluemke D, Bogaert JG, et al. Society for Cardiovascular Magnetic Resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson. 2009;11:5. doi: 10.1186/1532-429X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mordini FE, Haddad T, Hsu LY, et al. Diagnostic accuracy of stress perfusion CMR in comparison with quantitative coronary angiography: fully quantitative, semiquantitative, and qualitative assessment. J Am Coll Cardiol Img. 2014;7:14–22. doi: 10.1016/j.jcmg.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heydari B, Kwong RY, Jerosch-Herold M. Technical advances and clinical applications of quantitative myocardial blood flow imaging with cardiac MRI. Prog Cardiovasc Dis. 2015;57:615–22. doi: 10.1016/j.pcad.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 96.Shin T, Hu HH, Pohost GM, Nayak KS. Three dimensional first-pass myocardial perfusion imaging at 3T: feasibility study. J Cardiovasc Magn Reson. 2008;10:57. doi: 10.1186/1532-429X-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karamitsos TD, Leccisotti L, Arnold JR, et al. Relationship between regional myocardial oxygenation and perfusion in patients with coronary artery disease: insights from cardiovascular magnetic resonance and positron emission tomography. Circ Cardiovasc Imaging. 2010;3:32–40. doi: 10.1161/CIRCIMAGING.109.860148. [DOI] [PubMed] [Google Scholar]
- 98.Karamitsos TD, Arnold JR, Pegg TJ, et al. Patients with syndrome X have normal transmural myocardial perfusion and oxygenation: a 3-T cardiovascular magnetic resonance imaging study. Circ Cardiovasc Imaging. 2012;5:194–200. doi: 10.1161/CIRCIMAGING.111.969667. [DOI] [PubMed] [Google Scholar]
- 99.Sukpraphrute B, Drafts BC, Rerkpattanapipat P, et al. Prognostic utility of cardiovascular magnetic resonance upright maximal treadmill exercise testing. J Cardiovasc Magn Reson. 2015;17:103. doi: 10.1186/s12968-015-0208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–35. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 101.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:2993–9. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 102.Barbier CE, Bjerner T, Johansson L, Lind L, Ahlstrom H. Myocardial scars more frequent than expected: magnetic resonance imaging detects potential risk group. J Am Coll Cardiol. 2006;48:765–71. doi: 10.1016/j.jacc.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 103.Kim HW, Klem I, Shah DJ, et al. Unrecognized non-Q-wave myocardial infarction: prevalence and prognostic significance in patients with suspected coronary disease. PLoS Med. 2009;6:e1000057. doi: 10.1371/journal.pmed.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–6. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. J Am Coll Cardiol Img. 2013;6:806–22. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verhaert D, Thavendiranathan P, Giri S, et al. Direct T2 quantification of myocardial edema in acute ischemic injury. J Am Coll Cardiol Img. 2011;4:269–78. doi: 10.1016/j.jcmg.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McAlindon EJ, Pufulete M, Harris JM, et al. Measurement of myocardium at risk with cardiovascular MR: comparison of techniques for edema imaging. Radiology. 2015;275:61–70. doi: 10.1148/radiol.14131980. [DOI] [PubMed] [Google Scholar]
- 108.Ugander M, Oki AJ, Hsu LY, et al. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268–78. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mahmod M, Piechnik SK, Levelt E, et al. Adenosine stress native T1 mapping in severe aortic stenosis: evidence for a role of the intravascular compartment on myocardial T1 values. J Cardiovasc Magn Reson. 2014;16:92. doi: 10.1186/s12968-014-0092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berman DS, Kang X, Hayes SW, et al. Adenosine myocardial perfusion single-photon emission computed tomography in women compared with men. Impact of diabetes mellitus on incremental prognostic value and effect on patient management J Am Coll Cardiol. 2003;41:1125–33. doi: 10.1016/s0735-1097(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 111.Hachamovitch R, Berman DS, Kiat H, et al. Effective risk stratification using exercise myocardial perfusion SPECT in women: gender-related differences in prognostic nuclear testing. J Am Coll Cardiol. 1996;28:34–44. doi: 10.1016/0735-1097(96)00095-2. [DOI] [PubMed] [Google Scholar]
- 112.Amanullah AM, Kiat H, Friedman JD, Berman DS. Adenosine technetium-99m sestamibi myocardial perfusion SPECT in women: diagnostic efficacy in detection of coronary artery disease. J Am Coll Cardiol. 1996;27:803–9. doi: 10.1016/0735-1097(95)00550-1. [DOI] [PubMed] [Google Scholar]
- 113.Hachamovitch R, Berman DS, Kiat H, et al. Gender-related differences in clinical management after exercise nuclear testing. J Am Coll Cardiol. 1995;26:1457–64. doi: 10.1016/0735-1097(95)00356-8. [DOI] [PubMed] [Google Scholar]
- 114.Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(Suppl):S4–20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 115.Mieres JH, Shaw LJ, Arai A, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005;111:682–96. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 116.Arsanjani R, Hayes SW, Fish M, et al. Two-position supine/prone myocardial perfusion SPECT (MPS) imaging improves visual inter-observer correlation and agreement. J Nucl Cardiol. 2014;21:703–11. doi: 10.1007/s12350-014-9895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berman DS, Kang X, Nishina H, et al. Diagnostic accuracy of gated Tc-99m sestamibi stress myocardial perfusion SPECT with combined supine and prone acquisitions to detect coronary artery disease in obese and nonobese patients. J Nucl Cardiol. 2006;13:191–201. doi: 10.1007/BF02971243. [DOI] [PubMed] [Google Scholar]
- 118.Duvall WL, Croft LB, Corriel JS, et al. SPECT myocardial perfusion imaging in morbidly obese patients: image quality, hemodynamic response to pharmacologic stress, and diagnostic and prognostic value. J Nucl Cardiol. 2006;13:202–9. doi: 10.1007/BF02971244. [DOI] [PubMed] [Google Scholar]
- 119.Shaw LJ, Hendel RC, Cerquiera M, et al. Ethnic differences in the prognostic value of stress technetium-99m tetrofosmin gated single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol. 2005;45:1494–504. doi: 10.1016/j.jacc.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 120.Cerci MS, Cerci JJ, Cerci RJ, et al. Myocardial perfusion imaging is a strong predictor of death in women. J Am Coll Cardiol Img. 2011;4:880–8. doi: 10.1016/j.jcmg.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 121.Nandalur KR, Dwamena BA, Choudhri AF, Nandalur SR, Reddy P, Carlos RC. Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad Radiol. 2008;15:444–51. doi: 10.1016/j.acra.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 122.Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13:24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 123.Kay J, Dorbala S, Goyal A, et al. Influence of sex on risk stratification with stress myocardial perfusion Rb-82 positron emission tomography: results from the PET (Positron Emission Tomography) Prognosis Multicenter Registry. J Am Coll Cardiol. 2013;62:1866–76. doi: 10.1016/j.jacc.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 124.Neglia D, Rovai D, Caselli C, et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging. 2015;8:e002179. doi: 10.1161/CIRCIMAGING.114.002179. [DOI] [PubMed] [Google Scholar]
- 125.Danad I, Raijmakers PG, Harms HJ, et al. Impact of anatomical and functional severity of coronary atherosclerotic plaques on the transmural perfusion gradient: a [15O]H2O PET study. Eur Heart J. 2014;35:2094–105. doi: 10.1093/eurheartj/ehu170. [DOI] [PubMed] [Google Scholar]
- 126.Einstein AJ, Berman DS, Min JK, et al. Patient-centered imaging: shared decision making for cardiac imaging procedures with exposure to ionizing radiation. J Am Coll Cardiol. 2014;63:1480–9. doi: 10.1016/j.jacc.2013.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taqueti VR, Everett BM, Murthy VL, et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–35. doi: 10.1161/CIRCULATIONAHA.114.009716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making J Am Coll Cardiol. 2013;62:1639–53. doi: 10.1016/j.jacc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 130.Murthy VL, Lee BC, Sitek A, et al. Comparison and prognostic validation of multiple methods of quantification of myocardial blood flow with 82Rb PET. J Nucl Med. 2014;55:1952–8. doi: 10.2967/jnumed.114.145342. [DOI] [PubMed] [Google Scholar]
- 131.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 132.Khuddus MA, Pepine CJ, Handberg EM, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23:511–9. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–60. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin F, Shaw LJ, Berman DS, et al. Multi-detector computed tomography coronary artery plaque predictors of stress-induced myocardial ischemia by SPECT. Atherosclerosis. 2008;197:700–9. doi: 10.1016/j.atherosclerosis.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 136.Naya M, Murthy VL, Blankstein R, et al. Quantitative relationship between the extent and morphology of coronary atherosclerotic plaque and downstream myocardial perfusion. J Am Coll Cardiol. 2011;58:1807–16. doi: 10.1016/j.jacc.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liga R, Marini C, Coceani M, et al. Structural abnormalities of the coronary arterial wall—in addition to luminal narrowing—affect myocardial blood flow reserve. J Nucl Med. 2011;52:1704–12. doi: 10.2967/jnumed.111.091009. [DOI] [PubMed] [Google Scholar]
- 138.Anand DV, Lim E, Hopkins D, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27:713–21. doi: 10.1093/eurheartj/ehi808. [DOI] [PubMed] [Google Scholar]
- 139.Rozanski A, Gransar H, Wong ND, et al. Clinical outcomes after both coronary calcium scanning and exercise myocardial perfusion scintigraphy. J Am Coll Cardiol. 2007;49:1352–61. doi: 10.1016/j.jacc.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 140.Handberg E, Johnson BD, Arant CB, et al. Impaired coronary vascular reactivity and functional capacity in women: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol. 2006;47:S44–9. doi: 10.1016/j.jacc.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 141.Naya M, Murthy VL, Foster CR, et al. Prognostic interplay of coronary artery calcification and underlying vascular dysfunction in patients with suspected coronary artery disease. J Am Coll Cardiol. 2013;61:2098–106. doi: 10.1016/j.jacc.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779–88. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 143.Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308:1237–45. doi: 10.1001/2012.jama.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Douglas PS, Pontone G, Hlatky MA, et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFRCT: outcome and resource impacts study Eur Heart J. 2015;36:3359–67. doi: 10.1093/eurheartj/ehv444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cury RC, Kitt TM, Feaheny K, Akin J, George RT. Regadenoson-stress myocardial CT perfusion and single-photon emission CT: rationale, design, and acquisition methods of a prospective, multicenter, multivendor comparison. J Cardiovasc Compu Tomogr. 2014;8:2–12. doi: 10.1016/j.jcct.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 146.Techasith T, Cury RC. Stress myocardial CT perfusion: an update and future perspective. J Am Coll Cardiol Img. 2011;4:905–16. doi: 10.1016/j.jcmg.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 147.Ripley DP, Brown JM, Everett CC, et al. Rationale and design of the Clinical Evaluation of Magnetic Resonance Imaging in Coronary heart disease 2 trial (CE-MARC 2): a prospective, multicenter, randomized trial of diagnostic strategies in suspected coronary heart disease. Am Heart J. 2015;169:17–24. doi: 10.1016/j.ahj.2014.10.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hussain ST, Paul M, Plein S, et al. Design and rationale of the MR-INFORM study: stress perfusion cardiovascular magnetic resonance imaging to guide the management of patients with stable coronary artery disease. J Cardiovasc Magn Reson. 2012;14:65. doi: 10.1186/1532-429X-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Prescott E, Abildstrom SZ, Aziz A, et al. Improving diagnosis and treatment of women with angina pectoris and microvascular disease: the iPOWER study design and rationale. Am Heart J. 2014;167:452–8. doi: 10.1016/j.ahj.2014.01.003. [DOI] [PubMed] [Google Scholar]


