Abstract
Background
Targeting the N-methyl-d-aspartate receptor (NMDAR) is a major translational approach for treating negative symptoms of schizophrenia. Ketamine comprehensively produces schizophrenia-like symptoms, such as positive, cognitive and negative symptoms in healthy volunteers. The amplitude of the mismatch negativity (MMN) is known to be significantly reduced not only in patients with schizophrenia, but also in healthy controls receiving ketamine. Accordingly, it was the aim of the present study to investigate whether changes of MMN amplitudes during ketamine administration are associated with the emergence of schizophrenia-like negative symptoms in healthy volunteers.
Methods
We examined the impact of ketamine during an MMN paradigm with 64-channel electroencephalography (EEG) and assessed the psychopathological status using the Positive and Negative Syndrome Scale (PANSS) in healthy male volunteers using a single-blind, randomized, placebo-controlled crossover design. Low-resolution brain electromagnetic tomography was used for source localization.
Results
Twenty-four men were included in our analysis. Significant reductions of MMN amplitudes and an increase in all PANSS scores were identified under the ketamine condition. Smaller MMN amplitudes were specifically associated with more pronounced negative symptoms. Source analysis of MMN generators indicated a significantly reduced current source density (CSD) under the ketamine condition in the primary auditory cortex, the posterior cingulate and the middle frontal gyrus.
Limitations
The sample included only men within a tight age range of 20–32 years.
Conclusion
The MMN might represent a biomarker for negative symptoms in schizophrenia related to an insufficient NMDAR system and could be used to identify patients with schizophrenia with negative symptoms due to NMDAR dysfunction.
Introduction
Current pharmaceuticals for the treatment of schizophrenia — the typical and atypical antipsychotics — are dopamine D2 receptor antagonists with a satisfactory clinical benefit on positive symptoms, but with limited to no impact on negative symptoms of the disease. Therefore, there is an urgent need to improve pharmacotherapy of negative symptoms. Beside the dopaminergic system, dysfunctional glutamatergic neurotransmission has strongly been implicated in the etiology of schizophrenia,1–3 particularly a hypofunction of the N-methyl-d-aspartate-receptor (NMDAR).4 As NMDAR hypofunction is especially associated with negative symptoms,5 the NMDAR is continuously discussed as being a promising target for the introduction of new medications.
Subanesthetic doses of NMDAR antagonists, such as phencyclidine (PCP) and ketamine, elicit not only psychotomimetic effects reminiscent of positive, negative and cognitive symptoms of schizophrenia in healthy volunteers,6 but also exacerbate symptoms in patients with schizophrenia.7 Dopaminergic agents, such as amphetamines or serotonin (5HT)2A agonists induce effects reminiscent of only positive symptoms.8 Therefore, reduced NMDAR signalling may be a unique route to model negative symptoms reminiscent of schizophrenia. Investigating ketamine effects in healthy volunteers may be especially useful to describe neurophysiological mechanisms associated with negative symptoms.
To activate the NMDAR, not only the binding of glutamate, but also glycine is essential.9 Clinical trials of glycine site agonists (e.g. glycine itself, d-serine) or the glycine reuptake inhibitor sarcosine in patients with chronic schizophrenia have reported beneficial effects on the negative symptoms of schizophrenia.10,11 However, other studies have not reported significant changes in negative symptoms between glycine, d-serine or d-cycloserine and placebo, and thus were not able to replicate these findings.12,13 A reason for these inconsistent results may be the considerable heterogeneity among patients with schizophrenia.
Mismatch negativity (MMN) is an auditory event-related potential (ERP) generated when a deviant stimulus is presented that differs in some physical features, such as duration or frequency, from repeatedly presented standard stimuli.14 Reduced MMN amplitudes have been shown to be a robust finding in patients with schizophrenia in several studies.15–17 Mismatch negativity deficits, also known as prediction error signals, are suggested to be particularly severe in patients with chronic schizophrenia who have prominent negative symptoms.18 Furthermore, it is assumed that the neural activity underlying the MMN could be attributed to 2 sets of neural generators. Superior temporal generators are associated with the sensory memory part of change detection, and frontal generators are supposed to be responsible for triggering an attention shift upon change detection.19 The early main supratemporal generator is documented by several studies,20–22 whereas the exact cortical localization of later frontal MMN generators still remains challenging. Locations vary across studies and include parts of the medial frontal gyrus (MFG),23 inferior frontal gyrus,24 or cingulate cortex.25 In addition to MMN amplitudes, current source density (CSD) of temporal and frontal MMN generators has been shown to be reduced in patients with schizophrenia compared with healthy controls.26
Moreover, a reduction of the MMN amplitude suggests an association with glutamatergic NMDAR neurotransmission. Several electrophysiological studies have reported ketamine-induced reductions of MMN amplitudes.27,28 Additionally, studies with an acute stimulation of dopamine receptors show that dopaminergic modulation does not alter MMN in healthy individuals.29 Furthermore, it has been demonstrated that dopamine receptor–modulating drugs do not influence MMN in patients.30 Positive correlations between smaller MMN amplitude and a higher scoring in the negative symptom domain have been shown to be present in patients with chronic schizophrenia,17,31,32 although there are contrary findings.33
Therefore, the modulation of the NMDAR has emerged as an established model for some aspects of schizophrenia that has been used, for example, in developing new treatment strategies, especially with respect to negative symptoms of schizophrenia.34 Accordingly, we hypothesized both the emergence of schizophrenia-like negative symptoms and a reduction of the MMN during the administration of ketamine in healthy individuals. Moreover, based on previous reports of a significant association between MMN amplitudes and negative symptoms in patients with schizophrenia, we expected a specific association between negative symptoms during ketamine administration and the MMN amplitude.
Methods
Participants
Participants were recruited from the community through advertisement and word of mouth. Exclusion criteria were any acute or previous psychiatric disorders (assessed using the Mini International Neuropsychiatric Interview35) or treatment, family history of schizophrenia or bipolar disorder, neurologic disorders, heart or circulatory disease, thyroid disease, current strong mental or physical stress, ketamine intolerance and left-handedness (assessed using the empirically validated Edinburgh Handedness Inventory.36 Moreover, the Schizotypal Personality Questionnaire (SPQ)37 was used to exclude individuals with a schizotypal trait. All participants had normal IQ, as assessed using a vocabulary test,38 and hearing better than 30 dB at a pitch of 1000 Hz. The study was approved by the Ethics Committee of the Medical Association Hamburg and carried out in accordance with the latest version of the Declaration of Helsinki. Written informed consent was obtained from all participants after the nature of the procedures had been fully explained.
Study design
We used a single-blind, randomized, placebo-controlled crossover study design. All participants underwent 2 electroencephalography (EEG) recording sessions, during which either ketamine or placebo was administered. The order of sessions was randomized but counterbalanced overall with a time-lag of 1 week.
During the ketamine session, a subanesthetic dose of S-ketamine hydrochloride was administered intravenously in 0.9% sodium chloride (NaCl) solution using a syringe pump for a total duration of 75 minutes. The ketamine infusion was started with an initial bolus of 10 mg over 5 minutes followed by a maintenance infusion of 0.006 mg/kg/min. As ketamine plasma levels slowly increase with continuous infusion,39 the dosage was reduced by 10% every 10 minutes according to previously published protocols.40 Placebo was administered analogously as 0.9% NaCl infusion. After both sessions the participants stayed under constant supervision for 1 hour, until all drug effects had worn off. Finally, they were released into the custody of a friend or relative. Participants were not permitted to operate motor vehicles or to commute unaccompanied for the rest of the day.
Safety
A board-certified anesthesiologist (I.E. or L.E.) continuously monitored the heart rate, blood pressure and oxygen saturation of the participants during both sessions. Video surveillance during the study guaranteed safety. First aid drugs and oxygen supply were always ready to use. Adverse effects were high blood pressure, increase in heart rate, nausea and sweatiness.
Psychometric assessment
To assess the subjective effects of ketamine we administered the self-rating Altered State of Consciousness (5D-ASC) questionnaire with 94 items assessing 5 key dimensions: oceanic boundlessness (OBN), visionary restructuralization (VRS), dread of ego dissolution (DED), vigilance reduction (VIR) and auditory alterations (AUA).41 The scale was previously shown to be sensitive to the psychological effects of NMDAR antagonists in humans42 and was conducted after both EEG recording sessions.
We assessed psychiatric symptomatology using the Positive and Negative Syndrome Scale (PANSS).43 Prior to the first session (baseline) and immediately following the MMN acquisitions the PANSS was assessed by a trained rater (S.C.). The PANSS includes a positive symptom domain (7 items), a negative symptom domain (7 items) and a general pathology domain (16 items). We evaluated the PANSS scores using the 5-factor model by van der Gaag and colleagues.44
Stimuli
We used a 3-tone auditory oddball paradigm with 1800 stimuli, which had been shown earlier to be capable of detecting impaired MMN in patients with schizophrenia.45 As standard stimuli, 1560 tones (80% of all stimuli) at a pitch of 1000 Hz and a duration of 80 ms were presented via earphones at 75 dB SPL (closed system headphones, Sennheiser, HAD 200). Occasionally, 1 of 2 different deviant stimuli was presented. We considered 10% (180) of the stimuli duration deviants with a duration of 40 ms (pitch 1000 Hz). Another 10% of the stimuli were considered frequency deviants with a pitch of 1200 Hz (duration 80 ms). The paradigm lasted 15 minutes.
To minimize eye movements and to keep participants unattended to the auditory stimuli, participants had to perform a distracting visual task: a red circle was presented in the centre of a black screen in front of the participants. In intervals varying from 5 to 21 s (mean 13 s) the colour of the circle changed from red to green for a minimum of 60 ms and maximum of 450 ms. Participants were instructed to fixate on the circle and press a left mouse button whenever the colour changed to green (34 times in each of the sessions). The inter-stimulus interval between the presentation of an acoustic stimulus and a visual target stimulus (the green circle) was 200–400 ms (mean 300 ms).
Recording and preprocessing of EEG data
During performance of the oddball task, EEG recordings took place in a sound-attenuated and electrically shielded cabin. Participants were seated in a comfortable, slightly reclined chair to avoid muscle artifacts.
Continuous EEG activity was recorded using Ag/AgCl electrodes mounted in a 64-channel actiCAP system (recording apparatus: Brain Products GmbH). Electrodes were positioned in an extended 10/20 system, with additional electrodes placed at positions AF7, AF3, AF4, AF8, F5, F1, F2, F6, F10, FT9, FT7, FC3, FC4, FT8, FT10, C5, C1, C2, C6, TP7, CPz, TP8, P5, P1, P2, P6, PO3, POz and PO4. Eye movements were recorded by 2 horizontal electrooculography (EOG) and 2 vertical EOG channels. The reference electrode was positioned at FCz, and AFz served as ground. Electrode impedances were always kept below 5 kΩ. Data were collected at a sampling rate of 1000 Hz. To establish contact between the scalp and electrodes, we applied SuperVisc electrode gel (EASYCAP GmbH).
Preprocessing of EEG data was done using Brain Vision Analyzer software version 2.0 (Brain Products). Offline, the data were band-pass filtered from 1 to 35 Hz and down-sampled to 250 Hz. All data sets were corrected for eye-blink artifacts by applying an independent component analysis (ICA). The continuous EEG was segmented into epochs of 800 ms, starting 400 ms prior and ending 400 ms after an auditory stimulus. All channels were re-referenced to common average. Segments with electrical activity exceeding ± 95 μV were rejected46 (maximum number of rejected segments: 3 for standard tones, 1 for duration deviants, 2 for frequency deviants). To balance the number of trials used for averaging between different stimuli (standard and deviants), standard trials were randomly selected for each participant in such a way that the number of standard trials matched the number of duration- and frequency-deviant trials, respectively. For the whole group of participants, this yielded a mean of 149.15 ± 23.15 duration-deviant trials in the placebo condition and 156.29 ± 17.00 duration-deviant trials in the ketamine condition and a mean of 153.19 ± 27.07 frequency-deviant trials in the placebo condition and 159.21 ± 17.92 frequency-deviant trials in the ketamine condition (no significant differences between conditions). After baseline correction, using an interval of 200 ms before stimulus onset the selected epochs were averaged for each of the different stimuli and each of the participants separately.
Parameterization of MMN amplitude
To parameterize MMN effects in response to deviant stimuli, difference waves were calculated between ERPs evoked by standard stimuli and those evoked by duration and frequency deviants, respectively. Peak amplitudes of the MMN were determined at the frontal electrode Fz and defined as the amplitude of the maximum negative deflection of the difference waves occurring within 80–200 ms after stimulus onset.45
Source analysis of MMN
Source analyses were executed with the Low-resolution brain electromagnetic tomography (LORETA) KEY software package, as provided by The KEY Institute for Brain-Mind Research University Hospital Psychiatry, Zurich (www.uzh.ch/keyinst/LORETA.html; Appendix 1, available at jpn.ca). LORETA has been widely used to localize electrical generators of scalp EEG data.47,48 We conducted comparisons on LORETA source imaging between placebo and ketamine for the defined MMN interval. To become even more precise, the MMN timeframe was divided into 2 timeframes reflecting the early MMN (110–160 ms) and the late MMN (160–210 ms), as suggested by Fulham and colleagues.26
Statistical analysis
All data analyses were performed using SPSS software version 21. Comparisons of 5D-ASC scores and MMN amplitudes between the placebo and ketamine conditions were done using paired-sample t tests. The PANSS scores were analyzed using a 2-way repeated-measures analysis of variance (RM-ANOVA), with condition (3 levels: baseline, placebo, ketamine) as a within-subjects factor and order of administration (2 levels) as a between-subjects factor. Significant results were followed up with post hoc t tests. Correlational analyses were not optimal for the investigation of associations between MMN amplitudes and psychopathology ratings because of the large number of comparisons involving several intercorrelated variables (2 MMN deviants, 5 PANSS factors). Instead, we chose a multivariate linear regression analysis with MMN amplitudes for the duration deviant and the frequency deviant in the ketamine condition as dependent variables and the occurrence of schizophrenia-like symptoms in the ketamine condition (5 PANSS factors) as predictors. Significant results were followed up with univariate analyses. We repeated this analysis using the MMN amplitudes in the placebo condition as dependent variables. Moreover, we conducted a multivariate linear regression analysis with the difference between placebo condition MMN amplitudes and ketamine condition MMN amplitudes as dependent variables and the difference between the intensity of schizophrenia-like symptoms in the ketamine compared with the placebo condition (5 PANSS factors) as predictors. Comparisons between placebo and ketamine on LORETA source imaging were made using voxel × voxel paired t statistics (p < 0.05). The multiple comparisons were corrected by a randomized test based on statistical nonparametric mapping (SnPM; 5000 randomizations). In all analyses the significance level was set to α = 0.05. Bonferroni corrections were applied to adjust for multiple comparisons.
Results
Participants
Twenty-eight healthy, right-handed (mean EHI score 82.20 ± 16.89, range 40–100) men with a mean age of 25 ± 2.64 (range 20–32) years were enrolled in this study. Two volunteers dropped out owing to adverse events (strong dissociative effect/headache during the recording session). Two more participants withdrew consent shortly before the first recording session. Thus, 24 participants were included in our data analysis. All participants had normal IQ (mean 112.96 ± 5.95, range 99–125).
Ketamine-induced psychopathological symptoms
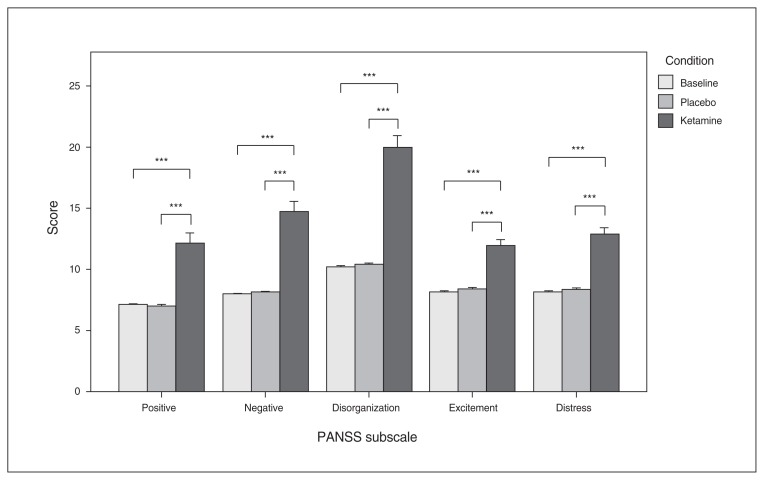
The RM-ANOVA revealed significant main effects of condition on the PANSS scores (total: F2,46 = 106.25, p < 0.001; positive: F2,46 = 34.04, p < 0.001; negative: F2,46 = 45.13, p < 0.001; disorganization: F2,46 = 73.28, p < 0.001; excitement: F2,46 = 40.43, p < 0.001; distress: F2,46 = 50.78, p < 0.001). No significant effect of the order of administration was present for any of the PANSS scores (p > 0.1). Post hoc t tests showed that the mean score and all subscores of the PANSS were significantly increased after ketamine administration compared with placebo and baseline values. There were no significant differences with respect to any PANSS score between the baseline and placebo conditions (Fig. 1). For means and standard deviations see Appendix 1, Table S1.
Fig. 1.
Mean values of the 5 Positive and Negative Syndrome Scale (PANSS) subscores, with error bars representing ± 1 standard errors or the mean. ***p < 0.001.
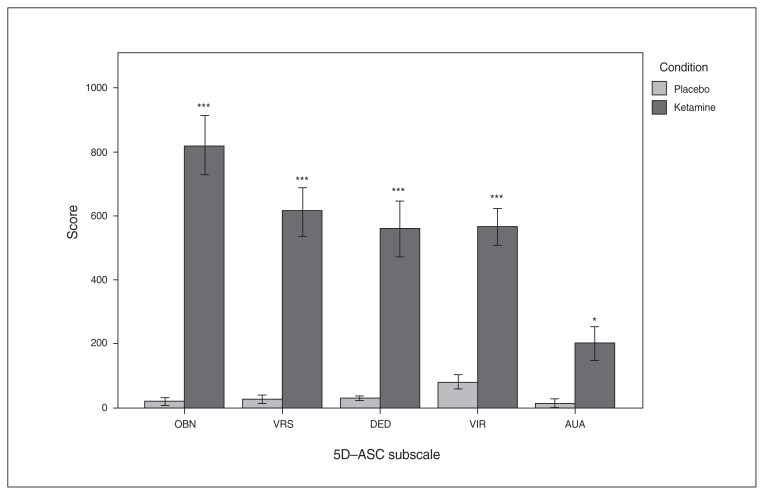
The 5D-ASC scores were significantly increased after ketamine administration compared with placebo (Fig. 2 and Appendix 1, Table S2).
Fig. 2.
Mean values of the 5 dimensions of the Altered State of Consciousness (5D-ASC) questionnaire subscores, with error bars representing ± 1 standard error of the mean. AUA = auditory alterations; DED = dread of ego dissolution; OBN = oceanic boundlessness; VIR = vigilance reduction; VRS = visionary restructuralization. *p < 0.05, ***p < 0.001, Bonferroni-corrected for multiple comparisons.
Ketamine-induced MMN effects
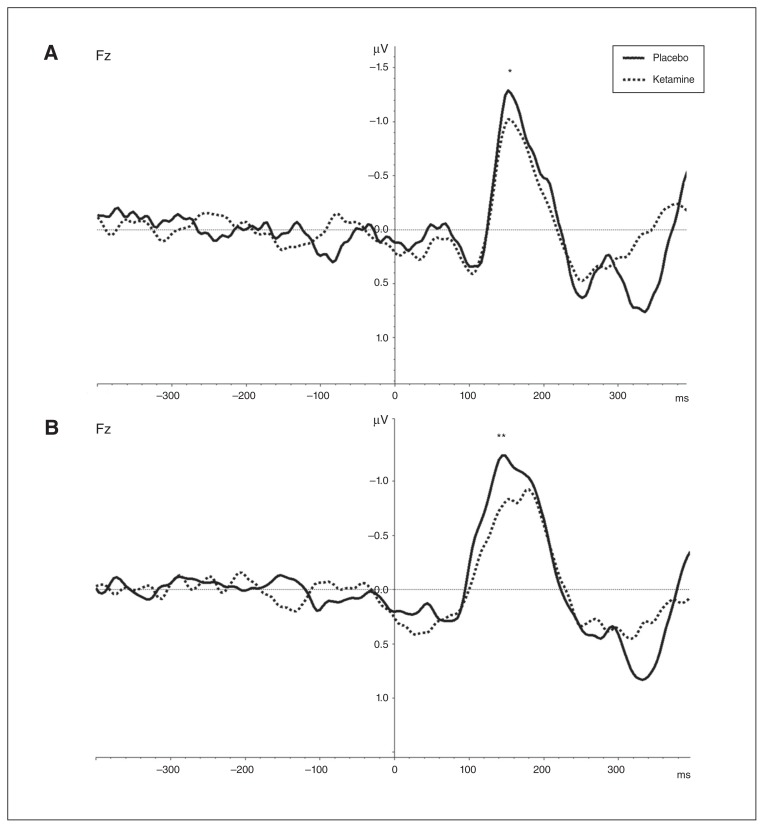
Ketamine significantly decreased the MMN amplitude at electrode Fz in comparison to placebo for the duration deviant (placebo: mean −1.54 ± 0.55; ketamine: mean −1.32 ± 0.59; t = −2.37, p = 0.026) and the frequency deviant (placebo: mean −1.68 ± 0.77; ketamine: mean −1.22 ± 0.50; t = −3.84, p = 0.001; Fig. 3). There were no significant changes in MMN latencies of the duration deviant (placebo: mean 159.3 ± 16.9 ms; ketamine: mean 161.8 ± 16.0 ms; t = −0.88, p = 0.39) and the frequency deviant (placebo: mean 155.0 ± 26.2 ms; ketamine: mean 166.0 ± 26.8 ms; t = −1.48, p = 0.15).
Fig. 3.
Grand averages (n = 24) of mismatch negativity (MMN) difference waves under ketamine administration (dashed lines) and placebo administration (solid lines). (A) Difference between standard and duration-deviant trials. (B) Difference between standard and frequency-deviant trials. A reduction of MMN peak amplitudes under ketamine administration at the frontal electrode Fz can be observed between 80 ms and 200 ms. *p < 0.05, **p < 0.01.
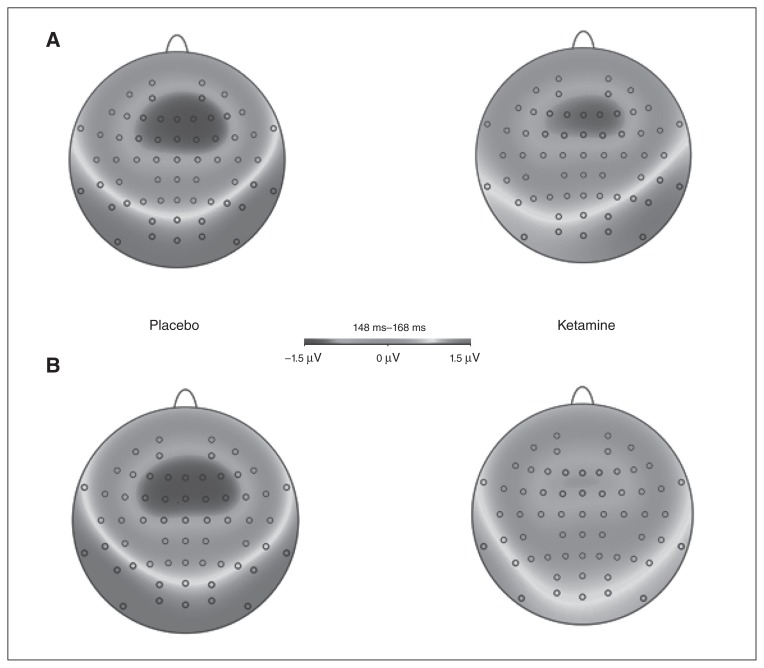
Figure 4 shows topographic maps of the MMN for both different deviant stimuli and conditions (placebo and ketamine).
Fig. 4.
Topographic maps (n = 24) derived from the mismatch negativity (MMN) difference waves of (A) the duration deviant and (B) the frequency deviant for the timeframe 148–168 ms after stimulus onset.
Association between MMN measures and psychopathological symptoms
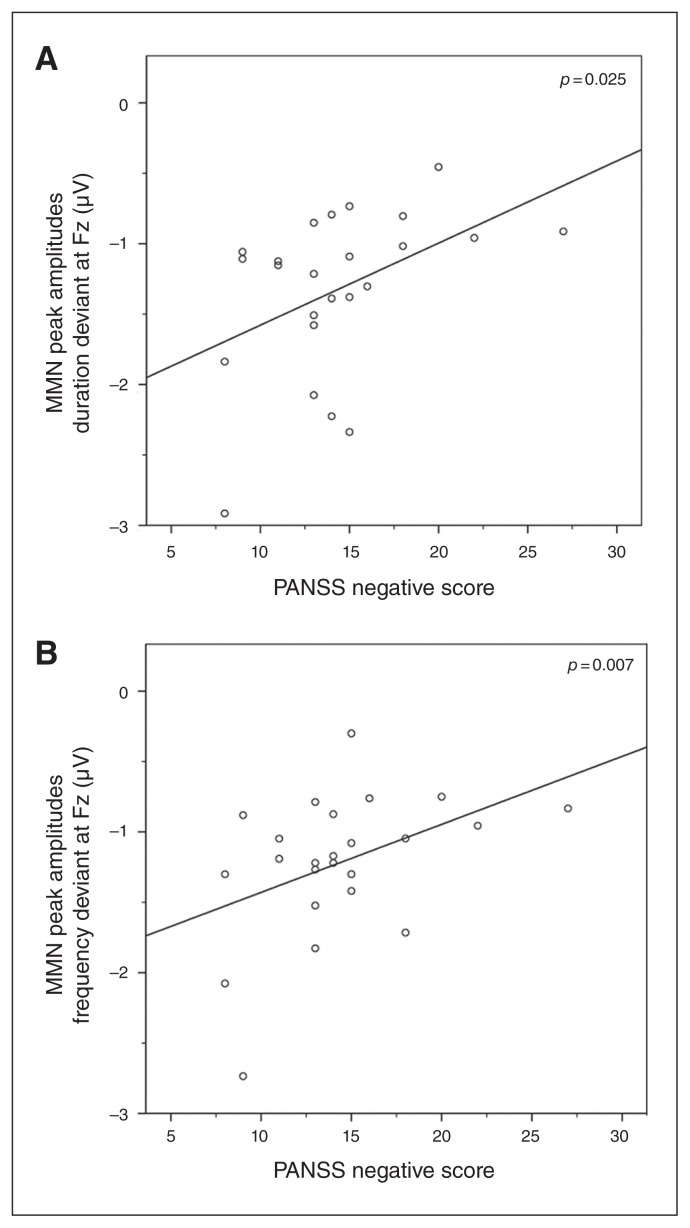
The multivariate linear regression analysis showed that PANSS negative scores were significant predictors of the MMN amplitude under the ketamine condition (F2,17 = 6.247, p = 0.009). Follow-up univariate tests indicated that this was the case for both duration deviants (F1,18 = 5.959, r = 0.566, p = 0.025) and frequency deviants (F1,18 = 9.093, r = 0.595, p = 0.007; Fig. 5). The effects of PANSS positive, disorganization, excitement and distress factors were not significant (all p > 0.39; Appendix 1, Fig. S1). The other multivariate linear regression analyses did not reveal significant effects.
Fig. 5.
Significant multivariate analysis of variance results, with (A) mismatch negativity (MMN) amplitude of the duration deviant and (B) MMN amplitude of the frequency deviant as dependent variables and the Positive and Negative Syndrome Scale (PANSS) negative factor as a predictor.
LORETA whole head analysis
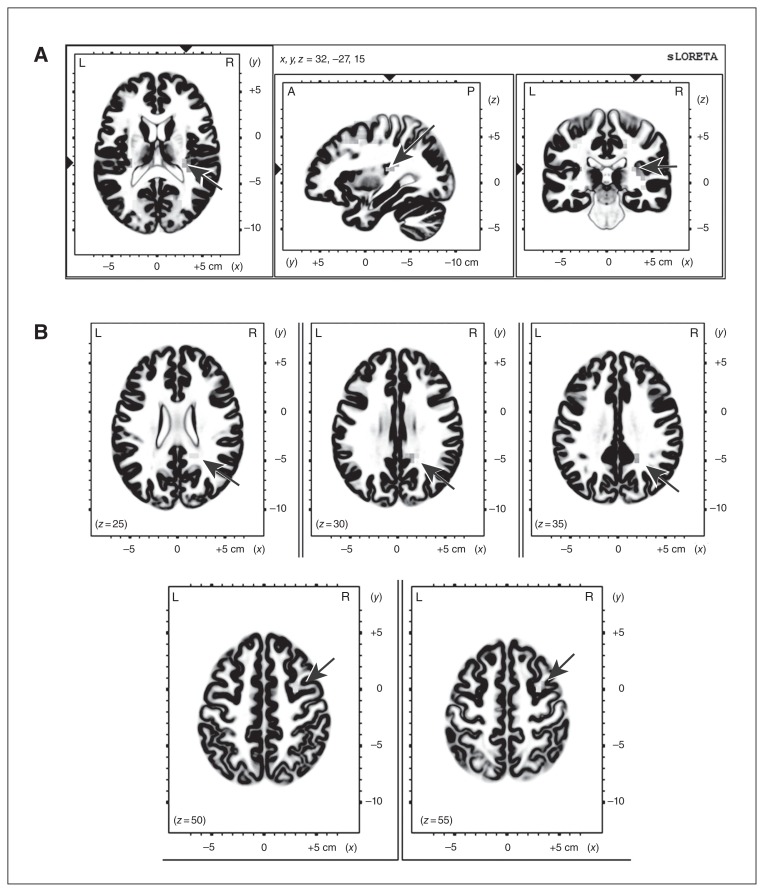
The comparison of the LORETA CSD between the placebo and ketamine conditions for the whole MMN interval showed a significantly lower CSD in voxels of the superior temporal gyrus (STG) within the area of the primary auditory cortex (PAC; Brodman area [BA] 41, temporal lobe, x, y, z = 35, −35, 15) in the ketamine condition. The frequency deviant MMN CSD was also significantly lower within the PAC area (x, y, z = 30, −25, 15) for the early MMN (110–160 ms) and within the middle frontal (BA6, x, y, z = 35, 0, 65) and the posterior cingulate gyrus (BA31, x, y, z = 20, −45, 25) for the late MMN (160–210 ms) in the ketamine condition (Fig. 6; for LORETA single condition localization results see Appendix 1, Fig. S2). There were no significant differences for the duration deviant and no brain areas with significantly lower CSD in the placebo compared with the ketamine condition.
Fig. 6.
Low-resolution brain electromagnetic tomography (LORETA) results comparing (A) early and (B) late mismatch negativity (MMN) of placebo and ketamine for the frequency deviant. Arrows show significantly higher current source density (CSD) in the placebo compared with the ketamine condition. *p < 0.05.
Discussion
In line with our hypothesis, the main finding of our study is the association between ketamine-induced PANSS negative symptoms and reduced MMN amplitudes under ketamine administration in healthy volunteers, suggesting an interdependence between MMN, negative symptoms of schizophrenia and glutamatergic neurotransmission.
Hence, the NMDAR seems to play a prominent role in the development of schizophrenia-like negative symptoms in healthy individuals. This hypothesis is supported by the fact that in healthy volunteers negative symptoms are not elicited by dopaminergic agents, such as amphetamines,8 but by glutamatergic agents, such as ketamine. In accordance with these findings, correlations of MMN deficits in patients with schizophrenia with negative symptoms have been reported.17,31,32 In the present study, this association is described for MMN deficits in healthy volunteers receiving ketamine, supposing that the development of negative symptoms underlies a deficit in glutamatergic neurotransmission at the NMDAR. A glutamateric deficit has also been reported to be associated with cognitive symptoms in patients with schizophrenia.28,49 The dysconnection hypothesis of schizophrenia has been linked to aberrant synaptic plasticity50 due to disturbed NMDAR functioning.49 Modelling approaches were able to show an association between ketamine effects on synaptic plasticity during the MMN and ketamine-induced MMN cognitive impairments.51 Our findings of an association between ketamine-induced MMN deficits and negative symptoms can be related to these findings in view of the MMN as a correlate of prediction errors. Negative symptoms might arise as a reaction to unpredictability due to a disturbed specification of expectations, which has been suggested to be mediated by NMDAR blockade.52
At first glance, our results with healthy controls seem to conflict with the conclusion from a meta-analysis by Umbricht and Krljes53 that suggested MMN does not correlate with clinical symptoms in patients with schizophrenia. This discrepancy might be explained by diverse test paradigms. Umbricht and Krljes,53 for example, showed that MMN deficits become more pronounced with decreasing probability of the deviant stimulus. Furthermore, there were many different participants included, from patients with first-episode to chronic schizophrenia. Studies reporting a smaller frontal MMN with greater negative symptoms included only patients with chronic illness, whereas studies with first-episode schizophrenia patients reported correlations in the reverse direction.32,33 Supposing MMN is dependent on NMDAR transmission, this suggests that the role of NMDAR functioning alters between different stages of schizophrenia. Consistent with this, magnetic resonance spectroscopy studies have demonstrated that glutamate levels may be elevated in early stages,54 but are reduced in patients with chronic illness.55 As MMN deficits are particularly associated with chronic illness and prominent negative symptoms,18 these results lead to the assumption of an association between MMN, negative symptoms and glutamate.
Besides MMN amplitudes, other studies were able to show that CSD of MMN in temporal and frontal brain regions are reduced in patients with schizophrenia compared with healthy controls.26 Mismatch negativity is mainly generated within the STG, but later frontal generators, including parts of the MFG23 and cingulate gyrus,25 are consistently discussed. In line with this, we found a reduced CSD in the STG under the ketamine compared with the placebo condition for the early MMN and a CSD reduction in the MFG and cingulate gyrus for the late MMN for the frequency deviant, with the finding in the cingulate cortex being more posterior than in previous studies.25,26 For the duration deviant there were no significant results. A reason for this could be that the administration of ketamine more likely resembles the MMN deficits found in chronic schizophrenia56 than in first-episode schizophrenia. Moreover, it has been shown that duration MMN deficits are seen early in the disease, whereas frequency MMN tends to be intact at early stages and is reduced only in chronic schizophrenia.57,58 This suggests that ketamine not only affects MMN amplitudes, but also MMN generators reminiscently as they are altered in patients with chronic schizophrenia.
Two other studies have reported correlations between ketamine-induced symptoms and MMN under a drug-free condition27,28 to predict ketamine effects. However, neither study investigated an association between MMN and negative symptoms. We could not replicate the findings of Umbricht and colleagues27 or Schmidt and colleagues,28 which could be explained by a variety of reasons, for instance because the reduction of MMN is dose-dependent. This has already been reported in subhuman primates16 and has been proven by several ketamine studies with different doses.59,60 One study using a low ketamine dose even reported no deficits of MMN due to drug intervention.61 As opposed to the present study, Umbricht and colleagues27 used a higher dose of ketamine (0.24 mg/kg over 5 minutes followed by 0.9 mg/kg/h). Moreover, male and female volunteers participated in contrast to only male participants being included in our study. Different rating scales were used to assess psychopathological status, and standard and deviant tones differed in duration and frequency between the studies. Schmidt and colleagues28 used a roving oddball MMN paradigm in contrast to our 3-tone odd-ball paradigm, and psychopathological status was evaluated only using a revised version of the ASC, not by a questionnaire detecting negative symptoms, such as the PANSS. Mismatch negativity also seems to be largely dependent on ketamine dose and test paradigm. The selected dose and test paradigm of the present study are able to corroborate the finding that in patients with schizophrenia a smaller frontal MMN is associated with greater negative symptoms.
Limitations
Limitations of the present study are related to the sample, as only male volunteers were included in order to avoid sex-specific or hormonal effects, and the mean age of participants was 25 years, with a tight range of 20–32 years. Nevertheless, this age range is reasonable because the early twenties are the typical age of onset for schizophrenia.62 Another limitation of our study might be that weak associations between MMN amplitudes and ketamine-induced symptoms (e.g., positive symptoms) were not detected owing to limited statistical power related to the sample size.
We did not find a prolongation of the latency of the MMN in the ketamine condition. However, a delayed latency of MMN is less consistently reported than the amplitude reduction.53 In our study, the PANSS was used for the investigation of ketamine-induced schizophrenia-like symptoms in healthy men, although it was initially designed and validated for the symptom evaluation in patients with schizophrenia. However, there is evidence of a similarity in symptom dimensions between ketamine-induced symptoms and schizophrenia psychosis, with the most consistent overlap for the negative symptom factor.63 Accordingly, the PANSS has been used in several recent studies investigating ketamine-induced schizophrenia-like symptoms in healthy volunteers.42,64,65
Conclusion
To the best of our knowledge, this is the first study exploring the association between reduced MMN amplitudes due to ketamine administration and ketamine-induced negative symptoms in healthy volunteers. Our results indicate that the emergence of negative symptoms in healthy individuals after ketamine administration is associated with brain changes, as assessed by the MMN. Accordingly, the MMN could potentially represent a biomarker for negative symptoms of schizophrenia elicited by insufficient NMDAR functioning. Investigating effects of ketamine in healthy individuals could be particularly useful for the identification of biomarkers that might enable the prediction of the treatment response to substances enhancing the function of NMDAR in patients with schizophrenia with prominent negative symptoms. Promising NMDAR-modulating agents could therefore be a novel treatment option for patients with schizophrenia with predominant negative symptoms and impaired MMN.
Acknowledgements
The authors thank Dr. Maja Maurer for providing the 5D-ASC questionnaire and thank the individuals who participated in this study. This research was performed within the collaborative research center grant SFB 936 C6 to C. Mulert and was supported by the German Research Foundation (DFG). J. Gallinat and I. Hanganu-Opatz were supported by DFG SFB 936 C7 and DFG SFB 936 B5, respectively. This work was prepared as part of S. Thiebes’ dissertation at the University of Hamburg.
Footnotes
Competing interests: None declared.
Contributors: S. Thiebes, G. Leicht, S. Curic, C. Zöllner, J. Gallinat and C. Mulert designed the study. S. Thiebes, S. Curic, N. Polomac, I. Eichler and L. Eichler acquired the data, which S. Thiebes, G. Leicht, S. Curic, S. Steinmann, C. Andreou, I. Hanganu-Opatz and C. Mulert analyzed. S. Thiebes wrote the article, which all authors reviewed and approved for publication.
References
- 1.Deutsch SI, Mastropaolo J, Schwartz BL, et al. Glutamatergic hypothesis of schizophrenia. Rationale for pharmacotherapy with glycine. Clin Neuropharmacol. 1989;12:1–13. [PubMed] [Google Scholar]
- 2.Mulert C, Scarr E. New treatment strategies in schizophrenia beyond dopamine: glutamatergic neurotransmission and more. Curr Pharm Biotechnol. 2012;13:1474–5. doi: 10.2174/138920112800784871. [DOI] [PubMed] [Google Scholar]
- 3.Gallinat J, McMahon K, Kuhn S, et al. Cross-sectional study of glutamate in the anterior cingulate and hippocampus in schizophrenia. Schizophr Bull. 2016;42:425–33. doi: 10.1093/schbul/sbv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghaddam B, Krystal JH. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull. 2012;38:942–9. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umbricht D, Alberati D, Martin-Facklam M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71:637–46. doi: 10.1001/jamapsychiatry.2014.163. [DOI] [PubMed] [Google Scholar]
- 6.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 7.Lahti AC, Koffel B, LaPorte D, et al. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 8.Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. Br J Psychiatry. 2004;185:196–204. doi: 10.1192/bjp.185.3.196. [DOI] [PubMed] [Google Scholar]
- 9.Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–7. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 10.Kantrowitz JT, Woods SW, Petkova E, et al. D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry. 2015;2:403–12. doi: 10.1016/S2215-0366(15)00098-X. [DOI] [PubMed] [Google Scholar]
- 11.Javitt DC, Zylberman I, Zukin SR, et al. Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry. 1994;151:1234–6. doi: 10.1176/ajp.151.8.1234. [DOI] [PubMed] [Google Scholar]
- 12.Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73:e728–34. doi: 10.4088/JCP.11m07031. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan RW, Javitt DC, Marder SR, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 14.Näätanen R. The mismatch negativity: a powerful tool for cognitive neuroscience. Ear Hear. 1995;16:6–18. [PubMed] [Google Scholar]
- 15.Shelley AM, Ward PB, Catts SV, et al. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–62. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- 16.Javitt DC, Steinschneider M, Schroeder CE, et al. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–7. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catts SV, Shelley AM, Ward PB, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–9. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- 18.Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–15. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- 19.Paavilainen P, Mikkonen M, Kilpelainen M, et al. Evidence for the different additivity of the temporal and frontal generators of mismatch negativity: a human auditory event-related potential study. Neurosci Lett. 2003;349:79–82. doi: 10.1016/s0304-3940(03)00787-0. [DOI] [PubMed] [Google Scholar]
- 20.Maurer U, Bucher K, Brem S, et al. Development of the automatic mismatch response: from frontal positivity in kindergarten children to the mismatch negativity. Clin Neurophysiol. 2003;114:808–17. doi: 10.1016/s1388-2457(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 21.Waberski TD, Kreitschmann-Andermahr I, Kawohl W, et al. Spatiotemporal source imaging reveals subcomponents of the human auditory mismatch negativity in the cingulum and right inferior temporal gyrus. Neurosci Lett. 2001;308:107–10. doi: 10.1016/s0304-3940(01)01988-7. [DOI] [PubMed] [Google Scholar]
- 22.Deouell LY, Parnes A, Pickard N, et al. Spatial location is accurately tracked by human auditory sensory memory: evidence from the mismatch negativity. Eur J Neurosci. 2006;24:1488–94. doi: 10.1111/j.1460-9568.2006.05025.x. [DOI] [PubMed] [Google Scholar]
- 23.Marco-Pallarés J, Grau C, Ruffini G. Combined ICA-LORETA analysis of mismatch negativity. Neuroimage. 2005;25:471–7. doi: 10.1016/j.neuroimage.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Rinne T, Alho K, Ilmoniemi RJ, et al. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000;12:14–9. doi: 10.1006/nimg.2000.0591. [DOI] [PubMed] [Google Scholar]
- 25.Jemel B, Achenbach C, Muller BW, et al. Mismatch negativity results from bilateral asymmetric dipole sources in the frontal and temporal lobes. Brain Topogr. 2002;15:13–27. doi: 10.1023/a:1019944805499. [DOI] [PubMed] [Google Scholar]
- 26.Fulham WR, Michie PT, Ward PB, et al. Mismatch negativity in recent-onset and chronic schizophrenia: a current source density analysis. PLoS One. 2014;9:e100221. doi: 10.1371/journal.pone.0100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umbricht D, Koller R, Vollenweider FX, et al. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51:400–6. doi: 10.1016/s0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt A, Bachmann R, Kometer M, et al. Mismatch negativity encoding of prediction errors predicts S-ketamine-induced cognitive impairments. Neuropsychopharmacology. 2012;37:865–75. doi: 10.1038/npp.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung S, Croft RJ, Baldeweg T, et al. Acute dopamine D(1) and D(2) receptor stimulation does not modulate mismatch negativity (MMN) in healthy human subjects. Psychopharmacology (Berl) 2007;194:443–51. doi: 10.1007/s00213-007-0865-1. [DOI] [PubMed] [Google Scholar]
- 30.Umbricht D, Javitt D, Novak G, et al. Effects of risperidone on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1999;2:299–304. doi: 10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- 31.Baldeweg T, Klugman A, Gruzelier J, et al. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr Res. 2004;69:203–17. doi: 10.1016/j.schres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Salisbury DF, Shenton ME, Griggs CB, et al. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–94. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 33.Umbricht DS, Bates JA, Lieberman JA, et al. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59:762–72. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 36.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 37.Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–64. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- 38.Herzfeld HD. WST-Wortschatztest. In: Karl-Heinz Schmidt, Metzler Peter., editors. Diagnostica. 40 Beltz Test GmbH; Weinheim: 1994. 1992. pp. 293–297. [Google Scholar]
- 39.Feng N, Vollenweider FX, Minder EI, et al. Development of a gas chromatography-mass spectrometry method for determination of ketamine in plasma and its application to human samples. Ther Drug Monit. 1995;17:95–100. doi: 10.1097/00007691-199502000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Umbricht D, Schmid L, Koller R, et al. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–47. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 41.Dittrich A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry. 1998;31(Suppl 2):80–4. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- 42.Musso F, Brinkmeyer J, Ecker D, et al. Ketamine effects on brain function–simultaneous fMRI/EEG during a visual oddball task. Neuroimage. 2011;58:508–25. doi: 10.1016/j.neuroimage.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 43.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 44.van der Gaag M, Hoffman T, Remijsen M, et al. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006;85:280–7. doi: 10.1016/j.schres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 45.Brockhaus-Dumke A, Tendolkar I, Pukrop R, et al. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr Res. 2005;73:297–310. doi: 10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–52. [PubMed] [Google Scholar]
- 47.Leicht G, Kirsch V, Giegling I, et al. Reduced early auditory evoked gamma-band response in patients with schizophrenia. Biol Psychiatry. 2010;67:224–31. doi: 10.1016/j.biopsych.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Mulert C, Leicht G, Pogarell O, et al. Auditory cortex and anterior cingulate cortex sources of the early evoked gamma-band response: relationship to task difficulty and mental effort. Neuropsychologia. 2007;45:2294–306. doi: 10.1016/j.neuropsychologia.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–27. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dys-connection in schizophrenia. Biol Psychiatry. 2006;59:929–39. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt A, Diaconescu AO, Kometer M, et al. Modeling ketamine effects on synaptic plasticity during the mismatch negativity. Cereb Cortex. 2013;23:2394–406. doi: 10.1093/cercor/bhs238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corlett PR, Honey GD, Krystal JH, et al. Glutamatergic model psychoses: prediction error, learning, and inference. Neuropsychopharmacology. 2011;36:294–315. doi: 10.1038/npp.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 54.De la Fuente-Sandoval C, Leon-Ortiz P, Favila R, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36:1781–91. doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rowland LM, Kontson K, West J, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–104. doi: 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosburg T, Kreitschmann-Andermahr I. The effects of ketamine on the mismatch negativity (MMN) in humans — a meta-analysis. Clin Neurophysiol. 2016;127:1387–94. doi: 10.1016/j.clinph.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 57.Nagai T, Tada M, Kirihara K, et al. Auditory mismatch negativity and P3a in response to duration and frequency changes in the early stages of psychosis. Schizophr Res. 2013;150:547–54. doi: 10.1016/j.schres.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Todd J, Michie PT, Schall U, et al. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Kreitschmann-Andermahr I, Rosburg T, Demme U, et al. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Brain Res Cogn Brain Res. 2001;12:109–16. doi: 10.1016/s0926-6410(01)00043-x. [DOI] [PubMed] [Google Scholar]
- 60.Heekeren K, Daumann J, Neukirch A, et al. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl) 2008;199:77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oranje B, van Berckel BN, Kemner C, et al. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 62.Gogtay N, Vyas NS, Testa R, et al. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37:504–13. doi: 10.1093/schbul/sbr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu K, Krystal JH, Ning Y, et al. Preliminary analysis of positive and negative syndrome scale in ketamine-associated psychosis in comparison with schizophrenia. J Psychiatr Res. 2015;61:64–72. doi: 10.1016/j.jpsychires.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagels A, Kirner-Veselinovic A, Krach S, et al. Neural correlates of S-ketamine induced psychosis during overt continuous verbal fluency. Neuroimage. 2011;54:1307–14. doi: 10.1016/j.neuroimage.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 65.D’Souza DC, Singh N, Elander J, et al. Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence. Neuropsychopharmacology. 2012;37:1036–46. doi: 10.1038/npp.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]