Abstract
α-Defensins are abundant antimicrobial peptides in polymorphonuclear leukocytes and play an important role in innate immunity. We have previously shown that α-defensin-1 can inhibit HIV-1 replication following viral entry. Here we examined the molecular mechanism(s) of α-defensin-1–mediated HIV-1 inhibition. α-Defensin-1 had a direct effect on HIV-1 virions at a low MOI in the absence of serum. The direct effect on HIV-1 virions was abolished by the presence of serum or an increase in virus particles. Studying the kinetics of the HIV life cycle revealed that α-defensin-1 inhibited steps following reverse transcription and integration. Analysis of PKC phosphorylation in primary CD4+ T cells in response to α-defensin-1 indicated that α-defensin-1 inhibited PKC activity. Pretreatment of infected CD4+ T cells with a PKC activator, bryostatin 1, partially reversed α-defensin-1–mediated HIV inhibition. Like α-defensin-1, the PKC isoform–selective inhibitor Go6976 blocked HIV-1 infection in a dose-dependent manner. Furthermore, kinetic studies and analysis of HIV-1 products indicated that α-defensin-1 and Go6976 blocked HIV-1 infection at similar stages in its life cycle, including nuclear import and transcription. Taken together, our studies demonstrate that, in the absence of serum, α-defensin-1 may act directly on the virus, but, in the presence of serum, its effects are on the cell, where it inhibits HIV-1 replication. At least 1 of the cellular effects associated with HIV inhibition is interference with PKC signaling in primary CD4+ T cells. Studying the complex function of α-defensin-1 in innate immunity against HIV has implications for prevention as well as therapeutics.
Introduction
The innate immune system provides the first line of defense for rapidly clearing a wide variety of microbes prior to the development of an adaptive immune response (1, 2). The effector mechanisms of innate immunity include the alternative complement pathway, phagocytes, and antimicrobial peptides (1, 2). In addition to the innate pathogen-recognition systems involving immune cells using pattern recognition receptors (3), antimicrobial peptides including defensins and cathelicidins play a significant role in protecting the host from the invasion of pathogens (4).
The importance of the innate immunity in controlling HIV infection is becoming increasingly appreciated (5–8). The inverse correlation between the level of viremia and the ability of NK cells to inhibit HIV replication is predominantly mediated through secretion of CC chemokines, including macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and RANTES, that inhibit HIV-1 entry via CCR5 (8). Similarly, antiviral activity of soluble factor(s) from CD8+ T cells, known as CD8+ antiviral factor(s) (CAF), is found very early in primary infection before the presence of antibodies against HIV (9) and correlates with delayed disease progression in HIV-1–infected people (10–12). CC chemokines contribute in part to CAF activity against HIV (13). Although CAF activity was attributed to α-defensins 1–3 (14), studies have now demonstrated that α-defensins are distinct from CAF (15, 16). Detection of α-defensins in CD8+ cells is most likely due to the uptake of defensins from cocultured cells that produce defensins (16, 17). Nevertheless, α-defensins clearly have anti–HIV-1 activity (14, 15) that warrants exploration of their role in innate immunity against HIV infection.
Defensins are small cysteine-rich, cationic peptides found in leukocytes and epithelial cells (18–21). The 3 types of mammalian defensins, α, β, and circular, have β-sheet structures stabilized by 3 disulfide bonds and differ in their distribution and connection of 6 cysteine residues (reviewed in ref. 20). They exhibit antimicrobial activity for a broad spectrum of organisms, including Gram-positive and Gram-negative bacteria, fungi, and enveloped as well as nonenveloped viruses (19, 22). In addition, all 3 classes of defensins exhibit anti–HIV-1 activity (14–16, 23–27).
α-Defensins are abundant in neutrophils (19) but are also found in NK cells, B cells, γδ T cells, monocytes/macrophages, and epithelial cells, which are important components of innate immunity (28). While high concentrations (high micromolar to millimolar) of α-defensins are toxic to mammalian cells in the absence of serum (29, 30), circulating levels of α-defensin range from 400 ng/ml (approximately 0.2 μM) in the plasma to 13 μg/ml (approximately 6.5 μM) in the blood (31, 32). Elevated levels of circulating α-defensins have been associated with sepsis, bacterial meningitis, endometritis, and intrauterine infections (31, 33–35). In addition to their direct antimicrobial role, α-defensins display immunostimulatory activities including a chemotactic effect for T lymphocytes, monocytes, and immature dendritic cells and the induction of cytokine production (reviewed in refs. 19, 20).
Inhibition of HIV replication by synthetic α-defensins from guinea pigs, rabbits, and rats was first reported in 1993 (23). Several recent studies demonstrate that human α-defensins display anti–HIV-1 activity (14–16). However, the mechanism of this anti-HIV activity of α-defensins is not well defined. Although a recent study suggests that α-defensins 1, 2, and 3 purified from neutrophils inhibit HIV-1 infection by directly inactivating virus particles and by targeting CD4+ T cells, the effective dose of 200 μg/ml (approximately 60 μM) is associated with cytotoxicity (16). We have shown that recombinant α-defensin-1 at a noncytotoxic concentration of 5 μg/ml (approximately 1.5 μM) inhibits HIV-1 infection in primary CD4+ T cells, macrophages, and HeLa-CD4 cells at a step following entry (15). The IC50 of recombinant α-defensin-1 is similar to that of native α-defensin-1 from human neutrophils in the range of 0.5–2.2 μM (14). Direct inactivation of virions does not appear to be required for its inhibitory effect.
Here, we analyze the mechanism(s) by which α-defensin-1 inhibits HIV-1 infection in primary CD4+ T cells. We show that, at a low dose and a low virion burden in the absence of serum, α-defensin-1 can inactivate the virus. In the presence of serum, α-defensin-1 acts on target cells and blocks HIV-1 infection at the steps of nuclear import and transcription. Furthermore, in primary CD4+ T cells, the PKC signaling pathway is involved in α-defensin-1–mediated HIV-1 inhibition. These studies promote understanding of the complexity of the innate immune response against HIV-1 infection, providing insights into prevention and the future therapeutic development of novel anti-HIV drug design.
Results
Effect of α-defensin-1 on HIV-1 virions.
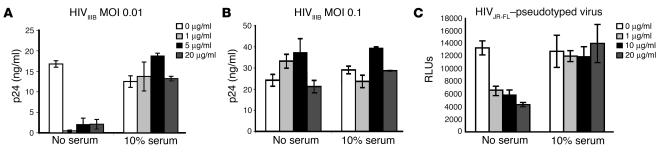
Our previous results suggest that direct inactivation of virions is not required for HIV-1 inhibition by α-defensin-1. We examined whether α-defensin-1 at low, noncytotoxic but physiologic concentrations can directly inactivate HIV-1 virions. The direct effect of α-defensin-1 on HIV-1 virions was examined by incubation of replication-competent HIV-1IIIB at an MOI of 0.01 or 0.1 with α-defensin-1 at initial concentrations of 0, 1, 5, and 20 μg/ml at 37°C for 1 hour. Samples were then diluted 100-fold in complete media to a final MOI of 0.0001 or 0.001 before infection of primary CD4+ T cells. These final concentrations of α-defensin-1 had no postentry effect on HIV. HIV-1 virus particles released into media were measured by HIV p24 assay. Because serum can block the direct effect of α-defensin-1 on virions (16, 36), we also examined whether the presence of serum affected the direct inactivation of HIV-1 virions. α-Defensin-1 at a concentration as low as 1 μg/ml displayed a direct inhibitory effect on the viruses at a low MOI in the absence of serum, whereas the inhibitory effect was abolished in the presence of serum (Figure 1A). Moreover, the inhibitory effect on HIV virions was abolished by a 10-fold increase in virus particles to an MOI of 0.1 in the absence of serum (Figure 1B). Increasing concentrations of α-defensin-1 up to 20 μg/ml did not restore its inhibitory effect. We further demonstrated that α-defensin-1 had a direct effect on the R5 HIV-1 virion using a single-cycle infection assay. Replication-defective recombinant HIV-1JR-FL–pseudotyped virus containing a luciferase reporter gene was produced in media without serum. Viruses were incubated with α-defensin-1 at concentrations of 0, 1, 10, and 20 μg/ml in the absence or presence of serum at 37°C for 1 hour and then diluted 100-fold with complete media before infection of HeLa-CD4 cells expressing CCR5 coreceptors. In the absence of serum, α-defensin-1 had a direct effect on HIV-1JR-FL–pseudotyped virus, and the inhibitory effect was abolished by serum (Figure 1C). No inhibition was observed when α-defensin-1 at 0.01, 0.1, and 0.2 μg/ml, the final concentrations present in samples after 100-fold dilution, was added during viral inoculation for 2 hours followed by wash out (data not shown). Therefore, the presence of residual low concentrations of α-defensin-1 during viral inoculation did not account for the inhibitory effect.
Figure 1.
Effect of α-defensin-1 on HIV-1 virions. (A and B) HIV-1IIIB virions at MOI 0.01 (A) or 0.1 (B) were incubated with α-defensin-1 at concentrations of 0, 1, 5, and 20 μg/ml in the absence or presence of serum at 37–C for 1 hour. The mixtures were then diluted 100-fold before addition to primary CD4+ T cells (5 × 105 per sample). The levels of HIV-1 p24 at day 10 after infection are shown. P < 0.05, α-defensin-1–treated samples at MOI 0.01 vs. nontreated controls, α-defensin-1–treated samples at MOI 0.1 vs. nontreated controls, calculated by Student’s t test. (C) HIV-1JR-FL–pseudotyped replication-defective luciferase virus was incubated with α-defensin-1 at concentrations of 0, 1, 10, and 20 μg/ml in the absence or presence of serum at 37–C for 1 hour. The samples were then diluted 100-fold before infection of HeLa-CD4 cells expressing CCR5 coreceptors. After 2 hours of incubation, cells were washed with PBS and incubated in the complete media for 48 hours before luciferase activity was measured (in RLUs). P < 0.05, α-defensin-1–treated virus vs. nontreated controls. Data are mean ± SD of triplicate samples and represent 3 independent experiments.
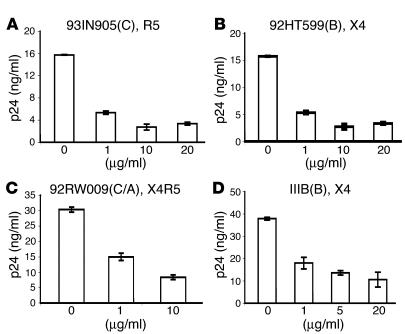
α-Defensin-1 inhibits X4 and R5 primary isolates following viral entry. We have previously shown that α-denfesin-1 inhibits HIV infection after viral entry. This was shown using replication-defective, HIVHxB2- or HIVVSV-pseudotyped luciferase-expressing viruses as well as replication-component HIVBaL (15). To determine whether α-defensin-1 can inhibit HIV primary isolates at a postentry level, primary CD4+ T cells were infected with HIV X4, R5, or X4R5 primary isolates at 37°C for 2 hours. Infected cells were then treated with α-defensin-1 at different concentrations, and virus production was measured by quantitation of HIV p24 antigens. As a control, cells were also infected with the laboratory-adapted strain HIVIIIB. As expected, HIVIIIB replication was inhibited when cells were treated with α-defensin-1 after viral infection (Figure 2D). More importantly, α-defensin-1 inhibited different subtypes of X4, R5, and dual-tropic HIV primary isolates following viral entry (Figure 2, A–C).
Figure 2.
α-Defensin-1 inhibited HIV-1 primary isolates following viral entry. (A–C) Primary CD4+ T cells were infected with HIV-1 primary isolates using R5, X4, or X4R5 coreceptors. The names and tropisms of virus isolates are indicated, and viral genotypes are shown in parentheses. (D) As a control, cells were also infected with a laboratory-adapted strain, HIVIIIB. After a 2-hour viral inoculation, cells were washed and treated with α-defensin-1 at different concentrations. HIV-1 production was measured, and the levels of HIV-1 p24 at day 10 after infection are shown. P < 0.05, α-defensin-1–treated infected cells vs. nontreated controls. Data are mean ± SD of triplicate samples and represent 2 independent experiments.
HIV-1 inhibition in α-defensin-1–pretreated CD4+ cells is not due to downregulation of CD4, CCR5, or CXCR4 surface expression.
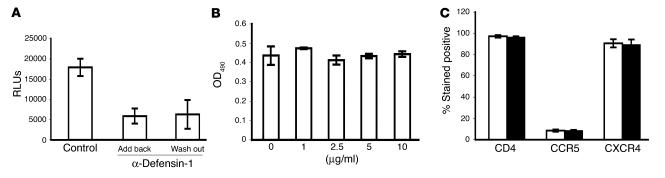
We have previously observed that pretreatment of HeLa-CD4 cells with α-defensin-1 blocks HIV-1 infection. To analyze whether pretreatment of CD4+ T cells with α-defensin-1 would also lead to HIV-1 inhibition during a single life cycle, cells were treated with α-defensin-1 at 5 μg/ml for 16 hours, washed, and cultured in media without or with the inhibitor during HIV-1 infection. In agreement with our previous finding, HIV-1 infection was inhibited by 70% in cells pretreated with α-defensin-1 even though the inhibitor was washed out before infection (Figure 3A). This result indicated that in the presence of serum α-defensin-1 had an antiviral effect on the target cell and that this effect persisted after wash-out of α-defensin-1.
Figure 3.
HIV-1 inhibition in α-defensin-1–pretreated primary CD4+ T cells and the effect of α-defensin-1 on CD4+ T cell proliferation and CD4, CCR5, and CXCR4 surface expression. (A) CD4+ T cells were pretreated with α-defensin-1 at 5 μg/ml for 16 hours. Cells were washed and infected with HIV-1VSV–pseudotyped replication-defective luciferase virus. Infected cells were then placed in complete media with (Add back) or without (Wash out) α-defensin-1 during viral infection. P < 0.05, α-defensin-1–treated cells vs. nontreated controls. Luciferase activity was measured at 48 hours after infection. Data are mean ± SD of triplicate samples and represent 2 independent experiments. (B) Cell viability of activated CD4+ T cells incubated with α-defensin-1 at different concentrations for 48 hours was measured by CellTiter 96 aqueous 1-solution cell proliferation assay (Promega Corp.). No significant difference was observed in cells in the absence or presence of α-defensin-1 (P > 0.05). (C) Activated CD4+ T cells were treated without (white bars) or with (black bars) α-defensin-1 at 5 μg/ml for 16 hours. Surface expression of CD4, CXCR4, and CCR5 in activated CD4 T cells was determined by flow cytometry. No significant difference was observed between α-defensin-1–treated cells and controls (P > 0.05). Results are mean ± SD of 2 independent experiments.
To ensure that the effect of α-defensin-1 on cells was not due to cytotoxicity, cell proliferation assay was performed. Activated CD4+ T cells were treated with α-defensin-1 at different concentrations for 48 hours, and cell proliferation was determined. α-Defensin-1 had no effect on CD4+ T cell proliferation up to 10 μg/ml for 48 hours (Figure 3B).
CD4, CXCR4, and CCR5 receptors are required for productive HIV infection of primary T cells (reviewed in ref. 37). A recent report shows that human β-defensin-2 downregulates cell surface CXCR4 but not CCR5 in unstimulated PBMCs in the absence of serum (24). We examined whether pretreatment of cells with α-defensin-1 altered the expression of these receptors, subsequently leading to HIV-1 inhibition. The effect of α-defensin-1 on expression of CD4, CXCR4, and CCR5 was analyzed by FACS analysis. Activated primary CD4+ T cells were incubated without or with α-defensin-1 at 5 μg/ml for 24 hours in complete media containing 10% FBS, and the expression of receptors on cell surfaces was determined. An isotype antibody was included as a control. α-Defensin-1 had no effect on expression of CD4, CXCR4, and CCR5 receptors (Figure 3C). Examination of the effect of α-defensin-1 on CD4 and CXCR4 expression in CD4+ T cells was also performed in the absence of serum. Activated primary CD4+ T cells were incubated in a serum-free medium, AIM-V, overnight before treatment with α-defensin-1. Cell surface CD4 and CXCR4 were analyzed by FACS analysis. Although expression of CXCR4 was downregulated in the serum-free condition, no significant change was observed in both CD4 and CXCR4 expression in cells treated with α-defensin-1 (data not shown). These results show that HIV-1 inhibition by α-defensin-1 pretreatment of CD4+ T cells was not due to downregulation of CD4, CXCR4, or CCR5 on cell surfaces.
α-Defensin-1 inhibits HIV-1 infection following reverse transcription and integration.
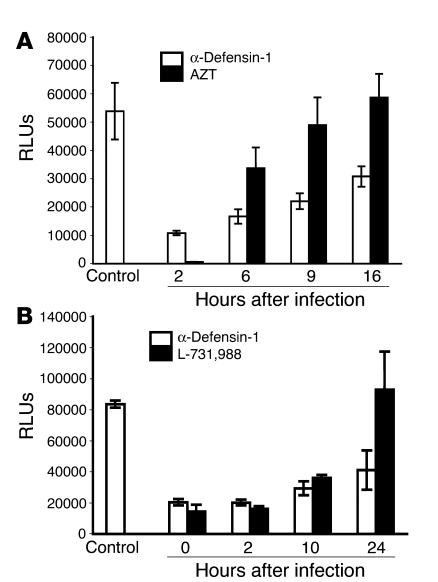
To dissect the stages of HIV-1 infection inhibited by α-defensin-1 after viral entry, we studied the kinetics of the HIV-1 life cycle in primary CD4+ T cells in the presence of α-defensin-1 using a single-cycle viral infection assay. Activated CD4+ T cells were infected with replication-defective recombinant HIV-1HxB2–pseudotyped virus containing a luciferase reporter gene. Infected cells were treated with α-defensin-1 at 2, 6, 9, and 16 hours after infection as indicated in Figure 4A. Samples treated with α-defensin-1 were compared with those treated with the reverse transcriptase inhibitor azidothymidine (AZT) at 5 μM. AZT or α-defensin-1 inhibited HIV-1 infection by 99% or 80%, respectively, when inhibitors were added at 2 hours after infection. AZT lost its inhibitory effect at 9 hours after infection, indicating that reverse transcription was complete, whereas the inhibitory effect of α-defensin-1 was sustained at this time point. The difference between levels of HIV infection in α-defensin-1–treated and AZT-treated cells at 9 and 16 hours was significant (P < 0.05, calculated by Student’s t test). This result suggests that the block in the HIV-1 life cycle by α-defensin-1 occurred after reverse transcription in primary CD4+ T cells.
Figure 4.
α-Defensin-1 inhibited HIV-1 infection following reverse transcription and integration. Activated CD4+ T cells infected with HIV-1HxB2–pseudotyped replication-defective luciferase virus were treated with α-defensin-1 at 5 μg/ml, or with AZT at 5 μM (A) or L-731,988 at 10 μM (B), at different time points after infection. Infected cells were collected at 48 hours after infection, and luciferase activity was measured. P < 0.05, α-defensin-1–treated cells vs. nontreated controls at different time points, α-defensin-1–treated vs. AZT-treated cells at 9 and 16 hours after infection, α-defensin-1–treated vs. L-731,988–treated cells at 24 hours after infection. Data are mean ± SD of triplicate samples and represent 3 independent experiments.
The anti-HIV activity of α-defensin-1 gradually decreased in the kinetic study in Figure 4A, suggesting that α-defensin-1 may affect more than 1 step in the HIV-1 life cycle. Therefore, we compared the kinetics of the HIV-1 life cycle in the presence of α-defensin-1 or an integrase inhibitor, L-731,988 (38). L-731,988 and α-defensin-1 inhibited HIV-1 infection by 75–80%, when inhibitors were added at 0 or 2 hours after infection (Figure 4B). At 24 hours after infection, L-731,988 lost its inhibitory effect, consistent with completion of integration, whereas α-defensin-1 blocked HIV-1 replication by 50%. Taken together, these results suggest that α-defensin-1 affects more than 1 step of the HIV-1 life cycle following reverse transcription, including a postintegration effect.
PKC signaling pathway(s) is involved in α-defensin-1–mediated HIV-1 inhibition in primary CD4+ T cells.
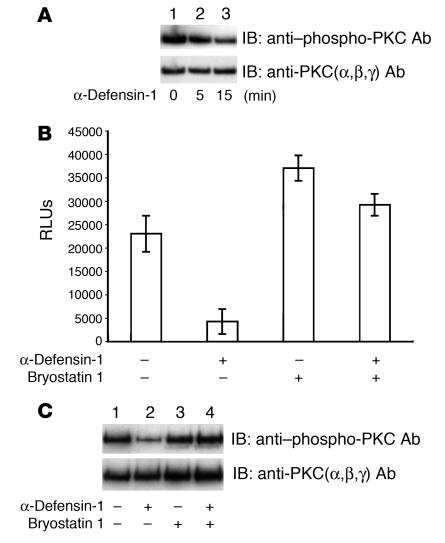
α-Defensin-1 can inhibit PKC in vitro (39). In addition, it is internalized and interacts with PKCα and β in smooth muscle cells (40). Because PKC plays an important role in HIV-1 infection (41–43), we investigated whether HIV-1 inhibition by α-defensin-1 is mediated through its effects on PKC activity. The activity of PKC is under the control of distinct serine/threonine phosphorylation (44). Therefore, we analyzed phosphorylated PKC proteins in whole-cell extracts from primary CD4+ T cells treated with α-defensin-1 using phospho-PKC–specific antibody. The blot was then stripped and reprobed with antibodies against PKCα, β, and γ as a control for equal loading. PKC phosphorylation was detected in activated CD4+ T cells without treatment (Figure 5A, lane 1). The level of PKC phosphorylation was decreased by 30% and 60% in cells treated with α-defensin-1 for 5 and 15 minutes, respectively (Figure 5A, lanes 2 and 3), indicating that α-defensin-1 inhibited PKC activity in primary CD4+ T cells.
Figure 5.
Involvement of PKC signaling pathway(s) in α-defensin-1–mediated HIV-1 inhibition. (A) Whole-cell extracts were prepared from cells treated without or with α-defensin-1 at 10 μg/ml for 0, 5, and 15 minutes. PKC phosphorylation was analyzed using a phospho-PKC antibody. The blot was then stripped and reprobed with an antibody against PKC as a control. (B) Activated CD4+ T cells were infected with HIV-1HxB2–pseudotyped replication-defective luciferase virus. Infected cells were then treated with bryostatin 1 at 10 nM for 30 minutes, washed, and incubated without or with α-defensin-1 for 48 hours before measurement of luciferase activity. Data are mean ± SD of triplicate samples and represent 2 independent experiments. (C) Primary activated CD4+ T cells were pretreated without (lanes 1 and 2) or with (lanes 3 and 4) bryostatin 1 for 30 minutes and then not treated (lanes 1 and 3) or treated (lanes 2 and 4) with α-defensin-1 for 15 minutes. Whole-cell extracts were prepared and PKC phosphorylation was analyzed as described above.
We then examined whether enhancement of PKC activity before treatment with α-defensin-1 would affect α-defensin-1–mediated HIV-1 inhibition during a single-cycle infection. HIV-1HxB2–infected primary CD4+ T cells were incubated with a PKC activator, bryostatin 1, at 10 nM for 30 minutes before treatment with α-defensin-1 for 48 hours. Pretreatment of infected cells with bryostatin 1 reduced the HIV-inhibitory effect of α-defensin-1 from 80% to 20% (Figure 5B). In addition, α-defensin-1 had no effect on PKC phosphorylation in bryostatin 1–treated cells (Figure 5C). These results suggest that PKC signaling pathways are involved in α-defensin-1–mediated HIV-1 inhibition in primary CD4+ T cells.
α-Defensin-1 and a PKC inhibitor, Go6976, block the HIV-1 life cycle at a similar stage.
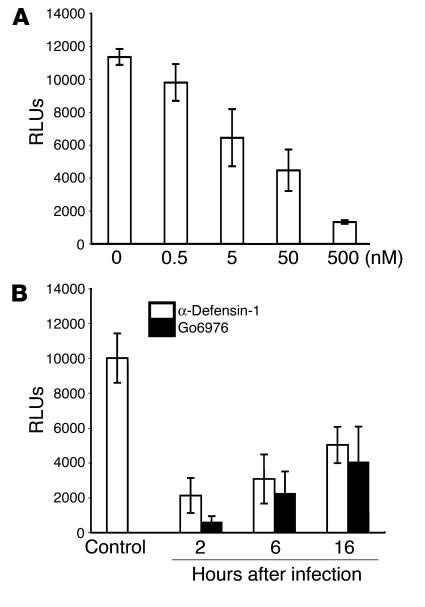
To examine the role of specific PKC isoforms in HIV-1 infection in primary CD4+ T cells, we studied HIV-1 infection in the presence of a PKC isoform–selective inhibitor, Go6976, which blocks activities of PKC isoforms α and β. Primary CD4+ T cells were infected with HIV-1HxB2–pseudotyped luciferase reporter virus, treated with Go6976 at 2 hours after infection, and incubated for 48 hours before measurement of luciferase activity. Go6976 inhibited HIV-1 infection in a dose-dependent manner (Figure 6A), indicating that PKCα and β were important for HIV-1 infection in primary CD4+ T cells.
Figure 6.
α-Defensin-1 and a PKC isoform–selective inhibitor, Go6976, blocked the HIV-1 life cycle at similar stages. (A) The effect of the PKCα and β inhibitor Go6976 on HIV-1 infection in primary CD4+ T cells was determined by a single-cycle infection assay. Cells infected with HIV-1HxB2–pseudotyped luciferase viruses were treated with Go6976 at different concentrations at 2 hours after infection. (B) The kinetics of the HIV-1 life cycle in primary CD4+ T cells in the presence of α-defensin-1 or Go6976 were studied as described in Figure 4. P < 0.05, inhibitor-treated cells vs. nontreated controls at different time points. Data are mean ± SD of triplicate samples and represent 3 independent experiments.
We then determined whether the PKC inhibitor Go6976 and α-defensin-1 blocked at a similar stage of HIV-1 infection by studying the kinetics of the HIV-1 life cycle in primary CD4+ T cells in the presence of these inhibitors. Infected cells were treated with α-defensin-1 at 5 μg/ml or Go6976 at 500 nM at 2, 6, and 16 hours after infection, and luciferase activity was measured at 48 hours after infection. Similar kinetics of HIV-1 inhibition were observed in cells treated with α-defensin-1 or Go6976, which suggests that the block in the HIV-1 life cycle may occur at the same stage(s) (Figure 6B).
To analyze whether α-defensin-1 or Go6976 also exhibited the anti–HIV-1 activity in transformed T cell lines, several transformed cell lines were infected with HIV-1HxB2–pseudotyped luciferase reporter virus and treated with α-defensin-1 or Go6976 at 2 hours after infection. Infected cells were incubated for 48 hours before measurement of luciferase activity. In contrast to the results found in primary CD4+ T cells, no effect of α-defensin-1 on HIV-1 infection was observed in transformed T cell lines including H9, CEM, and Jurkat cells. In these transformed T cells, Go6976 was found to actually enhance HIV-1 infection (data not shown). The results with Go6976, in parallel with those with α-defensin-1, suggest that signaling pathways involved with HIV infection in primary CD4+ T cells are not the same as those in transformed T cells.
Both α-defensin-1 and Go6976 inhibit HIV-1 infection at the steps of nuclear import and transcription.
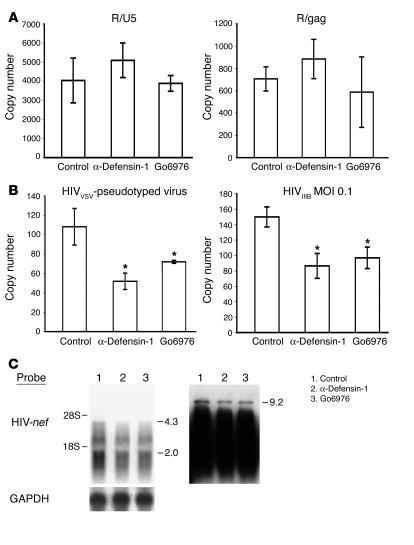
To confirm that Go6976 and α-defensin-1 inhibited HIV in primary CD4+ T cells following reverse transcription, real-time PCR analysis was performed to amplify HIV-1 strong-stop (R/U5) and full-length (R/gag) reverse-transcribed products. These represent early and late reverse-transcribed DNA, respectively (45). Activated primary CD4+ T cells were infected with HIV-1HxB2–pseudotyped luciferase reporter virus and then treated with α-defensin-1 at 5 μg/ml or Go6976 at 500 nM at 2 hours after infection. Genomic DNA was extracted at 48 hours after infection, and HIV reverse-transcribed DNA products were examined. There was no reduction of early and late HIV reverse-transcribed PCR products in primary CD4+ T cells in the presence of α-defensin-1 or Go6976 (Figure 7A), which suggests that the block occurred after reverse transcription.
Figure 7.
α-Defensin-1 and Go6976 inhibited HIV-1 infection at the steps of nuclear import and transcription. (A) CD4+ T cells were infected with HIV-1HxB2–pseudotyped viruses and then treated with α-defensin-1 or Go6976 for 48 hours. Quantitation of HIV-1 early strong-stop (R/U5) and late full-length (R/gag) reverse-transcribed products was performed. Data are mean ± SD of 3 independent experiments. No significant difference was observed between control (HIV-1–infected, no treatment) and α-defensin-1–treated cells, or between control and Go6976-treated cells, calculated by Student’s t test (P > 0.05). (B) To assess whether α-defensin-1 or Go6976 suppressed HIV-1 nuclear import, real-time PCR analysis was performed to measure c2-LTR circles in CD4+ T cells infected with replication-defective HIV-1VSV–pseudotyped virus (left panel) or replication-competent HIVIIIB at MOI 0.1 (right panel) upon treatment with inhibitors at 2 hours after infection. Samples were prepared at 24 h after infection, and c2-LTR circles were measured. *P < 0.05, control (HIV-1–infected, no treatment) vs. α-defensin-1–treated, control vs. Go6976-treated, calculated by Student’s t test. Data represent 2 independent experiments. (C) To determine whether α-defensin-1 or Go6976 inhibited HIV transcription, primary CD4+ T cells were infected with HIVIIIB at MOI 0.05 for 2 hours. Cells were washed and treated with Nelfinavir at 20 μM for 48 hours before exposure to inhibitors for 3 additional days. Total RNA was analyzed by Northern blot analysis using 32P-labeled HIV-nef DNA fragment. Longer exposure of the blot is shown in the right panel. The blot was stripped and then probed with GAPDH as a control.
To determine whether the inhibitory effect on HIV-1 infection occurred at nuclear import, real-time PCR analysis of closed 2–long-terminal repeat (c2-LTR) circles was performed. Extrachromosomal closed circular forms of HIV DNA (E-DNA), which form only after nuclear import of fully reverse-transcribed linear DNA and contain either a single or a tandem double copy of the LTR (c1-LTR or c2-LTR, respectively), are considered a marker of nuclear import (46). Primary CD4+ T cells were infected with replication-defective recombinant HIV-1VSV–pseudotyped luciferase-expressing virus and then treated with α-defensin-1 or Go6976 at 2 hours after infection. Genomic DNA was prepared at 24 hours after infection, and c2-LTR circles were analyzed. α-Defensin-1 and Go6796 inhibited nuclear import by 55% and 37%, respectively (Figure 7B, left panel). Similar inhibition of c2-LTR circle formation was observed when replication-component HIVIIIB was used to infect primary CD4+ T cells in the presence of the inhibitors (Figure 7B, right panel).
The kinetic study in Figure 4B indicated that α-defensin-1 had persistent inhibition of HIV-1 even after integration was complete. To examine whether the inhibitors affected HIV transcription in primary CD4+ T cells, cells were infected with replication-competent HIVIIIB and then treated with a protease inhibitor, Nelfinavir, to prevent new rounds of viral replication. Infected cells were incubated for 48 hours to allow completion of viral integration before treatment with α-defensin-1 or Go6976. Total RNA was prepared at 5 days after infection and analyzed by Northern blot analysis using a probe from the HIV-1 nef region. The level of all major species of viral RNAs was decreased in the presence of α-defensin-1 or Go6976 (Figure 7C). Longer exposure of the blot revealed that both inhibitors suppressed the full-length unspliced 9.2-kb mRNA by 43% (Figure 7C, right panel). These results and the kinetic studies demonstrated that α-defensin-1 and Go6976, a PKCα and β inhibitor, blocked HIV-1 infection at the steps of nuclear import and transcription.
Discussion
We demonstrated that α-defensin-1 at physiologic and nontoxic concentrations displayed a dual antiviral effect. α-Defensin-1 had a direct effect on HIV-1 virions, although this inhibitory effect was lost in the presence of serum or an increase in virus particles (Figure 1). In contrast, there was a postentry anti–HIV-1 activity of α-defensin-1 that was independent of serum and inhibited steps following reverse transcription. Both effects could function in vivo in an innate immune response to HIV. At the mucosal surface, α-defensins might work to inactivate the virions in the absence of serum; however, in the presence of serum, the inhibitory effect of α-defensin-1 would largely be on the susceptible cell. The HIV-1–inhibitory effect on cells was present following wash out of α-defensin-1 (Figure 3A), suggesting that the effect(s) on target cells persisted. We hypothesized that the cellular antiviral effect was mediated via cell signaling pathways that regulate HIV-1 replication. This study demonstrated that, in primary CD4+ T cells, PKC signaling pathways were involved in α-defensin-1–mediated HIV-1 inhibition.
PKC plays an important role in HIV-1 infection (41–43). At least 1 step in which PKC is involved in control of HIV replication is at the level of viral transcription. PKC upregulates transcription through NF-κB activation and Tat phosphorylation as well as JNK and MAPK signaling pathways (47–52). However, PKC also regulates other steps of the HIV-1 life cycle, including fusion, integration, and assembly (42, 53, 54). PKCβ is required for activation of HIV-1 transcription in the latently infected U1 cell lines (55, 56). However, the role of specific PKC isoforms in HIV infection in primary CD4+ T cells is not known. Our findings, using Go6796, which selectively inhibits the conventional PKC isoforms α and β, suggest that PKCα and PKCβ play a role in HIV-1 infection in primary CD4+ T cells by controlling the steps of nuclear import and transcription. It remains to be determined whether specific PKC isoforms control different stages of the HIV life cycle and whether PKC isoforms act individually or in concert to regulate HIV infection.
Several lines of evidence suggest that PKC signaling pathway(s) was involved in α-defensin-1–mediated HIV-1 inhibition in primary CD4+ T cells. First, α-defensin-1 blocked PKC phosphorylation, and enhancement of PKC phosphorylation interfered with α-defensin-1–mediated HIV-1 inhibition (Figure 5). In addition, both α-defensin-1 and the PKC inhibitor Go6796 blocked HIV-1 infection at similar stages of the viral life cycle (Figures 6 and 7). It remains to be determined which specific PKC isoform is involved in α-defensin-1–mediated HIV-1 inhibition in primary CD4+ T cells and whether α-defensin-1 is internalized and directly interacts with PKCα and β, as found in smooth muscle cells (40). While the presence or absence of anti-HIV activity of Go6976 paralleled α-defensin-1 in primary CD4+ T cells and transformed T cells, there were discordant effects in HeLa cells (data not shown), suggesting that the involvement of PKC signaling in α-defensin-1–mediated HIV-1 inhibition is cell specific.
The postentry effect of α-defensin-1 on HIV-1 infection occurred after reverse transcription in primary CD4+ T cells. The decrease in extrachromosomal closed circular HIV DNA (E-DNA), considered a marker of nuclear import (46), was observed in α-defensin-1– or Go6976-treated cells (Figure 7B), suggesting that a block occurred at the step prior to completion of nuclear import in the HIV-1 life cycle. The gradual trend of decreasing activity of α-defensin-1 or Go6976 in the kinetic studies suggested that α-defensin-1 and the PKC inhibitor Go6976 affect more than 1 step in the HIV-1 life cycle. Analysis of HIV-1 RNAs after viral integration indicated that both inhibitors also suppressed viral transcription (Figure 7C). The maximal anti–HIV-1 activity of α-defensin-1 was achieved when α-defensin-1 was added at an early stage of viral infection, which suggests that its effects on nuclear import and transcription are additive. Furthermore, α-defensin-1 may induce other factors such as cytokines that in turn inhibit HIV-1 replication. Although there is no report on the induction of cytokines by α-defensin-1 in primary CD4+ T cells, α-defensin-1 has been shown to upregulate IL-8 in the lung epithelial cell line A549 (29). Therefore, the indirect downstream effects may play a role in HIV inhibition.
α-Defensin-1 was originally reported to inhibit herpes simplex virus-1 (HSV-1) by directly inactivating virions, presumably interacting with and perturbing viral lipid envelopes (36). This anti–HSV-1 activity is blocked by serum. Similarly, a recent report indicates that a cytotoxic concentration of 200 μg/ml of α-defensin-1 purified from human neutrophils has a direct effect on HIV-1 virions. This direct effect on HIV-1 virions is also blocked by the presence of 5% serum (16). We observed that recombinant α-defensin-1 at the nontoxic and physiologic dose of 1 μg/ml had a direct effect on HIV-1 virions, which was blocked by the presence of serum. Since native α-defensin-1 purified from neutrophils has been shown to potently inhibit HIV-1 infection at a concentration of 0.5–2.2 μM (14), similar to what we found with recombinant proteins, the discrepancy in the effective anti-HIV dose cannot be easily attributed to the sources of α-defensin-1. The direct effect of α-defensin-1 on virions is not entirely nonspecific, as it does not efficiently suppress infection of several enveloped viruses (36). In addition, the antiviral effect is not limited to enveloped viruses; α-defensin-1 also inhibits infection of adenovirus, a nonenveloped virus (22). It is clear that, in the presence of serum, the primary anti-HIV effect of α-defensin-1 is on cells. In agreement with our report, other members of the α-defensin family, including guinea pig neutrophil peptide (GPNP), rabbit neutrophil peptide-1 (RbNP-1), and rat neutrophil peptide-1 (RatNP-1), inhibit HIV-1 replication following infection of cells. Their anti–HIV-1 activities are not affected by the presence of serum (23).
Our studies demonstrate that α-defensin-1 at physiologic concentrations inhibits HIV-1 by inactivating the virion in the absence of serum and inhibiting viral replication in target cells in the presence of serum. Other classes of defensins have been shown to use different routes to control HIV-1 infection. For example, human β-defensin-2 and HBD-3 inhibit HIV-1 replication by direct binding to virions and by downregulation of HIV-1 coreceptor CXCR4 (but not CCR5) in PBMCs in the absence of serum (24). Retrocyclin, a circular form of defensin, acts as a lectin and inhibits HIV-1 not through direct viral inactivation but at the step of viral entry (25–27). Taken together, our findings offer insights into the function of α-defensin-1 in innate immunity against HIV-1 infection. Understanding the mechanism by which defensins inhibit HIV-1 infection provides potential novel approaches to prevention and therapy.
Methods
Reagents.
Recombinant human α-defensin-1 (NP-1; produced in E. coli; greater than 95% purity by SDS-PAGE and HPLC analyses) was purchased from Chemicon International Inc. or Cell Sciences. The endotoxin level is less than 0.1 ng/mg of human α-defensin-1. Pan–phospho-PKC antibodies and antibodies against PKCα, β, and γ were purchased from Cell Signaling Technology Inc. and Upstate Biotechnology Inc., respectively. Nelfinavir was obtained from the NIH AIDS Research & Reference Reagent Program (ARRRP). Go6976 was purchased from BIOMOL Research Laboratories Inc., and L-731,988 was provided by Merck & Co. Go6976 and L-731,988 were dissolved in DMSO at a final concentration of 5 mM, and the final concentration of DMSO in the working solutions did not interfere with the infection assay.
Cell culture.
PBMCs from normal healthy blood donors were isolated by Ficoll-Hypaque gradient centrifugation. CD4+ T cells were isolated from PBMCs by negative selection using a CD4+ T cell isolation kit from Miltenyi Biotec Inc. The purity of cells is 98% based on flow cytometric analysis. CD4+ T cells were stimulated with phytohemagglutinin at 5 μg/ml and maintained in RPMI media supplemented with 10% FBS and IL-2 for 3 days at 37°C before viral infection. Transformed T cell lines H9, CEM, and Jurkat were maintained in RPMI media containing 10% FBS.
HIV-1 infection.
Replication-defective HIV-1HxB2, HIV-1JR-FL, and HIV-1VSV Env-pseudotyped, luciferase-expressing reporter viruses for a single-cycle infection assay were produced as described previously (57, 58). Briefly, HEK293T cells were cotransfected with a plasmid encoding the envelope-deficient HIV-1 NL4-3 virus with the luciferase reporter gene inserted into nef (pNL4-3.Luc.R-E-; gift of N. Landau, ARRRP) and a pSV plasmid expressing the HIV-1HxB2 and HIV-1JR-FL glycoprotein (gift of D. Littman, New York University, New York, New York, USA) or the VSV-G glycoprotein (gift of D. Trono, University of Geneva, Geneva, Switzerland). The supernatant medium was collected 48 hours after transfection, and filtered. Virus stocks were analyzed for HIV-1 p24 antigen concentration by ELISA (SAIC-Frederick Inc.). To produce HIV-1JR-FL–pseudotyped viruses in the absence of serum, transfection was performed as described above. Transfected cells were incubated for 24 hours, washed with PBS, and cultured in media without serum for an additional 24 hours before collection of viruses.
For a single-cycle infection assay, activated CD4+ T cells at 1 × 106 per sample were infected with HIV-1HxB2–pseudotyped luciferase reporter viruses for 2 hours at 37°C. Unbound virus was removed by washing, and infected cells were treated with α-defensin-1 or other inhibitors and incubated at 37°C before lysis with luciferase substrate buffer (Promega Corp.). Luciferase activity (in relative light units [RLUs]) was measured on an EG&G Berthold MiniLumat LB 9506 luminometer. Infection of HeLa-CD4-CCR5 cells was performed as described previously (15).
The direct effect of α-defensin-1 on HIV-1 virions was analyzed using replication-competent HIV-1IIIB virus (Advanced Biotechnologies Inc.) or HIV-1JR-FL–pseudotyped viruses. HIVIIIB virus at an MOI of 0.1 or 0.01 was incubated with α-defensin-1 at different concentrations at 37°C for 1 hour. Samples were diluted 100-fold before addition of the virus/inhibitor mix to cells, resulting in dilution of the α-defensin-1 to levels at which there was no postentry effect. After viral adsorption, viruses were washed out and cells were incubated at 37°C. HIV p24 levels in supernatants at days 3, 5, 7, and 10 after infection were measured by ELISA (SAIC-Frederick Inc.), and the data at day 10 after infection were presented.
To determine the effect of α-defensin-1 on infection of HIV-1 primary isolates following viral entry, phytohemagglutinin-activated primary CD4+ T cells were infected with HIV-1 primary isolates (ARRRP; and the UNAIDS Network for HIV Isolation and Characterization and the Division of AIDS, National Institute of Allergy and Infectious Diseases) at 37°C for 2 hours. Virus was washed out, and infected cells were treated with α-defensin-1 at different concentrations. HIV-1 p24 levels in the media were measured as described above.
Western blot analysis.
Whole-cell extracts were prepared by lysis of cells in 20 mM HEPES buffer (pH 7.9) with 0.2% NP-40, 10% glycerol, 200 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 1 mM sodium orthovanadate, 0.5 mM PMSF, and protease inhibitor cocktail (Roche Molecular Biochemicals). The protein concentration in whole-cell extracts was determined by the Bradford method using the Bio-Rad Protein Assay (Bio-Rad Laboratories). Proteins (30 μg per well) were separated by SDS-PAGE. After electrophoresis, proteins were transferred to PVDF membranes (Millipore Corp.). The membranes were blocked with 5% milk in rinse buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.1% Triton X-100) for 30 minutes at room temperature, then incubated overnight at 4°C with the appropriate primary antibodies in rinse buffer with 5% BSA fraction V. After 3 washes in rinse buffer for 15 minutes, the blots were incubated with HRP-linked secondary antibody (KPL) at a dilution of 1:10,000 for 1 hour at room temperature, and then washed 3 times with rinse buffer for 15 minutes. The immunoblotted proteins were visualized using the ECL chemiluminescent substrate, according to the manufacturer’s specifications (Amersham Biosciences Corp.). To reprobe blots, membranes were incubated in stripping buffer (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7) at 55°C for 1 hour and rinsed with PBS several times before a second Western blot analysis.
Flow cytometric analysis.
For cytofluorometric analysis (FACSCalibur; BD Biosciences), α-defensin-1–treated activated CD4+ T cells were stained with the appropriate mAbs conjugated with PE or FITC (BD Biosciences — Pharmingen). Appropriate anti-isotypic mAbs conjugated with PE or FITC were used as negative controls. Results were analyzed with CellQuest software (BD).
Detection of HIV-1 DNA by quantitative real-time PCR analysis.
DNA was extracted from cells with the QIAamp DNA Blood Mini Kit (QIAGEN Inc.), and 300 ng of genomic DNA (approximately 48,000 cells) was used for PCR amplification. Each PCR mix contained each primer at 0.2 μM, 2 mM MgCl2, and HotStarTag Master Mix (QIAGEN Inc.). The primer sequences for HIV reverse-transcribed DNA products were as follows: R/U5 forward, M667 (5′-GGCTAACTAGGGAACCCACTG-3′); R/U5 reverse, AA55 (5′-CTGCTAGAGATTTTCCACACTGAC-3′); R/gag forward, M667 (above); R/gag reverse, M661 (5′-CCTGCCTCGAGAGAGCTCCACACTGAC-3′) (45); RT-HEX probe [5′-(HEX)-ACGGGCACACACTACTTTGAGCACTCAAGGC-(BHQ-2)-3′]. The primer sequences for β-actin were as follows: actin-forward (5′-TGCGTGACATTAAGGAGAAG-3′), actin-reverse (5′-GCTCGTAGCTCTTCTCCA-3′), and actin-FAM probe [5′-(FAM)-CACGGCTGCTTCCAGCTCCTC-(BHQ-1)-3′]. The primer sequences for 2-LTR circles were as follows: 2-LTR circle forward, MH535 (5′-AACTAGGGAACCCACTGCTTAAG-3′); 2-LTR reverse, MH536 (5′-TCCACAGATCAAGGATATCTTGTC-3′); 2-LTR probe, MH603 [5′-(FAM)-ACACTACTTGAAGCACTCAAGGCAAGCTTT-(BHQ)-3′] (59). Plasmids pNL4-3.Luc and cc2-LTR (60) were used to generate standard curves for RT and 2-LTR circle primer-probe sets. In every experiment, a standard curve for RT products and 2-LTR circles derived from serial dilution of plasmids containing the target sequence and ranging from 100 to 106 copies was measured in triplicate. PCR cycling conditions included a 95°C denaturation for 10 minutes followed by 40 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. Reactions were carried out and analyzed using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems).
Northern blot analysis.
Total RNA (5 μg) prepared using TRIzol (GIBCO; Invitrogen Corp.) was separated on a 1% formaldehyde-agarose gel and transferred to a Zeta-Probe GT membrane (Bio-Rad Laboratories). The membrane was incubated with 32P-labeled probes overnight at 65°C in 0.25 M sodium phosphate buffer (pH 7.2) containing 7% SDS and 1 mM EDTA, and then washed for 15 minutes twice at room temperature and for 30 minutes once at 65°C with 40 mM sodium phosphate buffer (pH 7.2) containing 1% SDS and 1 mM EDTA. After washing, the membrane was exposed to Hyperfilm MP (Amersham Biosciences Corp.). To reprobe the blot, the membrane was incubated in 0.5% SDS and 0.1× SSC at 95°C for 15 minutes before probing with GAPDH.
Acknowledgments
We thank Fleur François for initiating the kinetic study and Mohammad Husain for providing the DNA fragment for HIV-1 nef, and Ben Chen and members of the Klotman laboratories for helpful discussions. This work was supported by NIH grants R01 AI43698 and P01 HD41763.
Footnotes
Nonstandard abbreviations used: ARRRP, NIH AIDS Research & Reference Reagent Program; AZT, azidothymidine; CAF, CD8+ antiviral factor(s); LTR, long-terminal repeat; RLU, relative light unit.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Hackett CJ. Innate immune activation as a broad-spectrum biodefense strategy: prospects and research challenges. J. Allergy Clin. Immunol. 2003;112:686–694. doi: 10.1016/S0091-6749(03)02025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway C., Jr Innate immunity. N. Engl. J. Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 4.Boman HG. Antibacterial peptides: basic facts and emerging concepts. J. Intern. Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 5.Lehner T. Innate and adaptive mucosal immunity in protection against HIV infection. Vaccine. 2003;21(Suppl. 2):S68–S76. doi: 10.1016/s0264-410x(03)00204-4. [DOI] [PubMed] [Google Scholar]
- 6.Levy JA, Scott I, Mackewicz C. Protection from HIV/AIDS: the importance of innate immunity. Clin. Immunol. 2003;108:167–174. doi: 10.1016/s1521-6616(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 7.Siegal FP, Spear GT. Innate immunity and HIV. AIDS. 2001;15(Suppl. 5):S127–S137. doi: 10.1097/00002030-200100005-00016. [DOI] [PubMed] [Google Scholar]
- 8.Kottilil S, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 2003;187:1038–1045. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 9.Mackewicz CE, Yang LC, Lifson JD, Levy JA. Non-cytolytic CD8 T-cell anti-HIV responses in primary HIV-1 infection. Lancet. 1994;344:1671–1673. doi: 10.1016/s0140-6736(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 10.Walker BD, Plata F. Cytotoxic T lymphocytes against HIV. AIDS. 1990;4:177–184. doi: 10.1097/00002030-199003000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J. Exp. Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackewicz CE, Ortega HW, Levy JA. CD8+cell anti-HIV activity correlates with the clinical state of the infected individual. J. Clin. Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocchi F, et al. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, et al. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- 15.Chang TL, Francois F, Mosoian A, Klotman ME. CAF-mediated human immunodeficiency virus (HIV) type 1 transcriptional inhibition is distinct from alpha-defensin-1 HIV inhibition. J. Virol. 2003;77:6777–6784. doi: 10.1128/JVI.77.12.6777-6784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackewicz CE, et al. α-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS. 2003;17:F23–F32. doi: 10.1097/00002030-200309260-00001. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Lopez P, He T, Yu W, Ho DD. Retraction of an interpretation. Science. 2004;303:467. doi: 10.1126/science.303.5657.467b. [DOI] [PubMed] [Google Scholar]
- 18.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu. Rev. Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 19.Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr. Opin. Immunol. 2002;14:96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 21.Schonwetter BS, Stolzenberg ED, Zasloff MA. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 22.Bastian A, Schafer H. Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 2001;101:157–161. doi: 10.1016/s0167-0115(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima H, Yamamoto N, Masuda M, Fujii N. Defensins inhibit HIV replication in vitro. AIDS. 1993;7:1129. doi: 10.1097/00002030-199308000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Quinones-Mateu ME, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 25.Cole AM, et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1813–1818. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munk C, et al. The theta-defensin, retro-cyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retroviruses. 2003;19:875–881. doi: 10.1089/088922203322493049. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J. Immunol. 2003;170:4708–4716. doi: 10.4049/jimmunol.170.9.4708. [DOI] [PubMed] [Google Scholar]
- 28.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 29.Van Wetering S, Mannesse-Lazeroms SP, Dijkman JH, Hiemstra PS. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J. Leukoc. Biol. 1997;62:217–226. doi: 10.1002/jlb.62.2.217. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein A. Mechanism of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J. Clin. Invest. 1991;88:93–100. doi: 10.1172/JCI115310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panyutich AV, Panyutich EA, Krapivin VA, Baturevich EA, Ganz T. Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J. Lab. Clin. Med. 1993;122:202–207. [PubMed] [Google Scholar]
- 32.Shiomi K, et al. Establishment of radio-immunoassay for human neutrophil peptides and their increases in plasma and neutrophil in infection. Biochem. Biophys. Res. Commun. 1993;195:1336–1344. doi: 10.1006/bbrc.1993.2190. [DOI] [PubMed] [Google Scholar]
- 33.Heine RP, Wiesenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin. Infect. Dis. 1998;27:513–518. doi: 10.1086/514691. [DOI] [PubMed] [Google Scholar]
- 34.Maffei FA, Heine RP, Whalen MJ, Mortimer LF, Carcillo JA. Levels of antimicrobial molecules defensin and lactoferrin are elevated in the cerebrospinal fluid of children with meningitis. Pediatrics. 1999;103:987–992. doi: 10.1542/peds.103.5.987. [DOI] [PubMed] [Google Scholar]
- 35.Wiesenfeld HC, et al. Association between elevated neutrophil defensin levels and endometritis. J. Infect. Dis. 2002;186:792–797. doi: 10.1086/342417. [DOI] [PubMed] [Google Scholar]
- 36.Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. J. Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore JP, Doms RW. The entry of entry inhibitors: a fusion of science and medicine. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10598–10602. doi: 10.1073/pnas.1932511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hazuda DJ, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000;287:646–650. doi: 10.1126/science.287.5453.646. [DOI] [PubMed] [Google Scholar]
- 39.Charp PA, et al. Inhibition of protein kinase C by defensins, antibiotic peptides from human neutrophils. Biochem. Pharmacol. 1988;37:951–956. doi: 10.1016/0006-2952(88)90187-6. [DOI] [PubMed] [Google Scholar]
- 40.Nassar T, et al. Human alpha-defensin regulates smooth muscle cell contraction: a role for low-density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Blood. 2002;100:4026–4032. doi: 10.1182/blood-2002-04-1080. [DOI] [PubMed] [Google Scholar]
- 41.Fields AP, Bednarik DP, Hess A, May WS. Human immunodeficiency virus induces phosphorylation of its cell surface receptor. Nature. 1988;333:278–280. doi: 10.1038/333278a0. [DOI] [PubMed] [Google Scholar]
- 42.Lenard J, Rabson A, Vanderoef R. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: inhibition of fusion and syncytia formation. Proc. Natl. Acad. Sci. U. S. A. 1993;90:158–162. doi: 10.1073/pnas.90.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degar S, et al. Inactivation of the human immunodeficiency virus by hypericin: evidence for photochemical alterations of p24 and a block in uncoating. AIDS Res. Hum. Retroviruses. 1992;8:1929–1936. doi: 10.1089/aid.1992.8.1929. [DOI] [PubMed] [Google Scholar]
- 44.Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr. Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- 45.Zack JA, et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 46.Bukrinsky MI, et al. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc. Natl. Acad. Sci. U. S. A. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 48.Kagnoff MF, Roebuck KA. Human immunodeficiency virus type 1 (HIV-1) infection and expression in intestinal epithelial cells: role of protein kinase A and C pathways in HIV-1 transcription. J. Infect. Dis. 1999;179(Suppl. 3):S444–S447. doi: 10.1086/314801. [DOI] [PubMed] [Google Scholar]
- 49.Han XM, Laras A, Rounseville MP, Kumar A, Shank PR. Human immunodeficiency virus type 1 Tat-mediated trans activation correlates with the phosphorylation state of a cellular TAR RNA stem-binding factor. J. Virol. 1992;66:4065–4072. doi: 10.1128/jvi.66.7.4065-4072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakobovits A, Rosenthal A, Capon DJ. Trans-activation of HIV-1 LTR-directed gene expression by tat requires protein kinase C. EMBO J. 1990;9:1165–1170. doi: 10.1002/j.1460-2075.1990.tb08223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roebuck KA, Gu DS, Kagnoff MF. Activating protein-1 cooperates with phorbol ester activation signals to increase HIV-1 expression. AIDS. 1996;10:819–826. doi: 10.1097/00002030-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Chen Y, Gabuzda D. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-kappaB. J. Biol. Chem. 1999;274:27981–27988. doi: 10.1074/jbc.274.39.27981. [DOI] [PubMed] [Google Scholar]
- 53.Farnet CM, et al. Human immunodeficiency virus type 1 cDNA integration: new aromatic hydroxylated inhibitors and studies of the inhibition mechanism. Antimicrob. Agents Chemother. 1998;42:2245–2253. doi: 10.1128/aac.42.9.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavie G, et al. Studies of the mechanisms of action of the antiretroviral agents hypericin and pseudohypericin. Proc. Natl. Acad. Sci. U. S. A. 1989;86:5963–5967. doi: 10.1073/pnas.86.15.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabbi MF, al-Harthi L, Saifuddin M, Roebuck KA. The cAMP-dependent protein kinase A and protein kinase C-beta pathways synergistically interact to activate HIV-1 transcription in latently infected cells of monocyte/macrophage lineage. Virology. 1998;245:257–269. doi: 10.1006/viro.1998.9158. [DOI] [PubMed] [Google Scholar]
- 56.Kim CH, Gollapudi S, Kim A, Lee T, Gupta S. Role of protein kinase C-beta isozyme in activation of latent human immunodeficiency virus type 1 in promonocytic U1 cells by phorbol-12-myristate acetate. AIDS Res. Hum. Retroviruses. 1996;12:1361–1366. doi: 10.1089/aid.1996.12.1361. [DOI] [PubMed] [Google Scholar]
- 57.Chen BK, Saksela K, Andino R, Baltimore D. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 1994;68:654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1–infected individuals. J. Exp. Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butler SL, Johnson EP, Bushman FD. Human immunodeficiency virus cDNA metabolism: notable stability of two-long terminal repeat circles. J. Virol. 2002;76:3739–3747. doi: 10.1128/JVI.76.8.3739-3747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cara A, et al. Circular viral DNA and anomalous junction sequence in PBMC of HIV-infected individuals with no detectable plasma HIV RNA. Virology. 2002;292:1–5. doi: 10.1006/viro.2001.1243. [DOI] [PubMed] [Google Scholar]