Abstract
Thrombotic thrombocytopenic purpura (TTP) is rare but life-threatening disease, characterized typically by microangiopathic hemolytic anemia (MAHA), profound peripheral thrombocytopenia and severe deficiency in the von Willebrand factor-cleaving prortease ADAMTS13. It has been reported that acquired immune TTP is closely associated with human immunodeficiency virus infection and influenza infection or vaccination. However, it has not been reported to be associated with Epstein Barr Virus infection or reactivation. We herein report a first case of acquired TTP associated with EBV reactivation in an otherwise healthy adult.
Keywords: Thrombocytopenic purpura (TTP), Epstein Barr Virus (EBV), Reactivation, ADAMTS13
1. Case report
A previously healthy 37-year-old man was referred to our hospital in September 2015 with fever (between 37 and 38 °C) for 10 days and general malaise. There was no history of recent travel, and the patient had no animals in his home. There were no remarkable features in the heart, lungs, or abdomen. A neurological examination revealed no abnormalities. Initial laboratory data showed anemia (hemoglobin, 10.5 g/dl), including red cell fragmentation (schistocytes) and thrombocytopenia (platelets, 1.4×109/l), and the Coombs test was negative. Further evidence of intravascular hemolysis included a lactate dehydrogenase (LDH) level of 748 U/L (normal range: 106–211 U/L) and decreased haptoglobin level of <10 mg/dL (normal range: 36–195 mg/dL). However, renal function was normal, with a blood urea nitrogen level of 12 mg/dL (normal range: 8–20 mg/dL) and creatinine level of 0.98 mg/dL (normal range: 0.6–1.1 mg/dL). His ADAMTS13 level was decreased (<0.5%, normal range: 50–150%), and autoantibodies against ADAMTS13 were positive (0.5 Bethesda unit/ml, normal range: <0.5 Bethesda unit/ml) in chromogenic act-ELISA [1]. All cultures were negative. Tests for a series of autoantibodies related to collagen diseases were negative. Hepatitis virus B and C and human immunodeficiency virus infections were ruled out. Cytomegalovirus DNA was absent and herpes simplex virus serology was negative. Anti-EBV viral capsid antigen IgG and IgM, early antigen IgM, and nuclear antigen IgG were positive, and the detection of a viral load of 7500 copies/ml suggested EBV reactivation. The features of MAHA, thrombocytopenia, decreased ADAMTS13, positivity for autoantibodies against ADAMTS13, fever, and altered sensorium without any evidence of disseminated intravascular coagulation (DIC) strongly suggested TTP associated with EBV reactivation.
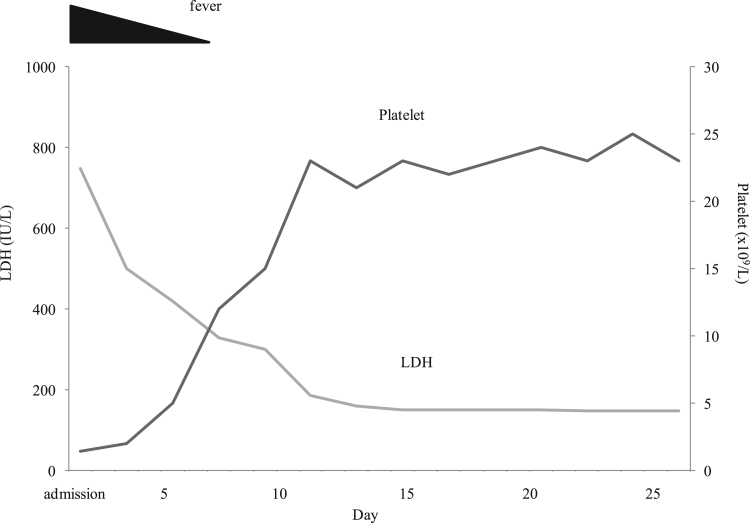
The patient recovered spontaneously without any treatment (Fig. 1). An ADAMTS13 activity assay was performed, and autoantibodies against ADAMTS13 were tested approximately one month after his discharge and gradually normalized (Table 1). Three months later, EBV IgM was no longer detectable and EBV-DNA in his serum had decreased. The remission of TTP has been maintained, and the condition of the patient has remained stable for 20 months with no recurrence of acquired TTP.
Fig. 1.
Clinical course of Epstein Barr virus-associated thrombotic thrombocytopenic purpura.
Table 1.
Clinical titers of plasma ADAMTS13 activity, the ADAMTS13 inhibitor and EBV viral load.
| Time (year/month) | 2015 Sep | 2015 Oct | 2015 Nor | 2015 Dec | 2016 Mar | 2016 Jun | 2016 Sep | 2016 Dec | 2017 Mar | 2017 Jun |
|---|---|---|---|---|---|---|---|---|---|---|
| ADAMTS13 activity (%) | < 0.5 | 4.4 | 22.8 | 31.9 | 47.8 | 59.8 | 69.9 | 70.3 | 71 | 74 |
| ADAMTS13 inhibitor (Bethesda units/ml) | 1 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| EBV-DNA (log copies/ml) | 7500 | 120 | 92 | 66 | 60 | 43 | 22 | < 20 | < 20 | < 20 |
TTP is a rare but life-threatening disease that is typically characterized by MAHA, profound peripheral thrombocytopenia, and a severe deficiency in the von Willebrand factor-cleaving protease ADAMTS13 [2]. A low baseline ADAMTS13 activity of <10%, with or without the presence of anti-ADAMTS13 autoantibodies, in a patient with thrombocytopenia and MAHA strongly supports a diagnosis of TTP [2], [9], [10]. In some cases, acquired TTP occurs in association with specific conditions that have to be identified for appropriate management: collagen disease, pregnancy, malignancies, treatment with antiplatelet agents, and infections [3]. Infections are well-known triggers of TTP episodes, and Shigera toxin-producing Escherichia coli, HIV, human parvovirus B19, hepatitis C virus, and influenza infection or vaccination are known to be associated with TTP [4], [5]. However, it currently remains unclear whether TTP is associated with EBV reactivation.
EBV is a human herpes virus with primary infections occurring in most adults in childhood. EBV mainly persists in B lymphocytes and shows four phases (types) of latency (latency 0–3) based on viral antigen expression. EBV causes persistent infection with a tight latency program in memory B-cells, which enables evasion from immune defenses. A number of immune escape mechanisms and immune-modulating proteins have been described for EBV [6]. These immune-modulating functions make EBV a good candidate for the initiation of autoimmune diseases and exacerbation of disease progression. EBV has long been associated with the induction of various cancers, including lymphoid malignancies and epithelial cell malignancies.
EBV occasionally reactivates to switch its replication mode from latent to lytic, producing a large number of infectious virions with the lysis of host cells [7]. Although reactivation is dangerous for the virus, its triggering factors have not yet been identified. EBV reactivation is associated not only with several autoimmune or cardiovascular diseases, but also with prolonged stress events among immunocompetent patients [8]. The pathophysiology of EBV reactivation currently remains unclear. Recent findings suggest that EBV triggers inflammation through the modulation of interleukin-6 [9]. Other studies indicated that EBV promotes an immune deficiency in T-cell responses [10]. Under conditions of immune perturbation, this deficit may contribute to counteracting immune responses.
EBV reactivation induces the production of immunoglobulins by host B cells. Although the exact triggers for lytic cycle reactivation have not yet been identified, the process is a dynamic interaction between the host's immune response to EBV and the infection state. Activation of the promoter for early lytic genes and, thus, the initiation of lytic replication are triggered by the differentiation of infected B-cells into plasma cells. Nagata et al. previously demonstrated that EBV rescues autoreactive B cells to produce autoantibodies, which contribute to the development and exacerbation of autoimmune disease [7]. The systemic EBV reactivation of B-cells and epithelial cells may occur, leading to the various overlapping systemic manifestations observed in systemic autoimmune diseases. The reactivation of EBV and, thus, an increased number of EBV-infected cells presumably result in increased amounts of cellular waste, which is followed by the stimulation of autoreactive B-cells and production of autoantibodies, leading to disease flares.
TTP has been associated with a deficiency in plasma ADAMTS13 activity, which is caused by genetic mutations in or acquired autoantibodies to this enzyme. In the idiopathic form, TTP is an autoimmune disease and, thus, may be triggered by immunological stimuli such as vaccination or viral infection. It has been hypothesized that a cross-reactive stimulation between vaccinal or virus antigens and ADAMTS13 protease or the bystander activation of a vaccination or virus with ADAMTS13-specific IgG causes a response. Furthermore, inflammatory responses caused by immunological factors represent another pathway linking endothelial injury and TTP [2]. EBV may rescue autoreactive B cells to produce autoantibodies that contribute to the development of TTP. The present case had a transient ADAMTS13 deficiency that was attributed to autoantibodies against ADAMTS13. The increased EBV load suggested active EBV lytic replication in the presence of TTP. Since the viral load was associated with ADAMTS13 activity, the reactivation of EBV may be associated with the development of TTP.
EBV is not a recognized trigger of TTP. Although the pathogenesis of TTP associated with EBV reactivation has not yet been elucidated, EBV may present with the features of TTP and need to be considered not only in immunocompromised patients, but also in immunocompetent patients. Therefore, further studies are warranted in order to improve clinical management and biological knowledge.
Conflict of interest
None.
Acknowledgement
The authors thank Professor Masanori Matsumoto (Department of Blood Transfusion Medicine, Nara Medical University) for his encouragement and valuable help in carrying out this study.
References
- 1.Kato S., Matsumoto M., Matsuyama T. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46(8):1444–1452. doi: 10.1111/j.1537-2995.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 2.Fujikawa K., Suzuki H., McMullen B. Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family. Blood. 2001;98(6):1662–1666. doi: 10.1182/blood.v98.6.1662. [DOI] [PubMed] [Google Scholar]
- 3.Coppo P. Thrombotic microangiopathies: from empiricism to targeted therapies. Presse Med. 2012;41(3 Pt 2):e101–e104. doi: 10.1016/j.lpm.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Bell W.R., Chulay J.D., Feinberg J.E. Manifestations resembling thrombotic microangiopathy in patients with advanced human immunodeficiency virus (HIV) disease in a cytomegalovirus prophylaxis trial (ACTG 204) Medicine. 1997;76(5):369–380. doi: 10.1097/00005792-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kosugi N., Tsurutani N., Y., Inonishi A. Influenza A infection triggers thrombotic thrombocytopenic purpura by producing the anti-ADAMTS13 IgG inhibitor. Intern. Med. 2010;49(7):689–693. doi: 10.2169/internalmedicine.49.2957. [DOI] [PubMed] [Google Scholar]
- 6.Draborg A.H., Duus K., Houen G. Epstein-Barr virus in systemic autoimmune diseases. Clin. Dev. Immunol. 2013;2013:535738. doi: 10.1155/2013/535738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagata K., Kumata K., Nakayama Y. Epstein-Barr virus lytic reactivation activates B Cells polyclonally and induces activation-induced Cytidine Deaminase expression: a mechanism underlying autoimmunity and its contribution to Graves' disease. Viral Immunol. 2017;30(3):240–249. doi: 10.1089/vim.2016.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donati D., Espmark E., Kironde F. Clearance of circulating Epstein-Barr virus DNA in children with acute malaria after antimalaria treatment. J. Infect. Dis. 2006;193(7):971–977. doi: 10.1086/500839. [DOI] [PubMed] [Google Scholar]
- 9.Waldman W.J., Williams M.V., Jr, Lemeshow S. Epstein-Barr virus-encoded dUTPase enhances proinflammatory cytokine production by macrophages in contact with endothelial cells: evidence for depression-induced atherosclerotic risk. Brain Behav. Immun. 2008;22(2):215–223. doi: 10.1016/j.bbi.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauce D., Larsen M., Curnow S.J. EBV-associated mononucleosis leads to long-term global deficit in T-cell responsiveness to IL-15. Blood. 2006;108(1):11–18. doi: 10.1182/blood-2006-01-0144. [DOI] [PubMed] [Google Scholar]