Abstract
Rationale
Health-related quality of life (HRQL) is impaired among patients with interstitial lung disease (ILD). Little is understood about HRQL in specific subtypes of ILD.
Objectives
The aim of this study was to characterize and identify clinical determinants of HRQL among patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD) and compare them to patients with idiopathic pulmonary fibrosis (IPF).
Methods
We identified patients with a diagnosis of RA-ILD and IPF from an ongoing longitudinal cohort of ILD patients. HRQL was measured at their baseline visit using the Short Form Health Survey (SF-36), versions 1 and 2. Regression models were used to characterize and understand the relationship between selected baseline clinical covariates, the physical component score (PCS) and mental component score (MCS) of the SF-36.
Measurements and Main Results
RA-ILD patients (n=50) were more likely to be younger and female compared to IPF patients (n=50). After controlling for age and pulmonary function, RA-ILD patients had a lower HRQL compared to IPF patients, as measured by the PCS (P=0.03), with significant differences in two of four PCS domains – bodily pain (P<0.01) and general health (P=0.01). Clinical covariates most strongly associated with a lower PCS in RA-ILD patients compared to IPF patients were the presence of joint pain or stiffness and dyspnea severity (P<0.01). Mental and emotional health, as measured by the MCS, was similar between RA-ILD and IPF patients.
Conclusion
The physical components of HRQL appear worse in RA-ILD patients compared to IPF patients as measured by the PCS of the SF-36. Differences in the PCS of the SF-36 can be explained in part by dyspnea severity and joint symptoms among patients with RA-ILD.
MeSH key words: rheumatoid arthritis, interstitial lung disease, quality of life, idiopathic pulmonary fibrosis, pain
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic inflammatory condition characterized by symmetric arthritis and synovial inflammation that leads to progressive joint erosion and eventual deformity1. Extra-articular manifestations of RA are common, affecting up to 40% of patients2. Pulmonary involvement in RA can manifest in many ways, including interstitial lung disease (ILD)3. Interstitial lung disease occurs in approximately 10% of patients with RA, leading to significant morbidity and mortality4.
Health-related quality of life (HRQL) is impaired among patients with ILD. In addition to the severity of underlying lung disease, factors such as older age, dyspnea severity, and depression appear to be associated with worse HRQL in ILD5, 6. Less is understood about HRQL among specific subtypes of ILD. A recent study demonstrated worse HRQL among patients with chronic hypersensitivity pneumonitis compared to patients with idiopathic pulmonary fibrosis (IPF)7. The difference in HRQL appeared to be explained, in part, by differences in sex, dyspnea severity, and fatigue.
The focus of this study was to characterize HRQL among patients with RA-ILD compared to patients with IPF and to identify any clinical determinants of HRQL among patients with RA-ILD. We hypothesized that patients with RA-ILD would report worse HRQL compared to patients with IPF, primarily due to the presence of articular manifestations of their disease.
MATERIALS AND METHODS
Study Design and Patient Population
Patients with RA-ILD and an equal number of patients with IPF were identified from an ongoing longitudinal cohort of patients with ILD seen at the University of California, San Francisco (UCSF) from March 2010 to September 2015. The diagnosis of RA-ILD was made prospectively by multidisciplinary discussion. The diagnosis of IPF was made using consensus criteria8, 9. Patients with IPF were matched to the RA-ILD patients by date of their initial ILD clinic visit due to a change in administration of the Short Form Healthy Survey (SF-36), from version 2 to version 1 in 2013. The parent study protocol was reviewed and approved by the UCSF Institutional Human Subject Review Committee (10-01592).
Patients were included in this study if they completed the SF-36 HRQL questionnaire at the time of enrollment. Patients were excluded from this study if they did not have pulmonary function tests (PFTs) within six months of completion of the HRQL self-assessment.
A standardized questionnaire was used to collect baseline patient demographic information, patient reported co-morbidities (e.g., sleep apnea, diabetes mellitus), and symptoms (e.g., fatigue, cough, weight loss, heartburn, joint pain or stiffness). Presence or absence (yes/no) of symptoms was determined based on survey responses to questions such as “Do you cough?” and “Do you experience joint pain or stiffness?”. The degree of dyspnea was measured using the University of California, San Diego Shortness of Breath Questionnaire (UCSD SOBQ), a validated numerical dyspnea-scoring tool in which a higher score corresponds to worse dyspnea10.
Health-Related Quality of Life Measurements
HRQL was measured using the SF-36, versions 1 and 2. The SF-36 is a validated instrument for assessing HRQL and has been applied to a variety of chronic medical conditions, including IPF7, 11, 12. The SF-36 is comprised of questions pertinent to eight domains of HRQL: physical functioning, role – physical, bodily pain, general health, vitality, social functioning, role – emotional, and mental health. The weighted averages of the domain scores are used to generate two summary scores: a physical component score (PCS) and a mental component score (MCS). The individual domain scores and summary scores are transformed to fit a norm-based scale on which the 1998 general U.S. population has a mean score of 50 with a standard deviation of 10. Higher scores indicate a better HRQL. Previous studies have confirmed that norm-based scores generated from version 1 are directly comparable to norm-based scores generated from version 213.
Statistical Analyses
Comparisons between RA-ILD and IPF patients were performed using an unpaired t-test or Chi-squared test. Univariate and multivariate linear regression models were applied to characterize the relationship between select covariates and the PCS and MCS scores. Standardized coefficients were generated to allow comparison between estimates. All multivariate models included age to adjust for potential confounding and percent predicted forced vital capacity (FVC%) to adjust for disease severity. A series of multivariate models were developed to examine the effects of potential covariates on HRQL in RAILD (with the goal of achieving the most parsimonious model that best described the observed data) and to identify potential variables that might explain some or all of the differences in HRQL between ILD subtypes (i.e., RA-ILD and IPF). Covariates were selected based on their performance in the univariate analyses (P≤0.10). The coefficient of determination (R2) was used to characterize model performance. All statistical analyses were performed using STATA version 11 (College Station, TX). Statistical significance was defined as a P value of <0.05.
RESULTS
Patient Characteristics and Clinical Symptoms
This study included 50 RA-ILD patients and 50 IPF patients. Compared to IPF patients, RA-ILD patients were more likely to be younger and female (Table 1). Lung function, based on FVC% and percent predicted diffusing capacity for carbon monoxide (DLCO%), and smoking were similar between groups.
Table 1.
Baseline Demographics and Clinical Characteristics of RA-ILD and IPF Patients
| Variable | RA-ILD (n=50) | IPF (n=50) | P Value |
|---|---|---|---|
| Age, years | 66.7 ± 9.8 | 70.8 ± 8.3 | 0.02 |
| Male sex | 18 (36%) | 39 (78%) | <0.01 |
| BMI* | 28.8 ± 5.6 | 28.1 ± 8.3 | 0.61 |
| Ever smoker | 31 (62%) | 31 (62%) | 1.00 |
| FVC% | 72.0 ± 20.5 | 70.3 ± 18.9 | 0.66 |
| DLCO%† | 51.0 ± 19.2 | 48.5 ± 16.6 | 0.49 |
| Prednisone use at baseline | 45 (90%) | 19 (38%) | <0.01 |
| Diabetes mellitus | 11 (22%) | 7 (14%) | 0.30 |
| Sleep apnea | 10 (20%) | 13 (26%) | 0.48 |
| Fatigue | 41 (82%) | 36 (72%) | 0.24 |
| Cough | 43 (86%) | 44 (88%) | 0.77 |
| Joint pain or stiffness | 46 (92%) | 22 (44%) | <0.01 |
| Weight loss | 18 (36%) | 14 (28%) | 0.39 |
| Heartburn | 23 (46%) | 18 (36%) | 0.31 |
| UCSD SOBQ score¶ | 49.8 ± 29.8 | 50.1 ± 32.4 | 0.96 |
Data are expressed as number (%) for categorical variables and mean ± standard deviation for continuous variables.
Body-mass index data missing in 3 RA-ILD patients
DLCO% data missing in 1 RA-ILD patient
UCSF SOBQ score data missing in 1 RA-ILD patient; higher scores indicate worse dyspnea
RA-ILD = rheumatoid arthritis-associated interstitial lung disease. IPF = idiopathic pulmonary fibrosis. FVC% = percent predicted forced vital capacity. DLCO% = percent predicted diffusing capacity for carbon monoxide. UCSD SOBQ = University of California, San Diego Shortness of Breath Questionnaire10.
RA-ILD patients were more likely than IPF patients to be taking prednisone at the time of HRQL assessment (90% vs. 38%, P<0.01). The presence of joint pain or stiffness was more common in RA-ILD patients (92% vs. 44%, P<0.01). Dyspnea severity, as measured by the USCD SOBQ, and presence of fatigue, cough, and weight loss were similar between patients with RA-ILD and IPF.
Health-Related Quality of Life
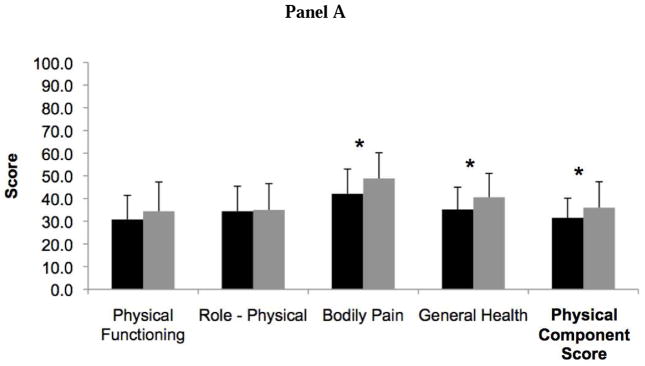
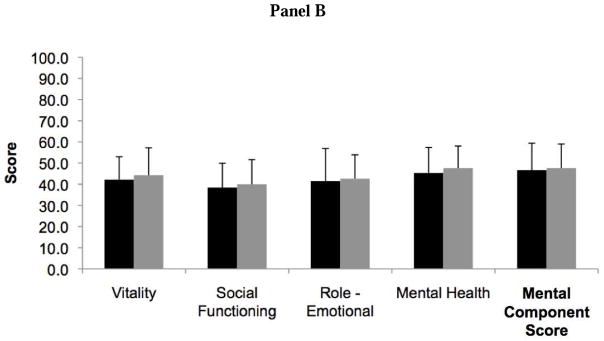
Patients with RA-ILD had more impaired HRQL than IPF patients as measured by the PCS of the SF-36 (31.5 ± 8.7 vs. 36.0 ± 11.4, P=0.03) (Figure 1). Two out of four domains that contribute to the PCS, bodily pain and general health, were significantly lower in RA-ILD compared to IPF. The overall MCS, including its four contributing domain scores, was similar between RA-ILD and IPF patients (46.7 ± 12.7 vs. 47.6 ± 12.0, P=0.72).
Figure 1.
Bar graph comparing Short Form Health Survey (SF-36) health-related quality of life scores in rheumatoid arthritis-associated interstitial lung disease (black) and idiopathic pulmonary fibrosis (gray). Panel A includes the physical component score and its associated domains, and Panel B includes the mental component score and its associated domains. Higher scores indicate better quality of life. *Indicates P value <0.05.
Clinical Predictors of Health-Related Quality of Life
Among the RA-ILD subgroup, covariates associated with a lower PCS on unadjusted analysis included presence of fatigue, joint pain or stiffness, and weight loss, history of sleep apnea, and dyspnea severity (e-Table 1). After adjustment for age and FVC%, fatigue and dyspnea severity were most closely associated with the PCS (as measured by the model R2), explaining an estimated 37% of the observed variance in PCS scores (Table 2A).
Table 2.
Relationship Between Clinical Covariates and the Physical and Mental Component Scores Among RA-ILD Patients: Multivariate Analysis
| A. Physical Component Score | |||||||
|---|---|---|---|---|---|---|---|
| Model R2 | Age | FVC% | Sleep Apnea | UCSD SOBQ Score | Fatigue | Weight Loss | Joint Pain or Stiffness |
| 0.02 | −0.07 (0.61) | 0.12 (0.40) | |||||
| 0.09 | −0.07 (0.60) | 0.19 (0.20) | 0.28 (0.06) | ||||
| 0.28 | 0.15 (0.26) | 0.04 (0.78) | −0.54 (<0.01) | ||||
| 0.23 | −0.09 (0.48) | 0.07 (0.58) | −0.47 (<0.01) | ||||
| 0.10 | −0.08 (0.59) | 0.06 (0.68) | −0.29 (0.05) | ||||
| 0.09 | −0.06 (0.66) | 0.19 (0.20) | −0.28 (0.06) | ||||
| 0.37 | 0.10 (0.45) | 0.02 (0.87) | −0.43 (<0.01) | −0.33 (0.02) | |||
| B. Mental Component Score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model R2 | Age | FVC% | UCSD SOBQ Score | Fatigue | Cough | Heartburn | Weight Loss | Joint Pain or Stiffness |
| 0.00 | 0.06 (0.71) | 0.02 (0.89) | ||||||
| 0.20 | 0.19 (0.19) | −0.06 (0.66) | −0.47 (<0.01) | |||||
| 0.17 | 0.04 (0.77) | −0.02 (0.86) | −0.41 (<0.01) | |||||
| 0.09 | 0.18 (0.25) | 0.09 (0.52) | 0.33 (0.04) | |||||
| 0.06 | 0.03 (0.82) | 0.05 (0.69) | −0.24 (0.11) | |||||
| 0.06 | 0.05 (0.71) | −0.03 (0.82) | −0.25 (0.09) | |||||
| 0.11 | 0.07 (0.62) | 0.10 (0.48) | −0.34 (0.02) | |||||
| 0.28 | 0.14 (0.33) | −0.08 (0.56) | −0.36 (0.02) | −0.31 (0.03) | ||||
| 0.34 | 0.23 (0.12) | 0.02 (0.89) | −0.37 (0.01) | −0.29 (0.04) | 0.25 (0.08) | |||
Data are presented as standardized β coefficients (P value).
RA-ILD = rheumatoid arthritis-associated interstitial lung disease. FVC% = percent predicted forced vital capacity. R2 = coefficient of determination. UCSD SOBQ = University of California, San Diego Shortness of Breath Questionnaire10.
In this subgroup, covariates associated with a lower MCS on unadjusted analysis included presence of fatigue, cough, joint pain or stiffness, weight loss, heartburn, and dyspnea severity (e-Table 1). After adjustment for age and FVC%, fatigue, dyspnea severity, and cough were the strongest predictors of MCS, explaining an estimated 34% of the observed variance (Table 2B). The addition of joint pain or stiffness, weight loss, and heartburn had little overall impact on model performance.
Individual predictors of the PCS and MCS score of IPF patients are provided in e-Table 2. While similar, key differences in individual predictors of the PCS included FVC%, DLCO%, and the presence of cough among IPF patients.
In the cohort as a whole, ILD subtype was associated with PCS (standardized coefficient −0.22, P=0.03) on unadjusted analysis. Other covariates associated with a lower PCS on unadjusted analysis included female sex, lower FVC%, lower DLCO%, fatigue, cough, weight loss, joint pain or stiffness, and dyspnea severity (Table 3); the covariates most strongly associated with PCS on unadjusted analysis were joint pain or stiffness (standardized coefficient −0.38, P<0.01) and dyspnea severity (standardized coefficient −0.54, P<0.01).
Table 3.
Unadjusted Associations Between Clinical Covariates and the Physical Component Score in RA-ILD and IPF Patients
| Variable | Standardized Coefficient | P Value |
|---|---|---|
| Age | 0.05 | 0.61 |
| Male sex | 0.24 | 0.02 |
| Body-mass index | −0.07 | 0.51 |
| Ever smoker | −0.11 | 0.27 |
| ILD subtype | −0.22 | 0.03 |
| FVC% | 0.29 | <0.01 |
| DLCO% | 0.29 | <0.01 |
| Prednisone use at baseline | −0.13 | 0.20 |
| Diabetes mellitus | −0.02 | 0.86 |
| Sleep apnea | 0.05 | 0.66 |
| Fatigue | −0.24 | 0.02 |
| Cough | −0.18 | 0.07 |
| Joint pain or stiffness | −0.38 | <0.01 |
| Weight loss | −0.27 | <0.01 |
| Heartburn | 0.09 | 0.36 |
| UCSD SOBQ score | −0.54 | <0.01 |
FVC% = percent predicted forced vital capacity. DLCO% = percent predicted diffusing capacity for carbon monoxide. ILD = interstitial lung disease. UCSD-SOBQ = University of California San Diego Shortness of Breath Questionnaire10.
After adjusting for age and FVC%, ILD subtype remained a significant predictor of PCS (P=0.02). A model that included ILD subtype, joint pain or stiffness, dyspnea severity, age, and FVC% explained an estimated 45% of the observed variance in PCS (Table 4). DLCO%, male sex, cough, fatigue, and weight loss were not included in the final model because they provided no additional explanatory power (e-Table 2). In the final model, ILD subtype was not an independent predictor of PCS (P=0.86). Sensitivity analyses were performed using the individual domains that comprise the PCS as the outcome variable. Joint pain or stiffness and dyspnea severity were the strongest clinical predictors of each of the bodily pain, general health, and role-physical domain scores (data not shown). For the physical functioning domain, dyspnea severity was the only independent predictor.
Table 4.
Relationship Between ILD Subtype and the Physical Component Score: Multivariate Analysis
| Model R2 | Diagnosis of RA-ILD vs. IPF | Age | FVC% | UCSD SOBQ Score | Joint Pain or Stiffness |
|---|---|---|---|---|---|
| 0.14 | −0.24 (0.02) | −0.02 (0.81) | 0.30 (<0.01) | ||
| 0.36 | −0.19 (0.03) | 0.07 (0.41) | 0.16 (0.08) | −0.50 (<0.01) | |
| 0.27 | −0.02 (0.85) | −0.05 (0.61) | 0.37 (<0.01) | −0.44 (<0.01) | |
| 0.45 | −0.02 (0.86) | 0.04 (0.59) | 0.23 (<0.01) | −0.45 (<0.01) | −0.36 (<0.01) |
| 0.45 | 0.05 (0.56) | 0.23 (<0.01) | −0.45 (<0.01) | −0.37 (<0.01) |
Data are presented as standardized β coefficients (P value).
RA-ILD = rheumatoid arthritis-associated interstitial lung disease. IPF = idiopathic pulmonary fibrosis. FVC% = percent predicted forced vital capacity. R2 = coefficient of determination. UCSD SOBQ = University of California, San Diego Shortness of Breath Questionnaire10.
DISCUSSION
Relative to the general U.S. population, patients with RA-ILD have reduced HRQL, as measured by the PCS and MCS of the SF-36. Impairments in the physical and mental health of RA-ILD patients can be explained, in part, by dyspnea severity and fatigue. The presence of cough also appears to impact mental health. Compared to patients with IPF, patients with RA-ILD have worse HRQL, as measured by the PCS, after adjusting for age and ILD severity. The differences in HRQL between these two ILD subtypes appear to be explained, in part, by dyspnea severity and the presence of joint pain or stiffness. However, our models explained, at most, only 45% of the observed variance in PCS scores; this suggests that HRQL is complex and multifactorial, with other unmeasured factors impacting HRQL in these patients.
Our data are consistent with the one prior publication reporting HRQL in RA-ILD14. In this study, the authors report SF-36 scores from seven RA-ILD patients obtained a median 94 days prior to lung transplant (inter-quartile range of 59 days): mean PCS 22.4 ± 8.1 and mean MCS 44.7 ± 15.3. The severity of physiological impairment was not reported but was severe enough to require transplantation. The investigators did not examine the determinants of HRQL in their patients.
One of the key determinants of HRQL in patients with ILD is dyspnea severity. Several authors have shown that increased severity of dyspnea correlates with worse HRQL among patients with ILD5, 6, 7, including patients with connective tissue disease-associated ILD15. Impairments in pulmonary function are associated with dyspnea severity11, 16, but it is well known that several other factors (e.g., limbic system activation17) contribute to the perception of dyspnea. For example, ILD patients with more severe dyspnea have higher rates of depression and a greater degree of functional impairment, which contribute to the sensation of breathlessness beyond their baseline pulmonary function18.
Another determinant of HRQL in this study was the presence of joint pain or stiffness. Although there was a trend toward statistical significance, while controlling for age and ILD severity, joint pain or stiffness was not an independent predictor of HRQL among RA-ILD patients alone. Not surprisingly, the presence of joint pain or stiffness was a driver of the difference in HRQL between RA-ILD and IPF patients. This highlights the impact of extra-pulmonary symptoms among patients with RA-ILD and the need for a multi-disciplinary approach to their care.
RA joint disease, in the absence of ILD, impairs physical and mental components of HRQL19. In a systematic review and meta-analysis, the authors analyzed SF-36 scores from 31 different studies including 22,355 RA patients and calculated a pooled mean PCS score of 34.1 (95% CI: 22.0–46.1) and MCS score of 45.6 (95% CI: 30.3–60.8). Predictors of HRQL in this meta-analysis included age, sex, and RA disease duration. Direct comparison of these SF-36 scores to our population is difficult given the differences in age (mean age range reported from 42.4 years to 64 years) and sex (female percentage range from 68–100%).
There are limitations to our study. All of our patients were drawn from a tertiary referral center, which may reflect a more severe and progressive disease population; this may limit the ability to generalize results to a broader population of RA-ILD patients. In addition, although our study looked specifically at the use of prednisone at baseline, the impact of other pharmacologic therapies (e.g., disease-modifying anti-rheumatic drugs) on HRQL could not be assessed. RA disease duration and RA-specific disease activity scores20 were also not available in this population. Last, although it was not an objective of this study, we do not have an RA group without ILD for comparison.
In summary, our study demonstrates that certain physical health determinants of HRQL are measurably worse in RA-ILD patients compared to IPF patients. In addition to providing therapies to target the underlying biology of RA-ILD and the accompanying joint pain and stiffness, our data suggest that dyspnea is a strong driver of HRQL impairment and respiratory symptom management should be an integral aspect of caring for patients with RA-ILD. Further research should be done to better understand and improve the HRQL impairments in patients with RA-ILD.
Supplementary Material
Highlights.
Health related quality of life (HRQL) is impaired in patients with interstitial lung disease.
The physical components of HRQL appear worse in rheumatoid arthritis associated interstitial lung disease (RA-ILD) compared to idiopathic pulmonary fibrosis (IPF).
Differences in the physical components of HRQL between RA-ILD and IPF can be explained in part by dyspnea severity and joint symptoms.
Acknowledgments
Funding: This work was supported by the National Center for Advancing Translational Science, NIH, grant number UCSF-CTI KL2TR000143, and the Nina Ireland Program.
JSL takes responsibility for the content of the manuscript, including the data and analysis. JN and JSL contributed to the conception and design, acquisition of data, analysis interpretation of the data. JM, JJS, AF, and HRC contributed to the analysis and interpretation of the data. BME and KDJ contributed to the acquisition of the data. All authors revised the manuscript for important intellectual content and have provided final approval of the version to be published
ABBREVIATION LIST
- DLCO
diffusing capacity for carbon monoxide
- FVC
forced vital capacity
- HRQL
health-related quality of life
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- MCS
mental component score
- PCS
physical component score
- PFT
pulmonary function test
- RA
rheumatoid arthritis
- RA-ILD
rheumatoid arthritis-associated interstitial lung disease
- SF-36
Short Form (36) Health Survey
- UCSD SOBQ
University of California, San Diego Shortness of Breath Questionnaire for Lung Health
- UCSF
University of California, San Francisco
Footnotes
Summary conflict of interest statements: JN has no conflicts of interest. JJS has no conflicts of interest. JM has no conflicts of interest. BME has no conflicts of interest. KDJ has no conflicts of interest. AF reports personal fees from Actelion, Bayer, Boehringer-Ingelheim, Genentech, Glaxo Smith Kline, Bristol Meyer Squibb, and Seattle Genetics, outside the submitted work. HRC has no conflicts of interest. JSL has no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations of rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62(8):722–7. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanoue LT. Pulmonary manifestations of rheumatoid arthritis. Clin Chest Med. 1998;19(4):667–85. doi: 10.1016/s0272-5231(05)70109-x. [DOI] [PubMed] [Google Scholar]
- 4.Olson AL, Swigris JJ, Sprunger DB, Fischer A, Fernandez-Perez ER, Solomon J, Murphy J, Cohen M, Raghu G, Brown KK. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372–8. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax. 2005;60(7):588–94. doi: 10.1136/thx.2004.035220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coelho AC, Knorst MM, Gazzana MB, Barreto SS. Predictors of physical and mental health-related quality of life in patients with interstitial lung disease: a multifactorial analysis. J Bras Pneumol. 2010;36(3):562–70. doi: 10.1590/s1806-37132010000500007. [DOI] [PubMed] [Google Scholar]
- 7.Lubin M, Chen H, Elicker BM, Jones KD, Collard HR, Lee JS. A comparison of health-related quality of life in idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. Chest. 2014;145(6):1333–8. doi: 10.1378/chest.13-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2):646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998;113(3):619–24. doi: 10.1378/chest.113.3.619. [DOI] [PubMed] [Google Scholar]
- 11.Tzanakis N, Samiou M, Lambiri I, Antoniou K, Siafakas N, Bouros D. Evaluation of health-related quality-of-life and dyspnea scales in patients with idiopathic pulmonary fibrosis. Correlation with pulmonary function tests. Eur J Intern Med. 2005;16(2):105–12. doi: 10.1016/j.ejim.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Swigris JJ, Brown KK, Behr J, du Bois RM, King TE, Raghu G, Wamboldt FS. The SF-36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med. 2010;104(2):296–304. doi: 10.1016/j.rmed.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ware JE. SF-36 Health Survey update. Spine. 2000;25(24):3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Yazdani A, Singer LG, Strand V, Gelber AC, Williams L, Mittoo S. Survival and quality of life in rheumatoid arthritis-associated interstitial lung disease after lung transplantation. J Heart Lung Transplant. 2014;33(5):514–20. doi: 10.1016/j.healun.2014.01.858. [DOI] [PubMed] [Google Scholar]
- 15.Swigris JJ, Yorke J, Sprunger DB, Swearingen C, Pincus T, du Bois RM, Brown KK, Fischer A. Assessing dyspnea and its impact on patients with connective tissue disease-related interstitial lung disease. Respir Med. 2010;104(9):1350–5. doi: 10.1016/j.rmed.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JA, Curtis JR, Patrick DL, Raghu G. Assessment of health-related quality of life in patients with interstitial lung disease. Chest. 1999;116(5):11175–82. doi: 10.1378/chest.116.5.1175. [DOI] [PubMed] [Google Scholar]
- 17.Faisal A, Alghamdi BJ, Ciavaglia CE, et al. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am J Respir Crit Care Med. 2016;193(3):299–309. doi: 10.1164/rccm.201504-0841OC. [DOI] [PubMed] [Google Scholar]
- 18.Ryerson CJ, Berkeley J, Carrieri-Kohlman VL, Pantilat SZ, Landefeld CS, Collard HR. Depression and functional status are strongly associated with dyspnea in interstitial lung disease. Chest. 2011;139(3):609–16. doi: 10.1378/chest.10-0608. [DOI] [PubMed] [Google Scholar]
- 19.Matcham F, Scott IC, Rayner L, Hotopf M, Kingsley GH, Norton S, Scott DL, Steer S. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):123–30. doi: 10.1016/j.semarthrit.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Gilek-Seibert K, Prescott K, Kazi S. Outcome assessments in rheumatoid arthritis. Curr Rheumatol Rep. 2013;15(11):370–6. doi: 10.1007/s11926-013-0370-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.