Abstract
Introduction
Since the identification of the dystrophin gene in 1986, a cure for Duchenne muscular dystrophy (DMD) has yet to be discovered. Presently, there are a number of genetic-based therapies in development aimed at restoration and/or repair of the primary defect. However, growing understanding of the pathophysiological consequences of dystrophin absence has revealed several promising downstream targets for the development of therapeutics.
Areas covered
In this review, we discuss various strategies for DMD therapy targeting downstream consequences of dystrophin absence including loss of muscle mass, inflammation, fibrosis, calcium overload, oxidative stress, and ischemia. The rationale of each approach and the efficacy of drugs in preclinical and clinical studies are discussed.
Expert opinion
For the last 30 years, effective DMD drug therapy has been limited to corticosteroids, which are associated with a number of negative side effects. Our knowledge of the consequences of dystrophin absence that contribute to DMD pathology has revealed several potential therapeutic targets. Some of these approaches may have potential to improve or slow disease progression independently or in combination with genetic-based approaches. The applicability of these pharmacological therapies to DMD patients irrespective of their genetic mutation, as well as the potential benefits even for advanced stage patients warrants their continued investigation.
Keywords: Duchenne muscular dystrophy, corticosteroids, myostatin, NF-κB, TGF-β, fibrosis, phosphodiesterase inhibitors, therapeutics, oxidative stress, calcium
1. Introduction
Duchenne muscular dystrophy (DMD) is an X-linked inherited progressive muscle-wasting disease caused by nonsense or frame shift loss of function mutations in the dystrophin gene. DMD affects approximately 1 in 3,500 male births, and is one of the most common and severe forms of muscular dystrophy. Diagnosis generally occurs between 2–4 years of age when children begin to demonstrate early hallmarks of the disease including delayed motor milestones and enlarged calf muscles. Independent ambulation is lost between 10–12 years of age, and premature death occurs in the late twenties to early thirties due to respiratory and/or cardiac failure1. Mutations that permit production of a partially functional dystrophin with reduced expression lead to the milder allelic variant, Becker muscular dystrophy (BMD)2.
Dystrophin is a critical component of the dystrophin-associated protein complex (DAPC), a multimeric assembly of proteins localized at the sarcolemma of skeletal, cardiac, and smooth muscle cells3. The DAPC is thought to primarily serve a mechanical role, functioning as a physical link between the internal cytoskeleton and extracellular matrix (ECM)4, 5, though roles in mediating signaling are emerging6, 7. In DMD, loss of functional dystrophin and the consequential loss of the DAPC enhances sarcolemma susceptibility to contraction-induced damage resulting in repetitive cycles of muscle degeneration and regeneration8. Continuous immune cell infiltration and necrosis ultimately results in replacement of muscle with adipose and fibrotic connective tissue, such that the muscle no longer possesses force-generating capacity and experiences functional ischemia9, 10.
Although advances in respiratory and cardiac supportive care in the last decades have improved quality of life and extended life expectancy considerably, further advances must come from therapeutic approaches to ameliorate the underlying pathophysiology of DMD. There are several genetic, cell-based, and pharmacological DMD treatment approaches under investigation aimed at replacement or repair of the primary DMD gene defect and restoration of the DAPC including viral delivery of mini-dystrophin, read-through of translation stop codons, and exon skipping to restore the reading frame (reviewed by Fairclough11 and Guiraud12). However, their widespread delivery at sufficiently high concentrations to elicit a meaningful effect without causing a systemic immune response has proved challenging. In addition, only a specific subset of patients can currently benefit from certain strategies based on their genetic mutation, and individualized medicine will be required to take full advantage of these potential therapies13, 14. Therefore, investigation of pharmacological therapies to improve pathology independently and/or to synergize with genetic-based therapies in a combinatorial approach is a worthwhile area of research.
Muscle fiber degeneration and regeneration, fibrosis, inflammation, Ca2+ dysregulation, oxidative stress, and ischemia are associated with the absence of dystrophin absence and contribute to DMD disease progression (Figure 1). Although correction of the primary defect and restoration of the sarcolemmal DAPC may ameliorate muscle pathology, it is likely that maximal benefit will be achieved by a multifaceted approach that addresses multiple aspects of the disease. In combination with supportive care, pharmacological interventions that counter the underlying pathological mechanisms and stimulate muscle growth, promote of blood flow, and reduce fibrosis and inflammation may be viable options for patients even at advanced stages of disease. This review will focus on pharmacological approaches that manipulate downstream targets that have emerged from our growing understanding of the pathological consequences of dystrophin absence in muscle.
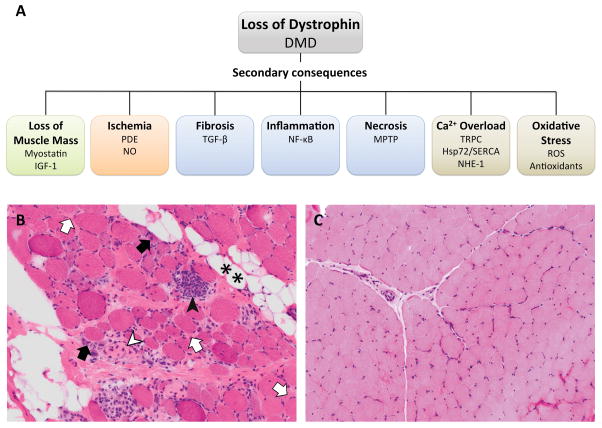
Figure 1.
Pathological features of DMD muscle. (a) Secondary consequences of dystrophin loss and their respective therapeutic targets grouped by closely related aspects of disease pathology. (b) Quadriceps muscle biopsy from a 6-year old DMD patient with scattered inflammatory cells in the endomysium and other dystrophic characteristics. **, fatty infiltration; white arrows, endomysial fibrosis; black arrows, regenerating fibers; white arrowhead, necrosis; black arrowhead, myophagocytosis. (c) Quadriceps muscle biopsy from a 7-year old with no identified neuromuscular disorder. Hematoxylin and eosin staining, 20x.
2. Corticosteroids
Currently, there is no cure for DMD, and the only standing treatment is long-term corticosteroid dosing with the anti-inflammatory glucocorticoids prednisone, prednisolone, and deflazacort. These drugs are thought to function primarily by inhibiting the NF-κB pathway14, 15, though there is also evidence that they activate the calcineurin/NF-AT pathway16. Corticosteroids have been shown to prolong independent ambulation, improve pulmonary function, and delay the onset of cardiomyopathy17, but they also have significant side effects including weight gain, hypertension, and vertebral compression fractures18. Although they target the same pathway, the incidence and severity of side effects differs among them. A direct comparison of deflazacort and prednisone in DMD in a multicenter, double-blind, randomized trial of 18 patients over one year of treatment showed that while there was no significant difference in motor outcomes, there was less weight gain in the group treated with deflazacort compared to prednisone (2.17 kg vs. 5.08 kg)19. Deflazacort has also been reported to decrease incidence of gastrointestinal pain19 and to prolong ambulation20 compared to prednisone, but it is not currently FDA approved and cannot be purchased in the U.S. making its acquisition difficult and costly for many families. However, Marathon Pharmaceuticals has recently submitted a deflazacort new drug application to the FDA with orphan drug and rare pediatric disease designation, and it has been granted fast track status21. Interestingly, it has also been shown that the addition of prednisolone treatment to mdx mice actually has a detrimental impact on positive cardiac outcomes from treatment with other drugs22. This finding emphasizes that care must be taken when introducing new therapeutics in patients already on a corticosteroid regimen to achieve a synergistic positive effect.
Efforts to develop a glucocorticoid alternative with fewer side effects are being investigated. Recently, ReveraGen has developed Vamorolone (previously known as VBP15), a novel glucocorticoid analogue that retains the anti-inflammatory characteristics of traditional DMD corticosteroid treatments, but without significant side effects. Mdx mice treated with this drug are reported to have less inflammation and increased grip strength, and did not cause stunted growth or immunotoxicity associated with prednisolone23. Vamorolone underwent successful phase 1 safety and tolerability clinical trials in healthy adult, and preparation for a phase 2a multiple ascending dose study in DMD boys ages 4–7 is currently underway, presenting a promising new glucocorticoid-based treatment option24.
3. Other approaches targeting NF-κB
NF-κB is a central transcription factor responsible for modulating immune and inflammatory responses. The NF-κB pathway is stimulated by pro-inflammatory cytokines TNF-α and interleukin 6 (IL-6), which permit and assembly of the I-κB kinase (IKK) catalytic complex subunits IKKα, IKKβ, and NEMO. Assembly of this complex permits NF-κB entry into the cell and subsequent transcription of genes encoding cytokines and other immune response factors25. Absence of dystrophin leads to increased sarcolemmal permeability and generation of reactive oxygen species, associated with chronic activation of NF-κB in DMD26, 27 and in mdx28 muscle fibers and macrophages, which is thought to be a significant contributor to muscle fiber necrosis and dystrophic pathology29, 30. Inhibition of NF-κB activation has been found to improve skeletal and cardiac dystrophic pathology in mdx mice28. Therefore, pharmacological approaches to block this pathway, including by preventing assembly of the catalytic complex or by inhibiting the stimulatory cytokines, have been investigated.
NEMO binding domain (NBD), an NF-κB inhibitory peptide, decreases activation of NF-κB by blocking phosphorylation of the IKKα and IKKβ subunits and subsequent binding to NEMO, preventing assembly of the IKK catalytic complex. Systemic administration of NBD in mdx mice has been shown to decrease necrosis and increase regeneration in hind limb and diaphragm muscles, and improve generation of specific force31. NBD also improved cardiac contractile function in the more severely affected mdx;utr−/− mice, although histopathology including collagen content and inflammatory markers remained unchanged compared to vehicle-treated mice32. NBD does not completely inhibit NF-κB activity, such that theoretically, other essential cellular processes are not disrupted, which may be important given the central role of this pathway in many tissues. Intravenous delivery of NBD to the Golden Retriever Muscular Dystrophy (GRMD) model over 4 months was found to improve pelvic limb muscle force and improve histopathologic indices for inflammation and necrosis. However, administration over time led to infusion reactions and an immune response33. Given the preclinical success of NBD in the mdx mouse model, NBD was poised to enter human trials, but safety concerns identified in the GRMD dog model warrant further assessment of doses and formulation of NBD for human application. Long-term preclinical toxicology studies in non-human primates are being pursued.
Catabasis has developed a NF-κB inhibitor, CAT-1004, that reduced muscle degeneration and improved muscle regeneration and function in skeletal, diaphragm, and cardiac muscle in mdx mice. In comparison with prednisolone, CAT-1004 yielded greater number of regenerating fibers and reduced muscle inflammation without reducing muscle weight. In GRMD dogs, a single dose of CAT-1004 reduced NF-κB activity and production of plasma TNF-α34. Phase 1 trials in healthy adults reported safety, tolerability, and significant reduction in NF-κB activity35. Recruitment for a CAT-1004 phase 1/2 clinical trial in DMD boys ages 4–7 with 3 different doses is ongoing36.
A repurposed drug under early investigation for DMD is infliximab (Remicade®), an antibody to human TNF-α that is commonly used for treatment of rheumatoid arthritis and Crohn’s disease. Long-term administration of infliximab in mdx mice was found to reduce skeletal and cardiac muscle fibrosis, but negatively impacted Akt activation and cardiac function. Interestingly, infliximab treatment also correlated with reduced cardiac myostatin mRNA levels37 (further expanded on in section 4).
Inhibition of NF-κB signaling has demonstrated positive effects in ameliorating dystrophic pathology. However, therapies delivered systemically could potentially lead to negative outcomes due the central role of NF-κB signaling in many other tissues. In addition, although it is generally observed that heightened NF-κB activity negatively impacts myogenic potential, there is also some evidence that NF-κB signaling is important for expression of antioxidant enzymes in response to oxidative stress38 (see section 7). Taking these considerations into account, targeted NF-κB inhibition in skeletal and cardiac muscle may be a desirable approach for DMD treatment given its consistent positive outcomes.
4. Promoting muscle mass
4.1 Myostatin inhibition
Myostatin is a member of the transforming growth factor-beta (TGF-β) superfamily of secreted growth and differentiation factors, and is a potent negative regulator of skeletal muscle growth. Its function is evident in animals with genetic mutations in the Mstn gene, including Belgian Blue cattle39, the Bully whippet dog40, and Mstn knockout mouse41, all of which exhibit dramatic muscle hypertrophy. In DMD, muscle mass is lost due to severe muscle fiber degeneration and eventual exhaustion of regenerative capacity. Thus, myostatin was implicated as an attractive target for DMD therapy, leading to research efforts directed at stimulating muscle growth via its inhibition.
Myostatin binds activin type II receptors (ActRII) which recruit, phosphorylate and activate activin type I receptor propagating signals via the receptor-associated proteins Smad 2 and Smad 342, 43. Smad 2/3 translocate into the nucleus, and activate the transcription of target genes through interaction with DNA and other nuclear factors muscle cell proliferation and differentiation. Several methods, including genetic knockdown44, blocking antibodies45, 46, and propeptide-mediated inhibition47, have been explored to inhibit its activity. Crossing myostatin null mice with mdx mice showed double knockout mstn−/−/mdx mice had reduced diaphragm fibrosis and improved grip strength compared to mdx mice. However, cycles of muscle degeneration and regeneration as well as fibers with centrally located nuclei and elevated serum creatine kinase were still evident48. Inhibition of myostatin by injection of anti-myostatin monoclonal antibodies into mdx mice muscles yielded more promising results. Three months of weekly injections increased muscle mass up to 35% due to fiber size enlargement, and strikingly, serum creatine kinases levels decreased to near normal levels45. Propeptide-mediated myostatin inhibition also yielded functional benefits including increased tetanic force production47. Interestingly, reduced myostatin in dystrophin-deficient Mstn+/− dogs did not yield beneficial results, instead causing unequal muscle growth and greater joint contractures49.
The success of myostatin inhibition in the mdx mouse model has led to multiple clinical trials, however, drug development in this effort has been challenging. Initial therapeutic strategies aimed to systemically abrogate myostatin/ActRIIB signaling to ensure widespread effect on musculature. However, there have been concerns regarding efficacy, interference with ActRIIB signaling in non-muscle tissues, and potential adverse side effects. MYO-029, a high-affinity myostatin binding antibody, was shown to increase muscle mass in mice by 30% over 3 months of treatment50. However, clinical studies of BMD, LGMD and FSHD patients treated with MYO-029 administered intravenously every 2 weeks for 6 months were ceased after they did not improve strength or function despite being well-tolerated50. Efforts to develop ACE-031, a recombinant pseudo ActIIB receptor that increased muscle mass and whole body pulling tension in mdx mice51, were terminated due to incidents of dilated blood vessels, nosebleeds, gum bleeding in treated DMD boys52. Subcutaneous ACE-031 delivery every 2–4 weeks to ambulatory DMD boys in a recent ascending dose trial was not associated with serious or severe adverse events, and demonstrated trends for pharmacodynamic effects on lean body mass and body mass density. However this study was also ceased due to safety concerns of epistaxis and telangiectasias53. A large phase 2 clinical study of over 100 DMD boys with monthly intravenous infused doses of PF-06252616, an anti-myostatin monoclonal antibody from Pfizer, is currently ongoing in the U.S., Canada, Europe, and Japan54. Unlike ACE-031, PF-06252616 is highly specific to myostatin, which may confer fewer negative side effects.
Despite variable clinical outcomes to increase muscle size and strength through myostatin inhibition, there is still active research in this pursuit. Fortunately, more directed approaches are now being developed to be delivered directly via intramuscular injection which may minimize negative side effects. Acceleron has developed ACE-083, a locally acting myostatin trap, to be delivered by single muscle injection. Phase 1 trials of ACE-083 in post-menopausal women showed safety and a 7–15% increase in muscle mass55. BMS-986089, a human anti-myostatin antibody developed by Bristol-Myers Squibb, yielded a 4.5–5% increase in muscle mass in healthy subjects following thigh muscle injection56. Recruitment for a phase 2 trial of BMS-986089 in ambulatory DMD boys ages 5–10 delivered weekly by subcutaneous injection is ongoing57. Given these positive preliminary results with highly specific antibodies and/or local delivery approach, myostatin inhibition as a target for DMD therapy may still be a viable treatment option.
4.2 Insulin growth factor-1
Insulin Growth Factor-1 (IGF-1) is a key mediator of skeletal myoblast differentiation that is also under investigation as a DMD therapy to increase muscle mass and retain strength. IGF-1 promotes muscle hypertrophy by activation of the PI3K/Akt/mTOR pathway stimulating protein synthesis and inhibiting protein degradation58. IGF-1 is well-tolerated in clinical studies for treatment of osteoporosis59 and Rett syndrome60 making it an attractive potential therapy. In addition, insulin-like growth factor was among a group of compounds found to increase tetanic force generation in an ex vivo bioartificial muscle mdx model61. Preclinical studies in mdx mice demonstrated that IGF-1 overexpression increases EDL and diaphragm muscle mass, enhances force generation, and reduces necrosis and fibrosis62. These promising mouse studies led to a controlled phase 2 clinical trial of Increlex (an FDA-approved recombinant IGF-1 delivered by daily subcutaneous injection) conducted in glucocorticoid-treated DMD boys to examine its efficacy to maintain muscle function. Preliminary analysis suggested that although the treatment improved height growth and measures of insulin resistance, there was no change observed in motor function following 6 months of treatment63. Although no follow-up studies have been reported, IGF-1 may be a valuable therapy in younger DMD patients to maintain muscle mass and strength.
4.3 Other drugs to promote muscle mass
Although traditionally used for treating asthma and COPD, β2-agonists have also been found to induce satellite cell proliferation and muscle protein production to increase lean body mass in models of muscle injury. In mdx mice, β2-agonists reduced muscle degeneration and increased muscle force64–66. The β2-agonist albuterol has been tested extensively in FSHD patients and showed only limited positive effects, but was well-tolerated67, 68. More recently, albuterol was shown to increase lean body mass in ambulatory DMD and BMD boys, but has not been pursued further. Urocortin, a corticoid releasing factor receptor agonist, functions similarly to β2-agonists by elevating cAMP to inhibit calapins. Urocortin 2 administered by 6 subcutaneous injections over 2 weeks rescued mdx diaphragm muscle force loss compared to saline treatment69, and was also found to improve mdx smooth muscle GI motility70. Urocortins have also shown efficacy in treating heart failure71, and thus may elicit both skeletal and cardiac muscle benefits in DMD.
DT-200 (formerly known as GLPG0492) is a non-steroidal selective androgen receptor modulator originally investigated to treat sarcopenia and cachexia72, 73. In mdx mice, DT-200 improved running performance and increased diaphragm force74. DT-200 had positive phase 1 clinical data in healthy subjects75 and has broad potential for multiple muscle wasting diseases.
A consideration for clinical trials aimed at promoting muscle mass remains that increased muscle mass may not necessarily translate to improved muscle strength41. In addition, later stage DMD patients may not have sufficient muscle tissue remaining to elicit meaningful outcomes with some of these therapies. Despite variable efficacy in earlier attempts to inhibit myostatin, some of the more recent therapeutics aimed at increasing muscle mass show promise.
5. Drugs targeting fibrosis
Fibrosis is defined as the deposition of ECM components, and in healthy muscle, it is critical for providing a scaffold on which to build and structure new tissue during growth and repair. However, even minor insults to this process can lead to detrimental pathological effects. In DMD, loss of dystrophin leads of sarcolemmal fragility, muscle fiber degeneration, and uncontrolled activation of profibrotic signaling pathways. Muscle tissue is progressively replaced by excessive accumulation of fibrotic and adipose tissue, which not only reduces contractile function, but also decreases the available muscle tissue for therapeutic intervention76. Thus, significant DMD research has been directed at preventing the development of this excessive fibrotic tissue.
Transforming growth factor-beta (TFG-β) is a potent profibrogenic factor that is expressed in regenerating muscle after injury and in dystrophic muscle of mdx mice77, GRMD dogs78 and DMD patients79, 80. TFG-β is normally stored in the ECM as a latent precursor, and upon activation by tissue damage or specific growth signals, binds to serine/threonine kinase receptors types I and II to initiate transcription of several profibrotic genes including collagen and fibronectin. TFG-β is also produced by infiltrating immune and inflammatory cells, and can decrease the production of enzymes that degrade the ECM81. Given that pathological fibrosis is a hallmark feature of dystrophic muscle and that TFG-β levels correlate with the extent of muscle fibrosis in DMD patients82, 83, the TFG-β pathway has emerged as an important therapeutic target to increase muscle regeneration and reduce excessive fibrotic tissue deposition in DMD.
One of the earliest investigated TFG-β inhibitors was losartan, which was an attractive candidate because of its extensive use as a safe antihypertensive drug. Losartan is oxidized in the liver and its metabolite acts to block angiotensin II receptor activation, which is required to initiate TFG-β signaling. Initial studies in mdx mice showed treatment with losartan for 6–9 months reduced fibrosis in the diaphragm and gastrocnemius muscles, increased grip strength84, decreased cardiac muscle fibrosis, and improved cardiac function85. However, subsequent studies showed minimal functional benefit86. Losartan entered a limited human clinical trial that showed one year of losartan treatment was safe and improved cardiac ejection fraction87.
Pirfenidone is another small molecule designed as an antifibrotic agent and used clinically for treatment of idiopathic pulmonary fibrosis88, and has received orphan drug status from the FDA tor treatment of scleroderma. Although the precise mechanism of action is unknown, it has been shown to suppress expression of TFG-β89, though its efficacy in mdx mouse studies has been mixed. One study showed 4-weeks of pirfenidone treatment in mdx mice increased matrix metalloproteinases MMP-2 and MMP-9 with minimal changes to fibrosis, independent of TFG-β levels90. In another study, 7-months of high-dose pirfenidone administration to 8-month old mice improved cardiac contractility, but not fibrosis91. However, when targeting therapeutic modulation of the TFG-β pathway, or any signaling pathway in the treatment of DMD, one must consider that timing of treatment may be critical in determining its efficacy. For example, TFG-β is elevated at 6 and 9 weeks of age, but not at 12 weeks in the mdx diaphragm77, suggesting that treatment with TFG-β inhibitors at this later stage may not be efficacious in this model. In DMD patient muscle derived fibroblasts, pirfenidone was shown to reduce synthesis and deposition of intracellular collagen92.
Two antineoplastic drugs, suramin and FDA-approved imatinib mesylate (Gleevec®), have been shown to act as TFG-β antagonists in several chronic fibrotic diseases including kidney disease93, myocarditis94, and liver disease95. Intraperitoneal injection of suramin in 6-month old mdx mice has been shown to increase MMP-2 and MMP-9 activity96, decrease creatine kinase, and to attenuate fibrosis in skeletal muscle, but not cardiac muscle97. In 8-month old mdx mice, however, suramin did improve cardiomyopathy and decrease myocardial fibrosis, inflammation, and myonecrosis98. Imatinib mesylate inhibited TFG-β pro-fibrogenic activity, decreased creatine kinase99, reduced fibrosis, and improve hind limb grip strength100 in mdx mice. Most recently, imatinib mesylate was found to ameliorate muscle pathology of the DBA/2-mdx mouse, a severe DMD mouse model that more closely recapitulates several human disease characteristics101. These results combined with the observation that these drugs are well tolerated in non-DMD clinical trials make them attractive candidates for DMD therapy.
Among the anti-TFG-β drugs under investigation, halofuginone has demonstrated some of the most promising results. Halofuginone is a low molecular weight plant alkaloid that plays dual roles – it inhibits TFG-β-mediated collagen synthesis signaling by impeding Smad 3 binding to DNA102, but also enhances Akt and MAPK/ERK signaling to promote myotube fusion in mdx and wild type primary myoblasts103. Ten weeks of halofuginone treatment in 4-week old mdx mice decreased diaphragm fibrosis, increased the number of revertant fibers104, and promoted satellite cell activation and survival105. The pro-proliferative features of halofuginone, in addition to its anti-fibrotic action, could have positive implications for muscle regeneration in DMD patients. Clinical trials in DMD boys with HT-100, a oral delayed-release halofuginone, showed positive trends in serum biomarkers of collagen degradation, and a 11.7% mean increase in total muscle strength compared to baseline (study entry) over 18–22 months106. However, dosing and enrollment was recently suspended due to the death of a study subject107.
A new approach under investigation is repurposing tamoxifen, a selective estrogen receptor modulator, to counter DMD-related fibrosis. Tamoxifen is most commonly known as an anticancer drug for treating and preventing breast cancer108, but is also used clinically for treating of retroperitoneal fibrosis109 and encapsulating peritoneal sclerosis110. In these diseases and others, tamoxifen demonstrates antifibrotic properties and is thought to work through inhibition of TGF-β111, 112, and preclinical application in dystrophin-deficient mice has shown promising results. Oral tamoxifen treatment of 3-week old mdx5cv mice for 15 months at a dose of 10mg/kg/day was found to diminish cardiac and diaphragm fibrosis, improved muscle structure and force, and conferred slower development of muscle phenotype113. In addition to its extensive use in adults, tamoxifen has also been tested for other conditions in adolescent boys ages 13–16 for up to 48 months and appears to be well tolerated and safe dosed up to 20mg twice daily114, 115. Of note, in these studies, tamoxifen did not cause pubertal delay, one of the side effects of long-term corticosteroid treatment in DMD boys116. The safety of tamoxifen in boys as young as 5 to 7 years old, the age at which early DMD treatment is often initiated, has not yet been established. However, 20mg/day of tamoxifen has been shown to be safe in young girls ages 3 to 10 over 12 months of treatment114. Given the safety of tamoxifen in children and its efficacy in decreasing fibrosis in other diseases, its repurposing in DMD boys is of considerable interest.
6. Calcium regulation and mitochondria
One of the earliest and most well studied hypotheses on the mechanisms leading to DMD muscle degeneration and myofiber necrosis is elevated cytosolic Ca2+. Stretch-induced membrane permeability due to dystrophin absence causes both passive and active Ca2+ entry through physical membrane tears and through stretch-activated channels, with subsequent mitochondrial dysfunction117 and activation of Ca2+-dependent degradative pathways118. Myofibers from dystrophic mice and DMD patients exhibit heightened resting and active Ca2+ levels119, 120, and also show defects in SR-Ca2+ handling and reuptake during relaxation121, 122. This excess cytosolic Ca2+ has been shown to cause pathology in several ways including by altering excitation contraction-coupling, causing mitochondrial rupture by overactivation of the mitochondrial permeability transition pore (MPTP), and by hyperactivation of calpains, which can exacerbate dystrophic pathology by cleaving critical intracellular proteins. Given that Ca2+ levels are influenced by several factors in dystrophic muscle, there have been pharmacological efforts to correct it on multiple levels including at the level of the plasma membrane, the SR, and the mitochondria.
6.1 Calcium channel blockers
Early investigation of the hypothesis of Ca2+-induced pathology in DMD led to a series of clinical trials involving various Ca2+ channel blockers including flunarizine, nifedipine, and verapamil123–125. Despite being well tolerated in DMD patients, there were no clinically significant effects, but other Ca2+ channel blockers have since been investigated. Streptomycin is a broad range antibiotic that primarily functions to inhibit protein synthesis, but also acts as a non-specific blocker of Ca2+ channels. Streptomycin has shown variable efficacy in mdx mice - initially it was demonstrated to decrease muscle degeneration, improve force and significantly reduce the uptake of procion orange dye into fibers from mdx muscles after eccentric contractions118, 126. However, a later study showed streptomycin did not show positive effects in diaphragm or heart muscle and actually worsened heart pathology127 and its suitability of streptomycin as a DMD therapeutic has been questioned due to the side effects associated with prolonged antibiotic use. An intriguing drug in this category recently gaining attention is AT-300 (previously known as GsMTx4), a peptide originally discovered in the venom of the Chilean Rose Tarantula, which was found to specifically block mechanosensitive Ca2+ channels128. AT-300 was first tested side-by-side with streptomycin in mdx mice and was shown to have similarly positive effects118, 126. Moreover, GsMTx4 was found to block transient receptor potential canonical 6 (TRPC6) channel activation in vitro129, and hyperactivation of TRPC6 channels has been linked to the adverse mechanical stress response and intracellular Ca2+ in dystrophic heart130. The rights to AT-300 were recently acquired by Akashi Therapeutics, and they are investigating its therapeutic potential for DMD131. AT-300 has been granted orphan drug designation by the FDA, but will require thorough preclinical testing before clinical application.
6.2 Repairing the plasma membrane
The absence of dystrophin permits Ca2+ to enter dystrophic muscle fibers through physical membrane tears. Mitsugumin 53 is a TRIM family protein that is principally expressed in skeletal and cardiac muscle, and plays an essential role in cell membrane repair evidenced by mitsugumin 53 knockout mice exhibiting a progressive muscular dystrophy132. In striated muscle, mitsugumin 53 facilitates vesicle translocation to sites of membrane injury to patch the membrane in damaged muscle cells133. Preclinical evidence showed that injection of recombinant human mitsugumin 53 decreased muscle pathology in mdx mice134.
Poloxamer 188 is a membrane sealant polymer that localizes into lipid monolayers and areas of damaged membranes. In cardiomyocytes from mdx mice, poloxamer 188 prevented stretch-mediated Ca2+ overload into the muscle cell,135 and chronic infusion in GRMD dogs was found to reduce myocardial fibrosis136. Most recently, chronic administration of national formulary grade poloxamer 188 in mdx and mdx/utr−/− dko mice was shown to improve diaphragm muscle including decreasing the number of centralized nuclei and variation in fiber size, improving fiber density, and decreasing collagen deposition137. However, contradictory evidence in mdx skeletal muscle shows that poloxamer 188 does not prevent exercise-induced membrane breakdown in quadriceps muscle138 and may actually increase susceptibility to contraction-induced injury in tibialis anterior muscle139. These studies present the possibility that poloxamer 188 may have differing effects in cardiac and diaphragm muscle versus limb muscles.
6.3 Clearing calcium overload
BGP-15 is a pharmacological inducer of Hsp72 that binds sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA). SERCA normally pumps Ca2+ out of the cytosol and into SR stores, but this function is perturbed in dystrophic muscle121. Subsequent studies showed that increasing the level of SERCA protein in mdx skeletal muscle using transgenic or viral delivery significantly mitigated the dystrophic pathology140, supporting SERCA as a DMD treatment target. Treatment of mdx mice with BGP-15 increased SERCA activity, improved muscle architecture, strength and contractile function in severely affected diaphragm muscles. In severely affected mdx;utrn−/− mice, BGP-15 decreased kyphosis, improved TA force to wild type levels, and extended lifespan141. In non-DMD mouse and rat models, BGP-15 was also shown to protect against heart failure and to alleviate ventilation-induced diaphragm dysfunction, respectively142, 143. It has yet to undergo clinical trials, but has strong preclinical evidence relevant to DMD.
In dystrophic muscle, ryanodine receptors (RyR1 in skeletal muscle and RyR2 in the heart) are overactive causing leaking of Ca2+ into the cytosol144, which can be stabilized by small molecules termed rycals. Rycal S107 has been shown to improve muscle strength in models of bone metastases145, lower SR Ca2+ leakage, and prevent cardiac arrhythmias in vivo in mdx mice146. ARM210/S48168, a proprietary rycal candidate from ARMGO Pharma, was found to improve exercise capacity, muscle specific force, grip strength and muscle histology in preclinical mouse models. It was recently advanced into a clinical stage program for DMD and received FDA orphan drug designation147.
Enhanced Na+/H+ exchanger type-1 (NHE-1) activity resulting in intracellular Na+ overload has been shown to contribute to dystrophic pathology148 and may contribute to Ca2+ overload by reducing Ca2+ extrusion through the Na+/Ca2+ exhanger149. NHE inhibitors cariporide and EIPA have been shown to elicit protective effects against muscle degeneration in dystrophic BIO14.6 hamsters and mdx mice that significantly reduce both elevated Na+ and Ca2+ in dystrophic myotubes148. Rimeporide, a selective Na+/H+ exchanger type-1 (NHE-1) inhibitor, was found to reduce cardiac necrosis in cardiopmyopathic hamsters150, and improve functional strength, and reduce diaphragm inflammation151. EspeRare Foundation is currently recruiting participants for a phase Ib clinical trial152.
6.4 Mitochondria
Intracellular Ca2+ overload and oxidative stress cause permeabilization of mitochondria by formation of large pore complexes termed mitochondrial permeability transition pores (MPTP) on the membrane, which is mediated by cyclophilin D. Subsequent disruption of cellular ATP generation causes mitochondrial swelling, rupture, and ultimately cell death153. Cyclosporin A is a fungal cyclic peptide that has been shown to inhibit cyclophilin D and prevent MPTP formation154 and is commonly used as an immunosuppressant. In mdx mice, cyclosporine A prevented force drop following exercise and reduced creatine kinase155. A small initial study in DMD boys showed that eight weeks of cyclosporin treatment elicited significant improvement in tetanic force and maximum voluntary contraction in TA muscles within two weeks of treatment156. However, a later study in a DMD myoblast transfer study showed no effect on strength157, and side effects associated with long-term immunosupression have dissuaded further investigation of this drug for use in DMD. To overcome this issue, Debio-25 (also known as alisporovir), a non-immunosupressive analogue of cyclosporine, has recently been produced. In mdx mice, Debio-025 has been shown to have similar efficacy to cyclosporine A158 and was reported to be more effective than prednisone in a side-by-side study in reducing fibrosis and infiltration of activated macrophages159. Debio-025 had a positive safety profile in clinical trials for hepatitis C, and Debiopharm International SA and Solid Biosciences have announced a collaboration to explore the use of Debio-025 in DMD160.
7. Countering oxidative stress
Inflammation, fibrosis, and Ca2+ dysregulation in dystrophic muscle are associated with production of reactive oxygen species (ROS), which lead to oxidative stress161, 162. The influx of Ca2+ through the dystrophin-deficient sarcolemma is thought to enhance ROS production by the mitochondria, which stimulates stretch-activated Ca2+ channels and further exacerbates Ca2+-induced mitochondrial dysfunction and necrosis126, 163. ROS are also generated by membrane-bound NADPH oxidase (NOX)164, and activate NF-κB signaling to trigger the inflammatory response165 and TGF-β signaling (see sections 2, 3, and 5). Thus, various antioxidant therapies have been investigated to decrease ROS in dystrophic muscle.
Coenzyme Q10 binds the inner mitochondrial membrane and functions as an electron acceptor in the respiratory chain that not only decreases oxidative stress, but also modulates the MPTP (see section 6.4) and could decrease mitochondrial Ca2+ accumulation166. Coenzyme Q10 has been reported to increase muscle strength 8.5% in a small trial of DMD boys167. Idebenone, a synthetic derivative of coenzyme Q10, was found to correct cardiac diastolic dysfunction, reduce inflammation and fibrosis, and improve voluntary running in mdx mice following a long-term 10-month treatment protocol168. Idebenone has recently shown promising results in DMD boys, and a phase III double-blind clinical trial in DMD patients on glucocorticoids to assess its efficacy in delaying the loss of respiratory function is in development169.
L-Arginine, a substrate of nitric oxide synthase, has been shown to reduce contraction-induced damage and myonecrosis in mdx mice suggesting partial restoration of Ca2+ handing170. In addition, L-Arginine decreases inflammation and enhances muscle regneration, and increases NF-kB in mdx muscle fibers171. L-Arginine has also demonstrated efficacy in combination with other pharmacological therapies. When combined with deflazacort, L-Arginine prevented exercise-induced damage and induced a persistent functional improvement in distance run in mdx mice. Recently, a small cohort of DMD boys aged 7–10 treated with L-Arginine and metformin for 16 weeks showed significantly increased mitochondrial protein expression and reduced in oxidative stress172. Other supplements investigated to improve muscle strength by providing metabolic support include creatine and glutamine, which have been shown to increase grip strength173 and decrease protein degradation174, respectively, in DMD boys. However, a CINRG phase II/III trial of creatine and glutamine in ambulant DMD boys did not show any statistically significantly effect on muscle strength175.
N-Acetylcysteine is an antioxidant that can directly scavenge ROS and was found to reduce creatine kinase by 88% and reduce stretch-induced damage in mdx mice176. It was also able to prevent mdx mice against exercise-induced damage and necrosis after only one week of treatment, improve Ca2+ handling, and decrease nuclear NF-κB177, 178. Despite promising preclinical data, N-Acetylcysteine has yet to be tested in DMD patients, but is currently recruiting participants for a clinical study of RyR1-related congenital myopathy179.
The antioxidant and anti-inflammatory properties of melatonin have led to its investigation as a DMD treatment. In mdx5cv mice, 10 days of melatonin treatment increased twitch force and tetanic force, decreased creatine kinase, and improved muscle redox status180. In a DMD clinical trial, 3 months of melatonin treatment decreased creatine kinase and reduced other hyperoxidative and inflammatory markers181, suggesting it may slow the degenerative process.
Green tea is an abundant source of free radical scavenging polyphenols, the predominant being epigallocatechin gallate (EGCg). Preclinical studies of EGCg in mdx mice showed improved histology, decreased necrosis, and improved muscle function182–184. Early treatment with green tea extract also decreased dystrophic muscle pathology, potentially by regulating NF-κB activity in regenerating fibers185. An advantage of EGCg is its availability and low potential for toxicity. A double-blind, placebo-controlled phase II/III of EGCg in 5–10 year old DMD boys to assess safety and efficacy is ongoing186.
As mentioned, ROS produced by NOX triggers the manifestation of dystrophic pathology, making NOX inhibition an attractive strategy. Diapocynin is a synthesized dimer of the NOX inhibitor apocynin, which unlike apocynin and other NOX inhibitors187, does not promote ROS production in some cell types. In dystrophic myotubes, diapocynin inhibited ROS production and reduced intracellular Ca2+ overload through stretch-activated and store-operated channels. Diapocynin also prevented force loss after eccentric contractions and reduced membrane damage in mdx muscle188. Given the role of NOX in promoting oxidative stress, it would be of interest to assess diapocynin or another potent, efficacious, and selective NOX inhibitor in DMD patients.
8. Phoshodiesterase inhibition
Neuronal nitric oxide synthase (nNOS) is a DAPC-associated protein that produces nitric oxide (NO), a key signaling molecule in regulation of skeletal muscle excitation-contraction coupling189, myogenesis, and muscle repair. NO stimulates the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), stimulating vasodilation. In healthy muscle, nNOS is enriched at the sarcolemma in fast twitch muscle fibers, but in mdx and DMD muscle, nNOS is mislocalized and its activity decreases by 80%105, 190, 191, implicating aberrant NO signaling in contributing to dystrophic pathology. Accordingly, mouse studies validate negative effects of low NO-induced ischemia on skeletal muscle contraction and fatigue192, 193. The therapeutic effects of restoring NO levels have been demonstrated by transgenic overexpression of nNOS in mdx skeletal and cardiac muscle, which corrected elevated serum creatine kinase, fibrosis, and macrophage infiltration194, 195. Further investigation of this pathway revealed that overexpression of guanylyl cyclase in the heart to enhance cGMP signaling without increased NO production were sufficient to elicit beneficial effects in dystrophic cardiomyopathy196. Therefore, pharmacological approaches to enhance cGMP levels have been pursued, specifically through inhibiting the activity of GMP-hydrolyzing phosphodiesterases (PDEs).
Pentoxifylline is a non-specific PDE inhibitor used clinically for the treatment of intermittent claudication197 and acts to suppress inflammation by inhibiting synthesis of pro-inflammatory cytokines and reactive oxygen species77. In mdx mice, pentoxifylline was shown to counteract hyperactive voltage-independent Ca2+ channels in myofibers77, increase tetanic tension in the diaphragm, and reduce creatine kinase198. Given these therapeutic effects, but without positive effects on fibrosis77, it is thought to act through PDE5 inhibition. Despite being well tolerated in adult populations, the liquid formulation of pentoxifylline caused intolerable gastrointestinal effects in DMD boys, requiring premature conclusion of the 2011 CINRG clinical study199. Thus, its efficacy in these subjects could not be assessed, and alternative routes of administration have been investigated. In a 12-month double-blind study of 64 DMD boys, treatment with a slow-release pentoxifylline formulation was better tolerated, but did not improve or halt the deterioration of muscle strength and function200.
There are also several PDE5 inhibitors currently used clinically including sildenafil (Viagra®), tadalafil (Cialis®) and vardenafil (Levitra®), which prevent cGMP breakdown and raise its concentration in muscle, promoting vasodilation201. Given that these PDE inhibitors are readily available, have minimal side effects, and have been tested in adult and pediatric subjects, they are an attractive treatment approach and have shown efficacy in preclinical studies. Sildenafil prevented onset of cardiomyopathy in young mdx mice, reversed cardiac dysfunction in older mdx mice following 3 months of treatment202, and reduced respiratory muscle weakness and fibrosis203. In addition, both sildenafil and tadalafil protect against mdx contraction-induced injury and reduce serum CK196, 204.
The success of the mdx studies led to clinical trials of sildenafil and tadalafil in DMD and BMD patients. A study of 10 DMD boys ages 8–13 and 10 healthy males treated with single doses of either tadalafil or sildenafil alleviated ischemia in a dose-dependent manner, and normalized exercise-induced increase in skeletal muscle blood flow, which is attenuated in DMD boys205. However, in a long-term study, 6-month treatment with sildenafil in DMD and BMD adults did not yield positive outcomes, with 29% of subjects actually experiencing worsening cardiac function, though it should be noted that the sample size in this study was relatively small (N=15)206. Most recently, Eli Lilly implemented a large phase 3 double blind clinical trial of tadalafil treatment in over 300 DMD patients. Unfortunately, tadalafil treatment did not elicit efficacy in the 6-minute walk distance or other assessments of motor function through 48 weeks of treatment, and the study was ended prematurely207. However, there is evidence that suggests that tadalafil treatment improves blood flow during exercise208, suggesting exercise may be necessary to observe an effect. In addition, it should be noted that the subjects in this study were older, ages 7 to 14, and there is precedence for investigating potential benefits of PDE inhibitors in younger DMD boys in future clinical studies. New evidence just published showed that GRMD dogs treated with tadalafil prior to detectable cardiomyopathy exhibited improved cardiac and skeletal muscle histopathological features, decreased TRPC6 levels, and delayed cardiomyopathy209.
9. Conclusion
Several potential targets for pharmacologic DMD therapies and the efficacy of relevant drugs tested in preclinical and clinical studies have been presented in this review (Table 1). The number of drugs under investigation is extensive, but while many of these therapies elicit meaningful benefits in the mdx mouse and GRMD dog models, this is not always recapitulated in human disease. Although the development of genetic-based therapies targeting DMD gene mutations is also a promising more direct approach, it has faced many hurdles in obtaining FDA approval and is not currently tailored to all patients. Therefore, pharmacological approaches that are applicable to all patients and may more quickly and easily enter the clinical setting are still an important area of DMD research.
Table 1.
Status of pharmacological therapies evaluated for DMD
| Target/Class | Drug | Mode of action | Therapeutic effects | Status reached in DMD research | Refs. |
|---|---|---|---|---|---|
|
| |||||
| Corticosteroid | Prednisone, Deflazacort, Prednisolone | Widespread, NF-κB inhibition |
 inflammation inflammation |
Standing long-term DMD therapy | 14, 15 |
| Vamorolone (prev. known as VBP15) | Novel glucocorticoid analogue that inhibits NF-κB |
 inflammation, inflammation,
 grip strength in mdx without side effects grip strength in mdx without side effects |
DMD Phase II ongoing | 23, 24 | |
|
| |||||
| NF-κB inhibition | NBD (NEMO binding domain) | Prevents assembly of the IKK catalytic complex |
 necrosis, necrosis,
 inflammation, inflammation,
 regeneration in mdx and GRMD regeneration in mdx and GRMD |
Non-human primates due to toxicity in GRMD | 31–33 |
| CAT-1004 | NF-κB inhibitor | Improved diaphragm muscle function in GRMD | DMD Phase II | 34–36 | |
| Infliximab (Remicade®) | Antibody to human TNF-α |
 skeletal and cardiac fibrosis in mdx skeletal and cardiac fibrosis in mdx
|
Preclinical | 37 | |
|
| |||||
| Myostatin inhibition | MYO-029 | High-affinity myostatin binding antibody | Ineffective but well tolerated in BMD, LGMD and FSHD | BMD, LGMD and FSHD Phase II | 50 |
| ACE-031 | Recombinant pseudo ActIIB receptor |
 muscle mass, muscle mass,
 whole body pulling force in mdx whole body pulling force in mdx
|
DMD Phase II | 51–53 | |
| PF-06252616 | Anti-myostatin monoclonal antibody |
 muscle mass in mdx muscle mass in mdx
|
DMD Phase II ongoing | 54 | |
| ACE-083 | Locally-acting myostatin trap |
 muscle mass and safe in Phase 1 trials in post-menopausal women muscle mass and safe in Phase 1 trials in post-menopausal women |
FSHD Phase II planned by Acceleron | 55 | |
| BMS-986089 | Anti-myostatin antibody |
 muscle mass in Phase I trial muscle mass in Phase I trial |
DMD Phase II | 56, 57 | |
|
| |||||
| IGF-1 | Increlex | Recombinant IGF-1 | No effect on motor function following 6 months of treatment in DMD | DMD Phase II | 63 |
|
| |||||
| Promoting muscle mass | Albuterol | β2-agonist, increases cAMP |
 lean body mass in DMD/BMD lean body mass in DMD/BMD |
DMD/BMD Phase II | 67, 68 |
| Urocortin | Increases cAMP |
 diaphragm force in mdx diaphragm force in mdx
|
Preclinical | 69, 70 | |
| DT-200 (prev. known as GLPG0492) | Androgen receptor modulator |
 running, running,
 diaphragm force in mdx diaphragm force in mdx
|
DMD Phase I | 74, 75 | |
|
| |||||
| TGF-β inhibition | Losartan | Blocks angiotensin II receptor activation |
 cardiac and diaphragm fibrosis, cardiac and diaphragm fibrosis,
 grip strength in mdx, inconclusive in DMD grip strength in mdx, inconclusive in DMD |
DMD Phase II | 84–87 |
| Pirfenidone | Suppress expression of TFG-β |
 cardiac contractility in mdx cardiac contractility in mdx
|
Preclinical | 89–92 | |
| Suramin | Inhibits TGF-β |
 CK, CK,
 skeletal muscle fibrosis, skeletal muscle fibrosis,
 necrosis in mdx necrosis in mdx
|
Preclinical | 96–98 | |
| Imatinib Mesylate (Gleevec®) | Inhibits TGF-β |
 CK, CK,
 fibrosis, fibrosis,
 hind limb grip strength in mdx hind limb grip strength in mdx
|
Preclinical | 99–101 | |
| Halofuginone | Impedes Smad 3 binding to DNA, enhances Akt and MAPK/ERK signaling |
 fibrosis in mdx, fibrosis in mdx,
 muscle strength in DMD muscle strength in DMD |
DMD Phase II | 102–105 | |
| Tamoxifen | Inhibits TGF-β |
 cardiac and diaphragm fibrosis, cardiac and diaphragm fibrosis,
 muscle force in mdx muscle force in mdx
|
Preclinical | 111–113 | |
|
| |||||
| Calcium regulation | Streptomycin | Non-specific Ca2+ channel blocker |
 muscle degeneration, muscle degeneration,
 muscle force in mdx muscle force in mdx
|
Preclinical | 118, 126 |
| AT-300 (prev. known as GsMtx4) | Blocks mechanosensitive Ca2+ channels |
 muscle degeneration, muscle degeneration,
 sarcolemmal stability in mdx sarcolemmal stability in mdx
|
Preclinical | 118, 126, 128–130 | |
| Recombinant Mitsugumin 53 | Facilitates membrane repair at sites of injury |
 muscle pathology in mdx muscle pathology in mdx
|
Preclinical | 133-, 134 | |
| Poloxamer 188 | Membrane sealant |
 Ca2+ overload in mdx cardiomyocytes, Ca2+ overload in mdx cardiomyocytes,
 myocardial fibrosis in GRMD, ineffective in mdx skeletal muscle myocardial fibrosis in GRMD, ineffective in mdx skeletal muscle |
Preclinical | 135–139 | |
| BGP-15 | Induces Hsp72 to enhance SERCA |
 diaphragm strength in mdx, diaphragm strength in mdx,
 lifespan in mdx;utrn−/− lifespan in mdx;utrn−/−
|
Preclinical | 141–143 | |
| Rycal 107 ARM210/S48168 |
RyR stabilizers |
 SR Ca2+ leakage, SR Ca2+ leakage,
 cardiac arrhythmias, cardiac arrhythmias,
 specific force in mdx specific force in mdx
|
Preclinical | 146 | |
| Rimeporide | NHE-1 inhibitor |
 Ca2+ and Na+ in dystrophic myotubes, Ca2+ and Na+ in dystrophic myotubes,
 muscle degeneration in BIO14.6 hamsters muscle degeneration in BIO14.6 hamsters |
DMD Phase Ib | 150–152 | |
|
| |||||
| Mitochondria | Cyclosporine A | Immunosupressant, inhibits cyclophilin D |
 force drop, force drop,
 CK in mdx, variable efficacy in DMD CK in mdx, variable efficacy in DMD |
DMD Phase II/III | 154–157 |
| Debio-025 | Inhibits cyclophilin D |
 fibrosis, fibrosis,
 macrophage infiltration macrophage infiltration |
DMD Phase I | 158–160 | |
|
| |||||
| Oxidative stress | Coenzyme Q10 | Antioxidant, metabolic support |
 muscle strength in DMD muscle strength in DMD |
DMD Phase III | 166. 167 |
| Idebenone (synthetic CoQ10) | Antioxidant |
 fibrosis, fibrosis,
 inflammation, inflammation,
 voluntary running in mdx voluntary running in mdx
|
DMD Phase III | 168, 169 | |
| L-Arginine | Metabolic support |
 NF-κB, NF-κB,
 NF-κB regeneration in mdx muscle fibers, NF-κB regeneration in mdx muscle fibers,
 oxidative stress in DMD oxidative stress in DMD |
DMD Phase I | 170–172 | |
| Creatine | Metabolic support |
 bone degeneration, bone degeneration,
 hand grip strength in DMD hand grip strength in DMD |
DMD Phase III (II/III with glutamine) | 173, 175 | |
| Glutamine | Metabolic support |
 protein degradation in DMD protein degradation in DMD |
DMD Phase III (II/III with creatine) | 174, 175 | |
| N-Acetylcysteine | Antioxidant, ROS scavenger |
 CK, CK,
 necrosis, necrosis,
 NF-κB in mdx NF-κB in mdx
|
Preclinical | 176–178 | |
| Melatonin | Antioxidant |
 CK, CK,
 oxidative stress in mdx5cv and DMD oxidative stress in mdx5cv and DMD |
DMD | 180, 181 | |
| Green tea extract/Epigallocatechin gallate | Antioxidant |
 necrosis, necrosis,
 muscle function muscle function |
DMD Phase II/III | 182–186 | |
| Diapocynin | Inhibits ROS production by NOX |
 ROS production in dystrophic myotubes, ROS production in dystrophic myotubes,
 force loss in mdx force loss in mdx
|
Preclinical | 188 | |
|
| |||||
| Phosphodiesterase inhibition | Pentoxifylline | Non-specific PDE inhibitor |
 diaphragm tension, diaphragm tension,
 CK in mdx, No effect on muscle strength and function in DMD CK in mdx, No effect on muscle strength and function in DMD |
DMD Phase II | 77, 198–200 |
| Sildenafil (Viagra®) | PDE5 inhibitor, increases cGMP |
 cardiomyopathy, cardiomyopathy,
 fibrosis in mdx, fibrosis in mdx,
 muscle ischemia, no effect in functional testing in DMD muscle ischemia, no effect in functional testing in DMD |
DMD Phase II | 196, 201–206 | |
| Tadalafil (Cialis®) | PDE5 inhibitor, increases cGMP |
 CK, CK,
 contraction-induced injury in mdx, contraction-induced injury in mdx,
 cardiomyopathy, cardiomyopathy,
 histopathology in GRMD histopathology in GRMD |
DMD Phase III | 196, 201, 204, 205, 207–209 | |
10. Expert opinion
Absence of dystrophin from the muscle sarcolemma triggers a number of pathological downstream events including inflammation, fibrosis, and necrosis. DMD is a multifaceted disease, and will likely require a multifaceted approach from several angles to address the many features of its pathology. Theoretically, restoration of dystrophin and the DAPC to the muscle sarcolemma would prevent further muscle damage and slow disease progression. Promising genetic-based approaches to achieve this include exon skipping and gene therapy, which have the potential to restore dystrophin in many tissues including the heart. However, they face challenges such as tailoring to specific DMD mutations and achieving widespread delivery, respectively, and would not necessarily reverse advanced pathology in older patients with little muscle mass remaining. The pharmacological therapeutics discussed above have potential to improve advanced pathology and would be applicable to all patients regardless of mutation, and would play a critical role in a combinatorial treatment approach for DMD.
Despite the benefits, there are also several challenges for pharmacologic treatment of DMD that must be considered. Given that corticosteroids are the only standing DMD drug therapy and that most patients are on a prednisone or deflazacort regimen, it is important to ensure candidate drugs act synergistically with them. With any combinatorial treatment approach, there is always the potential for decreased efficacy or side effects from multiple drug interference. As mentioned above regarding prednisolone, underlying glucocorticoid treatment may mask potential benefits from a candidate drug being tested. Significant improvement in quality of life would be achieved with a corticosteroid alternative that decreases inflammation without the side effects as a new standing DMD therapy. There are also meaningful benefits to be gained with an effective antifibrotic agent to decrease the connective tissue replacing force-generating muscle, an agent that promotes muscle growth, reducing oxidative stress, or by enhancing blood flow to optimize function of the muscle that remains.
With so many targets now known and so many potential drugs that appear promising in animal models, the number of clinical trials is growing rapidly. As we have discussed in this review, therapies that were highly effective in animal models have not necessarily been effective or well tolerated in human patients. Therefore, in this era of DMD therapeutics, the field must be especially thorough and selective in deciding which among the many options move forward to human trials (additional reviews210–212). DMD research has a growing number of clinical endpoints that includes ambulation (i.e. 6-minute walk test), ventilatory capacity, strength (i.e. gross motor, grip), MRI, and pharmacodynamic measures such as serum biomarkers to determine drug efficacy. It will be critical to carefully consider multiple outcomes as to not prematurely exclude a candidate that may elicit more subtle but meaningful benefits and may not necessarily translate into improved ambulation. Several drugs under investigation have been tested thoroughly in other diseases, which may hasten their approval for repurposing in DMD therapy.
Research of dystrophin-deficient animal models has greatly advanced our understanding of the pathophysiological mechanisms underlying dystrophic pathology, and has revealed viable DMD therapeutic targets beyond the primary genetic defect. In this review, we have presented several well-defined downstream targets for pharmacologic therapies for DMD and the efficacy of relevant therapeutics in ongoing preclinical and clinical trials. While there are certainly challenges associated with delivery, tolerance, and consistent efficacy, there are more potential treatments than ever before, some of which may have significant impact on disease progression.
Article highlights.
The standing treatment for DMD is corticosteroid therapy, which has been shown to slow disease progression, but is associated with significant side effects.
Genetic interventions including exon skipping and gene therapy to restore dystrophin expression are promising strategies, but face the challenges of tailoring for specific DMD mutations and achieving widespread delivery, respectively.
Pharmacological approaches are being investigated to address pathophysiological features of DMD including muscle fiber degeneration, inflammation, fibrosis, Ca2+ dysregulation, and oxidative stress.
These include targeting the NF-κB pathway, myostatin, TGF-β signaling, Ca2+ channels, reactive oxygen species, and phosphodiesterases. Several of these interventions involve repurposing drugs already used clinically for other diseases to treat DMD.
Drugs targeting the downstream pathology of DMD would be applicable to all patients irrespective of genetic mutation, and would play a significant role as part of a combinatorial treatment approach with genetic and palliative therapies.
Acknowledgments
The authors would like to thank Dr. Hart Lidov of Boston Children’s Hospital Department of Pathology for providing and analyzing the muscle biopsy images.
Funding
This paper was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH (R01AR064300).
Footnotes
Declaration of Interest
LM Kunkel has received a grant from Pfizer Inc. and consults with Summit Corporation PLC, Sarepta Therapeutics, and Claritas Genomics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Reference annotations
* Of interest
** Of considerable interest
- 1.Emery AE, Muntoni F, Quinlivan RC. Duchenne muscular dystrophy. OUP; Oxford: 2015. [Google Scholar]
- 2.Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2(1):90–5. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 3.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345(6273):315–9. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida M, Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem. 1990;108(5):748–52. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]
- 5.Ervasti JM, Campbell KP. Dystrophin-associated glycoproteins: their possible roles in the pathogenesis of Duchenne muscular dystrophy. Mol Cell Biol Hum Dis Ser. 1993;3:139–66. doi: 10.1007/978-94-011-1528-5_6. [DOI] [PubMed] [Google Scholar]
- 6.Rando TA. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve. 2001;24(12):1575–94. doi: 10.1002/mus.1192. [DOI] [PubMed] [Google Scholar]
- 7.Garbincius JF, Michele DE. Dystrophin-glycoprotein complex regulates muscle nitric oxide production through mechanoregulation of AMPK signaling. Proc Natl Acad Sci USA. 2015;112(44):13663–8. doi: 10.1073/pnas.1512991112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellorusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil. 2001;22(5):467–75. doi: 10.1023/a:1014587918367. [DOI] [PubMed] [Google Scholar]
- 9.Cros D, Harnden P, Pellissier JF, Serratrice G. Muscle hypertrophy in Duchenne muscular dystrophy. A pathological and morphometric study. J Neurol. 1989;236(1):43–7. doi: 10.1007/BF00314217. [DOI] [PubMed] [Google Scholar]
- 10.Marshall PA, Williams PE, Goldspink G. Accumulation of collagen and altered fiber-type ratios as indicators of abnormal muscle gene expression in the mdx dystrophic mouse. Muscle Nerve. 1989;12(7):528–37. doi: 10.1002/mus.880120703. [DOI] [PubMed] [Google Scholar]
- 11.Fairclough RJ, Wood MJ, Davies KE. Therapy for Duchenne muscular dystrophy: renewed optimism from genetic approaches. Nat Rev Genet. 2013;14(6):373–8. doi: 10.1038/nrg3460. [DOI] [PubMed] [Google Scholar]
- 12.Guiraud S, Aartsma-Rus A, Vieira NM, Davies KE, van Ommen G-JB, Kunkel LM. The pathogenesis and therapy of muscular dystrophies. Annu Rev Genomics Hum Genet 2015. 2015;16:281–308. doi: 10.1146/annurev-genom-090314-025003. [DOI] [PubMed] [Google Scholar]
- 13.Odom GL, Gregorevic P, Chamberlain JS. Viral-mediated gene therapy for the muscular dystrophies: successes, limitations and recent advances. Biochim Biophys Acta. 2007;1772(2):243–62. doi: 10.1016/j.bbadis.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, et al. Randomized, double-blind six-month trial of prednisone in Duchenne muscular dystrophy. N Engl J Med. 1989;320(24):1592–7. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 15.Biggar WD, Harris VA, Eliasoph L, Alman B. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord. 2006;16(4):249–55. doi: 10.1016/j.nmd.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 16.St-Pierre SJ, Chakkalakal JV, Kolodziejczyk SM, Knudson JC, Jasmin BJ, Megeney LA. Glucocorticoid treatment alleviates dystrophic myofiber pathology by activation of the calcineurin/NF-AT pathway. FASEB J. 2004;18(15):1937–9. doi: 10.1096/fj.04-1859fje. [DOI] [PubMed] [Google Scholar]
- 17**.Moxley RT, 3rd, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol. 2010;25(9):1116–29. doi: 10.1177/0883073810371004. Review of corticosteroids, the only current standing DMD therapy. [DOI] [PubMed] [Google Scholar]
- 18.Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2016;5:Cd003725. doi: 10.1002/14651858.CD003725.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonifati MD, Ruzza G, Bonometto P, Berardinelli A, Gorni K, Orcesi S, et al. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. 2000;23(9):1344–47. doi: 10.1002/1097-4598(200009)23:9<1344::aid-mus4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.Bello L, Gordish-Dressman H, Morgenroth LP, Henricson EK, Duong T, Hoffman EP, et al. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology. 2015;85(12):1048–55. doi: 10.1212/WNL.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marathon Pharmaceuticals announces submission of deflazacort new drug application to the FDA. Marathon Pharmaeuticals; Northbrook, IL: 2016. [Last accessed 29 July 2016]. Available from: www.marathonpharma.com/news/2016/06/marathon-pharmaceuticals-announces-submission-deflazacort-new-drug-application-fda/ [Google Scholar]
- 22.Janssen PM, Murray JD, Schill KE, Rastogi N, Schultz EJ, Tran T, et al. Prednisolone attenuates improvement of cardiac and skeletal contractile function and histopathology by lisinopril and spironolactone in the mdx mouse model of Duchenne muscular dystrophy. PLoS One. 2014;9(2):e88360. doi: 10.1371/journal.pone.0088360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, et al. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med. 2013;5(10):1569–85. doi: 10.1002/emmm.201302621. Potential new glucocorticoid alternative without side effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ReveraGen BioPharma Inc. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 3 June 2016]. A Study to assess vamorolone in boys with Duchenne muscular dystrophy (DMD) Available from: https://clinicaltrials.gov/ct2/show/NCT02760264. [Google Scholar]
- 25.Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107(1):3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PML, Carathers M, et al. Interplay of IKK/NF-κB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117(4):889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60(6):993–7. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- 28.Messina S, Bitto A, Aguennouz Mh, Minutoli L, Monici MC, Altavilla D, et al. Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in mdx mice. Exp Neurol. 2006;198(1):234–41. doi: 10.1016/j.expneurol.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Boriek AM. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17(3):386–96. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- 30.Messina S, Vita GL, Aguennouz M, Sframeli M, Romeo S, Rodolico C, et al. Activation of NF-kB pathway in Duchenne muscular dystrophy: relation to age. Acta Myologica. 2011;30(1):16–23. [PMC free article] [PubMed] [Google Scholar]
- 31.Reay DP, Yang M, Watchko JF, Daood M, O’Day TL, Rehman KK, et al. Systemic delivery of NEMO binding domain/IKKγ inhibitory peptide to young mdx mice improves dystrophic skeletal muscle histopathology. Neurobiol Dis. 2011;43(3):598–608. doi: 10.1016/j.nbd.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delfín DA, Xu Y, Peterson JM, Guttridge DC, Rafael-Fortney JA, Janssen PML. Improvement of cardiac contractile function by peptide-based inhibition of NF-κB in the utrophin/dystrophin-deficient murine model of muscular dystrophy. J Transl Med. 2011;9:68–68. doi: 10.1186/1479-5876-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornegay JN, Peterson JM, Bogan DJ, Kline W, Bogan JR, Dow JL, et al. NBD delivery improves the disease phenotype of the golden retriever model of Duchenne muscular dystrophy. Skelet Muscle. 2014;4:18. doi: 10.1186/2044-5040-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milne J, Donovan J, Sweeney L, Sleeper M, Hammers D, Jirousek M, et al. CAT-1004, a novel anti-inflammatory agent under development for treatment of Duchenne muscular dystrophy. Neuromuscul Disord. 2014;24(9):825. [Google Scholar]
- 35.Catabasis Pharmaceuticals. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2012. [Last accessed 14 June 2016]. Safety, tolerability, and pharmacokinetic study of CAT-1004 in healthy adult volunteers. Available from: https://clinicaltrials.gov/ct2/show/NCT01440166. [Google Scholar]
- 36.Catabasis Pharmaceuticals. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 3 June 2016]. Phase 1/2 study in boys with Duchenne muscular dystrophy (MoveDMD) Available from: https://clinicaltrials.gov/ct2/show/NCT02439216. [Google Scholar]
- 37.Ermolova NE, Martinez L, Vetrone SA, Jordan MC, Roos KP, Sweeney HL, et al. Long-term administration of the TNF blocking drug Remicade (cV1q) to mdx mice reduces skeletal and cardiac muscle fibrosis, but negatively impacts cardiac function. Neuromuscl Disord. 2014;24(7):583–95. doi: 10.1016/j.nmd.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou LZ, Johnson AP, Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31(11):1405–16. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]
- 39.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94(23):12457–61. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shelton GD, Engvall E. Gross muscle hypertrophy in whippet dogs is caused by a mutation in the myostatin gene. Neuromuscul Disord. 2007;17(9–10):721–2. doi: 10.1016/j.nmd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA. 2007;104(6):1835–40. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rios R, Carneiro I, Arce VM, Devesa J. Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol. 2002;282(5):C993–9. doi: 10.1152/ajpcell.00372.2001. [DOI] [PubMed] [Google Scholar]
- 43.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98(16):9306–11. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magee TR, Artaza JN, Ferrini MG, Vernet D, Zuniga FI, Cantini L, et al. Myostatin short interfering hairpin RNA gene transfer increases skeletal muscle mass. J Gene Med. 2006;8(9):1171–81. doi: 10.1002/jgm.946. [DOI] [PubMed] [Google Scholar]
- 45.Bogdanovich S, Krag TOB, Barton ER, Morris LD, Whittemore L-A, Ahima RS, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420(6914):418–21. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 46.Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300(4):965–71. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 47.Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 2005;19(6):543–9. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- 48.Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol. 2002;52(6):832–6. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- 49.Kornegay JN, Bogan DJ, Bogan JR, Dow JL, Wang J, Fan Z, et al. Dystrophin-deficient dogs with reduced myostatin have unequal muscle growth and greater joint contractures. Skelet Muscle. 2016;6:14. doi: 10.1186/s13395-016-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63(5):561–71. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 51.Cadena SM, Tomkinson KN, Monnell TE, Spaits MS, Kumar R, Underwood KW, et al. Administration of a soluble activin type IIB receptor promotes skeletal muscle growth independent of fiber type. J Appl Physiol (1985) 2010;109(3):635–42. doi: 10.1152/japplphysiol.00866.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acceleron Pharma Inc. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2013. [Last accessed 3 June 2016]. Study of ACE-031 in subjects with Duchenne muscular dystrophy. Available from: https://clinicaltrials.gov/ct2/show/NCT01099761. [Google Scholar]
- 53.Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2016 doi: 10.1002/mus.25268. [DOI] [PubMed] [Google Scholar]
- 54.Pfizer Inc. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 2 June 2016]. A phase 2 study to evaluate the safety, efficacy, pharmacokinetics and oharmacodynamics of PF-06252616 in Duchenne muscular dystrophy. Available from: http://clinicaltrials.gov/ct2/show/NCT02310763. [Google Scholar]
- 55.Acceleron Pharma Inc. At: ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 3 June 2016]. Phase 1 study of ACE-083 in healthy subjects. Available from: https://clinicaltrials.gov/ct2/show/NCT02257489. [Google Scholar]
- 56.Bristol-Meyers Squibb. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 3 June 2016]. Placebo-controlled, single and multiple ascending subcutaneous dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of BMS-986089 in healthy adult subjects. Available from: https://clinicaltrials.gov/ct2/show/NCT02145234. [Google Scholar]
- 57.Bristol-Meyers Squibb. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 3 June 2016]. Study of an investigational drug, BMS-986089, in ambulatory boys with DMD. Available from: https://clinicaltrials.gov/ct2/show/NCT02515669. [Google Scholar]
- 58.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. 2008;154(3):557–68. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Locatelli V, Bianchi VE. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int J Endocrinol. 2014:235060. doi: 10.1155/2014/235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pini G, Scusa MF, Congiu L, Benincasa A, Morescalchi P, Bottiglioni I, et al. IGF1 as a potential treatment for Rett Syndrome: Safety assessment in six Rett patients. Autism Res Treat. 2012;2012 doi: 10.1155/2012/679801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandenburgh H, Shansky J, Benesch-Lee F, Skelly K, Spinazzola JM, Saponjian Y, et al. Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts. FASEB J. 2009 Oct;23(10):3325–34. doi: 10.1096/fj.09-134411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157(1):137–48. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Children’s Hospital Medical Center Cincinnati. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2012. [Last accessed 3 June 2016]. Safety and efficacy study of IGF-1 in Duchenne muscular dystrophy. Available from: https://clinicaltrials.gov/ct2/show/NCT01207908. [Google Scholar]
- 64.Zeman RJ, Peng H, Danon MJ, Etlinger JD. Clenbuterol reduces degeneration of exercised or aged dystrophic (mdx) muscle. Muscle Nerve. 2000;23(4):521–8. doi: 10.1002/(sici)1097-4598(200004)23:4<521::aid-mus10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 65.Lynch GS, Hinkle RT, Faulkner JA. Power output of fast and slow skeletal muscles of mdx (dystrophic) and control mice after clenbuterol treatment. Exp Physiol. 2000;85(3):295–9. [PubMed] [Google Scholar]
- 66.Harcourt LJ, Schertzer JD, Ryall JG, Lynch GS. Low dose formoterol administration improves muscle function in dystrophic mdx mice without increasing fatigue. Neuromuscul Disord. 2007;17(1):47–55. doi: 10.1016/j.nmd.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Kissel JT, McDermott MP, Mendell JR, King WM, Pandya S, Griggs RC, et al. Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. 2001;57(8):1434–40. doi: 10.1212/wnl.57.8.1434. [DOI] [PubMed] [Google Scholar]
- 68.van der Kooi EL, Kalkman JS, Lindeman E, Hendriks JC, van Engelen BG, Bleijenberg G, et al. Effects of training and albuterol on pain and fatigue in facioscapulohumeral muscular dystrophy. J Neurol. 2007;254(7):931–40. doi: 10.1007/s00415-006-0432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burns D, Manning J, O’Malley D, O’Halloran K. Respiratory function in the mdx mouse model of Duchenne muscular dystrophy: Role of hypoxia, stress and immune factors. FASEB J. 2015:29. [Google Scholar]
- 70.Manning J, Buckley MM, O’Halloran KD, O’Malley D. In vivo neutralization of IL-6 receptors ameliorates gastrointestinal dysfunction in dystrophin-deficient mdx mice. Neurogastroenterol Motil. 2016;28(7):1016–26. doi: 10.1111/nmo.12803. [DOI] [PubMed] [Google Scholar]
- 71.Stirrat CG, Venkatasubramanian S, Pawade T, Mitchell AJ, Shah AS, Lang NN, et al. Cardiovascular effects of urocortin 2 and urocortin 3 in patients with chronic heart failure. Br J Clin Pharmacol. 2016 doi: 10.1111/bcp.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones A, Hwang DJ, Narayanan R, Miller DD, Dalton JT. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology. 2010;151(8):3706–19. doi: 10.1210/en.2010-0150. [DOI] [PubMed] [Google Scholar]
- 73.Blanque R, Lepescheux L, Auberval M, Minet D, Merciris D, Cottereaux C, et al. Characterization of GLPG0492, a selective androgen receptor modulator, in a mouse model of hindlimb immobilization. BMC Musculoskelet Disord. 2014;15:291. doi: 10.1186/1471-2474-15-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cozzoli A, Capogrosso RF, Sblendorio VT, Dinardo MM, Jagerschmidt C, Namour F, et al. GLPG0492, a novel selective androgen receptor modulator, improves muscle performance in the exercised-mdx mouse model of muscular dystrophy. Pharmacol Res. 2013;72:9–24. doi: 10.1016/j.phrs.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Galapagos NV. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2011. [Last accessed 8 August 2016]. Multiple ascending dose study of GLPG0492 in healthy subjects. Available from: https://clinicaltrials.gov/ct2/show/NCT01397370. [Google Scholar]
- 76.Muir LA, Chamberlain JS. Emerging strategies for cell and gene therapy of the muscular dystrophies. Expert Rev Mol Med. 2009;11:e18. doi: 10.1017/S1462399409001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gosselin LE, Williams JE. Pentoxifylline fails to attenuate fibrosis in dystrophic (mdx) diaphragm muscle. Muscle Nerve. 2006;33(6):820–3. doi: 10.1002/mus.20523. [DOI] [PubMed] [Google Scholar]
- 78.Passerini L, Bernasconi P, Baggi F, Confalonieri P, Cozzi F, Cornelio F, et al. Fibrogenic cytokines and extent of fibrosis in muscle of dogs with X-linked golden retriever muscular dystrophy. Neuromuscul Disord. 2002;12(9):828–35. doi: 10.1016/s0960-8966(02)00071-8. [DOI] [PubMed] [Google Scholar]
- 79.Zhou L, Porter JD, Cheng G, Gong B, Hatala DA, Merriam AP, et al. Temporal and spatial mRNA expression patterns of TGF-beta1, 2, 3 and TbetaRI, II, III in skeletal muscles of mdx mice. Neuromuscul Disord. 2006;16(1):32–8. doi: 10.1016/j.nmd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Vidal B, Serrano AL, Tjwa M, Suelves M, Ardite E, De Mori R, et al. Fibrinogen drives dystrophic muscle fibrosis via a TGFbeta/alternative macrophage activation pathway. Genes Dev. 2008;22(13):1747–52. doi: 10.1101/gad.465908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serrano AL, Munoz-Canoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res. 2010;316(18):3050–8. doi: 10.1016/j.yexcr.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 82.Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, et al. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96(2):1137–44. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun G, Haginoya K, Wu Y, Chiba Y, Nakanishi T, Onuma A, et al. Connective tissue growth factor is overexpressed in muscles of human muscular dystrophy. J Neurol Sci. 2008;267(1–2):48–56. doi: 10.1016/j.jns.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 84.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, et al. Angiotensin II type 1 receptor blockade attenuates TGF-[beta]-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13(2):204–10. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spurney CF, Sali A, Guerron AD, Iantorno M, Yu Q, Gordish-Dressman H, et al. Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin-deficient mdx mice. J Cardiovasc Pharmacol Ther. 2011;16(1):87–95. doi: 10.1177/1074248410381757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bish LT, Yarchoan M, Sleeper MM, Gazzara JA, Morine KJ, Acosta P, et al. Chronic losartan administration reduces mortality and preserves cardiac but not skeletal muscle function in dystrophic mice. PLoS One. 2011;6(6):e20856. doi: 10.1371/journal.pone.0020856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allen HD, Flanigan KM, Thrush PT, Dvorchik I, Yin H, Canter C, et al. A randomized, double-blind trial of lisinopril and losartan for the treatment of cardiomyopathy in Duchenne muscular dystrophy. PLoS Curr. 2013:5. doi: 10.1371/currents.md.2cc69a1dae4be7dfe2bcb420024ea865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noble PW, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47(1):243–53. doi: 10.1183/13993003.00026-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shimizu T, Fukagawa M, Kuroda T, Hata S, Iwasaki Y, Nemoto M, et al. Pirfenidone prevents collagen accumulation in the remnant kidney in rats with partial nephrectomy. Kidney Int Suppl. 1997;63:S239–43. [PubMed] [Google Scholar]
- 90.Gosselin LE, Williams JE, Personius K, Farkas GA. A comparison of factors associated with collagen metabolism in different skeletal muscles from dystrophic (mdx) mice: impact of pirfenidone. Muscle Nerve. 2007;35(2):208–16. doi: 10.1002/mus.20681. [DOI] [PubMed] [Google Scholar]
- 91.Van Erp C, Irwin NG, Hoey AJ. Long-term administration of pirfenidone improves cardiac function in mdx mice. Muscle Nerve. 2006;34(3):327–34. doi: 10.1002/mus.20590. [DOI] [PubMed] [Google Scholar]
- 92.Zanotti S, Bragato C, Zucchella A, Maggi L, Mantegazza R, Morandi L, et al. Anti-fibrotic effect of pirfenidone in muscle derived-fibroblasts from Duchenne muscular dystrophy patients. Life Sci. 2016;145:127–36. doi: 10.1016/j.lfs.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 93.Xiong C, Liu N, Fang L, Zhuang S, Yan H. Suramin inhibits the development and progression of peritoneal fibrosis. J Pharmacol Exp Ther. 2014;351(2):373–82. doi: 10.1124/jpet.114.215228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leipner C, Grun K, Muller A, Buchdunger E, Borsi L, Kosmehl H, et al. Imatinib mesylate attenuates fibrosis in coxsackievirus b3-induced chronic myocarditis. Cardiovasc Res. 2008;79(1):118–26. doi: 10.1093/cvr/cvn063. [DOI] [PubMed] [Google Scholar]
- 95.Yoshiji H, Noguchi R, Kuriyama S, Ikenaka Y, Yoshii J, Yanase K, et al. Imatinib mesylate (STI-571) attenuates liver fibrosis development in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288(5):G907–13. doi: 10.1152/ajpgi.00420.2004. [DOI] [PubMed] [Google Scholar]
- 96.Taniguti AP, Matsumura CY, Rodrigues-Simioni L, Santo Neto H, Marques MJ. Suramin affects metalloproteinase-9 activity and increases beta-dystroglycan levels in the diaphragm of the dystrophin-deficient mdx mouse. Muscle Nerve. 2012;46(5):810–3. doi: 10.1002/mus.23468. [DOI] [PubMed] [Google Scholar]
- 97.Taniguti AP, Pertille A, Matsumura CY, Santo Neto H, Marques MJ. Prevention of muscle fibrosis and myonecrosis in mdx mice by suramin, a TGF-beta1 blocker. Muscle Nerve. 2011;43(1):82–7. doi: 10.1002/mus.21869. [DOI] [PubMed] [Google Scholar]
- 98.De Oliveira Moreira D, Pereira JA, Taniguti AP, Matsumura CY, Ramos LA, Areas MA, et al. Suramin attenuates dystrophin-deficient cardiomyopathy in the mdx mouse model of duchenne muscular dystrophy. Muscle Nerve. 2013;48(6):911–9. doi: 10.1002/mus.23858. [DOI] [PubMed] [Google Scholar]
- 99.Bizario JC, Cerri DG, Rodrigues LC, Oliveira GL, Nomizo A, de Araujo DD, et al. Imatinib mesylate ameliorates the dystrophic phenotype in exercised mdx mice. J Neuroimmunol. 2009;212(1–2):93–101. doi: 10.1016/j.jneuroim.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Huang P, Zhao XS, Fields M, Ransohoff RM, Zhou L. Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J. 2009;23(8):2539–48. doi: 10.1096/fj.09-129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ito T, Ogawa R, Uezumi A, Ohtani T, Watanabe Y, Tsujikawa K, et al. Imatinib attenuates severe mouse dystrophy and inhibits proliferation and fibrosis-marker expression in muscle mesenchymal progenitors. Neuromuscul Disord. 2013;23(4):349–56. doi: 10.1016/j.nmd.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 102.Grudzien MM, Low P, Manning PC, Arredondo M, Belton RJ, Nowak RA. The antifibrotic drug Halofuginone inhibits proliferation and collagen production by human leiomyoma and myometrial smooth muscle cells. Fertil Steril. 2010;93(4):1290–8. doi: 10.1016/j.fertnstert.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roffe S, Hagai Y, Pines M, Halevy O. Halofuginone inhibits Smad3 phosphorylation via the PI3K/Akt and MAPK/ERK pathways in muscle cells: effect on myotube fusion. Exp Cell Res. 2010;316(6):1061–9. doi: 10.1016/j.yexcr.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Levi O, Genin O, Angelini C, Halevy O, Pines M. Inhibition of muscle fibrosis results in increases in both utrophin levels and the number of revertant myofibers in Duchenne muscular dystrophy. Oncotarget. 2015;6(27):23249–60. doi: 10.18632/oncotarget.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105*.Barzilai-Tutsch H, Bodanovsky A, Maimon H, Pines M, Halevy O. Halofuginone promotes satellite cell activation and survival in muscular dystrophies. Biochim Biophys Acta. 2016;1862(1):1–11. doi: 10.1016/j.bbadis.2015.10.007. TGF-β inhibitor showing with positive results in DMD. [DOI] [PubMed] [Google Scholar]
- 106.Akashi Reports Positive Clinical Data on HT-100. Akashi Therapeutics; Cambridge, MA: 2015. [Last accessed 3 June 2016]. Available from: http://akashirx.com/news/akashi-therapeutics-reports-positive-clinical-data-on-ht%E2%80%90100-in-patients-with-duchenne-muscular-dystrophy/ [Google Scholar]
- 107.Akashi Therapeutics. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 3 June 2016]. Safety, tolerability, and pharmacokinetics of single and multiple doses of HT-100 in Duchenne muscular dystrophy. Available from: https://clinicaltrials.gov/ct2/show/NCT01847573. [Google Scholar]
- 108.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339(22):1609–18. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 109.Moroni G, Gallelli B, Banfi G, Sandri S, Messa P, Ponticelli C. Long-term outcome of idiopathic retroperitoneal fibrosis treated with surgical and/or medical approaches. Nephrol Dial Transpl. 2006;21(9):2485–90. doi: 10.1093/ndt/gfl228. [DOI] [PubMed] [Google Scholar]
- 110.Korte MR, Fieren MW, Sampimon DE, Lingsma HF, Weimar W, Betjes MGH, et al. Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: results of the Dutch Multicentre EPS Study. Nephrol Dial Transpl. 2010;(26):691–97. doi: 10.1093/ndt/gfq362. [DOI] [PubMed] [Google Scholar]
- 111.Carthy JM, Sundqvist A, Heldin A, van Dam H, Kletsas D, Heldin CH, et al. Tamoxifen inhibits TGF-beta-mediated activation of myofibroblasts by blocking non-Smad signaling through ERK1/2. J Cell Physiol. 2015;230(12):3084–92. doi: 10.1002/jcp.25049. [DOI] [PubMed] [Google Scholar]
- 112.Kuhn MA, Wang X, Payne WG, Ko F, Robson MC. Tamoxifen decreases fibroblast function and downregulates TGFβ2 in Dupuytren’s Affected Palmar Fascia. Journal of Surgical Research. 2002;103(2):146–52. doi: 10.1006/jsre.2001.6350. [DOI] [PubMed] [Google Scholar]
- 113*.Dorchies OM, Reutenauer-Patte J, Dahmane E, Ismail HM, Petermann O, Patthey-Vuadens O, et al. The anticancer drug tamoxifen counteracts the pathology in a mouse model of duchenne muscular dystrophy. Am J Pathol. 2013;182(2):485–504. doi: 10.1016/j.ajpath.2012.10.018. Repurposing of a well-studied anti-cancer therapy in DMD. [DOI] [PubMed] [Google Scholar]
- 114.Eugster EA, Rubin SD, Reiter EO, Plourde P, Jou H-C, Pescovitz OH. Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial. J Peds. 2003;143(1):60–66. doi: 10.1016/S0022-3476(03)00128-8. [DOI] [PubMed] [Google Scholar]
- 115.Kreher NC, Eugster EA, Shankar RR. The use of tamoxifen to improve height potential in short pubertal boys. Pediatrics. 2005;116(6):1513–5. doi: 10.1542/peds.2005-0577. [DOI] [PubMed] [Google Scholar]
- 116.Merlini L, Gennari M, Malaspina E, Cecconi I, Armaroli A, Gnudi S, et al. Early corticosteroid treatment in 4 Duchenne muscular dystrophy patients: 14-year follow-up. Muscle Nerve. 2012;45(6):796–802. doi: 10.1002/mus.23272. [DOI] [PubMed] [Google Scholar]
- 117.Kelly-Worden M, Thomas E. Mitochondrial dysfunction in Duchenne muscular dystrophy. Open J Endocr Metab Dis. 2014;4:211–18. [Google Scholar]
- 118.Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord. 2006;16(12):845–54. doi: 10.1016/j.nmd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 119.Bertorini TE, Bhattacharya SK, Palmieri GM, Chesney CM, Pifer D, Baker B. Muscle calcium and magnesium content in Duchenne muscular dystrophy. Neurology. 1982;32(10):1088–92. doi: 10.1212/wnl.32.10.1088. [DOI] [PubMed] [Google Scholar]
- 120.Bodensteiner JB, Engel AG. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology. 1978;28(5):439–46. doi: 10.1212/wnl.28.5.439. [DOI] [PubMed] [Google Scholar]
- 121.Tutdibi O, Brinkmeier H, Rudel R, Fohr KJ. Increased calcium entry into dystrophin-deficient muscle fibres of mdx and ADR-mdx mice is reduced by ion channel blockers. J Physiol. 1999;515(Pt 3):859–68. doi: 10.1111/j.1469-7793.1999.859ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Divet A, Huchet-Cadiou C. Sarcoplasmic reticulum function in slow- and fast-twitch skeletal muscles from mdx mice. Pflugers Arch. 2002;444(5):634–43. doi: 10.1007/s00424-002-0854-5. [DOI] [PubMed] [Google Scholar]
- 123.Emery AE, Skinner R, Howden LC, Matthews MB. Verapamil in Duchenne muscular dystrophy. Lancet. 1982;1(8271):559. doi: 10.1016/s0140-6736(82)92063-3. [DOI] [PubMed] [Google Scholar]
- 124.Dick DJ, Gardner-Medwin D, Gates PG, Gibson M, Simpson JM, Walls TJ. A trial of flunarizine in the treatment of Duchenne muscular dystrophy. Muscle Nerve. 1986;9(4):349–54. doi: 10.1002/mus.880090412. [DOI] [PubMed] [Google Scholar]
- 125.Moxley RT, 3rd, Brooke MH, Fenichel GM, Mendell JR, Griggs RC, Miller JP, et al. Clinical investigation in Duchenne dystrophy. VI. Double-blind controlled trial of nifedipine. Muscle Nerve. 1987;10(1):22–33. doi: 10.1002/mus.880100106. [DOI] [PubMed] [Google Scholar]
- 126*.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562(Pt 2):367–80. doi: 10.1113/jphysiol.2004.075275. Selective mechanosensitive calcium channel blocker from tarantula venom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jorgensen LH, Blain A, Greally E, Laval SH, Blamire AM, Davison BJ, et al. Long-term blocking of calcium channels in mdx mice results in differential effects on heart and skeletal muscle. Am J Pathol. 2011;178(1):273–83. doi: 10.1016/j.ajpath.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F. Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: History, properties, mechanisms and pharmacology. Toxicon. 2007 Feb;49(2):249–70. doi: 10.1016/j.toxicon.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA. 2006;103(44):16586–91. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seo K, Rainer PP, Lee DI, Hao S, Bedja D, Birnbaumer L, et al. Hyperactive adverse mechanical stress responses in dystrophic heart are coupled to transient receptor potential canonical 6 and blocked by cGMP-protein kinase G modulation. Circ Res. 2014;114(5):823–32. doi: 10.1161/CIRCRESAHA.114.302614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Akashi Therapeutics acquires global rights to novel DMD treatment from Tonus Therapeutics. Akashi Therapeutics; Cambridge, MA: 2014. [Last accessed 10 August 2016]. Available from: http://akashirx.com/news/akashi-therapeutics-acquires-global-rights-novel-dmd-treatment-tonus-therapeutics/ [Google Scholar]
- 132.Waddell LB, Lemckert FA, Zheng XF, Tran J, Evesson FJ, Hawkes JM, et al. Dysferlin, annexin A1, and mitsugumin 53 are upregulated in muscular dystrophy and localize to longitudinal tubules of the T-system with stretch. J Neuropathol Exp Neurol. 2011;70(4):302–13. doi: 10.1097/NEN.0b013e31821350b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weisleder N, Takeshima H, Ma J. Mitsugumin 53 (MG53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Commun Integr Biol. 2009;2(3):225–6. doi: 10.4161/cib.2.3.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4(139):139ra85. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436(7053):1025–9. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 136.Townsend D, Turner I, Yasuda S, Martindale J, Davis J, Shillingford M, et al. Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs. J Clin Invest. 2010;120(4):1140–50. doi: 10.1172/JCI41329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Markham BE, Kernodle S, Nemzek J, Wilkinson JE, Sigler R. Chronic dosing with membrane sealant Poloxamer 188 NF improves respiratory dysfunction in dystrophic mdx and mdx/utrophin−/− mice. PLoS One. 2015;10(8):e0134832. doi: 10.1371/journal.pone.0134832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quinlan JG, Wong BL, Niemeier RT, McCullough AS, Levin L, Emanuele M. Poloxamer 188 failed to prevent exercise-induced membrane breakdown in mdx skeletal muscle fibers. Neuromuscul Disord. 2006 Dec;16(12):855–64. doi: 10.1016/j.nmd.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 139.Terry RL, Kaneb HM, Wells DJ. Poloxamer [corrected] 188 has a deleterious effect on dystrophic skeletal muscle function. PLoS One. 2014;9(3):e91221. doi: 10.1371/journal.pone.0091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morine KJ, Sleeper MM, Barton ER, Sweeney HL. Overexpression of SERCA1a in the mdx diaphragm reduces susceptibility to contraction-induced damage. Hum Gene Ther. 2010;21(12):1735–9. doi: 10.1089/hum.2010.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141*.Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, Church JE, et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 2012;484(7394):394–8. doi: 10.1038/nature10980. SERCA agonist with positive results in severely affected mdx;utr−/− mice. [DOI] [PubMed] [Google Scholar]
- 142.Sapra G, Tham YK, Cemerlang N, Matsumoto A, Kiriazis H, Bernardo BC, et al. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat Commun. 2014;5:5705. doi: 10.1038/ncomms6705. [DOI] [PubMed] [Google Scholar]
- 143.Salah H, Li M, Cacciani N, Gastaldello S, Ogilvie H, Akkad H, et al. The chaperone co-inducer BGP-15 alleviates ventilation-induced diaphragm dysfunction. Sci Transl Med. 2016;8(350):350ra103. doi: 10.1126/scitranslmed.aaf7099. [DOI] [PubMed] [Google Scholar]
- 144.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15(3):325–30. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat Med. 2015;21(11):1262–71. doi: 10.1038/nm.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, et al. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107(4):1559–64. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.ARMGO Pharma Inc; Tarrytown, NY: 2015. [Last accessed 5 August 2016]. ARMGO Pharma receives FDA Orphan Drug Designation and Rare Pediatric Disease Designation for ARM210/S48168 for the treatment of Duchenne muscular dystrophy. Available from: http://www.armgo.com/PDF/ARMGOPressRelease120915.pdf. [Google Scholar]
- 148.Iwata Y, Katanosaka Y, Hisamitsu T, Wakabayashi S. Enhanced Na+/H+ exchange activity contributes to the pathogenesis of muscular dystrophy via involvement of P2 receptors. Am J Pathol. 2007;171(5):1576–87. doi: 10.2353/ajpath.2007.070452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xiao XH, Allen DG. Role of Na(+)/H(+) exchanger during ischemia and preconditioning in the isolated rat heart. Circ Res. 1999;85(8):723–30. doi: 10.1161/01.res.85.8.723. [DOI] [PubMed] [Google Scholar]
- 150.Chahine M, Bkaily G, Nader M, Al-Khoury J, Jacques D, Beier N, et al. NHE-1-dependent intracellular sodium overload in hypertrophic hereditary cardiomyopathy: prevention by NHE-1 inhibitor. J Mol Cell Cardiol. 2005;38(4):571–82. doi: 10.1016/j.yjmcc.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 151.Translational development of Rimeporide for Duchenne muscular dystrophy. Action Duchenne; London, UK: 2014. [Last accessed 7 August 2016]. Available from: http://www.actionduchenne.org/wp-content/uploads/2014/11/Rimeporide-in-DMD-Esperare_-Action-Duchenne-Oct-2014-Port.pdf. [Google Scholar]
- 152.EspeRare Foundation. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 7 August 2016]. Rimeporide in patients with Duchenne muscular dystrophy (RIM4DMD) Available from: https://clinicaltrials.gov/ct2/show/NCT02710591. [Google Scholar]
- 153.Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14(4):442–7. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Andreeva L, Tanveer A, Crompton M. Evidence for the involvement of a membrane-associated cyclosporin-A-binding protein in the Ca(2+)-activated inner membrane pore of heart mitochondria. Eur J Biochem. 1995;230(3):1125–32. doi: 10.1111/j.1432-1033.1995.tb20664.x. [DOI] [PubMed] [Google Scholar]
- 155.De Luca A, Nico B, Liantonio A, Didonna MP, Fraysse B, Pierno S, et al. A multidisciplinary evaluation of the effectiveness of cyclosporine a in dystrophic mdx mice. Am J Pathol. 2005;166(2):477–89. doi: 10.1016/S0002-9440(10)62270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharma KR, Mynhier MA, Miller RG. Cyclosporine increases muscular force generation in Duchenne muscular dystrophy. Neurology. 1993;43(3 Pt 1):527–32. doi: 10.1212/wnl.43.3_part_1.527. [DOI] [PubMed] [Google Scholar]
- 157.Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, et al. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333(13):832–8. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- 158.Reutenauer J, Dorchies OM, Patthey-Vuadens O, Vuagniaux G, Ruegg UT. Investigation of Debio 025, a cyclophilin inhibitor, in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. Br J Pharmacol. 2008;155(4):574–84. doi: 10.1038/bjp.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159*.Wissing ER, Millay DP, Vuagniaux G, Molkentin JD. Debio-025 is more effective than prednisone in reducing muscular pathology in mdx mice. Neuromuscul Disord. 2010;20(11):753–60. doi: 10.1016/j.nmd.2010.06.016. Non-immunosuppressive althernative to cyclosporine A compared with prednisone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Debiopharm International SA and Solid Biosciences, LLC, announce a collaboration to explore the use of Alisporivir (Debio 025) in muscular dystrophy. DebioPharm Internation SA; Lausanne, Switzerland: 2015. [Last accessed 7 August 2016]. Available from: https://www.debiopharm.com/our-business/pipeline/item/3571-debiopharm-international-sa-and-solid-biosciences-llc-announce-a-collaboration-to-explore-the-use-of-alisporivir-debio-025-in-muscular-dystrophy.html. [Google Scholar]
- 161.Tidball JG, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J Appl Physiol (1985) 2007;102(4):1677–86. doi: 10.1152/japplphysiol.01145.2006. [DOI] [PubMed] [Google Scholar]
- 162.Kim JH, Kwak HB, Thompson LV, Lawler JM. Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy. J Muscle Res Cell Motil. 2013;34(1):1–13. doi: 10.1007/s10974-012-9330-9. [DOI] [PubMed] [Google Scholar]
- 163.Allen DG, Whitehead NP, Froehner SC. Absence of dystrophin disrupts skeletal muscle signaling: Roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev. 2016;96(1):253–305. doi: 10.1152/physrev.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Whitehead NP, Yeung EW, Froehner SC, Allen DG. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS One. 2010;5(12):e15354. doi: 10.1371/journal.pone.0015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Henríquez-Olguín C, Altamirano F, Valladares D, López JR, Allen PD, Jaimovich E. Altered ROS production, NF-κB activation and Interleukin-6 gene expression induced by electrical stimulation of in dystrophic mdx skeletal muscle cells. Biochim Biophys Acta. 2015;1852(7):1410–9. doi: 10.1016/j.bbadis.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, et al. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278(30):28220–8. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 167.Spurney CF, Rocha CT, Henricson E, Florence J, Mayhew J, Gorni K, et al. CINRG pilot trial of coenzyme Q10 in steroid-treated Duchenne muscular dystrophy. Muscle Nerve. 2011;44(2):174–8. doi: 10.1002/mus.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Buyse GM, Van der Mieren G, Erb M, D’Hooge J, Herijgers P, Verbeken E, et al. Long-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: cardiac protection and improved exercise performance. Eur Heart J. 2009;30(1):116–24. doi: 10.1093/eurheartj/ehn406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Santhera Pharmaceuticals. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 7 August 2016]. A Phase III double-blind study with Idebenone in patients with Duchenne muscular dystrophy (DMD) taking glucocorticoid steroids (SIDEROS) Available from: https://clinicaltrials.gov/ct2/show/NCT02814019. [Google Scholar]
- 170.Barton ER, Morris L, Kawana M, Bish LT, Toursel T. Systemic administration of L-arginine benefits mdx skeletal muscle function. Muscle Nerve. 2005;32(6):751–60. doi: 10.1002/mus.20425. [DOI] [PubMed] [Google Scholar]
- 171.Hnia K, Gayraud J, Hugon G, Ramonatxo M, De La Porte S, Matecki S, et al. Arginine decreases inflammation and modulates the Nuclear Factor-kB/Matrix Metalloproteinase cascade in mdx muscle fibers. Am J Pathol. 2008;172(6):1509–19. doi: 10.2353/ajpath.2008.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hafner P, Bonati U, Erne B, Schmid M, Rubino D, Pohlman U, et al. Improved muscle function in Duchenne muscular dystrophy through L-arginine and metformin: An investigator-initiated, open-label, single-center, proof-of-concept-study. PLoS One. 2016;11(1):e0147634. doi: 10.1371/journal.pone.0147634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tarnopolsky MA, Mahoney DJ, Vajsar J, Rodriguez C, Doherty TJ, Roy BD, et al. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology. 2004;62(10):1771–7. doi: 10.1212/01.wnl.0000125178.18862.9d. [DOI] [PubMed] [Google Scholar]
- 174.Mok E, Eleouet-Da Violante C, Daubrosse C, Gottrand F, Rigal O, Fontan JE, et al. Oral glutamine and amino acid supplementation inhibit whole-body protein degradation in children with Duchenne muscular dystrophy. Am J Clin Nutr. 2006;83(4):823–8. doi: 10.1093/ajcn/83.4.823. [DOI] [PubMed] [Google Scholar]
- 175.Escolar DM, Buyse G, Henricson E, Leshner R, Florence J, Mayhew J, et al. CINRG randomized controlled trial of creatine and glutamine in Duchenne muscular dystrophy. Ann Neurol. 2005;58(1):151–5. doi: 10.1002/ana.20523. [DOI] [PubMed] [Google Scholar]
- 176.Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586(Pt 7):2003–14. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Williams IA, Allen DG. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2007;293(3):H1969–77. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- 178.Terrill JR, Radley-Crabb HG, Grounds MD, Arthur PG. N-Acetylcysteine treatment of dystrophic mdx mice results in protein thiol modifications and inhibition of exercise induced myofibre necrosis. Neuromuscul Disord. 2012;22(5):427–34. doi: 10.1016/j.nmd.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 179.National Institute of Nursing Research. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 7 August 2016]. Antioxidant therapy in RYR1-related congenital myopathy. Available from: https://clinicaltrials.gov/ct2/show/NCT02362425. [Google Scholar]
- 180.Hibaoui Y, Reutenauer-Patte J, Patthey-Vuadens O, Ruegg UT, Dorchies OM. Melatonin improves muscle function of the dystrophic mdx5Cv mouse, a model for Duchenne muscular dystrophy. J Pineal Res. 2011 Sep;51(2):163–71. doi: 10.1111/j.1600-079X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 181.Chahbouni M, Escames G, Venegas C, Sevilla B, Garcia JA, Lopez LC, et al. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J Pineal Res. 2010;48(3):282–9. doi: 10.1111/j.1600-079X.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- 182.Dorchies OM, Wagner S, Vuadens O, Waldhauser K, Buetler TM, Kucera P, et al. Green tea extract and its major polyphenol (−)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol. 2006;290(2):C616–25. doi: 10.1152/ajpcell.00425.2005. [DOI] [PubMed] [Google Scholar]
- 183.Buetler TM, Renard M, Offord EA, Schneider H, Ruegg UT. Green tea extract decreases muscle necrosis in mdx mice and protects against reactive oxygen species. Am J Clin Nutr. 2002;75(4):749–53. doi: 10.1093/ajcn/75.4.749. [DOI] [PubMed] [Google Scholar]
- 184.Nakae Y, Dorchies OM, Stoward PJ, Zimmermann BF, Ritter C, Ruegg UT. Quantitative evaluation of the beneficial effects in the mdx mouse of epigallocatechin gallate, an antioxidant polyphenol from green tea. Histochem Cell Biol. 2012;137(6):811–27. doi: 10.1007/s00418-012-0926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Evans NP, Call JA, Bassaganya-Riera J, Robertson JL, Grange RW. Green tea extract decreases muscle pathology and NF-kappaB immunostaining in regenerating muscle fibers of mdx mice. Clin Nutr. 2010;29(3):391–8. doi: 10.1016/j.clnu.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Charite University. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 7 August 2016]. Sunphenon Epigallocatechin-Gallate (EGCg) in Duchenne muscular dystrophy (SUNIMUD) Available from: https://clinicaltrials.gov/ct2/show/NCT01183767. [Google Scholar]
- 187.Vejrazka M, Micek R, Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta. 2005;1722(2):143–7. doi: 10.1016/j.bbagen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 188.Ismail HM, Scapozza L, Ruegg UT, Dorchies OM. Diapocynin, a dimer of the NADPH oxidase inhibitor apocynin, reduces ROS production and prevents force loss in eccentrically contracting dystrophic muscle. PLoS One. 2014;9(10):e110708. doi: 10.1371/journal.pone.0110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hare JM. Nitric oxide and excitation-contraction coupling. J Mol Cell Cardiol. 2003;35(7):719–29. doi: 10.1016/s0022-2828(03)00143-3. [DOI] [PubMed] [Google Scholar]
- 190.Bia BL, Cassidy PJ, Young ME, Rafael JA, Leighton B, Davies KE, et al. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol. 1999;31(10):1857–62. doi: 10.1006/jmcc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 191.Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, et al. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci USA. 1996;93(17):9142–7. doi: 10.1073/pnas.93.17.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Percival JM, Anderson KN, Huang P, Adams ME, Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest. 2010;120(3):816–26. doi: 10.1172/JCI40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456(7221):511–5. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155(1):123–31. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wehling-Henricks M, Jordan MC, Roos KP, Deng B, Tidball JG. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum Mol Genet. 2005;14(14):1921–33. doi: 10.1093/hmg/ddi197. [DOI] [PubMed] [Google Scholar]
- 196.Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, et al. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci USA. 2008;105(19):7028–33. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McCarty MF, O’Keefe JH, DiNicolantonio JJ. Pentoxifylline for vascular health: a brief review of the literature. Open Heart. 2016;3(1):e000365. doi: 10.1136/openhrt-2015-000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Burdi R, Rolland JF, Fraysse B, Litvinova K, Cozzoli A, Giannuzzi V, et al. Multiple pathological events in exercised dystrophic mdx mice are targeted by pentoxifylline: Outcome of a large array of in vivo and ex vivo tests. J Appl Physiol (1985) 2009;106(4):1311–24. doi: 10.1152/japplphysiol.90985.2008. [DOI] [PubMed] [Google Scholar]
- 199.Zimmerman A, Clemens PR, Tesi-Rocha C, Connolly A, Iannaccone ST, Kuntz N, et al. Liquid formulation of pentoxifylline is a poorly tolerated treatment for Duchenne dystrophy. Muscle Nerve. 2011;44(2):170–3. doi: 10.1002/mus.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Escolar DM, Zimmerman A, Bertorini T, Clemens PR, Connolly AM, Mesa L, et al. Pentoxifylline as a rescue treatment for DMD: a randomized double-blind clinical trial. Neurology. 2012;78(12):904–13. doi: 10.1212/WNL.0b013e31824c46be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem. 1999;274(20):13729–32. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- 202.Adamo CM, Dai D-F, Percival JM, Minami E, Willis MS, Patrucco E, et al. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci. 2010;107(44):19079–83. doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol. 2012;228(1):77–87. doi: 10.1002/path.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JAJ, Yasuhara SE. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PloS One. 2007;2(8):e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nelson MD, Rader F, Tang X, Tavyev J, Nelson SF, Miceli MC, et al. PDE5 inhibition alleviates functional muscle ischemia in boys with Duchenne muscular dystrophy. Neurology. 2014;82(23):2085–91. doi: 10.1212/WNL.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Leung DG, Herzka DA, Thompson WR, He B, Bibat G, Tennekoon G, et al. Sildenafil does not improve cardiomyopathy in Duchenne/Becker muscular dystrophy. Ann Neurol. 2014;76(4):541–49. doi: 10.1002/ana.24214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Eli Lilly and Company. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2016. [Last accessed 3 June 2016]. A study of Tadalafil for Duchenne muscular dystrophy. Available from: https://clinicaltrials.gov/ct2/show/NCT01865084. [Google Scholar]
- 208.Martin EA, Barresi R, Byrne BJ, Tsimerinov EI, Scott BL, Walker AE, et al. Tadalafil alleviates muscle ischemia in patients with Becker muscular dystrophy. Science Translational Medicine 2012. 2012 Nov 28;4(162):162ra55. doi: 10.1126/scitranslmed.3004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hammers DW, Sleeper MM, Forbes SC, Shima A, Walter GA, Sweeney HL. Tadalafil treatment delays the onset of cardiomyopathy in dystrophin-deficient hearts. J Am Heart Assoc. 2016;5(8) doi: 10.1161/JAHA.116.003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kornegay JN, Spurney CF, Nghiem PP, Brinkmeyer-Langford CL, Hoffman EP, Nagaraju K. Pharmacologic management of Duchenne muscular dystrophy: target identification and preclinical trials. Ilar J. 2014;55(1):119–49. doi: 10.1093/ilar/ilu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Malik V, Rodino-Klapac L, Mendell JR. Emerging drugs for Duchenne muscular dystrophy. Expert Opin Emerg Drugs. 2012;17(2):261–77. doi: 10.1517/14728214.2012.691965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ruegg UT. Pharmacological prospects in the treatment of Duchenne muscular dystrophy. Curr Opin Neurol. 2013;26(5):577–84. doi: 10.1097/WCO.0b013e328364fbaf. [DOI] [PubMed] [Google Scholar]