Abstract
Background
HIV-1 enters the CNS within two weeks after peripheral infection and results in chronic neuroinflammation that leads to HIV associated neurocognitive disorders (HAND) in more than 50% of infected people. HIV enters the CNS by transmigration of infected monocytes across the blood brain barrier. Intravenous drug abuse is a major risk factor for HIV-1 infection, and opioids have been shown to alter the progression and severity of HAND. Methadone and buprenorphine are opioid derivates that are used as opioid maintenance therapies. They are commonly used to treat opioid dependency in HIV infected substance abusers, but their effects on monocyte migration relevant to the development of cognitive impairment are not well characterized.
Conclusion
Here, we will discuss the effects of opioids and opioid maintenance therapies on the inflammatory functions of monocytes and macrophages that are related to the development of neuroinflammation in the context of HIV infection.
Keywords: Buprenorphine, HIV, methadone, monocytes, neuroinflammation, opioid abuse
1. INTRODUCTION
There are 37 million people living with HIV worldwide [1]. Despite successful combined antiretroviral therapy (cART), a large percentage of infected people develop a spectrum of HIV associated neurocognitive disorders collectively termed HAND [2]. It is estimated that 13.1% of HIV infected people are intravenous drug users (IDU) [3]. Heroin, an opioid, is a drug abused intravenously [4]. Additionally, the abuse of prescription opioids has become a major health problem, as consumption of oxycodone increased nearly 500% between 1999 and 2011. In the last fourteen years, the number of individuals seeking treatment for prescription opioid abuse increased 900% [5]. Opioid abuse has a negative impact on cognitive functions [6]. In the context of HIV-1 infection, many studies in the pre-cART era showed that infected IDUs had worst neurocognitive outcomes than did non-abusers [7–9]. Post-cART, the contribution of opioid abuse to cognitive status is more nuanced [10–12], with some studies still demonstrating more cognitive impairment in HIV-1 infected people who abuse opioids despite successful antiretroviral therapy [13–15].
The mechanisms by which opioids may contribute to increased neurocognitive disorders in HIV-1 infected people are not fully understood, but the regulation of immune cell functions that participate in CNS inflammation is believed to play a role [16–19]. Chronic neuroinflammation persists despite cART in infected individuals [20, 21], in part, due to ongoing chemokine mediated transmigration of HIV infected and uninfected monocytes across the blood brain barrier (BBB) [22, 23]. Transmigrated monocytes differentiate into macrophages in the perivascular environment, constituting long-lived viral reservoirs and promoting low level neuroinflammation [24, 25].
Opioid maintenance therapies (OMT) with opioid derivates such as buprenorphine and methadone, are used to treat opioid dependency. In these therapies, opioids abusers substitute the use of buprenorphine or methadone for drugs of abuse, such as heroin or oxycodone [26, 27]. Some studies have shown that opioid abusers treated with buprenorphine have better cognitive outcomes than those who received methadone [28–30]. However, the effects of the therapeutics on cells of the immune system, and specifically on the monocyte chemotactic phenotype that facilitates transmigration across the BBB in the context of HIV infection, are not known. In this review we will discuss what is known about the effects of opioids and opioid maintenance therapies on the functions of monocytes and macrophages that contribute to neuroinflammation in the context of HIV neuropathogenesis. We will address how these therapies may impact on the development of neurocognitive disorders in HIV infected substance abusers.
2. MONOCYTE TRANSMIGRATION ACROSS THE BBB AND HIV INFECTION OF THE CNS
Transmigration of HIV-1 infected monocytes across the BBB plays a major role in HIV-1 infection of the CNS [31]. Within days after peripheral infection, infected monocytes transmigrate into the brain, bringing virus into the CNS [32, 33]. Once within the CNS, monocytes differentiate into macrophages [25, 34], where they remain as long-lived viral reservoirs [35, 36]. Viral replication in macrophages promotes the infection of resident microglia, perivascular macrophages, and to a lesser extent, astrocytes [37]. HIV-1 infection of these cells, as well as released viral proteins, including tat and gp120, promote their activation [38, 39]. This can induce the secretion of a wide range of inflammatory cytokines and other metabolic products, including IL-6, IL-1, TNF-α, CCL2, quinolinic acid, nitric oxide, and glutamate [40, 41]. These cytokines and metabolites by themselves or in conjunction with HIV-1 released proteins mediate the neuronal damage and loss characteristic of HAND [42]. Additionally, activated, uninfected CNS cells release these cytokines and metabolites [41]. CCL2 is a chemokine elevated in the CNS of HIV infected people with neurocognitive impairment, despite successful cART [43]. CCL2, as well as other chemokines, promotes the ongoing influx of monocytes into the CNS, contributing to chronic neuroinflammation [31, 41, 44, 45].
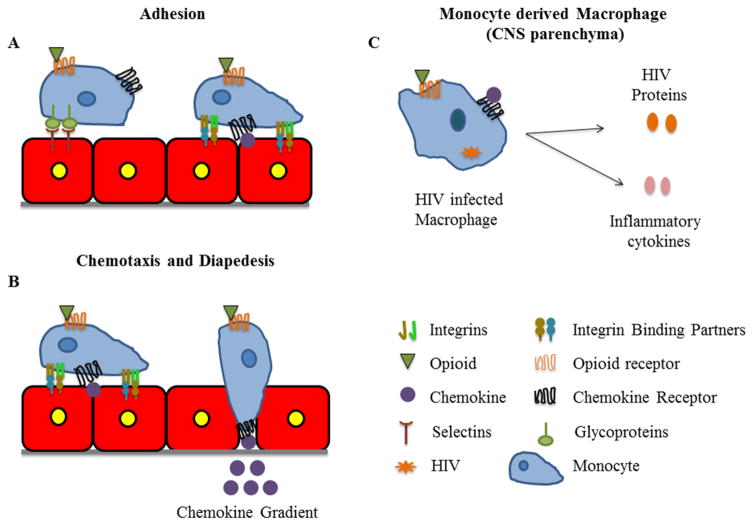
Transmigration of monocytes across the vasculature is a highly regulated process (Fig. 1). Initially, peripheral blood monocytes loosely adhere to the surface of the endothelial cells of the vasculature (Fig. 1A) [46]. If chemokines are not presented on the endothelial surface, monocytes will detach and re-enter the circulation. However, when chemokines, such as CCL2, are present, firm arrest is promoted by activation of integrins on the monocyte surface (Fig. 1A) [47]. Firm adhesion of monocytes facilitates their diapedesis across the endothelium into the parenchyma in response to a chemokine gradient (Fig. 1B) [48, 49]. Studies show that all of these steps can be altered by opioids (see section below). Therefore, examining the effects of opioids and OMT on the steps that facilitate monocyte transmigration across the vasculature and subsequent HIV-1 infection of macrophages is important for understanding how they can contribute to HIV neuropathogenesis in the context of opioid abuse.
Fig. 1.
Monocyte migration across the blood brain barrier (BBB) and consequences of HIV infection on monocyte derived macrophages. (A) Monocytes, both infected and uninfected, loosely adhere to the vasculature through the binding of their surface glycoproteins to selectins on the surface of endothelial cells. When a chemokine is presented on the endothelium, firm adhesion is promoted by the activation of integrins on the monocytes. (B) Monocytes then polarize and extend a leading edge that facilitates their diapedesis across the vasculature. (C) HIV infection of macrophages promotes the release of viral proteins and inflammatory cytokines that contribute to neuroinflammation. All of these processes can be regulated by opioids and opioid maintenance therapies.
3. OPIOID RECEPTOR EXPRESSION ON MONOCYTES AND MACROPHAGES
There are three main classes of opioid receptors, mu or MOR, kappa or KOR and delta or DOR. There is also another “non-classical” opioid receptor, NOP. All are G-protein coupled receptors (GPCR) [50]. Monocytes and macrophages express MOR, KOR and DOR [51, 52], and have also been shown to express the NOP receptor [53]. Sequencing of the opioid receptors in monocytes and macrophages showed that they are identical to the receptors expressed in neurons, where opioid receptors were first described [52].
Heroin, once injected, is metabolized into morphine [54], which is the main heroin metabolite in the CNS [54]. The effects of morphine, as well as other exogenous opioids, on immune cells, are mediated through opioid receptors, although some studies suggest that the effects of morphine are preferentially through activation of MOR [55]. In addition to exogenous opioids, many cell types also secrete endogenous opioids, which are divided into five families, the enkephalins, endorphins, dynorphin, nociceptin, endomorphins and morphiceptin [56]. Endogenous opioids also act through opioid receptors, and can bind to these receptors on immune cells, regulating their functions [57, 58]. In the next section we will review the roles of exogenous and endogenous opioids in regulating key functions of monocytes and macrophages, and how these effects may contribute to neuroAIDS.
4. REGULATION OF MONOCYTE MIGRATION AND MACROPHAGE INFLAMMATORY RESPONSES BY OPIOIDS
4.1. Opioid Effects on Monocytes
Monocyte transmigration requires their adherence to the vasculature (Fig. 1A). Treatment of primary human monocytes with a selective DOR agonist, DPDPE, induced α5β1 integrin mediated adhesion of these cells to fibronectin, and increased in vivo monocyte rolling on the endothelium. These effects were blocked by naltrindole, a DOR selective antagonist [59]. Additionally, in a separate study, treatment of the vasculature with morphine increased human monocyte adhesion in a process dependent on nitric oxide [60]. This effect was further increased when the vasculature was treated with morphine in the presence of HIV gp120 [60]. Morphine binds with high affinity to MOR, but it also can bind to DOR [50, 61]. Therefore, in the context of HIV infection and opioid abuse, activation of opioid receptors on the monocytes and endothelial cells could increase monocyte adherence to brain microvascular endothelial cells (BMVEC), facilitating their transmigration into the brain.
While the studies discussed above suggest that opioids increase monocyte adherence to the vasculature, other studies have shown that opioids decrease monocyte chemotaxis [62, 63]. Chemokine gradients mediate monocyte chemotaxis into the CNS during neuroinflammation [64]. Pretreatment of human primary monocytes with either heroin or morphine decreased their migration to endotoxin-activated serum [62]. Additionally, preincubation of human monocytes with morphine inhibited CCL3 stimulated chemotaxis in a process mediated through MOR and DOR [65]. Monocyte chemotaxis requires the formation of a leading edge of the cell (chemotactic protrusion), which is formed by the extension of the plasma membrane due to active polymerization of the actin cytoskeleton [66]. Treatment of human monocytes with morphine inhibited TNF- α or IL-1α stimulated leading edge formation [63]. Other studies using monocytes derived from monkeys showed that pretreatment with morphine inhibited CCL5 mediated chemotaxis in a concentration dependent manner [67].
Animal models have been used to study how opioids, in particular morphine, impact monocyte migration into the brain in vivo in the context of HIV infection. In a study using the simian immunodeficiency virus (SIV)/macaque model of HIV pathogenesis, it was shown that the brains of SIV infected monkeys treated with morphine had increased monocyte migration into the brain compared to the brains of SIV infected only monkeys [68]. A different study in mice, examining the effects of HIV tat and chronic morphine treatment on leukocyte diapedesis into the CNS, showed that intravenous injection of HIV tat induced a significant increase in inflammatory monocytes into the CNS. In contrast, chronic treatment with morphine alone did not alter monocyte trafficking [69]. This suggests that in the context of inflammation or cell activation that could be induced by opioid abuse, HIV infection may increase monocyte entry into the CNS. Interestingly, when mice were infected with S. pneumoniae to model comorbid bacterial infection, which is often seen in opioid abusers, morphine did increase monocyte trafficking into the CNS. Furthermore, when the infected mice were treated with both morphine and tat, an additional increase in monocyte transmigration into the CNS was shown, above that found with morphine alone [69]. This effect is important in the context of HIV, as comorbid infections such as tuberculosis, viral hepatitis, and S. pneumoniae often occur in HIV infected individuals, and even more so in opioid abusers [70]. These and other studies underscore that the effects of opioids on monocyte migration are complex. Thus, more studies are needed to understand how exogenous opioids regulate each step of the transmigration process, and how HIV infection and its associated comorbidities may contribute to this regulation.
Endogenous opioids also play a role in regulating monocyte chemotaxis. It has been shown that DADL, β-endorphin, dynorphin 1–13, and met-enkephalin induce monocyte migration [65, 71–73]. The effects of endogenous opioids on monocyte chemotaxis in the context of HIV-1 are not known. Increased secretion of these peptides in the CNS could lead to increased migration of monocytes to injured sites, promoting inflammatory responses [57, 74, 75]. Macrophages constitutively express and secrete β-endorphins [76]. It has been shown that inflammatory cytokines can induce the secretion β-endorphin [56]. Thus, inflammation in the CNS due to chronic HIV infection may regulate the secretion of endogenous opioids, increasing the recruitment of immune cells into the CNS [77].
The mechanisms by which opioids modulate monocyte transmigration are not fully characterized, but cross-regulation between chemokine receptors and opioid receptors is believed to play an important role [77, 78]. Both opioid receptors and chemokine receptors are GPCR and share similar structural and functional properties [79]. Different types of interactions between these receptors that affect monocyte migration in response to chemokines and opioids have been shown. These include receptor heterodimerization [80], heterologous desensitization [81, 82], and activation of common downstream regulatory pathways [83]. For example, heterodimerization between CXCR4 and DOR has been shown by co-immunoprecipitation from primary human monocytes and in the human monocyte cell line Mono Mac-1. Treatment with both CXCL12 and DPDPE, a DOR agonist, decreased Mono Mac-1 migration to CXCL12 in a process reversed by treatment with naltrindole, a DOR antagonist. This is due to the formation of heterodimers composed of CXCR4 and DOR. Co-stimulation of both receptors stabilizes the heterodimer, preventing CXCL12 mediated monocyte migration [80]. Heterologous desensitization has also been shown to mediate monocyte chemotaxis. Treatment of human monocytes with CCL5 decreased DPDPE and DAMGO mediated chemotaxis, involving MOR phosphorylation [73]. Activation of both opioid and chemokine receptors simultaneously on the surface of monocytes may occur in the context of HIV-1 infection and opioid abuse since inflammatory chemokines have been shown to be elevated in the plasma and CSF of HIV infected people [84].
Regulating monocyte influx into the CNS by exogenous and endogenous opioids may have important consequences for chronic CNS inflammation and neurocognitive impairment in HIV-1 infected people. The effect of the opioid on monocyte migration will likely depend upon whether the opioid is endogenous or exogenous. Additionally it will depend on the inflammatory context, on which different inflammatory cytokines and HIV proteins may influence the effects of opioids on migration [69, 84]. Further studies are needed to understand how the different classes of opioids regulate monocyte diapedesis across the vasculature in response to inflammatory mediators, in the context of HIV-1 infection.
4.2. Opioid Effect on Macrophages
Macrophages play an important role in HIV mediated neuropathogenesis [85]. Perivascular macrophages, derived from HIV infected monocytes that transmigrated across the BBB, remain as long-lived HIV reservoirs despite cART [25, 35, 36]. HIV infected macrophages release inflammatory cytokines and viral proteins that contribute to neuroinflammation and neuronal damage [86, 87]. It has been shown that morphine enhances HIV infection of and replication in macrophages and other CNS resident cells (Fig. 1C) [88–90]. Many factors have been proposed to contribute to this process. Morphine treatment of macrophages, derived from PBMC, increased surface expression of CXCR4 and CCR5, leading to increased susceptibility to infection by X4 and R5 viral strains [91]. In another study using human monocyte derived macrophages (hMDM), it was shown that morphine increased HIV infection of hMDM when an R5 strain was used [92]. These effects were mediated, in part, by increased surface CCR5, supporting the concept that morphine enhances HIV entry into macrophages by regulating the surface expression of its co-receptors [92]. Another proposed mechanism is the regulation of adhesion molecules that mediate pathogen-host interactions [90]. Galectin -1 is a soluble adhesion molecule secreted by many cell types including macrophages. Morphine treatment of macrophages increased the expression and secretion of galectin-1 [90], and treatment with both morphine and galectin-1 increased HIV infection of macrophages [90]. Morphine treatment has also been shown to down regulate anti-viral molecules in macrophages, including interferon alpha and beta [89], and anti-HIV miRNAs [93]. These studies show that activation of opioid receptors, through morphine treatment, regulates several aspects that mediate HIV infection of macrophages, resulting in increased numbers of infected cells. Opposite effects of MOR and KOR activation on HIV infection have also been observed [94]. Treatment of acutely HIV infected hMDM with the KOR agonist U50,488 decreased HIV p24 levels compared to untreated cells, indicating that activation of different opioid receptors could differentially impact HIV infection [94].
Morphine also enhances HIV transcription through activation of the HIV LTR [88]. Chronic morphine treatment increases adenylyl cyclase (AC) activity, which increases cyclic AMP (cAMP). Cyclic AMP activates PKA signaling pathways that result in the phosphorylation of CREB, that interacts with other proteins to enhance the transcription of genes that contain CRE elements in their promoters [88]. The HIV-LTR has been shown to contain such elements; therefore chronic treatment with morphine could activate MOR mediated cAMP signaling, increasing the activity of the HIV-LTR [88].
Increased HIV infection of and replication in macrophages by opioids is important in the context of CNS viral reservoirs and neuroinflammation. Activation of HIV in macrophage reservoirs in the presence of opioids could lead to increased infection of other CNS resident cells, including microglia and astrocytes, which will also contribute to inflammation [95, 96]. Additionally, viral infection of macrophages increases the secretion of inflammatory cytokines and HIV proteins [97]. It has been shown that morphine enhances HIV mediated secretion of IL-6 and CCL8 from treated hMDM [98]. In addition, MOR activation on macrophages induces CCL2, CCL5, and CXCL10 (Fig. 1C) [77]. All of these factors contribute to CNS inflammation in the context of HIV infection [99]. Increased chemokine secretion will recruit additional monocytes into the CNS [49]. Inflammatory cytokines in conjunction with HIV proteins will mediate neuronal damage exacerbating cognitive dysfunction in the context of opioid abuse [100].
5. OPIOID MAINTENANCE THERAPIES AND THEIR EFFECTS ON NEUROINFLAMMATION
5.1. Clinical Studies in Methadone and Buprenorphine Treated Substance Abusers and their Cognitive Outcomes
Opioid use disorders are defined as a problematic pattern of opioid use leading to clinically significant impairment or distress. This may be associated with tolerance, withdrawal, and impaired ability to fulfill obligations [101]. Opioid use disorders have been present for centuries, yet in recent years have become especially prevalent and problematic in the United States. An estimated 16,451 individuals died from opioid overdoses in 2010 in the United States [102]. Drug users may take prescription opioids illegally, inject non-medical opioids (heroin and opium), or may abuse medication they are prescribed for a chronic pain disorder [27]. Opioids of abuse are typically full agonists of MOR, which induces feelings of euphoria and alters neurochemical reward behavior in the user over time. Drug users ingest opioids orally, intranasally, or intravenously [27], and the spread of infectious disease is often tied to intravenous opioid use [103].
An often used approach for treatment of opioid dependent individuals is the use of an opioid maintenance therapy (OMT) such as methadone or buprenorphine [104, 105]. Although OMT may continue the cycle of opioid dependence, it decreases the pattern of intoxication and withdrawal that is so debilitating to opioid dependent people. The goal of OMT is to help such individuals to reduce illicit opioid use, reduce the spread of infectious diseases such as HIV and HCV, reduce the risk of overdose, and help the person to reintegrate into society. Methadone, like heroin and morphine, is a full agonist for MOR. However, it does not produce the same degree of euphoria as other MOR agonists when ingested orally, enabling it to be used in OMT to replace the cravings induced by drugs such as heroin. If injected intravenously, however, the effects of methadone are similar to those of morphine [27]. Therefore, individuals receiving methadone therapy must attend a maintenance clinic to receive their doses of methadone. However, individuals may continue illicit opioid use while enrolled in methadone treatment and the combination of illicit opioid use with their methadone dose may cause overdose and death. Use of methadone alone can cause overdose as well, specially if injected [27].
An alternative to methadone therapy is buprenorphine treatment. Unlike methadone and opioids of abuse, buprenorphine is a partial agonist of MOR and an antagonist of KOR. Although buprenorphine binds to MOR with high affinity, its partial agonism results in a maximal euphoric effect that is much lower than those produced by other MOR agonists. This “ceiling” to the dose dependent effects of buprenorphine makes buprenorphine safer, as it is more difficult for individuals to overdose when taking this therapeutic [106]. Buprenorphine is available sublingually as buprenorphine alone (subutex) or in combination with naloxone, an opioid receptor antagonist, in a 1:4 distribution (suboxone). The naloxone component of suboxone has minimal bioavailability when ingested sublingually, and the patient will not undergo withdrawal. However, if the individual injects suboxone intravenously, this will precipitate opioid withdrawal. Suboxone is the form of buprenorphine that is more often prescribed, as it prevents individuals from improperly using this drug. Buprenorphine in either form may be prescribed at a physician’s office rather than at select maintenance clinics.
Many HIV infected individuals contract the virus through intravenous drug use and are opioid dependent [103]. For HIV infected individuals, buprenorphine may be a superior therapeutic [27], as it does not interact with antiretrovirals whereas methadone does [107]. Liver function, however, can be affected by both buprenorphine and antiviral agents, which could cause a change of buprenorphine plasma levels in individuals taking both medications [27]. HIV induces cognitive deficits in half of infected individuals [2], which may be worsened by concomitant opioid use [10–12]. To our knowledge there are no studies on the impact of methadone compared to buprenorphine on cognitive dysfunction in HIV infected individuals, although there are many studies on the neurocognitive effects of buprenorphine treatment as compared to methadone in uninfected substance users [28–30, 108, 109]. These studies are described below.
Several observational and randomized control trials have compared methadone and buprenorphine effects on cognition. Some studies found no significant difference in levels of cognitive deficits between buprenorphine and methadone maintained people [110–113]. Others found that individuals treated with buprenorphine performed better in various cognitive tests [28–30, 108, 109]. Some of these studies are described below and in the Table 1. Limitations for each study are also detailed in the Table 1.
Table 1.
Summary of the clinical studies comparing the effects of buprenorphine and methadone on the cognitive function of opioid abusers.
| Author/Year/Number | Study Design | Patient Groups | Primary Outcomes | Results | Limitations |
|---|---|---|---|---|---|
| Bach et al./2012 [110] | Observational study | Methadone treated former opioid users, buprenorphine treated former opioid users, and healthy controls | Working memory performance and fMRI activation | Same working memory performance between treated people and controls but different fMRI activation | Task assessing working memory was too easy, a more difficult task could elucidate whether differences in fMRI activity correspond to more subtle cognitive dysfunction |
| Loeber et al./2008 [112] | Observational study | Opioid-dependent individuals, previously assigned according to their own preference to methadone (30) or buprenorphine (26) | Neuropsychological assessment looking at memory, response selection and control, selecting and focusing of sensory stimuli, vigilance and sustained attention | Same performance between treatment groups | Not randomized, is an observational study |
| Sokya et al./2011 [108] | Observational study | 22 buprenorphine treated, 24 methadone treated, 20 long-term heroin abusers and 25 healthy controls matched for age and education | Neuropsychological test battery related to driving skills, tested visual perception, selective attention, vigilance, reactivity, and stress tolerance | Heroin abusers did worse on several domains when compared with other groups and worse than healthy controls on almost all tests. Buprenorphine treated people performed better in decision and reaction tests than other treatment groups | Small sample size, not randomized (completely), heroin patients are very specific group (since not yet legal in Germany) and may therefore represent a more cognitively-impaired group |
| Pirastu et al./2006 [109] | Observational study | 20 methadone treated people, 19 buprenorphine treated people and 21 non-opioid dependent controls matched for age, education, employment level | Iowa gambling task measuring specific decision-making ability: ability to choose better for long-term benefit vs myopic decision-making, in addition general intelligence (WAIS-R), cognitive flexibility and abstract thinking (WCST), visual perception and memory (BVRT) | Buprenorphine treated people performed significantly better on WCST than methadone treated people, albeit worse than controls. Buprenorphine treated people performed better than methadone treated on Iowa gambling task. | Small sample size, observational study |
| Rapeli et al./2007 [29] | Observational study | 6 methadone, 17 buprenorphine/naloxone treated patients, and 17 healthy controls matched for sex and age | Neurocognitive assessment looking at attention, verbal memory, and working memory | Buprenorphine treated people outperformed methadone treated in several areas, both patient groups worse than controls | Small sample size, concomitant benzodiazepine use in all patients, observational study |
| Rapeli et al./2009 [114] | Observational study | 13 methadone and 15 buprenorphine or buprenorphine/naloxone treated people with benzodiazepine dependence or abuse disorder. The results were compared to 15 participants in the healthy control group | Within the first two months (T1) and between 6–9 months (T2) after opioid maintenance therapy admission, assessed working memory, immediate verbal memory, and memory consolidation | Both patient groups exhibited significant memory impairment with benzodiazepine use and opioid maintainance therapy combined, different patterns of memory deficits for methadone vs buprenorphine treated people | Small sample size, observational study, illicit drug use in patient populations, benzodiazepine use and interaction with opioid substitution therapeutics |
| Rapeli et al./2011 [30] | Observational study | 14 buprenorphine treated and 12 methadone treated people at T1, the patient sample was extended to include 36 people total at T2 and T3 | Neuropsychological assessment looking at attention, working memory, verbal memory | Buprenorphine treated people outperformed methadone treated in several areas | Observational study, other psychotropic medications and substance abuse present in treatment groups, while the opioid-dependent patient groups were comparable to each other in variables of interest |
| Darke et al./2012 [113] | Observational study | 94 individuals on methadone treatment, 31 on buprenorphine treatment, 50 abstinent former drug-users and 50 healthy controls | Neuropsychological tests measuring executive function, working memory, information processing speed, verbal learning and non-verbal learning | No difference between buprenorphine and methadone treated groups, worse than abstinent group | Observational study, (abstainers may have introduced selection bias), small sample size for buprenorphine group |
| Sokya et al./2008 [108] | Randomized control trial | People receiving OMT and healthy controls recruited from job centers matched for age, sex, and education level | Neuropsychological assessment looking at selective attention, verbal memory, motor/cognitive speed, and cognitive flexibility | Impairment in both buprenorphine and methadone treatment groups compared to controls, no difference in terms of each other | Small sample size, large amount of patients were also taking cannabis and other substances |
| Sokya et al./2005 [28] | Randomized control trial | 62 buprenorphine or methadone treated people | Performance on tests of visual perception, selective attention, vigilance, reactivity, and stress tolerance | Buprenorphine superior in psychomotor performance, decision and reaction test of executive function, no difference in other domains | Small sample size, large amount patients were also taking cannabis and other substances |
The effect of methadone and buprenorphine treatment on visuospatial working memory performance was examined in an observational study. Individuals receiving either methadone or buprenorphine and healthy controls were tested with the “Trail-Making Test”, a test of visuospatial working memory and verbal intelligence. While being tested, fMRI images were taken to assess neuronal activation. Different neural activity, with decreased blood oxygen level dependent signal (BOLD) response was found for the populations during spatial working memory tasks. However, individuals performed similarly to controls on these tests. The authors hypothesized that future studies with more complex tasks could identify whether the differences in neural activity correlate to more subtle cognitive deficits in the different populations [110].
Another observational study of 30 methadone and 24 buprenorphine treated individuals was performed. Opioid maintenance therapy chosen was based on individual preference. Areas tested included vigilance and sustained attention, selecting, and focusing of sensory stimuli, response selection and control, and memory function. No difference was found between the performance of methadone and buprenorphine treated people [112].
An observational study comparing cognition in heroin abusers, methadone and buprenorphine treated people was conducted. Twenty two buprenorphine and 24 methadone patients were recruited from a different randomized study for participation. Twenty non-randomized long-term heroin abusers were recruited as well as 25 healthy controls. A neuropsychological test battery assessing visual perception, selective attention, executive function, vigilance, reactivity, and stress tolerance was conducted. Heroin abusers performed worse than controls, as well than the other patient groups on most domains tested. Buprenorphine treated people performed better on tests of executive function than other groups [108].
In another observational study, decision-making ability of 20 methadone and 19 buprenorphine treated people, and 21 non-opioid dependent controls was compared. Executive function, cognitive flexibility, general intelligence, abstract thinking, and visual perception were tested. Buprenorphine treated people performed better than methadone treated individuals in tests of decision-making (executive function). Both groups performed similarly on tests of general intelligence, but buprenorphine treated were less impaired than methadone treated people in tests of cognitive flexibility and abstract thinking [109].
Another observational study assessing the differences in cognition between methadone and buprenorphine at an early time point in therapy was performed. Attention, working memory, and verbal memory were decreased. Sixteen methadone, 17 buprenorphine/naloxone, and 17 healthy controls were included. Benzodiazepine and other comedication use was both common and allowed to assess overall cognitive deficits between groups when typical comedication use was present. Verbal memory was found to be more preserved in buprenorphine treated people than in methadone treated individuals. Other cognitive domains tested were similar between patient groups. This underscores the importance of considering the exacerbating effects on memory of benzodiazepines in the setting of OMT [29].
A longitudinal observational study of 13 methadone, 15 buprenorphine or buprenorphine/naloxone treated people, all of which use benzodiazepines as well, was performed. Additionally, a control group with 15 participants was used for comparison. Since benzodiazepine use is common with OMT, this experimental study, similar to the one described above, was to demonstrate what effect on cognition benzodiazepine use in people on different opioid maintenance treatments. Working memory, immediate verbal memory and memory consolidation were tested. Both groups performed poorly on many tests of memory indicating concomitant benzodiazepine use with OMT may be associated with more memory problems than either therapeutic alone. The pattern of impairment differed between methadone and buprenorphine treated people [114].
Another longitudinal observational study on a sample of non-randomized buprenorphine and methadone treated people at various time points (2 months, 6–9 months, and 12–17 months) was performed. After the first time period the patient samples were extended to include a total of 36. Neuropsychological assessment of working memory, executive function, attention, and verbal memory was performed. Buprenorphine treated people outperformed methadone treated in several areas (combined attention performance and working memory). Both patient groups showed equal deficits in the other domains assessed [30]
An observational study was conducted of 94 methadone, 31 buprenorphine, 50 abstinent former opioid users and 50 healthy controls. Neuropsychological tests measuring executive function, working memory, information processing speed, verbal learning and non-verbal learning were administered. Poorer performance was found amongst both maintenance populations relative to healthy controls, without a significant difference between buprenorphine and methadone treated. Abstinent subjects performed similar to maintenance subjects in some domains, and similar to controls in others [113].
The effects on cognition of methadone and buprenorphine treatment were also examined through a randomized clinical trial. Fifty nine patients were randomized to either methadone or buprenorphine and 24 healthy control subjects were recruited for comparison. Participants were tested after 2 weeks of treatment, and again after 8–10 weeks. Neuropsychological tests assessed selective attention, cognitive flexibility, verbal memory, and motor/cognitive speed. No differences in performance were observed between the methadone and buprenorphine treated groups, but both patient groups scored lower on most cognitive domains when compared to healthy controls. Performance over time improved in both treated groups, indicating cognitive function may improve after stabilization on maintenance therapy [111].
Another study compared the cognitive effects of methadone and buprenorphine in a randomized clinical trial. Sixty two opioid dependent individuals were randomized to either buprenorphine or methadone treatment. Neuropsychological assessment of visual perception, selective attention, vigilance, reactivity, executive function and stress tolerance were performed. Patients randomized to buprenorphine performed better than those treated with methadone in psychomotor performance and executive function. Performance in other domains was similar between groups [28].
Several of the above mentioned studies showed that buprenorphine treated people have better cognitive outcomes than those treated with methadone, specifically in, verbal memory [29], working memory [30], executive functions [28, 108], and decision making [109]. These cognitive domains have also been shown to be significantly affected in HIV infected people on successful cART treatment [2, 115]. Therefore, when considering the most appropriate choice of therapeutic for HIV positive individuals, buprenorphine-based therapies such as suboxone may be superior to methadone due to its positive effects on cognition, specifically in the domains that have been shown to be most affected by HIV infection in the cART era, as well as to its safety profile. The differential effects of buprenorphine and methadone on the cognitive function of HIV infected individuals may have important implications for treatment success, adherence to antiretroviral medication and ability to reintegrate into society, and is an important area for future research.
5.2. Effects of Opioid Maintenance Therapies on Immune Cells
The effects of opioid maintenance therapies on immune cells, specifically on monocytes and macrophages, are important in the context of HIV infection and opioid abuse because they may impact chronic CNS inflammation that contributes to the development of cognitive impairment. This may provide mechanisms that explain the findings in the clinical studies described in the preceding section. Contradictory results have been found regarding the effects of opioid maintenance therapies on immune cells. In this section we will discuss the effects of buprenorphine and methadone on monocyte and macrophage functions related to chronic CNS inflammation. Additionally, we will briefly review the effect of these therapeutics on the function of other immune cells including, PBMC, NK cells, splenocytes and, T cells, although their role in neuroinflammation are not the focus of this review.
5.2.1. Effects of Opioid Maintenance Therapy on Monocytes and Macrophages
Chronic CNS inflammation is maintained by ongoing, low level monocyte influx into the CNS, mediated in part, by CCL2 [31]. We studied the effects of buprenorphine on the CCL2 mediated human monocyte migratory phenotype that facilitates their migration [116]. Human peripheral monocytes have a round morphology, but when treated with CCL2, they extend a leading edge, characterized by the colocalization of the actin and tubulin cytoskeleton [66]. When monocytes were treated with CCL2 in the presence of buprenorphine, the percentage of monocytes extending protrusions decreased, to similar numbers as base line [116]. CCL2 mediated chemotaxis was also decreased when monocytes were pretreated with both buprenorphine and CCL2 by a mechanism regulated, in part, by delayed CCR2 receptor recycling to the surface [116]. Additionally, we studied the effect of buprenorphine treatment on CCL2 mediated phosphorylation of p38, a MAP kinase associated with cell migration [117]. We found that buprenorphine inhibited CCL2 mediated p38 phosphorylation. This decrease involved the activation of MOR, because pretreatment of monocytes with CTAP, a MOR antagonist, reversed the effects [116]. Interestingly, when monocytes were treated with nor-BNI a KOR antagonist, CCL2 mediated p38 phosphorylation was decreased in the 60% of the experiments with primary human monocytes. As all of the studies were performed with primary cells from different people, this may reflect intrinsic variability in surface KOR expression from person to person [116]. Thus, KOR antagonist activity may also play a role in the effects of buprenorphine. Therefore, buprenorphine may decrease CCL2 mediated monocyte migration into the CNS in response to inflammatory chemokines by binding and signaling through MOR and KOR (Fig. 1A, B). It is important to note that buprenorphine treatment in the presence of CCL2 only decreased monocyte migration to baseline levels, suggesting that normal immune surveillance by monocytes remains intact. This further underscore the positive impact buprenorphine may have in HIV infected individuals [116].
Another recent study of human monocytes examined the effects of buprenorphine, methadone, and other opioid therapeutics on phagocytosis and oxidative burst in human monocytes. It found that at clinically relevant concentrations, none of these opioid therapeutics altered the in vitro phagocytic activity of human monocytes or their oxidative burst [118]. Thus, buprenorphine may have both positive and negative effects on monocyte function.
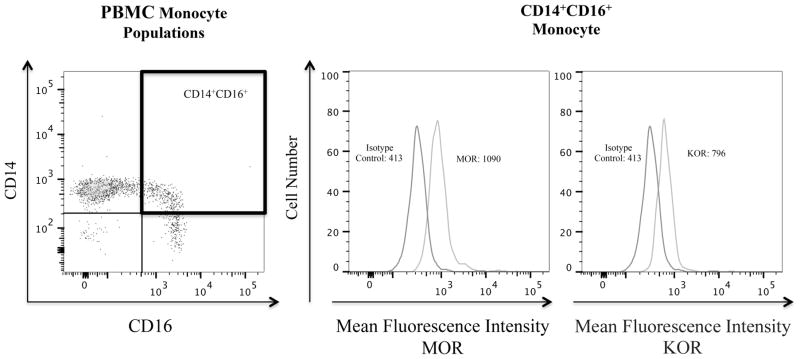
Peripheral blood monocytes are a heterogeneous population of cells that can be classified by the expression of two surface markers, CD14, the LPS receptor, and CD16, the FcγIII receptor [119]. CD14+CD16+ cells are believed to be a more mature population of monocytes than CD14+. This monocyte population increases in number with HIV-1 infection [119, 120]. We have shown that HIV infected substance abusers have a greater increase in CD14+CD16+ monocytes, compared to HIV infected non-abusers (Calderon, et al. submitted). CD14+CD16+ monocytes can be productively infected with HIV-1 and they transmigrate preferentially across an in vitro BBB model in response to CCL2 [121, 122]. Additionally, CD14+CD16+ monocytes mediate chronic inflammation [25]. Using flow cytometry we found that this monocyte subpopulation expresses both MOR and KOR opioid receptors on their surface (Fig. 2). Future research will examine how opioids and opioid maintenance therapies modulate the migratory properties of this inflammatory monocyte subset.
Fig. 2.
CD14+CD16+ monocytes in the PBMC obtained from a healthy individual express surface MOR and KOR. PBMC were isolated using Ficoll density gradient centrifugation. After isolation, PBMC were stained for surface markers CD14, CD16, and for the opioid receptors MOR and KOR, as well as for their respective isotype matched controls. Cells were analyzed using a BD Canto II flow cytometer. Left panel, PBMC were gated according to the expression of surface CD14 and CD16. Middle and right panels, histograms showing the surface expression of MOR and KOR on the CD14+CD16+ monocytes shown in the PBMC gate in the highlighted box.
Few studies have analyzed the effects of buprenorphine and methadone on macrophages. Using a mouse model of venous thrombosis (VT), an inflammatory process, it was shown that after treatment with buprenorphine, there was a significant decrease in the accumulation of vein wall macrophages, which is a characteristic response to blood vessels injury [123]. In another study, mice injected with methadone, showed decreased antibody responses to injected sheep red blood cells (SRBC). This was due, in part, by a decrease in macrophage dependent B cell activation [124]. A different study using hMDM found that acute treatment (30 minutes) with methadone and morphine inhibited phagocytosis in a dose dependent manner. In contrast, when hMDM were treated chronically (24hr) with morphine or methadone, no difference was observed in the phagocytic activity of these cells. Interestingly during the differentiation protocol of monocytes to macrophages used in the above referred study, methadone was present the entire time. This enabled the study to examine the effects of methadone withdrawal in vitro. When methadone was removed from the culture, an inhibition of phagocytosis was observed [125].
Therefore, decreasing monocyte migration and macrophage inflammatory functions by treatment with opioid maintenance therapies may be beneficial in the context of chronic inflammation during HIV infection. Not only would opioid abuse be controlled, but also the neuroinflammation characteristic of HIV infection in the cART era maybe reduced. Duration of the treatment with the therapeutics being studied, as well as the impact of removing those therapeutics, are important factors to consider for future studies analyzing their effect on monocytes and macrophages.
5.2.2. Effects of Opioid Maintenance Therapy on PBMC, Splenocytes, NK and T Cells
5.2.2.1. Effects on PBMC, Splenocytes, and NK Cells
Several studies have shown that methadone and buprenorphine have immune suppressive effects [126–131]. PBMC isolated from methadone treated people produced fewer oxygen radicals when stimulated with PMA as compared to PBMC from healthy people [127]. Additionally, methadone treatment decreased the secretion of IL-6 from IL-2 stimulated PBMC [118]. Acute (1hr) administration of buprenorphine was shown to decrease, in a dose dependent manner, the NK cytotoxic activity, as well as the ConA and LPS stimulated proliferation of splenocytes [131]. All of these effects were reversed by treatment with the general opioid receptor antagonist, naltrexone [131].
Other studies have suggested that methadone and buprenorphine do not have any effect on the immune response [132–136]. In a study analyzing PBMC from heroin abusers, proliferation of PBMC from these individuals was increased, as was secretion of IL-10, when compared to controls. In contrast, PBMC isolated from people chronically treated with methadone had responses similar to normal controls [137]. Another study compared proliferation and cytokine secretion after stimulation with PHA of PBMC isolated from current heroin abusers, buprenorphine or methadone treated people, and healthy non-abusing controls [138]. Only PBMC derived from current heroin abusers had decreased proliferative ability in response to PHA, as well as decreased secretion of IL-4, TNF, and IFN. PBMC from methadone and buprenorphine treated people showed no differences as compared to healthy controls [138]. When buprenorphine was administered acutely (1hr) or chronically (24hr or 72hr) to mice, it did not affect NK cytotoxicity, ConA mediated splenocyte proliferation, or IL-2 and IFN-γ secretion [135].
Discrepancies among the above mentioned studies could be attributed to many factors. Differences in experimental design and protocol, or the number of subjects used, could result in different outcomes. In some of the human studies, HIV infection status was not considered [132] and could be a confounding factor. Current drug abuse was often measured by self-reporting, which is not always reliable [126, 139]. Additionally, several pharmacologic factors, including doses of therapeutic used, duration of the treatment, and withdrawal of treatment, need to be considered to asses the specific contribution of these therapies to the regulation of the immune response [140].
5.2.2.2. Effects on T Cells
In a study of Jurkat T cells, chronic treatment (24hr) with methadone induced IL-4 mRNA, while treatment with morphine or buprenorphine had no significant effect on IL-4 message expression [141]. IL-4 is an important cytokine involved in skewing the T cell repertoire towards a less inflammatory Th2 subset [141]. In another study, treatment with buprenorphine and methadone, as well as the use of heroin, did not change the total number of CD4+ cells as compared to untreated controls. Additionally, buprenorphine and methadone did not change the composition of T-cell subpopulations, however, the population of CD4+CD25highFoxp3+ (Tregs) cells was increased in heroin abusers [142].
In studies focused on inflammatory diseases not caused by HIV infection, methadone treatment was shown to decrease T cell mediated inflammation. In a mouse model of diabetes induced by treatment with streptozotocin (STDZ), methadone prevented STDZ mediated inflammatory responses against insulin-producing beta pancreatic cells [143]. Methadone treatment also restored insulin secretion, as well as decreased pancreatic inflammatory cytokines [143]. In another study using an EAE mouse model of multiple sclerosis induced by MOG, daily treatment with methadone significantly decreased the severity of disease by decreasing T cell infiltration into the spinal cord [144]. These studies show methadone treatment could have an additional therapeutic benefit, which is limiting T cell mediated inflammatory responses.
CONCLUDING REMARKS
Opioid effects on monocyte and macrophages are complex, especially in the context of HIV infection. Many factors including the type of opioid receptor activated, the origin of the opioid, doses used, time of treatment, and opioid withdrawal significantly influence the effects of opioids on chemotaxis and diapedesis of monocytes in response to chemokines as well as on HIV infection of macrophages. The effects of buprenorphine on monocyte migration are not fully characterized but our studies suggest that buprenorphine treatment, in the context of the activation of immune cells with HIV infection may be used not only for opioid maintenance therapy, but also for limiting monocyte entry into the CNS in response to CCL2, decreasing chronic neuroinflammation and thereby neurocognitive impairment.
Future studies should be focused on studying the specific mechanisms by which opioids and opioid maintenance therapies alter the function of immune cells and more specifically on the multi step process of monocyte transmigration across the BBB, and the effects of opioid maintenance therapies on macrophages within the CNS. Characterizing the mechanisms we will be able to develop more effective treatments for opioid dependency, as well as for chronic activation of immune cells during HIV infection, which will limit the cognitive deficits of neuroAIDS.
Acknowledgments
MJB and RW contributed to the writing of the manuscript. LC and JWB contributed to writing and editing of the manuscript. Additionally, we thank Dr. Peter Gaskill for his helpful editing of this manuscript.
ABBREVIATIONS
- CNS
Central nervous system
- ConA
Concanavalin A
- CREB
cAMP response element-binding
- CSF
Cerebral spinal fluid
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- DADL
D-ala-D-leu-enkephalin
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]
- DOR
Delta opioid receptor
- DPDPE
[D-Pen2, 5] Enkephalin
- EAE
Experimental autoimmune encephalomyelitis
- fMRI
Functional magnetic resonance imaging
- gp
Glycoprotein
- HCV
Hepatitis C virus
- HIV
Human Immunodeficiency virus
- IFN
Interferon
- IL
Interleukin
- KOR
kappa opioid receptor
- LPS
Lipopolysaccharide
- MAP
Mitogen-activated protein
- MOG
Myelin oligodendrocyte glycoprotein
- MOR
mu opioid receptor
- NK
Natural Killer
- NOP
ORL-1 receptor
- nor-BNI
Norbinaltorphimine
- PBMC
Peripheral Blood Mononuclear Cells
- PHA
Polyclonal mitogen phytohemoagglutinin
- PKA
Protein kinase A
- PMA
Phorbol myristate acetate
- TNF
Tumor necrosis factor
- Tregs
T regulatory cell
- VT
Venous thrombosis
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest. The work described in this report was supported by National Institute of Health grant T32 AI007501 (to MJB) – Training in HIV/AIDS pathogenesis, basic and translational research. Additionally grants MH075679 and MH090958 (to JWB), and the center for AIDS research at the Albert Einstein College of Medicine and Montefiore Medical Center (CFAR/AI051519) supported this work.
References
- 1.UNAIDS. How AIDS Changed Eveything; UNAIDS Report on Global AIDS Epidemic. 2015. [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations Office on Drugs and Crime, World Drug Report 2014 (United Nations publication, Sales No. E.14.XI.7).
- 4.Diaz T, Chu SY, Byers RH, Jr, et al. The types of drugs used by HIV-infected injection drug users in a multistate surveillance project: implications for intervention. Am J Public Health. 1994;84(12):1971–5. doi: 10.2105/ajph.84.12.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–74. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 6.Anand P, Springer SA, Copenhaver MM, Altice FL. Neurocognitive impairment and HIV risk factors: a reciprocal relationship. AIDS Behav. 2010;14(6):1213–26. doi: 10.1007/s10461-010-9684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan V, Brettle RP, Goodwin GM. The Edinburgh cohort of HIV-positive drug users: pattern of cognitive impairment in relation to progression of disease. Br J Psychiatry. 1992;161:522–31. doi: 10.1192/bjp.161.4.522. [DOI] [PubMed] [Google Scholar]
- 8.Marder K, Stern Y, Malouf R, et al. Neurologic and neuropsychological manifestations of human immunodeficiency virus infection in intravenous drug users without acquired immunodeficiency syndrome. Relationship to head injury. Arch Neurol. 1992;49(11):1169–75. doi: 10.1001/archneur.1992.00530350083023. [DOI] [PubMed] [Google Scholar]
- 9.Bell JE, Donaldson YK, Lowrie S, et al. Influence of risk group and zidovudine therapy on the development of HIV encephalitis and cognitive impairment in AIDS patients. AIDS. 1996;10(5):493–9. doi: 10.1097/00002030-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Ryan LA, Brester M, Bohac D, Morgello S, Zheng J. Up-regulation of soluble tumor necrosis factor receptor two in plasma of HIV-seropositive individuals who use opiates. AIDS Res Hum Retroviruses. 2004;20(1):41–5. doi: 10.1089/088922204322749486. [DOI] [PubMed] [Google Scholar]
- 11.Applebaum AJ, Otto MW, Richardson MA, Safren SA. Contributors to neuropsychological impairment in HIV-infected and HIV-uninfected opiate-dependent patients. J Clin Exp Neuropsychol. 2010;32(6):579–89. doi: 10.1080/13803390903313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrd DA, Fellows RP, Morgello S, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58(2):154–62. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Applebaum AJ, Reilly LC, Gonzalez JS, Richardson MA, Leveroni CL, Safren SA. The impact of neuropsychological functioning on adherence to HAART in HIV-infected substance abuse patients. AIDS Patient Care STDS. 2009;23(6):455–62. doi: 10.1089/apc.2008.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer VJ, Rubin LH, Martin E, et al. HIV and recent illicit drug use interact to affect verbal memory in women. J Acquir Immune Defic Syndr. 2013;63(1):67–76. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Opiate drug use and the pathophysiology of neuroAIDS. Current HIV Res. 2012;10(5):435–52. doi: 10.2174/157016212802138779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121( Pt 11):2043–52. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- 17.Hauser KF, El-Hage N, Buch S, et al. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005;8(1–2):63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrd D, Murray J, Safdieh G, Morgello S. Impact of opiate addiction on neuroinflammation in HIV. J Neurovirol. 2012;18(5):364–73. doi: 10.1007/s13365-012-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdo TH, Katner SN, Taffe MA, Fox HS. Neuroimmunity, drugs of abuse, and neuroAIDS. J Neuroimmune Pharmacol. 2006;1(1):41–9. doi: 10.1007/s11481-005-9001-3. [DOI] [PubMed] [Google Scholar]
- 20.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 21.Gill AJ, Kolson DL. Chronic inflammation and the role for cofactors (hepatitis C, drug abuse, antiretroviral drug toxicity, aging) in HAND persistence. Curr HIV/AIDS Rep. 2014;11(3):325–35. doi: 10.1007/s11904-014-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa A, Liu H, Ling B, et al. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114(14):2917–25. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T. Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Current HIV Res. 2014;12(2):97–110. doi: 10.2174/1570162x12666140526114956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer-Smith T, Croul S, Sverstiuk AE, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7(6):528–41. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 26.Kreek MJ, Borg L, Ducat E, Ray B. Pharmacotherapy in the treatment of addiction: methadone. J Addict Dis. 2010;29(2):200–16. doi: 10.1080/10550881003684798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wesson DR, Smith DE. Buprenorphine in the treatment of opiate dependence. J Psychoactive Drugs. 2010;42(2):161–75. doi: 10.1080/02791072.2010.10400689. [DOI] [PubMed] [Google Scholar]
- 28.Soyka M, Hock B, Kagerer S, Lehnert R, Limmer C, Kuefner H. Less impairment on one portion of a driving-relevant psychomotor battery in buprenorphine-maintained than in methadone-maintained patients: results of a randomized clinical trial. J Clin Psychopharmacol. 2005;25(5):490–3. doi: 10.1097/01.jcp.0000178417.60426.60. [DOI] [PubMed] [Google Scholar]
- 29.Rapeli P, Fabritius C, Alho H, Salaspuro M, Wahlbeck K, Kalska H. Methadone vs buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Clin Pharmacol. 2007;7:5. doi: 10.1186/1472-6904-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapeli P, Fabritius C, Kalska H, Alho H. Cognitive functioning in opioid-dependent patients treated with buprenorphine, methadone, and other psychoactive medications: stability and correlates. BMC Clin Pharmacol. 2011;11:13. doi: 10.1186/1472-6904-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Current HIV Res. 2014;12(2):85–96. doi: 10.2174/1570162x12666140526114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis LE, Hjelle BL, Miller VE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42(9):1736–9. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 33.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams K, Burdo TH. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J Neuroimmune Pharmacol. 2012;7(2):363–71. doi: 10.1007/s11481-011-9330-3. [DOI] [PubMed] [Google Scholar]
- 35.Koenig S, Gendelman HE, Orenstein JM, et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233(4768):1089–93. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 36.Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179(4):1623–9. doi: 10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci. 2011;31(26):9456–65. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nath A. Pathobiology of human immunodeficiency virus dementia. Semin Neurol. 1999;19(2):113–27. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- 39.Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med. 2012;2(6):a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapshak P, Kangueane P, Fujimura RK, et al. Editorial neuroAIDS review. AIDS. 2011;25(2):123–41. doi: 10.1097/QAD.0b013e328340fd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Persidsky Y, Ghorpade A, Rasmussen J, et al. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155(5):1599–611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. doi: 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60(3):234–43. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anthony IC, Arango JC, Stephens B, Simmonds P, Bell JE. The effects of illicit drugs on the HIV infected brain. Front Biosci. 2008;13:1294–307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- 45.Conant K, Garzino-Demo A, Nath A, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95(6):3117–21. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Mello C, Riazi K, Le T, et al. P-selectin-mediated monocyte-cerebral endothelium adhesive interactions link peripheral organ inflammation to sickness behaviors. J Neurosci. 2013;33(37):14878–88. doi: 10.1523/JNEUROSCI.1329-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luscinskas FW, Gerszten RE, Garcia-Zepeda EA, et al. C-C and C-X-C chemokines trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Ann N Y Acad Sci. 2000;902:288–93. doi: 10.1111/j.1749-6632.2000.tb06324.x. [DOI] [PubMed] [Google Scholar]
- 48.Cambien B, Pomeranz M, Millet MA, Rossi B, Schmid-Alliana A. Signal transduction involved in MCP-1-mediated monocytic transendothelial migration. Blood. 2001;97(2):359–66. doi: 10.1182/blood.v97.2.359. [DOI] [PubMed] [Google Scholar]
- 49.Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91(3):401–15. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Standifer KM, Pasternak GW. G proteins and opioid receptor-mediated signalling. Cell Signal. 1997;9(3–4):237–48. doi: 10.1016/s0898-6568(96)00174-x. [DOI] [PubMed] [Google Scholar]
- 51.Sibinga NE, Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol. 1988;6:219–49. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]
- 52.Chuang TK, Killam KF, Jr, Chuang LF, et al. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995;216(3):922–30. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- 53.Trombella S, Vergura R, Falzarano S, Guerrini R, Calo G, Spisani S. Nociceptin/orphanin FQ stimulates human monocyte chemotaxis via NOP receptor activation. Peptides. 2005;26(8):1497–502. doi: 10.1016/j.peptides.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Hosztafi S. Heroin, part III: the pharmacology of heroin. Acta Pharm Hung. 2003;73(3):197–205. [PubMed] [Google Scholar]
- 55.Roy S, Barke RA, Loh HH. MU-opioid receptor-knockout mice: role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res. 1998;61(1–2):190–4. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- 56.Gein SV, Baeva TA. Endogenous opioid peptides in regulation of innate immunity cell functions. Biochemistry (Mosc) 2011;76(3):309–19. doi: 10.1134/s0006297911030035. [DOI] [PubMed] [Google Scholar]
- 57.Carr DJ. The role of endogenous opioids and their receptors in the immune system. Proc Soc Exp Biol Med. 1991;198(2):710–20. doi: 10.3181/00379727-198-43309b. [DOI] [PubMed] [Google Scholar]
- 58.Stefano GB, Kream R. Endogenous opiates, opioids, and immune function: evolutionary brokerage of defensive behaviors. Semin Cancer Biol. 2008;18(3):190–8. doi: 10.1016/j.semcancer.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Pello OM, Duthey B, Garcia-Bernal D, et al. Opioids trigger alpha 5 beta 1 integrin-mediated monocyte adhesion. J Immunol. 2006;176(3):1675–85. doi: 10.4049/jimmunol.176.3.1675. [DOI] [PubMed] [Google Scholar]
- 60.Stefano GB, Salzet M, Bilfinger TV. Long-term exposure of human blood vessels to HIV gp120, morphine, and anandamide increases endothelial adhesion of monocytes: uncoupling of nitric oxide release. J Cardiovasc Pharmacol. 1998;31(6):862–8. doi: 10.1097/00005344-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 61.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258(1):299–303. [PubMed] [Google Scholar]
- 62.Perez-Castrillon JL, Perez-Arellano JL, Garcia-Palomo JD, Jimenez-Lopez A, De Castro S. Opioids depress in vitro human monocyte chemotaxis. Immunopharmacology. 1992;23(1):57–61. doi: 10.1016/0162-3109(92)90009-2. [DOI] [PubMed] [Google Scholar]
- 63.Stefano GB, Digenis A, Spector S, et al. Opiate-like substances in an invertebrate, an opiate receptor on invertebrate and human immunocytes, and a role in immunosuppression. Proc Natl Acad Sci USA. 1993;90(23):11099–103. doi: 10.1073/pnas.90.23.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ransohoff RM. The chemokine system in neuroinflammation: an update. J Infect Dis. 2002;186(Suppl 2):S152–6. doi: 10.1086/344266. [DOI] [PubMed] [Google Scholar]
- 65.Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Wang JM, Oppenheim JJ. Opiate inhibition of chemokine-induced chemotaxis. Ann NY Acad Sci. 1998;840:9–20. doi: 10.1111/j.1749-6632.1998.tb09544.x. [DOI] [PubMed] [Google Scholar]
- 66.Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15(5):590–7. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 67.Miyagi T, Chuang LF, Lam KM, et al. Opioids suppress chemokine-mediated migration of monkey neutrophils and monocytes - an instant response. Immunopharmacology. 2000;47(1):53–62. doi: 10.1016/s0162-3109(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 68.Bokhari SM, Hegde R, Callen S, et al. Morphine potentiates neuropathogenesis of SIV infection in rhesus macaques. J Neuroimmune Pharmacol. 2011;6(4):626–39. doi: 10.1007/s11481-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dutta R, Roy S. Chronic morphine and HIV-1 Tat promote differential central nervous system trafficking of CD3+ and Ly6C+ immune cells in a murine Streptococcus pneumoniae infection model. J Neuroinflammation. 2015;12(1):120. doi: 10.1186/s12974-015-0341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutta R, Roy S. Mechanism(s) involved in opioid drug abuse modulation of HAND. Current HIV Res. 2012;10(5):469–77. doi: 10.2174/157016212802138805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Epps DE, Saland L. Beta-endorphin and met-enkephalin stimulate human peripheral blood mononuclear cell chemotaxis. J Immunol. 1984;132(6):3046–53. [PubMed] [Google Scholar]
- 72.Ruff MR, Wahl SM, Mergenhagen S, Pert CB. Opiate receptor-mediated chemotaxis of human monocytes. Neuropeptides. 1985;5(4–6):363–6. doi: 10.1016/0143-4179(85)90029-0. [DOI] [PubMed] [Google Scholar]
- 73.Szabo I, Chen XH, Xin L, et al. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci USA. 2002;99(16):10276–81. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chao CC, Gekker G, Hu S, Sheng WS, Portoghese PS, Peterson PK. Upregulation of HIV-1 expression in cocultures of chronically infected promonocytes and human brain cells by dynorphin. Biochem Pharmacol. 1995;50(5):715–22. doi: 10.1016/0006-2952(95)00176-z. [DOI] [PubMed] [Google Scholar]
- 75.Przewlocki R, Hassan AH, Lason W, Epplen C, Herz A, Stein C. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience. 1992;48(2):491–500. doi: 10.1016/0306-4522(92)90509-z. [DOI] [PubMed] [Google Scholar]
- 76.Mousa SA, Shakibaei M, Sitte N, Schafer M, Stein C. Subcellular pathways of beta-endorphin synthesis, processing, and release from immunocytes in inflammatory pain. Endocrinology. 2004;145(3):1331–41. doi: 10.1210/en.2003-1287. [DOI] [PubMed] [Google Scholar]
- 77.Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24(3):116–21. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 78.Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483(2–3):175–86. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 79.Steele AD, Szabo I, Bednar F, Rogers TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev. 2002;13(3):209–22. doi: 10.1016/s1359-6101(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 80.Pello OM, Martinez-Munoz L, Parrillas V, Serrano A, Rodriguez-Frade JM, Toro MJ, et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol. 2008;38(2):537–49. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- 81.Rogers TJ, Steele AD, Howard OM, Oppenheim JJ. Bidirectional heterologous desensitization of opioid and chemokine receptors. Ann NY Acad Sci. 2000;917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [DOI] [PubMed] [Google Scholar]
- 82.Zhang N, Hodge D, Rogers TJ, Oppenheim JJ. Ca2+-independent protein kinase Cs mediate heterologous desensitization of leukocyte chemokine receptors by opioid receptors. J Biol Chem. 2003;278(15):12729–36. doi: 10.1074/jbc.M300430200. [DOI] [PubMed] [Google Scholar]
- 83.Melik PS, Rivat C, Rostene W, Reaux-Le Goazigo A. Opioid and chemokine receptor crosstalk: a promising target for pain therapy? Nat Rev Neurosci. 2015;16(2):69–78. doi: 10.1038/nrn3858. [DOI] [PubMed] [Google Scholar]
- 84.Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37(3):542–8. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010;7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Porcheray F, Samah B, Leone C, Dereuddre-Bosquet N, Gras G. Macrophage activation and human immunodeficiency virus infection: HIV replication directs macrophages towards a pro-inflammatory phenotype while previous activation modulates macrophage susceptibility to infection and viral production. Virology. 2006;349(1):112–20. doi: 10.1016/j.virol.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 87.Brown A. Understanding the MIND phenotype: macrophage/microglia inflammation in neurocognitive disorders related to human immunodeficiency virus infection. Clinical and translational medicine. 2015;4:7. doi: 10.1186/s40169-015-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR. Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis. J Neurovirol. 2011;17(4):291–302. doi: 10.1007/s13365-011-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Wang X, Ye L, et al. Morphine suppresses IFN signaling pathway and enhances AIDS virus infection. PLoS One. 2012;7(2):e31167. doi: 10.1371/journal.pone.0031167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reynolds JL, Law WC, Mahajan SD, et al. Morphine and galectin-1 modulate HIV-1 infection of human monocyte-derived macrophages. J Immunol. 2012;188(8):3757–65. doi: 10.4049/jimmunol.1102276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309(1):99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 92.Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med. 2002;50(6):435–42. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, Ye L, Zhou Y, Liu MQ, Zhou DJ, Ho WZ. Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol. 2011;178(1):41–7. doi: 10.1016/j.ajpath.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chao CC, Gekker G, Sheng WS, Hu S, Peterson PK. U50488 inhibits HIV-1 expression in acutely infected monocyte-derived macrophages. Drug Alcohol Depend. 2001;62(2):149–54. doi: 10.1016/s0376-8716(00)00185-x. [DOI] [PubMed] [Google Scholar]
- 95.Purohit V, Rapaka RS, Rutter J, Shurtleff D. Do opioids activate latent HIV-1 by down-regulating anti-HIV microRNAs? J Neuroimmune Pharmacol. 2012;7(3):519–23. doi: 10.1007/s11481-012-9356-1. [DOI] [PubMed] [Google Scholar]
- 96.Li Y, Wang X, Tian S, Guo CJ, Douglas SD, Ho WZ. Methadone enhances human immunodeficiency virus infection of human immune cells. J Infect Dis. 2002;185(1):118–22. doi: 10.1086/338011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herbein G, Gras G, Khan KA, Abbas W. Macrophage signaling in HIV-1 infection. Retrovirology. 2010;7:34. doi: 10.1186/1742-4690-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dave RS. Morphine affects HIV-induced inflammatory response without influencing viral replication in human monocyte-derived macrophages. FEMS Immunol Med Microbiol. 2012;64(2):228–36. doi: 10.1111/j.1574-695X.2011.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rappaport J, Volsky DJ. Role of the macrophage in HIV-associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. J Neurovirol. 2015;21(3):235–41. doi: 10.1007/s13365-015-0346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hidalgo M, Atluri VS, Nair M. Drugs of Abuse in HIV infection and neurotoxicity. Front Microbiol. 2015;6:217. doi: 10.3389/fmicb.2015.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Savage SR, Joranson DE, Covington EC, Schnoll SH, Heit HA, Gilson AM. Definitions related to the medical use of opioids: evolution towards universal agreement. J Pain Symptom Manage. 2003;26(1):655–67. doi: 10.1016/s0885-3924(03)00219-7. [DOI] [PubMed] [Google Scholar]
- 102.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657–9. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 103.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–45. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 104.Sung S, Conry JM. Role of buprenorphine in the management of heroin addiction. Ann Pharmacother. 2006;40(3):501–5. doi: 10.1345/aph.1G276. [DOI] [PubMed] [Google Scholar]
- 105.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. The Cochrane database of systematic reviews. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55(5):569–80. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 107.McCance-Katz EF, Moody DE, Morse GD, et al. Interactions between buprenorphine and antiretrovirals. I. The nonnucleoside reverse-transcriptase inhibitors efavirenz and delavirdine. Clin Infect Dis. 2006;43(Suppl 4):S224–34. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- 108.Soyka M, Limmer C, Lehnert R, et al. A comparison of cognitive function in patients under maintenance treatment with heroin, methadone, or buprenorphine and healthy controls: an open pilot study. Am J Drug Alcohol Abuse. 2011;37(6):497–508. doi: 10.3109/00952990.2011.600381. [DOI] [PubMed] [Google Scholar]
- 109.Pirastu R, Fais R, Messina M, et al. Impaired decision-making in opiate-dependent subjects: effect of pharmacological therapies. Drug Alcohol Depend. 2006;83(2):163–8. doi: 10.1016/j.drugalcdep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 110.Bach P, Vollstadt-Klein S, Frischknecht U, et al. Diminished brain functional magnetic resonance imaging activation in patients on opiate maintenance despite normal spatial working memory task performance. Clin Neuropharmacol. 2012;35(4):153–60. doi: 10.1097/WNF.0b013e31825c38f5. [DOI] [PubMed] [Google Scholar]
- 111.Soyka M, Lieb M, Kagerer S, et al. Cognitive functioning during methadone and buprenorphine treatment: results of a randomized clinical trial. J Clin Psychopharmacol. 2008;28(6):699–703. doi: 10.1097/JCP.0b013e31818a6d38. [DOI] [PubMed] [Google Scholar]
- 112.Loeber S, Kniest A, Diehl A, Mann K, Croissant B. Neuropsychological functioning of opiate-dependent patients: a nonrandomized comparison of patients preferring either buprenorphine or methadone maintenance treatment. Am J Drug Alcohol Abuse. 2008;34(5):584–93. doi: 10.1080/00952990802308239. [DOI] [PubMed] [Google Scholar]
- 113.Darke S, McDonald S, Kaye S, Torok M. Comparative patterns of cognitive performance amongst opioid maintenance patients, abstinent opioid users and non-opioid users. Drug Alcohol Depend. 2012;126(3):309–15. doi: 10.1016/j.drugalcdep.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 114.Rapeli P, Fabritius C, Kalska H, Alho H. Memory function in opioid-dependent patients treated with methadone or buprenorphine along with benzodiazepine: longitudinal change in comparison to healthy individuals. Substance abuse treatment, prevention, and policy. 2009;4:6. doi: 10.1186/1747-597X-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10(6):350–7. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 116.Carvallo L, Lopez L, Che FY, et al. Buprenorphine decreases the CCL2-mediated chemotactic response of monocytes. J Immunol. 2015;194(7):3246–58. doi: 10.4049/jimmunol.1302647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ayala JM, Goyal S, Liverton NJ, Claremon DA, O’Keefe SJ, Hanlon WA. Serum-induced monocyte differentiation and monocyte chemotaxis are regulated by the p38 MAP kinase signal transduction pathway. J Leukoc Biol. 2000;67(6):869–75. [PubMed] [Google Scholar]
- 118.Boland JW, Foulds GA, Ahmedzai SH, Pockley AG. A preliminary evaluation of the effects of opioids on innate and adaptive human in vitro immune function. BMJ supportive & palliative care. 2014;4(4):357–67. doi: 10.1136/bmjspcare-2013-000573. [DOI] [PubMed] [Google Scholar]
- 119.Williams DW, Calderon TM, Lopez L, et al. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One. 2013;8(7):e69270. doi: 10.1371/journal.pone.0069270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allen JB, Wong HL, Guyre PM, Simon GL, Wahl SM. Association of circulating receptor Fc gamma RIII-positive monocytes in AIDS patients with elevated levels of transforming growth factor-beta. J Clin Invest. 1991;87(5):1773–9. doi: 10.1172/JCI115196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178(10):6581–9. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 122.Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol. 2011;267(2):109–23. doi: 10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hish GA, Jr, Diaz JA, Hawley AE, Myers DD, Jr, Lester PA. Effects of analgesic use on inflammation and hematology in a murine model of venous thrombosis. Journal of the American Association for Laboratory Animal Science: JAALAS. 2014;53(5):485–93. [PMC free article] [PubMed] [Google Scholar]
- 124.Filipczak-Bryniarska I, Nowak B, Sikora E, et al. The influence of opioids on the humoral and cell-mediated immune responses in mice. The role of macrophages. Pharmacol Rep. 2012;64(5):1200–15. doi: 10.1016/s1734-1140(12)70916-7. [DOI] [PubMed] [Google Scholar]
- 125.Delgado-Velez M, Lugo-Chinchilla A, Lizardo L, et al. Chronic exposure of human macrophages in vitro to morphine and methadone induces a putative tolerant/dependent state. J Neuroimmunol. 2008;196(1–2):94–100. doi: 10.1016/j.jneuroim.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 126.Tubaro E, Avico U, Santiangeli C, et al. Morphine and methadone impact on human phagocytic physiology. Int J Immunopharmacol. 1985;7(6):865–74. doi: 10.1016/0192-0561(85)90049-9. [DOI] [PubMed] [Google Scholar]
- 127.Peterson PK, Gekker G, Brummitt C, et al. Suppression of human peripheral blood mononuclear cell function by methadone and morphine. J Infect Dis. 1989;159(3):480–7. doi: 10.1093/infdis/159.3.480. [DOI] [PubMed] [Google Scholar]
- 128.Van Loveren H, Gianotten N, Hendriksen CF, Schuurman HJ, Van der Laan JW. Assessment of immunotoxicity of buprenorphine. Lab Anim. 1994;28(4):355–63. doi: 10.1258/002367794780745119. [DOI] [PubMed] [Google Scholar]
- 129.Hall TJ, Jagher B, Schaeublin M, Wiesenberg I. The analgesic drug buprenorphine inhibits osteoclastic bone resorption in vitro, but is proinflammatory in rat adjuvant arthritis. Inflamm Res. 1996;45(6):299–302. doi: 10.1007/BF02280995. [DOI] [PubMed] [Google Scholar]
- 130.Piersma FE, Daemen MA, Bogaard AE, Buurman WA. Interference of pain control employing opioids in in vivo immunological experiments. Lab Anim. 1999;33(4):328–33. doi: 10.1258/002367799780487887. [DOI] [PubMed] [Google Scholar]
- 131.Carrigan KA, Saurer TB, Ijames SG, Lysle DT. Buprenorphine produces naltrexone reversible alterations of immune status. Int Immunopharmacol. 2004;4(3):419–28. doi: 10.1016/j.intimp.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 132.McLachlan C, Crofts N, Wodak A, Crowe S. The effects of methadone on immune function among injecting drug users: a review. Addiction. 1993;88(2):257–63. doi: 10.1111/j.1360-0443.1993.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 133.Gomez-Flores R, Weber RJ. Differential effects of buprenorphine and morphine on immune and neuroendocrine functions following acute administration in the rat mesencephalon periaqueductal gray. Immunopharmacology. 2000;48(2):145–56. doi: 10.1016/s0162-3109(00)00198-3. [DOI] [PubMed] [Google Scholar]
- 134.D’Elia M, Patenaude J, Hamelin C, Garrel DR, Bernier J. No detrimental effect from chronic exposure to buprenorphine on corticosteroid-binding globulin and corticosensitive immune parameters. Clin Immunol. 2003;109(2):179–87. doi: 10.1016/s1521-6616(03)00177-3. [DOI] [PubMed] [Google Scholar]
- 135.Martucci C, Panerai AE, Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain. 2004;110(1–2):385–92. doi: 10.1016/j.pain.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 136.Hugunin KM, Fry C, Shuster K, Nemzek JA. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock. 2010;34(3):250–60. doi: 10.1097/shk.0b013e3181cdc412. [DOI] [PubMed] [Google Scholar]
- 137.Zajicova A, Wilczek H, Holan V. The alterations of immunological reactivity in heroin addicts and their normalization in patients maintained on methadone. Folia Biol (Praha) 2004;50(1):24–8. doi: 10.14712/fb2004050010024. [DOI] [PubMed] [Google Scholar]
- 138.Sacerdote P, Franchi S, Gerra G, Leccese V, Panerai AE, Somaini L. Buprenorphine and methadone maintenance treatment of heroin addicts preserves immune function. Brain Behav Immun. 2008;22(4):606–13. doi: 10.1016/j.bbi.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 139.Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1(2):182–91. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- 140.Budd K. Pain management: is opioid immunosuppression a clinical problem? Biomed Pharmacother. 2006;60(7):310–7. doi: 10.1016/j.biopha.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 141.Borner C, Lanciotti S, Koch T, Hollt V, Kraus J. mu opioid receptor agonist-selective regulation of interleukin-4 in T lymphocytes. J Neuroimmunol. 2013;263(1–2):35–42. doi: 10.1016/j.jneuroim.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 142.Riss GL, Chang DI, Wevers C, et al. Opioid maintenance therapy restores CD4+ T cell function by normalizing CD4+CD25(high) regulatory T cell frequencies in heroin user. Brain Behav Immun. 2012;26(6):972–8. doi: 10.1016/j.bbi.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 143.Amirshahrokhi K, Dehpour AR, Hadjati J, Sotoudeh M, Ghazi-Khansari M. Methadone ameliorates multiple-low-dose streptozotocin-induced type 1 diabetes in mice. Toxicol Appl Pharmacol. 2008;232(1):119–24. doi: 10.1016/j.taap.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 144.Kafami L, Etesami I, Felfeli M, et al. Methadone diminishes neuroinflammation and disease severity in EAE through modulating T cell function. J Neuroimmunol. 2013;255(1–2):39–44. doi: 10.1016/j.jneuroim.2012.10.015. [DOI] [PubMed] [Google Scholar]