Abstract
Background
Schizophrenia is associated with important disturbances in empathy that are related to everyday functioning. Empathy is classically defined as including affective (sharing others’ emotions) and cognitive (taking others’ cognitive perspectives) processes. In healthy individuals, studies on empathy for pain revealed specific brain systems associated with these sets of processes, notably the anterior middle cingulate (aMCC) and anterior insula (AI) for affective sharing and the bilateral temporoparietal junction (TPJ) for the cognitive processes, but the integrity of these systems in patients with schizophrenia remains uncertain.
Methods
Patients with schizophrenia and healthy controls performed a pain empathy task while undergoing fMRI scanning. Participants observed pictures of hands in either painful or nonpainful situations and rated the level of pain while imagining either themselves (self) or an unknown person (other) in these situations.
Results
We included 27 patients with schizophrenia and 21 healthy controls in our analyses. For the pain versus no pain contrast, patients showed overall typical activation patterns in the aMCC and AI, with only a small part of the aMCC showing reduced activation compared with controls. For the other versus self contrast, patients showed an abnormal modulation of activation in the TPJ bilaterally (extending to the posterior superior temporal sulcus, referred to as the TPJ/pSTS).
Limitations
The design included an unnecessary manipulation of the visual perspective that reduced the number of trials for analysis. The sample size may not account for the heterogeneity of schizophrenia.
Conclusion
People with schizophrenia showed relatively intact brain activation when observing others’ pain, but showed abnormalities when asked to take the cognitive perspectives of others.
Introduction
Patients with schizophrenia often present with important derficits in their social functioning even when their psychotic symptoms have abated. There is thus an increased interest in understanding the obstacles to proper functioning in patients with schizophrenia, particularly their deficits in social cognition. One aspect of social cognition that is now garnering increasing attention in schizophrenia research is empathy (i.e., the capacity to share and understand the emotional states of others).1,2 Traditionally, affective and cognitive processes are thought to contribute to the complex multifaceted experience of empathy. The former set of processes is called upon when the affective states of others are shared (affective sharing), whereas the latter reflects effortful processes of inferring the emotional states and taking the cognitive perspectives of others.3,4 The relative importance of each set of processes in the experience of pain empathy has been recently debated. Some authors propose that empathy for pain is based mainly on processes “functionally equivalent to those engaged by first-hand experience of pain” (i.e., shared representations between self and others),5 whereas others argue that empathy relies instead on our capacity to adopt others’ perspectives and infer their emotional states.6
Previous questionnaire-based research in schizophrenia has suggested abnormalities both in the cognitive and the affective processes, as evidenced respectively by lower perspective taking and increased personal distress scores compared with controls on the Interpersonal Reactivity Index (IRI7).1,8–11 Recent work has highlighted important associations between impaired cognitive processes underlying empathy and reduced social functioning in individuals with schizophrenia10,11 as well as between impaired affective sharing and reduced insight.12 Despite the functional impact of empathy impairments in individuals with schizophrenia, the exploration of their neural bases is relatively recent. Yet, neuroimaging could help us to better understand the processes underlying empathy deficits in patients with this disorder.
In healthy individuals, most neuroimaging studies of empathy have focused on empathy for physical pain. This is likely because pain is one of the rare affective states with a strong physiologic component in addition to the representational dimension. Furthermore, it is relatively easy to find experimental situations that trigger pain in another person and thus elicit empathy. These neuroimaging studies have identified the bilateral anterior insula (AI) and the anterior middle cingulate cortex (aMCC), often extending to the supplementary motor area, as the core neural network underlying the affective set of empathy processes.13,14 The cognitive processes involved in pain empathy have been consistently associated with activation in the bilateral temporoparietal junction (TPJ),15–18 sometimes together with the precuneus16,17 or the medial prefrontal cortex (mPFC).15,19
The few previous neuroimaging studies of empathy in patients with schizophrenia used various tasks and stimuli, asking participants to infer the emotional states of characters presented in verbal stories,20 comic strips,21–23 photographs/static pictures11,24,25 or video clips.26 Overall, previous neuroimaging studies reported abnormal activations in patients with schizophrenia in a variety of brain regions that authors interpreted as underlying both the affective and cognitive processes contributing to empathy. These results thus suggest alterations of both sets of empathy processes in patients with this disorder. However, there is an important heterogeneity among studies in the brain regions showing abnormal activation in patients with schizophrenia, which is not surprising given the variety of tasks used. Moreover, previous studies have often focused solely on the affective or the cognitive processes underlying empathy,11,20,24 have investigated both of them but using different sets of stimuli,21,23,25 or have used tasks that did not enable researchers to disentangle the activation associated with each set of processes.26 Importantly, although tasks using physical pain stimuli are the most commonly used tasks for investigating empathy in healthy people, fMRI studies in patients with schizophrenia have typically focused on empathy for emotions such as sadness, anger or happiness. Yet, the breadth of studies in healthy individuals in the past 10 years led to the development of many validated tasks and to the accumulation of findings on the processes and brain activations underlying the experience of empathy for pain. This growing body of knowledge is relevant to guide our interpretation and understanding of findings in patients with schizophrenia.
To our knowledge, the only fMRI study assessing empathy for pain in schizophrenia conducted to date was the recent study by Horan and colleagues.27 Their study relied on the task developed by Lamm and colleagues17 in which participants were presented with aversive tones and then with video clips showing individuals listening to such tones (presented as medical patients receiving a treatment involving the painful sounds). Horan and colleagues27 manipulated perspective taking by asking participants to imagine themselves or another person experiencing the pain in the video clips. They also manipulated cognitive appraisal by telling participants that the treatment had been effective or not for each individual. The authors performed region of interest (ROI) analyses, focusing on the aMCC and bilateral AI. Group comparisons across all task conditions revealed no significant group difference in these brain regions. However, a significant perspective × group interaction emerged, such that patients exhibited greater activation for the other condition than for the self condition in these regions, whereas the control group showed the reverse pattern of activation. Additionally, in whole brain analyses, controls exhibited greater activation than patients in the posterior cingulate and precuneus for the self versus other contrast. Conversely, for the other versus self contrast, patients showed greater activation than controls in the bilateral frontal poles. These results led the authors to conclude that although patients show relatively intact sensitivity to the pain of others, between-group differences were observed when the modulatory impact of perspective taking was considered. Although TPJ activation is often observed for other versus self contrasts,15–18 neither controls nor patients showed differences in activation in that brain region in the study by Horan and colleagues.27 In addition, their study included only stimuli depicting pain, which did not allow the observation of painful versus nonpainful situations to be compared.
The present fMRI study aimed to improve our understanding of empathy dysfunctions in patients with schizophrenia by investigating the neural correlates associated with both the affective and cognitive processes contributing to empathy. To benefit from the knowledge acquired studying healthy indivdiuals, we chose a well-known pain empathy task based on the presentation of hands in painful or non-painful situations (to manipulate the affective processes) while instructing participants to imagine either themselves or an unknown person in these situations (to manipulate cognitive perspective taking).15,16,18 This task thus provides a means to examine both the affective and cognitive processes involved in empathy separately using the same set of stimuli as well as the interaction between them. Moreover, we recently showed in a group of healthy participants (also included in this study) that a pseudodynamic version of this task elicited typical bilateral AI/aMCC and TPJ activations associated respectively with the affective and cognitive processes constituting the experience of pain empathy.18
Given previous knowledge on the neural bases of empathy for physical pain in healthy people and findings from questionnaire-based and fMRI studies in schizophrenia, we expected that patients would show abnormal activations compared with controls in the core regions associated with both the affective and the cognitive processes involved in pain empathy. More specifically, in line with the increased scores on the personal distress scale of the IRI,1 we expected patients to show greater activations than controls in the bilateral AI and aMCC (typically associated with affective sharing13,14). We also hypothesized that patients would show decreased activations in the bilateral TPJ (involved in the cognitive processes underlying empathy15–18) compared with controls given previous findings of lower perspective taking scores on the IRI.1 Then, we expected that patients’ activations in the bilateral AI and aMCC would correlate with their personal distress scores on the IRI while activations in the bilateral TPJ would correlate with their perspective taking scores. Finally, we sought to explore associations between brain activations in these regions and patients’ symptomatology and level of functioning.
Methods
Participants
We recruited adults with schizophrenia or schizoaffective disorder (DSM-IV-TR) and healthy controls, matched for age, sex and parental socioeconomic status28 to participate in this study. To be included, healthy controls had to have no personal or familial history of psychotic disorders. All participants underwent a semistructured diagnostic interview based on the Structured Clinical Interview for DSM-IV-TR (SCID)29 that also included the Positive and Negative Syndrome Scale (PANSS)30 as an assessment of patients’ symptomatology. Questions about functioning were also integrated in order to rate the Schizophrenia Objective Functioning Instrument (SOFI).31 All participants completed the matrix reasoning and the vocabulary subtests of the Wechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV)32 to estimate their IQ. All participants were informed of the study requirements and provided written consent before their participation. All patients were stabilized outpatients recruited via their treating psychiatrists, who judged that they were able to participate in the study and provide their informed consent. The local ethics committee of the Centre de recherche CERVO, formerly the Centre de Recherche de l’Institut Universitaire en Santé Mentale de Québec, approved this study.
Measures of empathic traits
We used the IRI, a 28-item self-report questionnaire previously used in a schizophrenia population,1 to assess personal traits associated with the cognitive (perspective taking and fantasy subscales) and affective (personal distress and empathic concern subscales) processes contributing to empathy experience.7 We also considered the recent proposal of Koller and Lamm,33 who subdivided the perspective taking subscale into “cognitive-comparative” and “self-projection and simulation” dimensions.
Functional MRI task
Stimuli
The stimuli used during fMRI all depict a right hand performing a daily life action. As shown in Appendix 1, Figure S1, available at jpn.ca, our validated stimuli consisted of pseudodynamic scenarios, each comprising 3 coloured pictures presented in a rapid succession (150 ms, 150 ms and 5500–6100 ms) to reinforce the impression of an ecological movement.18,34–36 The present task involved 3 manipulations.
Stimulus outcome (pain v. no pain): Each stimulus resulted in either a painful or nonpainful event. Pain and no pain conditions were visually similar, with only the last picture differing.
Instruction (self v. other): Instructions required participants to imagine that the hand was either their own (self) or that of an unknown person (other).
Visual perspective (first v. third person): Stimuli were acquired by capturing the same scenario using 2 different cameras — 1 positioned according to a first person visual perspective (arm at a 0° ± 45° angle) and 1 positioned according to a third person visual perspective (arm at 180° ± 45° angle).
The task thus followed a 2 (pain v. no pain) × 2 (self v. other) × 2 (first person v. third person visual perspective) design resulting in 8 conditions: PS1 (pain, self, first person visual perspective), PS3 (pain, self, third person visual perspective), PO1 (pain, other, first person visual perspective), PO3 (pain, other, third visual perspective) and 4 no pain (NP) equivalents (Appendix 1, Table S1).
Procedure
During the task, participants were asked to imagine and rate the level of pain they (self) or an unknown person (other) would feel in each situation by using a button pad with their left hand to move a cursor along a visual analogue scale ranging from “no pain” to “maximum pain.” The initial cursor position was randomized across the trials.
Participants completed 2 fMRI runs, each including 32 pain and 32 no pain stimuli presented either in a first person or a third person visual perspective (counterbalanced). The self and other conditions were presented in blocks, with 4 blocks of each condition for each run. Before each block, the self/other instruction appeared on the screen for 10 seconds (the words “self” or “other” were also displayed below the image during the rating period). Eight stimuli and 3 fixation crosses of jittered duration (2500–8500 ms) were presented in each block.
Behavioural analyses
For each scenario, the final cursor position on the scale was converted into scores ranging from 0 (no pain) to 100 (maximum pain). Response time was defined as the time between the onset of the scale and participants’ final ratings. Despite a floor effect on no pain ratings, the effects tested by a 2 (controls v. patients) × 2 (pain v. no pain) × 2 (self v. other) analysis of variance (ANOVA) were normally distributed.
Functional MRI data acquisition and processing
We used a Philips Achieva 3 T scanner to measure changes in blood oxygen–level dependent (BOLD) signal (T2*-weighted) using a gradient echo-planar imaging (EPI) sequence (repetition time [TR] 3000 ms, echo time [TE] 35 ms, field of view [FOV] 230 mm, flip angle 90°, 128 × 128 matrix, 45 slices of 3 mm, no gap, voxel size 1.8 mm × 1.8 mm × 3 mm). In each session 195 EPI volumes were acquired along the anterior–posterior commissure (AC–PC) plane. Structural images were acquired using a MPRAGE sequence (TR 8.2 ms, TE 3.7 ms, FOV 250 mm, flip angle 8°, 256 × 256 matrix, 180 slices/volume, slice thickness 1 mm, no gap, voxel size 1 mm3).
Statistical analysis
Data were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience). Images were corrected for slice timing and realigned to correct for head movements. Larger volume-to-volume movements (> 0.5 mm between 2 adjacent volumes) were corrected using the ArtRepair toolbox. Structural images were then coregistered to the mean realigned EPI image using a linear rigid transformation (6 motion parameters) and segmented. Segmentation parameters were used to normalize functional data into a standard anatomic space based on the ICBM-152 template (Montreal Neurological Institute [MNI]). Functional images were resampled at a resolution of 2 mm3 and then spatially smoothed using an 8 mm isotropic Gaussian kernel. For each participant, we then defined a design matrix including a separate regressor for each of our 8 conditions (Appendix 1, Table S1) corresponding to the time interval between the onset of the first image and the end of the response period. The design matrix also included 7 regressors of noninterest (instructions and 6 movement regressors). Low-frequency noise was removed using a 440 s high-pass filter (minimum intertrials within a single condition interval).
Whole-brain analyses were conducted to isolate brain regions related to both the affective and the cognitive sets of empathy processes at the participant and group levels. For brain activations associated with affective processes, we computed the pain > no pain contrast: (PS1 + PS3 + PO1 + PO3) > (NPS1 + NPS3 + NPO1 + NPO3). To isolate the activations associated with the cognitive perspective taking manipulation, we computed the PO1 > PS1 contrast. This choice was based on our recent observation that in healthy participants, the brain areas related to the cognitive processes are specifically recruited when hands are presented in a first person visual perspective.18 These first-level contrasts (pain > no pain and PO1 > PS1) were then used to assess regions of common activation between patients and controls by computing conjunctions between the 1-sample t tests of both groups (we used the masking procedure as well as 1-way ANOVAs, as implemented in SPM, to perform this conjunction). We then examined between-group differences in both directions using 2-sample t tests.
We also examined the effect of the cognitive perspective taking manipulation for the no pain scenarios and compared it to the pain conditions with the (PO1 > PS1) > (NPO1 > NPS1) contrasts to investigate the specificity to the pain condition.
We performed all analyses using a voxel threshold of p < 0.001 with a cluster size (k) of 90 voxels, corresponding to a whole-brain corrected p < 0.05 based on Monte Carlo simulation. In addition, for brain regions where we had our a priori hypotheses (i.e., aMCC and bilateral AI for the pain > no pain contrast and bilateral TPJ for the PO1 > PS1 contrast), we also reported activations at a cluster threshold of k ≥ 10. This less conservative threshold corresponds to a previous standard in neuroimaging studies that is still used in some recent work37 and allows for a desirable balance between type I and type II errors.38
Correlations
In the patients group, we investigated associations between brain activations and empathic traits for the brain regions where we had a priori hypotheses and where we observed between-group differences. We used the MarsBaR toolbox to define ROIs using 6 mm (3 voxels) radius spheres centred on the peak coordinates and extracted mean β values for each ROI. For the ROI related to the pain > no pain contrast (see the whole-brain investigation of pain observation section in Results), we extracted mean β values specifically for the pain condition. Similarly, for ROIs associated with the PO1 > PS1 contrast (i.e., bilateral TPJ/pSTS; see the whole-brain investigation of the cognitive perspective taking manipulation section in Results), mean β values were extracted specifically for the PO1 condition. We considered the left and right TPJ separately, as we could not assume the functional homogeneity of these 2 brain regions.39,40 We computed Pearson correlations between these mean β values and IRI subscores of interest, perspective taking that targets the cognitive processes contributing to empathy, and personal distress that targets the affective processes. These specific subscales were selected because they are representative of the construct and because previous questionnaire-based studies of schizophrenia reported abnormal scores on these specific subscales.1 We also explored the association between the mean β values and patients’ symptomatology (PANSS 5-factor model41) as well as patients’ level of functioning (each of the 4 SOFI subscales31).
Results
Study sample
We included 27 adults with schizophrenia or schizoaffective disorder and 21 matched healthy controls in our analyses. All participants were right-handed and had no history of head injury or neurologic disorders. Table 1 summarizes the demographic and clinical characteristics of the 2 groups.
Table 1.
Demographic and clinical characteristics of participants
| Group; no. or mean ± SD | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Schizophrenia (n = 27) | Control (n = 21) | Statistical comparison | p value |
| Sex, male:female | 23:4 | 17:4 | χ2 = 0.151 | p = 0.70 |
| Age, yr | 29.7 ± 8.6 | 29.2 ± 7.9 | t = −0.198 | p = 0.84 |
| Parental socioeconomic status* | 45.8 ± 15.5 | 41.6 ± 14.9 | t = −0.922 | p = 0.36 |
| IQ† | 99.9 ± 15.2 | 109.3 ± 15.4 | t = 2.121 | p = 0.039 |
| Schizophrenia:schizoaffective disorder | 18:9 | — | — | — |
| Illness duration, yr | 7.6 ± 7.9 | — | — | — |
| Antipsychotic medication, atypical:typical:atypical + typical | 24:0:3 | — | — | — |
| Chlorpromazine equivalent, mg | 547.7 ± 474.7 | — | — | — |
| PANSS‡ | ||||
| Positive | 13.8 ± 6.5 | — | — | — |
| Negative | 16.0 ± 6.3 | — | — | — |
| Cognitive/disorganization | 9.6 ± 3.0 | — | — | — |
| Depression/anxiety | 8.5 ± 3.1 | — | — | — |
| Excitement/hostility | 6.6 ± 3.0 | — | — | — |
| SOFI§ | ||||
| Living situation | 74.3 ± 16.4 | — | — | — |
| Instrumental activities of daily living | 68.1 ± 17.5 | — | — | — |
| Productive activities and role functioning | 44.1 ± 26.7 | — | — | — |
| Social functioning | 59.7 ± 16.4 | — | — | — |
PANSS = Positive and Negative Syndrome Scale; SD = standard deviation; SOFI = Schizophrenia Objective Functioning Instrument.
Assessed using the Hollingshead index.
Assessed using the matrix reasoning and the vocabulary subtests of the Wechsler Adult Intelligence Scale – Fourth Edition.
Five-factor model by Lehoux and colleagues.41
Data available for 26 patients.
Empathic traits
As reported in Table 2, patients showed reduced perspective taking (t46 = 2.27, p = 0.028) and elevated personal distress scores (t46 = −2.13, p = 0.039) on the IRI compared with controls. No significant difference was found for empathic concern (t46 = −0.18, p = 0.86) or fantasy scores (t46 = −0.53, p = 0.60). Considering the subdivision of the perspective taking subscale proposed by Koller and Lamm,33 controls presented with mean scores of 8.57 ± 1.12 for the “cognitive-comparative” dimension and 7.00 ± 1.55 for the “self-projection and simulation” dimension. Patients showed mean scores of 7.93 ± 2.09 and 7.00 ± 1.84 for the “cognitive-comparative” and the “self-projection and simulation” dimensions, respectively. The groups did not differ significantly for the “cognitive-comparative” dimension (t46 = 1.37, p = 0.18) and showed the same mean scores for the “self-projection and simulation” dimension (t46 = 0.00, p > 0.99). As we used a different version of the IRI in the present study (the original 28-item version) than that used by Koller and Lamm33 (16-item version), we computed the 2-sample t test this time for the mean scores on items that were specific to the 28-item version of the IRI and found a significant group difference (t46 = 3.66, p = 0.001).
Table 2.
Empathic traits and behavioural results on the fMRI task
| Group; mean ± SD | ||||
|---|---|---|---|---|
|
|
||||
| Trait/result | Schizophrenia (n = 27) | Control (n = 21) | Statistical comparison | p value |
| Empathic traits (IRI) | ||||
| Empathic concern | 26.4 ± 3.9 | 26.2 ± 4.0 | t = −0.18 | p = 0.86 |
| Personal distress | 18.0 ± 5.2 | 14.9 ± 4.8 | t = −2.13 | p = 0.039 |
| Fantasy | 21.4 ± 6.1 | 20.6 ± 4.3 | t = −0.53 | p = 0.60 |
| Perspective taking | 25.4 ± 4.3 | 27.9 0 ± 2.9 | t = 2.27 | p = 0.028 |
| Mean pain ratings (out of 100)* | p < 0.001 | |||
| Pain other | 63.4 ± 15.0 | 65.5 ± 10.9 | ||
| Pain self | 61.5 ± 13.3 | 63.5 ± 15.2 | ||
| No pain other | 4.9 ± 7.3 | 3.5 ± 5.6 | ||
| No pain self | 4.7 ± 5.3 | 3.5 ± 5.6 | ||
| Mean response time, ms* | p < 0.001 | |||
| Pain other | 2470.5 ± 465.3 | 2453.5 ± 440.9 | ||
| Pain self | 2501.6 ± 404.9 | 2412.3 ± 393.5 | ||
| No pain other | 2002.0 ± 407.0 | 1842.7 ± 234.6 | ||
| No pain self | 2168.4 ± 431.7 | 1886.9 ± 267.7 | ||
IRI = Interpersonal Reactivity Index; SD = standard deviation.
Data available for 26 patients.
Behavioural results
Behavioural data were missing for 1 patient owing to technical problems. Mean pain ratings and response times are reported in Table 2. There was a main pain/no pain effect on ratings (F1,45 = 990.99, p < 0.001) and response times (F1,45 = 69.76, p < 0.001). For response times, trends emerged toward a main effect of self/other instruction (F1,45 = 3.2, p = 0.08), a group × instruction interaction (F1,45 = 3.01, p = 0.09) and a stimulus outcome × instruction interaction (F1,45 = 3.42, p = 0.07). All other effects or interactions were nonsignificant (all F < 2.24, all p > 0.14).
Functional MRI results
Whole-brain investigation of pain observation (pain v. no pain)
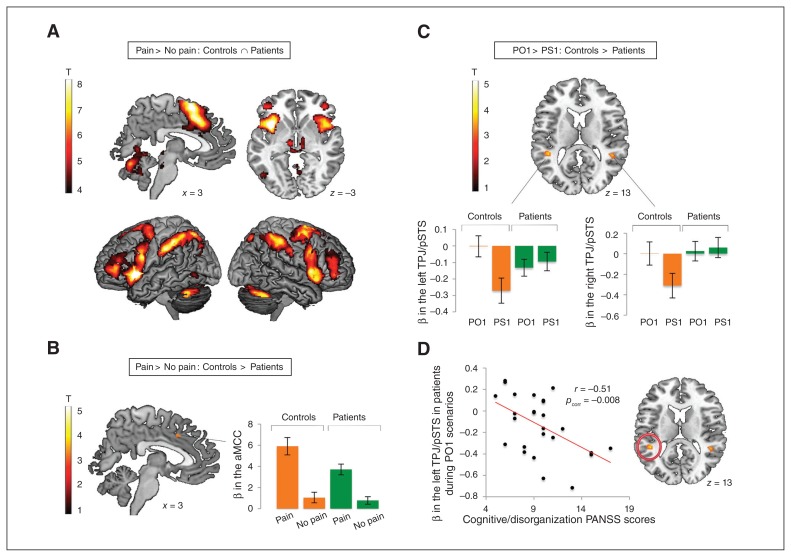
As reported in Fig. 1A and Table 3, the controls ∩ patients conjunction for the pain > no pain contrast revealed common activations in large clusters encompassing the aMCC extending to the supplementary motor area, the bilateral AI extending to the inferior and middle frontal gyrus, and in other frontal and parietal regions. The controls > patients comparison for this contrast (Fig. 1B and Table 3) revealed a difference in activation in a small portion of the aMCC, such that patients showed significant activation in that region that was of lesser magnitude than that in healthy controls. We found no significant group difference in the AI. No regions showed more activation in patients than controls.
Fig. 1.
Functional MRI results for the observation of pain (v. no pain) and the manipulation of cognitive perspective taking (other v. self). A) Conjunction of the 2 groups for the pain > no pain contrast. B) Between-groups comparison for the pain > no pain contrast and β values extracted for each condition and group in the region showing a significant between-groups difference. C) Between-groups comparison for the PO1 > PS1 contrast and β values for each condition and group in brain regions showing a significant between-groups difference. D) Correlation between patients’ left temporoparietal junction/posterior superior temporal sulcus (TPJ/pSTS) activation during the PO1 condition and cognitive/disorganization symptoms. The threshold used for significance was p < 0.001, uncorrected, with k = 90. A less conservative threshold (k = 10) was used for regions with a priori hypotheses (anterior middle cingulate cortex [aMCC], bilateral anterior insula [AI] and TPJ). PANSS = Positive and Negative Syndrome Scale.
Table 3.
Brain regions reaching statistical significance for each analysis of interest*
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Brain region | Lat. | Cluster size (k) | x | y | z | t value |
| Pain > no pain: controls ∩ patients | ||||||
| Superior parietal sulcus | R | 750 | 16 | −72 | 52 | 6.4 |
| Superior occipital gyrus | R | 26 | −64 | 34 | 4.71 | |
| Superior parietal sulcus/precuneus | R | 14 | −70 | 42 | 4.49 | |
| Inferior occipital/occipito-temporal cortex | L | 233 | −50 | −68 | −4 | 4.65 |
| Cerebellum (crus1) | R | 3707 | 36 | −54 | −32 | 7.41 |
| Cerebellum (crus1) | L | −40 | −56 | −32 | 7.2 | |
| Cerebellum (declive 6) | R | 28 | −66 | −30 | 7.18 | |
| Inferior parietal/intraparietal sulcus | L | 3862 | −46 | −36 | 44 | 8.33 |
| Inferior parietal/supramarginal gyrus | L | −54 | −26 | 34 | 8.12 | |
| Superior parietal sulcus | L | −22 | −66 | 58 | 8.01 | |
| Inferior parietal/supramarginal gyrus | R | 2386 | 54 | −32 | 46 | 7.91 |
| Inferior parietal/intraparietal sulcus | R | 44 | −36 | 40 | 7.27 | |
| Inferior parietal/posterior parietal gyrus | R | 48 | −40 | 50 | 7.19 | |
| Anterior insula/inferior frontal cortex | L | 22 920 | −36 | 14 | −4 | 10.45 |
| Supplementary motor area | R | 2 | 10 | 60 | 9.46 | |
| Anterior middle cingulate cortex | R | 4 | 16 | 44 | 8.98 | |
| Pain > no pain: controls > patients | ||||||
| Anterior middle cingulate cortex/anterior cingulate cortex | R | 24 | 6 | 16 | 38 | 3.56 |
| PO1 > PS1: controls > patients | ||||||
| TPJ/pSTS | R | 35 | 46 | −44 | 14 | 4.33 |
| TPJ/pSTS | L | 92 | −44 | −42 | 12 | 4.47 |
| TPJ/pSTS | L | −44 | −38 | 4 | 3.84 | |
L = left; MNI = Montreal Neurological Institute; pSTS = posterior superior temporal sulcus; R = right; TPJ = temporoparietal junction.
The threshold used for significance was p < 0.001, uncorrected, with k = 90. A less conservative threshold (k = 10) was used for regions with a priori hypotheses (middle cingulate cortex, bilateral anterior insula and TPJ).
Whole-brain investigation of the cognitive perspective taking manipulation (other v. self)
No significant common activation was observed for the controls ∩ patients conjunction for the PO1 > PS1 contrast. In controls, the PO1 > PS1 contrast revealed significant activation in the bilateral TPJ at the border of the posterior part of the superior temporal sulcus (TPJ/pSTS), as reported in the study by Vistoli and colleagues18 (the present study included the same healthy participants). Patients showed significant activation in the precuneus, but not in the TPJ/pSTS. As shown in Table 3 and Fig. 1C, the controls > patients comparison for the PO1 > PS1 contrast accordingly revealed a significant difference in activation in the bilateral TPJ/pSTS. This interaction remained significant when we included the between-groups difference in IQ as a covariate. The patients > controls comparison was not significant.
Effect of self/other instruction on no pain scenarios
As reported in more detail in Appendix 1, the NPO1 > NPS1 contrast revealed no activation in the TPJ/pSTS for either group. The (PO1 > PS1) > (NPO1 > NPS1) contrast notably revealed significant activations in the bilateral TPJ in controls, but not in patients.
Correlations
Correlations with empathic traits
Activation in the aMCC during the pain condition in patients did not significantly correlate with the IRI (personal distress: r = −0.33, p = 0.09; perspective taking: r = 0.04, p = 0.83). For the right TPJ/pSTS, activation shown by patients during the PO1 condition was positively correlated with their perspective taking score on the IRI (r = 0.46, p = 0.015). However, when considering the 2 dimensions of the perspective taking subscale of the IRI proposed by Koller and Lamm,33 no significant associations were found for either of the 2 dimensions (cognitive–comparative dimension: r = 0.206, p = 0.30; self-projection and simulation dimension: r = 0.312, p = 0.11). Patients’ activation in the right TPJ/pSTS during the PO1 condition did not correlate with their personal distress scores (r = −0.28, p = 0.15). Finally, no significant correlation was found for the left TPJ/pSTS (personal distress: r = 0.01, p = 0.98; perspective taking: r = 0.12, p = 0.56).
Exploratory correlations with symptomatology and level of functioning
No correlation was found between the aMCC or right TPJ/pSTS and the symptomatology or the level of functioning (all r < −0.32, all p > 0.12). Similarly, we found no correlation between the left TPJ/pSTS and the level of functioning (all r < −0.27, all p > 0.19). For the left TPJ/pSTS, patients’ activation during the PO1 condition showed negative correlations with both the positive (r = −0.39, p = 0.046) and the cognitive/disorganization factors (r = −0.51, p = 0.008) from the PANSS. Although these correlations would not survive a strict correction for multiple comparisons (9 scores × 3 ROIs [aMCC, right and left TPJ/pSTS], corrected α = 0.05/27 = 0.002), the correlation with the cognitive/disorganization symptoms nonetheless showed a large effect according to the guidelines by Cohen42 and approached the corrected α threshold. In addition, this correlation was still significant when the between-groups difference in IQ was taken into account (Fig. 1D). The same correlation using activation in the PS1 condition was not significant (r = −0.18, p = 0.37), and the 2 correlations (using β values from the PO1 and PS1 conditions) were significantly different, as tested using a Fisher r to z transformation for dependent groups (z = −1.84, p = 0.033, 1-tailed).
Discussion
This study used a classic pain empathy paradigm based on the observation of hands in either painful or nonpainful everyday life situations and required participants to imagine either themselves or an unknown person in these situations.15,16,18 Patients with schizophrenia presented with the typical pattern of activation for pain observation in brain regions including in the AI and aMCC; only a small portion of the aMCC showed significantly reduced activation compared with healthy controls. Furthermore, there was no difference between the groups in pain intensity ratings. In contrast, for the other > self contrast patients showed significantly less activation in the bilateral TPJ/pSTS than controls, with significant TPJ/pSTS activation observed only in controls. Additionally, in patients left TPJ/pSTS activation was negatively correlated with their cognitive/disorganization symptoms. Overall, these results suggest that sharing others’ pain, which is related to the affective processes contributing to empathy, seems relatively preserved in patients with schizophrenia, which is consistent with the find-ings of Horan and colleagues.27 In contrast, these patients seem to present with difficulties in perspective taking — a major cognitive process involved in the experience of empathy.
Brain activations linked to the affective processes involved in pain empathy are partially preserved in patients with schizophrenia
During the observation of painful (compared with nonpainful) actions, both groups activated the bilateral AI/aMCC network, which is often associated with the affective processes contributing to empathy.13,14 Though a small portion of the aMCC was significantly less activated in patients than in controls, this effect reflected a difference in the extent of the observed activation, such that patients significantly activated the aMCC, but a small part of this region showed less important activation than that observed in controls (Fig. 1B).
Based on previous studies that used actual nociceptive stimulation,43–45 it was proposed that when empathizing with others’ pain, the AI is involved in representing and integrating others’ sensory and emotional feelings, predicting affective consequences and comparing them with the observed cues (e.g., facial expressions), whereas the aMCC would support the motivation and coordination of appropriate responses (prosocial behaviour or withdrawal from pain).19,46–49 Our results thus suggest that patients with schizophrenia normally represent and integrate others’ pain, corroborating recent fMRI27 and EEG50 evidence showing that this affective aspect of empathy is preserved in schizophrenia.
The reduced aMCC activation that we observed in patients with schizophrenia could reflect a less efficient coordination of motivated responses to others’ pain in individuals with this disorder, but other roles have also been suggested for the aMCC, including in attention and salience processing.51 Our result could thus reflect the previously suggested abnormal salience processing in patients with schizophrenia.52 However, Eisenberger53 recently reviewed arguments that clearly challenge the salience processing hypothesis for the aMCC (e.g., salient stimuli, such as negative [nonpainful] emotional faces, do not activate the ACC or AI when compared with neutral faces54). It thus seems unlikely that salience processing impairments in patients with schizophrenia entirely account for the present between-groups difference in the aMCC.
Finally, the conjunction analysis for the pain > no pain contrast showed that both groups also exhibited activation in large clusters encompassing the inferior frontal and the inferior and superior parietal cortices. These brain regions, classically described as the human “mirror neuron system,”55 are consistently activated during empathy paradigms based on the presentation of pictures of body parts14 and are thought to reflect action understanding and motor resonance.56 Our results thus suggest that these aspects could also be preserved in patients with schizophrenia, reinforcing recent evidence showing that mirroring others’ experiences seems relatively intact in individuals with this disorder.57
Brain bases of cognitive perspective taking involved in empathy for pain are altered in patients with schizophrenia
In line with previous studies in healthy participants,15–17 our control group exhibited bilateral TPJ/pSTS activation when imagining pain in others (compared with pain in oneself; PO1 > PS1 contrast), which is similar to the findings reported by Vistoli and colleagues.18 In contrast, patients showed no suprathreshold activation for this contrast, leading to significant between-group differences in the TPJ/pSTS bilaterally. Regarding the role of the TPJ in taking the perspective of others during pain empathy,15–18 this result suggests a specific difficulty with cognitive perspective taking in patients with schizophrenia. This is in line with the results of previous fMRI investigations targeting the cognitive processes involved in empathy,11,21–23,25,26 although between-group differences in some of these previous studies were observed in different brain regions.
Behaviourally, there was no effect of the self/other instruction on pain ratings in healthy controls.18 Accordingly, no significant group × self/other instruction interaction was observed. However, patients reported reduced perspective taking scores on the IRI (related to the cognitive aspect of empathy) compared with controls, consistent with previous studies.1,8,11 This group difference on perspective taking scores was no longer significant when considering the 2 dimensions of the perspective taking subscale on the IRI proposed by Koller and Lamm.33 This absence of significant difference can be explained by the use of different versions of the IRI between the present study (the original 28-item version) and that of Koller and Lamm,33 who used a 16-item version. When focusing on the items included only in the original 28-item version, we found a significant group difference. Overall, patients with schizophrenia seem to have difficulty taking the cognitive perspective of others.
Taking the perspective of others is at the very heart of our capacity to infer others’ mental states, also referred to as “theory of mind” (ToM) or “mentalizing.” Using a different fMRI task, we recently showed that the inferior TPJ supports social inferences, whereas the superior TPJ supports context-sensitive ToM judgments.58 In the present study, the abnormal activation in patients with schizophrenia was in this inferior part of the TPJ, consistent with a difficulty in making social inferences about others’ pain. This result extends previous findings of aberrant TPJ activation in patients with schizophrenia during the inference of others’ nonemotional mental states, such as beliefs or intentions.59
An alternative role of the TPJ in reorienting attention to relevant stimuli has been proposed.60 However, it seems unlikely that this hypothesis accounts for the present TPJ/pSTS activation and its alteration in patients with schizophrenia, as there is no reason to believe that reorienting attention was more involved when participants were asked to imagine others’ pain (PO1 condition) than their own pain (PS1 condition). Also, because we presented the self and other conditions in pseudorandom blocks, one might suspect that the repeated task switching could have activated attention networks in controls more so than in patients. However, this hypothesis does not seem plausible, as task switching paradigms appear to involve more dorsal parietal and frontal regions.60 Overall, the bilateral TPJ/pSTS activation was observed when participants were asked to change their cognitive perspective from the self to that of others to imagine how much pain they would feel in the situations depicted. The TPJ/pSTS activation thus seems to reflect cognitive perspective taking.
Recent fMRI findings by Horan and colleagues27 suggested impairments in taking others’ cognitive perspective and self/other distinction in patients with schizophrenia during a pain empathy task in which participants observed videos of faces expressing pain. The authors observed abnormal modulation of activity in the aMCC and bilateral AI linked to the self/other manipulation in patients with schizophrenia. We did not replicate this finding in the aMCC and AI, which could be explained by the use of different tasks and stimuli (faces v. limbs). For instance, it is possible that the self/other manipulation may have a different impact depending on the kind of stimuli (e.g., faces naturally encourage the observer to think about another person, whereas hands might require more cognitive effort to take the perspective of others).
Interestingly, we observed that the between-group differences in TPJ/pSTS activation were specific to the painful stimuli, with neither group showing activation related to the self/other manipulation for the no pain condition (Appendix 1). This specificity may reflect that participants were engaged in pain inference and hence cognitive perspective taking only when the situations required understanding others’ pain.
Finally, the negative association we observed between activation in the left TPJ/pSTS and patients’ cognitive/disorganization symptoms is in line with recent findings showing the negative impact of cognitive deficits (processing speed and mentalizing) on empathic abilities in patients with schizophrenia.61 This result is also coherent with the negative association reported between social cognition performance and disorganization symptoms in patients with schizophrenia.62 However, we did not replicate the correlation reported by Smith and colleagues11 between activation in the aMCC and patients’ social functioning. This discrepancy may reflect the use of different empathy tasks (social empathy task v. pain empathy task) and different measures of functioning.
Limitations
A first limitation of this study is that our material included hands viewed from a first-person or a third-person visual perspective, leaving fewer trials to include in the PO1 > PS1 contrast of interest. Although including the third-person perspective trials initially seemed interesting, we recently showed that the TPJ activation related to the self/other manipulation was strictly observed in the first-person condition in healthy participants,18 and we accordingly restricted our self/other analyses to the first-person visual perspective. Another limitation is that even if our sample size compares favourably with those of other fMRI studies, the 27 patients in our sample are unlikely to be representative of the schizophrenia population as a whole, given the highly heterogeneous nature of this disorder.
Conclusion
The observation of painful scenarios led to relatively preserved brain activation and pain intensity ratings in patients with schizophrenia, but these patients did not show TPJ/pSTS activation when asked to take the cognitive perspective of others. It thus seems that taking the cognitive perspective of others, contributing to the experience of empathy, could be more specifically altered in patients with schizophrenia, with pain observation linked to the affective set of empathy processes being relatively preserved.
By refining our knowledge on empathy alteration in patients with schizophrenia, the present findings identified potential treatment targets for future therapeutic approaches, such as cognitive remediation. The efficiency of this kind of cognitive therapy is highly associated with the specificity of the cognitive target. The present findings suggest that empathy alteration in people with schizophrenia is particularly linked to difficulties in taking the cognitive perspective of others and inferring others’ experiences. We thus propose that efficient cognitive remediation techniques for empathy in patients with schizophrenia should focus on these cognitive processes. The association observed between TPJ/pSTS activation and cognitive/disorganization symptoms suggests that patients with these symptoms may further benefit from such interventions, though this result certainly deserves replication. By contrast, we provided evidence suggesting that emotion sharing and mirroring others’ behaviours are relatively intact in people with schizophrenia and thus may not require as much attention to improve empathy, at least at the group level.
Acknowledgements
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC #435556-2013) and the Fonds de Recherche du Québec Santé (FRQ-S) to A. Achim, and from the Brain and Behavior Foundation to P. Jackson. The FRQ-S also supported the team through salary grants (A. Achim and P. Jackson), a studentship (M.-A. Lavoie) and a postdoctoral award (D. Vistoli). S. Sutliff was supported by studentships from the Réseau de Bio-Imagerie du Québec (RBIQ) and the Centre Thématique de Recherche en Neurosciences (CTRN). Support was also provided by the Consortium d’imagerie en neuroscience et santé mentale de Québec (CINQ) for protocol development and MRI acquisition via a Platform Support Grant (PSG-3456) from the Brain Canada Foundation. The authors thank the health professionals in Québec who collaborated on the project, notably the professionals from the Clinique Notre-Dame-des-Victoires, the Centre de Traitement et de Réadaptation de Nemours and the External clinic, all linked to the Institut Universitaire en Santé Mentale de Québec. Finally, the authors also thank FE and PEM for their technical help.
Footnotes
Competing interests: None declared.
Contributors: D. Vistoli, M.-A. Lavoie, P.L. Jackson and A.M. Achim designed the study, and acquired and analyzed the data. S. Sutliff also contributed to data acquisition. D. Vistoli, P.L. Jackson and A.M. Achim wrote the article, which all authors reviewed and approved for publication.
References
- 1.Achim AM, Ouellet R, Roy MA, et al. Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatry Res. 2011;190:3–8. doi: 10.1016/j.psychres.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Green MF, Horan W, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16:620–31. doi: 10.1038/nrn4005. [DOI] [PubMed] [Google Scholar]
- 3.Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- 4.Zaki J, Ochsner KN. The neuroscience of empathy: progress, pitfalls and promise. Nat Neurosci. 2012;15:675–80. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
- 5.Rutgen M, Seidel E-M, Silani G, et al. Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proc Natl Acad Sci USA. 2015;112:E5638–E5646. doi: 10.1073/pnas.1511269112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan A, Woo C-W, Chang LJ, et al. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. eLife. 2016;5:e15166. doi: 10.7554/eLife.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–26. [Google Scholar]
- 8.Abramowitz AC, Ginger EJ, Gollan JK, et al. Empathy, depressive symptoms, and social functioning among individuals with schizophrenia. Psychiatry Res. 2014;216:325–32. doi: 10.1016/j.psychres.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Michaels TM, Horan WP, Ginger EJ, et al. Cognitive empathy contributes to poor social functioning in schizophrenia: evidence from a new self-report measure of cognitive and affective empathy. Psychiatry Res. 2014;220:803–10. [PubMed] [Google Scholar]
- 10.Smith MJ, Horan WP, Karpouzian TM, et al. Self-reported empathy deficits are uniquely associated with poor functioning in schizophrenia. Schizophr Res. 2012;137:196–202. doi: 10.1016/j.schres.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Smith MJ, Schroeder MP, Abram SV, et al. Alterations in brain activation during cognitive empathy are related to social functioning in schizophrenia. Schizophr Bull. 2015;41:211–22. doi: 10.1093/schbul/sbu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pijnenborg GH, Spikman JM, Jeronimus BF, et al. Insight in schizophrenia: associations with empathy. Eur Arch Psychiatry Clin Neurosci. 2013;263:299–307. doi: 10.1007/s00406-012-0373-0. [DOI] [PubMed] [Google Scholar]
- 13.Fan Y, Duncan NW, de Greck M, et al. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev. 2011;35:903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Chen C, Lin CP, et al. Love hurts: an fMRI study. Neuroimage. 2010;51:923–9. doi: 10.1016/j.neuroimage.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 16.Jackson PL, Brunet E, Meltzoff AN, et al. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- 18.Vistoli D, Achim AM, Lavoie MA, et al. Changes in visual perspective influence brain activity patterns during cognitive perspective-taking of other people’s pain. Neuropsychologia. 2016;85:327–36. doi: 10.1016/j.neuropsychologia.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Zaki J, Ochsner KN, Hanelin J, et al. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2007;2:276–91. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KH, Brown WH, Egleston PN, et al. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. Am J Psychiatry. 2006;163:1926–33. doi: 10.1176/ajp.2006.163.11.1926. [DOI] [PubMed] [Google Scholar]
- 21.Benedetti F, Bernasconi A, Bosia M, et al. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res. 2009;114:154–60. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Langdon R, Coltheart M, Ward PB. Empathetic perspective-taking is impaired in schizophrenia: evidence from a study of emotion attribution and theory of mind. Cogn Neuropsychiatry. 2006;11:133–55. doi: 10.1080/13546800444000218. [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Kang do H, Kim CW, et al. Multi-level comparison of empathy in schizophrenia: an fMRI study of a cartoon task. Psychiatry Res. 2010;181:121–9. doi: 10.1016/j.pscychresns.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Modi S, Goyal S, et al. Functional and structural abnormalities associated with empathy in patients with schizophrenia: an fMRI and VBM study. J Biosci. 2015;40:355–84. doi: 10.1007/s12038-015-9509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derntl B, Finkelmeyer A, Voss B, et al. Neural correlates of the core facets of empathy in schizophrenia. Schizophr Res. 2012;136:70–81. doi: 10.1016/j.schres.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey PO, Zaki J, Lee J, et al. Neural substrates of empathic accuracy in people with schizophrenia. Schizophr Bull. 2013;39:617–28. doi: 10.1093/schbul/sbs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horan WP, Jimenez AM, Lee J, et al. Pain empathy in schizophrenia: An fMRI study. Soc Cogn Affect Neurosci. 2016;11:783–92. doi: 10.1093/scan/nsw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollingshead AA. Four-factor index of social status. Yale University; New Haven, CT: 1975. Unpublished data. [Google Scholar]
- 29.First MB, Spitzer RL, Miriam G, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 30.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 31.Kleinman L, Lieberman J, Dube S, et al. Development and psychometric performance of the schizophrenia objective functioning instrument: an interviewer administered measure of function. Schizophr Res. 2009;107:275–85. doi: 10.1016/j.schres.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 33.Koller I, Lamm C. Item response model investigation of the (german) Interpersonal Rreactivity Index empathy questionnaire. Eur J Psychol Assess. 2015;31:211–21. [Google Scholar]
- 34.Canizales DL, Voisin JI, Michon PE, et al. The influence of visual perspective on the somatosensory steady-state response during pain observation. Front Hum Neurosci. 2013;7:849. doi: 10.3389/fnhum.2013.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcoux LA, Michon PE, Voisin JI, et al. The modulation of somatosensory resonance by psychopathic traits and empathy. Front Hum Neurosci. 2013;7:274. doi: 10.3389/fnhum.2013.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcoux LA, Michon PE, Lemelin S, et al. Feeling but not caring: empathic alteration in narcissistic men with psychopathic traits. Psychiatry Res. 2014;224:341–8. doi: 10.1016/j.pscychresns.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Carp J. The secret lives of experiments: methods reporting in the fMRI literature. Neuroimage. 2012;63:289–300. doi: 10.1016/j.neuroimage.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igelström KM, Webb TW, Graziano MS. Neural processes in the human temporoparietal cortex separated by localized independent component analysis. J Neurosci. 2015;35:9432–45. doi: 10.1523/JNEUROSCI.0551-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol. 2012;108:3382–92. doi: 10.1152/jn.00674.2012. [DOI] [PubMed] [Google Scholar]
- 41.Lehoux C, Gobeil MH, Lefèbvre AA, et al. The five-factor structure of the PANSS: a critical review of its consistency accross studies. Clin Schizophr Relat Psychoses. 2009;3:103–10. [Google Scholar]
- 42.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 43.Craig AD. How do you feel now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 44.Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–49. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat rev Neurosci. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu X, Liu X, Guise KG, et al. Functional dissociation of the fronto-insular and anterior cingulate cortices in empathy for pain. J Neurosci. 2010;30:3739–44. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Yesudas EH, Lee TMC. The role of cingulate cortex in vicarious pain. BioMed Res Int. 2015 doi: 10.1155/2015/719615. 719615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCall C, Singer T. Empathy and the brain. In: Baron Cohen S, Tager-Flusberg H, Lombardo MV, editors. Understanding other minds, perspective from developmental social neuroscience. New York: Oxford University Press; pp. 195–213. [Google Scholar]
- 50.Corbera S, Ikezawa S, Bell MD, et al. Physiological evidence of a deficit to enhance the empathic response in schizophrenia. Eur Psychiatry. 2014;29:463–72. doi: 10.1016/j.eurpsy.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Iannetti GD, Salomons TV, Moayedi M, et al. Beyond metaphor: contrasting mechanisms of social and physical pain. Trends Cogn Sci. 2013;17:371–8. doi: 10.1016/j.tics.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–85. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisenberger NI. Social pain and the brain: controversies, questions, and where to go from here. Annu Rev Psychol. 2015;66:601–29. doi: 10.1146/annurev-psych-010213-115146. [DOI] [PubMed] [Google Scholar]
- 54.Sabatinelli D, Fortune EE, Li Q, et al. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–33. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 56.Lamm C, Majdandžic J. The role of shared neural activations, mirror neurons, and morality in empathy — a critical comment. Neurosci Res. 2015;90:15–24. doi: 10.1016/j.neures.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Horan WP, Pineda JA, Wynn JK, et al. Some markers of mirroring appear intact in schizophrenia: evidence from mu suppression. Cogn Affect Behav Neurosci. 2014;14:1049–60. doi: 10.3758/s13415-013-0245-8. [DOI] [PubMed] [Google Scholar]
- 58.Lavoie MA, Vistoli D, Sutliff S, et al. Social representations and contextual adjustments as two distinct components of the theory of mind brain network: evidence from the REMICS task. Cortex. doi: 10.1016/j.cortex.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Brunet-Gouet E, Achim AM, Vistoli D, et al. The study of social cognition with neuroimaging methods as a means to explore future directions of deficit evaluation in schizophrenia? Psychiatry Res. 2011;190:23–31. doi: 10.1016/j.psychres.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 60.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konstantakopoulos G, Oulis P, Ploumpidis D, et al. Self-rated and performance-based empathy in schizophrenia: the impact of cognitive deficits. Soc Neurosci. 2014;9:590–600. doi: 10.1080/17470919.2014.934395. [DOI] [PubMed] [Google Scholar]
- 62.Fett AK, Matt A GROUP Investigators. Social cognitive impairments and psychotic symptoms: what is the nature of their association? Schizophr Bull. 2013;39:77–85. doi: 10.1093/schbul/sbr058. [DOI] [PMC free article] [PubMed] [Google Scholar]