Abstract
In the U.S., colorectal cancer (CRC) incidence and mortality have declined due to screening and improvements in early detection; however, racial/ethnic disparities in screening and mortality persist. Patient navigation has been shown to be effective in increasing CRC screening prevalence. This systematic review answered three questions about navigation in federally qualified community health centers (FQHCs): 1) Which navigation activities increased CRC screening prevalence? 2) What were the challenges to implementing these programs in FQHCs? 3) Which clinic protocols supported screening completion? Findings suggest that navigation services must be tailored to the specific screening test provided. Federally qualified community health centers report difficulty maintaining a current electronic medical records system and sustaining funding; they should establish excellent patient tracking systems (for follow-up and annual rescreening) and establish multiple protocols to facilitate screening completion. With the movement toward patient-centered care models, patient navigation will be integral to FQHCs and their clients.
Keywords: Patient navigation, early detection, community health centers, health care disparities, minority health, patient-centered care
Colorectal cancer (CRC) is the third-most commonly diagnosed cancer among men and women in the U.S., with an estimated 95,270 new cases of colon and 39,220 cases of rectum cancer in 2016. Colorectal cancer is the second-leading cause of cancer deaths among both men and women in the U.S., with an estimated 49,190 deaths due to colorectal cancer in 2016.1 In the U.S., the economic burden of colorectal cancer has been estimated between $5.3 and $6.5 billion.2 A recent study analyzing the linked Surveillance Epidemiology and End Results (SEER)-Medicare database estimated that the total CRC-related health care cost for insurers and patients was $28,626 more for Medicare recipients with CRC compared with similarly aged non-cancer patients.3 Increased efforts to improve screening and early detection may help reduce the personal and economic burden of CRC in the U.S.
Nationally, both CRC incidence and mortality have been on the decline due to effective screening and improvements in early detection.4 However, disparities in CRC screening and mortality remain among racial and ethnic minorities, despite these advances in screening. Among non-Hispanic White Americans, screening prevalence is 61.5% compared with only 55.5% among Black Americans. This disparity in CRC screening is seen across other racial/ethnic minorities, with prevalence at 48.1% among American Indian/Alaskan Natives, 47% among Hispanic/Latinos, and 45.9% among Asians.4 Looking more closely at the Asian Americans and Pacific Islanders, CRC screening is the lowest among Korean Americans (32.7%) followed by Filipino Americans (41.7%), Pacific Islanders (42.2%), and South Asians (42.3%).5
Several systematic literature reviews already have identified successful approaches to improve CRC screening prevalence. Currently, screening interventions have been targeted at the patient, provider, health system, or combination of the three.6–8 For patient-level interventions, one-on-one education, client screening reminders, and reducing structural barriers have been effective in increasing CRC screening.6 One-on-one education was described as interventions provided either in person or over the phone by a health care provider that educates on CRC, emphasizes the importance of screening, and encourages clients to get screened. Another systematic review found that these one-on-one interactions improved CRC screening by 15 to 42 percentage points.7
Another patient-level intervention shown to improve CRC screening prevalence focused on improving access through the elimination of structural barriers. The Community Preventive Task Force defines structural barriers as “non-economic burdens or obstacles” that prevent people from accessing screenings.6 Examples of efforts to reduce structural barriers included decreasing the time or distance between patients and the screening method. For CRC screening, these interventions focused on directly mailing fecal occult blood test (FOBT) kits to patients. This direct mailing of FOBT kits was shown to be effective in increasing CRC screening prevalence by 15 to 42 percentage points.6,7
Client reminders have been shown to be another successful patient-level intervention. Client reminders include letters, emails, postcards, or telephone calls that alert clients that they were due or overdue for recommended CRC screening. These interventions reported an increase of CRC screening by 5 to 15 percentage points.7 Another systematic review observed an 11.5 percentage point increase in guaiac-based FOBT screening through use of client reminders.8
Provider-level interventions also have been shown to be modestly effective in increasing CRC screening prevalence. The literature describes three types of interventions that have been effective in increasing CRC screening: chart audits and feedback, electronic provider reminders, and training on communicating with low health literate patients. Chart audits and feedback systems are mechanisms that inform providers of their performance in providing screening services. Sabatino et al.6 observed that these interventions increased screening by 12.3 and 23.0 percentage points. Electronic reminders prompt providers to recommend screening to eligible patients through flags on their medical record. Holden et al.7 observed a modest increase of 5 percentage points in CRC screening through use of provider-level interventions. Among racial and ethnic minorities, interventions that trained providers to communicate with low health literate patients were effective at increasing CRC screening by 10 to 15%.9
A promising system-level intervention discussed in the literature was the provision of a patient navigator to help patients access screening services. Patient navigators are specialized health care workers who identify and anticipate patient barriers and help patients overcome barriers to quality cancer screening, diagnosis, and treatment.10 These interventions have been shown to increase screening prevalence between 7 and 28 percentage points.7 Naylor et al.9 found that navigated minority group members improved screening between 10 and 15 percentage points. Authors also discovered that when navigator had repeated or intense contacts with patients and when navigation was combined with other patient-level interventions, CRC screening prevalence could increase by 15 percentage points compared with one-on-one education alone.9
Although literature reviews have demonstrated the effectiveness of patient navigation in increasing CRC screening, there is limited information on which specific activities provided by patient navigators lead to increases in CRC screening. Further, the literature does not describe which factors must be in place for navigation to be implemented successfully in federally qualified community health centerss (FQHCs), a health care venue that serves low-income individuals, many of whom are racial or ethnic minorities. There also has been limited research on the role of screening navigation in FQHCs. Sarfaty et al.11 asserts that FQHCs are uniquely situated to address cancer health disparities by reaching a large number of minority clients who may be in need of CRC screening.
The purpose of this systematic literature review was to investigate the role of the navigator in CRC screening among minority populations and explore the key navigation activities that make up an effective screening navigation program. This review also sought to identify the barriers and facilitators to implementing CRC screening navigation FQHCs. Three specific research questions were addressed:
Which navigation activities increased CRC screening prevalence?
What were the challenges to implementing these programs in FQHCs?
Which clinic protocols supported screening completion?
These findings will help inform public health programming for CRC screening navigation in FQHCs serving minority populations.
Methods
Search strategy
Between September 2014 and October 2014, PubMed MEDLINE, CINAHL, and PsychINFO were searched for peer-reviewed studies and dissertations conducted in the U.S. between 2005 and 2014 using combinations of the following key search terms: colorectal cancer, early detection and screening, patient navigat*, community health centers, fecal occult blood test, and colonoscopy. A manual review of reference listings of relevant articles also was conducted to capture additional studies that did not appear in previous database searches. This review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12
Patient navigation was the primary intervention of interest. Navigation was defined as a clinic-based service in which trained professionals or paraprofessionals provided individualized assistance to a patient to help him/her obtain CRC screening. Articles were included if they tracked and reported CRC screening completion among navigated and non-navigated federally qualified health center (FQHC) clients through experimental and quasi-experimental study designs. Colorectal cancer (CRC) screening outcomes needed to be evidenced by completion of a fecal occult blood test (FOBT), flexible sigmoidoscopy, or a colonoscopy.
Using the definition of navigation by Dr. Harold P. Freeman, we excluded articles if the intervention focused solely on education without an assessment of a patient’s barriers to CRC screening.10 This exclusion is adopted because barriers assessment is a key component of cancer patient navigation. To understand the impact of navigation activities on CRC screening, we excluded articles if they were descriptive or qualitative studies and did not provide any screening outcome data. Further, we excluded articles if the majority (more than 50%) of the study population were non-Hispanic Whites. Studies were excluded if they did not take place in FQHC settings. Further, articles were excluded if they were not written in English and/or focused on diagnostic or treatment interventions.
After duplicate citations were removed, the titles and abstracts were reviewed, and obviously irrelevant articles were excluded. The remaining articles then were read in full, and assessed against the inclusion and exclusion criteria. Citations were managed in EndNote X7.13 Once relevant articles were identified, a data abstraction form was used to collect study characteristics and pertinent data points to answer the research questions.
Quality assessment
The quality of each study was assessed using a modified form of The Community Preventive Task Force’s assessment tool. Each study was scored based on 9 domains, including intervention description, sampling frame, eligibility criteria, population sampling, intervention exposure, valid and reliable outcome measures, appropriate statistical analysis, participant completion, and controlling for confounders. Scores ranged from 0–9 based on the number of criteria met and were rated as good (8–9 criteria met), fair (5–7 met), or limited (<5 met).14
Results
Article selection
A total of 620 articles were identified through a combined search of three databases (Figure 1). Duplicates (n = 160) and non-relevant articles based on title and abstract (n = 279) were removed. Of the 184 remaining, 84 did not describe a navigation intervention, 18 were not focused on increasing CRC screening, four were conducted outside the U.S., and four were systematic literature reviews. The remaining 74 were read in full, and three additional articles were found through manual review of reference lists. Upon application of inclusion and exclusion criteria, 63 more were excluded: 19 studies did not meet the definition of navigation; 12 were descriptive, 11 did not report screening outcomes, two were not focused on CRC screening, eight did not target minorities, 10 were not located in a FQHC, and three reported on the same intervention. As a result, eight articles were selected for data abstraction and assessed for quality.
Figure 1.
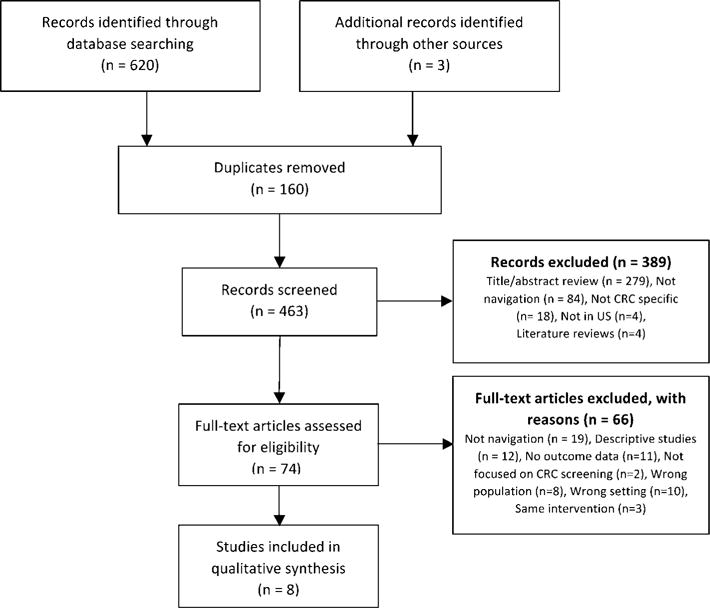
PRISMA Flow Chart.
Study characteristics and outcomes
Table 1 details the intervention setting and type, study design, ethnic breakdown of population, sample size, comparison groups, and CRC screening outcomes of the studies included in this review. Six of the eight articles described randomized control trials, and two described quasi-experimental studies. Five of the published studies were conducted in FQHCs located on the East coast, two in the Midwest, and one in the West coast. Four of the articles included a majority of African Americans, and the other four targeted Latino/Hispanic populations. No studies were found with Asian American, Native American, or Pacific Islander groups.
Table 1.
STUDY CHARACTERISTICS AND OUTCOMES
| Reference | Study Location | Study Design | Ethnicity Majority | Intervention Type | Screening Modality | Sample Size | Intervention Outcome | Control Outcome | Percentage Point Difference in Posttest Screening Prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Baker et al.20 | Erie Family Health Center, Chicago, IL | RCT | 89.3% Latino | PN vs. Usual Care (chart prompts, standing orders, clinic feedback) | FOBT | N = 450 | 84.0% (191) | 40.0% (90) | 44% |
| Christie et al.15 | Community Health Center in Seattle, WA | RCT | 71% Latino | PN vs. non-PN (MD referral + GI scheduler follow-up) | CS | N = 21 | 53.8% (7) | 13% (1) | 40.8% (ns) |
| Dietrich et al.14 | Community Health Center in New York City, NY | RCT | 62.8% Latino | PN vs. Usual Care (CRC Edu brochure + follow-up phone call) | FOBT, FS, CS | N = 1413 | 63% (438) | 50% (347) | 13% |
| Katz et al.19 | Community Health Center in Columbus, OH | RCT | 72.2% African American | PN vs. CRC Education only | FOBT | N = 270 | 19.6% (27) | 9.9% (13) | 9.7% |
| Lasser et al.16 | Community Health Centers in Cambridge, MA | RCT | 39.7% African American | PN vs. Usual Care | FOBT or CS | N = 465 | 33.6% (79) | 20% (46) | 13.6% |
| Percac-Lima et al.17 | Community Health Center in MA | RCT | 40.1% Latino | PN vs. Usual Care | CS | N = 1223 | 27.4% (112) | 11.9% (97) | 15.5% |
| Honeycutt et al.21 | Community Health Centers in Southwest, GA | Quasi-experimental | 62.9% African American | PN Program vs. No CRC Screening Program | CS | N = 809 | 42.6% (123) | 10.8% (56) | 31.8% |
| Davis et al.18 | Community Health Centers in North Louisiana | Quasi-experimental | 67% African American | PN vs. CRC Education | FOBT | N = 961 | 60.6% (245) | 38.6% (106) | 22.0% |
RCT = Randomized Control Trial; PN = Patient Navigation; FOBT = Fecal Occult Blood Test; FS = Flexible Sigmoidoscopy; CS = Colonoscopy
All eight articles varied in navigation interventions and comparison groups. Four of the eight articles compared a patient navigation intervention with varying definitions of usual care. The authors of Dietrich et al.15 described usual care as the provision of a CRC screening brochure and a follow-up phone call. Christie et al.16 defined usual care as a physician referral to colonoscopy plus follow-up call from a gastrointestinal clinic scheduler. Lasser et al.17 and Percac-Lima et al.18 did not detail what constituted usual care at their study settings. The remaining four studies looked at comparing patient navigation with CRC education19,20 and established clinic protocols alone.21 Honeycutt et al.22 compared health centers with a patient navigation program to health centers without a program. One study16 had a sample of 21, another20 had a sample of 207, but the rest had larger samples, from 450–1,413 participants.
Navigation was used to promote CRC screening by different modalities. Three interventions promoted screening by colonoscopy,16,18,22 and three focused on increasing screening by FOBT.19–21 One intervention17 promoted screening by either FOBT or screening colonoscopy. The remaining intervention15 promoted any recommended form of CRC screening (colonoscopy, flexible sigmoidoscopy, or FOBT).
Despite the variations in interventions and methods of screening promotion, all eight articles concluded that patient navigation was effective in improving CRC screening among minority populations served by FQHCs. All but one article observed a statistically significant difference between groups receiving patient navigation and usual care when measured post-intervention; difference in screening prevalence ranged from 9.7% to 44%. The one article that did not show significant differences between intervention and control still reported an increase in screening prevalence from baseline.16 In fact, there was a 40.8% difference between the navigated and non-navigated groups in post-intervention prevalence of CRC screening, but the sample size (n = 21) was too small to yield statistical significance.
Study quality assessment
Table 2 displays the results of each study’s quality assessment score. Quality assessment scores ranged from five to nine, with a median score of eight. Six of the eight studies rated as “good”, with three meeting all nine criteria. The remaining two of the eight studies were rated as fair, with one scoring a seven, and one scoring a five. All articles sampled well, explicitly stated specified screening criteria for the study sample, and utilized valid and reliable exposure and outcome variables. Six of the eight studies described the intervention with enough detail to identify specific navigation tasks. Six of the eight studies retained more than 80% of enrolled participants at the end of the study.
Table 2.
STUDY QUALITY ASSESSMENT
| Reference | Intervention well described? | Sample well? Specify Sampling frame? | Screening criteria for study eligibility specified? | Entire eligible population or probability sample? | Measure exposure to intervention? | Exposure & outcome valid and reliable? | Conduct appropriate analysis by stats testing, controlling? | >80% of enrolled participants complete study? | Correct for controllable confounders? | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Davis et al.18 | X | X | X | X | X | X | X | X | X | 9 |
| Katz et al.19 | X | X | X | X | X | X | X | X | X | 9 |
| Baker et al.20 | X | X | X | X | X | X | X | X | X | 9 |
| Dietrich et al.14 | X | X | X | X | X | X | X | X | 8 | |
| Lasser et al.16 | X | X | X | X | X | X | X | X | 8 | |
| Percac-Lima et al.17 | X | X | X | X | X | X | X | X | 8 | |
| Honeycutt et al.21 | X | X | X | X | X | X | 7 | |||
| Christie et al.15 | X | X | X | X | X | 5 | ||||
| Total | 6 (75.0%) | 7 (87.5%) | 8 (100%) | 6 (75.0%) | 7 (87.5%) | 8 (100%) | 7 (87.5%) | 6 (75.0%) | 7 (87.5%) |
The results of the literature review are presented to address the three research questions.
Which navigation activities increased colorectal cancer screening prevalence?
The findings suggest that navigation activities fell into eight categories. Table 3 details the navigation activities reported in each article and the observed difference in CRC screening prevalence between intervention and control groups at posttest. By inclusion criteria, all eight interventions assessed patient barriers to CRC screening. In addition to this, common navigation activities were providing CRC screening education, reminding patients they were due for screening, motivating and supporting patients, scheduling appointments, providing translation assistance, arranging transportation, counseling patients to overcome barriers, and teaching patients how to prepare for and complete the screening test.
Table 3.
NAVIGATION ACTIVITIES AND OBSERVED DIFFERENCE IN CRC SCREENING OUTCOMES
| Reference | CRC Screening Test | CRC screening education | Client reminders | Motivating/Supporting Patients | Scheduling appointments | Translation assistance | Arranging transport | Ongoing barriers counseling | Teaching test instructions | Percentage point difference between groups at posttest |
|---|---|---|---|---|---|---|---|---|---|---|
| Christie et al.15 | CS | X | X | X | X | X | X | 40.80%a | ||
| Honeycutt et al.21 | CS | X | X | X | X | X | 31.80% | |||
| Percac-Lima et al.17 | CS | X | X | X | X | X | X | 15.50% | ||
| Baker et al.20 | FOBT | X | X | X | X | X | X | 44.00% | ||
| Davis et al.18 | FOBT | X | X | X | X | X | 22.00% | |||
| Katz et al.19 | FOBT | X | X | X | X | 9.70% | ||||
| Lasser et al.16 | FOBT or CS | X | X | X | X | X | X | X | 13.60% | |
| Dietrich et al.14 | FOBT, FS, CS | X | X | X | X | X | X | X | 13.00% | |
| Total | 8 (100%) | 8 (100%) | 6 (75.0%) | 5 (62.5%) | 5 (62.5%) | 4 (50.0%) | 6 (75.0%) | 4 (50.0%) |
not statistically significant
CRC = colorectal cancer; FOBT = Fecal Occult Blood Test; CS = Colonoscopy
Regardless of the screening test (e.g., FOBT, colonoscopy) to which patients were navigated, navigators provided education regarding CRC screening and procedures and reminded clients that they were due for screening by phone or letter. In all but two studies, navigators motivated and supported clients to receive their CRC screening. In studies targeted at limited English speaking population, navigators assisted with translation.15–18,21
For the three interventions that promoted colonoscopies, scheduling appointments and arranging patient transportation were key activities provided by the navigator.16,18,22 For the three studies promoting FOBTs only, findings suggest that the effect on CRC screening prevalence was enhanced if navigators provided ongoing counseling to overcome screening barriers and taught patients how to complete the FOBT.19–21
For example, both Davis et al.19 and Katz et al.20 promoted CRC screening among a predominantly African American group and compared a navigation intervention with those who received CRC education only. In both studies, navigators provided CRC screening education, reminded clients, offered motivation and support, and counseled patients to overcome barriers. However, in the study by Davis et al.,19 navigators also taught patients how to complete the FOBT, leading to an observed 22% difference in screening prevalence between the intervention and control groups at posttest. In contrast, in the article by Katz et al.,20 navigators did not teach patients to complete the FOBT, and the researchers only detected a 9.7% difference in CRC screening completion between the navigated group and the control group at posttest.
Only three of the eight studies measured the frequency of navigator contact and the time spent on each patient’s case.15,17,18 However, all three studies differed in how they reported these measures. Percac-Lima et al.18 reported that the navigator contacted the patients an average of 3.1 times. In the article by Dietrich et al.,15 authors reported that the mean number of navigator contacts was four, ranging from one to 20 contacts per patient. Only two of these studies measured the time the navigator spent per patient. Lasser et al.17 reported that navigators recorded an average of 107 minutes per patient, ranging from four to 335 minutes. The study by Dietrich et al.15 only timed a portion of navigators’ calls, reporting the initial call averaging 17 minutes per patient with each subsequent phone call averaging 14 minutes per patient. However, Dietrich et al.15 did not report the total time spent with patients.
What were the challenges to implementing these programs in FQHCs?
All eight articles suggest that challenges lie in maintaining an updated electronic medical records system and sustaining funding to support a navigator position.15–22
Seven studies suggested that an electronic medical records system with updated records was an important factor in increasing CRC screening rates. Three of the seven studies reported a relatively stable patient population, so patient contact information did not change frequently. These authors recommended that community health centers establish an up-to-date and reliable electronic medical records system.15,21,22 Baker et al.21 recommended that community health centers work closely with their information technology departments to establish a system to maintain current patient information. Dietrich et al.15 echoed this recommendation, stating that a systematic approach to update and maintain patient information is necessary. The other four studies reported difficulties in maintaining updated contact information in their electronic medical records systems, resulting in being unable to make or maintain contact with a significant number of patients.16–18,20 Katz et al.20 reported that the navigator was unable to reach 31.4% of the patients because of incorrect contact information.
Four out of the eight studies suggested that continued funding is needed to sustain CRC navigation interventions.16,17,21,22 Honeycutt et al.22 and Christie et al.16 cited sustainability concerns because of the costs associated with hiring a navigator and subsidizing costs for colonoscopy preparation and transportation. Lasser et al.17 and Baker et al.21 also noted these challenges, but suggested expanding the scope of the navigator to justify the investment. Lasser et al.17 recommended that navigator assist with screening for multiple cancers and detail those responsibilities in the navigator’s job description. Baker et al.21 suggested that the navigator’s role should start in outreach to screening and follow the patient through diagnostic resolution.
Only two of the eight studies assessed cost-effectiveness. Both studies focused on promoting screening by FOBT and varied in how they measured cost-effectiveness.19,21 Baker et al.21 found that the intervention cost $43.13 per completed FOBT test. In the study by Davis et al.,19 nurses took on patient navigator tasks and the cost was $1,337 per person screened. The authors also noted that intervention costs could be reduced to $389 per person screened if less expensive staff were assigned navigator duties. However, all eight studies recognized the need for more research on assessing the cost-effectiveness of patient navigation.
Two studies reported additional system challenges related to ordering and completing the screening tests.16,20 In the study by Christie et al.,16 navigators reported challenges in lost referral paperwork to gastroenterology clinics. Further, authors noted that the time between referral and the colonoscopy could have been attributed to the high number of patients lost to follow-up. Katz et al.20 also noted that only 30.6% of eligible patients in their study reported receiving a CRC screening recommendation from their provider.
Which clinic protocols supported CRC screening completion?
Seven of the eight articles suggested that six clinic protocols facilitated CRC screening completion (Table 4). These protocols included following up with high risk and diagnostic patients, establishing standing orders for clinic staff, tracking referrals and follow-up appointments, tracking test results, facilitating provider feedback, and establishing quality improvement mechanisms.
Table 4.
CLINIC PROTOCOLS TO SUPPORT CRC SCREENING COMPLETION
| Reference | Tracking referrals & follow-up appointments | Follow-up for high risk & diagnostic patients | Tracking results | Standing orders | Provider feedback | Quality Improvement | Protocols in place | % change observed |
|---|---|---|---|---|---|---|---|---|
| Baker et al.20 | X | X | X | X | X | X | 6 | 44.00% |
| Honeycutt et al.21 | X | X | X | X | X | 5 | 31.80% | |
| Davis et al.18 | X | X | X | X | 4 | 22.00% | ||
| Lasser et al.16 | X | X | X | 3 | 13.60% | |||
| Percac-Lima et al.17 | X | X | 2 | 15.50% | ||||
| Christie et al.15 | X | 1 | 40.80%a | |||||
| Dietrich et al.14 | X | 1 | 13.00% | |||||
| Katz et al.19 | 0 | 9.70% | ||||||
| Total | 6 (75.0%) | 4 (50.0%) | 4 (50.0%) | 3 (37.5%) | 3 (37.5%) | 2 (33.3%) |
Not significant.
All but two of the interventions reported having a tracking system in place to monitor referrals and follow-up appointments for patients.18,20 Half of the studies described an established follow-up clinic protocol for high risk patients or those in need of diagnostic tests.18,19,21,22 Only three had standing orders for CRC screening.17,19,21 Standing orders allowed any member of the health care team to provide overdue patients with an FOBT kit or schedule them for a screening colonoscopy. Four studies kept track of client’s results using an external database, log-sheet, or electronic medical records.18,19,21,22 Additionally, three studies provided feedback to clinicians on their performance in recommending CRC screening.17,21,22 Two studies established a quality improvement system that audited medical records and provided regular feedback to clinic administration.21,22
Findings suggest that the more screening policies and tracking mechanisms established, the greater the increase in CRC screening prevalence following the intervention, as shown in Table 4. Of the eight studies, Baker et al.21 was the only study that established all six systems, and this study yielded that greatest difference at posttest in CRC screening prevalence between the intervention and comparison groups. Katz et al.20 did not report the establishment of any clinic protocols, and this study yielded the lowest difference in posttest screening prevalence between intervention and control groups. These findings suggest that the number of clinic protocols impacts CRC screening completion in these populations.
In addition to establishing clinic protocols, five of the eight interventions were designed to reduce system barriers to CRC screening completion. These interventions subsidized costs for transportation, provided direct access to colorectal cancer screening tests, and adjusted the navigator’s work schedule to accommodate patient’s work schedules. Three of these five interventions also established partnerships with gastroenterology (GI) clinics in their communities.17,18,22 In these three studies, the difference in screening prevalence at post-intervention ranged from 13.6% to 31.8%. Honeycutt et al.22 noted that the partnership with GI facilities enabled them to provide reduced-cost colonoscopies and cover the cost of colonoscopy preparation and transportation fees. Percac-Lima et al.18 also worked with the gastroenterology clinic to provide free screening colonoscopies. Additionally, researchers established a point of contact at the gastroenterology clinic dedicated to working with the navigators to schedule appointments for study participants. Lasser et al.17 also worked closely with gastroenterology clinic nurses to schedule colonoscopies. The navigators also were instrumental in communicating a patient’s CRC screening preference to providers. Once the navigator identified the patient’s preference, providers were alerted immediately via the electronic medical record, which allowed them to place orders for the preferred screening modality. Further, this study increased access to CRC screening by reducing structural barriers through extended clinic hours for the navigator to nights and weekends, allowing more flexibility for patients to contact the navigator.
Discussion
This systematic literature review identified eight studies that tested the impact of CRC screening navigation among minorities served by FQHCs in the U.S. All of the articles included a majority of African Americans or targeted Latino/Hispanic populations. No studies were found with Asian American, Native American, or Pacific Islander groups. Further, the majority of published studies were conducted in FQHCs in the Midwest or East coast. Of the eight articles included in this review, the majority were good quality studies, with only two rated as fair.
Consistent with the Naylor et al.9 systematic review, findings confirm that patient navigation is an effective intervention to increase CRC screening prevalence among minority populations. The literature suggests that navigation may increase CRC screening prevalence between 9.7% and 44% in FQHCs. Adding to results of Naylor et al.,9 this review explored the role of navigators working with minority populations served by FQHCs. Further, this review explored the challenges to implementing navigator programs at FQHCs and the clinic protocols necessary to support a patient navigation program.
The findings suggest three navigation activities are essential to navigation programs. Following assessment of their CRC screening barriers, navigators educated clients regarding CRC screening procedures, reminded them that they were due for screening, and offered motivation and support. These activities remained constant regardless of the screening test to which patients were navigated. Escoffery et al.23 noted similar findings when surveying grantees of the Centers for Disease Control and Prevention’s Colorectal Cancer Control Program. Authors reported the most common navigation activities included assessing patient barriers to screening, providing education on CRC screening tests, and scheduling screening appointments. Natale-Perreira et al.24 confirmed that motivating and supporting minority clients are key functions of a navigator. Authors asserted that navigation plays a crucial role in mediating language and cultural barriers by fostering trust and empowering ethnic minorities to access cancer services.
Findings also suggest that navigation services must be tailored to specific CRC screening modalities. For example, navigators assisting patients with screening colonoscopies scheduled appointments for patients and arranged transportation. Escoffery et al.23 noted similar findings and reported that navigators also helped patients obtain colonoscopy preparation materials. For studies that promoted screening by FOBT, the combination of ongoing barriers counseling plus test instruction impacted FOBT screening completion (9.7% without instruction vs. 22% with instruction).
Findings from this review also suggest that clinics with established and operational screening protocols observed a greater impact of navigation on CRC screening prevalence. This further suggests a moderating effect of clinic protocols on navigation and screening prevalence. Roetzheim et al.25 examined the impact of the addition of cancer screening protocols on screening prevalence in primary care clinics serving disadvantaged populations in Florida. Authors concluded that a combination of checklists and chart prompts and the distribution of screening promotion tasks to a variety of clinic staff were effective in increasing screening compliance among underserved groups. These protocol changes resulted in a 2.5 greater odds of completing an FOBT. In an investigation of CRC protocol and screening prevalence at 49 FQHCs in the Midwest, Daly et al.26 similarly found a significant correlation between the number of protocols employed and the percentage of patients that were screening compliant. In addition to protocol changes, DeGroff et al.27 proposed 10 key features to establish a successful CRC screening navigation program: 1) define the patient population and its unique barriers, 2) apply a theoretical framework, 3) establish entry and exit points for navigation services, 4) determine a location where navigation services will be performed, 5) provide communication training, 6) identify the navigation services offered and navigator responsibilities, 7) determine qualifications for the navigator, 8) design navigator training, 9) identify navigation supervision, and 10) evaluate navigation services.
The reviewed studies suggested that challenges to implementing and sustaining a patient navigator program within federally qualified health centers (FQHCs) lie in maintaining an updated electronic medical records system and sustaining funding to support a navigator position. These findings are consistent with the literature. Fiscella et al.28 stated that, despite the number of evidence-based interventions, facilities serving low-income and minority patients oft en are limited in funding to support and sustain these types of interventions. FQHCs may be more inclined to invest in screening navigation if the navigator assists with multiple cancers, outreaches to patients to get them to screening, and follows them through to diagnostic resolution. Fiscella et al.28 extended this recommendation, suggesting that primary care practices engage in intensive patient outreach, focus on patient-centered communication, and integrate navigation into the patient-centered medical home model. Further, Sarfaty et al.11 suggested that FQHC need to develop a CRC screening program that applies CRC screening modalities that fit their needs of their target population and “the realities of delivering the tests available on a programmatic scale” [p. 223]. Authors also stressed the importance of improving communication with other health care facilities and coordinating strategies with ongoing local initiatives. Smith and Brawley29 advocated for an organized program for all cancer screenings recommended by the U.S. Preventive Services Task Force, proposing the development of a program like CDC’s National Breast and Cervical Cancer Early Detection Program that would include all cancer screenings. Doing so would centralize and streamline invitations to cancer screening, reducing the onus of developing and maintaining tracking systems at each specific primary care setting.
The Affordable Care Act has created a unique opportunity to integrate screening navigation into FQHCs. By design, screening navigation complements many of the national quality initiatives currently underway. For example, the National Committee for Quality Assurance (NCQA) puts forth standards and criteria necessary for facilities to receive the patient-centered medical home designation. National Committee for Quality Assurance (NCQA) standards include enhanced access and continuity of care, integrating a population management system for patients, managing patient care, supporting self-care support and access to community resources, tracking and coordinating care, and assuring ongoing quality improvement. Sarfaty et al.30 proposed that screening navigation would help to meet the standards related to self-care and access to resources. Further, the Centers for Medicare and Medicaid Services (CMS) has amended their regulations to provide reimbursement for preventive services recommended by health care providers and non-licensed providers (such as patient navigators).31
As with all systematic literature reviews, this review has limitations. Publication bias is possible, as studies with negative outcomes usually do not move forward to publication. Although three databases were searched, there may be other sources of relevant literature. The inclusion criteria were narrow, excluding descriptive or qualitative studies. Inclusion of these studies may have discovered other key features and challenges to implementing navigation programs. However, the barriers described in each of the included studies may have highlighted the most salient barriers. Further, 19 articles were excluded because the patient navigation model being tested did not appear to include the assessment of CRC screening barriers; following Harold Freeman, the father of cancer patient navigation, we note that barriers assessment is a key tenet of navigation.10 This underscores the need to create and disseminate standards in cancer patient navigation.
The services offered to the navigated and the comparison groups also varied across studies. Therefore, it would be difficult to compare effect sizes and draw definitive conclusions. The diversity in comparison groups and study populations made it difficult to pinpoint the specific navigation activities that led to increases in CRC screening. Additionally, only two major racial/ethnic groups were represented in this review, so the findings may not be relevant with other ethnic groups who experience cancer health disparities. Further, only three of the eight studies measured the frequency of navigator contact and the time spent on each patient’s case.15,17,18 Therefore, it was difficult to determine the minimum “dosage” of navigation needed to affect CRC screening outcomes.
Based on these findings, future interventions focused on improving FOBT completion should definitely task navigators to provide ongoing barriers counseling and instructions to complete the test. Future colorectal cancer screening programs should include a system to maintain updated contact information for patient population, especially in FQHCs where patient contact information may change frequently. Clinics also should establish multiple CRC screening protocols to enhance and support the role of the patient navigator and screening integrity. Additionally, to justify investing in a position, the scope of the navigators should be expanded to apply their skills across the cancer care continuum, to assist with multiple cancer screenings, to outreach to patients in need of screening, and to maintain contact with patients through diagnostic resolution. Finally, future research should focus on assessing the cost-effectiveness of patient navigation programs and interventions.
With the advent of health care reform and the movement toward patient-centered care models, patient navigation will become integral to primary care settings. Patient navigators can facilitate and coordinate access to cancer screenings through ongoing assistance to overcome barriers to quality care. More research should be focused on effective ways to implement and sustain these programs to ensure that patients are receiving timely access to recommended cancer screenings.
Acknowledgments
The authors would like to acknowledge Dr. Cheryl Albright and Dr. Tetine Sentell of the University of Hawai‘i at Mānoa for reviewing and providing feedback on this manuscript and Angela Lee from the University of Hawai‘i John A. Burns School of Medicine’s Health Science Library for assisting with the search strategy. This work is supported by grants from the National Cancer Institute’s Center to Reduce Health Disparities (U54CA153459, 3U54CA153459–05S1) to ‘Imi Hale Native Hawaiian Cancer Network, a program of Papa Ola Lōkahi.
Contributor Information
Jermy-Leigh B. Domingo, University of Hawai‘i at Mānoa’s Office of Public Health Studies.
Kathryn L. Braun, University of Hawai‘i at Mānoa’s Office of Public Health Studies and ‘Imi Hale Native Hawaiian Cancer Network, a program of Papa Ola Lōkahi.
References
- 1.American Cancer Society. Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society; 2016. Available at: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. [Google Scholar]
- 2.Redaelli A, Cranor CW, Okano GJ, et al. Screening, prevention and socioeconomic costs associated with the treatment of colorectal cancer. Pharmacoeconomics. 2003;21(17):1213–38. doi: 10.2165/00019053-200321170-00001. https://doi.org/10.2165/00019053-200321170-00001. [DOI] [PubMed] [Google Scholar]
- 3.Lang K, Lines LM, Lee DW, et al. Lifetime and treatment-phase costs associated with colorectal cancer: evidence from SEER-Medicare data. Clin Gastroenterol Hepatol. 2009 Feb;7(2):198–204. doi: 10.1016/j.cgh.2008.08.034. https://doi.org/10.1016/j.cgh.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Colorectal Cancer Facts & Figures 2014–2016. Atlanta, GA: American Cancer Society; 2014. Available at: http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.pdf. [Google Scholar]
- 5.Lee HY, Lundquist M, Ju E, et al. Colorectal cancer screening disparities in Asian Americans and Pacific Islanders: which groups are most vulnerable? Ethn Health. 2011 Dec;16(6):501–18. doi: 10.1080/13557858.2011.575219. Epub 2011 Jun 21. https://doi.org/10.1080/13557858.2011.575219. [DOI] [PubMed] [Google Scholar]
- 6.Sabatino SA, Lawrence B, Elder R, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012 Jul;43(1):97–118. doi: 10.1016/j.amepre.2012.04.009. https://doi.org/10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Holden DJ, Jonas DE, Porterfield DS, et al. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010 May 18;152(10):668–76. doi: 10.7326/0003-4819-152-10-201005180-00239. Epub 2010 Apr 13. https://doi.org/10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 8.Baron RC, Rimer BK, Breslow RA, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening: a systematic review. Am J Prev Med. 2008 Jul;35(1 Suppl):S34–55. doi: 10.1016/j.amepre.2008.04.002. https://doi.org/10.1016/j.amepre.2008.04.002 https://doi.org/10.1016/j.amepre.2008.04.001 https://doi.org/10.1016/j.amepre.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: a systematic review. J Gen Intern Med. 2012 Aug;27(8):1033–46. doi: 10.1007/s11606-012-2044-2. https://doi.org/10.1007/s11606-012-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006 Mar;83(2):139–41. doi: 10.1007/s11524-006-9030-0. https://doi.org/10.1007/s11524-006-9030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarfaty M, Doroshenk M, Hotz J, et al. Strategies for expanding colorectal cancer screening at community health centers. CA Cancer J Clin. 2013 Jul-Aug;63(4):221–31. doi: 10.3322/caac.21191. https://doi.org/10.3322/caac.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007. Epub 2010 Feb 18. https://doi.org/10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Thomson Reuters. EndNote for windows. Philadelphia, PA: Thomson Reuters; 2013. (version X7). [Google Scholar]
- 14.Zaza S, Wright-De Aguero LK, Briss PA, et al. Data collection instrument and procedure for systematic reviews in the Guide to Community Preventive Services. Task Force on Community Preventive Services. Am J Prev Med. 2000 Jan;18(1 Suppl):44–74. doi: 10.1016/s0749-3797(99)00122-1. https://doi.org/10.1016/S0749-3797(99)00122-1. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich AJ, Tobin JN, Cassells A, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann Intern Med. 2006 Apr;144(8):18. 563–71. doi: 10.7326/0003-4819-144-8-200604180-00006. https://doi.org/10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie J, Itzkowitz S, Lihau-Nkanza I, et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278–84. doi: 10.1016/s0027-9684(15)31240-2. https://doi.org/10.1016/S0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 17.Lasser KE, Murillo J, Lisboa SL, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011 May;171(10):23. 906–12. doi: 10.1001/archinternmed.2011.201. https://doi.org/10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 18.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211–7. doi: 10.1007/s11606-008-0864-x. https://doi.org/10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis T, Arnold C, Rademaker A, et al. Improving colon cancer screening in community clinics. Cancer. 2013 Nov;119(21):1. 3879–86. doi: 10.1002/cncr.28272. Epub 2013 Aug 20. https://doi.org/10.1002/cncr.28272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz ML, Fisher JL, Fleming K, et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45–52. doi: 10.1158/1055-9965.EPI-11-0815. Epub 2011 Nov 8. https://doi.org/10.1158/1055-9965.EPI-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker DW, Brown T, Buchanan DR, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1235–41. doi: 10.1001/jamainternmed.2014.2352. https://doi.org/10.1001/jamainternmed.2014.2352. [DOI] [PubMed] [Google Scholar]
- 22.Honeycutt S, Green R, Ballard D, et al. Evaluation of a patient navigation program to promote colorectal cancer screening in rural Georgia, USA. Cancer. 2013 Aug;119(16):15. 3059–66. doi: 10.1002/cncr.28033. Epub 2013 May 29. https://doi.org/10.1002/cncr.28033. [DOI] [PubMed] [Google Scholar]
- 23.Escoffery C, Fernandez ME, Vernon SW, et al. Patient navigation in a colorectal cancer screening program. J Public Health Manag Pract. 2015 Sep-Oct;21(5):433–40. doi: 10.1097/PHH.0000000000000132. https://doi.org/10.1097/PHH.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natale-Pereira A, Enard KR, Nevarez L, et al. The role of patient navigators in eliminating health disparities. Cancer. 2011 Aug;117(15 Suppl):3543–52. doi: 10.1002/cncr.26264. https://doi.org/10.1002/cncr.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roetzheim RG, Christman LK, Jacobsen PB, et al. A randomized controlled trial to increase cancer screening among attendees of community health centers. Ann Fam Med. 2004 Jul-Aug;2(4):294–300. doi: 10.1370/afm.101. https://doi.org/10.1370/afm.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daly JM, Levy BT, Moss CA, et al. System strategies for colorectal cancer screening at Federally Qualified Health Centers. Am J Public Health. 2015 Jan;105(1):212–19. doi: 10.2105/AJPH.2013.301790. https://doi.org/10.2105/AJPH.2013.301790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeGroff A, Coa K, Morrissey KG, et al. Key considerations in designing a patient navigation program for colorectal cancer screening. Health Promot Pract. 2014 Jul;15(4):483–95. doi: 10.1177/1524839913513587. https://doi.org/10.1177/1524839913513587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiscella K, Humiston S, Hendren S, et al. Eliminating disparities in cancer screening and follow-up of abnormal results: what will it take. J Health Care Poor Underserved. 2011 Feb;22(1):83–100. doi: 10.1353/hpu.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith RA, Brawley OW. The National Breast and Cervical Cancer Early Detection Program: toward a system of cancer screening in the United States. Cancer. 2014 Aug 15;120(Suppl 16):2617–9. doi: 10.1002/cncr.28828. https://doi.org/10.1002/cncr.28828. [DOI] [PubMed] [Google Scholar]
- 30.Sarfaty M, Wender R, Smith R. Promoting cancer screening within the patient cen-tered medical home. CA Cancer J Clin. 2011 Nov-Dec;61(6):397–408. doi: 10.3322/caac.20125. https://doi.org/10.3322/caac.20125. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Medicare and Medicaid Services. Update on preventive services initiatives. Baltimore, MD: Centers for Medicare and Medicaid Services; 2013. Available at: https://www.medicaid.gov/Federal-Policy-Guidance/Downloads/CIB-11-27-2013-Prevention.pdf. [Google Scholar]


