Abstract
Nitric oxide (NO) is a signaling molecule with multiple facets and involved in numerous pathological process, including cancer. Among the different pathways where NO has a functionally relevant participation, is the control of mitochondrial respiration and biogenesis. NO is able to inhibit the electron transport chain, mainly at Complex IV, regulating oxygen consumption and ATP generation, but at the same time, can also induce increase in reactive oxygen and nitrogen species. The presence of reactive species can induce oxidative damage or participate in redox signaling. In this review, we discuss how NO affects mitochondrial respiration and mitochondrial biogenesis, and how it influences the development of mitochondrial deficiency and cancer.
Keywords: nitric oxide, nitric oxide synthase, mitochondria, cancer, respiratory chain
Introduction
Nitric oxide (NO) has been studied in several areas of health sciences and it has been recognized as an important signaling molecule in several cellular pathways. However, due to its nature as a free radical, NO can also produce highly damaging reactive species, such as peroxynitrite, inducing oxidative or nitrosative stress. While the whole gamut of processess where NO is involved are still being elucidated, it has become clear that the interactions of NO in metabolic networks are complex and involve specific aspects, such as NO concentration at the site of action, source (NO synthase, NOS, isoform responsible for its synthesis), site of synthesis, interactions with other reactive species and post-translational modifications (1).
NO has a short half-life (0.1–2.0 s) and can react with metal and free radicals, but as a highly diffusible molecule it can also reach other cells in a short period of time (2), supporting the relevance of NO in physiological processes. NO has multiple facets and can have relevant roles in several pathological conditions, such as cardiovascular, pulmonary, neurodegenerative, infectious diseases and cancer (3). Specifically in cancer, NO is considered to have dual effects because it can be pro-tumorigenesis, but at the same time, depending on the circumstances it can also have anti-tumorigenesis effects, such as inducing apoptosis, reducing invasion and metastasis and supressing DNA synthesis (4, 5) (6) (7). When NO acts with a tumor promoting role, several mechanisms have been involved, including: genotoxic mechanisms, anti-apoptotic effects, induction of angiogenesis, reduction of host immune response against tumor or promoting metastasis (5).
One of the factors that can explain such differences in the outcome of the disease is NO concentration, which affects proteins regulated by NO in a concentration-dependent manner and modifies cell phenotypes (2).
Besides all these effects, NO has also important roles in mitochondria physiology, such as the control of mitochondrial respiration, mitochondrial biogenesis, redox signaling and oxidative damage. These effects in mitochondria need to be considered when NO synthesis is altered, as they influence normal and tumor metabolism (8).
In this review we discuss the roles of NO in mitochondria, focusing in the control of mitochondrial respiration, mitochondrial biogenesis and free radical generation.
NO and NO synthases
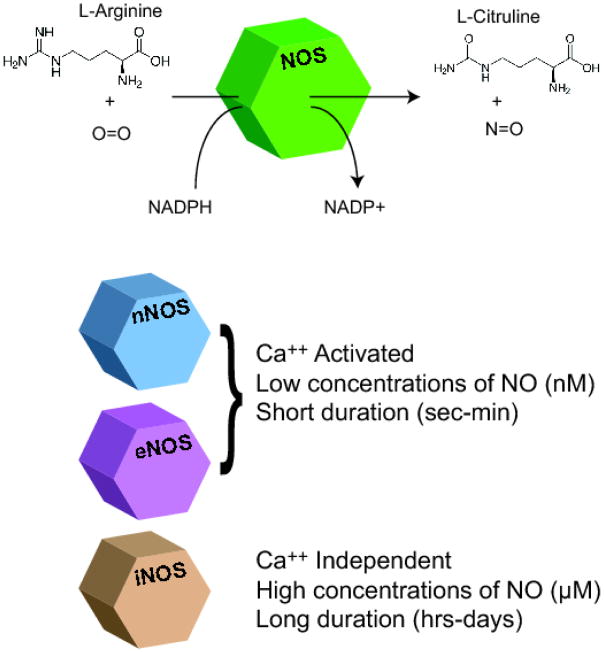
NO is a gaseous and uncharged molecule, highly lipophilic and diffusible. With these features NO can reach a target at some distance from where it is synthesized (9–11). It is endogenously produced by the enzyme NOS, which catalyzes the conversion of L-arginine to L-citrulline (11). There are three NOS isoforms: neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS) (12). Neuronal and endothelial isoforms were originally named according to the tissues from where they were first purified (neuronal and endothelial) but they can be expressed in several other tissues and cell types (13–16). The neuronal isoform is highly expressed in skeletal and cardiac muscles but is also found in other tissues, such as aorta, bladder, colon, corpus cavernosum, lung, prostate, small intestine, testis and uterus (14). Besides the endothelial cells, eNOS is highly expressed in spleen, kidney and lung (16). Both isoforms are constitutive and are activated by interactions with calcium and calmodulin, while iNOS is calcium independent and is usually activated in defense responses such as infection and inflammation (13). Unlike eNOS and nNOS, iNOS generates a sustained NO flux, that can vary from seconds to days (Fig.1) and this prolonged time of NO exposure can influence the type of effect that this iNOS will induce in the cell (17).
Figure 1.
Nitric oxide synthases. NO is synthesized by nitric oxide synthase (NOS), that uses molecular oxygen to oxidize the guanidinium nitrogen atom of L-arginine to produce NO and L-citrulline. There are three NOS isoforms: neuronal (nNOS), endothelial (eNOS) and inducible (iNOS). nNOS and eNOS are constitutive and Calcium activated, while iNOS is not activated by calcium and is only activated in certain circunstamces. The constitutive isoforms synthesize low concentrations of NO with short duration, while iNOS synthesizes higher amounts of NO for longer periods. These are relevant differences that will determine the type of effect that will be induced by NO.
NOS isoforms are products of three separate genes but the proteins have similar structures that differ mainly in the N-terminal, with the presence of a PDZ domain in nNOS and myristoylation and palmitoylation sites in eNOS (18). The expression of NOS isoforms in cancer cells depends on the tumor cell type. Abnormal expression of the three NOS isoforms has been demonstrated in several types of cancer, but iNOS and eNOS are the most frequently affected.
NOS has a large variety of functions that are dependent on the type of isoform, type of cell or tissue where it is located and the local conditions, such as hypoxia and inflammation. For instance, the neuronal isoform is involved in several synaptic signaling events in the brain and mediates smooth muscle relaxation in corpus cavernosum; whereas eNOS mediates vascular effects such as vasodilation, inhibition of platelet agregation and stimulation of angiogenesis (19).
Due to the large range of actions of NO in physiological and disease situations, the mechanisms involved in the regulation of NOS are very complex. Although nNOS and eNOS are classically described as constitutive and iNOS as inducible, in fact, all isoforms can exhibit inducible and constitutive patterns of expressions depending on the tissue environment (9). There are several mechanisms that control the expression, localization and activity of the three NOS isoforms. They can be summarized in alternative mRNA splicing, protein-protein interactions (Ca2+/calmodulin complex, PDZ domains, PIN, caveolin-1 and 3, Hsp90, ENAP-1, kalirin), covalent modifications and redox signaling (18).
The localization of the two constitutive NOS isoforms is determined by mechanisms involving protein-protein interactions ans covalent modifications. In muscle, one of the main features is the presence of PDZ domain in nNOS which determines the interactions through the binding to syntrophin, localyzed in the dystrophin complex at the skeletal muscle membrane. The importance of this binding is demonstrated when nNOS changes the localization to the cytosol when PDZ cannot bind to syntrophin, such as in conditions where the dystrophin complex is absent, as in Duchenne muscular dystrophy (20). On the other hand, the localization of eNOS in the caveolae of endothelial cells requires the processes of myristoylation and palmitoylation (acylation with the fatty acids myristate and palmitate) (21, 22). Some subtypes of nNOS and eNOS are generated through alternate mRNA splicing, which determines the expression in some tissues, such as in the case of an nNOS expressed in testis (23) NOSs are mainly activated through Ca-calmodulin binding, but protein interactions, covalent modifications and redox modifications can also modulate the enzyme activity (9, 24). The dimer structure is essencial for NOS activity, so factors that interfere in this structure can inhibit the enzyme. For instance, Kalirin prevents dimer formation causing inhibition of iNOS or any factor compromising the BH4 and Zinc binding, which are important for the stabilization of the dimer structure (25, 26). Other modifications that can also affect eNOS activity include phosphorylation, glutathionylation, glycosylation and nitrosylation (9, 27).
It is important to remember that NO can also be generated through non-enzymatic sources, such as the reduction of nitrite to NO in physiological or disease states, as in ischemia or under acidic and highly reduced conditions (28). So when evaluating NO effects at the cellular level, both enzymatic and non-enzymatic sources of NO should be considered.
A mitochondrial NOS?
Although NO is a very diffusable molecule, able to cross membranes and reach mitochondria, there are evidences that mitochondria produce NO (29) and that there is a mitochondrial NOS, which strongly supports that NO has a relevant participation in mitochondrial regulatory pathways. However, the identity of this mitochondrial NOS is still debated (18, 30). Several studies have demonstrated mitochondrial co-localization of the immunoreactivity using antibodies against iNOS (31), eNOS (32–34) and nNOS (35) in various tissues, such as skeletal muscle (32, 33, 35), heart (34), kidney (34), brain (33) and liver (31, 33). Furthermore, Gao (36)and colleagues were able to better define the localization of eNOS on the cytoplasmic surface of the outer mitochondrial membrane in endothelial cells (37). Elfering and colleagues characterized the sequence of rat mitochondrial NOS as an nNOS isoform alpha, that could be modified by acylation, was calcium responsive and had phosphorylation sites (38). They hypothesized that these pos-translational modifications could be responsible for the targeting of the NOS to the inner mitochondrial membrane.
Supporting the results by Elfering and colleagues, Kanai and colleagues showed that mitochondria isolated from heart of mice that had the nNOS isoform alpha knocked out did not produce NO, while eNOS−/− and iNOS−/− knocked out mice mantained NO production in isolated mitochondria (39). Lacza and colleagues were able to detect NO production, NOS activity and low amounts of eNOS protein in mitochondria, but NOS activity was intact in eNOS knockout mice (40). Furthermore, NO production in mitochondria was not affected by nNOS and iNOS knockout mice (40).
Venkatakrishnan and colleagues could not find NOS activity or expression of the endothelial and neuronal NOS isoforms in rat liver mitochondria, questioning the existence of a mitochondrial NOS, at least in the liver (41).
Regardless of this controversy on the existence or identity of the mitochondrial NOS, there are recent studies showing a closer relationship between mitochondrial NOS and the respiratory chain. Parihar and colleagues suggested that the respiratory chain Complex I regulates mitochondrial NOS activity by showing mitochondrial NOS activity associated to Complex I in isolated rat liver and brain mitochondria (42). Furthermore, the study of heart mitochondrial preparations demonstrated that Complex I enriched fractions had higher mitochondrial NOS (nNOS-alpha isoform), suggesting that mitochodrial NOS is physically close to Complex I (43).
The conflicting results regarding the existence and identity of the mtNOS can be explained by several methodological problems, which was extensively reviewed by Brookes(36). Some of these problems include mitochondrial purity, presence of other cell types in the tissues (e.g. endothelial cells from vessels, inflammatory cells), detection of NO production in mitochondria and immunodectection of NOS isoforms. If mtNOS does exist, despite the methodological issues, the identity of mtNOS may also be related to the tissue of origin, which could explain the inconsistency of the isoform identified, for instance, mtNOS could be the neuronal isoform in the brain but iNOS in the liver. The existence of an mtNOS can be supported by the relevance of NO in the control of mitochondrial respiration, as when produced in mitochondria, NO would have a rapid and easy acessibility to its target, the cytochrome c oxidase (COX), which is inhibitted by NO (44). However, it is also argued that considering that NO is highly diffusable, it is not essential that it is produced close to its site of action (36). For instance, NO could be produced by eNOS in endothelial cells of vessels or by eNOS located in the cytosol face of the outer mitochondrial membrane, then diffusing across the other compartments to reach the respiratory chain.
NO, the respiratory chain and control of respiration
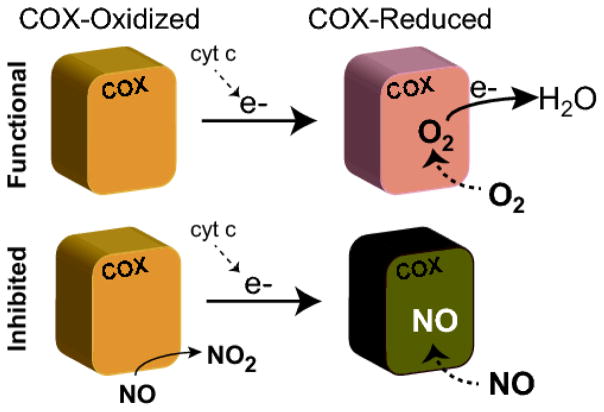
NO has a direct inhibitory effect on the respiratory chain at three sites: COX (Complex IV) (45, 46), complex I (NADH-Dehydrogenase)(47–49) and complex III (ubiquinol-cytochrome c reductase) (10, 50, 51). NO concentration and how long NO remains on these sites are important to determine the reversibility and effects of this inhibition (Fig.2). The most important sensitive site to NO is COX, which is the terminal electron acceptor of the respiratory chain and is the enzyme complex that reduces molecular oxygen to H2O, helping generate a proton gradient that drives Complex V and ATP synthesis. The kind of NO inhibition is dependent on COX redox state (52–54). COX is reduced after receiving electrons from cytochrome c and the reduction of the metals (Fe2+ and Cu+) when molecular oxygen binds to a heme-copper binuclear active site (53). When local oxygen concentration is adequate, COX is oxidized, but when oxygen is low, COX becomes reduced. When COX is in the reduced state, NO binds to the heme a3 domain, through a competitive binding at the O2 site. (52). Low concentrations of NO induce a reversible inhibition of COX, reducing oxygen consumption and ATP formation, due to an inhibition of electron flux at Complex IV (44, 55). This inhibition allows the redistribution of oxygen to other sites with low concentrations of oxygen and may be a mechanism to preserve certain areas that have critical oxygen concentration (56).
Figure 2.
Inhibition of cytochrome c oxidase by NO. The most sensitive site to NO in the electron transport chain is cytochrome c oxidase (COX). When COX in the reduced state NO binds to de Oxygen binding site with a competitive and reversible nature. While when COX is in the oxidized state, NO binds at the copper moiety of the heme-copper center, but it is rapidly converted to nitrite leading to an increase in O2 consumption and contributing to NO metabolism.
When COX is in the oxidized state, NO binding occurs at the copper moiety of the binuclear (heme-copper) center (52). However, differently from what happens when NO competes with the oxygen binding site, after NO binding to COX, NO is converted to nitrite leading to an increase in O2 consumption (52).
When there is prolonged exposure to NO, other sites of the respiratory chain can be inhibitted, such as Complex I and III. Complex I inhibition was demonstrated in murine macrophage cultured cells with a concomitant decrease in intracelullar reduced glutathione (47). The reversibility of this inhibition depends on the duration of the exposure to NO. Short exposures to NO provoke a reversible inhibition of COX, but when the exposure is persistent, an irreversible inhibition can be induced. The mechanisms of NO induced inhibition of Complex I are still not clear (48). One possible mechanism involves S-nitrosation because Complex I inhibition was reverted by light or reduced thiols, which are known to revert S-nitrosation (47, 57).
Chouchani and colleagues showed that treatment with a mitochondria-selective S-nitrosating agent led to a Complex I inhibition in a model of cardiac ischemia. Complex I inhibition was due to a selective S-nitrosation of Cys39 on the ND3 subunit and they proposed that this modification would slow the electron transfer at Complex I, decreasing the generation of free radicals and acting as a protective mechanism to ischemic injury (58). Galkin and coleagues showed that the mechanism involved in this type of inhibition is even more complex as nitrosation of Complex I depends on the conformational state (active or deactivated) (59). They showed that the active form (A-form) of Complex I is insensitive to nitrosothiols and peroxinitrite and proposed that transitions of active/deactivated states are important in the regulation of complex I activity and control of the cellular respiration by NO (59).
The second proposed mechanism is tyrosine nitration, due to the observation of tyrosine nitration when Complex I was inhibited by peroxynitrite in tissues such as heart, liver and brain (49) (60). Although Complex I and III are the major sites reported as sensitive to NO chemical modifications, there are reports pointing to other sensitive sites in the electron transport chain (51). Poderoso and coleagues reported that NO induced reversible inhibition of succinate-cytochrome c reductase (Complex II) and NADH-cytochrome c reductase (Complex I) (51). In this study, these enzymes had a reduction in activity of 63% and 74% after exposure of 0.1μM •NO, while cytochrome c oxidase was the most sensitive with a 100% reduction. The same study also showed that NO was also able to induce an inhibition of electron transfer at the ubiquinone-cytochrome b region of the respiratory chain (Complex III) (10, 51).
Besides the direct effect of NO on signaling events, and as described above, NO can also promote nitrosative and oxidative modifications which will act in physiological responses through signaling or promoting mitochondrial damage. Basically, NO can react to superoxide anions, transition metals (heme, iron-sulfur centers) and reduced thiols (61). The combination of NO with superoxide can generate peroxinitrite, a potentially damaging radical, that promotes oxidation and nitration of biomolecules. Inhibition of Complex I can be an effect of NO interaction with iron-sulfur clusters, while the reaction with the heme iron of the soluble guanylyl cyclase activates the enzyme and increases the levels of cyclic guanosine monophosphate (cGMP)(62, 63).
Although the most known NO effect on the respiratory chain is the inhibition of Complex IV, NO can also exert this control through the action in different other sites, such as Complex I, III and II, involving modifications such as S-nitrosation and nitration. These interactions are important in the regulation of the efficiency of the respiratory chain and thus can regulate the energy balance of the cell. The metabolic state of cancer cells is one of the key elements that are altered in tumorigenesis.
Normally, cells depend mostly on the oxidative phosphorylation to generate ATP, but certain cancer cell types have a metabolic shift, which makes the cell more dependent on the glycolitic pathway (Warburg effect). Although at first, defective mitochondrial respiration could be the most plausible explanation for this effect, several tumors with a glycolytic state had a normal mitochondrial respiration (64). Thus it was proposed that this metabolic shift occurs mainly to provide other nutrients such as carbohydrates, lipids and aminoacids, that could not be supplied by the oxidative phosphorylation system and that were required for cancer cell proliferation (65, 66).
The exact mechanisms involved in mitochondrial bioenergetics and signaling in tumorigenesis still need to be elucidated, and will enable the elaboration of better strategies for treatment. Metformin is one example of a drug that has a potential use in cancer and has specific action in the mitochondria (67, 68). It has been demonstrated that metformin can inhibit the growth of cancer cells through the inhibition of Complex I and cellular respiration (67, 68), so similar approaches can lead to other options in the treatment of cancer.
NO and Mitochondrial Biogenesis
Mitochondrial biogenesis is the process by which the mitochondrial network is maintained and expanded (69). Although mitochondria have their own genome, this process requires the cross-talk between nuclear and mtDNA because the majority of mitochondrial components are controlled by nuclear genes. Biogenesis of new mitochondria can be induced when there is energy demand such as in skeletal muscle during exercise (70) (71) and in rodent adipocytes during thermogenesis (72). The molecular pathway of mitochondrial biogenesis involves the activation of PGC-1α, a co-activator of nuclear transcription factors (73). PGC-1α induces the expression of nuclear respiratory factors (ERRα, PPARs, NRF-1 and NRF-2), which activate the expression of nuclear encoded mitochondrial genes, including mitochondrial transcription factor A (mtTFA). Expression of mtTFA will induce replication and transcription of mtDNA, allowing the increase in mtDNA copy number and mtDNA encoded respiratory chain subunits (73).
Alterations in PGC-1α expression have been reported in various types of cancer, such as melanoma, breast, colon, kidney and liver cancer (74). Some studies indicate that PGC-1α has a pro-tumorigenesis activity (74, 75), while in others, reduced expression of PGC-1α was reported in tumor samples and correlated with poor outcome (74, 76, 77). PGC-1 co-activators are emerging with relevant roles in almost every step of tumorigenesis, by balancing mitochondrial energy production with demands for cell proliferation (74) and protecting against cell death (78). The main function of PGC-1α is the control of energetic balance in the cell, so altered PGC-1α expression determines the metabolic state of the cell. The relevance of of PGC-1α in the process of carcinogenesis and tumor progression is supported by the findings showing decreased expression of PGC-1α in tumour tissues in patients with colon (76)and breast (79) cancer, together with an association of reduced levels of PGC-1α with poor clinical outcomes (77). On the other hand, there are studies showing opposite effect, with PGC-1α activation associated to tumor growth and metastasis in breast cancer (80). In this study, LeBleu and coleagues (80) found higher levels of oxidative phosphorylation transcripts in circulating cancer cells, compatible with PGC-1α-mediated mitochondrial biogenesis, which was important for the formation of distant metastases and poor outcome. They suggested that these effects are essential for the process of metastasis, including mantaining functional motility of cancer cells. Conversely, a recent work showed an opposite effect in melanoma, with PGC-1α inducing suppression of metastasis through a pathway not related with bioenergetic funcions, but involving integrins, which are related to invasion and metastasis (81). A better understanding on how PGC-1α affects the tumoral cell is still needed, but it probably depends on several tumor features, such as metabolic state, tumor heterogeneity, tissue type, microenvironment and tumor stage (74, 82, 83).
Several studies have demonstrated that cells treated with an NO-donor were able to induce biogenesis of functional mitochondria (84–87), indicating the participation of NO in the process of mitochondrial biogenesis. The link between NO and mitochondrial biogenesis was also observed in primary malignant cells in a study by Carew and colleagues that showed a highly significant relationship between the endogenous NO levels and the mitochondrial mass in chronic lymphocytic leukemia (CLL) cells (88). Furthermore, exposure to exogenous NO was also able to increase the mitochondrial mass in two types of human B cell lines (Raji B cell lymphoma and a clone of EBV- transformed B lymphocytes) (88).
A study by Nisoli and colleagues showed important features of NO-induced mitochondrial biogenesis. Using brown adipocytes treated with an NO-donor and NOS-antagonist, they could confirm that NO activated PGC-1α (87). Treatment with an NO-donor was able to increase mitochondrial content shown as increased Mitotracker fluorescence signal, increased expression of mitochondrial proteins (COX subunit IV and cytochrome c) and increased number of mitochondria per cell (87).
These cells had increased expression of PGC-1α, NRF-1 and mtTFA, confirming that NO activated mitochondrial biogenesis via PGC-1α pathway. The same results were obtained with white fat 3T3-L1 cells and human monocytic U937 cells, showing that NO effects were not restricted to adipocyte cells (87). Another important finding was that NO-induction of mitochondrial biogenesis was also obtained with a guanosine 3′,5′-monophosphate (cGMP) analog, demonstrating that this process is dependent on cGMP (87). The mechanism involved in mitochondrial biogenesis is probably independent of NO-induced Complex IV inhibition, because this inhibition involves direct NO binding and does not require cGMP (53, 89).
The exact molecular mechanisms of NO-induced mitochondrial biogenesis are still being elucidated. There are evidences that these effects are mediated by 5′-AMP-activated protein kinase (AMPK) in skeletal muscle cells (84). AMPK is a heterotrimeric kinase, which is activated by AMP and inhibited by ATP. In conditions with low energy (low ATP/ high AMP), such as exercise, starvation or mitochondrial dysfunction, AMPK activity is increased and mediates the induction of mitochondrial biogenesis (90–92).
Aquilano and colleagues have demonstrated that nNOS is required in the process of mitochondrial biogenesis by studying the effects of full length nNOS and nNOS lacking the PDZ domain in C2C12 and HeLa cells (93). They showed that nNOS induced mitochondrial biogenesis, which was abolished in the absence of PGC-1α, showing that indeed NO is acting in PGC-1α pathway. Furthermore, this process involves the direction of nNOS to the nucleus, where nNOS induces S-nitrosylation of CREB to activate mitochondrial biogenesis. Control of mitochondrial dynamics, such as the balance between fission and fusion of mitochondria, has also been influenced by S-nitrosylation. Cho and colleagues demonstrated that the pathogenesis of Alzheimer disease involves increased production of NO and S-nitrosylation of dynamin related protein 1 (DRP-1), a protein that induces mitochondrial fission (94). S-nitrosilated DRP-1 increases mitochondrial fragmentation, affecting energy production and contributing to mitochondrial damage. The balance between fusion and fission of mitochondria has also gained importance in cancer, as disruption of the mitochondrial network was found in lung adenocarcinoma cells due to impared fusion and enhanced fission, with increased expression of DRP-1 (95).
In summary, NO induces mitochondrial biogenesis via PGC-1α pathway in a process that involves cGMP, AMPK and nNOS. Furthermore, NO can also influence mitochondrial fragmentation (fission) through S-nitrolisation of DRP-1. Thus, action in NO synthesis can also be used to influence cancer biology, as mitochondrial biogenesis and dynamics are important elements in the regulation of energic state in cancer cells.
NO synthesis and mitochondrial dysfunction
As described above, NO can regulate the oxidative phosphorylation through the action in specific sites in the respiratory chain and by different mechanisms. However, little is known about how NO synthesis is affected when there is a mitochondrial dysfunction. Studies using muscle biopsies from patients with mitochondrial diseases have demonstrated that muscle fibers with mitochondrial deficiency have alterations in NOS activity or expression. Mitochondrial diseases are good models to study NO synthesis in a scenario with dysfunctional mitochondria as we can correlate specific respiratory chain defects with induction of mitochondrial biogenesis. These diseases are caused by mutations in nuclear or mtDNA genes and have extremely variable phenotypes ranging from mild exercise intolerance to severe infantile encephalomyopathies. Skeletal muscle is frequently affected and has a very typical pattern of abnormality characterized by muscle fibers with marked mitochondrial proliferation. Because fibers with mitochondrial proliferation also have a mitochondrial deficiency, such as in Complex IV, it is suggested that mitochondrial biogenesis is induced to compensate the energy deficiency (96). Interestingly, skeletal muscle fibers express the two constitutive NOS isoforms, but with distinct localizations. The nNOS is localized in the muscle fiber membrane (sarcolemma) while eNOS can be found in the intermyofibrilar region and small muscle vessels (32, 97). The fact that each isoform has specific localizations in the muscle fiber, suggest that they are involved in distinct functions in the cell. Regarding nNOS expression in muscle fibers of patients with mitochondrial diseases, there are studies showing increased expression in muscle fibers with mitochondrial proliferation in patients with mtDNA deletions or point mutations (m.3243A>G; m.4369_4370insA)(98) (97).
There was a good correlation between the expression of nNOS and SDH-Fp (a Complex II subunit), supporting the idea that nNOS is responsible for the NO signaling in mitochondrial biogenesis in these muscle fibers. Studies using histochemical stainings also support that nNOS is upregulated as increased NOS activity was found in several types of mtDNA mutations and the activity was higher in muscle fibers with mitochondrial proliferation (99).
Interestingly, a similar situation with activation of mitochondrial biogenesis was also found in thyroid oncocytomas, but involving the PGC-1 related coactivator (PRC1) instead of PGC-1α (100). PRC1 is a member of the PGC-1 family, which includes PGC-1α and PGC-1β. PRC1 is a relevant regulator of mitochondrial function in cells where PGC-1α is absent and mediates the activation of respiratory genes through transcription factors (101). However, the physiological role of PRC1 is still debated (102). Similar to PGC-1α, PRC1 also binds to nuclear transcription factors implicated in the expression of mitochondrial function, including NRF-1, cAMP response element-binding protein 1 (CREB) and estrogen-related receptor alpha (ERRalpha) (103). Thyroid oncocytomas are characterized by cells with abnormal and abundant mitochondria with deficiency in ATP synthesis (104, 105). A study on gene expression profiles showed that thyroid oncocytomas have increased expression of genes related to mitochondrial biogenesis, such as NRF-1, mitochondrial respiratory chain genes and eNOS (106). In these cells, PRC1 was increased in response to an NO-donor treatment and was also associated with an increase in expression of mitochondrial genes (100). However, the same response was not obtained with another type of thyroid tumor cells, such as the BCPAP cell line derived from a papillary thyroid carcinoma, which has a glycolytic phenotype. The relevance of mitochondrial proliferation in the pathogenesis of the oncocytic tumors is still no clear, but it has been suggested that a defective ATP synthesis triggers the up-regulation of mitochondrial biogenesis to compensate a deficiency in the energy production in these cells (106). But as we have described above, in skeletal muscle the isoform related to mitochondrial proliferation was nNOS while in oncocytomas, increased eNOS was reported. Thus depending on the cell type, we can have different isoforms acting in similar pathways. More recently, it was found that PRC1 and c-MYC can act in concert to reprogram gene expression in response to mitochondrial stress, providing an additional link between PRC1 and cancer (102)(Fig.3).
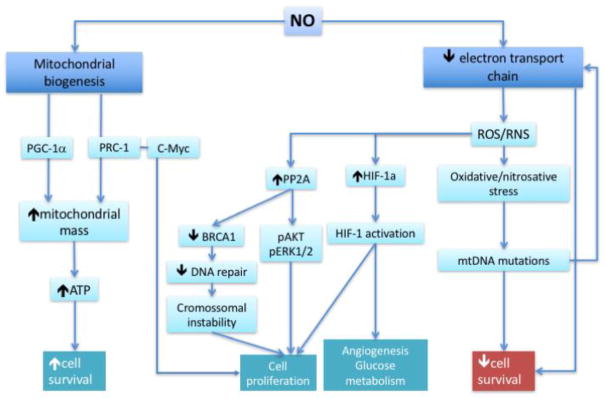
Figure 3.
Mitochondrial effects of NO related to cancer. In cancer cells, NO may induce mitochondrial biogenesis, increasing mitochondrial mass and energy supply, which results in increase of the cell survival. Electron transport chain can be inhibitted by NO, mainly at Complex IV, which will reduce ATP generation and decrease cell survival. This inhibition can also increase the generation of reactive oxygen or nitrogen species, inducing oxidative/nitrosative stress or redox signaling. mtDNA mutations can be originated from the oxidative stress, inducing mitochondrial dysfunction and reducing cell survival. On the other hand, two examples are shown of activation of pathways by ROS (HIF-1) and Tyr nitration of PP2A, inducing cell proliferation, angiogenesis and glucose metabolism. NO: nitric oxide; ROS: reactive oxygen species; RNS: reactive nitrogen species; BRCA1: breast cancer type 1 susceptibility protein; PP2A: protein phosphatase 2A; pAKT: phosphorylated protein kinase B; pERK1/2: phosphorylated extracellular signal-regulated protein kinases 1 and 2; HIF-1: hypoxia inducible factor; mtDNA: mitochondrial DNA.
In mitochondrial diseases associated with mtDNA mutations, skeletal muscle fibers with mitochondrial proliferation also have a Complex IV (cytochrome-c-oxidase, COX) deficiency. Fibers with COX deficiency express eNOS but had low NOS activity in the sarcoplasm, indicating that eNOS is down-regulated probably as a regulatory mechanism to increase ATP generation (97). This down-regulation was only found in the presence of COX deficiency, as other patients with Complex II or I deficiency did not have alterations in NOS activity (99). However, these mechanisms may be more complex because a study with cultured osteosarcoma derived cybrid cells with a mitochondrial deficiency due to the m.3243A>G mutation, showed an increase of intracellular NO and nitrate/nitrite in cultured media when compared the control cells (107). On the other hand, another study with SH-SY5Y (neuroblastoma derived) cybrid cells, carrying the same mutation (m.3243A>G) showed a significant reduction of nitrite/nitrate concentration, which suggested that these cells had a reduced NOS activity (108). In another study with cybrids generated from a neuroblastoma cell line also carrying the m.3243A>G mutation, the basal nitrite concentration were similar in mutant and control cell lines (109). It is still unclear why the levels of NO are so divergent in these studies, but it is possible that they are influenced by the cell type, in one study the cells were derived from an osteosarcoma cell line while in the other, they were from neuroblastoma. Another possibility is the method used for detection of NO. Measurements of NO levels are challenging and most of the methods are indirect, as the measure of nitrate/nitrite concentration, which is done in culture medium and it may not reflect the intracellular NO content.
Osteosarcoma cells express eNOS and iNOS, but the latter in very low levels (110). In addition, we do not know which NOS isoforms are activated or deactivated in these cultured cells carrying an mtDNA mutation.
NO, mitochondrial dysfunction and cancer
Numerous studies have focused on the role of NO in tumor pathophysiology but the results show that NO can be a pro-malignant or anti-cancer agent. The understanding of the mechanisms involved in the participation of NO in tumorigenesis or as an anti-cancer agent is very complex and needs to consider the source of NO generation, local concentration and tumor microenvironment (17). One important factor is the local NO concentration, as low concentrations are related to tumor progression and metastasis and high concentrations with tumor regression and cell death (17). Which NOS isoform is responsible for NO synthesis is also important, because constitutive isoforms (eNOS and nNOS) produces lower concentrations of NO for short periods, while iNOS is able to produce high amounts of NO during long periods, which would be more related to a pro-apoptotic environment. All three NOS isoforms were reported in several types of cancer cells, but iNOS is the most frequently found. The effect of NO depends on the level of expression of iNOS, duration and timing of NO delivery, microenvironment, genetic background and the cell type (4). Another interesting point that showed the relevance of mitochondria in cancer cells is the study of Fantappie and colleagues that showed that iNOS is constitutively expressed in the inner mitochondrial membrane in hepatocellular carcinoma cells with multi-drug-resistant phenotype (111). The presence of iNOS in mitochondria could indicate a more aggressive behaviour of cancer cells and it was suggested that it could be an advantage for cancer cells to survive in hypoxia, as cancer cells frequently grow in hypoxic conditions. Under hypoxia, a sequence of cellular events is initiated, including the signaling pathway via hypoxia-inducible factors (HIFs). NO is an important regulator of HIF-1, which promotes the up-regulation of genes involved in glucose metabolism, angiogenesis and hematopoieses (Fig.3). HIF is a heterodimer composed by subunits HIF-1α and HIF-1β. HIF-1α is constantly degraded under normal conditions, but under hypoxic environment its degradation is inhibited and it dimerizes with HIF-1 β, constituting HIF-1 (112). Reactive oxygen species generated in mitochondria are essential in the stabilization of HIF-1α under hypoxia as in the absence of respiratory chain electron flux or with the use of antioxidants, the expression of HIF-1α is reduced (113). The exact mechanisms involved in the participation of NO in this pathway still needs to be clarified but it is certainly very complex, which is dependent on NO local concentration and interactions with reactive species (114, 115).
Corroborating the idea that iNOS in mitochondria is related to a more aggressive cancer, Sen and colleagues showed that the treatment with an NOS antagonist was able to reduce the rate of proliferation in malignant cells expressing mitochondrial-associated NOS, but had not effect on the normal breast epithelial cells. They showed that mitochondrial NOS activity was associated with inhibition of Complex IV, increased production of hydrogen peroxide, which inhibited protein phosphatase 2A activity, resulting in cell proliferation due to maintenance of Akt and ERK1/2 in a phosphorylated stated (116) (Fig.3).
Oxidative damage is an important contributor to carcinogenesis and increased reactive species induce genomic instability and cell proliferation (8). Considering mitochondrial respiratory chain as the main source of reactive oxygen radical, mtDNA is more susceptible to mutations, and in fact, numerous mtDNA mutations have been reported in several types of tumors and cancer cells (117). Regarding the effects of increased nitrogen species, Vitto and colleagues studied the effect of increasing concentrations of an NO donor in a breast cancer cell line (BT-20), using DNA sequencing to detect mtDNA mutations. They found mutations in the Complex I coding and the control regions and interestingly, the same mutations were also found in breast cancer samples from patients, which exhibited increased eNOS expression (118). The relevance of mitochondria and redox environment on the modulation of cancer activity was demonstrated by Wilson and colleagues (8). They showed that inhibition of ROS production from mitochondria, generating mitochondrial-electron-transport-deficient cell lines or inhibiting NADPH oxidase activiy, resulted in reduction of the Tyr nitration of the protein phosphatase 2A catalytic subunit (PP2Ac). Tyr nitration of PP2Ac, promotes the activity of protein phosphatase 2A, which activates several steps ending in the reduction of DNA repair in double-strand breaks due to inhibition of BRCA1 expression (8)(Fig.3).
As we expand the knowledge on the mechanisms related to NO effects in cancer cells, NO has also been studied as a therapeutic agent. Fantappie and colleagues showed that it is possible to modulate iNOS expression with drugs, such as celecoxib, a drug that inhibits cyclooxygenase-2 (111). This modulation was dependent on the drug concentrations, as low concentrations (2.5 or 5 μmol/l) were able to increase iNOS expression and reduce multi-drug-resistant related proteins such as P-glycoprotein (P-gp), while the opposite effect was obtained with 10 μmol/l and no effect was seen with a concentration of 50 μmol/l. The mechanism involved in this modulation is still unclear, and it is not related to the inhibition of cyclooxigenase-2, as iNOS expression was not affected in a concentration that was able to inhibit cyclooxigenase 2.
Other strategies of therapy based on NO have been studied, such as iNOS inhibitors proposed as adjunct therapy to photodynamic therapy (PDT) in prostate cancer (119) and an NO-donor as a radiosensitizer (120). PDT is an anti-tumor intervention that is based on the use of a sensitizer, light and molecular oxygen, which will act focally in the ablation of the tumor. Fahey & Girotti found that the exposure of breast cancer cell lines to a PDT-like chalenge caused a rapid an prolonged upregulation of iNOS and the cells that survived the challenge were found to be more aggressive, with increased proliferation, migration and invasion. These findings suggest that iNOS inhibitors should be used as adjunct therapy to PDT (119). Cellular hypoxia is one the reasons for resistance to radiotherapy and NO has been used as a radiosensitizer, but the mechanisms of its action is very complex, due to the multiple actions of NO in diverse microenvironments. NO can induce vasodilation, increasing tumor oxygenation, but it can also induce apoptosis and nitrosative stress, all improving the sensibility to radiotherapy. However, the use o NO donors still need adjustments to reduce side effects, thus novel agents are being developed to deliver NO only to the tumor tissue, sparing normal regions and reducing side effects (120).
Concluding remarks
Despite decades of investigation, the physiological and pathological roles of NO in mitochondrial function, mitochondrial diseases and cancer are still obscure. Nitric oxide (NO) and its derivative peroxynitrite (ONOOQ) have been shown to inhibit the activity of respiratory chain complexes, either by competing with oxygen or by nitrosylating or oxidising mitochondrial components. Mitochondria may also contain a NO synthase and can produce NO to regulate energy metabolism. In the nucleus, NO is involved in the control of mitochondrial biogenesis, and this regulation at the transcription levels by PGC-1α and PRC1 shows potential links to tumor modulation. The interplay between oxygen, ROS and NOS levels, OXPHOS function and tumor progression is just beginning to be understood, but current knowledge places NO at the center of this complex metabolic control.
Highlights.
iNOS synthesizes higher amounts of NO for long periods, while constitutive NOS (eNOS and nNOS) produce low concentrations of NO for short time.
NO inhibits the respiratory chain at complex IV, I and III
NO induces mitochondrial biogenesis
The biological effects of NO depends on local concentration of NO, source of NO generation, duration of NO exposure, microenvironment (ROS)
Acknowledgments
Funding: This work was supported by research grants from São Paulo Research Foundation [FAPESP, 2007/03134-9] the National Institutes of Health [grant numbers 1R01NS079965, 1R01AG036871, 5R01EY010804 and 1R21ES025673 ]; and the Muscular Dystrophy Association.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vasudevan D, Bovee RC, Thomas DD. Nitric oxide, the new architect of epigenetic landscapes. Nitric oxide: biology and chemistry / official journal of the Nitric Oxide Society. 2016;59:54–62. doi: 10.1016/j.niox.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DD. Breathing new life into nitric oxide signaling: A brief overview of the interplay between oxygen and nitric oxide. Redox biology. 2015;5:225–33. doi: 10.1016/j.redox.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free radical research. 1999;31(6):577–96. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 4.Vannini F, Kashfi K, Nath N. The dual role of iNOS in cancer. Redox biology. 2015;6:334–43. doi: 10.1016/j.redox.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhari SK, Chaudhary M, Bagde S, Gadbail AR, Joshi V. Nitric oxide and cancer: a review. World journal of surgical oncology. 2013;11:118. doi: 10.1186/1477-7819-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basudhar D, Somasundaram V, de Oliveira GA, Kesarwala A, Heinecke JL, Cheng RY, et al. Nitric Oxide Synthase-2-Derived Nitric Oxide Drives Multiple Pathways of Breast Cancer Progression. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6813. [DOI] [PMC free article] [PubMed]
- 7.Hussain SP, Wang J. NO and Pancreatic Cancer: A complex interaction with therapeutic potential. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson A, Yakovlev VA. Cells redox environment modulates BRCA1 expression and DNA homologous recombination repair. Free radical biology & medicine. 2016;101:190–201. doi: 10.1016/j.freeradbiomed.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annual review of pharmacology and toxicology. 2006;46:235–76. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 10.Boveris A, Costa LE, Poderoso JJ, Carreras MC, Cadenas E. Regulation of mitochondrial respiration by oxygen and nitric oxide. Annals of the New York Academy of Sciences. 2000;899:121–35. doi: 10.1111/j.1749-6632.2000.tb06181.x. [DOI] [PubMed] [Google Scholar]
- 11.Toledo JC, Jr, Augusto O. Connecting the chemical and biological properties of nitric oxide. Chemical research in toxicology. 2012;25(5):975–89. doi: 10.1021/tx300042g. [DOI] [PubMed] [Google Scholar]
- 12.Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. British journal of pharmacology. 2015;172(24):6024–109. doi: 10.1111/bph.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78(6):915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 14.Larsson B, Phillips SC. Isolation and characterization of a novel, human neuronal nitric oxide synthase cDNA. Biochemical and biophysical research communications. 1998;251(3):898–902. doi: 10.1006/bbrc.1998.9578. [DOI] [PubMed] [Google Scholar]
- 15.Marsden PA, Schappert KT, Chen HS, Flowers M, Sundell CL, Wilcox JN, et al. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS letters. 1992;307(3):287–93. doi: 10.1016/0014-5793(92)80697-f. [DOI] [PubMed] [Google Scholar]
- 16.Janssens SP, Shimouchi A, Quertermous T, Bloch DB, Bloch KD. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. The Journal of biological chemistry. 1992;267(21):14519–22. [PubMed] [Google Scholar]
- 17.Singh S, Gupta AK. Nitric oxide: role in tumour biology and iNOS/NO-based anticancer therapies. Cancer chemotherapy and pharmacology. 2011;67(6):1211–24. doi: 10.1007/s00280-011-1654-4. [DOI] [PubMed] [Google Scholar]
- 18.Tengan CH, Rodrigues GS, Godinho RO. Nitric oxide in skeletal muscle: role on mitochondrial biogenesis and function. International journal of molecular sciences. 2012;13(12):17160–84. doi: 10.3390/ijms131217160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. European heart journal. 2012;33(7):829–37. 37a–37d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84(5):757–67. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 21.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, et al. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. The Journal of biological chemistry. 1996;271(11):6518–22. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Garcia-Cardena G, Sessa WC. Biosynthesis and palmitoylation of endothelial nitric oxide synthase: mutagenesis of palmitoylation sites, cysteines-15 and/or -26, argues against depalmitoylation-induced translocation of the enzyme. Biochemistry. 1995;34(38):12333–40. doi: 10.1021/bi00038a029. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Goligorsky MS, Lin M, Wilcox JN, Marsden PA. A novel, testis-specific mRNA transcript encoding an NH2-terminal truncated nitric-oxide synthase. The Journal of biological chemistry. 1997;272(17):11392–401. [PubMed] [Google Scholar]
- 24.Maron BA, Michel T. Subcellular localization of oxidants and redox modulation of endothelial nitric oxide synthase. Circulation journal: official journal of the Japanese Circulation Society. 2012;76(11):2497–512. doi: 10.1253/circj.cj-12-1207. [DOI] [PubMed] [Google Scholar]
- 25.Ratovitski EA, Alam MR, Quick RA, McMillan A, Bao C, Kozlovsky C, et al. Kalirin inhibition of inducible nitric-oxide synthase. The Journal of biological chemistry. 1999;274(2):993–9. doi: 10.1074/jbc.274.2.993. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Poulos TL. Structure-function studies on nitric oxide synthases. Journal of inorganic biochemistry. 2005;99(1):293–305. doi: 10.1016/j.jinorgbio.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Heiss EH, Dirsch VM. Regulation of eNOS enzyme activity by posttranslational modification. Current pharmaceutical design. 2014;20(22):3503–13. doi: 10.2174/13816128113196660745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochimica et biophysica acta. 1999;1411(2–3):250–62. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 29.Giulivi C, Poderoso JJ, Boveris A. Production of nitric oxide by mitochondria. The Journal of biological chemistry. 1998;273(18):11038–43. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 30.Figueira TR, Barros MH, Camargo AA, Castilho RF, Ferreira JC, Kowaltowski AJ, et al. Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid Redox Signal. 2013;18(16):2029–74. doi: 10.1089/ars.2012.4729. [DOI] [PubMed] [Google Scholar]
- 31.Tatoyan A, Giulivi C. Purification and characterization of a nitric-oxide synthase from rat liver mitochondria. The Journal of biological chemistry. 1998;273(18):11044–8. doi: 10.1074/jbc.273.18.11044. [DOI] [PubMed] [Google Scholar]
- 32.Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372(6506):546–8. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 33.Bates TE, Loesch A, Burnstock G, Clark JB. Immunocytochemical evidence for a mitochondrially located nitric oxide synthase in brain and liver. Biochemical and biophysical research communications. 1995;213(3):896–900. doi: 10.1006/bbrc.1995.2213. [DOI] [PubMed] [Google Scholar]
- 34.Bates TE, Loesch A, Burnstock G, Clark JB. Mitochondrial nitric oxide synthase: a ubiquitous regulator of oxidative phosphorylation? Biochemical and biophysical research communications. 1996;218(1):40–4. doi: 10.1006/bbrc.1996.0008. [DOI] [PubMed] [Google Scholar]
- 35.Frandsen U, Lopez-Figueroa M, Hellsten Y. Localization of nitric oxide synthase in human skeletal muscle. Biochemical and biophysical research communications. 1996;227(1):88–93. doi: 10.1006/bbrc.1996.1472. [DOI] [PubMed] [Google Scholar]
- 36.Brookes PS. Mitochondrial nitric oxide synthase. Mitochondrion. 2004;3(4):187–204. doi: 10.1016/j.mito.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Gao S, Chen J, Brodsky SV, Huang H, Adler S, Lee JH, et al. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. The Journal of biological chemistry. 2004;279(16):15968–74. doi: 10.1074/jbc.M308504200. [DOI] [PubMed] [Google Scholar]
- 38.Elfering SL, Sarkela TM, Giulivi C. Biochemistry of mitochondrial nitric-oxide synthase. The Journal of biological chemistry. 2002;277(41):38079–86. doi: 10.1074/jbc.M205256200. [DOI] [PubMed] [Google Scholar]
- 39.Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, et al. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(24):14126–31. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacza Z, Snipes JA, Zhang J, Horvath EM, Figueroa JP, Szabo C, et al. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free radical biology & medicine. 2003;35(10):1217–28. doi: 10.1016/s0891-5849(03)00510-0. [DOI] [PubMed] [Google Scholar]
- 41.Venkatakrishnan P, Nakayasu ES, Almeida IC, Miller RT. Absence of nitric-oxide synthase in sequentially purified rat liver mitochondria. The Journal of biological chemistry. 2009;284(30):19843–55. doi: 10.1074/jbc.M109.003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parihar MS, Nazarewicz RR, Kincaid E, Bringold U, Ghafourifar P. Association of mitochondrial nitric oxide synthase activity with respiratory chain complex I. Biochemical and biophysical research communications. 2008;366(1):23–8. doi: 10.1016/j.bbrc.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bombicino SS, Iglesias DE, Zaobornyj T, Boveris A, Valdez LB. Mitochondrial nitric oxide production supported by reverse electron transfer. Archives of biochemistry and biophysics. 2016;607:8–19. doi: 10.1016/j.abb.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Giulivi C. Characterization and function of mitochondrial nitric-oxide synthase. Free radical biology & medicine. 2003;34(4):397–408. doi: 10.1016/s0891-5849(02)01298-4. [DOI] [PubMed] [Google Scholar]
- 45.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS letters. 1994;356(2–3):295–8. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 46.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS letters. 1994;345(1):50–4. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 47.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(13):7631–6. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochimica et biophysica acta. 2004;1658(1–2):44–9. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Riobo NA, Clementi E, Melani M, Boveris A, Cadenas E, Moncada S, et al. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. The Biochemical journal. 2001;359(Pt 1):139–45. doi: 10.1042/0264-6021:3590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cadenas E. Mitochondrial free radical production and cell signaling. Molecular aspects of medicine. 2004;25(1–2):17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Archives of biochemistry and biophysics. 1996;328(1):85–92. doi: 10.1006/abbi.1996.0146. [DOI] [PubMed] [Google Scholar]
- 52.Taylor CT, Moncada S. Nitric oxide, cytochrome C oxidase, and the cellular response to hypoxia. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(4):643–7. doi: 10.1161/ATVBAHA.108.181628. [DOI] [PubMed] [Google Scholar]
- 53.Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: Molecular mechanism and tissue physiology. American journal of physiology Cell physiology. 2007;292(6):C1993–2003. doi: 10.1152/ajpcell.00310.2006. [DOI] [PubMed] [Google Scholar]
- 54.Pannala VR, Camara AK, Dash RK. Modeling the Detailed Kinetics of Mitochondrial Cytochrome c Oxidase: Catalytic Mechanism and Nitric Oxide Inhibition. Journal of applied physiology. 2016 doi: 10.1152/japplphysiol.00524.2016. jap 00524 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghafourifar P, Cadenas E. Mitochondrial nitric oxide synthase. Trends in pharmacological sciences. 2005;26(4):190–5. doi: 10.1016/j.tips.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Giulivi C, Kato K, Cooper CE. Nitric oxide regulation of mitochondrial oxygen consumption I: cellular physiology. American journal of physiology Cell physiology. 2006;291(6):C1225–31. doi: 10.1152/ajpcell.00307.2006. [DOI] [PubMed] [Google Scholar]
- 57.Beltran B, Orsi A, Clementi E, Moncada S. Oxidative stress and S-nitrosylation of proteins in cells. British journal of pharmacology. 2000;129(5):953–60. doi: 10.1038/sj.bjp.0703147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nature medicine. 2013;19(6):753–9. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galkin A, Moncada S. S-nitrosation of mitochondrial complex I depends on its structural conformation. The Journal of biological chemistry. 2007;282(52):37448–53. doi: 10.1074/jbc.M707543200. [DOI] [PubMed] [Google Scholar]
- 60.Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA. Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. The Journal of biological chemistry. 2003;278(39):37223–30. doi: 10.1074/jbc.M305694200. [DOI] [PubMed] [Google Scholar]
- 61.Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta physiologica Scandinavica. 1998;162(3):401–9. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- 62.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78(6):931–6. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 63.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258(5090):1898–902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 64.Viale A, Corti D, Draetta GF. Tumors and mitochondrial respiration: a neglected connection. Cancer research. 2015;75(18):3685–6. doi: 10.1158/0008-5472.CAN-15-0491. [DOI] [PubMed] [Google Scholar]
- 65.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinnaird A, Zhao S, Wellen KE, Michelakis ED. Metabolic control of epigenetics in cancer. Nat Rev Cancer. 2016;16(11):694–707. doi: 10.1038/nrc.2016.82. [DOI] [PubMed] [Google Scholar]
- 67.Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer & metabolism. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cherry AD, Piantadosi CA. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antioxid Redox Signal. 2015;22(12):965–76. doi: 10.1089/ars.2014.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gollnick PD, King DW. Effect of exercise and training on mitochondria of rat skeletal muscle. The American journal of physiology. 1969;216(6):1502–9. doi: 10.1152/ajplegacy.1969.216.6.1502. [DOI] [PubMed] [Google Scholar]
- 71.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annual review of physiology. 1976;38:273–91. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 72.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–39. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 73.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 74.Luo C, Widlund HR, Puigserver P. PGC-1 Coactivators: Shepherding the Mitochondrial Biogenesis of Tumors. Trends in cancer. 2016;2(10):13. doi: 10.1016/j.trecan.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salem AF, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Mitochondrial biogenesis in epithelial cancer cells promotes breast cancer tumor growth and confers autophagy resistance. Cell Cycle. 2012;11(22):4174–80. doi: 10.4161/cc.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feilchenfeldt J, Brundler MA, Soravia C, Totsch M, Meier CA. Peroxisome proliferator-activated receptors (PPARs) and associated transcription factors in colon cancer: reduced expression of PPARgamma-coactivator 1 (PGC-1) Cancer letters. 2004;203(1):25–33. doi: 10.1016/j.canlet.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 77.Jiang WG, Douglas-Jones A, Mansel RE. Expression of peroxisome-proliferator activated receptor-gamma (PPARgamma) and the PPARgamma co-activator, PGC-1, in human breast cancer correlates with clinical outcomes. Int J Cancer. 2003;106(5):752–7. doi: 10.1002/ijc.11302. [DOI] [PubMed] [Google Scholar]
- 78.Girnun GD. The diverse role of the PPARgamma coactivator 1 family of transcriptional coactivators in cancer. Semin Cell Dev Biol. 2012;23(4):381–8. doi: 10.1016/j.semcdb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The localisation and reduction of nuclear staining of PPARgamma and PGC-1 in human breast cancer. Oncol Rep. 2004;12(2):483–8. [PubMed] [Google Scholar]
- 80.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003. 1–15. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo C, Lim JH, Lee Y, Granter SR, Thomas A, Vazquez F, et al. A PGC1alpha-mediated transcriptional axis suppresses melanoma metastasis. Nature. 2016;537(7620):422–6. doi: 10.1038/nature19347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vyas S, Zaganjor E, Haigis MC. Mitochondria and Cancer. Cell. 2016;166(3):555–66. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015;282(4):647–72. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 84.Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH, et al. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. The Journal of physiology. 2010;588(Pt 18):3551–66. doi: 10.1113/jphysiol.2010.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McConell GK, Phillips M, Ruan Z, Macaulay SL, Wadley GD. Central role of nitric oxide synthase in AICAR and caffeine-induced mitochondrial biogenesis in L6 myocytes. J Appl Physiol. 2010;108(3):589–95. doi: 10.1152/japplphysiol.00377.2009. [DOI] [PubMed] [Google Scholar]
- 86.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(47):16507–12. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299(5608):896–9. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 88.Carew JS, Nawrocki ST, Xu RH, Dunner K, McConkey DJ, Wierda WG, et al. Increased mitochondrial biogenesis in primary leukemia cells: the role of endogenous nitric oxide and impact on sensitivity to fludarabine. Leukemia. 2004;18(12):1934–40. doi: 10.1038/sj.leu.2403545. [DOI] [PubMed] [Google Scholar]
- 89.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(12):2524–31. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 90.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. American journal of physiology Endocrinology and metabolism. 2001;281(6):E1340–6. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 91.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiological reviews. 2009;89(3):1025–78. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 92.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. The Journal of physiology. 2006;574(Pt 1):33–9. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aquilano K, Baldelli S, Ciriolo MR. Nuclear recruitment of neuronal nitric-oxide synthase by alpha-syntrophin is crucial for the induction of mitochondrial biogenesis. The Journal of biological chemistry. 2014;289(1):365–78. doi: 10.1074/jbc.M113.506733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324(5923):102–5. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, et al. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012;26(5):2175–86. doi: 10.1096/fj.11-196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wenz T. PGC-1alpha activation as a therapeutic approach in mitochondrial disease. IUBMB life. 2009;61(11):1051–62. doi: 10.1002/iub.261. [DOI] [PubMed] [Google Scholar]
- 97.Tengan CH, Kiyomoto BH, Godinho RO, Gamba J, Neves AC, Schmidt B, et al. The role of nitric oxide in muscle fibers with oxidative phosphorylation defects. Biochemical and biophysical research communications. 2007;359(3):771–7. doi: 10.1016/j.bbrc.2007.05.184. [DOI] [PubMed] [Google Scholar]
- 98.Ohkoshi N, Mizusawa H, Fujita T, Shoji S. Histological determination of nitric oxide synthase (NOS) and NADPH-diaphorase in ragged-red fibers from patients with mitochondrial encephalomyopathies. Journal of the neurological sciences. 1997;149(2):151–6. doi: 10.1016/s0022-510x(97)05385-9. [DOI] [PubMed] [Google Scholar]
- 99.Rodrigues GS, Godinho RO, Kiyomoto BH, Gamba J, Oliveira AS, Schmidt B, et al. Integrated analysis of the involvement of nitric oxide synthesis in mitochondrial proliferation, mitochondrial deficiency and apoptosis in skeletal muscle fibres. Scientific reports. 2016;6:20780. doi: 10.1038/srep20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raharijaona M, Le Pennec S, Poirier J, Mirebeau-Prunier D, Rouxel C, Jacques C, et al. PGC-1-related coactivator modulates mitochondrial-nuclear crosstalk through endogenous nitric oxide in a cellular model of oncocytic thyroid tumours. PloS one. 2009;4(11):e7964. doi: 10.1371/journal.pone.0007964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vercauteren K, Gleyzer N, Scarpulla RC. Short hairpin RNA-mediated silencing of PRC (PGC-1-related coactivator) results in a severe respiratory chain deficiency associated with the proliferation of aberrant mitochondria. The Journal of biological chemistry. 2009;284(4):2307–19. doi: 10.1074/jbc.M806434200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gleyzer N, Scarpulla RC. Concerted Action of PGC-1-related Coactivator (PRC) and c-MYC in the Stress Response to Mitochondrial Dysfunction. The Journal of biological chemistry. 2016 doi: 10.1074/jbc.M116.719682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends in endocrinology and metabolism: TEM. 2012;23(9):459–66. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Savagner F, Franc B, Guyetant S, Rodien P, Reynier P, Malthiery Y. Defective mitochondrial ATP synthesis in oxyphilic thyroid tumors. The Journal of clinical endocrinology and metabolism. 2001;86(10):4920–5. doi: 10.1210/jcem.86.10.7894. [DOI] [PubMed] [Google Scholar]
- 105.Bonora E, Porcelli AM, Gasparre G, Biondi A, Ghelli A, Carelli V, et al. Defective oxidative phosphorylation in thyroid oncocytic carcinoma is associated with pathogenic mitochondrial DNA mutations affecting complexes I and III. Cancer research. 2006;66(12):6087–96. doi: 10.1158/0008-5472.CAN-06-0171. [DOI] [PubMed] [Google Scholar]
- 106.Baris O, Savagner F, Nasser V, Loriod B, Granjeaud S, Guyetant S, et al. Transcriptional profiling reveals coordinated up-regulation of oxidative metabolism genes in thyroid oncocytic tumors. The Journal of clinical endocrinology and metabolism. 2004;89(2):994–1005. doi: 10.1210/jc.2003-031238. [DOI] [PubMed] [Google Scholar]
- 107.Gamba J, Gamba LT, Rodrigues GS, Kiyomoto BH, Moraes CT, Tengan CH. Nitric oxide synthesis is increased in cybrid cells with m.3243A>G mutation. International journal of molecular sciences. 2012;14(1):394–410. doi: 10.3390/ijms14010394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Desquiret-Dumas V, Gueguen N, Barth M, Chevrollier A, Hancock S, Wallace DC, et al. Metabolically induced heteroplasmy shifting and l-arginine treatment reduce the energetic defect in a neuronal-like model of MELAS. Biochimica et biophysica acta. 2012;1822(6):1019–29. doi: 10.1016/j.bbadis.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sandhu JK, Sodja C, McRae K, Li Y, Rippstein P, Wei YH, et al. Effects of nitric oxide donors on cybrids harbouring the mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) A3243G mitochondrial DNA mutation. The Biochemical journal. 2005;391(Pt 2):191–202. doi: 10.1042/BJ20050272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.MacPherson H, Noble BS, Ralston SH. Expression and functional role of nitric oxide synthase isoforms in human osteoblast-like cells. Bone. 1999;24(3):179–85. doi: 10.1016/s8756-3282(98)00173-2. [DOI] [PubMed] [Google Scholar]
- 111.Fantappie O, Sassoli C, Tani A, Nosi D, Marchetti S, Formigli L, et al. Mitochondria of a human multidrug-resistant hepatocellular carcinoma cell line constitutively express inducible nitric oxide synthase in the inner membrane. Journal of cellular and molecular medicine. 2015;19(6):1410–7. doi: 10.1111/jcmm.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Galkin A, Higgs A, Moncada S. Nitric oxide and hypoxia. Essays Biochem. 2007;43:29–42. doi: 10.1042/BSE0430029. [DOI] [PubMed] [Google Scholar]
- 113.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8(6):425–37. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ball KA, Nelson AW, Foster DG, Poyton RO. Nitric oxide produced by cytochrome c oxidase helps stabilize HIF-1alpha in hypoxic mammalian cells. Biochemical and biophysical research communications. 2012;420(4):727–32. doi: 10.1016/j.bbrc.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berchner-Pfannschmidt U, Tug S, Kirsch M, Fandrey J. Oxygen-sensing under the influence of nitric oxide. Cell Signal. 2010;22(3):349–56. doi: 10.1016/j.cellsig.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 116.Sen S, Kawahara B, Chaudhuri G. Mitochondrial-associated nitric oxide synthase activity inhibits cytochrome c oxidase: implications for breast cancer. Free radical biology & medicine. 2013;57:210–20. doi: 10.1016/j.freeradbiomed.2012.10.545. [DOI] [PubMed] [Google Scholar]
- 117.Carew JS, Huang P. Mitochondrial defects in cancer. Molecular cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Vitto H, Mendonca BS, Elseth KM, Vesper BJ, Portari EA, Gallo CV, et al. Part II. Mitochondrial mutational status of high nitric oxide adapted cell line BT-20 (BT-20-HNO) as it relates to human primary breast tumors. Tumour Biol. 2013;34(1):337–47. doi: 10.1007/s13277-012-0555-4. [DOI] [PubMed] [Google Scholar]
- 119.Fahey JM, Girotti AW. Accelerated migration and invasion of prostate cancer cells after a photodynamic therapy-like challenge: Role of nitric oxide. Nitric oxide: biology and chemistry / official journal of the Nitric Oxide Society. 2015;49:47–55. doi: 10.1016/j.niox.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Scicinski J, Oronsky B, Ning S, Knox S, Peehl D, Kim MM, et al. NO to cancer: The complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001. Redox biology. 2015;6:1–8. doi: 10.1016/j.redox.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]