Abstract
Background and Purpose
Rapid recognition of those at high-risk for malignant edema after stroke would facilitate triage for monitoring and potential surgery. Admission data may be insufficient for accurate triage decisions. We developed a risk prediction score using clinical and radiographic variables within 24 hours of ictus to better predict potentially lethal malignant edema (PLME).
Methods
Patients admitted with diagnosis codes of “Cerebral Edema” and “Ischemic Stroke,” NIHSS ≥ 8, and head CTs within 24 hours of stroke-onset were included. Primary outcome of PLME was defined as death with midline shift (MLS) ≥ 5mm or Decompressive Hemicraniectomy. We performed multivariate analyses on data available within 24 hours of ictus. Bootstrapping was used to internally validate the model and a risk score was constructed from the results.
Results
33% of 222 patients developed PLME. The final model c-statistic was 0.76 (CI 0.68-0.82) in the derivation cohort, and 0.75 (0.72-0.77) in the bootstrapping validation sample. The EDEMA score was developed using the following independent predictors: Basal cistern effacement (=3); Glucose ≥150 (=2); No tPA or thrombectomy (=1), MLS >0-3 (=1), 3-6 (=2), 6-9 (=4); >9 (=7); No prior stroke (=1). A score over 7 was associated with 93% positive predictive value.
Conclusion
The EDEMA score identifies patients at high risk for PLME. While it requires external validation, this scale could help expedite triage decisions in this patient population.
Keywords: Brain Edema, Decompressive Surgery, Hemispheric Infarct, Risk prediction, Risk Score
Introduction
Rapid recognition of patients who will develop life-threatening edema after large hemispheric stroke (LHI) is essential for appropriate triage to Comprehensive Stroke Centers, and possible decompressive hemicraniectomy (DHC). Several studies have attempted to ascertain predictors of cerebral edema based on demographic, clinical and radiographic features.1-6 However, baseline clinical variables and advanced (and less easily accessible) imaging measures of infarct volume have limited predictive accuracy.3-6 While early CT imaging has limited resolution, sequential imaging demonstrating evolving infarction and early signs of edema may be beneficial. The purpose of this study was to develop a practical risk prediction tool that can aid in rapidly and accurately triaging patients at high risk for PLME within the first 24 hours of stroke with high positive predictive value.
Methods
Study Population
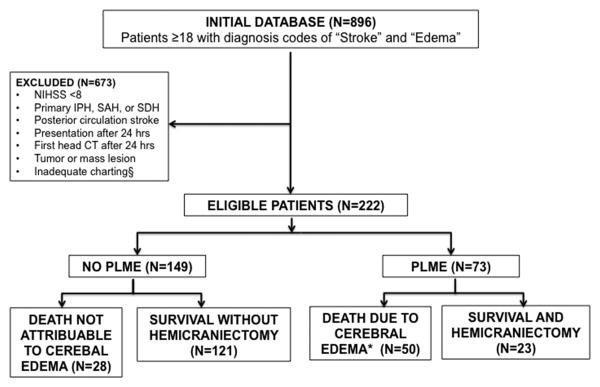
We assembled a retrospective cohort of patients with cerebral edema admitted to Barnes-Jewish Hospital between 2006 and 2015 with diagnosis codes of “Cerebral Edema” and “Ischemic Stroke” from the Clinical Investigation Data Exploration Repository (CIDER) maintained by Washington University's Center for Biomedical Informatics.7 This was limited to: patients over age 18 with confirmed acute anterior circulation stroke and NIHSS ≥ 8 who received at least one head CT within 24 hours of last-known normal. (Figure 1) The Washington University Human Studies Committee approved the study.
Figure 1.
Eligibility Criteria: Abbreviations: IPH=Intraparenchymal Hemorrhage; SAH= Subarachnoid Hemorrhage; SDH= Subdural Hemorrhage; PLME= Potentially Lethal Malignant Edema. *Death due to cerebral edema is defined as midline shift ≥ 5mm at the level of the septum pellucidum on any scan during admission. § Inadequate charting of either outcome or missing values for final model variables.
Primary Outcome
Potentially lethal malignant edema (PLME) was defined as death with ≥ 5mm midline shift (MLS) or need for DHC. Patients are selected for DHC at our institution based on the criteria outlined in previously published trials.8
Selection of Predictor Variables
Prior literature informed the selection of potentially predictive clinical and radiologic variables.1, 2, 9 Data was abstracted from medical records available within 24 hours of onset. When NIHSS was not specifically recorded (35%) it was calculated from documented neurologic exams. Authors (CJO, JG, OL-S) independently reviewed head CTs: MLS was measured at the septum-pellucidum, cisternal effacement was recorded (as present/absent) based on basal cisterns narrowing. A randomly selected 10% sample yielded a kappa of 0.78 and absolute agreement of 96%, indicating good inter-rater reliability.
Statistical Analysis
We employed binary logistic regression to construct a multivariable model, with input of variables if p-value < 0.2 and backward elimination if p-value > 0.10. The ability of the model to discriminate those with PLME was evaluated using the c-statistic. Bootstrapping was used for internal validation.10 To construct a clinically relevant risk score, we assigned integer point values to each independent predictor.11 We performed statistical analyses with SAS (v. 9.4) and R software (Version 0.99.893) packages.12, 13
Results
Of 896 potential subjects. 222 met final eligibility (Figure 1). Seventy-three (33%) developed PLME (50 died, 23 survived with DHC). Patients with PLME were younger (63 vs. 71, p=0.049), more likely to have concurrent ACA or PCA territory infarction (22 v 5% and 10 v 2%, p=0.0005, 0.019), had greater early MLS (2.88 vs. 0.5-mm, p<0.001), and effacement of basal cisterns (19% vs. 1%, p=0.0002). Admission glucose (143 vs. 130 mg/dL, p=0.008) and white blood cell count (10.2 vs. 9.2 cells/mcL) were higher. Blood pressure and temperature did not differ. While NIHSS on arrival did not differ (18 vs. 17), the PLME group had higher max NIHSS (23 vs. 21, p=0.037) signifying greater early neurological deterioration. The PLME group was less likely to receive an acute intervention. Mortality of the group overall was 35% (Table I).
Table 1. EDEMA Score.
| Component | EDEMA Score Point |
|---|---|
| Effacement | |
| Yes | 3 |
| No | 0 |
| Midline Shift | |
| 0 | 0 |
| 0-3 mm | 1 |
| 3-6 mm | 2 |
| 6-9 mm | 4 |
| >9 mm | 7 |
| Glucose | |
| <150 | 0 |
| ≥150 | 2 |
| Previous Stroke | |
| No | 1 |
| Yes | 0 |
| Intervention (tPA or thrombectomy) | |
| No | 1 |
| Yes | 0 |
Independent predictors in the final multivariable model included glucose (OR 1.056 CI [1.104-1.01]), midline shift (1.299 [1.11-1.50]), basilar cistern effacement (5.27 [0.94-29.5]), absence of previous stroke (2.02 [0.99-4.13]) and absence of acute intervention like tPA or thrombectomy (1.94 [1.08-3.74]) (Supplemental Table II). While basal cistern effacement and MLS were correlated (rho 0.48), each still significantly contributed to the model so both were retained. The c-statistic for this model in the derivation dataset was 0.76 (CI 0.68, 0.82). Statistical resampling using 1000 bootstrapped samples revealed a validation AUC of 0.75.
The EDEMA Score
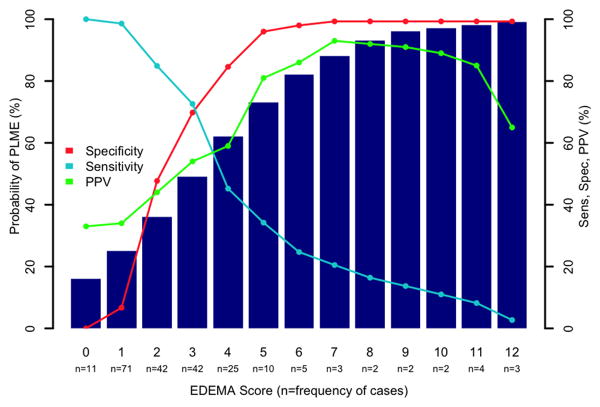
The EDEMA score was constructed with points weighted towards characteristics with greater influence on outcome. Glucose was dichotomized to ≥ 150 and <150 based on previous literature.5 Probabilities of PLME development for each score allocation are shown in Figure 1. Although our score had a maximum possible total of 14 points, the highest observed score was 12. In patients who scored 7 or greater, positive predictive value was 93%, with a specificity of 99% (Supplemental Table III).
Discussion
In this study of 222 patients with LHI and cerebral edema, we identified several independent predictors of PLME, many of which were consistent with other studies, including hyperglycemia5 and absence of recanalization.1, 2 Age and NIHSS, found to have significant associations in some studies,2-4 were not independent predictors in our final model.
This study incorporated additional CT imaging variables (MLS and basilar cistern effacement up to 24 hours) to improve predictive accuracy. These have been shown to be associated with level of arousal, neurologic deterioration and poor outcome.1, 14 The association between previous stroke and edema may be related to greater atrophy, which could be protective against malignant edema development.6 Radiographic variables at 24-hours were more useful than baseline clinical stroke severity or imaging markers such as ASPECTS or hyperdense vessel sign.
Other robust predictors from the literature include radiographic markers obtained from CTAs or MRI, like infarct volume,3, 6 poor collateral status,2 and proximal or internal carotid artery occlusion.3, 5 We did not collect data from MRI or CTA which may not be as widely available, to maintain the generalizability of our model.
Our model performs comparably to others in the literature, whose c-statistics range from 0.69-0.91 depending on whether MRI findings were used.3-5 Because MRI may not be feasible for all centers, we did not include advanced imaging in our score. We also relied on data readily available to most practitioners within the first 24 hours of stroke onset, as limiting predictor variables to those collected within the first 6 hours3, 4, 6 may not be widely generalizable.
Given that the EDEMA score's more intuitive endpoints of death or DHC might be used to facilitate discussion and communication with other members of the healthcare team for prognostication purposes, transfer or surgical decision-making, positive predictive value is of particular importance. In patients who received a score ≥ 7, the positive predictive value was 93%. (Figure 2)
Figure 2. EDEMA Score.
Probability of PLME. Positive predictive value (PPV) approaches 90% at a score of 7.
Limitations
Our study has important limitations. Its retrospective nature means that clinical, imaging and long-term functional data was limited to what was available in clinical practice. 16.5% of participants did not have fully documented neurologic exams—in these cases only listed findings were scored. While our use of the composite outcome of death or DHC has precedent,15 it could introduce bias as practitioners may have different thresholds for surgical intervention. Practice variability is arguably less at a single institution, it may nevertheless limit the score's generalizability. To increase its specificity we required at least 5 mm of MLS at time of death or DHC on CT imaging. Ideally, detection of patients at risk for PLME would occur even prior to radiographic signs, our score depended on these signals. Further external validation of our model is necessary to assess its predictive power. Presumably not all of the patients who expired would have survived had they underwent DHC, and therefore our score only identifies those who develop PLME, not who would benefit from surgery. Despite these weaknesses, our study makes important contributions. To our knowledge, it describes the largest single cohort of patients with malignant edema in the literature. Moreover it is an attempt to assign concrete scores to important variables that influence an important and clinically relevant outcome.
Conclusion
The EDEMA score is a grading scale that predicts PLME development in patients with moderate to severe LHI in the first 24 hours with high positive predictive value. Identifying these patients can inform management including transfer to tertiary care centers, family discussions, surgery, and future research studies.
Supplementary Material
Acknowledgments
Sources of Funding: Research was supported by: 1) Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, KL2TR000450, National Center for Advancing Translational Sciences (NCATS) of the NIH, and 2) R01 NS085419 & R01 NS084028 awards from the NINDS of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Conflict of Interest/Disclosures: The authors report no conflicts of interest.
References
- 1.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant' middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Jin ST, Kim YW, Kim SR, Park IS, Jo KW. Predictors of malignant brain edema in middle cerebral artery infarction observed on ct angiography. J Clin Neurosci. 2015;22:554–560. doi: 10.1016/j.jocn.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Thomalla G, Hartmann F, Juettler E, Singer OC, Lehnhardt FG, Kohrmann M, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Ann Neurol. 2010;68:435–445. doi: 10.1002/ana.22125. [DOI] [PubMed] [Google Scholar]
- 4.Asuzu D, Nystrom K, Sreekrishnan A, Schindler J, Wira C, Greer D, et al. Turn score predicts 24-hour cerebral edema after iv thrombolysis. Neurocrit Care. 2016;24:381–388. doi: 10.1007/s12028-015-0198-6. [DOI] [PubMed] [Google Scholar]
- 5.Shimoyama T, Kimura K, Uemura J, Yamashita S, Saji N, Shibazaki K, et al. The dash score: A simple score to assess risk for development of malignant middle cerebral artery infarction. J Neurol Sci. 2014;338:102–106. doi: 10.1016/j.jns.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Beck C, Kruetzelmann A, Forkert ND, Juettler E, Singer OC, Kohrmann M, et al. A simple brain atrophy measure improves the prediction of malignant middle cerebral artery infarction by acute dwi lesion volume. J Neurol. 2014;261:1097–1103. doi: 10.1007/s00415-014-7324-9. [DOI] [PubMed] [Google Scholar]
- 7.Clinical Investigation Data Exploration Repository. St. Louis, MO: Washington University in St. Louis Center for Biomedical Informatics; 2010. [Google Scholar]
- 8.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. The Lancet Neurology. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 9.Hofmeijer J, Algra A, Kappelle LJ, van der Worp HB. Predictors of life-threatening brain edema in middle cerebral artery infarction. Cerebrovasc Dis. 2008;25:176–184. doi: 10.1159/000113736. [DOI] [PubMed] [Google Scholar]
- 10.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan LM, Dukes KA, Losina E. Tutorial in biostatistics. An introduction to hierarchical linear modelling. Stat Med. 1999;18:855–888. doi: 10.1002/(sici)1097-0258(19990415)18:7<855::aid-sim117>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 13.Harrell Frank E., Jr . Regression modeling strategies. Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 14.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 15.Sheth KN, Kimberly WT, Elm JJ, Kent TA, Yoo AJ, Thomalla G, et al. Exploratory analysis of glyburide as a novel therapy for preventing brain swelling. Neurocrit Care. 2014;21:43–51. doi: 10.1007/s12028-014-9970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.