Abstract
Objectives
To determine the effects on weight loss of three abbreviated behavioral weight loss interventions with and without coaching and mobile technology.
Methods
Randomized controlled efficacy study of three six-month weight loss treatments delivered to 96 adults with obesity: 1) self-guided [SELF], 2) standard [STND], or 3) technology-supported [TECH]. STND and TECH received 8 in-person group treatment sessions. SELF and STND used paper diaries to self-monitor diet, activity, and weight; TECH used a smartphone application with social networking features and wireless accelerometer.
Results
Weight loss was greater for TECH and STND than SELF at 6 months [−5.7kg (95% CI: −7.2, −4.1) vs. −2.7kg (95% CI: −5.1, −0.3), p<.05]), but not 12 months. TECH and STND did not differ except that more STND (59%) than TECH (34%) achieved ≥5% weight loss at 6 months (P < 0.05). Self-monitoring adherence was greater in TECH than STND (P <0.001), greater in both interventions than SELF (P <0.001), and covaried with weight loss (r(84) = 0.36 − 0.51, P<.001).
Conclusions
Abbreviated behavioral counseling can produce clinically meaningful weight loss regardless of whether self-monitoring is performed on paper or smartphone, but long-term superiority over standard of care self-guided treatment is challenging to maintain.
Keywords: mobile technology, obesity, weight loss, technology intervention, obesity treatment
Introduction
Intensive lifestyle interventions produce clinically meaningful sustained weight loss, but their required minimum 16 in-person treatment sessions renders them too burdensome and costly to reach the 70% of adults who remain overweight/obese.1,2 However, attempts to decrease treatment sessions have yielded greatly diminished weight loss.3,4 The unmet challenge of behavioral weight loss implementation remains how to reduce treatment intensity without excising the regular social support, accountability, and feedback needed to maintain adherence to diet and activity goals. The E-Networks Guiding Adherence to Goals in Exercise and Diet (ENGAGED) trial tested whether in-person treatment sessions could be reduced by half but weight loss preserved by using mobile technology to deliver effective treatment components more efficiently.5,6
Smartphones offer a promising intervention channel and self-regulation tool, particularly as ownership continues to rise: from 46% in 2012 to 67% in 2015.7 Smartphones hold potential to reduce treatment burden and increase reach by replacing some in-person contact with telephonic or digital communication.8,9 Most weight loss applications (apps) provide a control system10 whose feedback reinforces self-monitoring of diet, physical activity, and weight by visualizing progress toward goals.6,11,12 Patients perceive apps as an acceptable behavior change tool that becomes less predictable and more engaging through the use of passively transmitted worn sensor data.13 Transmitting the participant’s data to a coach extends the control system beyond the individual to a facilitator who tracks progress, conveys accountability, and tailors support provision without requiring a face-to-face meeting.11
The ENGAGED Study extended the control system still further into social space by incorporating features known to produce weight loss: group competition for financial incentives14 and social media.15,16 A group weight loss competition motivated participants to help their teammates because they would benefit financially from belonging to the group that lost the most weight. The technology included social networking features to help team members track and support each other’s weight management efforts remotely. The smartphone app showed status icons for each member of a participant’s weight loss group. Icons changed color throughout the day to depict overall wellbeing, dietary self-monitoring and accelerometer wear (tracking physical activity). A private chatroom on the app let participants communicate digitally with team members.
The ENGAGED study examined the efficacy of two abbreviated (8 in-person session) versions of the Diabetes Prevention Program (DPP), as compared to a self-guided version. The control self-guided program (SELF) provided DPP sessions on DVD and utilized paper self-monitoring diaries. Having produced modest weight loss in a prior primary care trial,17 SELF has been recommended as low cost weight loss standard of care.18,19 One abbreviated intervention (technology-supported: TECH) used a custom-designed smartphone app for diet and weight self-monitoring, and integrated social media and passively monitored physical activity data from an accelerometer. The other abbreviated intervention (standard: STND) used paper and pencil self-monitoring. Both TECH and STND were hypothesized to produce greater weight loss than SELF because they included in-person group treatment sessions.20 Based on previous research showing greater self-monitoring with technology relative to paper methods,21 TECH was hypothesized to yield better self-monitoring adherence and therefore greater weight loss than STND.
Methods
Setting and Study Participants
The ENGAGED 3-group randomized controlled trial examined the efficacy at end of treatment (6 months) and 12 month follow-up of an abbreviated smartphone-supported weight loss program among adults with obesity. A detailed description of the study design, methods, and technology has been published.5,6 Northwestern University’s Institutional Review Board approved all study procedures. Chicago area participants were recruited via flyers and transit advertisements between July, 2011 and February, 2012. Eligible participants were between 18 and 60 years old, with body mass index (BMI) between 30–40 kg/m2, no weight gain or loss exceeding 11.3 kg for the past 6 months, and not participating in another weight loss program. Individuals who were pregnant, nursing, had an unstable medical condition, contraindications to moderate intensity physical activity, or took medications known to cause weight gain or loss were excluded.
Those meeting entry criteria via online questionnaire were telephoned by staff who explained the study and conducted screening. Eligible candidates attended an in-person orientation and equipoise induction where staff and participants discussed pros and cons of the three conditions to reduce differential attrition after randomization.22 Interested enrollees completed the informed consent process, in-person screening, and baseline assessments. Figure 1 illustrates participant flow through the trial. Of 776 individuals screened online, 96 were randomized.
Figure 1.
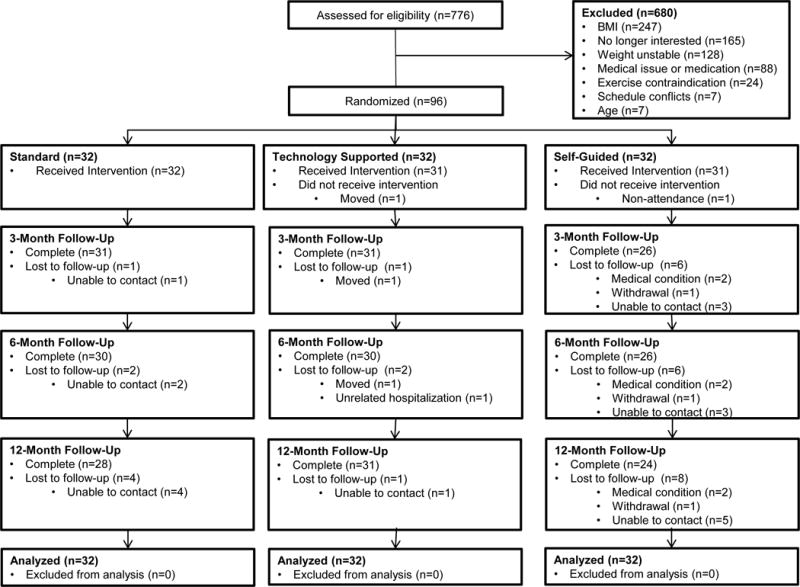
Flow of Participants Through the ENGAGED Study
Randomization
Participants were recruited in two cohorts, each including 6 groups of 8 participants. Half of the groups within each cohort were constructed to include only women (homogenous); half included both men and women (heterogeneous), with the latter with the latter including at least 2 men to prevent marginalization. Group assignments were based on when participants were available for in-person treatment. Randomization, stratified by group type (homogenous/heterogeneous) occurred at the group level. Once all eligible participants of a cohort were assigned to a group, the 3 groups within each stratum were randomized by a statistician using a randomly permuted block with 3 cells. The statistician notified the project staff who then revealed the treatment assignment [STND, TECH, or SELF] to participants during the first in-person group session. Although treatment condition was evident to participants and interventionists, outcome assessment and data analysis were blinded.
Intervention
All study participants had a 7% weight loss goal and were encouraged to lose approximately 0.5–1.0 kg/week. Calorie and fat gram allowances ranged from 1200–2000 kcal/day and 32–55 grams/day based upon initial body weight. Physical activity goals progressed from 45 to 175 minutes/week of moderate-to-vigorous intensity physical activity (MVPA). Figure 2 depicts the timeline of events for each intervention condition.
Figure 2.
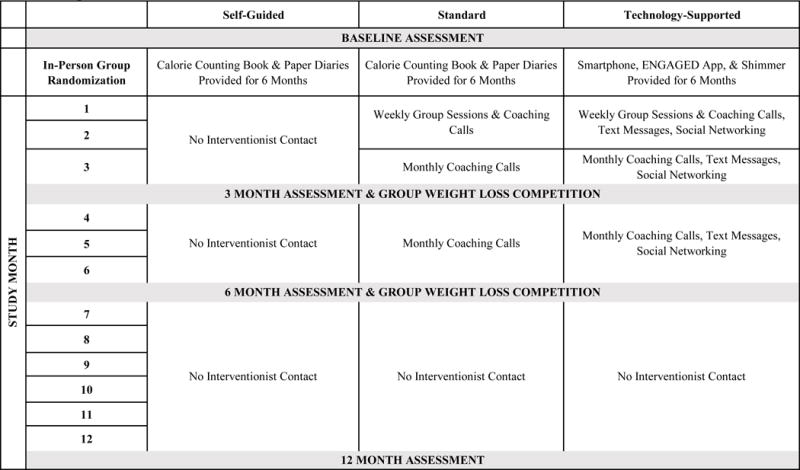
ENGAGED Intervention and Assessment Timeline
Those randomized to SELF attended one 60 minute group session at which treatment assignment was revealed and participants received their weight loss target, a calorie and fat gram counting book (The Complete Book of Food Counts23), and 6 months of daily paper self-monitoring diaries. They also received Group Lifestyle Balance24 DVDs presenting 12 mock group treatment sessions adapted from the original DPP curriculum.25 SELF participants received no additional in-person sessions or coaching calls.
In contrast, for the first 8 weeks, those assigned to STND or TECH treatment attended weekly 90-minute group sessions led by a psychologist or exercise physiologist and focused on nutrition, MVPA, and behavior change strategies. A 30-minute guided walking exercise was offered after group sessions. STND and SELF participants received the same calorie counting book and paper diaries.
TECH participants were lent an Android smartphone with study-designed ENGAGED app and accelerometer for 6 months. They used the app to self-monitor dietary intake and body weight and wore the accelerometer to objectively measure MVPA; these data transmitted wirelessly to their coach. The app’s dietary intake “fans” showed traffic light colors depicting calorie and fat gram allowances remaining for that day. MVPA data, transmitted by the accelerometer, automatically populated an app display, visualizing the remaining MVPA needed to reach the weekly goal. TECH participants used the app’s team tab to track their group members’ self-monitoring adherence, post messages to the team, or message individual group members directly. Additionally, TECH participants received 2–4 personalized messages/week for 6 months.
Weekly for the first 8 weeks and monthly from months 3–6, TECH and STND participants received 10–15 minutes calls during which trained coaches with at least a bachelor’s degree reviewed self-monitoring and goal attainment, and helped participants solve problems. Participants returned the Android phone with the ENGAGED app and accelerometer at the 6 month visit and had no further contact with coaches.
Groups competed for financial incentives in weight loss competitions held at the 3- and 6-month assessments. STND and TECH participants competed in their original 8-person groups; SELF participants competed in groups of 8 that were assigned at their in-person meeting. The team with the highest average percent weight loss won and each of its members received $50.
All coaching calls were audio recorded and treatment fidelity was assessed using a 6-item checklist covering required call topics: 1) reviewed weight loss goal; 2) evaluated diet self-monitoring; 3) evaluated physical activity self-monitoring; 4) set calorie and fat goals; 5) set physical activity goal; 6) set new SMART behavioral goal. Fidelity was assessed biweekly during weeks 1–8 and then monthly until month 6. A 10% sample of each coach’s sessions was randomly selected and rated. If fidelity fell below 90%, the coach was retrained by a doctoral level staff member.
Outcomes
Primary outcomes were weight loss and behavioral adherence. Weight loss was measured both continuously and as the attainment of clinically meaningful ≥5% weight loss.26 The time that coaches spent administering the intervention was an exploratory outcome. Body weight was measured without shoes on a calibrated balance beam scale at baseline, 3-, 6-, and 12-months. Behavioral adherence, operationalized by self-monitoring of diet, physical activity, and weight, was examined during months 1–6. Diet self-monitoring adherence was measured as the percent of days reporting energy intake of ≥ 1000 calories in the paper diary (STND and SELF) or on the ENGAGED smartphone application (TECH). Physical activity monitoring adherence was assessed as the percent of days when any activity was reported in the paper diary (STND and SELF) or when any physical activity was detected on the accelerometer (TECH). Weight self-monitoring was assessed as the percent of days when a body weight was recorded in the paper diaries (STND and SELF) or on the ENGAGED smartphone application (TECH).
Statistical Analysis
Data were analyzed on an intent-to-treat basis. Linear mixed models for longitudinal data were used to test for differences between treatments on weight loss measured continuously over time; mixed effects logistic models were used to test for differences in the percent achieving 5% weight loss.27 This class of models does not assume that subjects are measured at all time points, and therefore can include subjects with missing data across time. Changes across time were examined by treating time as a factor variable with baseline as the reference cell. In addition to the effect of time, the main independent variable is treatment condition, a between-subjects factor with 3 levels: standard care, technology-assisted, and self-guided. Helmert contrasts were used to test two comparisons; H1: the experimental conditions (STND & TECH) vs. the self-guided condition, and H2: the standard care condition vs technology-assisted. Condition by time interactions were examined at 3, 6, and 12 months to test whether change in outcomes across time differed by condition, in terms of these two contrasts. Finally, since subjects were nested within groups and groups were randomized to treatment conditions, a random group effect was included in the model to account for any cluster effects attributable to groups (though this group variance was estimated to be zero).
Power analyses were based on data from our previous +Mobile Trial,11 where standard deviations of 3.8 and 4.9 kg. were seen at months 3 and 6, respectively, with a correlation of 0.86 between the two time points. With 80% power and n=30 at the final endpoint in both the STND and TECH groups, a difference of 3 kg. between these two groups is expected to be able to be detected. The power calculations were based on the second Helmert contrast: TECH vs. STND because this contrast contains the fewest number of subjects (30 in each group) and it is expected that this contrast will have the smallest effect sizes. The first Helmert contrast: STND and TECH vs. SELF has a larger sample size (60 vs. 30) and larger effects sizes were expected in this contrast due to the limited intensity of the SELF intervention. Therefore, by powering the study based on the second contrast, it is expected that there will be more than adequate power for the first contrast.
Results
Study Participants
At baseline, participants had a mean (SD) age of 39.3 (11.7) years and BMI of 34.6 (3.0) kg/m2. Eighty-four percent were female; 57.3% were white, 31.3% were black. Baseline participant characteristics appear in Table 1. The treatment groups showed no baseline differences on age, sex, race, ethnicity, marital status, education, or weight. Attrition at the final 12 month follow-up assessment was greater for SELF (25.0%) than either STND (12.5%) or TECH (3.1%) treatments (p = .02), and was not differential between the STND and TECH treatments (p=.20) (Figure 1).
Table 1.
Baseline Participant Characteristics by Intervention Condition
|
All Participants (n=96) |
Self-Guided (n=32) |
Standard (n=32) |
Technology-Supported (n=32) |
|
|---|---|---|---|---|
| Age, mean (SD) (y) | 39.3 (11.7) | 40.1 (11.1) | 37.3 (13.3) | 40.4 (10.7) |
| Female, No. (%) | 84.4 | 84.4 | 81.3 | 87.5 |
| Ethnicity, No. (%) | ||||
| Hispanic or Latino | 19.8 | 15.6 | 31.3 | 12.5 |
| Not Hispanic or Latino | 80.2 | 84.4 | 68.8 | 87.5 |
| Race, No. (%) | ||||
| White | 57.3 | 53.1 | 62.5 | 56.3 |
| Black or African American | 31.3 | 34.4 | 18.8 | 40.6 |
| College Graduate or above (%) | 68.8 | 71.9 | 65.6 | 68.8 |
| Weight (kg) | 94.8 (12.4) | 93.5 (11.0) | 96.0 (14.6) | 94.7 (11.6) |
| BMI (kg/m2) | 34.6 (3.0) | 34.3 (3.2) | 34.8 (3.0) | 34.8 (2.8) |
| Waist Circumference (cm) | 96.0 (8.7) | 95.6 (9.9) | 96.2 (8.3) | 96.3 (8.1) |
Abbreviations: BMI, body mass index
Treatment Fidelity
Across the study’s duration, 105 coaching calls were assessed for treatment fidelity from 46 participants. Overall, fidelity was 95.0%; three coach re-trainings were held.
Weight Loss
At 12 months, participants showed a mean ± 95% CI weight change from baseline of −5.6(−8.5, −2.8) kg in STND, −3.1(−5.9, −0.3) kg in TECH, and −2.7(−5.7, 0.4) kg in SELF (Table 2). When TECH and STND were combined, weight change was significantly greater than SELF at 3 (P < 0.005) and 6 months (P < 0.05), but not 12 months (Table 2 and Figure 3). When measured as a continuous variable, there was no difference in weight change between TECH and STND at any time point. At 6 months, weight loss of at least 5% occurred more often in the experimental treatments (47%) than in SELF (13%) (P < 0.005) and more often in STND (59%) than TECH (34%) (P <0.05). At 12 months, weight loss of at least 5% was observed in 47% of STND, 28% of TECH and 25% of SELF participants; these differences were not significant.
Table 2.
Weight Change at 3, 6, and 12 Months by Intervention Condition
| Mean & 95%CI | P Value | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Self-Guided (n=32) |
Standard (n=32) |
Technology-Supported (n=32) |
TECH+STND Combined (n=64) |
TECH+STND Combined vs. SELF | TECH vs. STND | |
| Weight Change (kg) | ||||||
| 3 Months | −1.8(−3.4, −0.1) | −5.9(−7.5, −4.4) | −4.1(−5.7, −2.6) | −5.0(−6.1, −3.9) | <.005 | n.s. |
| 6 Months | −2.7(−5.1, −0.3) | −6.6(−8.8, −4.4) | −4.7(−6.7, −2.5) | −5.7(−7.2, −4.1) | <.05 | n.s. |
| 12 Months | −2.7(−5.7, 0.4) | −5.6(−8.5, −2.8) | −3.1(−5.9, −0.3) | −4.4(−8.8, −4.4) | n.s. | n.s. |
STND: Standard Weight Loss Program; TECH: Technology-Supported Weight Loss Program; SELF: Self-Guided Weight Loss Program
Figure 3.
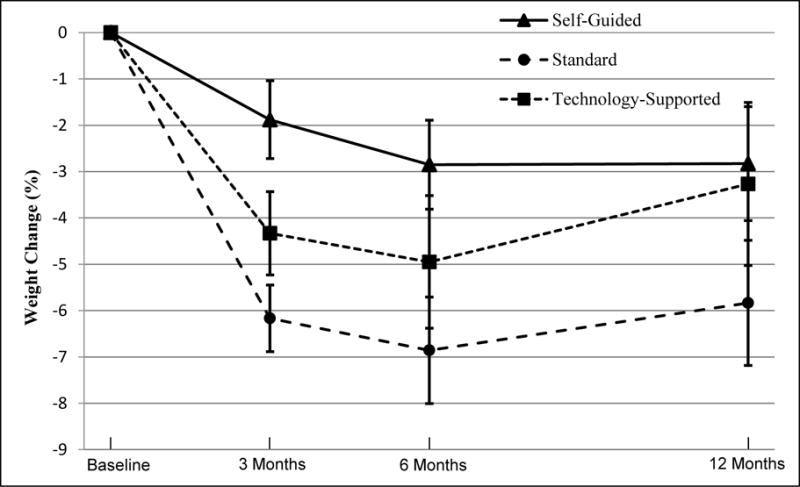
Percent Weight Change at 3, 6, and 12 Months by Intervention Condition
Self-Monitoring Adherence
Table 3 compares adherence to diet, physical activity, and weight self-monitoring across the three treatment conditions during the 6 months of the intervention. Diet, activity, and weight self-monitoring were greater in TECH and STND than SELF (P <0.001). Self-monitoring of all behavioral outcomes also was greater in TECH than STND: diet (P <0.05), activity (P<0.001), and weight (P <0.001). The amount of weight loss at 6 months covaried with the amount of self-monitoring of diet [r(84)=.509, p<.001], physical activity [r(84)=.460, p<.001], and weight [r(84)=.364, p<.001]; the correlations did not differ as a function of treatment condition.
Table 3.
Adherence to Dietary, Physical Activity, and Weight Self-Monitoring Across 6 months by Intervention Condition
| Mean + 1 SE | P Value | ||||
|---|---|---|---|---|---|
| Self-Guided (n=32) | Standard (n=32) | Technology-Supported (n=32) | TECH+STND Combined vs. SELF | TECH vs. STND | |
| Adherence (% days) | |||||
| Diet | 18.4(5.3) | 32.9(3.9) | 48.0(4.1) | <.001 | <.05 |
| Physical Activity | 9.8(2.4) | 30.5(4.4) | 56.8(4.8) | <.001 | <.001 |
| Weight | 16.0(4.6) | 38.4(5.0) | 90.4(1.7) | <.001 | <.001 |
Coaching Time
The time throughout months 1–6 that lifestyle coaches spent preparing for and conducting calls was greater for TECH participants [285.71 (SD 83.9) minutes] than STND [202.8 (SD 89.4) minutes] [F(1, 61) = 14.39, p < 0.001].
Adverse Events
The treatment groups did not differ on adverse events and no severe adverse events were considered study-related.
Discussion
The ENGAGED study demonstrated that two abbreviated DPP versions (TECH and STND) yielded clinically significant and greater weight losses than a self-guided version at 3 and 6 months even though they decreased treatment intensity from 16 in-person sessions to 8. The time needed to deliver the ENGAGED hybrid in-person group plus telephone intervention was less than 4 hours per participant, as compared to 8–16 hours needed to deliver the DPP in-person curriculum. This reduction in needed interventionist time should make ENGAGED less burdensome than the DPP program whose first year of intervention costs approximately $1,400.28 The interventionist time needed to deliver the ENGAGED treatment over 6 months is similar to that needed to deliver the POWER intervention, a version of the DPP provided entirely remotely by telephone and internet.9 Likewise, ENGAGED, POWER, and a recent remotely delivered DPP translation providing equivalent interventionist contact29 all produced approximately 6 kg weight loss in 6 months. Between 44–53% of participants in all three trials lost at least 5% of initial body weight. This magnitude of weight loss has been shown to result in significant health benefits30 and, although it is about 1 kg less than that seen in more intensive weight loss treatment programs,2,31 the difference is not large. Reducing or replacing some in-person sessions with telephone coaching may increase program scalability while still producing modest weight losses capable of improving health.
Although the abbreviated interventions produced meaningful weight loss at 6 months, differences from self-guided treatment dissipated by 12 months. Once self-monitoring tools were no longer provided and coach contact ceased at month 7, weight loss halted in all three treatment conditions. Individuals in all intervention conditions regained some weight, but those in TECH appeared most affected, regaining on average nearly 2% of initial body weight. Presumably, a trigger for the TECH group’s regain was loss of the study-owned smartphone, an implementation challenge that could be avoided now that the majority of adults own a smartphone and can access many available weight loss apps. Given this and other evidence32 that withdrawing intervention components at 6 months can undermine behavioral gains, ongoing treatment provision beyond 6 months appears warranted because weight regain after weight loss is normative, and continued lifestyle intervention is moderately effective for weight loss maintenance.33
The current findings, like those from another recent trial,34 contradict the premise that technology-supported weight loss interventions always outstrip standard treatment.35,36 Although weight loss did not differ as a function of whether self-monitoring was performed on an app or on paper, the percent of individuals achieving at least 5% weight loss was greater among those recording on paper. TECH not only tended to lose less weight than STND but they also regained more, dispelling differences between the abbreviated in-person treatments and SELF at 12 months.
Unlike differences between TECH and STND in the magnitude of weight loss, self-monitoring adherence differed across treatments in the manner predicted: i.e., greater for TECH than STND and greater for STND than SELF. Technology has been widely reported to bolster self-monitoring adherence.21,37,38 The puzzle raised by the present results is why the TECH group’s more diligent self-monitoring not only failed to translate into greater weight loss but in fact, translated to somewhat lesser weight loss. Notably a recent review found that only 53% of studies comparing technology-supported to standard weight loss interventions reported differences in weight outcomes, with even fewer smartphone studies detecting differences.39 Like the present study, two others showed enhanced self-monitoring with mobile technology compared to paper, but no treatment difference in weight loss.21,38 It is possible that a level of self-monitoring adherence between 30–50% represents a functional ceiling, beyond which no further enhancement of weight loss is discernable. An alternative explanation we consider more plausible is that the mobile technologies used in these ground breaking, early studies presented a perfect storm of attractive and frustrating features.21,38,40 On the positive side, they were engaging enough to reinforce participants for self-monitoring and generate an attachment to the devices. On the negative side they had technical glitches that are to be expected with first generation technologies. For example, the accelerometer used in ENGAGED was, at the time, the only one available with wireless transmission capabilities. However, the device sometimes lost power without warning and was difficult to restart, as is reflected by the greater coaching time utilized by participants in TECH as compared to STND. Additionally, digital dialogue among the 8 members of each weight loss group was difficult to sustain.
Weight loss in SELF was slightly less than in a prior study by Ma et al17 that utilized the DPP-based Group Lifestyle Balance DVD’s. SELF participants in that study showed a decrease in body weight of −4.7% at 6 months, in contrast to −2.7% in the current study. The smaller weight loss in the present study may be because our SELF intervention was less intensive, involving a single in-person session and DVDs that participants were to view on their own. In contrast, Ma et al.17 gave participants a scale and pedometer, trained them to use an online portal, and emailed them biweekly reminder messages to self-monitor. Even though the weight loss produced by SELF did not reach the recommended 5% weight loss, intervention burden was low which may make this a scalable way to reach more of the overweight and obese population.
Although highly innovative, the study is not without limitations. Enrollees were highly motivated. Retention in TECH may have benefited from the fact that participants were provided with a smartphone with an unlimited calling, text, and data package. Despite this benefit, retention was comparable for the TECH and STND conditions. Drop-out was increased for SELF, which may have biased comparisons between the experimental treatments and control. However, it is unlikely that drop-outs in SELF lost more weight than SELF participants who remained in the study. Thus, if our reported treatment effects involving the control group are in fact biased, the direction of the bias is that the treatment effect may have been underestimated. Finally, the control condition in the study was not inert, which also may have led to underestimation of treatment effects.
CONCLUSIONS
The ENGAGED study was a highly innovative randomized controlled trial examining the efficacy of three abbreviated versions of the DPP. Two abbreviated interventions combining face-to-face and telephone treatment but differing on whether self-monitoring was done via smartphone versus paper, yielded more weight loss than a self-guided intervention at 3 and 6 months. Results suggest that, in a weight loss intervention including other evidence based components, use of either technology or paper tracking holds potential to provide lower burden alternatives to the full DPP lifestyle intervention. The findings also accord with other results indicating that mobile technologies can enhance self-monitoring and engagement with a weight loss intervention. Finally, the results raise a provocative question about boundary conditions affecting whether or not enhanced technology-facilitated self-monitoring translates into greater weight loss.
Study Importance Questions.
1. What is already known about this subject?
Intensive lifestyle intervention for obesity has proved effective in yielding clinically meaningful sustained weight loss, but the large number of needed in-person treatment sessions is costly and difficult to scale.
However, reducing treatment intensity below 12–16 sessions yields greatly diminished weight loss.
2. What does your study add?
This is the first trial to test whether in-person treatment sessions can be reduced to 8 but weight loss efficacy preserved by using a unique mobile technology (combining smartphone, wireless accelerometer, and social media) to deliver intervention components more efficiently.
We found that abbreviated 8-session in-person treatment using either technology or paper and pencil recording increased weight loss at 6 months more than a standard of care self-guided control intervention. Even though technology-supported intervention yielded markedly greater self-monitoring of diet, physical activity, and weight than did paper and pencil, the difference did not translate into greater weight loss.
Acknowledgments
The authors express our appreciation to Harold Snyder and Tom McTavish for design and programming of the ENGAGED application.
Funding Agencies: Supported in part by grants RC1DK087126 and R01DK097364 from the National Institute of Diabetes and Digestive and Kidney Diseases and by the Robert Lurie Comprehensive Cancer Center Support Grant (P30CA60553) and the Northwestern University Clinical Translational Science Award (UL1TR001422).
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT01051713
Disclosures: BS serves on scientific advisory boards for Actigraph and Arivale. The other authors declare that they have no conflicts of interest to disclose.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer JS, Sibley RF, Bartlett DP, Kahn LS, Loffredo L. An adaptation of the diabetes prevention program for use with high-risk, minority patients with type 2 diabetes. Diabetes Educ. 2007;33(3):503–508. doi: 10.1177/0145721707301680. [DOI] [PubMed] [Google Scholar]
- 4.ter Bogt NC, Bemelmans WJ, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing weight gain: one-year results of a randomized lifestyle intervention. Am J Prev Med. 2009;37(4):270–277. doi: 10.1016/j.amepre.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrini CA, Duncan JM, Moller AC, et al. A smartphone-supported weight loss program: design of the ENGAGED randomized controlled trial. BMC public health. 2012;12(1):1041. doi: 10.1186/1471-2458-12-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spring B, Gotsis M, Paiva A, Spruijt-Metz D. Healthy apps: mobile devices for continuous monitoring and intervention. IEEE pulse. 2013;4(6):34–40. doi: 10.1109/MPUL.2013.2279620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith A. Pew Research Center: US Smartphone Use in 2015. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/. Accessed June 27, 2016.
- 8.Coons MJ, DeMott A, Buscemi J, et al. Technology interventions to curb obesity: a systematic review of the current literature. Curr Cardiovasc Risk Rep. 2012;6:120–134. doi: 10.1007/s12170-012-0222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carver C, Scheier M. Attention and Self-Regulation: A Control Theory Approach to Human Behavior. New York: Springer-Verlag; 1981. [Google Scholar]
- 11.Spring B, Duncan JM, Janke EA, et al. Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med. 2013;173(2):105–111. doi: 10.1001/jamainternmed.2013.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spring B, Schneider K, McFadden HG, et al. Multiple behavior changes in diet and activity: a randomized controlled trial using mobile technology. Arch Intern Med. 2012;172(10):789–796. doi: 10.1001/archinternmed.2012.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsch LA, Lord SE, Dallery J. Behavioral Healthcare and Technology. New York, NY: Oxford University Press; 2014. [Google Scholar]
- 14.Kullgren JT, Troxel AB, Loewenstein G, et al. Individual- versus group-based financial incentives for weight loss: a Randomized, Controlled Trial. Ann Intern Med. 2013;158(7):505–514. doi: 10.7326/0003-4819-158-7-201304020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashrafian H, Toma T, Harling L, Kerr K, Athanasiou T, Darzi A. Social networking strategies that aim to reduce obesity have achieved significant although modest results. Health Aff. 2014;33(9):1641. doi: 10.1377/hlthaff.2014.0370. [DOI] [PubMed] [Google Scholar]
- 16.Poncela-Casasnovas J, Spring B, McClary D, et al. Social embeddedness in an online weight management programme is linked to greater weight loss. J Roy Soc Interface. 2015;12:20140686. doi: 10.1098/rsif.2014.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma J, Yank V, Xiao L, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao G, Kirley K. The future of obesity treatment: comment on “integrating technology into standard weight loss treatment: a randomized controlled trial”. JAMA Intern Med. 2013;173(2):111–112. doi: 10.1001/jamainternmed.2013.1232. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Preventive Services Task Force. Final update summary. Obesity in Adults: Screening and Management. 2016 https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obesity-in-adults-screening-and-management. Accessed September 12, 2016.
- 20.Wing RR. Behavioral weight control. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York: Guilford Press; 2002. pp. 301–316. [Google Scholar]
- 21.Burke LE, Conroy MB, Sereika SM, et al. The effect of electronic self-monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity (Silver Spring) 2011;19(2):338–344. doi: 10.1038/oby.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg JH, Kiernan M. Innovative techniques to address retention in a behavioral weight-loss trial. Health Educ Res. 2005;20(4):439–447. doi: 10.1093/her/cyg139. [DOI] [PubMed] [Google Scholar]
- 23.Netzer C. The complete book of food counts. 7th. New York: Dell Pub; 2006. [Google Scholar]
- 24.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37(6):505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Eaglehouse YL, Schafer GL, Arena VC, Kramer MK, Miller RG, Kriska AM. Impact of a community-based lifestyle intervention program on health-related quality of life. Qual Life Res. 2016;25(8):1903–1912. doi: 10.1007/s11136-016-1240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan D, Heaner M. Guidelines (2013) for managing overweight and obesity in adults. Preface to the full report. Obesity. 2014;22(Suppl 2):S1–3. doi: 10.1002/oby.20819. [DOI] [PubMed] [Google Scholar]
- 27.Hedeker D, Gibbons RD. Longitudinal Data Analysis. New York: Wiley; 2006. [Google Scholar]
- 28.Hernan WH, Brandle M, Zhang P, et al. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care. 2003;26(1):36–47. doi: 10.2337/diacare.26.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross KM, Wing RR. Impact of newer self-monitoring technology and brief phone-based intervention on weight loss: A randomized pilot study. Obesity. 2016;24(8):1653–1659. doi: 10.1002/oby.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkelstein E, Haaland B, Bilger M, et al. Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):983–995. doi: 10.1016/S2213-8587(16)30284-4. [DOI] [PubMed] [Google Scholar]
- 33.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6(1):67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 34.Jakicic JM, Davis KK, Rogers RJ, et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: the idea randomized clinical trial. JAMA. 2016;316(11):1161–1171. doi: 10.1001/jama.2016.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchesson MJ, Rollo ME, Callister R, Collins CE. Self-monitoring of dietary intake by young women: online food records completed on computer or smartphone are as accurate as paper-based food records but more acceptable. J Acad Nutr Diet. 2015;115(1):87–94. doi: 10.1016/j.jand.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 36.Young MD, Morgan PJ, Plotnikoff RC, Callister R, Collins CE. Effectiveness of male-only weight loss and weight loss maintenance interventions: a systematic review with meta-analysis. Obes Rev. 2012;13(5):393–408. doi: 10.1111/j.1467-789X.2011.00967.x. [DOI] [PubMed] [Google Scholar]
- 37.Pellegrini CA, Verba SD, Otto AD, Helsel DL, Davis KK, Jakicic JM. The comparison of a technology-based system and an in-person behavioral weight loss intervention. Obesity. 2012;20(2):356–363. doi: 10.1038/oby.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner-McGrievy GM, Beets MW, Moore JB, Kaczynski AT, Barr-Anderson DJ, Tate DF. Comparison of traditional versus mobile app self-monitoring of physical activity and dietary intake among overweight adults participating in an mHealth weight loss program. J Am Med Inform Assoc. 2013;20(3):513–518. doi: 10.1136/amiajnl-2012-001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen JK, Stephens J, Patel A. Technology-assisted weight management interventions: systematic review of clinical trials. Telemed J E Health. 2014;20(12):1103–1120. doi: 10.1089/tmj.2014.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svetkey LP, Batch BC, Lin P-H, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity. 2015;23(11):2133–2141. doi: 10.1002/oby.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]


