Abstract
The molecular mechanisms underlying major depressive disorder remain poorly understood, and current antidepressant treatments have many shortcomings. The recent discovery that a single intravenous infusion of ketamine at a subanesthetic dose had robust, rapid and sustained antidepressant effects in individuals with treatment-resistant depression inspired tremendous interest in investigating the molecular mechanisms mediating ketamine’s clinical efficacy as well as increased efforts to identify new targets for antidepressant action. We review the clinical utility of ketamine and recent insights into its mechanism of action as an antidepressant, including the roles of N-methyl-d-aspartate receptor inhibition, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor upregulation, activation of downstream synaptogenic signalling pathways and the production of an active ketamine metabolite, hydroxynorketamine. Emerging knowledge of the molecular mechanisms underlying both ketamine’s positive therapeutic and detrimental side effects will aid the development of a new generation of much-needed superior antidepressant agents.
Introduction
Despite decades of research, the molecular mechanisms underlying major depressive disorder (MDD) remain poorly understood, and current antidepressant treatments have many shortcomings. In the early 2000s, an initial report announced that a single intravenous infusion of ketamine at a subanesthetic dose had robust, rapid and sustained antidepressant effects in individuals with treatment-resistant depression (TRD). This exciting discovery has inspired tremendous interest over recent years in investigating the molecular mechanisms mediating ketamine’s clinical efficacy. This was accompanied by increased efforts to identify new targets for antidepressant action, with a particular focus on the glutamatergic system. In this paper we review the clinical utility of ketamine and recent insights into its mechanism of action as an antidepressant, including the roles of N-methyl-d-aspartate receptor (NMDAR) inhibition, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) upregulation, activation of downstream synaptogenic signalling pathways (e.g., brain-derived neurotrophic factor [BDNF], mechanistic target of rapamycin [mTOR]), as well as the production of an active ketamine metabolite (hydroxynorketamine [HNK]). Emerging knowledge of the molecular mechanisms underlying ketamine’s positive therapeutic and detrimental side effects will inform the development of a new generation of much-needed superior antidepressant agents.
Depression and current antidepressants
Depression is a prevalent and debilitating mental disorder that affects up to 350 million people globally and is associated with an extremely high personal and socioeconomic burden, with an estimated annual cost of $210 billion world-wide as a result of treatment expenditure and loss of productivity.1,2 Current treatment options for people with MDD rely heavily on the use of antidepressant medications, most of which are monoaminergic agents, such as selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), selective norepinephrine or dual serotonin–norepinephrine reuptake inhibitors and monoamine oxidase inhibitors (MAOIs).3 The efficacy of these agents is based on the idea that monoamine neurotransmitter systems (predominantly serotonin, norepinephrine and dopamine) are hypoactive, especially in brain regions strongly implicated in the pathophysiology of MDD, such as the hippocampus and prefrontal cortex (PFC).3–6 Unfortunately, current anti-depressants have several important shortcomings, including a delayed onset of therapeutic effects and limited efficacy.7 Monoamine drugs are associated with a substantial time lag of several weeks to months before a therapeutic effect is observed, which is a major concern, considering individuals with depression can be at high risk for suicide.8,9 In addition, clinical trials have reported that more than 60% of patients fail to obtain clinically important or sustained remission with a traditional antidepressant, with approximately one-third of all depressed patients failing to respond to 2 or more first-line antidepressant treatments and thus being characterized as having treatment-resistant depression (TRD).1,2 Despite decades of research, the fundamental neurochemical and molecular mechanisms underlying the disorder are still poorly understood, while current antidepressant treatments have limited clinical efficacy. This highlights a clear, unmet clinical need for safer, superior antidepressants with higher response rates and a more rapid onset of action.
Clinical efficacy and implications of ketamine
Owing to the therapeutic limitations of current antidepressants as well as the lack of strong or direct evidence to support the monoamine deficiency hypothesis of depression, understandably there has been considerable and growing interest in new targets for antidepressant action, with a particular focus on the glutamatergic system.1,9,10 Ketamine hydrochloride is a noncompetitive, nonsubtype selective NMDAR antagonist, which has been used primarily as an anesthetic agent (at doses in the range of 1–3mg/kg) since the 1960s.3 One of the most exciting discoveries in the field of depression treatment strategies came in 2000, when a seminal pilot trial first reported a rapid and robust antidepressant effect following a single intravenous (IV) infusion of ketamine at a sub-anesthetic dose in individuals with TRD.11 Following this initial finding, several additional placebo-controlled randomized clinical trials and meta-analyses have been conducted, supporting the utility of ketamine as a novel and potentially superior antidepressant.12–14 Most of these trials used a single 40-minute IV infusion of (R,S)-ketamine (racemic mixture) at a dose of 0.5 mg/kg, resulting in response rates of 50%–70% in TRD populations.1,14,15 Following a single infusion of ketamine, the majority of patients experienced considerable symptomatic relief (i.e., reductions in depressed mood, anhedonia and suicidal thoughts) as early as 2 hours after ketamine administration, with therapeutic effects peaking at 24 hours and lasting up to 2 weeks.14,16
Ketamine’s rapid antidepressant effects are in stark contrast to the substantial delay in the onset of therapeutic effects following administration of traditional antidepressants. These rapid effects with an NMDA antagonist provide support for an alternative to the monoamine deficiency hypothesis of depression. Although monoaminergic systems do not appear to represent a convergent pathway regulating mood and mediating antidepressant response, emerging evidence suggests they may modulate downstream signalling pathways more directly targeted by ketamine.17 The duration of therapeutic effects initiated by a single ketamine infusion, which can last up to 2 weeks, is maintained long after the complete metabolism and elimination of the drug (which has a plasma half-life of 2.5 hours). Accordingly, the sustained antidepressant response cannot be attributed to the direct receptor effects of ketamine, but instead may be mediated by activation of crucial downstream signalling cascades.2,12,15 Not surprisingly, ketamine’s unprecedented rapid and sustained antidepressant effects as well as its high response rates, especially in TRD populations, have inspired massive efforts to uncover the molecular mechanisms mediating the drug’s unique therapeutic profile.
Despite these positive features, it is important to note that even at subanesthetic doses, ketamine administration is associated with mild and transient dissociative effects, neurocognitive and sensorimotor disturbances as well as short-lasting elevations in heart rate and blood pressure.2,9 In addition, ketamine, or “Special K,” is used as a recreational drug and thus has the potential to be abused, and prolonged use may cause neurotoxic effects.1,3 Hopefully, the identification of the mechanism of action underlying ketamine’s anti-depressant effects will assist in the development of novel rapid-acting antidepressants that lack the features noted above that currently limit ketamine’s wider clinical use.
Preclinical studies and insights into the mechanism of action of ketamine
Behavioural effects
These encouraging clinical findings of ketamine’s utility in the treatment of depression inspired a wave of preclinical research designed to shed light on the molecular mechanisms underlying the robust antidepressant effects of ketamine. Numerous studies have successfully duplicated the positive effects of ketamine in rodent tests or models of depression, including the forced swim test (FST; the most commonly used preclinical screen for antidepressant activity) and the chronic mild stress paradigm (CMS; the most commonly used preclinical model of depression).18 A single systemic injection of ketamine at an intraperitoneal dose of 10mg/kg produces a significant reduction in FST immobility shortly (30 minutes to 1 hour) after administration, which has been shown to persist for an average of 7 days in both rats and mice.19–22 In addition, although rodents exposed to chronic stress exhibit depressive-like behaviours, including despair/helplessness (as in the FST) and anhedonia (as in the sucrose preference test [SPT]), ketamine is able to reverse these effects.22,23
In addition to mirroring the rapid and sustained anti-depressant effects of ketamine seen in the clinic, animal studies have also yielded further intriguing observations. Despite the prevalence of MDD in women being roughly twice as high as in men, preclinical research investigating the mechanisms underlying ketamine’s antidepressant effects has been conducted almost exclusively in male rodents.5 Interestingly, preclinical studies that include both sexes report that ketamine produces more robust antidepressant responses in female than in male rodents in the FST, so that lower doses are required to obtain significant reductions in depression-related behaviours in females.22,24 On the other hand, there is also emerging evidence that repeated ketamine treatment (daily injections of 10 mg/kg for 21 days) effectively sustains the antidepressant response in male mice, whereas it may actually worsen depression- and anxiety-related phenotypes in female mice.25 Therefore, further research into the sex-specific effects of ketamine is warranted. It is also important to note that such preclinical studies involving prolonged exposure to ketamine over several days have also reported neurotoxicity in animal models of schizophrenia.26 Thus, approaches that extend the therapeutic effects of ketamine without the onset of severe neurotoxicity and other side effects are much needed. In addition, whereas ketamine is usually administered as a racemic mixture, animal studies have shown that S-ketamine is approximately 3–4 times more potent than R-ketamine at inducing both anesthesia and psychotomimetic side effects.27 Importantly, the R-enantiomer is more effective than the S-enantiomer at reducing depressive-related behaviours, apparently without many of the side effects.15,22,27 These findings highlight the potential of enantiomer-specific therapy as a new approach for treating MDD.
Compelling clinical and preclinical evidence support ketamine’s utility in treating depression and inspire many efforts to uncover the molecular mechanisms responsible for ketamine’s apparently superior antidepressant effects, which holds promise for a new generation of much-needed, superior antidepressant agents.
Cellular and molecular effects
For almost 2 decades, the mechanisms mediating the anti-depressant effects of ketamine were attributed to the direct inhibition of glutamatergic NMDA receptors. Until recently, the widely accepted model of ketamine’s actions involved a cascade of events initiated by the preferential blockade of NMDA receptors located on γ-aminobutyric acid (GABA)-ergic interneurons, whose tonic firing is driven by these NMDA receptors.12 This in turn results in pyramidal cell disinhibition and a surge of glutamate release, and subsequently in the activation of AMPA receptors along with release of BDNF and activation of downstream synaptogenic signalling pathways (e.g., mTOR).12,28–30
Role of NMDA receptors
The promising clinical findings concerning ketamine’s utility in treating depression prompted further investigation into the efficacy of other glutamatergic agents, with a particular focus on nonketamine NMDAR antagonists.13 However, a growing body of evidence casts some doubt on the assumption that ketamine’s antidepressant effects are simply due to NMDAR blockade. First, as mentioned previously, R-ketamine has more potent and sustained antidepressant properties than S-ketamine in several rodent tests of depression.22,27 However, S-ketamine is known to be approximately 4 times more potent than R-ketamine at inhibiting NMDAR, which challenges the NMDAR hypothesis of ketamine action.22,27,31 Furthermore, animal studies indicate that treatment with the NMDAR antagonist MK-801, which binds to the same receptor site as ketamine, failed to produce sustained antidepressant effects.22,29 Moreover, although the selective NMDA 2B blocker Ro25–6981 has antidepressant effects in rodents, these effects have been reported to be less robust and/or shorter-lasting than ketamine.23,29,32 In addition, more recent human clinical trials indicate that alternative NMDAR antagonists lack ketamine’s robust, rapid and/or sustained antidepressant effects.13,22,27 Also, in contrast to ketamine, other NMDAR antagonists, such as memantine, AZD6765 and CP-101 606, have smaller effect sizes and produce shorter-lasting symptomatic improvements.13 Furthermore, 2 partial agonists at the NMDA glycine site, d-cycloserine and rapastinel (GLYX-13), appear to reduce depressive symptoms without clinically important psychotomimetic or dissociative side effects.13,27 However, to date, ketamine is the only NMDAR antagonist to consistently show antidepressant efficacy in multiple trials. Altogether, the hypothesis that NMDAR blockade is mainly responsible for the clinical efficacy of ketamine appears to be effectively challenged. Clearly, further investigation is warranted in order to clarify the contribution of NMDAR blockade to ketamine’s unique therapeutic profile and side effects.
Role of AMPA receptors
In contrast to the ambivalent findings concerning NMDAR inhibition, accumulating evidence supports the crucial involvement of AMPA receptors in mediating the antidepressant effects of ketamine.16,29,33,34 Studies in rodents show that ketamine produces a rapid and transient rise in glutamate release and cycling in the medial prefronal cortex (mPFC), which in turn leads to the acute activation of AMPA receptors, properties proposed to mediate the rapid antidepressant effects of ketamine.35,36 Subanesthetic doses of ketamine facilitate AMPAR-mediated synaptic transmission in the mPFC, as shown by intracellular recordings of AMPA -induced currents in rat mPFC pyramidal neurons37 as well as in the hippocampus, as measured by extracellular recordings and microiontophoresis of rat CA3 pyramidal neurons.38 One study found that ketamine applied to hippocampal slices potentiated CA1 AMPAR-mediated evoked neurotransmission within 30 minutes, which could not be attributed to increased presynaptic release probability.39 Interestingly, when the same experiment was performed in GluA2 knockout mice, both the ketamine-induced synaptic potentiation and behavioural antidepressant effects were absent.39 Moreover, quantitative electroencephalography (qEEG) performed in both humans and rodents within hours after ketamine administration has shown that this drug induces substantial increases in γ-band power, which depends on the activation of fast iono-tropic excitatory receptors, mainly AMPARs.22 In addition, ketamine treatment in rats can lead to upregulation of AMPAR subunit (GluA1 and GluA2) total or surface expression in the PFC and hippocampus, as measured by Western blot22,33,40,41 or by surface biotinylation assays,39 along with increased hippocampal AMPAR binding, as measured by autoradiography.42 Interestingly, postmortem studies have reported reductions in the mRNA expression levels of AMPA receptor subunits GluA1 and GluA3 in the perirhinal cortex,43 CA1 and dentate gyrus44 of patients with depression, and several animal studies similarly found that exposure to chronic stress leads to a decrease in the expression of AMPAR subunits in these brain regions.44–46 Site-specific AMPAR phosphorylation at serine, threonine or tyrosine residues in the intracellular C-terminal domain by various protein kinases is known to modulate the channel kinetics, localization, subunit composition and protein–protein interactions of AMPARs.28 In addition to regulating the number of AMPA receptors on the surface, ketamine has been shown to affect the phosphorylation state of AMPAR subunits. However, conflicting reports show both increased47 and decreased29 phosphorylation of the GluA1 subunit at a key residue (serine 845) in rodents hours after systemic injection of the drug, making it hard to draw any conclusions.
Importantly, several studies show that pretreating rats or mice with the AMPAR antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) 10 minutes before ketamine injection blocks its rapid and sustained antidepressant effect in the FST without affecting other behaviours, such as the ketamine-induced hyperloco-motion.21,22,29,33 Furthermore, NBQX administration 23.5 hours after ketamine treatment blocked its anti-depressant effects in the FST at 24 hours.22,33 Interestingly, preclinical studies indicate that group II metabotropic glutamate (mGlu2/3) receptor antagonists, such as MGS0039 and LY341495, possess ketamine-like anti-depressant effects, which can also be blocked by NBQX pretreatment and thus similarly involve AMPAR stimulation.33,48 In addition, the SSRI fluoxetine and the TCA imipramine also are associated with upregulation of AMPARs in the hippocampus or mPFC, whereas their antidepressant action in the FST can be abolished by NBQX, indicating that facilitation of AMPAR-mediated signalling might represent a downstream point of convergence in the antidepressant action of ketamine and classical anti-depressants.45,49 Taken together, these data indicate that activation of AMPA receptors, both at the time of injection and at later time points, is required for both the rapid and sustained antidepressant actions of ketamine.
Such findings encourage the investigation of drugs that enhance AMPAR function for their potential utility in treating depression. Preclinically, AMPAR-positive allosteric modulators (or AMPAkines, such as LY392098, LY451646 and Org 26576),6,28,50,51 the AMPAR agonist CX54620 and AMPA itself52 have dose-dependent antidepressant activity in rodents and can enhance the effects of ketamine when coadministered as an adjunctive. In addition, the AMPAkine Org 26576 showed some (although not significant) efficacy, good tolerability and even cognitive enhancement in depressed patients in a small pilot phase 1b clinical trial, but further investigation of AMPAkines for depression is needed.53 Therefore, convergent evidence reveals that postsynaptic AMPA receptor activation and upregulation is emerging as a crucial factor in mediating ketamine’s antidepressant effects29 —findings that are particularly important at a time when the NMDA receptor blockade hypothesis of ketamine’s antidepressant actions is hotly debated.
Downstream signalling pathways, synaptic and structural plasticity
Consensus appears to be growing as to the key downstream cascades mediating ketamine’s clinical efficacy. The most consistent structural changes associated with MDD involve neuronal atrophy and brain volume loss in the hippocampus and PFC, 2 brain regions highly implicated in the pathophysiology of depression and in mediating antidepressant responses.10,12,54 These findings are supported by electrophysiological experiments performed in rat hippocampal slices showing that acute stress causes an imbalance of synaptic plasticity favouring long-term depression (LTD) over long-term potentiation (LTP), which in turn can lead to synaptic hypofunction, destabilization and neuronal loss.5,55,56 In fact, chronic stress is known to induce reductions in spine density and in the length and number of dendritic branches in the rat hippocampus and PFC. Importantly, all these dysfunctions in synaptic and structural plasticity can be reversed by ketamine.23,54 We contend that the involvement of several neuroplasticity-related processes acting synergistically to induce an upregulation of AMPARs and other synaptic proteins, accompanied by an increase in synaptic strength and synaptogenesis, leading in turn to a rapid reversal of stress- and depression-induced neuronal dysfunction and atrophy, provide a compelling argument for a focus on AMPARs as a key to understanding the antidepressant actions of ketamine.
Chronic stress in animals and depression in humans are associated with decreases in the expression and/or function of BDNF in the PFC and hippocampus along with reports of lower BDNF blood levels in patients with MDD.5,9,57,58 On the other hand, many studies report that ketamine consistently causes an increase in BDNF translation and secretion in rodents, again in the hippocampus and PFC,2,20,30 although 1 study reported that ketamine’s antidepressant efficacy was preserved in bdnf+/− heterozygous null mice.51 In addition, BDNF is associated with antidepressant responses to classical antidepressants (e.g., SSRIs, TCAs), which lack efficacy in BDNF knockout mice.2,5,57 Although it takes several weeks for these agents to trigger BDNF-mediated synaptic plasticity, such changes can occur within hours following ketamine administration.2,5
Another key downstream convergence pathway implicated in depression susceptibility and ketamine action is the mTOR signalling pathway, which regulates activity-dependent synaptic plasticity and translation of synaptic proteins.41,59 In addition, mTOR activation is accompanied by inhibition of eukaryotic elongation factor 2 (eEF2) kinase, desuppression of eEF2 and increased synaptic protein synthesis (e.g., BDNF, GluA1–2, PSD95); promotes synapse formation and maturation; and is believed to underlie the synaptogenic effects of ketamine.20,41,59 Consistent with this, both the antidepressant and synaptogenic effects of ketamine are blocked by preadministration of rapamycin, a selective mTOR inhibitor.41 Importantly, mTOR activation is not seen following chronic treatment with any class of traditional anti-depressant drugs.5
Furthermore, consistent with the role of AMPAR in the actions of ketamine, the ketamine-induced increases in mTOR, BDNF and GluR1 levels can be prevented by pretreatment with the AMPA antagonist NBQX.20,29 Moreover, administration of AMPAkines appears to mimic these key molecular effects of ketamine and can be abolished by NBQX.6,20,30,33,34,48,51,52 Accumulating evidence indicates that ketamine enhances synaptogenesis and connectivity in the hippocampus, prefrontal cortex and associated regions via the activation of key signalling pathways, including those involving BDNF and mTOR.5 At the level of neural circuits, both animal and human imaging studies suggest that by activating these signalling cascades, ketamine is able to effectively reverse the loss of connectivity between the PFC and other limbic structures (e.g., amygdala) in depressed individuals. Through these actions, ketamine appears to restore appropriate top–down inhibitory control of hypothalamic–pituitary–adrenal (HPA) axis reactivity, negative emotion and anxiety.5,9
Ketamine metabolite
The latest piece of the ketamine puzzle was revealed in 2016 in a pivotal paper by Zanos and colleagues22 published in Nature showing that an active ketamine metabolite exhibits similar efficacy without key receptor binding properties or side effects of its parent compound.20 Ketamine is stereo-selectively metabolized into a wide range of metabolites, many of which are presumed to be clinically inactive owing to the absence of anesthetic properties resulting from NMDAR antagonism.31 Importantly, 1 ketamine metabolite, HNK, was observed to have 3-fold higher brain concentrations in female than in male rats, and this difference was postulated to underlie the enhanced potency of ketamine in females.20,22 Furthermore, this pivotal study showed that a metabolically inert form of ketamine with the same receptor binding properties but not metabolized into HNK lacked the sustained antidepressant actions of ketamine in several depression-relevant tasks in rodents.22 Interestingly, human data indicate a positive link between plasma HNK metabolite levels and antidepressant response to ketamine.60 Next, Zanos and colleagues22 showed that the HNK metabolite itself has robust rapid and sustained antidepressant action similar to ketamine, with (R,R)-HNK being more potent than (S,S)-HNK, mirroring the enantiomer-specific effects of the parent compound.
Similar to findings with ketamine, the AMPAR antagonist NBQX administered either before HNK injection or at the time of behavioural testing, blocked the rapid and sustained antidepressant effects of the metabolite.22 Intriguingly, HNK does not bind to or functionally inhibit NMDARs, but instead causes a dramatic increase in AMPAR-mediated synaptic transmission in hippocampal CA1 slices, a rapid upregulation of GluA1 and GluA2 AMPAR subunits in hippocampal synaptosomes (within an hour) and elevated levels of hippocampal BDNF (24 hours after treatment).22 However, to date the mechanisms underlying the actions of ketamine or its metabolite on AMPAR-mediated transmission remain unclear. Importantly, Zanos and colleagues22 did not evaluate the effects of HNK or ketamine on GluA1 and GluA2 phosphorylation, a process known to regulate channel trafficking and function, which limits any conclusion on the role of AMPAR in the effects of the ketamine metabolite. Finally, in contrast to ketamine, in preclinical tests HNK lacked sensory dissociation and hyperlocomotion effects, or reinforcing properties, and thus lacked the potential for abuse.22
Overall, these exciting findings indicate that this key metabolite of ketamine is necessary and sufficient in rats for antidepressant actions of the prodrug. As HNK does not inhibit NMDARs, questions remain as to the molecular target responsible for its behavioural and synaptic effects. One report claims that HNK can inhibit α7-nicotinic acetylcholine receptor function (at concentrations of 1 μM),31 but future studies are needed to define HNK’s exact mechanism of action. In addition, although these findings with HNK represent important progress in the field, the claim that ketamine’s clinical efficacy is exclusively due to an active metabolite should be considered with caution. As ketamine is administered intraperitoneally in rats, it undergoes extensive first-pass metabolism, so whereas the bioavailability of the parent compound can be as low as 20%, plasma and brain concentrations of its metabolites are relatively high.12 In contrast, ketamine is administered clinically as an IV infusion, thereby greatly reducing the first-pass liver metabolism, resulting in a different profile of ketamine/metabolite concentrations in the blood and central nervous system. Although important questions remain as to whether or not HNK is exclusively responsible for ketamine’s therapeutic actions, it is clear that it represents a new promising candidate antidepressant compound with an ability to modulate AMPAR function, which warrants further preclinical and clinical investigation.
Conclusion
A growing body of preclinical data challenges the prominent hypothesis that NMDA inhibition mediates the striking clinical effects of ketamine in the treatment of depression. An emerging hypothesis proposes that ketamine has the unique ability to increase the AMPA–NMDA receptor throughput by directly blocking NMDA receptors and indirectly enhancing AMPAR density and/or function, which in turn activates downstream synaptogenic signalling pathways (e.g., BDNF, mTOR) that restore synaptic strength and connectivity in the hippocampus and prefrontal cortex12,20,28,29 (see Fig. 1 for a convergent model61). Accordingly, we propose that future pre-clinical and clinical research should focus on several promising avenues, including more direct enhancement of AMPAR function as a therapeutic strategy, enantiomer-specific ketamine therapy, the efficacy and mechanism of action of ketamine metabolites, repeated administration of optimized doses of ketamine to extend therapeutic effects without exacerbating its side effects, and possibly combination therapy with ketamine and classical antidepressants. Importantly, there is every expectation that emerging knowledge of the molecular mechanisms underlying ketamine’s therapeutic and side effects will inform the development of much needed new, superior antidepressant agents.
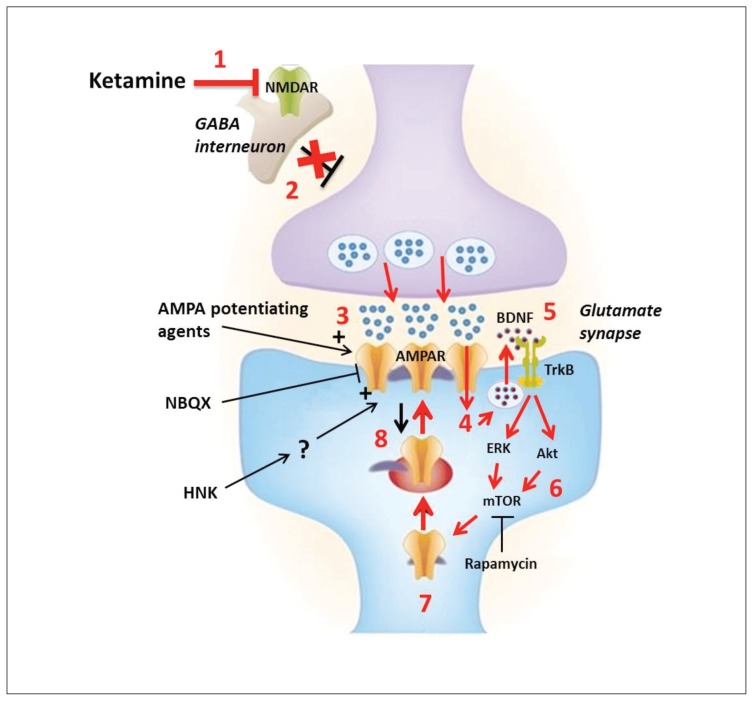
Fig. 1.
Convergent theory of ketamine’s mechanism of action in depression. 1) At low doses ketamine is thought to preferentially bind to and inhibit N-methyl-d-aspartate receptors (NMDARs) on γ-aminobutyric acid (GABA)ergic interneurons. 2) This results in reduced excitability of these inhibitory interneurons, which in turn causes disinhibition of glutamatergic neurons. 3) Increased depolarization of the presynaptic neuron leads to a surge of glutamate release, as reported in the medial prefrontal cortex (mPFC). 3) Released glutamate binds to and activates postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which conduct Na+ and Ca2+ into the cell. 4) The calcium influx and depolarization activates voltage-gated calcium channels (VDCCs). 5) The high local intracellular concentration of Ca2+ triggers the activity-dependent vesicular release of brain-derived neurotrophic factor (BDNF) into the synaptic space. 6) BDNF binds to and activates its surface receptor, tropomyosin receptor kinase B (TrkB), which activates 2 major downstream signalling cascades involving MEK–ERK and PI3K-Akt. These 2 pathways converge onto the mechanistic target of rapamycin (mTOR), which is a key regulator of protein synthesis and synaptic plasticity. 7) These events lead to disinhibition of synaptic protein translation (e.g., GluR1–2, PSD95, synapsin1) as well as BDNF, in part through the supressed phosphorylation of eukaryotic elongation factor 2 (eEF2). 8) These newly synthesized proteins are then inserted into the postsynaptic density, leading to further increases in AMPAR-mediated synaptic transmission and dendritic spine density, thus causing massive synaptogenesis. This series of events is believed to restore normal connectivity between key brain regions, such as the PFC and limbic structures like the hippocampus and amygdala. The AMPAR antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) completely abolishes ketamine’s antidepressant actions, supporting the role of AMPA receptors in mediating these effects. On the other hand, AMPA potentiating agents could represent a more targeted strategy of obtaining ketamine-like antidepressant effects. The ketamine metabolite, hydroxynorketamine (HNK), has been recently shown to facilitate AMPAR-mediated transmission, but the exact molecular target and mechanism of action of HNK remain unknown. Figure adapted with permission from from Sanacora and Schatzberg.61
Footnotes
Competing interests: No financial interest or any direct conflict of interest exists. Y.T. Wang and A.G Phillips declare a patent related to glutamate receptor function (A Peptide that Specifically Blocks Regulated AMPA Receptor Endocytosis and Hippocampal CA1 Long-term Depression; Europe 04789721.0, and United States 13/066,700). A.G Phillips also declares a pending patent for the use of d-Govadine in treatment of cognitive deficits.
Contributors: All authors designed the study. L.R. Aleksandrova acquired and analyzed the data and wrote the article, which all authors reviewed and approved for publication.
References
- 1.Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21:454–64. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz J, Murrough JW, Iosifescu DV. Ketamine for treatment-resistant depression — recent developments and clinical applications. Evid Based Ment Health. 2016;19:35–8. doi: 10.1136/eb-2016-102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Réus GZ, Abelaira HM, Tuon T, et al. Glutamatergic NMDA receptor as therapeutic target for depression. Adv Protein Chem Struct Biol. 2016;103:169–202. doi: 10.1016/bs.apcsb.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Hindmarch I. Expanding the horizons of depression beyond the monoamine hypothesis. Hum Psychopharmacol. 2001;16:203–18. doi: 10.1002/hup.288. [DOI] [PubMed] [Google Scholar]
- 5.Duman RS, Aghajanian GK, Sanacora G, et al. Synaptic plasticity and depression: new insights from stress and rapid-acting anti-depressants. Nat Med. 2016;22:238–49. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schechter LE, Ring RH, Beyer CE, et al. Innovative approaches for the development of antidepressant drugs — current and future strategies. NeuroRx. 2005;2:590–611. doi: 10.1602/neurorx.2.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huynh NN, McIntyre R. What are the implications of the STAR D trial for primary care — a review and synthesis. Prim Care Companion J Clin Psychiatry. 2008;10:91–6. doi: 10.4088/pcc.v10n0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duman RS, Li N, Liu RJ, et al. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–23. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 12.Abdallah CG, Adams TG, Kelmendi B, et al. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33:689–97. doi: 10.1002/da.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172:950–66. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 14.Kishimoto T, Chawla JM, Hagi K, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46:1459–72. doi: 10.1017/S0033291716000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller J, Pentyala S, Dilger J, et al. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 2016;6:185–92. doi: 10.1177/2045125316631267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park M, Niciu MJ, Zarate CA., Jr Novel glutamatergic treatments for severe mood disorders. Curr Behav Neurosci Rep. 2015;2:198–208. doi: 10.1007/s40473-015-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machado-Vieira R, Salvadore G, Diazgranados N, et al. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–50. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abelaira HM, Reus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Rev Bras Psiquiatr. 2013;35(Suppl 2):S112–20. doi: 10.1590/1516-4446-2013-1098. [DOI] [PubMed] [Google Scholar]
- 19.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Wang N, Yang C, et al. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014;29:419–23. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–11. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Thelen C, Sens J, Mauch J, et al. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav Brain Res. 2016;312:305–12. doi: 10.1016/j.bbr.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 26.Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77:357–67. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, Shirayama Y, Zhang JC, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du J, Machado-Vieira R, Maeng S, et al. Enhancing AMPA to NMDA throughput as a convergent mechanism for antidepressant action. Drug Discov Today Ther Strateg. 2006;3:519–26. doi: 10.1016/j.ddstr.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Yang C, Hu YM, Zhou ZQ, et al. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci. 2013;118:3–8. doi: 10.3109/03009734.2012.724118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moaddel R, Abdrakhmanova G, Kozak J, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in alpha7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698:228–34. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiménez-Sanchez L, Campa L, Auberson YP, et al. The role of GluN2A and GluN2B subunits on the effects of NMDA receptor antagonists in modeling schizophrenia and treating refractory depression. Neuropsychopharmacology. 2014;39:2673–80. doi: 10.1038/npp.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 2014;271:111–5. doi: 10.1016/j.bbr.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 34.Machado-Vieira R, Henter ID, Zarate CA., Jr New targets for rapid antidepressant action. Prog Neurobiol. 2015 doi: 10.1016/j.pneuro-bio.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdhury GM, Zhang J, Thomas M, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2016;22:120–6. doi: 10.1038/mp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Björkholm C, Jardemark K, Schilstrom B, et al. Ketamine-like effects of a combination of olanzapine and fluoxetine on AMPA and NMDA receptor-mediated transmission in the medial prefrontal cortex of the rat. Eur Neuropsychopharmacol. 2015;25:1842–7. doi: 10.1016/j.euroneuro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 38.El Iskandrani KS, Oosterhof CA, El Mansari M, et al. Impact of subanesthetic doses of ketamine on AMPA-mediated responses in rats: an in vivo electrophysiological study on monoaminergic and glutamatergic neurons. J Psychopharmacol. 2015;29:792–801. doi: 10.1177/0269881115573809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nosyreva E, Szabla K, Autry AE, et al. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang B, Zhang JC, Han M, et al. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2016 Aug 4; doi: 10.1007/s00213-016-4399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tizabi Y, Bhatti BH, Manaye KF, et al. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beneyto M, Kristiansen LV, Oni-Orisan A, et al. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 44.Duric V, Banasr M, Stockmeier CA, et al. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;16:69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freudenberg F, Celikel T, Reif A. The role of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: Central mediators of pathophysiology and antidepressant activity? Neurosci Biobehav Rev. 2015;52:193–206. doi: 10.1016/j.neubiorev.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Toth E, Gersner R, Wilf-Yarkoni A, et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–32. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- 47.Burgdorf J, Zhang XL, Nicholson KL, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–42. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karasawa J, Shimazaki T, Kawashima N, et al. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042:92–8. doi: 10.1016/j.brainres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 49.Du J, Suzuki K, Wei Y, et al. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Griffey K, Clay M, et al. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001;40:1028–33. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 51.Lindholm JS, Autio H, Vesa L, et al. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf(+)/(−) heterozygous null mice. Neuropharmacology. 2012;62:391–7. doi: 10.1016/j.neuropharm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 52.Akinfiresoye L, Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology (Berl) 2013;230:291–8. doi: 10.1007/s00213-013-3153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nations KR, Dogterom P, Bursi R, et al. Examination of Org 26576, an AMPA receptor positive allosteric modulator, in patients diagnosed with major depressive disorder: an exploratory, randomized, double-blind, placebo-controlled trial. J Psychopharmacol. 2012;26:1525–39. doi: 10.1177/0269881112458728. [DOI] [PubMed] [Google Scholar]
- 54.Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: What are the connections? Neuroscience. 2013;251:108–19. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Dong Z, Cao J, et al. NR2B-containing N-methyl-D-aspartate subtype glutamate receptors regulate the acute stress effect on hippocampal long-term potentiation & long-term depression in vivo. Neuroreport. 2006;17:1343–6. doi: 10.1097/01.wnr.0000227994.07799.6c. [DOI] [PubMed] [Google Scholar]
- 56.Xiong W, Wei H, Xiang X, et al. The effect of acute stress on LTP and LTD induction in the hippocampal CA1 region of anesthetized rats at three different ages. Brain Res. 2004;1005:187–92. doi: 10.1016/j.brainres.2004.01.051. [DOI] [PubMed] [Google Scholar]
- 57.Björkholm C, Monteggia LM. BDNF — a key transducer of anti-depressant effects. Neuropharmacology. 2016;102:72–9. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murakami S, Imbe H, Morikawa Y, et al. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–39. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Ignácio ZM, Reus GZ, Arent CO, et al. New perspectives on the involvement of mTOR in depression as well as in the action of anti-depressant drugs. Br J Clin Pharmacol. 2016;92:1280–90. doi: 10.1111/bcp.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zarate CA, Jr, Brutsche N, Laje G, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–8. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanacora G, Schatzberg AF. Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology. 2015;40:259–67. doi: 10.1038/npp.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]