Abstract
Objective
Loneliness is a well-established risk factor for poor physical health. Much less is known about how loneliness affects patient-reported outcomes (PROs), such as somatic symptoms, which are increasingly important for guiding symptom management and assessing quality of patient care. The current study investigates whether (a) loneliness and social isolation predict cold symptoms independent of each other, and (b) whether loneliness is a more robust risk factor than objective social isolation for experiencing cold symptoms.
Methods
As part of a larger parent study, 213 healthy participants completed the Short Loneliness Scale (LON) and the Social Network Index (SNI) at baseline. They were given nasal drops containing rhinovirus 39 (RV39; i.e., a common cold virus), then quarantined for 5 days during which they reported on subjective cold symptoms in addition to being monitored for objective indicators of infection. Data from 160 of the participants (who were infected with the virus) were used in the present analyses.
Results
A hierarchical multiple regression revealed that baseline loneliness predicted self-reported cold symptoms over time (assessed via area under the curve), over and above demographic variables, season of participation, and depressive affect. Interestingly, social network size and diversity did not predict cold symptoms.
Conclusions
These findings suggest that the perception of loneliness is more closely linked to self-reported illness symptoms than objectively measured social isolation. Assessing psychosocial factors such as loneliness when treating and evaluating the common cold could contribute to health care practitioners’ understanding of their patients’ experiences with acute illness.
Keywords: loneliness, social network size, common cold, viral challenge, somatic symptoms, cold symptoms
Loneliness, an aversive psychological state experienced when a discrepancy exists between desired and achieved quality of an individual’s social relationships (Perlman & Peplau, 1981), enhances risk for a wide range of health problems. For example, those who were lonelier reported worse physical health and were more likely to develop serious health conditions such as coronary heart disease, than their less lonely counterparts (Thurston & Kubzansky, 2009). Those who are lonely have a 26% increase in odds of premature mortality, an outcome that rivals the negative health effects of obesity and inactivity (Holt-Lunstad, Smith, Baker, Harris, & Stephenson, 2015). Importantly, the link between loneliness and mortality remains after accounting for a variety of health-relevant risk factors (e.g., physical inactivity, smoking, income, age, & sex; Luo, Hawkley, Waite, & Cacioppo, 2012; Perissinotto, Cenzer, & Covinsky, 2012).
Although loneliness is a well-established risk factor for poor physical health, much less is known about how loneliness affects patient-reported outcomes (PROs), such as somatic symptoms. PROs are increasingly important for guiding symptom management and assessing quality of patient care as evidenced by the increased use of PROs in daily clinical practice to identify and monitor symptoms, evaluate treatment outcomes, and support shared decision making (Fagundes et al., 2015; Santana et al., 2015). Recent work suggests that loneliness may be a key risk factor for chronic somatic symptoms, including pain and fatigue (Jaremka et al., 2014). Somatic symptoms are higher among lonelier cancer survivors, dementia caregivers, and older adults compared with those who are less lonely (Jaremka et al., 2014; Jaremka et al., 2013). The current study extends this small but growing body of literature in an important and clinically relevant direction by examining acute self-reported somatic symptoms after a viral challenge. Suffering from illness symptoms can affect quality of life, limiting an individual’s ability to fulfill normal social roles and daily activities. For example, the common cold is responsible for over 20 million days of absence from work and school (Adams, Hendershot, & Marano, 1999). The current study will fill in the gap in the literature on loneliness and PROs in the context of acute illness.
Like loneliness, social isolation has also been consistently associated with poor physical health outcomes (Cohen, 2004; Cohen & Wills, 1985). Unlike loneliness, which focuses on perceptions of social connectedness with others, social isolation is an objective and quantifiable reflection of reduced social network size and paucity of social contact. Socially isolated individuals are at increased risk for the development of cardiovascular disease (Case, Moss, Case, McDermott, & Eberly, 1992) and mortality (Sugisawa et al., 1994). Greater levels of social isolation are also associated with increased depressive symptomology (Cohen & Wills, 1985).
Research linking social isolation to acute self-reported illness-related outcomes is limited. Within this sparse literature, most studies focused primarily on objective measures of infection rather than patient-reported outcomes. One notable exception demonstrated that those with less social network diversity (i.e., participation in different types of social relationships) experienced more objectively measured cold symptoms than those with more social network diversity (Cohen, Doyle, Skoner, Rabin, & Gwaltney, 1997). In contrast, a separate study reported that having a more diversified network was not associated with the incidence of upper respiratory illness symptoms (Hamrick, Cohen, & Rodriguez 2002).
As described above, both loneliness and social isolation are risk factors for a variety of health outcomes. Interestingly, although loneliness and social isolation are similar in this way, they are distinct and separable phenomenological experiences. Specifically, individuals with small social networks may be socially isolated, but are not necessarily lonely (Wenger, Davies, Shahtahmasebi, & Scott, 1996). Likewise, lonely people can have small or large social networks; loneliness relies on the perception of the quality of one’s social relationships, and high quality relationships can occur within any size social network. These phenomenological differences suggest that loneliness and social isolation may also have different health consequences. Accordingly, an important next step is to begin unpacking the boundary conditions for the global similarities between loneliness, social isolation, and health. In other words, it would be beneficial to investigate whether (a) loneliness and social isolation predict somatic symptoms independent of each other, and whether (b) perceived social isolation (i.e., loneliness) is a more robust risk factor than objective social isolation (or vice versa) for experiencing somatic symptoms. The current study accomplishes these goals by examining loneliness, social network size, and social network diversity as predictors of self-reported cold symptoms after exposure to rhinovirus 39 (RV39; a common cold virus).
The Present Study
The viral challenge paradigm provides an excellent way to examine links between psychosocial factors and self-reported acute illness-related symptoms in a controlled environment. Using this paradigm, researchers expose individuals to a virus and examine how psychological factors are associated with susceptibility to the virus, after accounting for previous exposure to the virus (Cohen et al., 1997; Luecken & Gallo, 2007). This paradigm also provides a well-controlled framework to examine patient reported outcomes such as cold symptoms post-exposure.
The aims of the current study are to: (1) Examine the link between loneliness and self-reported cold symptoms over time. (2) Evaluate the link between social isolation (i.e., social network size [2a], social network diversity [2b]) and self-reported cold symptoms over time. (3) Determine whether loneliness predicts self-reported cold symptoms over and above social isolation and whether this link is explained by demographic variables, season of participation, or depressive affect. Importantly, we expected the social variables to predict self-reported cold symptoms over and above the covariates. A research question of secondary interest was whether individuals who were lonely were more likely to get infected with the virus. The current study extends existing research in a novel new direction by simultaneously examining both loneliness and social isolation, and by focusing on patient reported outcomes (i.e., cold symptoms) in the context of acute illness within the same investigation of these social factors. Further, by testing whether both loneliness and social isolation predict self-reported cold symptoms independent of one another, this study will establish whether one is a more robust risk factor for self-reported symptoms during acute illness.
Method
Participants and Procedure
Potential participants who responded to advertisements were screened for eligibility over the phone, and then medically examined by a study physician (see also Cohen et al., 2013; Janicki-Deverts, Cohen, Doyle, Marsland, & Bosch, 2014). Participants were required to be between the ages of 18–55, not married or in a marriage-like relationship with another participant in the trial, and available to stay in quarantine for 5–6 days. In addition, participants were required to be in good health as determined by their medical history and the physical exam, be proficient in English, and have no evidence of immunity to the challenge virus (i.e., viral specific antibody titers ≤ 4). Those who had a history of psychiatric illness within one year prior to study enrollment, chronic physical illness (e.g., diabetes, asthma, hepatitis), egg allergies, or nasal or specific otologic surgeries were excluded from the study. Also excluded were individuals who had an abnormal urinalysis, abnormal complete blood count (CBC) or blood enzymes, or were pregnant, lactating, seropositive for the human immunodeficiency virus (HIV), or were taking regular medication (especially use of steroids or immunosuppressants). Participants could not be participating in another study involving psychological questionnaires and/or investigational products, or have had a cold or flu-like illness within 30 days prior to the trial. This study was approved by both the Carnegie Mellon University and University of Pittsburgh Internal Review Boards. Participants gave oral consent prior to their phone screening, and if eligible, completed a thorough consent process prior to enrolling in the study.
The parent-study sample consisted of 213 healthy individuals. Because the primary goal was to understand illness symptoms, we conducted analyses (Aims 1–3) on the portion of the sample who met criteria for infection (75.1%) after being exposed to the common cold (Cohen et al., 1997). An additional participant was removed due to incomplete data, yielding a final sample of 159 participants (59.4% male) aged 18 to 55 years (M = 30.15, SD = 11.08) who were predominantly Caucasian (68.8%) and African American (24.4%). After completing psychosocial measures and biological assessments (including testing for viral specific antibody titers) at baseline, participants were given nasal drops containing rhinovirus 39 (RV39), a common cold virus (Cohen et al., 2013). Participants were then quarantined for 5 days and monitored for development of infection. In quarantine, participants stayed at a hotel with meal service three times per day; participants were not allowed to have visitors and were required to stay on the floors of the hotel designated for the study. The only socialization permitted were brief interactions with other study participants in passing; participants were prohibited to enter other participants’ rooms and maintained a distance of 3 feet from one another to avoid transference of infection. Approximately 28 days after exposure to the virus, blood was collected for serological testing. Each participant was paid $1,060 for completion of all study procedures.
Measures
Demographics
During the phone screening, participants reported their marital status. Age, sex, income, and education data was obtained during a pre-quarantine study visit. Participants’ height and weight were measured in order to calculate body mass index (BMI = weight (kg)/[height (m)]2) between 3 days and 3 weeks prior to being exposed to the virus.
Season of Participation
Similar to past studies on the common cold (e.g., Cohen et al., 1997), we included the season of the year in which participants completed the study as a covariate. Because the incidence of the common cold peaks during the fall, with a smaller peak following in the spring (Gwaltney, Hendley, Simon, & Jordan, 1966), we coded those who participated in the fall or spring (i.e., “cold season”) as 1 and all others as 0.
Depressive Affect
Participants were asked by interviewers to rate the extent to which a series of 14 mood adjectives (e.g., cheerful, angry, sad, unhappy) described how they had been feeling that day, on a scale of 0 to 4, with 0 meaning “you haven’t felt that way at all today” and 4 meaning “you’ve felt that way a lot today.” A depressive affect composite score was calculated by taking a mean of one “sad” and one “unhappy” item, which of the series of mood adjectives, best reflected depressive affect (Cohen, 2007–2011).
Loneliness
Participants indicated their perceived loneliness using the Short Loneliness Scale (LON) (Hughes, Waite, Hawkley, & Cacioppo, 2004). This measure asks participants to think about their relationship with others (i.e., friends, neighbors, or family members) when answering three items on a 1(Never) to 4(Very often) scale. These items include, “In general, how often do you feel that you lack companionship?” “In general, how often do you feel left out?” and “In general, how often do you feel isolated from others?” A total score is computed by taking the sum of the three items. This measure has been validated for use with adults (Matthews-Ewald & Zullig, 2013), and demonstrated adequate reliability in the current sample (α = .79).
Social Isolation
Social network size and social network diversity were measured using the Social Network Index (SNI; Cohen et al., 1997) to reflect participant’s social isolation. The SNI assesses participation in 12 types of social relationships (e.g., spouse, parents, children, friends, workmates, etc.). Social network size was computed as the sum of all individuals with whom an individual had contact with at least once every two weeks (Brissette, Cohen, & Seeman, 2000). The SNI also assesses the number of high contact social roles (e.g., spouse, parent, friend, etc.) individuals engage in at least once every two weeks. Total social network diversity was computed as the sum of all high contact roles (Cohen et al., 1997).
Cold incidence
To determine whether participants had been infected with RV39 (i.e., a common cold virus), evidence of viral replication (i.e., shedding) and/or changes in virus-specific antibody titers were analyzed. As part of the larger parent study procedures, participants were considered to have a clinical cold if they displayed either recovery of the challenge virus in nasal secretions on any of the five days postchallenge or a 4 fold or greater increase in serum level virus-specific antibody titers between the pre-viral challenge baseline and 28 days post-challenge (Cohen et al., 1997; Cohen, Tyrrell, & Smith, 1991).
Self-reported cold symptoms
Participants self-reported daily cold symptoms (i.e., runny nose, sneezing, sore throat, nasal congestion, headache, chills, malaise) at baseline and over the course of the five days in quarantine. Participants completed their daily self-reports using a modified version of the Jackson scoring system, a symptom scoring system used to differentiate adults with viral upper respiratory infections from adults with other conditions (Jackson, Dowling, Spiesman, & Boand, 1958). This scale has been used in previous investigations of RV39 and similar viruses (Cohen et al., 1997; Gwaltney, Moskalski, & Hendley, 1980). Using a 5-point scale, 0 (none) to 4 (very severe), participants rated the severity of the 8 symptoms during the past 24 hours.
Analytic Strategy
Prior to conducting linear regression analyses, we examined assumptions of normality, linearity, and homoscedasticity, in addition to evaluating collinearity statistics (i.e., Tolerance and VIF). For Aim 1, we conducted a simple linear regression investigating whether loneliness predicted self-reported cold symptoms. We followed the same strategy for Aim 2a and 2b, but replaced loneliness with social network size and social network diversity (respectively) as the key predictor. To investigate Aim 3, a five step hierarchical multiple regression was conducted to determine whether loneliness predicted self-reported cold symptoms over and above social isolation (i.e., social network size and social network diversity) and vice versa. This analysis also examined whether the effects held after controlling for demographic variables, season of participation, and depressive affect. The covariates were selected based on previous literature reporting a relationship among these variables and loneliness, social network size and diversity, or common cold-related outcomes (Cohen et al., 1997; Cohen, Doyle, Turner, Alper, & Skoner, 2003; Essex & Nam, 1987; Hawkley & Cacioppo, 2007; Berg & Peplau, 1982; Pinquart & Sorensen, 2001). Based on previous research suggesting that varying affective states may impact symptom complaints (e.g., Cohen, Doyle, Skoner, Fireman, Gwaltney, & Newsom, 1995), we adjusted for depressive affect to rule out the possibility that depressive affect explained the association between loneliness and self-reported symptoms.
Consistent with prior research examining the cold symptom trajectory (Heikkinen & Järvinen, 2003), the number of cold symptoms reported reached the highest level at day three and then decreased over time. In this type of non-linear repeated data, the distance from the ground (i.e., the intensity or magnitude of the response) and its distance from its neighboring time point (i.e., changes over time) can be calculated.
Both pieces of this information are captured through the use of area under the curve (AUC). We utilized this approach in order to reduce the number of statistical comparisons between groups, which minimized the need for multiple comparison adjustments. Our analyses were specific to area under the curve with respect to increase from baseline (AUCI) (Fekedulegn et al., 2007). Importantly, AUCI does not assume baseline measurements are zero and thus provides a more precise measurement of the true area under the curve compared to AUC relative to the ground, which assumes baseline measurements are zero. In this sample, baseline self-reported cold symptoms were on average, slightly greater than zero (M=.86, SD=1.30), making AUCI a more precise calculation choice. AUCI was calculated by subtracting the AUC with respect to baseline from the AUC with respect to the ground (Fekedulegn et al., 2007), and is used to reflect self-reported cold symptoms over time.
Results
Out of the total sample (N=213), 160 (75.1%) participants met criteria for infection with the virus and 53 (24.9%) participants did not. After adjusting for demographics, season of participation, depressive affect, and social isolation, a logistic regression revealed that participants who were lonely were no more likely to get infected with the virus than less lonely participants (OR=0.994, p=.941). As more and less lonely people were equally as likely to get infected and because our goal was to understand self-reported cold symptoms post-infection, we focused our analyses on those who were infected.
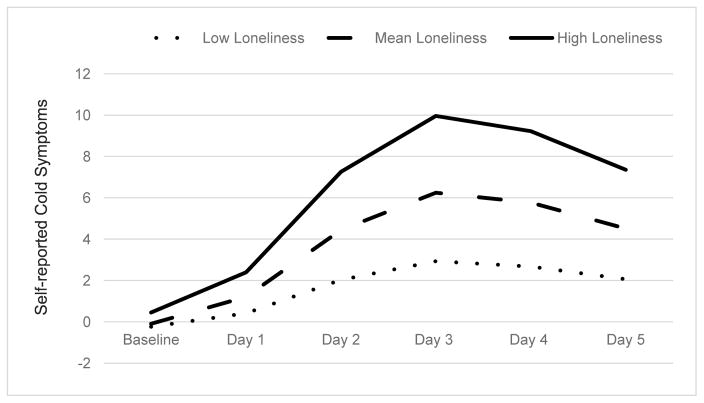
Descriptive statistics for all study variables are presented in Table 1 and zeroorder correlations are presented in Table 2. A simple linear regression determined that loneliness at baseline predicted greater cold symptom severity after being exposed to the common cold, such that lonelier individuals reported more severe cold symptoms than less lonely people (Aim 1; Table 3). In contrast, a second simple linear regression determined that social network size failed to predict cold symptom severity (Aim 2a; Table 3). Similarly, number of social roles (i.e., social network diversity) also failed to predict symptom severity (Aim 2b; Table 3). A five step hierarchical multiple regression (Aim 3; Table 4) revealed that at Step 1, the demographic covariates accounted for 9.5% of the variability in self-reported cold symptom severity, (F (6, 150) = 2.62, p = 0.02). At Step 2, introducing season of participation into the model explained an additional 8.6% of variability in self-reported cold symptom severity (F (1, 149) = 15.55, p < 0.001). At Step 3, introducing depressive affect into the model explained an additional 2.5% of variation in cold symptoms, (F (1, 148) = 4.66, p = 0.03). At Step 4, introducing objective measures of social isolation (i.e., social network size and diversity) explained an additional .2% of variation in cold symptom severity, but failed to reach significance (F (2, 146) = .20, p = 0.82). Finally, at Step 5, loneliness explained an additional 2.4% of variation in cold symptom severity, (F (1, 145) = 4.56, p = 0.03) and the R squared change was significant (p = .02). See Figure 1 depicting self-reported cold symptom severity as a function of high, average, and low levels of loneliness.
Table 1.
Study sample characteristics stratified into subsamples.
| Variable | Number (%) or mean (SD) | ||
|---|---|---|---|
|
| |||
| Overall | Infected | Not Infected | |
| Age | 30.13 (10.85) | 30.15 (11.08) | 30.08 (.50) |
| Ethnicity | |||
| Caucasian | 142 (66.7) | 110 (68.8) | 32 (60.4) |
| African-American | 58 (27.2) | 39 (24.4) | 19 (35.8) |
| Asian | 4 (1.9) | 4 (2.5) | none |
| Native American, Eskimo, Aleut | 1 (.5) | 1 (0.6) | none |
| Hispanic | 3 (1.4) | 1 (0.6) | 2 (3.8) |
| Other | 5 (2.3) | 5 (3.1) | none |
| Sex | |||
| Male | 123 (57.7) | 95 (59.4) | 28 (52.8) |
| Female | 90 (42.3) | 65 (40.6) | 25 (47.2) |
| Education | |||
| < High School | 7 (3.3) | 6 (3.6) | 1 (1.9) |
| High School graduate | 44 (20.7) | 32 (20.0) | 12 (22.6) |
| Completed High School & Vocational/technical program | 15 (7.0) | 11 (6.9) | 4 (7.5) |
| < 2 years of college | 42 (19.7) | 32 (20.0) | 10 (19.0) |
| > 2 years of college | 51 (23.9) | 38 (23.8) | 13 (24.5) |
| College degree or higher (Bachelor’s, Master’s, PhD, MD, or other higher degree) | 54 (25.4) | 41 (25.7) | 13 (24.5) |
| Marital Status | |||
| Currently married or living together | 32 (15) | 26 (16.2) | 6 (11.3) |
| Never married | 155 (72.8) | 112 (70.0) | 43 (81.1) |
| Separated | 10 (4.7) | 7 (4.4) | 3 (5.7) |
| Divorced | 14 (6.6) | 13 (8.1) | 1 (1.9) |
| Widowed | 2 (.9) | 2 (1.3) | none |
| Income | $22,205.19 (25,251.14) | $22,091 ($25,251) | $22,547.17 ($24,614.78) |
| Body Mass Index (BMI) | 27.36 (6.47) | 27.39 (6.46) | 27.26 (6.57) |
| Depressive Affect | .32 (.59) | .32 (.56) | .33 (.70) |
| Loneliness | 5.39 (1.92) | 5.41 (2.04) | 5.36 (1.52) |
| Social Network Size (total # network members) | 16.48 (8.94) | 15.94 (8.76) | 18.09 (9.38) |
| Social Network Diversity (total # of high contact social roles) | 5.15 (1.88) | 5.04 (1.85) | 5.53 (1.96) |
| Self-reported Cold Symptoms | 3.06 (2.63) | 3.61 (2.71) | 1.38 (1.41) |
| Season of Participation (“Cold Season”) | 70 (32.9) | 54 (33.8) | 16 (30.2) |
Note. The mean and standard deviation for self-reported cold symptoms reflect the mean symptom level over the 5 days in quarantine. The percentage reported for season of participation reflects the number of participants who participated in the study during “cold season” (i.e., fall or spring). Total N=213 (missing 1 participant’s income information); Infected Subsample n=160 (missing 1 participant’s income information); Not infected subsample n=53.
Table 2.
Zero-order correlations.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Loneliness | -- | |||||||||||
| 2. SNS | − .16* | -- | ||||||||||
| 3. SND | −.25** | .79** | -- | |||||||||
| 4. Depressive Affect | .23** | .02 | −.02 | -- | ||||||||
| 5. Cold Symptoms | .20* | − .07 | .16* | .16* | -- | |||||||
| 6. Age | .12 | −.14 | −.24** | −.01 | −.01 | -- | ||||||
| 7. Sex | .02 | .05 | .003 | .24** | .04 | .04 | -- | |||||
| 8. Income | −.16* | .03 | .05 | −.03 | .16 | −.08 | −.04 | -- | ||||
| 9. Education | .10 | .18* | .11 | −.02 | .08 | .09 | .21** | −.08 | -- | |||
| 10. Marital Status | .18* | −.14 | −.07 | .17* | .36** | .11 | −.04 | .04 | .21** | -- | ||
| 11. BMI | −.07 | −.09 | −.22 | .11 | .33** | .14 | −.04 | −.10 | .06 | −.10 | -- | |
| 12. Season | −.07 | .05 | .11 | −.07 | −.30** | −.07 | −.11 | −.06 | −.001 | −.06 | −.03 | -- |
Note. SNS= Social Network Size; SND= Social Network Diversity; Cold symptoms (selfreported) calculated by area under the curve; Sex coded as 0 = male and 1 = female; Income coded in $5000 increments from 1 ($2500) to 13 ($162,500); Education coded as 1 = didn’t finish high school, 2 = less than high school, completed vocational/technical program, 3 = completed high school, 4 = high school and vocational/technical program, 5 = less than 2 years of college, 6 = more than 2 years of college, 7 = Bachelor’s degree, 8 = Master’s degree, 9 = PhD, MD, or other higher degree; Marital status coded as 1 = currently married or living together, 2 = never married, 3 = separated, 4 = divorced, 5 = widowed; BMI= Body Mass Index; Season= Season of participation in the study, coded as 1= fall or spring (i.e., “cold season”), 0= winter or summer.
p < .05
p < .01
Table 3.
Simple linear regression results depicting self-reported cold symptoms as a function of variables of interest.
| Predictor Variable | β | b | SE | t | Sig | CI | d |
|---|---|---|---|---|---|---|---|
| Loneliness (Aim 1) | .20 | 1.12 | .46 | 2.60 | .01* | [.28, 2.09] | .41 |
| Size of Social Network (Aim 2a) | −.07 | −.09 | .11 | −.86 | .39 | [−.31, .12] | −.13 |
| Social Network Diversity (Aim 2b) | −.06 | −.37 | .51 | −.73 | .47 | [−1.39, .64] | −.11 |
Note. df (1, 158) for all models reported above; N= 159;
p < .05.
CI 95%
Table 4.
Hierarchical multiple regression results depicting self-reported cold symptoms as a function of variables of interest at each step of analysis.
| Variable | β | SE | t | Sig | CI | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| Step 1 | .095 | .095* | |||||
| Sex | .20 | 1.95 | 2.52 | .01* | [1.05, 8.74] | ||
| Age | −.11 | .10 | −1.121 | .23 | [−31, .08] | ||
| Income | .02 | <.001 | .26 | .80 | [.000, .000] | ||
| Education | −.013 | .56 | −.16 | .88 | [−1.20, 1.03] | ||
| Marital Status | .18 | 1.26 | 2.18 | .03* | [.24, 5.23] | ||
| BMI | .13 | .16 | 1.47 | .14 | [−.08, .55] | ||
| Step 2 | .18 | .09** | |||||
| Season of Participation | −.30 | 1.90 | −3.94 | <.001** | [−11.26, −3.74] | ||
| Step 3 | .21 | .03* | |||||
| Depressive Affect | .17 | 1.66 | 2.16 | .03* | [.30, 6.84] | ||
| Step 4 | .21 | .002 | |||||
| Social Network Size | −.07 | .17 | −.60 | .55 | [−.43, .23] | ||
| Social Network Diversity | .04 | .78 | .34 | .73 | [−1.28, 1.82] | ||
| Step 5 | .23 | .02* | |||||
| Loneliness | .48 | .50 | 2.14 | .03* | [.08, 1.97] |
Note. N= 159;
p < .05
p < .001
CI 95%
Figure 1.
Self-reported cold symptoms as a function of high, mean, and low levels of loneliness.
Ancillary Analyses
We tested our primary research questions (Aims 1–3) using individuals who were infected in order to make clinically relevant conclusions about loneliness in the context of acute illness. To ensure that acute illness was the context driving our effects, we repeated our analyses using only participants who were not infected with the virus. If acute illness was a critical contextual factor, the link between loneliness and cold symptoms would not be present among those uninfected with a cold. Indeed, loneliness, social network size, and social network diversity did not predict cold symptom severity among those who were not infected (all p values > .55). In addition, because individuals with neuroticism (i.e., emotional instability) may overreport symptoms (e.g., Cohen, Doyle, Turner, Alper, & Skoner, 2003), we tested whether the effect persisted when including neuroticism as a covariate in the model. Loneliness remained significantly associated with self-reported cold symptoms after controlling for neuroticism (p=.045). Lastly, we ran an ancillary analysis to examine whether loneliness was also associated with an objective common cold indicator (i.e., mucus weight). When we replaced self-reported symptoms with mucus weight for each model (Aims 1–3), none were significant (all p values > .56).
Discussion
The current results indicate that loneliness is an important risk factor for more severe self-reported cold symptoms after infection. Compared to those who were less lonely, lonely individuals were more likely to report experiencing higher levels of cold symptoms over the course of five days in quarantine after being exposed to RV39, a common cold virus. These results held after accounting for age, sex, education, income, marital status, BMI, season of participation, and depressive affect. Furthermore, loneliness predicted self-reported cold symptoms independent of social network size and social network diversity. Interestingly, social network size and social network diversity did not predict self-reported cold symptoms across our analyses, suggesting that loneliness may be a more important social factor linked to the experience of acute illness compared to social isolation. Specifically, loneliness may be an important risk factor for suffering from cold symptoms after getting infected. Interestingly, individuals who were lonely were no more likely than less lonely individuals to get infected with the common cold, but upon infection, their self-reported cold symptoms were more severe. These findings are in line with a wide range of research suggesting that loneliness is predictive of health-related outcomes (Adam, Hawkley, Kudielka, & Cacioppo, 2006; Hawkley, Masi, Berry, & Cacioppo, 2006; Hawkley, Thisted, & Cacioppo, 2009).
Put simply, lonelier people feel worse when they are sick than less lonely people. Based on the current study findings, it is critical for clinicians and researchers to consider the perceived quality of peoples’ social relationships (i.e., the experience of loneliness), which may be an even more powerful predictor of acute illness-related symptoms than the quantity of relationships and social roles. More broadly, our findings contribute to a growing literature on social factors and susceptibility to acute illness and disease (Cohen, Doyle, Turner, Alper, & Skoner, 2003) by expanding previous research on social networks and susceptibility to illness to the somatic experience of acute illness.
Previous research demonstrated that people with less social network diversity (i.e., participation in different types of social relationships) experienced more selfreported cold symptoms than those with more social network diversity (e.g., Cohen, Doyle, Skoner, Rabin, & Gwaltney, 1997). In contrast, others reported that having a more diversified network was not associated with upper respiratory illness symptoms (Hamrick, Cohen, & Rodriguez, 2002). The current results shed light on these inconsistent findings and suggest that social isolation may not be reliably related to selfreported cold symptoms. More research is needed to conclusively establish the role of social isolation across different illnesses, with different samples, and within different contexts. For example, studies investigating illness symptoms produced naturally without a viral challenge (e.g., Hamrick, Cohen, & Rodriguez, 2002) may differ in their results compared to those among individuals inoculated with an acute illness in a more controlled environment, as was the case in the current study. Likewise, inoculation of RV39 may facilitate a different illness experience than other strains (e.g., Hanks Strain; Cohen, Doyle, Skoner, Rabin, & Gwaltney, 1997).
The viral challenge employed in the current study provides a well-controlled framework to rule out potential confounds. In the current study, we ruled out the possibility that the association between loneliness and self-reported cold symptoms was due to social isolation. Indeed, the current findings support the notion that loneliness is a potential risk factor for self-reported symptoms surrounding acute illness. Assessing psychosocial factors such as loneliness when treating and evaluating the common cold could contribute to health care practitioners’ understanding of their patients’ experiences with acute illness. Further, helping lonely individuals establish satisfying interpersonal ties could reduce the severity of their cold symptoms when infected.
The way a patient feels when infected can have a profound impact on their daily life, regardless of objective indicators of illness. For example, “not feeling well” is more likely the culprit for the more than 20 million days of absence from work and school per year the common cold is responsible for (Adams, Hendershot, & Marano, 1999), rather than objective indicators (e.g., an individual’s production of mucus). Thus, these findings also have important economic implications; the economic cost of lost productivity due to the common cold approaches 25 billion (Bramley, Lerner, & Sames, 2002).
Interventions and treatments that may reduce feelings of loneliness may also reduce an individual’s risk of experiencing acute illness symptoms. Fewer illness symptoms may translate into fewer visits to see a physician and fewer absences from the workplace, reducing both health care costs and overall economic burden. These results support the utility of interventions designed to improve maladaptive social cognitions that enhance feelings of loneliness, social skills, and opportunities for high quality social interaction rather than interventions that simply increase social network size (Masi, Chen, Hawkley, & Cacioppo, 2010).
The current investigation has limitations. Loneliness was only assessed at baseline; future studies should investigate whether fluctuations in loneliness over time also predict self-reported cold symptoms throughout the cold trajectory. In addition, other unexamined variables could account for the relationship between loneliness and the severity of cold symptoms. For example, lonely individuals report poorer sleep than non-lonely individuals (Cacioppo et al., 2002), and this may in turn affect their response to a viral challenge and the subsequent experience of symptoms. In addition, we did not directly test specific mechanisms that may explain the relationship between loneliness and self-reported cold symptoms, limiting our conclusions about how loneliness is driving these differences in symptoms. Future research should investigate potential mechanisms that may explain these effects. In addition, future research should include stronger measures in which multiple symptom domains of depression are evaluated. Lastly, participants in the current sample were largely middle-aged, and were considered healthy at the time of the experiment. Future investigations of loneliness and acute illness symptoms should target the aging; loneliness is associated with a broad range of negative health outcomes among older adults (Holmén & Furukawa, 2002; Luo et al., 2012; Shankar, McMunn, Banks, & Steptoe, 2011). Studying how loneliness influences acute illness symptoms is important among the elderly as they are more likely to have compromised immune systems (Miller, 1996) and may have a higher susceptibility to experiencing loneliness than the age group of the current sample (Qualter et al., 2015).
This study suggests that loneliness is an important factor in predicting selfreported cold symptoms associated with acute illness above and beyond a host of demographic factors and depressive affect. Interestingly, social network size and social network diversity failed to predict cold symptoms altogether, while loneliness predicted symptoms independent of social network size and diversity suggesting that loneliness may be a more important social factor linked to the experience of acute illness. Consequently, the present study suggests that loneliness enhances risk for increased severity of cold symptoms. These data also provide insight into the pathways through which loneliness may impact physical well-being.
Acknowledgments
Funding: The data set used in this article was made available through a grant from the National Center for Complementary and Integrative Health (AT006694); the study was supported by a grant from the National Institute of Allergy and Infectious Disease (R01 AI066367); supplementary support was provided by Linked Specialized Center Cooperative Agreement Grants (NIH UL1 RR024153 & UL1 TR000005) awarded to the University of Pittsburgh Clinical and Translational Science Institute. Preparation of the manuscript was supported by a grant from the National Heart, Lung, and Blood Institute (R01HL127260-01) to C. P. Fagundes and a Ruth L. Kirschstein National Research Service Award to K. W. Murdock (1 F32 HL131353-01). The data were collected by the Laboratory for the Study of Stress, Immunity and Disease at Carnegie Mellon University under the directorship of Sheldon Cohen, PhD; and were accessed via the Common Cold Project http://www.cmu.edu/common-cold-project/ website (grant number NCCIH AT006694).
Footnotes
Conflicts of Interest: None
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Vital and Health Statistics. Series 10, Data from the National Health Survey. 1999;(200):1–203. [PubMed] [Google Scholar]
- Berg JH, Peplau LA. Loneliness: The relationship of self-disclosure and androgyny. Personality and Social Psychology Bulletin. 1982;8(4):624–630. doi: 10.1177/0146167282084004. [DOI] [Google Scholar]
- Bramley TJ, Lerner D, Sames M. Productivity losses related to the common cold. Journal of Occupational and Environmental Medicine. 2002;44(9):822829. doi: 10.1097/00043764-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Brissette I, Cohen S, Seeman TE. Measuring social integration and social networks. In: Cohen S, Underwood LG, Gottlieb BH, editors. Social support measurement and intervention: A guide for health and social scientists. New York, NY: Oxford University Press; 2000. pp. 53–85. [DOI] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford E, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: Potential mechanisms. Psychosomatic Medicine. 2002;64(3):407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychology and Aging. 2006;21(1):140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Case RB, Moss AJ, Case N, McDermott M, Eberly S. Living alone aftermyocardial infarction: A prospective, population based study of the elderly. Journal of the American Medical Association. 1992;267(4):515–519. doi: 10.1001/jama.1992.03480040063031. [DOI] [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59(8):676–684. doi: 10.1037/0003-066x.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen. Pittsburgh Cold Study 3 [Code book] 2007–2011 Retrieved from http://www.cmu.edu/common-cold-project/data/codebooks/index.html.
- Cohen S, Doyle WJ, Skoner DP, Fireman P, Gwaltney JM, Jr, Newsom JT. State and trait negative affect as predictors of objective and subjective symptoms of respiratory viral infections. Journal of personality and Social Psychology. 1995;68(1):160–169. doi: 10.1037/0022-3514.68.1.159. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. The Journal of the American Medical Association. 1997;277(24):1940–1944. doi: 10.1001/jama.278.15.1231b. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner R, Alper CM, Skoner DP. Sociability and susceptibility to the common cold. Psychological science. 2003;14(5):389–395. doi: 10.1111/1467-9280.01452. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Emotional style and susceptibility to the common cold. Psychosomatic medicine. 2003;65(4):652–657. doi: 10.1097/01.psy.0000077508.57784.da. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Turner RB, Marsland AL, Casselbrant ML, LiKorotky HS, Epel ES, Doyle WJ. Childhood socioeconomic status, telomere length, and susceptibility to upper respiratory infection. Brain, Behavior, and Immunity. 2013;34:31–38. doi: 10.1016/j.bbi.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. New England Journal of Medicine. 1991;325(9):606–612. doi: 10.1056/nejm199108293250903. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98(2):310–357. doi: 10.1037/0033-2909.98.2.310. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Nam S. Marital status and loneliness among older women: The differential importance of close family and friends. Journal of Marriage and the Family. 1987;49(1):93–106. doi: 10.2307/352674. [DOI] [Google Scholar]
- Fagundes CP, Shi Q, Vaporciyan AA, Rice DC, Popat KU, Cleeland CS, Wang XS. Symptom recovery after thoracic surgery: Measuring patient reported outcomes with the MD Anderson Symptom Inventory. The Journal of Thoracic and Cardiovascular Surgery. 2015;150(3):613–619. doi: 10.1016/j.jtcvs.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine. 2007;69(7):651–659. doi: 10.1097/psy.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Gwaltney JM, Hendley JO, Simon G, Jordan WS., Jr Rhinovirus infections in an industrial population: the occurrence of illness. New England Journal of Medicine. 1966;275(23):1261–1268. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- Gwaltney JM, Moskalski PB, Hendley JO. Interruption of experimental rhinovirus transmission. Journal of Infectious Diseases. 1980;142(6):811–815. doi: 10.1093/infdis/142.6.811. [DOI] [PubMed] [Google Scholar]
- Hamrick N, Cohen S, Rodriguez MS. Being popular can be healthy or unhealthy: Stress, social network diversity, and incidence of upper respiratory infection. Health Psychology. 2002;21(3):294–298. doi: 10.1037//0278-6133.21.3.294. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Aging and loneliness downhill quickly? Current Directions in Psychological Science. 2007;16(4):187–191. doi: 10.1111/j.1467-8721.2007.00501.x. [DOI] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychology and Aging. 2006;21(1):152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Thisted RA, Cacioppo JT. Loneliness predicts reduced physical activity: cross-sectional & longitudinal analyses. Health Psychology. 2009;28(3):354–363. doi: 10.1037/a0014400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Järvinen A. The common cold. The Lancet. 2003;361(9351):51–59. doi: 10.1016/s0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmén K, Furukawa H. Loneliness, health and social network among elderly people—a follow-up study. Archives of Gerontology and Geriatrics. 2002;35(3):261–274. doi: 10.1016/s0167-4943(02)00049-3. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspectives on Psychological Science. 2015;10(2):227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys: Results from two population-based studies. Research on Aging. 2004;26(6):655–672. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions: I. The common cold as a clinical entity. American Medical Association Archives of Internal Medicine. 1958;101(2):267–278. doi: 10.1001/archinte.1958.00260140099015. [DOI] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Doyle WJ, Marsland AL, Bosch J. Childhood environments and cytomegalovirus serostatus and reactivation in adults. Brain, Behavior, and Immunity. 2014;40:174–181. doi: 10.1016/j.bbi.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Andridge RR, Fagundes CP, Alfano CM, Povoski SP, Lipari AM, … Yee LD. Pain, depression, and fatigue: Loneliness as a longitudinal risk factor. Health Psychology. 2014;33(9):948. doi: 10.1037/a0034012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: Understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38(8):1310–1317. doi: 10.1016/j.psyneuen.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, Gallo LC. Handbook of physiological research methods in health psychology. Sage Publications; 2007. [Google Scholar]
- Luo Y, Hawkley LC, Waite LJ, Cacioppo JT. Loneliness, health, and mortality in old age: A national longitudinal study. Social science & medicine. 2012;74(6):907–914. doi: 10.1016/j.socscimed.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi CM, Chen HY, Hawkley LC, Cacioppo JT. A meta-analysis of interventions to reduce loneliness. Personality and Social Psychology Review. 2010;15(3):219–266. doi: 10.1177/1088868310377394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews-Ewald MR, Zullig KJ. Evaluating the performance of a short loneliness scale among college students. Journal of College Student Development. 2013;54(1):105–109. doi: 10.1353/csd.2013.0003. [DOI] [Google Scholar]
- Miller RA. The aging immune system: Primer and prospectus. Science. 1996;273(5271):70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Perissinotto CM, Cenzer IS, Covinsky KE. Loneliness in older persons: A predictor of functional decline and death. Archives of Internal Medicine. 2012;172(14):1078–1084. doi: 10.1001/archinternmed.2012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D, Peplau LA. Toward a social psychology of loneliness. Personal Relationships. 1981;3:31–56. [Google Scholar]
- Pinquart M, Sorensen S. Influences on loneliness in older adults: A meta analysis. Basic and applied social psychology. 2001;23(4):245–266. doi: 10.1207/s15324834basp2304_2. [DOI] [Google Scholar]
- Qualter P, Vanhalst J, Harris R, Van Roekel E, Lodder G, Bangee M, Maes M, Verhagen M. Loneliness across the life span. Perspectives on Psychological Science. 2015;10(2):250–264. doi: 10.1177/1745691615568999. [DOI] [PubMed] [Google Scholar]
- Santana MJ, Haverman L, Absolom K, Takeuchi E, Feeny D, Grootenhuis M, Velikova G. Training clinicians in how to use patient-reported outcome measures in routine clinical practice. Quality of Life Research. 2015;24(7):1707–1718. doi: 10.1007/s11136-014-0903-5. [DOI] [PubMed] [Google Scholar]
- Shankar A, McMunn A, Banks J, Steptoe A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychology. 2011;30(4):377–385. doi: 10.1037/e513892012-009. [DOI] [PubMed] [Google Scholar]
- Sugisawa H, Liang J, Liu X. Social networks, social support, and mortality among older people in Japan. Journal of Gerontology. 1994;49(1):S3–S13. doi: 10.1093/geronj/49.1.s3. [DOI] [PubMed] [Google Scholar]
- Thurston RC, Kubzansky LD. Women, loneliness, and incident coronary heart disease. Psychosomatic Medicine. 2009;71(8):836–842. doi: 10.1097/psy.0b013e3181b40efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger GC, Davies R, Shahtahmasebi S, Scott A. Social isolation and loneliness in old age: Review and model refinement. Ageing and Society. 1996;6:333358. doi: 10.1017/s0144686x00003457. [DOI] [Google Scholar]