Abstract
Background
Although people with schizophrenia (PSZ) frequently exhibit reduced working memory capacity relative to healthy comparison subjects (HCS), the mechanisms that underlie this impairment are not yet known. The present study aimed to assess one putative mechanism: impaired suppression of alpha and beta frequency bands during the delay period of a visual working memory task.
Methods
The electroencephalogram (EEG) was recorded from 30 PSZ and 31 HCS while they completed a change detection task in which they were required to remember a briefly presented array of colored squares over an 1800-ms delay interval.
Results
Overall, we found that PSZ had significantly reduced alpha and beta-band suppression during the delay interval compared to HCS, and that these significant differences emerged early (100-200 ms after the onset of the memory array). Furthermore, the magnitude of suppression was significantly associated with task performance across both groups. Finally, the magnitude of suppression in alpha and beta frequency bands was significantly associated with a range of cognitive measures among HCS, but not PSZ.
Conclusions
These results implicate impaired alpha/beta suppression during the consolidation period of working memory tasks as a promising neural mechanism that constrains capacity in PSZ.
Keywords: schizophrenia, visual, working memory, EEG, alpha suppression, beta suppression
Introduction
Despite many reports of robust working memory (WM) capacity deficits among people with schizophrenia (PSZ), the precise mechanism of impairment has not yet been identified. In an attempt to isolate the capacity-limiting process, various investigations have probed different stages of WM storage, including selection (1; 2), consolidation (3; 4), and maintenance (5). Overall, the pattern of results from these studies implicates abnormalities in early stages of WM formation, such as encoding or consolidation; there is little evidence that capacity limitations are a consequence of accelerated decay during maintenance. These conclusions are consistent with a meta-analysis (5) that revealed robust effect sizes of WM impairment that did not change as a function of the length of the delay period. It is now increasingly recognized that the primary impairment likely takes place during the first several hundred milliseconds of WM formation and storage, though the specific neural mechanisms that support this process remain unclear.
In the present study, we used electroencephalography (EEG) to examine one candidate mechanism of WM formation and maintenance—alpha and beta frequency band suppression—as a potential constraint on WM capacity among PSZ. Recent work has revealed that the magnitude of suppression of alpha (8-13 Hz) and beta (14-30 Hz) frequency bands during the delay period of WM tasks is associated with the number of items that are later recalled in healthy control subjects (HCS; 6; 7). This mechanism appears to serve an important role for prioritizing and attending to items within the visuospatial domain. One study reported that alpha suppression contralateral to the attended item in a visual array was associated with a blood-oxygen level dependent (BOLD) signal increase in object-specific ventral processing stream cortex (8). By contrast, alpha enhancement contralateral to the ignored hemifield was associated with a BOLD decrease in associated ventral processing stream cortex. This and other evidence revealing attention-modulated suppression of alpha during WM (9) suggest a causal relationship between alpha modulation and encoding and/or consolidation of items into WM for further processing. Similarly, delay-period beta suppression has been linked to WM capacity, although the mechanism by which this occurs has received less attention (see 10 for review). Despite enthusiasm for these neural substrates of WM capacity among HCS, the integrity of this mechanism has been little explored in PSZ.
The present study used a change-detection visual WM task to test the hypothesis that delay-period alpha/beta suppression is impaired among PSZ, and that suppression failure is associated with impaired WM performance in this population. We predicted that the magnitude of suppression would be significantly associated with successful recall of items from the memory array. Given previous reports suggesting that WM deficits do not worsen as the delay period is increased, we also expected that alpha/beta suppression impairments would emerge early and be sustained throughout the maintenance period. Such evidence would (1) provide converging evidence for the hypothesis that capacity deficits among PSZ are a consequence of early encoding/consolidation impairments, and (2) implicate impaired delay-period alpha and beta suppression as a primary route by which this impairment emerges.
Methods
Participants
Thirty individuals meeting DSM-IV-TR criteria for schizophrenia or schizoaffective disorder (21 male) and 31 psychiatrically healthy controls (20 male) were included in the present study (see Supplementary Table 1)1. The groups were matched on age (t=0.01; p=1.00), gender (χ2=0.21; p=0.79), race (χ2=0.60; p=0.74), and parental education, a proxy measure of socioeconomic status (t=0.29; p=0.77); however, they differed significantly on IQ (t=2.87; p<0.01) and education level (t=3.40; p <0.01). Diagnosis was confirmed using the Structured Clinical Interview for the DSM-IV (SCID-I/P; 11), as well as a review of medical records and informant reports when appropriate. All PSZ were clinically stable, and had not received any changes in medication for at least four weeks prior to testing. Chlorpromazine dose equivalents were calculated according to the formula recommended by Andreasen and colleagues (12). HCS had no current Axis I diagnoses or schizotypal personality disorder, were not taking psychiatric medications, and reported no family history of psychosis. All participants were between the ages of 18-55, and reported no history of neurological injury. PSZ were recruited from the Maryland Psychiatric Research Center and other community clinics, whereas HCS were recruited by advertisement from the community. All recruiting methods and experimental procedures were approved by the University of Maryland Institutional Review Board.
Neuropsychological & Symptom Measures
The following neuropsychological measures were administered to examine current and premorbid cognitive functioning in PSZ and HCS: the MATRICS Consensus Cognitive Battery (MCCB; 13); the Wide Range Achievement Test 4 (WRAT-4; 14); the Wechsler Test of Adult Reading (WTAR; 15); and the Wechsler Abbreviated Scale of Intelligence (WASI; 16). Additionally, current symptom severity level was measured using the Brief Psychiatric Rating Scale (BPRS; 17) and the Scale for the Assessment of Negative Symptoms (SANS; 18). Finally, engagement in social and occupational activities was measured using the total value of these two subscales from the Level of Functioning Scale (LOFS; 19).
Experimental Paradigm
Stimuli were presented on a LCD monitor with a gray background (x=0.324, y=0.283, 32.76 cd/m2) and a continuously visible central fixation cross at a nominal viewing distance of 100 cm. On each trial, a sample array was presented for 200 ms that contained either 1, 2, 4, or 6 colored squares arranged around an invisible circle with a radius of 4.1°, each of which measured 0.66 × 0.66° visual angle and was separated from the other squares in the sample array with a minimum distance of 2.12° (see Figure 1A). The colors of the squares in the sample array were highly discriminable, and selected randomly and without replacement from a list of red, white, black, blue, purple, green, and yellow. Following an 1800 ms delay interval, a test array was presented. This array was identical to the sample array on 50% of the trials, and on the other 50% one of the items changed to a color that was not present in the sample array. Participants were asked to indicate by button-press response whether the test array was the same as or different from the sample array. Each participant received 96 trials of each set size.
Figure 1.
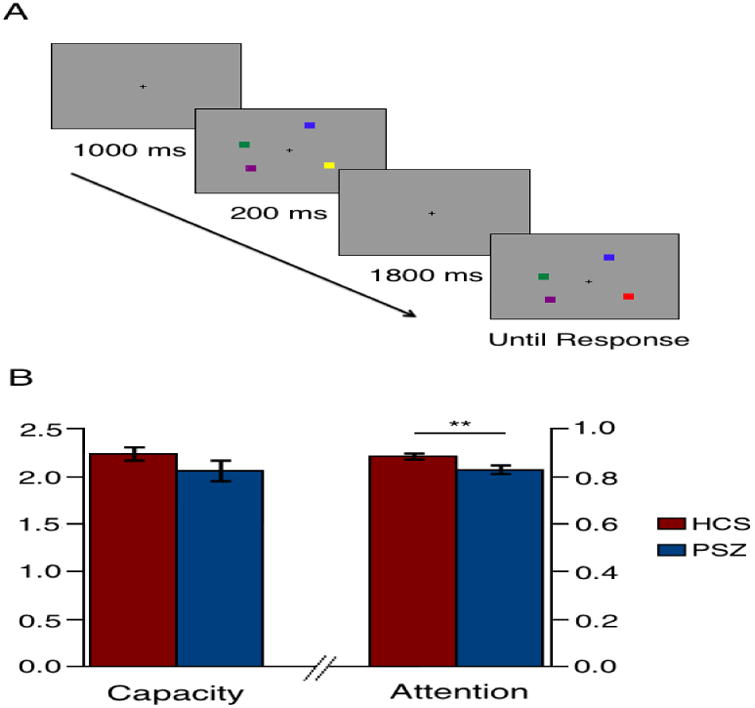
Example sequence of stimuli at set size 4 in the change detection task (A); KMAX and Attention by group (± standard error; B).
Electrophysiological Recording and Analysis
The electroencephalogram (EEG) was recorded continuously from 32 scalp electrodes (Fp1, Fp2, Fz, F3, F4, F7, F8, FCz, Cz, C3, C4, T7, T8, Pz, P1, P2, P3, P4, P5, P6, P7, P8, P9, P10, POz, PO3, PO4, PO7, PO8, Oz, O1, and O2) at 1024 samples/second with a Biosemi ActiveTwo EEG recording system. EEG was low-pass filtered online with a half-power cutoff at 1/5 sampling rate. Vertical and horizontal electrooculogram (EOG) were also recorded with four external electrodes: two above and below the left eye and two lateral to the external canthi.
Offline data processing was conducted in Matlab using the EEGLAB (20) and ERPLAB toolboxes (21). Data were referenced to the average of the left and right mastoid electrodes and down-sampled to 512 Hz. Data were then segmented into 4-second epochs (1500 ms pre-stimulus to 2500 ms post-stimulus), and baseline corrected to the mean pre-stimulus voltage. An independent components analysis (ICA) was conducted to remove components from the data that were associated with eyeblinks. Following ocular correction, artifact rejection was performed by using a series of algorithms built into ERPLAB. We rejected any epochs with (1) amplitudes exceeding ±200 μV at any point in the epoch, or (2) peak-to-peak amplitudes that exceeded ±150 μV within a 200-ms moving window. Finally, a visual inspection of the data was conducted to remove any remaining artifacts. HCS retained 72% of trials and PSZ retained 71% of trials (p=0.73). See Supplementary Table 2 for a breakdown of trials retained by set size.
To measure the task-related frequency content of the EEG signal, time-frequency analysis was conducted on single trials by convolving a Hanning-tapered 3-cycle Morlet wavelet with the EEG from each channel. Posterior activity was measured by taking the average single-trial power of all 19 occipital and parietal electrode sites, and frontal activity was measured by taking the average power of the three frontal electrodes. Power was measured from each of four a priori frequency bands—theta (4-8 Hz), alpha (9-12 Hz), beta (13-30 Hz), and gamma (31-50 Hz)—and was baseline corrected to the proportional change in post-stimulus power relative to the 1500 ms prior to the onset of the sample array on a logarithmic scale (dB). Baseline power was not significantly different between PSZ and HCS for alpha, beta, or gamma in either frontal or posterior electrode sites (t's<1.27; p's>0.21); however, PSZ exhibited significantly elevated prestimulus theta power in both regions (t's>3.18; p's<0.01; see Supplementary Figure 1), a difference that may be an effect of clozapine (22; 23) as 12 out of the 30 PSZ included in the present study were taking clozapine at the time of testing.
Results
Task performance and cognitive variables
Behavioral performance in this task can be decomposed into three conceptually independent factors: WM storage capacity (KMAX); the probability that the participant was paying attention on a given trial (A); and the bias to make a “change” guess (G) when the participant is uncertain. We used a parameter estimation process (see Supplementary Materials (24)) to estimate these three parameters from the entire set of behavioral data for each participant. KMAX was numerically but not significantly reduced in PSZ (t=1.34; p=0.19; Cohen's d=0.34), and A was significantly reduced in PSZ (t=2.90; p<0.01; Cohen's d=0.74), indicating a higher probability of lapses of attention (see Figure 1B). G is not typically theoretically meaningful, and it was not significantly different between the two groups (t=0.58; p=0.56). Among HCS, KMAX was significantly associated with WASI IQ (r=0.54; p<0.01), as well as MATRICS total score (r=0.44; p<0.05; see Supplementary Table 3). Similarly, KMAX was significantly associated with WASI IQ (r=0.42; p<0.05) and WRAT (r=0.37; p<0.05) in PSZ. Interestingly, A was not significantly associated with cognitive performance for either HCS or PSZ; however, A was significantly associated with better social/occupational functioning (r=0.41; p<0.05) and lower negative symptoms in the patient group (r=-0.57; p<0.01), suggesting that decreased attentional engagement is associated with poorer outcomes among PSZ
Visual evoked potentials
Early visual evoked potentials (VEP's) were measured by taking the average of PO7/PO8, where P1, N1, and P2 were maximal. The waveforms are depicted in Supplementary Figure 2; the two groups did not significantly differ in mean amplitude for any of the three components that were measured (P1, N1, P2; p's>0.18).
Time-frequency analysis
The time-frequency transformation of the EEG data from posterior electrode sites is depicted in Figure 2 for HCS (top panel) and PSZ (middle panel; for frontal electrode sites, see Supplementary Figure 3). The scalp distributions for delay-period (200-2000 ms post-stimulus) alpha and beta activity are depicted in Figure 3A. The time courses of posterior alpha and beta activity are shown Figure 3B (see Supplementary Figure 4 for frontal sites). As can be seen in Figures 2 and 3B, both groups exhibited a clear suppression of alpha activity from approximately 250 to 750 ms after the onset of the sample array, henceforth referred to as the suppression peak. This suppression peak is followed by maintenance suppression, which was observed for the remainder of the delay period.
Figure 2.
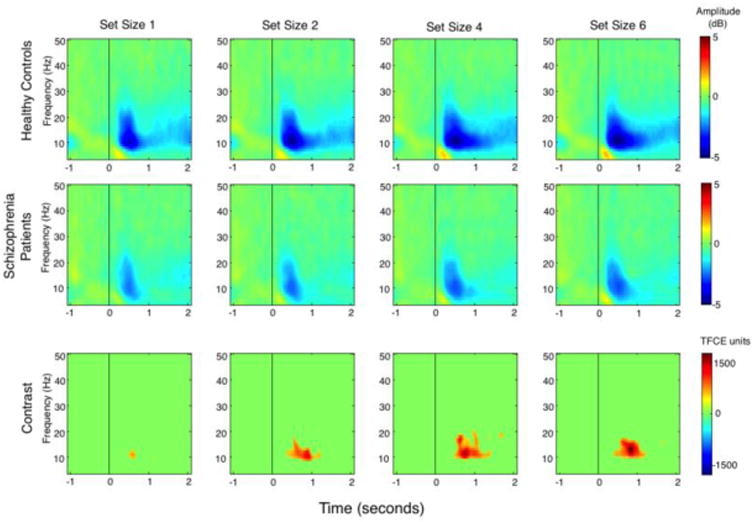
Time × frequency plots for average posterior electrodes, corrected to pre-stimulus baseline period. Top panel = healthy control subjects; middle panel = schizophrenia patients; bottom panel = contrast in arbitrary units using threshold-free cluster enhancement.
Figure 3.
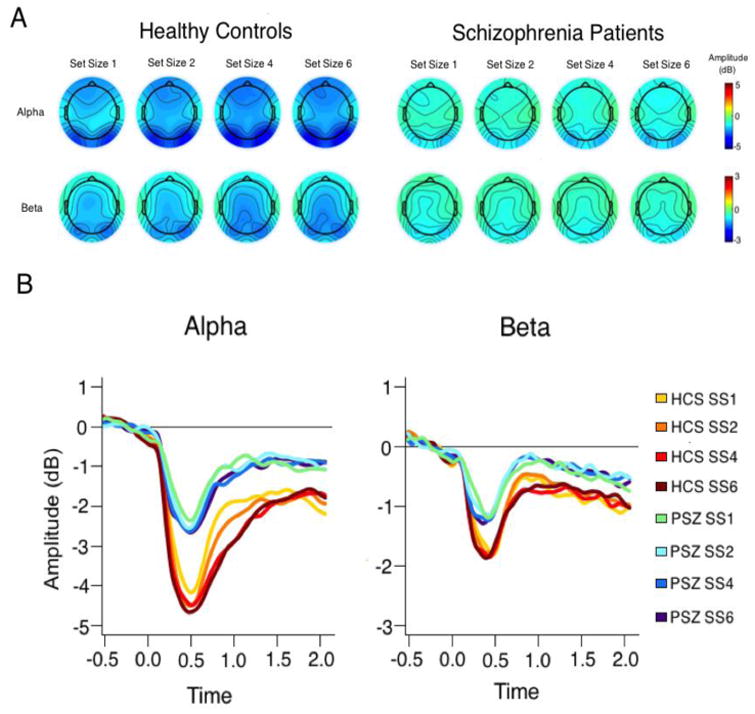
Scalp distributions of alpha (top) and beta (bottom) suppression during the delay period (A); time course of alpha and beta suppression in healthy controls and schizophrenia patients in posterior electrode channels (B).
We begin by describing alpha band activity and then move on to beta band activity. In HCS, alpha suppression was maintained fairly strongly throughout the delay period, and was larger for larger set sizes. Alpha suppression was weaker in PSZ during both the suppression peak and maintenance suppression phase, and set size had little impact on alpha suppression in PSZ.
These descriptions were supported by a repeated-measures ANOVA with factors of group and set size. Collapsed across groups, the alpha activity from 200-2000 ms was significantly different from zero (i.e., suppressed) at all four set sizes at posterior electrode sites (t's>12.07; p's<0.001), and follow-up analyses indicated that the suppression was significant in both PSZ (t's>7.80; p's<0.001) and HCS (t's>10.54; p's<0.001). The alpha suppression was significantly greater in HCS than in PSZ (group effect: F1,59=24.01; p<0.001; Cohen's d=1.28). The increase in alpha suppression with increasing set size was also significant (set size effect: F3,177=14.08; p<0.001), and the stronger set size effect in HCS than in PSZ led to a significant group-by-set size interaction (F3,177=3.03; p<0.05). Despite this interaction, separate one-way ANOVAs revealed that the main effect of set size was significant for both HCS (F3,90=11.58; p<0.001) and PSZ (F3,87=4.20; p<0.01). Post hoc analyses revealed that PSZ exhibited significant impairment in both alpha suppression peak and maintenance suppression components (t's > 3.03; p's < 0.01; see Supplementary Materials).
We now turn to beta band activity, which was also measured from 200-2000 ms and analyzed in a group-by-set size ANOVA. As shown in Figures 2-3, the beta activity from 200-2000 ms was significantly different from zero at all four set sizes at posterior electrode sites (t's>12.68; p's<0.001). Follow-up analyses indicated that suppression was significant at all four set sizes in both PSZ (t's>8.10; p's<0.001) and HCS (t's>10.25; p's<0.001). Beta suppression was significantly greater in HCS (F1,59=15.40; p<0.001; Cohen's d =1.02); however, in contrast to the alpha suppression findings, there was no significant main effect of set size (F3,177=1.19; p=0.32), nor was there a group-by-set size interaction (F3,177=1.17; p=0.32). Again, post hoc analyses revealed that PSZ exhibited a significant impairment in both the beta suppression peak and maintenance suppression components (t's>3.43; p's<0.01; see Supplementary Materials).
The two groups did not differ in the magnitude of theta or gamma suppression during the delay period (F's<0.14; p's>0.71). A similar pattern of results was observed at frontal electrode sites, although group-by-set size interactions only reached trend-level significance (Supplementary Figure 3).
Time course of alpha/beta suppression
The bottom panel of Figure 2 depicts the points of significant group differences, corrected for multiple dependent comparisons using threshold-free cluster enhancement (TFCE; 25; 26). The weighting parameters for extent and height, E and H, were set to 2/3 and 2, respectively (26). It is noteworthy that the most robust differences appear within the first 500 milliseconds following stimulus onset. Similar effects were observed at frontal sites (Supplementary Figure 3). T-test comparisons on the mean alpha and beta band activity revealed that the earliest significant group differences emerged approximately 100-200 milliseconds after the onset of the memory array in both alpha and beta frequency bands at posterior and frontal electrode sites.
Alpha/beta suppression and WM capacity
We next ask the question of whether the average magnitude of alpha/beta suppression is associated with capacity separately for each group. There are two parts to this question. First, is the overall magnitude of suppression associated with capacity across participants? Second, is the relative increase in suppression across set sizes for a given participant associated with that participant's capacity?
To answer the first question, we calculated composite alpha and beta suppression scores by taking the average suppression values across all four set sizes. KMAX was significantly associated with alpha and beta suppression for HCS at both posterior and frontal electrode sites (r's=-0.38 to -0.55; p's<0.05; Figure 4A), whereas KMAX was not significantly associated with suppression at either frontal or posterior electrode sites in PSZ (r's=-0.11 to -0.26; p's>0.16). Despite these numeric differences in the magnitude of correlations across groups, Fisher's r-to-z transformation indicated that these differences were not significant (all p's>0.13).
Figure 4.
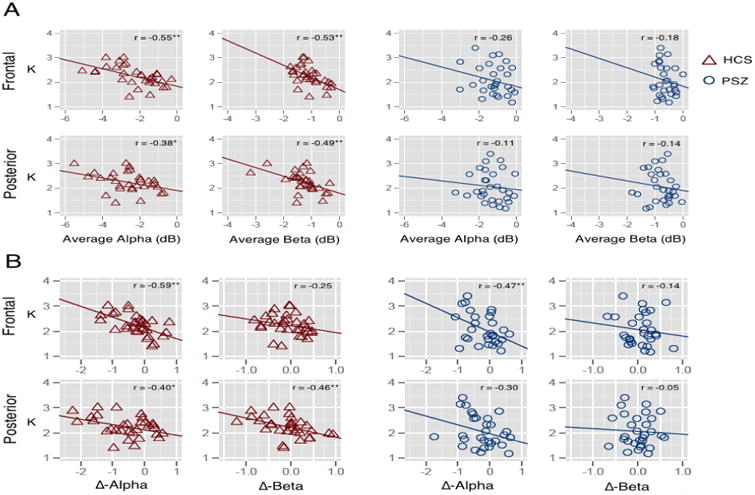
Scatterplot depicting the relationship between KMAX and alpha and beta suppression, averaged across set sizes, in frontal and posterior electrode sites for HCS (red triangles) and PSZ (blue circles; A); Scatterplot depicting the relationship between KMAX and Δ-alpha and Δ-beta in frontal and posterior electrode sites for HCS (red triangles) and PSZ (blue circles; B).
To address the second question, we examined the relationship between KMAX and modulation of alpha/beta suppression across set sizes. This analysis is based on the idea that participants who can effectively modulate alpha activity in accordance with the demands of the current trial will achieve a greater WM capacity. Suppression modulation was calculated as the subtraction between suppression at set size 6 and suppression at set size 1 (Δ-alpha and Δ-beta, respectively). More negative Δ-alpha and Δ-beta values would indicate a more effective modulation of alpha/beta to accommodate task demands for a given trial. The results of this analysis are presented in Figure 4B. Among HCS, Δ-alpha and Δ-beta were significantly associated with overall capacity at posterior electrode sites (r's=-0.40 to -0.46; p's<0.05) and in the alpha band at frontal electrode sites (r=-0.59; p <0.01). Among PSZ, the relationship between KMAX and Δ-alpha was significant in frontal electrode sites (r=-0.47; p <0.01); KMAX was not significantly associated with Δ-beta at frontal sites (r=-0.14; p=0.47). In contrast to controls, neither Δ-alpha nor Δ-beta at posterior sites were significantly correlated with KMAX (r's=-0.05 to -0.30; p's>0.11). Fisher's r-to-z transformation revealed that posterior Δ-beta correlations with KMAX were larger in HCS at the level of a trend (z=1.65; p<0.10). All other relationships between KMAX and Δ-alpha/beta were not significantly different between the two groups (all z's<0.62; p's>0.53).
Alpha/beta suppression and attention
The above results indicate that KMAX is significantly associated with (1) the overall magnitude of alpha/beta suppression, and (2) modulation of alpha/beta suppression according to the WM load. We next examined the relationship between alpha/beta suppression and the attention parameter. We found that there were no significant correlations between A and alpha/beta suppression in either the posterior or frontal electrode sites for HCS (r's=-0.02 to 0.15; p's>0.42) or for PSZ (r's=-0.03 to 0.29; p's>0.13). To rule out the possibility that impaired suppression is a consequence of group differences in attention, we matched a subset of HCS and PSZ on the A parameter (N=18 per group; A t=0.02; p=0.98). With these subgroups of matched participants, the alpha/beta suppression impairment in PSZ remained significant at both posterior sites (F's=6.21-10.05; p's<0.05) and frontal sites (F's=10.26-10.64; p's<0.01). Interestingly, A was significantly correlated with the prestimulus alpha power in PSZ (r=-0.38; p <0.05), indicating that a greater propensity for attentional lapses was associated with higher alpha power prior to the onset of the memory array. These observations suggest that group differences in attentional lapse rate may impact estimates of WM capacity when lapses are not factored out from capacity estimates with the type of method used in the present study. Moreover, differences between groups in attentional lapses and in WM capacity appear to arise from different mechanisms (prestimulus alpha levels for lapses, and poststimulus alpha/beta suppression for capacity).
Alpha/beta suppression and cognitive function
Our final analyses examined the relationship between alpha/beta suppression and measures of cognitive function and symptoms. Specifically, we computed separate Pearson correlations between the alpha and beta suppression values at the posterior electrode sites (averaged across set sizes) and each of our neuropsychological and symptom measures. Because these analyses were exploratory rather than hypothesis-driven, a correction for false discovery rate (FDR; 27) was used. The correlations are shown in Supplementary Table 4 (see Supplementary Table 5 for frontal electrode sites). Overall, larger delay-period suppression in the alpha and beta frequency bands tended to be associated with better cognitive functioning in HCS but not in PSZ. By contrast, alpha/beta suppression was not significantly correlated with outcome, symptom, or medication variables in PSZ.
This lack of correlation between suppression and clinical and cognitive measures in PSZ may reflect the fact that PSZ were so uniformly unable to exhibit substantial suppression of alpha or beta (see Figure 4A).
Discussion
We found that delay-period alpha and beta suppression was significantly reduced in PSZ, at all set sizes. Furthermore, these suppression differences emerged early—approximately 100-200 ms after stimulus onset—lending support to the hypothesis that capacity in SZ is constrained by early encoding/consolidation processes. We next sought to determine whether suppression impairment was associated with poorer task performance. We found two results that link this suppression mechanism to WM capacity: first, consistent with prior studies in healthy individuals (7; 28), we found that greater alpha/beta suppression was associated with higher capacity (but not attentional lapse rate). Second, we found that higher capacity is associated with greater modulation of suppression as a function of memory load. Interestingly, some studies have reported alpha enhancement (29) or attenuated suppression (30) in healthy individuals during the maintenance period with increased WM load. Though the precise reason for these seemingly inconsistent findings is not yet clear, one speculation is that alpha enhancement represents active suppression of distractor encoding, which was a feature of both of these previous studies (29; 30). Future investigations will be critical for examining conditions under which alpha is suppressed and enhanced to facilitate WM storage.
The above observations implicate impaired delay-period alpha/beta suppression as a promising candidate mechanism for altered WM function in PSZ. These results expand upon recent work that found impaired lateralized alpha suppression during a task of visuospatial attention and WM in PSZ (31). That study suggested that poor spatial memory for the cue location was a consequence of impoverished alpha modulation in patients; here, we extend these results by revealing that suppression abnormalities can be observed during feature-based memory tasks as well. Furthermore, the magnitude of alpha/beta suppression in the present paradigm was associated with better cognitive function among HCS, indicating that this mechanism has broader functional implications beyond WM consolidation and maintenance. The lack of an association between alpha/beta suppression and cognitive function in PSZ may indicate that alpha/beta suppression impairment is relatively consistent across PSZ, a conclusion that is supported by the narrower range of suppression values in PSZ.
Given that alpha/beta suppression appears to be functionally related to WM consolidation and maintenance, a sensible question that emerges from these observations is how. That is, how does suppression of alpha and beta frequency bands result in successful WM storage? Two non-mutually exclusive hypotheses have been proposed: first, it has been suggested that task-related reduction in alpha/beta power improves the richness of the visual representation (see 10 for a review). This view suggests that highly synchronous neural firing yields a reduction in the richness of the information that can be stored, as synchronous signals are inherently redundant. By contrast, desynchronization of the EEG increases the entropy of the signal, thereby increasing the amount of information that can be encoded by neuronal populations.
A second hypothesis aims to account for the functional role of alpha suppression. In this view, items are encoded into WM by gamma bursts during the oscillatory trough of the ongoing alpha rhythm. During the encoding/consolidation period, alpha is asymmetrically suppressed, such that the oscillatory peaks become smaller and the amount of time spent in the oscillatory trough is lengthened (see 32 for a review). From this perspective, alpha suppression functions as a gatekeeper for the encoding/consolidation process; after this process is concluded, alpha power rebounds to prevent accidental encoding of task-irrelevant items while the memory array is maintained through the delay period. To date, evidence from studies with healthy individuals has not directly compared these two hypotheses.
The present results provide compelling evidence that impaired alpha/beta suppression is a critical mechanism by which WM performance is constrained in health and illness. One caveat that deserves mention is that the observed reduction in storage capacity in PSZ relative to HCS did not reach statistical significance (effect size=0.34). By contrast, PSZ did exhibit a significant impairment in sustained attention. Given known vigilance deficits in this patient group (e.g., 33), the attention impairment is perhaps not surprising; however, the present findings draw attention to the need to evaluate the separable contributions of attention and capacity to memory performance in future studies.
Altogether, the present results suggest that this mechanism plays an important role in constraining WM performance. Interestingly, whereas the relationship between suppression and task accuracy and cognitive ability is clearly observable among HCS, these same relationships are qualitatively weaker in PSZ. It may therefore be concluded that impaired suppression plays a role in WM capacity constraints among PSZ, but the coupling between this phenomenon and behavior is altered by some feature of the illness or medication exposure. Future experiments may explore additional routes by which capacity is constrained by early encoding/consolidation processes in PSZ.
Supplementary Material
Acknowledgments
None.
Financial Disclosures: This work was supported by the National Institute of Mental Health (R01 MH065034 to J. M.G. and S.J. Luck and T32 MH067533-10).
Footnotes
In total, 37 HCS and 41 PSZ participated. Of this total, 5 HCS and 10 PSZ were removed from analysis due to noisy EEG data (fewer than 50% of trials retained, which was our a priori criterion for exclusion). Also, one participant from each group was removed due to near-chance performance on set size 1 trials, indicating poor effort. All demographic information provided here exclude these participants.
All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. Journal of Abnormal Psychology. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- 2.Erickson MA, Hahn B, Leonard CJ, Robinson B, Gray B, Luck SJ, Gold J. Impaired working memory capacity is not caused by failures of selective attention in schizophrenia. Schizophrenia Bulletin. 2015;41:366–373. doi: 10.1093/schbul/sbu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired visual working memory consolidation in schizophrenia. Neuropsychology. 2009;23:71–80. doi: 10.1037/a0013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuller RL, Luck SJ, McMahon RP, Gold JM. Working Memory Consolidation Is Abnormally Slow in Schizophrenia. Journal of Abnormal Psychology. 2005;114:279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Park S. Working Memory Impairments in Schizophrenia: A Meta- Analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 6.Bashivan P, Bidelman GM, Yeasin M. Spectrotemporal dynamics of the EEG during working memory encoding and maintenance predicts individual behavioral capacity. Eur J Neurosci. 2014;40:3774–3784. doi: 10.1111/ejn.12749. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda K, Mance I, Vogel EK. Power Modulation and Event-Related Slow Wave Provide Dissociable Correlates of Visual Working Memory. J Neurosci. 2015;35:14009–14016. doi: 10.1523/JNEUROSCI.5003-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zumer JM, Scheeringa R, Schoffelen JM, Norris DG, Jensen O. Occipital Alpha Activity during Stimulus Processing Gates the Information Flow to Object-Selective Cortex. In: Vogel E, editor. PLoS Biol. Vol. 12. 2014. pp. e1001965–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dijk H, van der Werf J, Mazaheri A, Medendorp WP, Jensen O. Modulations in oscillatory activity with amplitude asymmetry can produce cognitively relevant event-related responses. Proc Natl Acad Sci USA. 2010;107:900–905. doi: 10.1073/pnas.0908821107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanslmayr S. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. 2012:1–12. doi: 10.3389/fnhum.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.First MB, Spitzer RL, Gibbon M, Williams J. Clinical Interview for DSM-IV- TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- 12.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic Dose Equivalents and Dose-Years: A Standardized Method for Comparing Exposure to Different Drugs. BPS. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. The American Journal of Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson GS, Robertson GJ. WRAT 4: Wide Range Achievement Test; professional manual 2006 [Google Scholar]
- 15.Wechsler D. Wechsler Test of Adult Reading: WTAR 2001 [Google Scholar]
- 16.Wechsler D. Wechsler abbreviated scale of intelligence 1999 [Google Scholar]
- 17.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological reports. 1962;10:799–812. [Google Scholar]
- 18.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) The British Journal of Psychiatry. 1989 [PubMed] [Google Scholar]
- 19.Hawk AB, Carpenter WT, Jr, Strauss JS. Diagnostic criteria and five-year outcome in schizophrenia: A report from the International Pilot Study of Schizophrenia. Arch Gen Psychiatry. 1975;32:343. doi: 10.1001/archpsyc.1975.01760210077005. [DOI] [PubMed] [Google Scholar]
- 20.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 2014;8:1–14. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malow BA, Reese KB, Sato S, Bogard PJ, Malhotra AK, Su TP, Pickar D. Spectrum of EEG abnormalities during clozapine treatment. Electroencephalogr Clin Neurophysiol. 1994;91:205–211. doi: 10.1016/0013-4694(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 23.Knott V, Labelle A, Jones B, Mahoney C. Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophrenia Research. 2001;50:41–53. doi: 10.1016/s0920-9964(00)00165-1. [DOI] [PubMed] [Google Scholar]
- 24.Rouder JN, Morey RD, Cowan N, Zwilling CE, Morey CC, Pratte MS. An assessment of fixed-capacity models of visual working memory. Proc Natl Acad Sci USA. 2008;105:5975–5979. doi: 10.1073/pnas.0711295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mensen A, Khatami R. Advanced EEG analysis using threshold-free cluster- enhancement and non-parametric statistics. Neuroimage. 2013;67:111–118. doi: 10.1016/j.neuroimage.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Pernet CR, Latinus M, Nichols TE, Rousselet GA. Cluster-based computational methods for mass univariate analyses of event-related brain potentials/fields: A simulation study. Journal of Neuroscience Methods. 2015;250:85–93. doi: 10.1016/j.jneumeth.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995:289–300. [Google Scholar]
- 28.Fukuda K, Kang MS, Woodman G. Electrophysiology reveals distinct neural mechanisms for lateralized and spatially global visual working memory representations. J Vis. 2015;15:1114. doi: 10.1152/jn.00991.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. 2012;32:12411–12420. doi: 10.1523/JNEUROSCI.0421-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manza P, Hau CLV, Leung HC. Alpha power gates relevant information during working memory updating. J Neurosci. 2014;34:5998–6002. doi: 10.1523/JNEUROSCI.4641-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kustermann T, Rockstroh B, Kienle J, Miller GA, Popov T. Deficient attention modulation of lateralized alpha power in schizophrenia. Psychophysiology. 2016:n/a–n/a. doi: 10.1111/psyp.12626. [DOI] [PubMed] [Google Scholar]
- 32.Jensen O, Gips B, Bergmann TO, Bonnefond M. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends in Neurosciences. 2014;37:357–369. doi: 10.1016/j.tins.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Nuechterlein KH, Green MF, Calkins ME, Greenwood TA, Gur RE, Gur RC, et al. Attention/vigilance in schizophrenia: Performance results from a large multi-site study of the Consortium on the Genetics of Schizophrenia (COGS) Schizophrenia Research. 2015;163:38–46. doi: 10.1016/j.schres.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


