Abstract
Objective
The objective of the study is to investigate in the hypertensive population the possible differential association between increased aortic and/or carotid stiffness and organ damage in multiple districts, such as the kidney, the vessels, and the heart.
Methods
In 314 essential hypertensive patients, carotid–femoral pulse wave velocity (cfPWV, by applanation tonometry) and carotid stiffness (from ultrasound images analysis), together with left ventricular hypertrophy, carotid intima–media thickness, urinary albumin–creatinin ratio, and glomerular filtration rate were measured. Increased cfPWV and carotid stiffness were defined according to either international reference values or the 90th percentile of a local control group (110 age and sex-matched healthy individuals).
Results
When considering the 90th percentile of a local control group, increased cfPWV was associated with reduced glomerular filtration rate, either when carotid stiffness was increased [odds ratio (OR) 13.27 (confidence limits (CL) 95% 3.86–45.58)] or not [OR 7.39 (CL95% 2.25–24.28)], whereas increased carotid stiffness was associated with left ventricular hypertrophy, either when cfPWV was increased [OR 2.86 (CL95% 1.15–7.09)] or not [OR 2.81 (CL95% 1.13–6.97)]. No association between increased cfPWV or carotid stiffness and target organ damage was found when cutoffs obtained by international reference values were used. The concomitance of both increased cfPWV and carotid stiffness did not have an additive effect on organ damage.
Conclusion
Aortic and carotid stiffness are differentially associated with target organ damage in hypertensive patients. Regional arterial stiffness as assessed by cfPWV is associated with renal organ damage and local carotid stiffness with cardiac organ damage.
Keywords: carotid arteries, hypertension, organ damage, pulse wave velocity, risk factors
INTRODUCTION
Stiffening of large arteries, mainly because of ageing, is a key feature of arteriosclerosis and cardiovascular disease [1]. Though carotid–femoral pulse wave velocity (cfPWV) is the most used and reliable method to measure aortic stiffness and is able to improve cardiovascular event prediction [2], the evaluation of local arterial stiffness on the carotid arteries is of particular interest, as it can be directly determined without the need for any assumption from models of circulation [3], it is easily accessed by ultrasound and it is evaluated in a classical site of atherosclerosis development. Moreover, the prognostic role of carotid mechanical properties has been demonstrated in patients with end-stage renal disease [4] as well as in the general population [5]. Despite hypertension being a major determinant of elastic properties of the carotid artery, the contribution of other cardiovascular risk factors in hypertensive patients [6] has been poorly investigated.
The development of target organ damage may represent a mechanism underlying the association of arterial stiffness and adverse cardiovascular events. This hypothesis is supported by a number of cross-sectional studies showing a significant association between cfPWV and subclinical coronary, peripheral arterial and cerebral target organ damage [7], as well as renal damage [8], whereas the association with left ventricular hypertrophy (LVH) is more controversial [9]. On the other side, the relationship between carotid stiffness and hypertensive target organ damage has been poorly studied [10,11]. To our knowledge, the role of combined stiffening of carotid and aortic arterial districts on target organ damage and cardiovascular prognosis has never been explored. Multisite large artery stiffness might incrementally increase hemodynamic load on target organs, thus favoring the development of extensive damage. This aspect is of relevance as cardiovascular risk factors have a differential effect on carotid and aortic stiffness. Furthermore, reference values have recently become available for both parameters [12,13], but their reliability has never been tested against established intermediate endpoints.
The aims of the study were to evaluate the prevalence of increased aortic and carotid stiffness in a cohort of hypertensive patients, defined according to the Reference Values for Arterial Measurements Collaboration, as well as in comparison with a control group enrolled on purpose in our center; and to investigate whether the presence of increased carotid stiffness, cfPWV, or both are associated with hypertensive cardiac, vascular, and renal organ damage.
METHODS
Study population
A total of 314 essential hypertensive patients consecutively referring to the Hypertension Outpatient Clinic of the University Hospital of Pisa, Italy, for target organ damage evaluation, were enrolled. Inclusion criteria were diagnosis of essential hypertension defined on the basis of SBP at least 140 mmHg, DBP at least 90 mmHg, or use of antihypertensive drugs, according to current guidelines [14]. Exclusion criteria were known secondary forms of hypertension, end-stage renal disease, and any other major comorbidities, including active neoplasm, severe hepatic insufficiency and chronic heart failure, and any other disease reducing life expectancy at less than 1 year. A group of 110 healthy, normotensive individuals, comparable for age and sex, were also recruited as controls by advertising in the hospital. The institutional ethics committee approved the study and all patients provided written informed consent before entering the study.
Experimental protocol
Study participants were requested to refer to the Hypertension Outpatient Clinic in the morning after an overnight fasting for collection of medical history and anthropometric parameters as well as blood and urine samples, brachial blood pressure (BP) measurement, cardiac and vascular ultrasound, and arterial tonometry. Patients under pharmacological treatment were asked to assume their medications as usual on the day of the experimental session.
Experimental procedures
Cardiovascular risk factors estimation
Medical history was collected for parental and personal history of cardiovascular disease and in first-degree relatives. Smoking status (current smoker/nonsmoker) was also recorded. In all patients, height, weight, and waist circumference were measured: obesity was defined when BMI more than 30 kg/m2, abdominal obesity when waist circumference was more than 102 cm for men and more than 88 cm for women. Patients were classified as having diabetes if fasting glucose was at least 7.0 mmol/l, or in the presence of glucose-lowering treatment. Dyslipidemia was defined as total cholesterol at least 5.0 mmol/l, or low density lipoprotein (LDL) cholesterol more than 3.0 mmol/l, high density lipoprotein (HDL) less than 1.2 mmol/l and less than 1.0 mmol/l for women and men, respectively, triglycerides more than 1.7 mmol/l, or current use of lipid-lowering drugs. Metabolic syndrome in hypertensive patients was defined as the concomitant presence of two or more risk factors among abdominal obesity, altered fasting plasma glucose (between 5.6 and 6.9 mmol/l) or diabetes, low HDL, or high triglycerides, as defined above. Glomerular filtration rate (eGFR) was estimated by the simplified Modification of Diet in Renal Disease equation [15] and renal function was considered impaired when eGFR was less than 60 ml/min per 1.73 m2. Microalbuminuria was defined according to European Society of Hypertension/European Society of Cardiology (ESH/ESC) 2013 Guidelines with albumin-to-creatinine ratio more than 30 mg/g [14]. Previous cardiovascular events were defined according to ESH/ESC 2013 Guidelines for hypertension management: myocardial infarction or coronary revascularization, stroke or transient ischemic events, heart failure, and symptomatic peripheral artery disease [14].
Blood pressure measurement
Brachial BP was measured with the patient resting in supine position for at least 10 min under quiet environmental conditions, performing three measurements at 2-min intervals by an automatic oscillometric device (OMRON 705IT, HEM-759-E; Omron Corporation, Kyoto, Japan). BP was taken as the average of the last two measurements.
Applanation tonometry
Arterial tonometry was performed using standardized procedures [16] according to international guidelines [3]. cfPWV was assessed by SphygmoCor device (AtCor Medical, Sydney, New South Wales, Australia), recording waveforms sequentially at the femoral and carotid site. cfPWV was calculated as the ratio of the surface distance between the two recording sites – obtained subtracting carotid–suprasternal notch from femoral–suprasternal notch distance – and pulse transit time. Two consecutive measurements were recorded and averaged.
Carotid ultrasound
Common carotid artery scans (25 frames/s) were obtained by high-resolution ultrasound with a 10 MHz linear array transducer (MyLab25; ESAOTE, Florence, Italy) by a trained operator. Longitudinal scans were acquired from each common carotid artery (1 cm proximal to the carotid bulb in a region 1-cm wide and free of plaques) and automatically analyzed with Carotid Studio (Cardiovascular Suite, Quipu srl, Pisa, Italy), a well validated system based on contour tracking algorithm [17]. Arterial interfaces were automatically detected, with estimation of instantaneous mean diameter as the distance between far and near media–adventitia interfaces. Carotid measurements were validated for accuracy and precision against the gold standard approach by radiofrequency [17]. The following parameters were obtained: carotid distension (ΔD), that is the stroke change in diameter, calculated as the difference between the systolic and diastolic diameter values; carotid distensibility coefficient = ΔA/(A * ΔP), where A represents the diastolic lumen area, evaluated from the diameter values (assuming the cross-section of the artery to be circular), ΔA represents the stroke change in lumen area, ΔP the local pulse pressure obtained by tonometry. Carotid stiffness was calculated according to the Moens–Korteweg equation [3]. In particular, carotid distensibility was converted into carotid stiffness by using the equation: PWV = [(ΔP * A)/(ΔA * r)](1/2) where r is the blood density. This formula allows carotid stiffness to be obtained as carotid stiffness = (r * carotid distensibility)(−1/2), expressed in m/s, allowing a direct comparison with cfPWV. Finally, common carotid intima–media thickness (IMT) was simultaneously measured on the same image sequences of carotid stiffness, by the same computerized automatic system [17]. All parameters were then calculated as the average of the right and left common carotid values. For the definition of increased IMT, two different thresholds were considered: 0.9 mm, as indicated in the ESH/ESC Guidelines for the management of arterial hypertension [14]; the 90th percentile of age and sex-specific reference intervals obtained in healthy individuals by echotracking techniques and calculated by the Reference Values for Arterial Measurements Collaboration [18].
Echocardiography
A single trained operator performed echocardiography to measure left ventricular (LV) dimensions, wall thickness, and mass in accordance with current American Society of Echocardiography (ASE) guidelines [19]. LV mass was indexed to body surface area. Study participants with ejection fraction less than 40% were excluded from the analysis, because of the known confounding effect of systolic performance on cfPWV [20]. LVH was defined according to ESH/ESC 2013 Guidelines with a LV mass index more than 95 g/mq in women and more than 115 g/mq in men [14].
Statistical analysis
All statistical analyses were performed using NCSS 8 (NCSS; Kaysville, Utah, USA). For normally distributed data, results were expressed as mean ± SD, whereas median value and 25–75% interquartile range was used for not normally distributed data. Differences in means between hypertensive and normotensive individuals were analyzed using analysis of variance (ANOVA) for normally distributed variables, or Kruskal–Wallis Z test for not normally distributed variables; differences in categorical variables were analyzed by χ2 test. Analysis of variance or covariance was used to compare cfPWV and carotid stiffness in the presence or absence of cardiovascular risk factors; for cfPWV, mean BP was considered as covariate. For the definition of increased cfPWV and carotid stiffness, two different thresholds were considered: first, they were considered increased when they were over the 90th percentile of the distribution obtained in the sample of healthy study participants enrolled in the present study; second, cfPWV and carotid stiffness values were also stratified according to age categories, as suggested by the Reference Values for Arterial Measurements Collaboration [12,13]. For this purpose, brachial pulse pressure (PP) instead of carotid PP was used in the formula for carotid stiffness calculation [carotid stiffness (brach)].
Multiple logistic regression was performed to identify risk factors associated with an increased cfPWV and carotid stiffness. The hypertensive population was then divided in four groups according to the presence or absence of increased cfPWV and/or carotid stiffness and logistic regression analysis was used to establish the relationship between the presence of increased cfPWV and/or carotid stiffness and target organ damage. A multiple logistic regression model, adjusted for risk factors independently associated with increased cfPWV and carotid stiffness, was then built.
RESULTS
Clinical and vascular variables
Table 1 shows the clinical characteristics of the study population. The majority of hypertensive patients (68%) were on treatment with BP-lowering drugs (median number of drugs 2 [1–4]; 30% angiotensin-converting enzyme (ACE) inhibitors, 31% AT1 receptor blockers, 27% diuretics, 23% calcium channel blockers, 13% β-blockers, 4% others). Moreover, 18% of patients were treated with statins, 13% with antiplatelet drugs, and 20% with glucose-lowering drugs.
TABLE 1.
Clinical characteristics of the study population
| Parameter | Normotensive Individuals (n = 110) | Hypertensive Patients (n = 314) |
|---|---|---|
| Age (years) | 56.4 ± 10.4 | 57.9 ± 16.1 |
| Men, number (%) | 71 (64.5) | 191 (60.8) |
| Smokers, number (%) | 24 (21.8) | 47 (15.0) |
| SBP (mmHg) | 121.7 ± 10.7 | 143.5 ± 16.3* |
| DBP (mmHg) | 70.7 ± 8.7 | 82.0 ± 10.0* |
| Pulse pressure (mmHg) | 51.0 ± 8.8* | 61.5 ± 14.3 |
| Heart rate (bpm) | 66.0 ± 8.8 | 68.5 ± 11.8 |
| BMI (kg/m2) | 23.4 (22.1–25.7) | 27.8 (25.5–31.2)* |
| Waist circumference (cm) | 94.0 ± 11.2 | 103.3 ± 13.0 |
| Blood glucose (mmol/l) | 5.1 (4.7–5.4) | 5.4 (4.8–6.7)* |
| Total cholesterol (mmol/l) | 5.6 ± 0.9 | 5.3 ± 0.9 |
| HDL cholesterol (mmol/l) | 1.7 ± 0.4 | 1.3 ± 0.4* |
| LDL cholesterol (mmol/l) | 3.3 ± 0.9 | 3.2 ± 0.9 |
| Triglycerides (mmol/l) | 1.0 (0.8–1.3) | 1.4 (1.0–2.0)* |
| Serum creatinine (mg/dl) | 0.80 ± 0.29 | 0.93 ± 0.25 |
| Estimated GFR (ml/min per 1.73 m2) | 83.1 ± 19.4 | 79.1 ± 17.7 |
| UACR (mg/g) | 2.8 (0.5–4.9) | 4.0 (0.2–12) |
BP, blood pressure; GFR, glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio.
P < 0.001 versus normotensive study participants.
Regarding vascular parameters (Table 2), hypertensive patients had higher cfPWV than normotensive individuals. PP, carotid diameter, and carotid stiffness were significantly greater in hypertensive patients as compared with healthy study participants. Common carotid IMT was also significantly increased in the patients’ group as compared with controls.
TABLE 2.
Central blood pressure and vascular parameters in the study population
| Parameter | Normotensive individuals | Hypertensive patients |
|---|---|---|
| Central SBP (mmHg) | 113.8 ± 10.4 | 131.5 ± 16.9** |
| Central DBP (mmHg) | 72.7 ± 8.6 | 83.0 ± 10.5** |
| Central pulse pressure (mmHg) | 41.1 ± 8.6 | 48.5 ± 14.0** |
| Carotid–femoral pulse wave velocity (m/s) | 7.37 ± 1.76 | 9.36 ± 2.13** |
| Carotid intima–media thickness (mm) | 0.68 ± 0.12 | 0.74 ± 0.16* |
| Mean diastolic diameter (mm) | 6.57 ± 1.45 | 7.21 ± 1.12** |
| Carotid distensibility coefficient (kPA−1 × 10−3) | 28.1 ± 7.7 | 23.7 ± 9.3* |
| Carotid stiffness (m/s) | 6.19 ± 1.13 | 6.91 ± 1.34** |
P <0.01.
P <0.001 versus normotensive study participants.
Prevalence of increased carotid stiffness and carotid–femoral pulse wave velocity
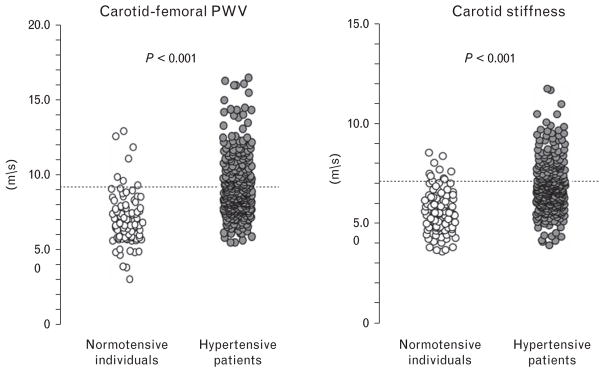
The 90th percentile of cfPWV and carotid stiffness in healthy individuals enrolled in the present study was 9.1 and 7.1 m/s, respectively (Fig. 1). Using this cutoff, we found that 46.8% of the hypertensive population had increased cfPWV and 40.8% had increased carotid stiffness. The 90th percentile of carotid stiffness (brach) in healthy individuals enrolled in the present study was 8.0 m/s: according to this cutoff, 27.2% of hypertensive patients had increased carotid stiffness (brach).
FIGURE 1.
Dot plot shows aortic pulse wave velocity and carotid stiffness in normotensive study participants and essential hypertensive patients. The dotted lines represent the values of the 90th percentile of stiffness parameters, calculated on the healthy population sample.
When increased cfPWV and carotid stiffness (brach) were defined according to the age-specific 90th percentiles of the Reference Values for Arterial Measurements Collaboration, increased cfPWV was found in 34.0% and increased carotid stiffness (brach) in 9.6% of hypertensive patients.
Association of carotid stiffness and carotid–femoral pulse wave velocity with cardiovascular risk factors and treatments
Hypertensive patients with diabetes mellitus and metabolic syndrome had higher carotid stiffness than those without these risk factors (Table 3). Carotid stiffness was significantly higher in women as compared with men but similar in patients treated or not with BP-lowering drugs (6.9 ± 1.5 versus 6.8 ± 1.3 m/s, P = 0.43) or statins (7.1 ± 1.2 versus 6.9 ± 1.4 m/s, P = 0.13).
TABLE 3.
Prevalence of cardiovascular risk factors in the hypertensive population and aortic and carotid stiffness parameters in the absence (no) or presence (yes) of cardiovascular risk factors
| Risk factors | Prevalence | cfPWV (m/s) | CS (m/s) | ||
|---|---|---|---|---|---|
|
|
|
||||
| (%) | No | Yes | No | Yes | |
| Male sex | 60.8 | 9.5 ± 2.2 | 9.3 ± 2.2 | 7.2 ± 1.5 | 6.7 ± 1.3* |
|
| |||||
| Type 2 diabetes | 22.6 | 8.9 ± 2.1 | 10.7 ± 2.2* | 6.8 ± 1.4 | 7.4 ± 1.3* |
|
| |||||
| Obesity | 33.1 | 9.1 ± 2.1 | 9.8 ± 2.4* | 6.9 ± 1.5 | 7.0 ± 1.2 |
|
| |||||
| Dyslipidemia | 76.7 | 8.9 ± 2.1 | 9.5 ± 2.2 | 6.9 ± 1.3 | 7.0 ± 1.4 |
|
| |||||
| Metabolic syndrome | 54.8 | 8.7 ± 1.8 | 9.8 ± 2.3* | 6.8 ± 1.4 | 7.2 ± 1.3* |
|
| |||||
| Current smoking | 15.0 | 9.4 ± 2.2 | 9.3 ± 2.2 | 6.9 ± 1.4 | 6.8 ± 1.4 |
|
| |||||
| Family history of CV disease | 24.2 | 9.2 ± 2.0 | 9.8 ± 2.2* | 6.9 ± 1.4 | 7.0 ± 1.3 |
|
| |||||
| Previous CV events | 7.3 | 9.2 ± 2.1 | 10.7 ± 3.0* | 6.9 ± 1.4 | 7.2 ± 1.8 |
CV, cardiovascular; cfPWV, carotid to femoral pulse wave velocity; CS, carotid stiffness.
P < 0.05 or less versus ‘no.’
Multiple logistic regression, including mean BP, mean carotid diameter, and heart rate (HR) as continuous variables, and age, sex, diabetes mellitus, metabolic syndrome, antihypertensive drugs, and statin use as discrete variables, showed that age [odds ratio (OR) 3.10 (confidence limits (CL) 95% 1.56–6.17)], mean BP [OR 1.08 (CL95% 1.05–1.11)], statin use [OR 2.60 (CL95% 1.09–6.20)], and female sex [male sex = 1; OR 0.16 (CL95% 0.08–0.32)] were independently associated to an increased carotid stiffness, when defined according to the 90th percentile of the healthy subpopulation of the present study. When the same model was built considering increased carotid stiffness (brach) according to the 90th percentile of our healthy subpopulation, age [OR 2.31 (CL95% 1.14–4.69)], mean BP [OR 1.05 (CL95% 1.02–1.08)], female sex [male sex = 1; 1; OR 0.30 (CL95% 0.15–0.59)], and diabetes [OR 3.67 (CL95% 1.56–8.59)] were independently associated to an increased carotid stiffness. Increased carotid stiffness (brach) according to the age and sex-specific international reference values, was independently associated with diabetes mellitus [OR 6.63 (CL95% 1.88–23.35)], HR [OR 1.04 (CL95% 1.01–1.08)], and mean BP [OR 1.07 (CL95% 1.02–1.12)].
Hypertensive patients with previous cardiovascular events, family history of premature cardiovascular disease, diabetes mellitus, obesity, and metabolic syndrome had higher cfPWV as compared with patients without these risk factors (Table 3). Furthermore, hypertensive patients on chronic treatment with BP-lowering drugs (9.6 ± 2.1 versus 8.7 ± 2.0 m/s, P = 0.001) or with statins showed higher cfPWV than their untreated counterparts. Multiple logistic regression, including mean BP as continuous variable and age, sex, previous cardiovascular events, family history of premature cardiovascular disease, diabetes mellitus, obesity, metabolic syndrome, and statin use as discrete variables, demonstrated that only diabetes mellitus (OR 3.26, CL95% 1.50–7.07), age [OR 4.67 (CL95% 2.55–8.55)] and mean BP [OR 1.03 (CL95% 1.01–1.06)] were independently associated to an increased cfPWV defined according to the 90th percentile of the healthy subpopulation. In the same analysis, increased cfPWV defined according the age-specific reference values, was associated to mean BP (OR 1.05, CL95% 1.02–1.08) and obesity (OR 2.64, CL95% 1.27–5.47).
Association of carotid stiffness and carotid–femoral pulse wave velocity with target organ damage in the hypertensive population
Within the hypertensive population (n = 314), the prevalence of reduced eGFR (<60 ml/min) was 11.7% (out of 291 patients), albuminuria (albumin-to-creatinine ratio >30 mg/g) was 10.4% (out of 183 patients), and LVH (>95 g/mq in women; >115 g/mq in men) was 62.8% (out of 261 patients). The prevalence of increased common carotid IMT (>0.9 mm) was 14.0% (out of 314 patients), whereas, considering age and sex-stratified reference values for IMT, 147 individuals (47%) showed IMT greater than 90th percentile.
The hypertensive population was divided into four groups according to the presence or not of increased carotid and/or aortic stiffness according to 90th percentile of our healthy population: 38.8% presented neither carotid nor aortic stiffness (PWV−C−), 24.0% presented isolated increased cfPWV (PWV+CS−), 13.8% presented isolated increased carotid stiffness (PWV−CS+), and 23.4% presented both (PWV+CS+). Microalbuminuria was equally prevalent in the four groups, whereas significant differences were found for the other kinds of target organ damage considered (Table 4a). Increased carotid stiffness, with or without increased cfPWV (groups PWV−CS+ and PWV+CS+), was associated to an increased probability of having LVH in comparison with PWV−CS−, both in the unadjusted model and in the model adjusted for age, mean BP, and diabetes. As sex-specific cutoffs were used for LVH definition, sex was not included in the model. Conversely, reduced GFR was associated to increased cfPWV, in the presence or absence of increased carotid stiffness (groups PWV+CS− and PWV+CS+), both in the adjusted and unadjusted model. PWV+CS+ was associated with an increased IMT (either defined according to the 0.9-mm cutoff or according to age-specific reference values), though significance was lost in the adjusted model (Table 4a).
TABLE 4.
Logistic regression analysis of the association between target organ damage and increased carotid–femoral pulse wave velocity and/or carotid stiffness, defined according to the 90th percentile of the healthy population (a) and the Reference Values of Arterial Measurement Collaboration, in the hypertensive population (b)
| (a) | Unadjusted model OR (CL95%) | Adjusted model OR (CL95%) (age, sex, mean BP, diabetes) | |
|---|---|---|---|
| Microalbuminuria | PWV−CS− | 1.00 | NA |
| PWV+CS− | 1.02 (0.30–3.47) | ||
| PWV−CS+ | 1.00 (0.24–4.20) | ||
| PWV+CS+ | 0.80 (0.22–2.92) | ||
|
| |||
| Reduced eGFRa | PWV−CS− | 1.00 | 1.00 |
| PWV+CS− | 6.19 (1.92–19.9) | 7.39 (2.25–24.28) | |
| PWV−CS+ | 1.36 (0.24–7.77) | 1.80 (0.31–10.48) | |
| PWV+CS+ | 7.42 (2.34–23.5) | 13.27 (3.86–45.58) | |
|
| |||
| LVHb | PWV−CS− | 1.00 | 1.00 |
| PWV+CS− | 1.69 (0.86–3.33) | 1.28 (0.62–2.62) | |
| PWV−CS+ | 3.29 (1.36–7.93) | 2.81 (1.13–6.97) | |
| PWV+CS+ | 4.49 (1.97–10.2) | 2.86 (1.15–7.09) | |
|
| |||
| Increased IMT (cutoff: 0.9 mm) | PWV−CS− | 1.00 | 1.00 |
| PWV+CS− | 1.20 (0.46–3.14) | 0.79 (0.29–2.21) | |
| PWV−CS+ | 1.03 (0.31–3.44) | 0.85 (0.24–2.98) | |
| PWV+CS+ | 3.60 (1.59–8.14) | 2.18 (0.83–5.67) | |
|
| |||
| Increased IMT (cutoff: 90th percentile reference values)c | PWV−CS− | 1.00 | 1.00 |
| PWV+CS− | 1.48 (0.81–2.70) | 1.21 (0.64–2.26) | |
| PWV−CS+ | 1.04 (0.50–2.18) | 0.84 (0.39–1.82) | |
| PWV+CS+ | 2.89 (1.54–5.37) | 1.87 (0.95–4.72) | |
|
| |||
| (b) | Unadjusted model OR (CL95%) | Adjusted model OR (CL95%) (age, mean BP, diabetes) | |
|
| |||
| Microalbuminuria | PWV−CS− | 1.00 | NA |
| PWV+CS− | 1.07 (0.35–3.26) | ||
| PWV−CS+ | 2.29 (0.43–12.2) | ||
| PWV+CS+ | 1.31 (0.15–11.7) | ||
|
| |||
| Reduced eGFR | PWV−CS− | 1.00 | NA |
| PWV+CS− | 1.09 (0.23–5.18) | ||
| PWV−CS+ | 1.36 (0.24–7.77) | ||
| PWV+CS+ | 1.42 (0.29–6.93) | ||
|
| |||
| LVH | PWV−CS− | 1.00 | NA |
| PWV+CS− | 0.64 (0.36–1.14) | ||
| PWV−CS+ | 5.72 (0.72–45.6) | ||
| PWV+CS+ | 4.16 (0.51–34.2) | ||
|
| |||
| Increased IMT (cutoff: 0.9 mm) | PWV−CS− | 1.00 | NA |
| PWV+CS− | 0.81 (0.37–1.77) | ||
| PWV−CS+ | 0.39 (0.05–3.08) | ||
| PWV+CS+ | 1.95 (0.49–7.68) | ||
|
| |||
| Increased IMT (cutoff: 90th percentile reference values)c | PWV−CS− | 1.00 | 1.00 |
| PWV+CS− | 1.80 (1.06–3.06) | 1.43 (0.81–2.50) | |
| PWV−CS+ | 0.61 (0.20–1.83) | 0.35 (0.11–1.13) | |
| PWV+CS+ | 2.68 (0.68–9.24) | 1.47 (0.39–5.56) | |
BP, blood pressure; CS, carotid stiffness; eGFR, estimated glomerular filtration rate; IMT, intima-media thickness; LVH, left ventricular hypertrophy; PWV, pulse wave velocity.
As sex and age are used for the calculation of eGFR, they were not included in the model.
As sex-specific cutoffs were used for LVH definition, sex was not included in the model.
As age-specific cutoffs were used for IMT definition, age was not included in the model.
When considering the 90th percentile of our healthy population, 60.3% of the studied population was classified in the PWV−CS (brach)− group, 26.1% PWV+CS (brach)−, 12.5% PWV−CS (brach)+, and 1.0% PWV+CS (brach)+.
LVH was more prevalent in patients with PWV−CS (brach)+ in comparison with PWV−CS (brach) − (OR 2.47, CL95% 1.21–5.07) when considering the 90th percentile of our healthy population. The association was present even after adjustment for mean BP and diabetes (OR 2.16, CL95% 1.04–4.49). For the latter analysis, we excluded the group PWV+CS(brach)+ because it was constituted of only four patients.
When the hypertensive population was divided in four groups according to Reference Values for Arterial Measurements Collaboration, 61.0% of the studied population was classified in the PWV−CS (brach)− group, 28.5% PWV+CS(brach)−, 5.6% PWV−CS(brach)+, and 4.2% PWV+CS(brach)+. No significant association between the presence/absence of carotid/aortic stiffness and any kind of target organ damage was found (Table 4b).
DISCUSSION
In the present study, we investigated the association of carotid and aortic stiffness with target organ damage in a population of hypertensive patients referring to our center. The main finding of our study is that arterial stiffening of the two examined conductance arteries is differentially associated with target organ damage. Indeed, increased cfPWV was associated with renal target organ damage, whereas increased carotid stiffness was associated with cardiac organ damage. In contrast, the simultaneous presence of increased cfPWV and increased carotid stiffness did not result in an additive effect in terms of organ damage accrual. Furthermore, our results confirm that metabolic factors, such as obesity or diabetes, are the only risk factors showing an additive effect on arterial stiffening in addition to hypertension. Finally, our results indicated that prevalence of increased cfPWV and carotid stiffness, as well as association with target organ damage, varies considerably when a fixed cutoff obtained from a local control group or age-specific international reference values are used.
Association of carotid stiffness and carotid–femoral pulse wave velocity with target organ damage in the hypertensive population
The identification of a specific association between different measures of arterial stiffness and hypertensive target organ damage represents a novelty of the study. We found that increased cfPWV was specifically associated with renal target organ damage, whereas increased carotid stiffness correlated with cardiac organ damage. These observations have important clinical implication considering the role of hypertension on LVH and the fact that reduced eGFR is considered both a risk factor and target organ damage by ESH/ESC Hypertension Guidelines [14]. Although the cross-sectional design of our study did not allow us to determine the causal effect of arterial stiffness on target organ damage, we recently found that higher cfPWV in newly diagnosed hypertensive patients at baseline resulted in a significant GFR decline at 4 years of follow-up [21]. These data suggest that chronic kidney disease (CKD) might be an intermediate mechanism by which cfPWV induces cardiovascular events. A similar predictive value of cfPWV on GFR decline was observed in diabetic patients [22], but not in the general population [23], whereas conflicting data exist for CKD patients [24,25].
Conversely, increased carotid stiffness is associated with cardiac organ damage, expressed as LVH. In agreement to our results, some evidence exists relating distensibility of large, elastic arteries, such as aortic arch and its main branches, evaluated by MRI and LVH [26,27]. It is well known that a marked heterogeneity exists along the arterial tree in terms of molecular, cellular, and histological characteristics, leading to nonnegligible differences in local elasticity even among large elastic arteries, such as the common carotid artery and the thoracic aorta. On the other hand, as cfPWV is a measure of regional stiffness, including segments with heterogeneous wall composition, from central, elastic arterial segments, such as aorta and carotid artery, to muscular arteries, such as femoral artery [28], the lack of relationship with LVH is not surprising and confirmed by current literature [9,29]. Indeed, aortic characteristic impedance (Zc), the ratio between the pulsatile change in pressure and flow in the proximal aorta, rather than cfPWV, reflecting stiffness of the whole aorta, is related to LVH in hypertensive individuals [30]. It should also be noted that, while hypertension induces a similar remodeling (enlargement) in both aorta and carotid arteries, as a consequence of elastin fiber fracture induced by increased pulsatile mechanical load, the stiffening effect of hypertension involves mainly the aorta [28]. However, bearing in mind the abovementioned limitations, our data suggest that carotid stiffness is a more adequate proxy of thoracic aorta (which can be studied only by MRI in research settings) than cfPWV for the noninvasive study of the contribution of large artery stiffness to LVH development, even in the clinical setting.
Taken together, our results suggest that aortic and carotid stiffness could not be used interchangeably as predictors of cardiovascular and renal risk in hypertensive patients. This hypothesis is reinforced by the fact that the simultaneous presence of increased cfPWV and increased carotid stiffness is not associated with further worsening of target organ damage.
Association of carotid stiffness and carotid–femoral pulse wave velocity with cardiovascular risk factors
In our hypertensive population, metabolic factors emerged as major determinants of carotid stiffness and cfPWV, and this effect was independent of the classification chosen [6,16,31]. Indeed, accumulating evidence support the hypothesis that metabolic factors, such as type 2 diabetes mellitus [6,16] and metabolic syndrome [32,33], contribute to increase cfPWV of hypertensive patients. Interestingly, carotid stiffness (brach) but not carotid stiffness is associated with diabetes, because of the fact that PP amplification is increased with increasing levels of blood glucose [34].
Prevalence of increased carotid stiffness and carotid–femoral pulse wave velocity according to different cutoffs
Finally, our results highlight remarkable differences in prevalence of increased cfPWV and carotid stiffness, as well as in their association with target organ damage, when a fixed cutoff obtained from a local control group or age-specific international reference values are used. This striking discrepancy might be explained by several factors. First, to compare with reference values, carotid stiffness was calculated using brachial PP instead of local PP. Thus, the significant association between LVH and carotid stiffness might be driven by a tighter association of LVH with central BP than with brachial BP [35]. Indeed, the discrepancy is reduced, even though not abolished, when carotid stiffness was calculated using brachial PP and dichotomized using a fixed cutoff from our local healthy population, probably as a consequence of the selective impact of some risk factors, such as diabetes, on PP amplification [34]. Second, genetic and environmental factors such as ethnicity, latitude, outdoor temperature, and lifestyle are established determinants of BP [36,37] and vascular stiffness [10]. Therefore, the single-center nature of our study might have reduced the bias potentially introduced by the enrollment of patients and controls from diverse geographic areas as in multicentric studies. In line with this possibility, we observed greater discrepancies between the two classifications for carotid stiffness than for cfPWV, possibly indicating a greater impact of geographical and/or genetic factors on the carotid vessel than the aorta. Finally, the use of age-specific reference values might be a more appropriate way for controlling for the effect of aging on arterial stiffness and target organ damage parameters. The use of age and sex-specific cutoffs seems also useful to take into account for the different impact of HR on central and brachial BP [38], as sex is a crucial determinant of both carotid stiffness and HR. Indeed, in our study, HR remained an independent determinant of carotid stiffness only in the model considering age and sex-specific cutoffs, whereas, when a single cutoff was used for men and women, female sex rather than HR remained an independent determinant.
Limitations
Possible limitations of the present study should be acknowledged. First, the cross-sectional design does not allow drawing any conclusion on a causal relationship of the observed associations. Furthermore, our hypertensive patients, recruited among those performing a target organ damage evaluation in an ESH Excellence Center, might not be representative of the general hypertensive population, as well as the control group, which has been recruited on purpose. Finally, subtracted rather than direct distance was used for cfPWV calculation, while the latter is currently preferred [39]. However, appropriate cutoffs for cfPWV calculated with subtracted distance, provided as Supplemental material by the Reference Values for Arterial Stiffness Collaboration [13], were used for the present analysis.
Perspectives
Arterial stiffening in hypertension is a systemic process, which seems to proceed differently at the aortic and carotid level in hypertensive patients and leading to different consequences on target organ damage. Thus, despite growing evidence supporting the importance of carotid stiffness evaluation as an independent predictor of cardiovascular events [5,40], whether carotid stiffness provides additional or complementary information to cfPWV remains largely undetermined. Our data indicate that aortic and carotid stiffness provide complementary information on target organ damage accrual in hypertensive patients, but its predictive role for organ damage development needs to be assessed in large prospective clinical trials.
Acknowledgments
Conflicts of interest
L.G. and E.B. are cofounders of QUIPU s.r.l., a spin-off company of the National Research Council and the University of Pisa, Italy. C.G. is supported by the NIH, National Heart, Lung, and Blood Institute grant K23HL111339, and by the Department of Medicine of the Icahn School of Medicine at Mount Sinai, Multidisciplinary Research Development Initiative. The remaining authors declare no conflicts of interest.
Abbreviations
- ACE
angiotensin-converting enzyme
- ANOVA
analysis of variance
- ASE
American Society of Echocardiography
- BP
blood pressure
- CATOD
Carotid/Aortic stiffness and Target Organ Damage
- cfPWV
carotid–femoral pulse wave velocity
- CL
confidence limits
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- ESH/ESC
European Society of Hypertension/European Society of Cardiology
- HDL
high density lipoprotein
- HR
heart rate
- IMT
intima–media thickness
- LV
left ventricular
- LVH
left ventricular hypertrophy
- LDL
low density lipoprotein
- MDRD
Modification of Diet in renal Disease
- OR
odds ratio
- PP
pulse pressure
- ΔD
carotid distension
References
- 1.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 4.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998;32:570–574. doi: 10.1161/01.hyp.32.3.570. [DOI] [PubMed] [Google Scholar]
- 5.van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, et al. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol. 2014;63:1739–1747. doi: 10.1016/j.jacc.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 6.Paini A, Boutouyrie P, Calvet D, Tropeano AI, Laloux B, Laurent S. Carotid and aortic stiffness: determinants of discrepancies. Hypertension. 2006;47:371–376. doi: 10.1161/01.HYP.0000202052.25238.68. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho T, Turner ST, Kullo IJ. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc Imaging. 2011;4:754–761. doi: 10.1016/j.jcmg.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triantafyllidi H, Tzortzis S, Lekakis J, Ikonomidis I, Arvaniti C, Trivilou P, et al. Association of target organ damage with three arterial stiffness indexes according to blood pressure dipping status in untreated hypertensive patients. Am J Hypertens. 2010;23:1265–1272. doi: 10.1038/ajh.2010.156. [DOI] [PubMed] [Google Scholar]
- 9.Libhaber E, Woodiwiss AJ, Libhaber C, Maseko M, Majane OH, Makaula S, et al. Gender-specific brachial artery blood pressure-independent relationship between pulse wave velocity and left ventricular mass index in a group of African ancestry. J Hypertens. 2008;26:1619–1628. doi: 10.1097/HJH.0b013e328302ca27. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi N, Bulpitt CJ, Leggetter S, Schiff R, Nihoyannopoulos P, Strain WD, et al. Ethnic differences in vascular stiffness and relations to hypertensive target organ damage. J Hypertens. 2004;22:1731–1737. doi: 10.1097/00004872-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Blaha MJ, Budoff MJ, Rivera JJ, Katz R, O’Leary DH, Polak JF, et al. Relationship of carotid distensibility and thoracic aorta calcification: multiethnic study of atherosclerosis. Hypertension. 2009;54:1408–1415. doi: 10.1161/HYPERTENSIONAHA.109.138396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelen L, Bossuyt J, Ferreira I, van Bortel LM, Reesink KD, Segers P, et al. Reference values for local arterial stiffness. Part A: carotid artery. J Hypertens. 2015;33:1981–1996. doi: 10.1097/HJH.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 13.Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 15.Hallan S, Asberg A, Lindberg M, Johnsen H. Validation of the Modification of Diet in Renal Disease formula for estimating GFR with special emphasis on calibration of the serum creatinine assay. Am J Kidney Dis. 2004;44:84–93. doi: 10.1053/j.ajkd.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Bruno RM, Penno G, Daniele G, Pucci L, Lucchesi D, Stea F, et al. Type 2 diabetes mellitus worsens arterial stiffness in hypertensive patients through endothelial dysfunction. Diabetologia. 2012;55:1847–1855. doi: 10.1007/s00125-012-2517-1. [DOI] [PubMed] [Google Scholar]
- 17.Bianchini E, Bozec E, Gemignani V, Faita F, Giannarelli C, Ghiadoni L, et al. Assessment of carotid stiffness and intima-media thickness from ultrasound data: comparison between two methods. J Ultrasound Med. 2010;29:1169–1175. doi: 10.7863/jum.2010.29.8.1169. [DOI] [PubMed] [Google Scholar]
- 18.Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S. Reference intervals for common carotid intima-media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013;34:2368–2380. doi: 10.1093/eurheartj/ehs380. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Weber T, Auer J, Lamm G, O’Rourke MF, Eber B. Arterial stiffness, central blood pressures, and wave reflections in cardiomyopathy-implications for risk stratification. J Card Fail. 2007;13:353–359. doi: 10.1016/j.cardfail.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Bruno RM, Salvati A, Barzacchi M, Raimo K, Taddei S, Ghiadoni L, et al. Predictive value of dynamic renal resistive index (DRIN) for renal outcome in type 2 diabetes and essential hypertension: a prospective study. Cardiovasc Diabetol. 2015;14:63. doi: 10.1186/s12933-015-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchi R, Babazono T, Mugishima M, Yoshida N, Nyumura I, Toya K, et al. Arterial stiffness is associated with incident albuminuria and decreased glomerular filtration rate in type 2 diabetic patients. Diabetes Care. 2011;34:2570–2575. doi: 10.2337/dc11-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upadhyay A, Hwang SJ, Mitchell GF, Vasan RS, Vita JA, Stantchev PI, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol. 2009;20:2044–2053. doi: 10.1681/ASN.2009010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010;55:1110–1115. doi: 10.1161/HYPERTENSIONAHA.109.143024. [DOI] [PubMed] [Google Scholar]
- 25.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, et al. Arterial remodeling associates with CKD progression. J Am Soc Nephrol. 2011;22:967–974. doi: 10.1681/ASN.2010080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohyama Y, Ambale-Venkatesh B, Noda C, Chugh AR, Teixido-Tura G, Kim JY, et al. Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2016:9. doi: 10.1161/CIRCIMAGING.115.004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, et al. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:194–201. doi: 10.1161/ATVBAHA.107.156950. [DOI] [PubMed] [Google Scholar]
- 28.Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007–1021. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 29.Matsui Y, Ishikawa J, Shibasaki S, Shimada K, Kario K. Association between home arterial stiffness index and target organ damage in hypertension: comparison with pulse wave velocity and augmentation index. Atherosclerosis. 2011;219:637–642. doi: 10.1016/j.atherosclerosis.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Pucci G, Hametner B, Battista F, Wassertheurer S, Schillaci G. Pressure-independent relationship of aortic characteristic impedance with left ventricular mass and geometry in untreated hypertension. J Hypertens. 2015;33:153–160. doi: 10.1097/HJH.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 31.Giannarelli C, Bianchini E, Bruno RM, Magagna A, Landini L, Faita F, et al. Local carotid stiffness and intima-media thickness assessment by a novel ultrasound-based system in essential hypertension. Atherosclerosis. 2012;223:372–377. doi: 10.1016/j.atherosclerosis.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Schillaci G, Pirro M, Vaudo G, Mannarino MR, Savarese G, Pucci G, et al. Metabolic syndrome is associated with aortic stiffness in untreated essential hypertension. Hypertension. 2005;45:1078–1082. doi: 10.1161/01.HYP.0000165313.84007.7d. [DOI] [PubMed] [Google Scholar]
- 33.Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Penno G, Pucci L, et al. Peripheral wave reflection and endothelial function in untreated essential hypertensive patients with and without the metabolic syndrome. J Hypertens. 2008;26:1216–1222. doi: 10.1097/HJH.0b013e3282fa7158. [DOI] [PubMed] [Google Scholar]
- 34.Herbert A, Cruickshank JK, Laurent S, Boutouyrie P Reference Values for Arterial Measurements Collaboration. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014;35:3122–3133. doi: 10.1093/eurheartj/ehu293. [DOI] [PubMed] [Google Scholar]
- 35.Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension. 2016;67:183–190. doi: 10.1161/HYPERTENSIONAHA.115.06066. [DOI] [PubMed] [Google Scholar]
- 36.Bruno RM, Taddei S. ‘tis bitter cold and I am sick at heart’: establishing the relationship between outdoor temperature, blood pressure, and cardiovascular mortality. Eur Heart J. 2015;36:1152–1154. doi: 10.1093/eurheartj/ehv024. [DOI] [PubMed] [Google Scholar]
- 37.Brook RD, Weder AB, Rajagopalan S. ‘Environmental hypertensionology’ the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens (Greenwich) 2011;13:836–842. doi: 10.1111/j.1751-7176.2011.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams B, Lacy PS CAFE and the ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) Investigators. Impact of heart rate on central aortic pressures and hemodynamics: analysis from the CAFE (Conduit Artery Function Evaluation) study: CAFE-Heart Rate. J Am Coll Cardiol. 2009;54:705–713. doi: 10.1016/j.jacc.2009.02.088. [DOI] [PubMed] [Google Scholar]
- 39.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 40.Bianchini E, Giannarelli C, Bruno RM, Armenia S, Landini L, Faita F, et al. Functional and structural alterations of large arteries: methodological issues. Curr Pharm Des. 2013;19:2390–2400. doi: 10.2174/1381612811319130007. [DOI] [PubMed] [Google Scholar]