Abstract
Transcranial direct current stimulation (tDCS)1 has been reported to be a promising technique for consciousness improvement for patients with disorders of consciousness (DOC).2 However, there has been no direct electrophysiological evidence to demonstrate the efficacy of tDCS on patients with DOC. Therefore, we aim to measure the cortical excitability changes induced by tDCS in patients with DOC, to find electrophysiological evidence supporting the therapeutic efficacy of tDCS on patients with DOC. In this study, we enrolled sixteen patients with DOC, including nine vegetative state (VS)3 and seven minimally conscious state (MCS)4 (six females and ten males). TMS-EEG was applied to assess cortical excitability changes after twenty minutes of anodal tDCS of the left dorsolateral prefrontal cortex. Global cerebral excitability were calculated to quantify cortical excitability in the temporal domain: four time intervals (0–100, 100–200, 200–300, 300-400 ms). Then local cerebral excitability in the significantly altered time windows were investigated (frontal, left/right hemispheres, central, and posterior). Compared to baseline and sham stimulation, we found that global cerebral excitability increased in early time windows (0–100 and 100-200 ms) for patients with MCS; for the patients with VS, global cerebral excitability increased in the 0-100 ms interval but decreased in the 300-400 ms interval. The local cerebral excitability was significantly different between MCS and VS. The results indicated that tDCS can effectively modulate the cortical excitability of patients with DOC; and the changes in excitability in temporal and spatial domains are different between patients with MCS and those with VS.
Keywords: tDCS, Disorder of consciousness, TMS-EEG, Cortical excitability
Highlights
-
•
TDCS was used to alter cerebral excitability in patients of DOC.
-
•
TMS-EEG was used to evaluate cortical excitability changes in patients of DOC.
-
•
TDCS could induce significant cortical excitability changes in patients of DOC.
-
•
TDCS induced different temporal-spatial excitability changes between MCS and VS.
Introduction
Due to severe brain injury, many patients fail to recover and develop disorders of consciousness (DOC). Patients with preserved arousal but absence of any behavioral signs of awareness are diagnosed as vegetative state (Laureys et al. 2004) (Jennett and Plum 1972); while patients of minimally conscious state (MCS) are defined as having preserved arousal and non-reflexive and purposeful behaviors (Giacino et al. 2002). In the clinic, it is a challenge to diagnose and treat patients with DOC, and due to the costs of prolonged intensive care (Laureys and Schiff 2012), DOC patients place great financial strain on families and medical structures (Monti and Sannita 2016). It has been reported that there are no evidence-based guidelines regarding the treatment of patients with disorders of consciousness (Bernat, 2006, Gosseries et al., 2011), even though many potential pharmacological, as well as non-pharmacological interventions have been evaluated in the last decade. The promising and growing field of neuromodulation has been proposed as a source of non-pharmacological therapeutic techniques for DOC patients. Invasive neuro-stimulation techniques, such as deep brain stimulation, have been reported to induce behavioral improvement in MCS and VS patients (Giacino et al., 2012, Schiff et al., 2007). Recently, due to ethical and procedural limitations on the use of invasive stimulation techniques, non-invasive brain stimulation, including transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), have been investigated for treating DOC patients (Angelakis et al., 2014, Guerra et al., 2014, Thibaut et al., 2014).
It has been reported that tDCS can modulate cortical excitability in healthy people, and it is safer, less uncomfortable, easier to handle, and less expensive than TMS (Romero Lauro et al. 2014). Recently, it was found that tDCS is effective in treating a variety of psychiatric and neurological conditions, including depression (D'Urso et al., 2013, Kuo et al., 2014), Parkinson's disease (Li et al., 2015, Pereira et al., 2013), autism (D'Urso et al. 2014), and epilepsy (Fregni et al., 2006, Nitsche and Paulus, 2009). Also, the application of tDCS facilitates neuro-rehabilitation, such as post-stroke recovery (Kang et al., 2009, Tanaka et al., 2011). At the same time, tDCS has been applied for consciousness improvement of DOC patients (Angelakis et al., 2014, Thibaut et al., 2014). A double-blind sham-controlled crossover design study with anodal and sham tDCS delivered over the left DLPFC indicated a significant treatment effect in MCS patients, and several patients with MCS, as well as VS, showed post-anodal tDCS related signs of consciousness (Thibaut et al. 2014). A long duration tDCS protocol applied to MCS and VS patients showed that all patients with MCS had clinical improvement immediately after tDCS, but no patient in VS showed immediate improvement (Angelakis et al. 2014). Another study demonstrated that tDCS can boost cortical connectivity and excitability in MCS and VS patients (Naro et al. 2015a).
In the clinic, behavioral assessment is usually applied to detect conscious awareness. Thus, the possible mechanisms of change of tDCS in DOC patients have not been studied, and the electrophysiological effects of tDCS of DOC patients are unclear. TMS-EEG has been proposed to obtain real-time and direct information about cortical reactivity as TMS-EEG can detect changes in cortical excitability/inhabitation. It has been used to assess cortical excitability in different consciousness states (Casula et al., 2014, Ferrarelli et al., 2010, Massimini et al., 2005). Motor system (Pellicciari et al. 2013) and central cortex (Romero Lauro et al. 2014) excitability induced by tDCS in healthy subjects have been effectively assessed using TMS-EEG. TMS evoked potential showed that, in healthy subjects, tDCS over the left primary motor cortex induced an enhancement of cortical excitability, whereas cathodal stimulation produced a reduction (Pellicciari et al. 2013). And the anodal tDCS over parietal cortex also induced an excitability enhancement (Romero Lauro et al. 2014).
In this study, we propose to take advantage of TMS-EEG for assessing the cortical excitability of patients with DOC treated with tDCS, comparing before and after stimulation. We aim to demonstrate the effectiveness of tDCS on patients with DOC, which will support the use of tDCS in clinical practice.
Materials and methods
Participants
We enrolled 18 patients with disorder of consciousness (9 VS and 9 MCS, 7 females and 11 males) in this study. Two of them were excluded with suspected of EEG epileptiform activity in EEG evaluation. The clinical characteristics of the others are shown in Table 1. Clinical status of each patient was assessed with the JFK Coma Recovery Scale (JFK CRS-R) (Giacino et al. 2004). And based on their CRS-R scores, patients were diagnosed as MCS and VS. All the patients had no focal lesions in frontal lobes at MRI scans, epileptic history, pacemarker, aneurysms clips, neurostimulator, or brain/ subdural electrodes. Patient VS5 and VS8 have received craniotomy surgery to reduce intracranial pressure. No one suffered craniotomy plastic surgery. All patients were stabilized with no consciousness improvement for more than one month. Patients with complications such as acute pneumonia during two weeks before the experiment were excluded. Any other treatment and drug which modifying cortical-excitability were excluded. Written informed consent to participate in the study was obtained from the patients' caregivers and the patients. The present study was approved by the ethics committee of the PLA Army General Hospital (registration number: 2015–023).
Table 1.
Demographic details for the patients.
| Patient | Age&Sex | Etiology | Moths Post-injury |
CRS-R |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Auditory | Visual | Motor | Oro-motor | Comm | Arousal | Total | ||||
| VS1 | 17 m | TBI | 7 | 1 | 1 | 2 | 1 | 0 | 2 | 7 |
| VS2 | 50 f | Anoxia | 8 | 1 | 0 | 2 | 1 | 0 | 2 | 6 |
| VS3 | 46 m | Haemorrhage | 9 | 1 | 1 | 2 | 1 | 0 | 0 | 5 |
| VS4 | 35 m | Anoxia | 12 | 0 | 0 | 2 | 1 | 0 | 2 | 5 |
| VS5 | 52 m | Ischemic stroke | 8 | 1 | 1 | 2 | 1 | 0 | 0 | 5 |
| VS6 | 39 m | Haemorrhage | 8 | 1 | 1 | 2 | 1 | 0 | 2 | 7 |
| VS7 | 60 m | Anoxia | 13 | 1 | 0 | 2 | 1 | 0 | 2 | 6 |
| VS8 | 43 m | TBI | 8 | 1 | 0 | 1 | 0 | 0 | 2 | 4 |
| VS9 | 70 f | Anoxia | 30 | 1 | 1 | 2 | 1 | 0 | 2 | 7 |
| MCS1 | 53 m | Haemorrhage | 8 | 3 | 3 | 2 | 1 | 0 | 2 | 11 |
| MCS2 | 68 m | TBI | 12 | 3 | 3 | 5 | 1 | 1 | 2 | 15 |
| MCS3 | 31 f | Anoxia | 35 | 2 | 3 | 3 | 1 | 0 | 2 | 11 |
| MCS4 | 29 f | Anoxia | 28 | 1 | 2 | 3 | 1 | 0 | 2 | 9 |
| MCS5 | 49 f | TBI | 13 | 3 | 3 | 3 | 1 | 0 | 2 | 12 |
| MCS6 | 52 m | Anoxia | 6 | 3 | 0 | 2 | 1 | 0 | 2 | 8 |
| MCS7 | 47 f | Haemorrhage | 6 | 1 | 1 | 3 | 1 | 0 | 2 | 8 |
Comm = communication; TBI = traumatic brain injury; CRS-R = Coma recovery scale-revised; f = female; m = male.
TDCS.
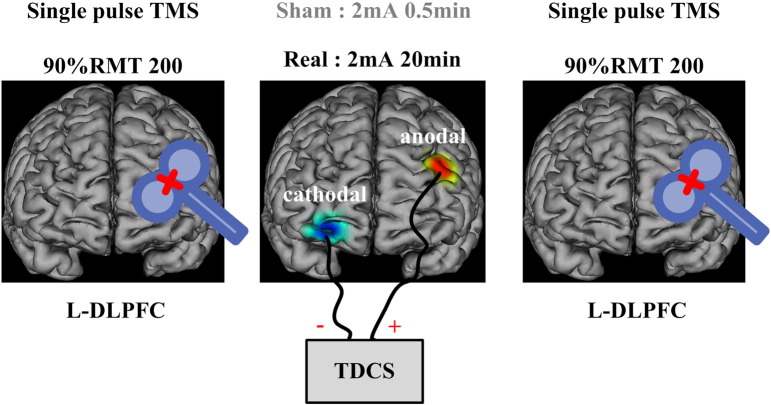
Each patient received two sessions of tDCS stimulation: one real and one sham session. The two sessions were separated by at least three days and the order was counterbalanced across patients. TDCS was delivered by an Eldith DC-stimulator (neuroConn GmbH, Ilmenau, Germany). The stimulation electrodes (25 cm2) were covered by sponges soaked with saline solution. The anodal tDCS electrode was placed over the left dorsolateral prefrontal cortex (DLPFC) centered at F3 (International 10–10 system), and the cathodal electrode was placed over the right supraorbital area, centered at FP2. For real tDCS, a constant current of 2 mA was applied for 20 min, with 15 s of fade-in/fade-out period. For sham tDCS, the same electrode arrangement and parameters for stimulation were employed, except that the stimulator was turned off after 30s (Gandiga et al. 2006).
TMS-EEG.
As shown in Fig. 1, patients received 200 single pulses of TMS in the left DLPFC before and immediately after the tDCS protocol with intensity of 90%RMT. The magnetic stimulation was administered in accordance with safety guidelines (Wassermann 1998). TMS pulses were delivered using a Magstim R2 stimulator with a 70 mm figure-of-eight coil (Magstim Company Limited, Whitland, UK). We used a biphasic waveform with a pulse width of ~ 0.1 ms. Stimulation intensity varied across this experiment and was determined relative to the resting motor threshold (RMT), defined as the lowest TMS intensity which can evoke in at least five out of ten trials an EMG with an amplitude > 50μV peak-to-peak in the relaxed first dorsal interosseous muscle of the right hand. To avoid contamination of TMS-evoked potentials by auditory potentials evoked by the click associated with the TMS discharge, patients wore inserted earplugs which continuously played a masking noise. Bone conduction was attenuated by placing a thin layer of foam between coil and scalp. The details of the TMS-EEG procedure and setup could be found in previous study (Massimini et al. 2005). And this TMS setup has been used to evaluate cerebral excitability of a patient with DOC in her consciousness recovery (Bai et al. 2016).
Fig. 1.
A schematic representation of the experimental procedure. Each experimental session began with a TMS-EEG block, 200 single pulse TMS delivered to L-DLPFC with 90%RMT intensity. Then, real or sham anodal tDCS was delivered to L-DLPFC with cathode over the right supraorbital area. Stimulation lasted 20 min, and a TMS-EEG block immediately followed.
In this experiment, we used a TMS-compatible EEG recorder (BrainAmp 64 MRplus, BrainProducts). EEG was continuously acquired from 62 channels at positions of the international 10–20 system. The equipment used TMS-compatible sintered Ag/AgCl-pin electrodes. We set a band-pass filter at DC to 1000 Hz in the recorder, and the EEG signal was digitized at a sampling rate of 2.5 kHz. During the experiment, the skin/electrode impedance was maintained below 5kΩ. EEG recordings were carried out while patients were behaviorally awake (eyes open, EO), and if a patient showed signs of sleepiness (prolonged eye closure, EC), the CRS-R arousal facilitation protocol was applied, or the experiment was suspended.
Analysis methods.
(1) Preprocessing.
Off-line analysis was performed with EEGLAB 12.0.2.5b, running in a MATLAB environment (Version 2013b, MathWorks Inc., Natick, USA). The continuous EEG signal was segmented into epochs starting 300 ms before TMS pulse onset and ending 500 ms (Ferrarelli et al., 2010, Ferreri et al., 2011, Massimini et al., 2005) after it. After this, data 10 ms before to 20 ms after the TMS pulse were removed from each trial to exclude the TMS artifact, using the cubic interpolation function of MATLAB (Thut et al. 2011). Independent component analysis (ICA) was used to identify the TMS unrelated artifacts (such as eye movement and muscle artifacts), by visually inspecting in terms of scalp distribution, frequency, timing, and amplitude. The components deemed as artifact were removed with ICA (Casula et al. 2014). The 50 Hz power-line artifact was removed from remaining trials using a notch filter. Then, EEG data were common-average referenced; down-sampled to 500 Hz, band-pass filtered (1-80 Hz), and baseline corrected over 300 ms pre-stimulus. Single trials were carefully inspected to ensure absence of residual TMS artifacts. Each TMS-evoked response was obtained by averaging 150 to 200 artifact-free trials.
(2) Global and local mean field amplitude.
We used global mean field amplitude (GMFA) to describe the overall TMS induced activities. The GMFA can be expressed by
where Vi(t) is the signal averaged over trials measured on EEG channel i at time t, is the signal averaged over trials and channels at time t, and N is the number of channels. The GMFA identifies the maximum amplitude of the evoked field and is used to index the effect of TMS on global brain activity (Komssi et al. 2004).
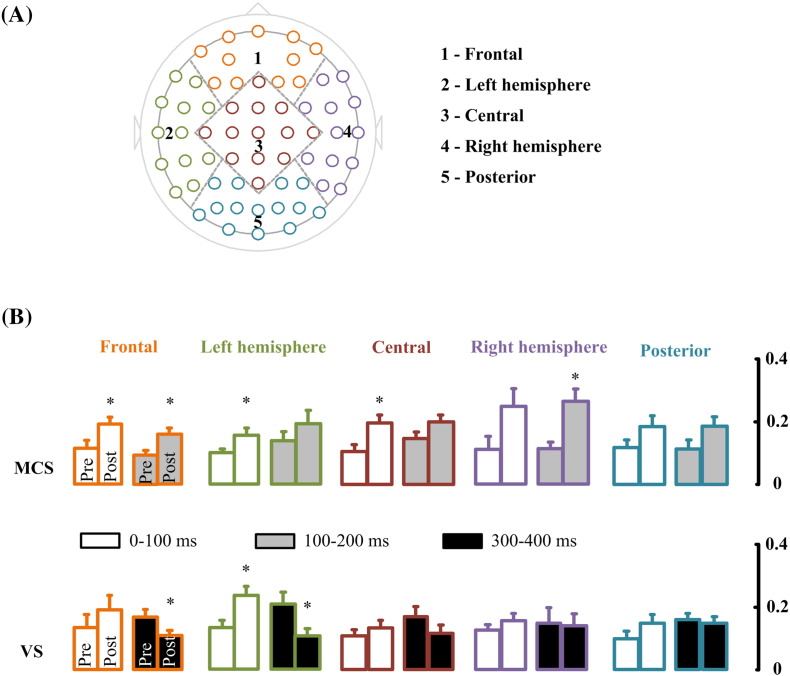
Local mean field amplitude (LMFA) was calculated to investigate the change in local cortical regions. This used the same procedure as used for calculating the GMFA, except that only the channels over a cortical region were included. The brain was divided into five cortical regions: frontal (FPz, FP1, FP2, AF7, AF8, AF3, AF4, F1, F2, F3, F4), left hemisphere (F5, F7, FC3, FC5, FT7, C5, T7, CP3, CP5, TP7, P5, P7), central (Fz, FCz, FC1, FC2, Cz, C1, C2, C3, C4, CPz, CP1, CP2, Pz), right hemisphere (F6, F8, FC4, FC6, FT8, C6, T8, CP4, CP6, TP8, P6, P8), and posterior (P1, P2, P3, P4, POz, PO3, PO4, PO5, PO6, PO7, PO8, Oz, O1, O2) (See Fig. 4 A). Four intervals (0-100 ms, 100-200 ms, 200-300 ms, 300-400 ms) of GMFA and LMFA, for both real and sham stimulation, were calculated.
Fig. 4.
Mean LMFA for real stimulation. (A) Brain was divided into five regions for LMFA calculation: frontal, left hemisphere, central, right hemisphere, and posterior. (B) Mean LMFA of MCS patients in time windows of 0-100 ms and 100-200 ms for the five regions. And mean LMFA of VS patients in time windows of 0-100 ms and 300-400 ms for the five regions. * indicates p < 0.05.
Results
(1) Example of different TMS evoked potentials between a MCS and a VS patients
Fig. 2 shows the average EEG response to TMS pulses of one MCS and one VS patient, recorded at the 60 electrodes. Comparing the MCS with the VS patient (see Fig. 2. A-a, B-a), the TMS evoked potentials (TEP) are different before tDCS (pre-tDCS), with significant different TEP in the interval of 150–300 ms after the TMS pulse. The TEP before tDCS was different from the TEP after tDCS for both the MSC and VS patient (see Fig. 2 A-a,b and B-a,b). For the MCS patient, the TEP within 150 ms after the TMS pulse was increased after tDCS. However the change is very weak for the VS patient, which showed some weak changes after 300 ms. Consistent with single channel signal trends, the overall GMFA of the MCS patient indicates significant activation in the first 150 ms after the TMS pulse, while power significantly decreased around 250 ms (Fig. 2 (A-c)), as compared to pre-tDCS. The changes to TEP also occurred in the VS patient, and analysis of significant activation shows expanded regions for signal peaks in the first 100 ms after the TMS pulse (see Fig. 2 B-c), compared to pre-tDCS. Comparison of the GMFA shows significant power increase around 100 ms and decrease after 300 ms (Fig.2 B-c).
Fig. 2.
EEG response to TMS pulses by one MCS and one VS patient. (A) Average TEP pre-tDCS and significant activation spatial patterns at each peak. (B) Average TEP post-tDCS and significant activation spatial patterns at each peak. (C) GMFA traces for pre-tDCS (blue) and post-tDCS (red). Shadow areas show plus and minus one standard deviation. Black lines above the traces indicate significance with paired t-test, p < 0.05;
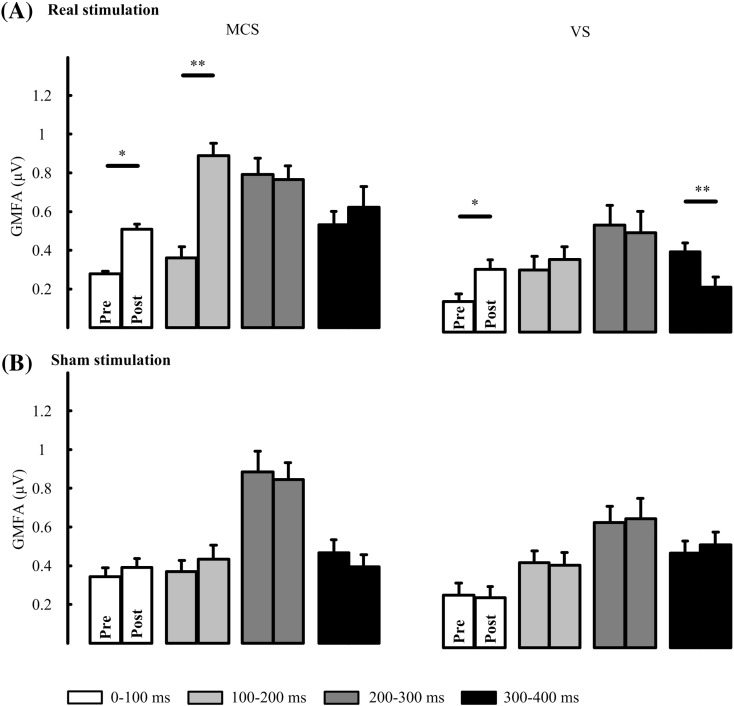
(2) Different global excitability induced by tDCS for MCS and VS patients
Plots of GMFA averaged over patients are shown in Fig. 3. Two-way repeated measures ANOVA was performed in MCS and VS with time windows (4 levels: 0-100 ms, 100-200 ms, 200-300 ms and 300-400 ms) and condition (2 levels: pre-tDCS and post-tDCS) as within-subject factors, and the GMFA value as the dependent variable. Correction of the degrees of freedom was performed by using the Greenhouse-Geisser correction. Main effect of condition was found (F(1,6) = 57.88, p = 0.00) and interaction between condition with time windows was found (F(1.24,7.45) = 12.04, p = 0.008) in MCS group. Significant changes between pre- and post-tDCS were found for real tDCS stimulation in both the MCS and VS groups, while no significant changes could be found for sham stimulation. For MCS patients, pairwise comparison with multiple correction in each time windows showed significantly higher values for post-tDCS in the 0-100 ms (p = 0.020, FDR correction) and 100-200 ms (p = 0.006, FDR correction), as compared to pre-tDCS. For VS patients, significantly higher values post-tDCS compared to pre-tDCS in the 0-100 ms (p = 0.013, FDR correction) and significantly lower values in 300-400 ms (p = 0.004, FDR correction) were found.
Fig. 3.
Mean GMFA in different time windows. (A) GMFA of MCS (left column) and VS (right column) for real stimulation. (B) GMFA of MCS (left column) and VS (right column) for sham stimulation. * indicates p < 0.05 and ** indicates p < 0.01.
(3) Different local excitability induced by tDCS for MCS and VS patients
In order to specify the spatial distribution of the tDCS induced changes in each group during the time windows which has significant changes of GMFA, the brain was divided into five regions and LMFA was calculated in each region. Fig. 4 shows LMFA averaged over patients for the time windows in each group. For the MCS group, two-way repeated measures ANOVA was performed with regions (5 levels: frontal, left/right hemispheres, central, and posterior) and condition (2 levels: pre-tDCS and post-tDCS) as within-subject factors, and the LMFA value as the dependent variable. Correction of the degrees of freedom was performed by using the Greenhouse-Geisser correction. We found significant main effects of condition in the 0-100 ms (F(1,6) = 12.75, p = 0.012), 100-200 ms (F(1,6) = 30.74, p = 0.001). There was no significant main effect of regions and no interaction between regions and condition. Pairwise comparisons with multiple correction between pre-tDCS and post-tDCS in each region and time window showed significant excitability increase in frontal (p = 0.03, FDR correction), left hemisphere (p = 0.037, FDR correction), central (p = 0.03, FDR correction) during 0-100 ms; increase in frontal (p = 0.02, FDR correction) and right hemisphere (p = 0.033, FDR correction) during 100-200 ms.
For the VS group, we found significant main effects of condition (F(1,8) = 8.37, p = 0.020) and region (F(1.1,9.1) = 16.12, p = 0.002) in the 0-100 ms time window. Furthermore, there was significant interaction between regions and conditions in the 300-400 ms (F(2.4,10.4) = 5.11, p = 0.012) time windows. As shown in Fig. 4 (B), pairwise comparisons with multiple correction between pre-tDCS and post-tDCS in each region and time window showed significant excitability increase in left hemisphere (p = 0.045, FDR correction) during the 0-100 ms time window. However, there was significantly decreased excitability in frontal (p = 0.02, FDR correction) and left hemisphere (p = 0.037, FDR correction) during 300-400 ms.
Under sham stimulation, for all time windows, the main effect of condition and interaction of regions with condition were not significant in both the MCS and VS groups. But, it should be noted that all the comparison results above are only indicative trends which based on the samples in this study, and larger samples should be included confirm these trends in future studies.
Discussion
Previous studies have reported effectiveness of using tDCS for clinical improvement of DOC (Angelakis et al., 2014, Thibaut et al., 2014), but this therapy is still far from becoming an established clinical guideline. Assessment methods need to be developed to evaluate its treatment effects, and these methods will also facilitate the understanding of the mechanisms behind how tDCS alters consciousness states. In this study, TMS-EEG was used to assess the cortical excitability changes induced by tDCS on patients with DOC. In tDCS studies, left DLPFC are mainly considered site to be modulated in patients of DOC, and results suggested the patients could significantly benefit from the tDCS modulation at left DLPFC (Angelakis et al., 2014, Thibaut et al., 2014). Besides, for patients of DOC, left DLPFC is always selected as target in other non-invasive modulation such as repetitive TMS (Louise-Bender Pape et al., 2009, Naro et al., 2015b). Therefore, in this study, we targeted anodal tDCS at left DLPFC to explore its effects on cerebral excitability. Compared with sham stimulation, we found that 20mins of anodal tDCS of DLPFC could effectively modulate the GMFA of TEP both in MCS and VS patents, and these changes occurred in different time windows and brain regions for MCS and VS patients. For MCS patients, the GMFA were significantly increased within 200 ms after the TMS pulse, and all regions but posterior had significant reactivity. For VS patients, the tDCS affects mainly the 0-100 ms and 300-400 ms post-TMS time intervals. Changes mainly occurred in the frontal and left hemisphere. Comparing with sham stimulation allowed us to exclude the confounding influence of unspecific effects, such as the cortical state fluctuation of patients.
For the healthy subjects, anodal tDCS induced significant enhancement of cortical excitability, which was also represented by higher amplitude in TEP and GMFA (Pellicciari et al., 2013, Romero Lauro et al., 2014). When anodal tDCS delivered at right parietal, temple and spatial analysis showed that the enhancement significantly changed within 100 ms following TMS pulse and the altering could be found in bilateral frontal, parietal and right temporal (Romero Lauro et al. 2014). Consistent with the healthy subjects, we suggest that the significant change in TEP within the early time window (100 ms) for MCS and VS patients is directly caused by changes in the excitability of the DLPFC, and the changes occurring in other time windows may be caused by excitability changes in remote regions which interact with the DLPFC through ipsilateral and contralateral connectivity. This theory is supported by the following findings. The GMFA increases within early time windows were due to resting membrane potential increases caused by anodal tDCS stimulation (Batsikadze et al., 2013, Furubayashi et al., 2008). Similar modulation of early TEP components have been demonstrated after rTMS (Esser et al. 2006), anesthesia (Ferrarelli et al. 2010), and electroconvulsive therapy (Casarotto et al. 2013). The cortical excitability decreases in the later time window of 300-400 ms for VS patients could be related to the overall cortical state or consciousness level. Considering the differences in clinical response to tDCS between patients with MCS and VS, this excitability decrease may be a marker of consciousness level. But, the statistic results were based on the limited samples in this study. Considering to confirm these trends, larger samples should be investigated in future studies.
Previous studies demonstrated that tDCS can be applied to modulate brain connectivity. In (Keeser et al., 2011, Pena-Gomez et al., 2012), it was demonstrated that prefrontal tDCS could induce network connectivity change in healthy subjects; after anodal stimulation, increase of cortical response has been found not only over the stimulated cortex but also propagated to the contralateral homotopic areas (Stagg et al., 2012, Stagg et al., 2009b). It has also been reported that tDCS can modulate cortico-subcortical networks such as thalamo-cortical circuits (Polanía et al. 2012). Unilateral anodal tDCS can affect inter-hemispheric interactions measured by TMS-EEG (Pellicciari et al., 2013, Romero Lauro et al., 2014). Consistent with previous studies (Keeser et al., 2011, Romero Lauro et al., 2014, Stagg et al., 2013), the GMFA and LMFA changes observed in this study indicate that the effects of tDCS can diffuse from underneath the stimulated area to other brain regions for patients with DOC. Previous research has demonstrated widespread impairment of fronto-central cortices in VS patients (Laureys 2005), and severe impairment of functional inter-regional connectivity has also been demonstrated in DOC patients (Rosanova et al. 2012). Since it is likely that cortical impairment may disrupt some cortico-cortical and cortico-subcortical connectivity, when we apply tDCS to the DLPFC of VS patients, we cannot obtain remote effects similar to healthy subjects.
Considering that patients with MCS generally have relatively less severe brain injury than VS, especially patient0s with MCS in better consciousness states, we may observe significant differences between MCS and VS patients in terms of excitability induced by tDCS. These differences depend on the injured connections in brain regions. Furthermore, we believe that tDCS modulation of the brain may be dependent on the underlying structural integrity of the cortical network.
Studies have demonstrated that short term tDCS can induce modulation of neuronal resting membrane potential and neuronal recruitment (Komssi et al., 2004, Nitsche et al., 2005). Long term tDCS can induce after-effects which are mediated by synaptic long-term potentiation and depression mechanisms, likely mediated by N-methyl-d-aspartate (NMDA) receptors and altering GABAergic activity and intra-cellular CA2 + concentration (Liebetanz et al., 2002, Stagg et al., 2009a). The modulation is polarity dependent: anodal stimulation increases the spontaneous firing rate and cathodal stimulation decreases cortical excitability. The TEP in this study reflects reactivity of cortical neuronal populations to stimulation and is dependent on the complex interactions between both excitatory glutamatergic and inhibitory GABAergic neurotransmitters (Macdonell 2012). Hence, by observing the amplitude variation in TEP, which represents a direct measure of the neuronal changes induced by tDCS, we infer that tDCS of DLPFC effectively modulates cortical excitation or depression circuits in patients with DOC by changing neurotransmitter activity.
TMS-EEG is a robust functional neuroimaging technique which can assess brain reactivity and connectivity with high temporal resolution in a relatively objective manner (Daskalakis et al., 2012, Rogasch et al., 2013), and this method has been applied in research on sleep (Massimini et al. 2005), anesthesia (Ferrarelli et al. 2010), and DOC (Rosanova et al. 2012). In this study, we used TMS-EEG to evaluate the effects of tDCS on patients with DOC. TMS-EEG has the capability to shed light on both local and global brain network changes, which makes it valuable for understanding the underlying effects of and mechanisms behind tDCS of patients with DOC. It is known that current density and stimulation duration influence tDCS effects (Nikulin et al. 2003). Anatomical differences between patients and depth and orientation of cells within the cortex are also influencing factors on the modulatory effects (Bikson et al., 2013, Bikson et al., 2004). Thus, more investigation is needed to optimize tDCS parameters, including current density, current output time, and position of anode and cathode. This study gives an example of using TMS-EEG to assess the electrophysiological effects of tDCS on patients with DOC. We believe that TMS-EEG is a promising technique for assessing the effectiveness of various tDCS parameters settings and target brain regions, as well as the effectiveness of other non-invasive brain stimulation techniques, such as transcranial alternating current stimulation, transcranial random noise stimulation. And repetitive transcranial magnetic stimulation.
Conclusion
This is the first study to provide electrophysiological reactivity evidence that tDCS can modulate the cortical excitability of patients with DOC. We found differences in tDCS induced cortical excitability changes between MCS and VS patients using TMS-EEG. We believe TMS-EEG assessment can contribute to therapeutic trials for tDCS of patients with DOC. Lastly, this study provides an example of combining TMS-EEG with non-invasive brain stimulation to evaluate neuro-modulatory effects of stimulation in patients with DOC.
Conflict of interest statement
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research was supported by the National Natural Science Foundation of China (No.61273063, No.81230023), Innovation Cultivation Fund of the PLA Army General Hospital (No. 2015-LC-09), Beijing Municipal Science & Technology Commission (No. Z141107002514111) and the commercialization of research findings was supported by Beijing Municipal Commission of Education.
Acknowledgments
The authors thank the assistance of all persons and volunteers whose participation was essential in the successful completion of this study, and thank Zheng Li for his manuscript editing.
Footnotes
TDCS = transcranial direct current stimulation
DOC = disorders of consciousness
VS = vegetative state
MCS = minimally conscious state
Contributor Information
Jianghong He, Email: he_jianghong@sina.cn.
Xiaoli Li, Email: xiaoli@bnu.edu.cn.
References
- Angelakis E., Liouta E., Andreadis N., Korfias S., Ktonas P., Stranjalis G., Sakas D.E. Transcranial direct current stimulation effects in disorders of consciousness. Arch. Phys. Med. Rehabil. 2014;95:283–289. doi: 10.1016/j.apmr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Bai Y., Xia X., Kang J., Yin X., Yang Y., He J., Li X. Evaluating the effect of repetitive transcranial magnetic stimulation on disorders of consciousness by using TMS-EEG. Front. Neurosci. 2016;10:473. doi: 10.3389/fnins.2016.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsikadze G., Moliadze V., Paulus W., Kuo M.F., Nitsche M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013;591:1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat J.L. Chronic disorders of consciousness. Lancet. 2006;367:1181–1192. doi: 10.1016/S0140-6736(06)68508-5. [DOI] [PubMed] [Google Scholar]
- Bikson M., Inoue M., Akiyama H., Deans J.K., Fox J.E., Miyakawa H., Jefferys J.G. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M., Dmochowski J., Rahman A. The "quasi-uniform" assumption in animal and computational models of non-invasive electrical stimulation. Brain Stimul. 2013;6:704–705. doi: 10.1016/j.brs.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto S., Canali P., Rosanova M., Pigorini A., Fecchio M., Mariotti M., Lucca A., Colombo C., Benedetti F., Massimini M. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26:326–337. doi: 10.1007/s10548-012-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula E.P., Tarantino V., Basso D., Arcara G., Marino G., Toffolo G.M., Rothwell J.C., Bisiacchi P.S. Low-frequency rTMS inhibitory effects in the primary motor cortex: Insights from TMS-evoked potentials. NeuroImage. 2014;98:225–232. doi: 10.1016/j.neuroimage.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Daskalakis Z.J., Farzan F., Radhu N., Fitzgerald P.B. Combined transcranial magnetic stimulation and electroencephalography: its past, present and future. Brain Res. 2012;1463:93–107. doi: 10.1016/j.brainres.2012.04.045. [DOI] [PubMed] [Google Scholar]
- D'Urso G., Mantovani A., Micillo M., Priori A., Muscettola G. Transcranial direct current stimulation and cognitive-behavioral therapy: evidence of a synergistic effect in treatment-resistant depression. Brain Stimul. 2013;6:465–467. doi: 10.1016/j.brs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- D'Urso G., Ferrucci R., Bruzzese D., Pascotto A., Priori A., Altamura C.A., Galderisi S., Bravaccio C. Transcranial direct current stimulation for autistic disorder. Biol. Psychiatry. 2014;76:e5–e6. doi: 10.1016/j.biopsych.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Esser S.K., Huber R., Massimini M., Peterson M.J., Ferrarelli F., Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res. Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F., Massimini M., Sarasso S., Casali A., Riedner B.A., Angelini G., Tononi G., Pearce R.A. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri F., Pasqualetti P., Maatta S., Ponzo D., Ferrarelli F., Tononi G., Mervaala E., Miniussi C., Rossini P.M. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. NeuroImage. 2011;54:90–102. doi: 10.1016/j.neuroimage.2010.07.056. [DOI] [PubMed] [Google Scholar]
- Fregni F., Thome-Souza S., Nitsche M.A., Freedman S.D., Valente K.D., Pascual-Leone A. A controlled clinical trial of cathodal DC polarization in patients with refractory epilepsy. Epilepsia. 2006;47:335–342. doi: 10.1111/j.1528-1167.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- Furubayashi T., Terao Y., Arai N., Okabe S., Mochizuki H., Hanajima R., Hamada M., Yugeta A., Inomata-Terada S., Ugawa Y. Short and long duration transcranial direct current stimulation (tDCS) over the human hand motor area. Exp. Brain Res. 2008;185:279–286. doi: 10.1007/s00221-007-1149-z. [DOI] [PubMed] [Google Scholar]
- Gandiga P.C., Hummel F.C., Cohen L.G. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Ashwal S., Childs N., Cranford R., Jennett B., Katz D.I., Kelly J.P., Rosenberg J.H., Whyte J., Zafonte R. The minimally conscious state definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Kalmar K., Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Giacino J., Fins J.J., Machado A., Schiff N.D. Central thalamic deep brain stimulation to promote recovery from chronic posttraumatic minimally conscious state: challenges and opportunities. Neuromodulation. 2012;15:339–349. doi: 10.1111/j.1525-1403.2012.00458.x. [DOI] [PubMed] [Google Scholar]
- Gosseries, O., Vanhaudenhuyse, A., Bruno, M.-A., Demertzi, A., Schnakers, C., Boly, M.M., Maudoux, A., Moonen, G., Laureys, S., 2011. Disorders of consciousness: coma, vegetative and minimally conscious states. States of consciousness. Springer, pp. 29–55.
- Guerra A., Costantini E.M., Maatta S., Ponzo D., Ferreri F. Disorders of consciousness and electrophysiological treatment strategies: a review of the literature and new perspectives. Curr. Pharm. Des. 2014;20:4248–4267. [PubMed] [Google Scholar]
- Jennett B., Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet. 1972;1:734–737. doi: 10.1016/s0140-6736(72)90242-5. [DOI] [PubMed] [Google Scholar]
- Kang E.K., Baek M.J., Kim S., Paik N.J. Non-invasive cortical stimulation improves post-stroke attention decline. Restor. Neurol. Neurosci. 2009;27:645–650. doi: 10.3233/RNN-2009-0514. [DOI] [PubMed] [Google Scholar]
- Keeser D., Meindl T., Bor J., Palm U., Pogarell O., Mulert C., Brunelin J., Moller H.J., Reiser M., Padberg F. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 2011;31:15284–15293. doi: 10.1523/JNEUROSCI.0542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komssi S., Kahkonen S., Ilmoniemi R.J. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum. Brain Mapp. 2004;21:154–164. doi: 10.1002/hbm.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M.F., Paulus W., Nitsche M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage. 2014;85(Pt 3):948–960. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn. Sci. 2005;9:556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Laureys S., Schiff N.D. Coma and consciousness: paradigms (re)framed by neuroimaging. NeuroImage. 2012;61:478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Laureys S., Owen A.M., Schiff N.D. Brain function in coma, vegetative state, and related disorders. The Lancet Neurology. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- Li H., Lei X., Yan T., Li H., Huang B., Li L., Xu L., Liu L., Chen N., Lu L., Ma Y., Xu L., Li J., Wang Z., Zhang B., Hu X. The temporary and accumulated effects of transcranial direct current stimulation for the treatment of advanced Parkinson's disease monkeys. Sci Rep. 2015;5:12178. doi: 10.1038/srep12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D., Nitsche M.A., Tergau F., Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Louise-Bender Pape T., Rosenow J., Lewis G., Ahmed G., Walker M., Guernon A., Roth H., Patil V. Repetitive transcranial magnetic stimulation-associated neurobehavioral gains during coma recovery. Brain Stimul. 2009;2:22–35. doi: 10.1016/j.brs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Macdonell R.A. Cortical excitability and neurology: insights into the pathophysiology. Funct. Neurol. 2012;27:131. [PMC free article] [PubMed] [Google Scholar]
- Massimini M., Ferrarelli F., Huber R., Esser S.K., Singh H., Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Monti M.M., Sannita W.G. Springer; 2016. Brain Function and Responsiveness in Disorders of Consciousness. [Google Scholar]
- Naro A., Calabrò R.S., Russo M., Leo A., Pollicino P., Quartarone A., Bramanti P. Can transcranial direct current stimulation be useful in differentiating unresponsive wakefulness syndrome from minimally conscious state patients? Restor. Neurol. Neurosci. 2015;33:159–176. doi: 10.3233/RNN-140448. [DOI] [PubMed] [Google Scholar]
- Naro A., Russo M., Leo A., Bramanti P., Quartarone A., Calabro R.S. A single session of repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex in patients with unresponsive wakefulness syndrome: preliminary results. Neurorehabil. Neural Repair. 2015;29:603–613. doi: 10.1177/1545968314562114. [DOI] [PubMed] [Google Scholar]
- Nikulin V.V., Kicic D., Kahkonen S., Ilmoniemi R.J. Modulation of electroencephalographic responses to transcranial magnetic stimulation: evidence for changes in cortical excitability related to movement. Eur. J. Neurosci. 2003;18:1206–1212. doi: 10.1046/j.1460-9568.2003.02858.x. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Paulus W. Noninvasive brain stimulation protocols in the treatment of epilepsy: current state and perspectives. Neurotherapeutics. 2009;6:244–250. doi: 10.1016/j.nurt.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M.A., Seeber A., Frommann K., Klein C.C., Rochford C., Nitsche M.S., Fricke K., Liebetanz D., Lang N., Antal A., Paulus W., Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J. Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicciari M.C., Brignani D., Miniussi C. Excitability modulation of the motor system induced by transcranial direct current stimulation: a multimodal approach. NeuroImage. 2013;83:569–580. doi: 10.1016/j.neuroimage.2013.06.076. [DOI] [PubMed] [Google Scholar]
- Pena-Gomez C., Sala-Lonch R., Junque C., Clemente I.C., Vidal D., Bargallo N., Falcon C., Valls-Sole J., Pascual-Leone A., Bartres-Faz D. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimul. 2012;5:252–263. doi: 10.1016/j.brs.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J.B., Junque C., Bartres-Faz D., Marti M.J., Sala-Llonch R., Compta Y., Falcon C., Vendrell P., Pascual-Leone A., Valls-Sole J., Tolosa E. Modulation of verbal fluency networks by transcranial direct current stimulation (tDCS) in Parkinson's disease. Brain Stimul. 2013;6:16–24. doi: 10.1016/j.brs.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Polanía R., Paulus W., Nitsche M.A. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum. Brain Mapp. 2012;33:2499–2508. doi: 10.1002/hbm.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch N.C., Daskalakis Z.J., Fitzgerald P.B. Mechanisms underlying long-interval cortical inhibition in the human motor cortex: a TMS-EEG study. J. Neurophysiol. 2013;109:89–98. doi: 10.1152/jn.00762.2012. [DOI] [PubMed] [Google Scholar]
- Romero Lauro L.J., Rosanova M., Mattavelli G., Convento S., Pisoni A., Opitz A., Bolognini N., Vallar G. TDCS increases cortical excitability: direct evidence from TMS-EEG. Cortex. 2014;58:99–111. doi: 10.1016/j.cortex.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Rosanova M., Gosseries O., Casarotto S., Boly M., Casali A.G., Bruno M.A., Mariotti M., Boveroux P., Tononi G., Laureys S., Massimini M. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain. 2012;135:1308–1320. doi: 10.1093/brain/awr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff N.D., Giacino J.T., Kalmar K., Victor J.D., Baker K., Gerber M., Fritz B., Eisenberg B., Biondi T., O'Connor J., Kobylarz E.J., Farris S., Machado A., McCagg C., Plum F., Fins J.J., Rezai A.R. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Best J.G., Stephenson M.C., O'Shea J., Wylezinska M., Kincses Z.T., Morris P.G., Matthews P.M., Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., O'Shea J., Kincses Z.T., Woolrich M., Matthews P.M., Johansen-Berg H. Modulation of movement-associated cortical activation by transcranial direct current stimulation. Eur. J. Neurosci. 2009;30:1412–1423. doi: 10.1111/j.1460-9568.2009.06937.x. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Bachtiar V., O'Shea J., Allman C., Bosnell R.A., Kischka U., Matthews P.M., Johansen-Berg H. Cortical activation changes underlying stimulation-induced behavioural gains in chronic stroke. Brain. 2012;135:276–284. doi: 10.1093/brain/awr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Lin R.L., Mezue M., Segerdahl A., Kong Y., Xie J., Tracey I. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J. Neurosci. 2013;33:11425–11431. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Takeda K., Otaka Y., Kita K., Osu R., Honda M., Sadato N., Hanakawa T., Watanabe K. Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabil. Neural Repair. 2011;25:565–569. doi: 10.1177/1545968311402091. [DOI] [PubMed] [Google Scholar]
- Thibaut A., Bruno M.A., Ledoux D., Demertzi A., Laureys S. tDCS in patients with disorders of consciousness: sham-controlled randomized double-blind study. Neurology. 2014;82:1112–1118. doi: 10.1212/WNL.0000000000000260. [DOI] [PubMed] [Google Scholar]
- Thut G., Veniero D., Romei V., Miniussi C., Schyns P., Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr. Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann E.M. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5-7, 1996. Electroencephalogr. Clin. Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]