Abstract
Background
Rapid improvements in hepatitis C virus (HCV) therapy have led to the approval of multiple oral direct-acting antiviral (DAA) regimens by the U.S. Food and Drug Administration (FDA) for treatment of chronic HCV infection.
Purpose
To summarize published literature on the efficacy and safety of oral DAAs for treatment of persons with chronic HCV infection.
Data Sources
MEDLINE and EMBASE from inception through 1 November 2016.
Study Selection
42 English-language studies from controlled and single-group registered clinical trials of adults with HCV infection that evaluated at least 8 weeks of an FDA-approved interferon-free HCV regimen that included at least 2 DAAs.
Data Extraction
Two investigators abstracted data on study design, patient characteristics, and virologic and safety outcomes sequentially and assessed quality independently.
Data Synthesis
Six DAA regimens showed high sustained virologic response (SVR) rates (>95%) in patients with HCV genotype 1 infection without cirrhosis, including those with HIV co-infection. Effective treatments for HCV genotype 3 infection are limited (2 DAA regimens). Patients with hepatic decompensation, particularly those with Child–Turcotte–Pugh class C disease, had lower SVR rates (78% to 87%) than other populations. The addition of ribavirin was associated with increased SVR rates for certain DAA regimens and patient groups. Overall rates of serious adverse events and treatment discontinuation were low (<10% in the general population); regimens that included ribavirin had more mild or moderate adverse events than those without.
Limitations
Twenty-three studies had moderate risk of bias (10 were open-label single-group trials, 11 had limited information on concealment of the allocation scheme, and 5 had selective outcome reporting). All but 1 of the studies were industry-funded. Heterogeneity of interventions precluded pooling.
Conclusion
Multiple oral DAA regimens show high rates of safety, tolerability, and efficacy for treatment of HCV genotype 1 infection, particularly among persons without cirrhosis.
Primary Funding Source
Patient-Centered Outcomes Research Institute. (PROSPERO: CRD42014009711)
In the United States, 3.2 to 5 million people are chronically infected with hepatitis C virus (HCV) and are at risk for cirrhosis, liver cancer, and death if untreated (1,2). Infection with HCV is the primary indication for liver transplantation and causes more deaths than all other notifiable infectious diseases in the United States combined (3, 4). Cure of this infection, defined as the absence of detectable HCV RNA in the blood at least 12 weeks after treatment completion (sustained virologic response [SVR]), is strongly associated with reduced liver-related morbidity and mortality (5, 6). The development of drugs that directly inhibit key steps in viral replication has led to availability of several oral HCV treatment regimens (7). We systematically reviewed the efficacy and safety of oral interferon-free HCV treatment regimens that have been approved by the U.S. Food and Drug Administration (FDA) and include at least 2 direct-acting antivirals (DAAs). We also assessed the effect of ribavirin on rates of SVR and adverse events. We reviewed phase 2 and 3 clinical trial data for patients infected with HCV genotypes 1 to 6 and patients previously considered difficult to cure with decompensated cirrhosis, HIV infection, renal failure, or liver transplantation.
Methods
Data Sources and Searches
We developed a protocol for this systematic review and registered it in PROSPERO (CRD42014009711). We searched MEDLINE and EMBASE for literature published in English from inception through 1 November 2016. The search strategy included terms for HCV infection and the medications of interest (Figure 1). We also searched ClinicalTrials.gov and hand-searched the reference lists of included articles and related systematic reviews.
Figure 1. Summary of evidence search and selection.
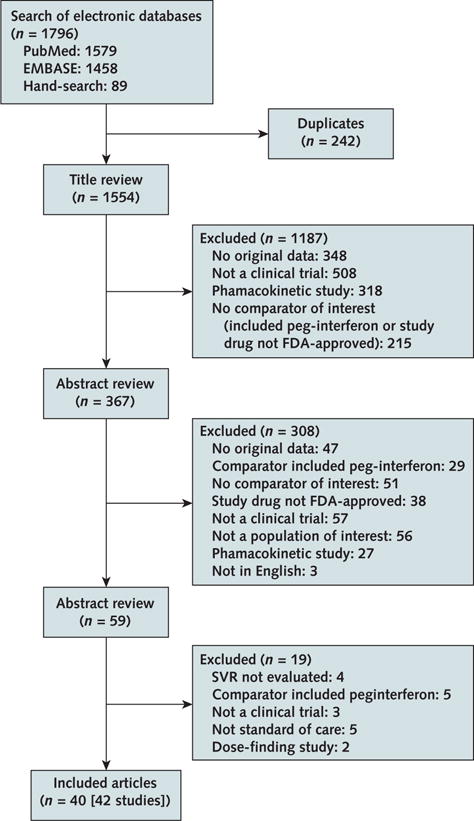
FDA = U.S. Food and Drug Administration; SVR = sustained virologic response.
Study Selection
We included English-language, single-group, randomized, controlled trials (RCTs) of adults with chronic HCV infection that evaluated at least 8 weeks of an FDA-approved interferon-free HCV regimen that included at least 2 DAAs. We included trials that used DAA combinations—including inhibitors of HCV NS3 protease (grazoprevir, paritaprevir, and simeprevir), NS5A (daclatasvir, elbasvir, ledipasvir, ombitasvir, and velpatasvir), and NS5B polymerase (sofosbuvir and dasabuvir), as well as the oral antiviral ribavirin—and for which the primary outcome was SVR. We excluded studies published only as abstracts; dose-finding studies; those in which the primary outcome was pharmacokinetics; or those in which the regimens included interferon, DAAs that were not FDA-approved, or only 1 DAA (for example, sofosbuvir plus ribavirin). Trials were included regardless of participants’ cirrhosis, HIV, or liver transplantation status, but trials of limited populations (for example, DAA-experienced patients or those of a single race) were excluded.
Data Extraction and Quality Assessment
Two reviewers independently screened titles and abstracts and then the full text of potentially eligible articles to identify studies meeting inclusion criteria. Using standardized forms, 1 reviewer extracted information from the selected studies about study characteristics, design, outcomes, and the funding source. A second reviewer confirmed the accuracy of the extractions. Differences were resolved through consensus. Two reviewers independently assessed risk of bias for each selected study by using 5 items from the Cochrane risk-of-bias tools for RCTs and a Cochrane tool for assessment of risk of bias in nonrandomized trials and observational studies (8, 9).
Data Synthesis and Analysis
Detailed evidence tables were generated, and studies were summarized by outcomes. The results were organized by genotype and then by the specific population studied. The heterogeneity of the interventions precluded quantitative pooling of results.
Role of the Funding Source
The Patient-Centered Outcomes Research Institute (PCORI) funded the study and reviewed the report but did not participate in the formulation of the review’s questions, data searches, study appraisals, evidence interpretation, or the preparation or approval of the manuscript for publication.
Results
Study and Quality Characteristics
Of 1796 citations evaluated, we included 42 studies published in 40 articles (Figure 1). All but 1 of the studies were funded by industry (10). Ten were open-label, single-group studies (10–19); 5 had a placebo group with deferred treatment (20–24); 11 evaluated different durations of therapies and the addition of ribavirin (for example, 8 vs. 12 weeks or 12 vs. 24 weeks of therapy with or without ribavirin) (25–35); 5 evaluated the same duration of therapy with and without ribavirin (36–39); 6 evaluated different durations with ribavirin (40–45); and 3 evaluated different durations of therapy without ribavirin (46–48). Only 2 studies had an active comparator group receiving an HCV treatment regimen other than that being evaluated in the trial (49). Three studies had 48 weeks of posttreatment follow-up, whereas the remainder had 12 or 24 weeks of follow-up.
Of the 42 studies, 19 had low risk of bias and 23 had moderate risk. Sources of possible bias included single-group design (n = 10), lack of information on sequence generation or concealment of the allocation scheme (n = 11), and selective reporting of outcomes (n = 5). Because SVR is a highly objective outcome measure, lack of blinding was not considered an important threat to validity. Rates of loss to follow-up were low (<10% for all studies).
HCV Genotype 1 Infection
Thirty-two studies enrolled persons with HCV genotype 1 infection (Table; Figure 2; and Table 1 of the Supplement, available at Annals.org).
Table.
Summary of Clinical Trial Outcomes, by Genotype and Regimen
| Regimen, by Genotype | Studies Included | Summary of Findings | Risk of Bias |
|---|---|---|---|
| HCV-1 | |||
| GZP–EBV | 4 RCTs (6 articles) (n = 1644) | SVR with 12 wk ≥92% in TN and TE patients with and without cirrhosis, HIV co-infection, and chronic kidney disease. SVR with 12 wk in patients with genotype 1a infection varied according to baseline NS5A RAS status (present, 58%–60%; absent, 98%–99%). | Low (n = 3) Moderate (n = 3) |
| PTV–r–OBV–DAV ± RBV | 10 RCTs (n = 2702) | SVR with 12 wk of 97%–100% in TN and TE patients with genotype 1b infection with and without cirrhosis. In noncirrhotic patients with genotype 1a infection, SVR with 12 wk varied according to RBV use (with, 97%; without, 90%). In cirrhotic patients, SVR with 12 wk varied according to treatment duration (12 wk, 87%; 24 wk, 94%). SVR with 12 wk ± RBV in patients with HIV infection, 94%; a liver transplant, 97%; and chronic kidney disease, 85%. | Low (n = 6) Moderate (n = 4) |
| SIM + SOF ± RBV | 2 RCTs (n = 478) 1 single-group study (n = 103) |
SVR with 12 wk varied according to cirrhosis and treatment experience (TN and no cirrhosis, 97%; TN and cirrhosis, 91%; TE and cirrhosis, 79%). SVR was lower in patients with cirrhosis and the NS3 RAS Q80K at baseline. | Low (n = 1) Moderate (n = 2) |
| DCV + SOF | 2 RCTs (n = 238) 1 single-group study (n = 168) |
SVR with 12 or 24 wk in TN and TE patients, including those with HIV co-infection, 95%–100%. SVR with 12 wk + RBV in patients with a liver transplant, 95%; in those with decompensated cirrhosis, 82%. | Moderate (n = 3) |
| LDV–SOF ± RBV | 7 RCTs (n = 2718) 1 single-group study (n = 327) |
SVR with 12 wk ≥95% in TN patients with and without cirrhosis. In TE patients with cirrhosis, SVR varied according to RBV use and treatment duration (12 wk + no RBV, 86%; 12 wk + RBV, 97%; 24 wk + no RBV, 96%). SVR with 12 wk + RBV in patients with decompensated cirrhosis, 85%–87%. | Low (n = 4) Moderate (n = 4) |
| VEL–SOF ± RBV | 2 RCTs (n = 600) | SVR with 12 wk >98% in TN and TE patients with and without cirrhosis. SVR with 12 wk + RBV in patients with decompensated cirrhosis, 94%. | Low (n = 1) Moderate (n = 1) |
| HCV-2 | |||
| DCV + SOF | 2 RCTs (n = 45) 1 single-group study (n = 5) |
SVR with 12 or 24 wk of 92%–100% in TN and TE patients, including those with HIV co-infection. SVR with 12 wk + RBV in 5 patients with decompensated cirrhosis, 80%. | Moderate (n = 3) |
| VEL–SOF ± RBV | 3 RCTs (n = 407) | SVR with 12 wk of 99%–100% in TN and TE patients with and without cirrhosis. SVR with 12 wk ± RBV in 8 patients with decompensated cirrhosis, 100%. | Low (n = 2) Moderate (n = 1) |
| HCV-3 | |||
| DCV + SOF ± RBV | 3 RCTs (n = 107) 2 single-group studies (n = 169) |
SVR with 12 wk varied according to cirrhosis status (TN/TE + no cirrhosis, 94%–97%; TN/TE + cirrhosis, 58%–69%). SVR with 12 or 16 wk + RBV in patients with cirrhosis, 83%–89%. | Moderate (n = 5) |
| LDV–SOF ± RBV | 1 RCT (n = 26) | SVR varied according to RBV use (12 wk + RBV, 100%; 12 wk + no RBV, 64%). | Low (n = 1) |
| VEL–SOF ± RBV | 2 RCTs (n = 591) | SVR with 12 wk of 95% in TN and TE patients with and without cirrhosis. SVR in patients with decompensated cirrhosis varied according to RBV use (12 wk + RBV, 85%; 12 wk + no RBV, 50%; 24 wk + no RBV, 50%). | Low (n = 1) Moderate (n = 1) |
| HCV-4 | |||
| GZP–EBV ± RBV | 2 RCTs (n = 63) 1 single-group study (n = 28) |
SVR with 12 wk of 96%–100% in TN and TE patients with and without cirrhosis, including those with HIV co-infection. | Moderate (n = 3) |
| PTV–r–OBV ± RBV | 1 RCT (n = 135) | SVR varied according to RBV use in TN and TE patients without cirrhosis (12 wk + RBV, 100%; 12 wk + no RBV, 91%). | Low (n = 1) |
| SIM + SOF | 1 RCT (n = 63) | SVR with 12 wk of 100% in TN and TE patients with and without cirrhosis. | Moderate (n = 1) |
| LDV–SOF ± RBV | 2 RCTs (n = 41) 3 single-group studies (n = 74) |
SVR with 12 wk of 93%–95% in TN and TE patients with and without cirrhosis. SVR with 12 wk + RBV in 7 patients with decompensated cirrhosis before and after liver transplantation, 0%–100%. | Low (n = 2) Moderate (n = 3) |
| VEL–SOF ± RBV | 2 RCTs (n = 146) | SVR with 12 wk of 100% in TN and TE patients with and without cirrhosis. SVR with 12 wk ± RBV in 6 patients with decompensated cirrhosis, 100%. | Low (n = 1) Moderate (n = 1) |
| HCV-5 | |||
| LDV–SOF | 1 single-group study (n = 41) | SVR with 12 wk of 95% in TN and TE patients with and without cirrhosis. | Moderate (n = 1) |
| VEL–SOF | 1 RCT (n = 35) | SVR with 12 wk of 97% in TN and TE patients with and without cirrhosis. | Moderate (n = 1) |
| HCV-6 | |||
| LDV–SOF | 1 single-group study (n = 25) | SVR with 12 wk of 96% in TN and TE patients with and without cirrhosis. | Moderate (n = 1) |
| VEL–SOF | 2 RCTs (n = 42) | SVR with 12 wk of 100% in TN and TE patients with and without cirrhosis. SVR with 24 wk in 1 patient with decompensated cirrhosis, 100%. | Low (n = 1) Moderate (n = 1) |
DAV = dasabuvir; DCV = daclatasvir; EBV = elbasvir; GZP = grazoprevir; HCV = hepatitis C virus; LDV = ledipasvir; OBV = ombitasvir; PTV–r = paritaprevir–ritonavir; Q80K = position 80 of the NS3 region; RAS = resistance-associated substitution; RBV = ribavirin; RCT = randomized, controlled trial; SIM = simeprevir; SOF = sofosbuvir; SVR = sustained virologic response; TE = treatment-experienced; TN = treatment-naive; VEL = velpatasvir.
Figure 2. HCV genotype 1a and 1b SVR12 rates and 95% CIs, by oral DAA regimen and clinical trial.
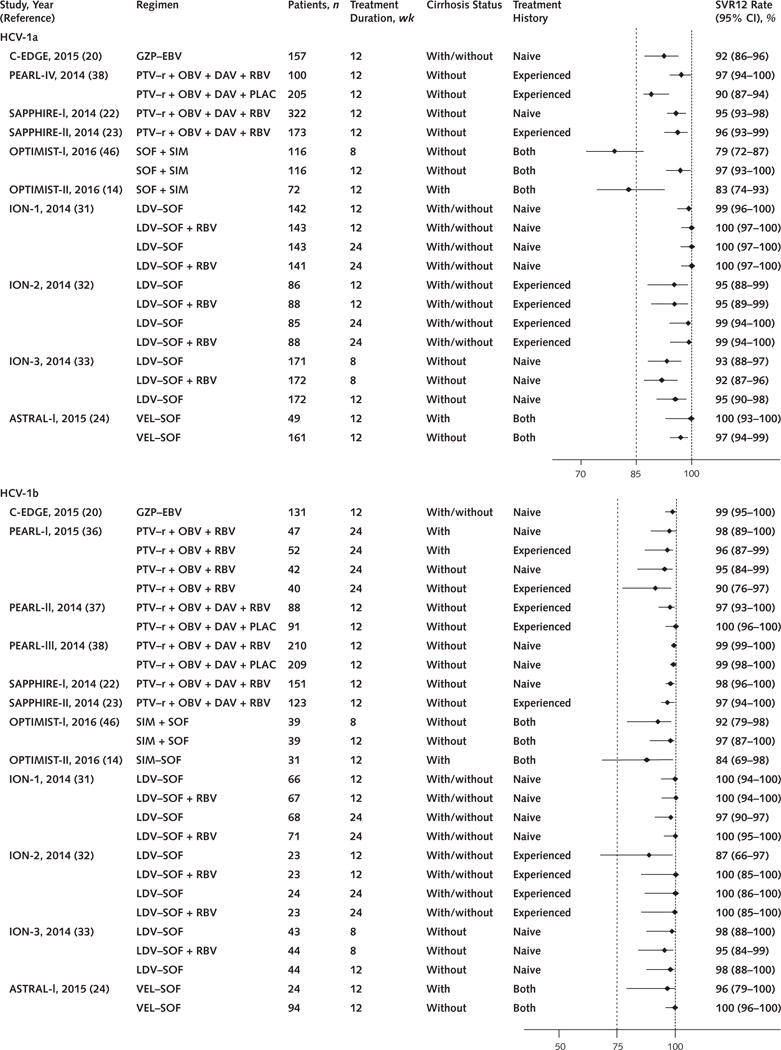
DAA = direct-acting antiviral; DAV = dasabuvir; DCV = daclatasvir; EBV = elbasvir; GZP = grazoprevir; HCV = hepatitis C virus; LDV = ledipasvir; OBV = ombitasvir; PLAC = placebo; PTV–r = paritaprevir–ritonavir; RBV = ribavirin; SIM = simeprevir; SOF = sofosbuvir; SVR12 = sustained virologic response at 12 wk; VEL = velpatasvir.
Regimens That Include NS3/4A Protease Inhibitors
Grazoprevir–Elbasvir
Grazoprevir is an NS3 protease inhibitor that is available in a fixed-dose combination with elbasvir, an NS5A inhibitor. This regimen was studied in 4 multicenter randomized trials published in 6 articles (11, 20, 21, 25–27). Risk of bias was moderate in 3 of these studies due to lack of a comparator group (n = 1) and selective reporting (n = 2). Daily grazoprevir-elbasvir for 12 weeks was associated with SVR rates of 92% and 99% to 100% in treatment-naive and treatment-experienced patients with genotype 1a and 1b infection, respectively (20, 26, 27). Among patients with genotype 1a but not genotype 1b infection, lower SVR rates were associated with pretreatment presence of naturally occurring resistance-associated substitutions (RASs) at positions 28, 30, 31, and 93 of the NS5A region (20, 27). Prolongation of therapy to 16 weeks and addition of ribavirin led to SVR among 49 treatment-experienced patients, including all 6 patients with baseline NS5A RASs (27). Ribavirin was associated with greater incidence of anemia (3% to 16% vs. 0%), fatigue, and nausea (25–27). With the exception of patients with genotype 1a infection with baseline RASs, the SVR rate was similar in those treated with or without ribavirin. Cirrhosis was not associated with lower SVR rates (14, 16).
Paritaprevir–Ritonavir–Ombitasvir and Dasabuvir
Paritaprevir is an NS3 protease inhibitor that is coformulated with ritonavir (to provide pharmacologic boosting) and ombitasvir (an NS5A inhibitor). For patients with genotype 1 infection, dasabuvir (a non-nucleoside NS5B polymerase inhibitor) was added. We identified 1 study with low risk of bias that used the two-DAA regimen without dasabuvir (45) and 9 studies (5 with low risk of bias and 4 with moderate risk of bias) that used the three-DAA regimen for 12 or 24 weeks (12, 13, 22, 23, 37, 38, 40, 41). Moderate risk of bias was due to lack of a comparator group (n = 2) and unclear sequence generation and allocation scheme concealment (n = 2). The three-DAA regimen without ribavirin yielded lower SVR rates in persons with genotype 1a infection (90%) than those with genotype 1b infection (99%); however, with the addition of ribavirin, the SVR rate among noncirrhotic patients with genotype 1a infection increased to 97% (38). Compared with placebo, ribavirin was associated with more anemia, fatigue, insomnia, and rash (22, 38). Among cirrhotic patients with genotype 1a infection, the three-DAA regimen plus ribavirin for 24 weeks led to higher SVR rates than 12 weeks of treatment (94.2% vs. 88.6%) (41). High rates of SVR were seen among cirrhotic and noncirrhotic patients with genotype 1b infection treated for 12 weeks with the three-DAA regimen alone or with ribavirin (97% to 100%) (22, 23, 37, 38, 41, 45).
Simeprevir and Sofosbuvir
Simeprevir is an NS3 protease inhibitor that is used once daily in combination with sofosbuvir, a nucleoside analogue NS5B polymerase inhibitor. We identified 3 studies using this regimen (14, 28, 46). Risk of bias was moderate in 2 studies due to unclear sequence generation (n = 1) and lack of a comparator group (n = 1). When used for 12 weeks, the regimen was associated with high rates of SVR (97%) in persons with HCV genotype 1a or 1b infection without cirrhosis (46). In this population, pretreatment presence of naturally occurring simeprevir RASs at position 80 of the NS3 region (Q80K) was not associated with lower SVR rates (46). However, lower SVR rates were observed among patients with cirrhosis (79% to 88%) and, in this population, the presence of the Q80K RAS was associated with lower SVR rates in patients with genotype 1a infection (74% with Q80K and 92% without) (14).
Regimens That Do Not Include NS3/4A Protease Inhibitors
Daclatasvir and Sofosbuvir
Daclatasvir is an NS5A inhibitor used with sofosbuvir. Clinical trial data on this combination are limited but suggest high SVR rates with 12- and 24-week treatment (96% to 100%), based on data from 2 studies with moderate risk of bias (29, 48). Among patients with advanced liver disease, SVR rates were lower (82%) (15).
Ledipasvir–Sofosbuvir
Ledipasvir, an NS5A inhibitor, is coformulated with sofosbuvir as a once-daily tablet. Eight studies (4 with low risk of bias and 4 with moderate risk of bias) evaluated different treatment durations (8, 12, and 24 weeks) and the addition of ribavirin (17, 30–34, 43, 44). Moderate risk of bias was due to lack of a comparator (n = 1) and unclear sequence generation or allocation scheme concealment (n = 3). In treatment-naive patients, SVR rates were greater than 95% with 12 weeks of treatment, and longer treatment did not yield higher rates (30, 31, 33). Although 8 weeks of therapy was assessed in 1 RCT and was found to lead to high SVR rates in noncirrhotic persons with pretreatment HCV RNA levels less than 6 × 106 IU/mL (33), the most data on efficacy are for 12 weeks. In treatment-naive patients, ribavirin was not associated with higher SVR rates regardless of cirrhosis status, whereas in treatment-experienced patients, either longer therapy (24 weeks) with ledipasvir–sofosbuvir or the addition of ribavirin to the regimen for 12 weeks was associated with higher SVR rates in patients with cirrhosis (97% vs. 96%) (34). The addition of ribavirin led to more adverse events, notably anemia, fatigue, and insomnia (31–33).
Velpatasvir–Sofosbuvir
Velpatasvir, a pangenotypic NS5A inhibitor, is coformulated with sofosbuvir as a once-daily tablet. This regimen for 12 weeks was associated with high SVR rates (97% to 99%) in patients with HCV genotype 1a or 1b infection, including those with cirrhosis and prior treatment experience (24). In this placebo-controlled, double-blind trial with low risk of bias, the incidence of adverse events was similar in patients receiving velpatasvir–sofosbuvir and those receiving placebo.
HCV Genotype 2 Infection
Six studies enrolled patients with HCV genotype 2 infection (Table and Figure 3); 3 studies (2 with low risk of bias and 1 with moderate risk of bias) evaluated the fixed-dose combination of velpatasvir–sofosbuvir (24, 35, 49), and 3 with moderate risk of bias evaluated daclatasvir plus sofosbuvir (15, 29, 48).
Figure 3. HCV genotype 2 to 6 SVR12 rates and 95% CIs, by oral DAA regimen and clinical trial.
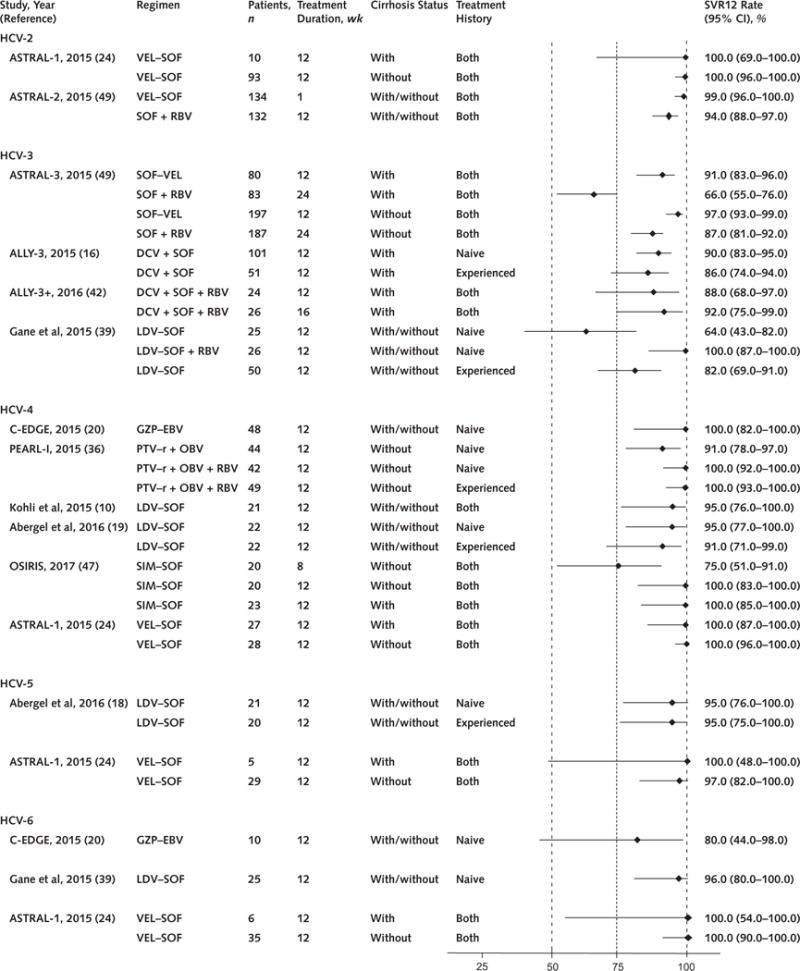
DAA = direct-acting antiviral; DAV = dasabuvir; DCV = daclatasvir; EBV = elbasvir; GZP = grazoprevir; HCV = hepatitis C virus; LDV = ledipasvir; OBV = ombitasvir; PTV–r = paritaprevir–ritonavir; RBV = ribavirin; SIM = simeprevir; SOF = sofosbuvir; SVR12 = sustained virologic response at 12 wk; VEL = velpatasvir.
Daclatasvir and Sofosbuvir
In the ALLY-2 study, all 13 HIV-infected patients with genotype 2 infection who were treated for 12 weeks achieved SVR (48). In another study, 24 of 26 (92%) treatment-naive, noncirrhotic, HIV-seronegative patients treated for 24 weeks with or without ribavirin achieved SVR; 2 patients were lost to follow-up (29).
Velpatasvir–Sofosbuvir
The ASTRAL-1 and ASTRAL-2 studies reported SVR in 237 of 238 patients (99%) with genotype 2 infection who received velpatasvir–sofosbuvir for 12 weeks; 1 patient was lost to follow-up (24, 49). Rates of SVR were not affected by cirrhosis or prior treatment experience. In an RCT, velpatasvir–sofosbuvir was superior to sofosbuvir plus ribavirin (SVR of 99% vs. 94%) and was associated with fewer adverse events (49).
HCV Genotype 3 Infection
Eight studies enrolled patients with HCV genotype 3 infection (Table and Figure 3).
Daclatasvir and Sofosbuvir
In a phase 2 study, 16 of 18 noncirrhotic patients treated with or without ribavirin for 24 weeks achieved SVR (29). In the single-group ALLY-3 trial, which had moderate risk of bias, 94% to 97% of noncirrhotic treatment-naive and treatment-experienced patients achieved SVR with 12 weeks of treatment (16). In the same study, cirrhosis was associated with a marked reduction in SVR (58% to 69%) (16). The addition of ribavirin to the regimen for 12 or 16 weeks in patients with advanced liver disease led to SVR in 86% of cirrhotic patients (n = 36) in the ALLY-3+ study, which had moderate risk of bias due to unclear sequence generation and allocation scheme (42).
Ledipasvir–Sofosbuvir
In a single-center study with low risk of bias, all 26 treatment-naive patients treated with ledipasvir– sofosbuvir plus ribavirin for 12 weeks achieved SVR (39). The SVR rate was lower without ribavirin (64%) and in treatment-experienced patients (82%) (39).
Velpatasvir–Sofosbuvir
In a phase 3 RCT with 552 patients and low risk of bias, velpatasvir–sofosbuvir for 12 weeks (95%) was superior to sofosbuvir plus ribavirin for 24 weeks (80%) and was associated with fewer adverse events, particularly less anemia (49). Lower SVR rates were observed in patients with pretreatment presence of velpatasvir NS5A RASs, particularly at position 93 (88%), compared with those without RASs (97%).
HCV Genotype 4 Infection
Twelve studies enrolled persons with HCV genotype 4 infection (Table and Figure 3).
Grazoprevir–Elbasvir
In the C-EDGE study, efficacy of grazoprevir– elbasvir was demonstrated among 18 of 18 treatment-naive patients with genotype 4 infection (SVR of 100%) who received the regimen for 12 weeks; baseline presence of NS5A RASs did not affect SVR (20). Among treatment-experienced patients in a randomized trial of 12 or 16 weeks of the regimen with or without ribavirin, SVR rates were below 95% in all groups except patients who received 16 weeks of the regimen with ribavirin (27).
Paritaprevir–Ritonavir–Ombitasvir
In 1 trial with low risk of bias, paritaprevir– ritonavir–ombitasvir plus ribavirin resulted in high efficacy (SVR of 100%) in both treatment-naive (n = 42) and treatment-experienced (n = 44) patients with genotype 4 infection (36). The absence of ribavirin was associated with a lower SVR rate (91%).
Simeprevir and Sofosbuvir
In an RCT with moderate risk of bias due to unclear sequence generation and allocation scheme concealment, simeprevir plus sofosbuvir was associated with SVR in all 43 patients (100%) treated for 12 weeks, including those with cirrhosis (n = 23); however, SVR rates were lower in 20 patients treated for 8 weeks (75%) (47).
Ledipasvir–Sofosbuvir
In a single-group trial of 21 patients, 95% who received 12 weeks of ledipasvir–sofosbuvir achieved SVR; the study included few patients with cirrhosis (n = 7) or prior treatment experience (n = 8) (10). In a similar trial conducted in France, 41 of 44 patients (93%) who were treated for 12 weeks achieved SVR (19). No serious adverse events were reported in these studies (10, 19).
Velpatasvir–Sofosbuvir
In the ASTRAL-1 RCT, which had low risk of bias, velpatasvir–sofosbuvir led to SVR in all 116 treatment-naive and treatment-experienced patients (100%) who were treated, including those with cirrhosis (24).
HCV Genotype 5 and 6 Infection
Six studies enrolled persons with HCV genotype 5 and/or 6 infection (18, 20, 24, 27, 35, 39) (Figure 3).
Ledipasvir–Sofosbuvir
This combination led to high SVR rates in persons with genotype 5 (n = 41; SVR of 95%) and genotype 6 (n = 25; SVR of 96%) infection (18, 39). Although the numbers of patients in these subgroups were small, SVR rates were high in treatment-experienced patients (≥95%) and those with cirrhosis (89%) (18).
Velpatasvir–Sofosbuvir
In 1 RCT with low risk of bias, patients with genotype 5 (n = 35) and genotype 6 (n = 41) infection achieved high rates of SVR (97% and 100%, respectively) with 12 weeks of treatment; only 1 patient did not achieve SVR (death unrelated to treatment) (24).
Subpopulations
Patients With HIV Co-infection
Direct-acting antiviral regimens used for 12 or 24 weeks showed high SVR rates (91% to 98%) and low adverse event rates (<10%). These rates were similar to those observed in persons without HIV (11, 17, 26, 40, 48). Shorter therapy (8 weeks) was evaluated in 1 RCT of daclatasvir plus sofosbuvir and led to lower rates of SVR (76%) than 12 weeks of therapy (97%) (48).
Patients With Decompensated Cirrhosis
Relatively few patients with decompensated liver disease (for example, those with jaundice, ascites, encephalopathy, or variceal hemorrhage) have been enrolled in DAA trials. Because of impaired metabolism, NS3 protease inhibitors are not recommended (simeprevir) or are contraindicated (paritaprevir or grazoprevir) in patients with Child–Turcotte–Pugh class B and C disease. These patients have been treated in trials of sofosbuvir plus NS5A inhibitors, including daclatasvir, ledipasvir, and velpatasvir (15, 35, 43, 44). In 1 RCT, velpatasvir–sofosbuvir with ribavirin for 12 weeks was more effective than velpatasvir–sofosbuvir alone for 12 or 24 weeks; however, ribavirin was associated with more treatment discontinuation due to adverse events (35). Across all studies, rates of serious adverse events were higher in patients with decompensated cirrhosis (10% to 52%) than in the general HCV patient populations (<10%).
Patients After Liver Transplantation
Four trials evaluated DAAs in patients who had undergone liver transplantation. Overall, SVR rates observed in these trials were similar to those reported in patients without a transplant (12, 15, 43, 44). However, among liver transplant patients with decompensated liver disease due to recurrent HCV infection, SVR rates were lower (50% to 80%) and adverse event rates were higher (16% to 75%) than those observed in liver transplant patients with compensated cirrhosis or those with minimal liver disease (6% to 21%) (12, 43, 44).
Patients With Chronic Kidney Disease
In 2 studies of patients with advanced renal dysfunction, including those receiving hemodialysis, high SVR rates were reported in those with HCV genotype 1 infection (13, 21). In 1 study with low risk of bias, grazoprevir–elbasvir for 12 weeks resulted in SVR in 94% of patients (n = 111) (21). In a smaller study with moderate risk of bias due to lack of a comparator group, a regimen of paritaprevir–ritonavir–ombitasvir and dasabuvir was effective (SVR of 90%), but ribavirin, which was used for patients with genotype 1a infection, was poorly tolerated and was discontinued due to adverse events in 8 of 14 patients (48).
Discussion
Multiple interferon-free, oral DAA regimens are available for treatment of chronic HCV infection. We found high SVR rates for all FDA-approved DAA regimens, with some evidence of variable response influenced by specific patient and virus characteristics. Rates of serious adverse events (<10%), loss to follow-up (<10%), and treatment discontinuation (<5%) were low even in patients with comorbid conditions, such as HIV infection and cirrhosis.
The evidence was robust for persons with genotype 1 infection, which is the most common genotype worldwide, infecting approximately 84 million persons (50). We reviewed 6 distinct DAA regimens for genotype 1 infection, with SVR rates greater than 95% for most drug combinations and patient populations. Our findings represent an important update of other systematic reviews of DAA regimens with and without interferon for treatment of HCV genotype 1 infection, which reported SVR rates in the range of 95% (50, 51) and 92% (52). The high treatment response rates in persons with genotype 1 infection are particularly important in light of the historically poor SVR rates observed with interferon in this population.
In contrast, fewer DAA regimens are available and effective for the treatment of HCV genotype 3 infection, which is the second most prevalent HCV genotype globally, infecting approximately 54 million persons. Our findings indicate that the most effective DAA regimens for patients who have genotype 3 infection without cirrhosis are sofosbuvir plus the NS5A inhibitors velpatasvir or daclatasvir for 12 weeks, whereas higher SVR rates were observed with velpatasvir–sofosbuvir in patients with cirrhosis. This agrees with recent systematic reviews, identified through MEDLINE searches from 2014 to 2016, that identified velpatasvir–sofosbuvir as the most effective treatment for genotype 3 infection (51, 52). Our findings also suggest that lower SVR rates were achieved in patients with compensated and decompensated cirrhosis, prior treatment experience, or NS5A RASs; the addition of ribavirin and longer treatment duration were associated with higher SVR rates in these patient groups (42, 53).
Although relatively few studies enrolled patients with genotype 2, 4, 5, or 6 infection, high rates of SVR (>92%) were observed for all regimens administered for at least 12 weeks. Rates of SVR were particularly high (99%) for patients with genotype 2, 4, 5, or 6 infection treated with velpatasvir–sofosbuvir (24). For treatment of genotype 4 infection, all but 1 of the DAA regimens (paritaprevir–ritonavir–ombitasvir) led to high SVR rates (93% to 100%) without ribavirin and were associated with minimal adverse effects in treatment-naive patients.
Oral DAA regimens also showed high SVR rates and minimal adverse events in patient populations that were poorly responsive or could not be treated with interferon, including those with HIV co-infection, decompensated cirrhosis, severe chronic kidney disease, and a liver transplant. Patients co-infected with HIV and HCV and those receiving immunosuppressive agents after liver transplantation had SVR rates similar to those of persons without immune dysfunction, suggesting that oral DAAs mitigate the effect of an impaired HCV immune response (54–56). Direct-acting antiviral options for persons with severe chronic kidney disease remain limited, and although high SVR rates (85% to 100%) were observed in 2 RCTs for persons with HCV genotype 1 infection, no trials were identified in persons with genotype 2 or 3 infection, for whom interferon is still recommended (57). Treatment options also remain limited in patients with decompensated liver disease. Current NS3 protease inhibitors are hepatically metabolized and are contraindicated in this population; as such, trials have been restricted to sofosbuvir plus NS5A inhibitors. The evidence indicates that these regimens provide high rates of SVR (>85%), but serious adverse events are common (10% to 52%). In addition, questions remain with regard to the long-term clinical benefit of cure of HCV infection in persons with severe liver dysfunction.
Across multiple trials, our findings indicate that ribavirin continues to have a role in maximizing SVR rates in certain patients, including those with genotype 1a or 3 infection, cirrhosis, or prior treatment experience. Clinical trials for patients with decompensated cirrhosis and a liver transplant have also largely included ribavirin. Although ribavirin was associated with an increase in anemia, fatigue, and insomnia, the rates of serious adverse events and treatment discontinuation were similar in patients treated with and without it.
Limitations of this study include the fact that safety data from clinical trials may not fully represent patient experience in clinical practice. Persons with chronic hepatitis B virus infection were excluded from trials, and the risk for hepatitis B virus reactivation was not examined. We also included noncontrolled trials; however, spontaneous cure of HCV infection is rare. Most of the studies were industry-funded; such studies are more likely to be published if results are favorable (58), but we are not aware of large, unpublished studies in this field and the risk of bias with the objective outcome of SVR is low. The heterogeneity of the interventions studied also prevented quantitative pooling of results, and the relatively short follow-up limits our ability to comment on late relapse of HCV infection. Several studies were also population-specific, thus limiting generalizability of findings to all patients. Given the multitude of effective oral DAA regimens with similar rates of SVR and adverse events, RCTs will be needed to determine the best HCV treatments for different patient populations. One such trial, the PRIORITIZE study (ClinicalTrials.gov: NCT02786537), is under way in persons with genotype 1 infection (59).
Finally, our systematic review is limited by the rapidly evolving HCV treatment landscape and the inability to include all DAA regimens in ongoing or recently completed clinical trials that we identified on ClinicalTrials.gov (Table 7 of the Supplement). These ongoing clinical trials include 2 novel nucleotide analogue NS5B polymerase inhibitors, MK-3682 and AL-335, which are being evaluated in combination with approved NS3 protease inhibitors and novel NS5A inhibitors (ruzasvir and odalasvir), as well as 2 novel pangenotypic NS3 protease inhibitors, voxilaprevir and glecaprevir, which are being evaluated in combination with approved (sofosbuvir–velpatasvir–voxilaprevir) and novel (glecaprevir–pibrentasvir) DAAs (60).
In conclusion, oral DAA regimens that are highly efficacious, well-tolerated, and relatively short in duration are now available for all HCV genotypes and for patient populations historically considered difficult to cure. The ease of dosing, safety profile, and effectiveness of these agents provide an opportunity to expand the number of patients who can be treated for HCV infection and the pool of treating providers. Rapid developments in oral DAA therapies can be beneficial only if they are linked to efforts to improve rates of HCV detection, linkage to care, and access to DAA therapy.
Supplementary Material
Acknowledgments
Disclaimer: This work was partially supported through a research contract from PCORI (HPC-1503-27891). The statements presented in this article are solely the responsibility of the authors and do not necessarily represent the views of PCORI, its Board of Governors, or its Methodology Committee.
Financial Support: In part by a research contract from PCORI (HPC-1503-27891). Dr. Falade-Nwulia was supported by a Johns Hopkins Clinician Scholar Award and a National Institutes of Health patient-oriented research career development award (K23 DA041294). Dr. Sulkowski was supported by a National Institutes of Health midcareer mentor award (K24 DA034621).
Dr. Nelson reports grants from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceutical, and Merck outside the submitted work. Dr. Fried reports grants and personal fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, and Merck outside the submitted work. Dr. Segal reports a grant from the Patient-Centered Outcomes Research Institute during the conduct of the study. Dr. Sulkowski reports a grant from the National Institutes of Health during the conduct of the study; grants from AbbVie, Gilead Sciences, Janssen Pharmaceutical, and Merck outside the submitted work; and personal fees from AbbVie, Cocrystal Pharma, Gilead Sciences, Janssen Pharmaceutical, Merck, and Trek outside the submitted work.
Footnotes
Disclosures: Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M16-2575.
Authors not named here have disclosed no conflicts of interest.
Current author addresses and author contributions are available at Annals.org.
Author Contributions: Conception and design: O. Falade-Nwulia, C. Suarez-Cuervo, D.R. Nelson, M.W. Fried, J.B. Segal, M.S. Sulkowski.
Analysis and interpretation of the data: O. Falade-Nwulia, C. Suarez-Cuervo, D.R. Nelson, M.W. Fried, J.B. Segal, M.S. Sulkowski.
Drafting of the article: O. Falade-Nwulia, C. Suarez-Cuervo, M.S. Sulkowski.
Critical revision of the article for important intellectual content: O. Falade-Nwulia, C. Suarez-Cuervo, D.R. Nelson, M.W. Fried, J.B. Segal, M.S. Sulkowski.
Final approval of the article: O. Falade-Nwulia, C. Suarez-Cuervo, D.R. Nelson, M.W. Fried, J.B. Segal, M.S. Sulkowski.
Provision of study materials or patients: C. Suarez-Cuervo.
Statistical expertise: C. Suarez-Cuervo, J.B. Segal.
Obtaining of funding: M.S. Sulkowski.
Administrative, technical, or logistic support: C. Suarez-Cuervo, M.S. Sulkowski.
Collection and assembly of data: O. Falade-Nwulia, C. Suarez-Cuervo, J.B. Segal, M.S. Sulkowski.
References
- 1.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Hughes KN, Ly EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003–2013. Clin Infect Dis. 2016;62:1287–8. doi: 10.1093/cid/ciw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell O, Gurakar A. Management of hepatitis C post-liver transplantation: a comprehensive review. J Clin Transl Hepatol. 2015;3:140–8. doi: 10.14218/JCTH.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308:370–8. doi: 10.1001/jama.2012.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 7.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–17. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 9.Sterne J, Higgins JPT, Reeves B, Development Group for ROBINS-I A tool for assessing Risk Of Bias in Non-randomized Studies of Interventions. Version. 2016 Jul;5 Accessed at www.riskofbias.info on 3 August 2016. [Google Scholar]
- 10.Kohli A, Kapoor R, Sims Z, Nelson A, Sidharthan S, Lam B, et al. Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis. 2015;15:1049–54. doi: 10.1016/S1473-3099(15)00157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2(15):e319–27. 00114–9. doi: 10.1016/S2352-3018. [DOI] [PubMed] [Google Scholar]
- 12.Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R, Jr, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371:2375–82. doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 13.Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, et al. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology. 2016;150:1590–8. doi: 10.1053/j.gastro.2016.02.078. [DOI] [PubMed] [Google Scholar]
- 14.Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: a phase 3 study (OPTIMIST-2) Hepatology. 2016;64:360–9. doi: 10.1002/hep.28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493–505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. ALLY-3 Study Team All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127–35. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. ION-4 Investigators Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:705–13. doi: 10.1056/NEJMoa1501315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abergel A, Asselah T, Metivier S, Kersey K, Jiang D, Mo H, et al. Ledipasvir-sofosbuvir in patients with hepatitis C virus genotype 5 infection: an open-label, multicentre, single-arm, phase 2 study. Lancet Infect Dis. 2016;16(15):459–64. 00529–0. doi: 10.1016/S1473-3099. [DOI] [PubMed] [Google Scholar]
- 19.Abergel A, Metivier S, Samuel D, Jiang D, Kersey K, Pang PS, et al. Ledipasvir plus sofosbuvir for 12 weeks in patients with hepatitis C genotype 4 infection. Hepatology. 2016;64:1049–56. doi: 10.1002/hep.28706. [DOI] [PubMed] [Google Scholar]
- 20.Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4 or 6 infection: a randomized trial. Ann Intern Med. 2015;163:1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 21.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H, Jr, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–45. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 22.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 23.Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604–14. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 24.Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, et al. ASTRAL-1 Investigators Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5 and 6 Infection. N Engl J Med. 2015;373:2599–607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 25.Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(14):1075–86. 61795–5. doi: 10.1016/S0140-6736. [DOI] [PubMed] [Google Scholar]
- 26.Sulkowski M, Hezode C, Gerstoft J, Vierling JM, Mallolas J, Pol S, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(14):1087–97. 61793–1. doi: 10.1016/S0140-6736. [DOI] [PubMed] [Google Scholar]
- 27.Kwo P, Gane EJ, Peng CY, Pearlman B, Vierling JM, Serfaty L, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology. 2017;152:164–175. doi: 10.1053/j.gastro.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 28.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384(14):1756–65. 61036–9. doi: 10.1016/S0140-6736. [DOI] [PubMed] [Google Scholar]
- 29.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. AI444040 Study Group Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–21. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 30.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383(13):515–23. 62121–2. doi: 10.1016/S0140-6736. [DOI] [PubMed] [Google Scholar]
- 31.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. ION-1 Investigators Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 32.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. ION-2 Investigators Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 33.Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370:222–32. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
- 34.Bourlière M, Bronowicki JP, de Ledinghen V, Hézode C, Zoulim F, Mathurin P, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS) Lancet Infect Dis. 2015;15(15):397–404. 70050–2. doi: 10.1016/S1473-3099. [DOI] [PubMed] [Google Scholar]
- 35.Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. ASTRAL-4 Investigators Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373:2618–28. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 36.Hézode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385(15):2502–9. 60159–3. doi: 10.1016/S0140-6736. [DOI] [PubMed] [Google Scholar]
- 37.Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–365. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 38.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. PEARL-III Study ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 39.Gane EJ, Hyland RH, An D, Svarovskaia E, Pang PS, Brainard D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology. 2015;149:1454–61. doi: 10.1053/j.gastro.2015.07.063. [DOI] [PubMed] [Google Scholar]
- 40.Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313:1223–31. doi: 10.1001/jama.2015.1328. [DOI] [PubMed] [Google Scholar]
- 41.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–82. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 42.Leroy V, Angus P, Bronowicki JP, Dore GJ, Hezode C, Pianko S, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3+) Hepatology. 2016;63:1430–41. doi: 10.1002/hep.28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr, et al. SOLAR-1 Investigators Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–59. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, et al. SOLAR-2 investigators Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685–97. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 45.Lawitz E, Makara M, Akarca US, Thuluvath PJ, Preotescu LL, Varunok P, et al. Efficacy and safety of ombitasvir, paritaprevir, and ritonavir in an open-label study of patients with genotype 1b chronic hepatitis C virus infection with and without cirrhosis. Gastroenterology. 2015;149:971–80. doi: 10.1053/j.gastro.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Kwo P, Gitlin N, Nahass R, Bernstein D, Etzkorn K, Rojter S, et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016;64:370–80. doi: 10.1002/hep.28467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Raziky M, Gamil M, Ashour MK, Sameea EA, Doss W, Hamada Y, et al. Simeprevir plus sofosbuvir for eight or 12 weeks in treatment-naïve and treatment-experienced hepatitis C virus genotype 4 patients with or without cirrhosis. J Viral Hepat. 2017;24:102–110. doi: 10.1111/jvh.12625. [DOI] [PubMed] [Google Scholar]
- 48.Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, et al. ALLY-2 Investigators Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373:714–25. doi: 10.1056/NEJMoa1503153. [DOI] [PubMed] [Google Scholar]
- 49.Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, et al. ASTRAL-2 Investigators Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–17. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 50.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berden FA, Aaldering BR, Groenewoud H, IntHout J, Kievit W, Drenth JP. Identification of the best direct-acting antiviral regimen for patients with hepatitis C virus genotype 3 infection: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 52.Gimeno-Ballester V, Buti M, San Miguel R, Riveiro M, Esteban R. Interferon-free therapies for patients with chronic hepatitis C genotype 3 infection: a systematic review. J Viral Hepat. 2016 doi: 10.1111/jvh.12660. [DOI] [PubMed] [Google Scholar]
- 53.Majumdar A, Kitson MT, Roberts SK. Systematic review: current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2016;43:1276–92. doi: 10.1111/apt.13633. [DOI] [PubMed] [Google Scholar]
- 54.Rosenthal ES, Kottilil S, Polis MA. Sofosbuvir and ledipasvir for HIV/HCV co-infected patients. Expert Opin Pharmacother. 2016;17:743–9. doi: 10.1517/14656566.2016.1157580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swallow E, Song J, Yuan Y, Kalsekar A, Kelley C, Mu F, et al. Daclatasvir + sofosbuvir versus standard of care for hepatitis C genotype 3: a matching-adjusted indirect comparison. J Comp Eff Res. 2016;5:129–39. doi: 10.2217/cer.15.49. [DOI] [PubMed] [Google Scholar]
- 56.Swallow E, Song J, Yuan Y, Kalsekar A, Kelley C, Peeples M, et al. Daclatasvir and sofosbuvir versus sofosbuvir and ribavirin in patients with chronic hepatitis C coinfected with HIV: a matching-adjusted indirect comparison. Clin Ther. 2016;38:404–12. doi: 10.1016/j.clinthera.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 57.American Association for the Study of Liver Diseases; Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. doi: 10.1002/hep.31060. Accessed at www.hcvguidelines.org on 16 January 2017. [DOI] [PMC free article] [PubMed]
- 58.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326:1167–70. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patient-Centered Outcomes Research Institute. The PRIORITIZE study: a pragmatic, randomized study of oral regimens for hepatitis C: transforming decision-making for patients, providers, and stakeholders. Accessed at www.pcori.org/research-results/2015/prioritize-study-pragmatic-randomized-study-oral-regimens-hepatitis-c on 17 June 2016.
- 60.Kwo PY, Wyles DL, Wang S, Poordad F, Gane E, Maliakkal B, et al. 100% SVR12 with ABT-493 and ABT-530 with or without ribavirin in treatment-naive HCV genotype 3-infected patients with cirrhosis. Presented at 51st Annual Meeting of the European Association for the Study of the Liver; Barcelona, Spain. 13–17 April 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


