Abstract
Invariant Natural Killer T-cells (iNKT-cells) are an attractive target for immune response modulation, as upon CD1d-mediated stimulation with KRN7000, a synthetic α-galactosylceramide, they produce a vast amount of cytokines. Here we present a synthesis that allows swift modification of the phytosphingosine side chain by amidation of an advanced methyl ester precursor. The resulting KRN7000 derivatives, termed α-galactosylsphingamides, were evaluated for their capacity to stimulate iNKT-cells. While introduction of the amide-motif in the phytosphingosine chain is tolerated for CD1d binding and TCR recognition, the studied α-galactosylsphingamides showed compromised antigenic properties.
Introduction
Invariant natural killer T-cells (iNKT-cells) are a small set of glycolipid reactive T-cells that bridge innate and adaptive immunity. They display characteristics of both the NK cell lineage (mouse NK1.1, human CD161) and conventional thymus-derived T-cells1. Their semi-invariant T-cell receptor (TCR) is composed of an invariant Vα14-Jα18 chain in mice (homologous Vα24-Jα18 chain in humans), preferentially paired with Vβ8.2, Vβ7 or Vβ2 (Vβ11 in humans). Whereas conventional T-cells are activated by peptide antigens presented by MHC proteins, iNKT-cells typically recognize glycolipid antigens presented by MHC class 1 like CD1d molecules on antigen presenting cells2.
The prototypical antigen for iNKT-cell stimulation is KRN7000, also known as α-GalCer (1), a synthetic glycolipid derived from agelasphins found in marine sponge extracts (Fig. 1)3, 4. Structural data illustrated that the lipid chains of α-GalCer are bound in two hydrophobic pockets deeply buried in CD1d5, 6. The larger A’-pocket binds the fatty acid part while the linear F’-pocket accommodates the phytosphingosine chain. Consequently, the galactose sugar is positioned at the surface of the CD1d binding groove and is available for recognition by the TCR of iNKT-cells7.
Figure 1.
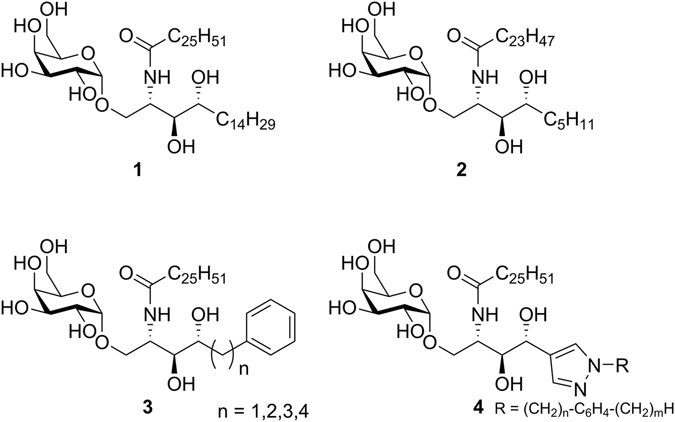
Structures of α-GalCer (1) and OCH (2) and known phytosphingosine-modified α-GalCer derivatives 3 and 4.
Upon stimulation, iNKT-cells rapidly secrete large amounts of pro-inflammatory Th1- (e.g., IFN-γ) and anti-inflammatory Th2-cytokines (e.g., IL-4). These cytokines mediate immune responses against tumors8, microbial infections1, 9 and auto-immunity10. In addition, iNKT-cells transactivate other immune cells including dendritic cells, B- and T-cells, macrophages and NK-cells and are thus able to orchestrate complex immune functions1. Despite the potent immune response triggered by α-GalCer, this antigen showed limited therapeutic efficacy in several clinical studies11. The lack of therapeutic effect is attributed to the antagonizing activities of the secreted pro-inflammatory Th1- and anti-inflammatory Th2-cytokines. Accordingly, the search for improved α-GalCer analogues capable of inducing a biased cytokine release is ongoing.
To date, the growing library of α-GalCer analogues mainly consists of glycolipids with an altered fatty acid chain12, 13 and/or carbohydrate part14–17. In addition, a vast amount of non-glycosidic analogues, including C-glycosides18–20 and cyclitol derivatives21 exist. However, analogues with modifications in the phytosphingosine chain remain relatively scarce, due to the more challenging syntheses required to access these analogs.
A common strategy for the synthesis of α-GalCer analogues modified in the phytosphingosine moiety relies on the Wittig olefination on L-Garner’s aldehyde, followed by stereoselective dihydroxylation of the Z-double bond furnishing the required d-ribo stereochemistry14, 22. Although this approach may afford high stereoselectivity, it inevitably leads to minor unwanted isomers thereby lowering the overall yield and forcing demanding purification steps ahead in the synthesis.
Hitherto, a small number of phytosphingosine modified analogues have been reported, some of which exhibit interesting biological properties. Most notably, OCH (2), a glycolipid with a truncated phytosphingosine chain and a slightly trimmed fatty acid residue, became a prototypical Th2-polarizing iNKT-cell ligand23, 24. Wong and coworkers reported derivatives with a terminal phenyl group in the sphingosine chain (3)25. Interestingly, the immunological properties of these analogues were strongly dependent on the relative position of the aromatic ring12, 22, 26. More recently, Park et al. demonstrated that selected derivatives bearing a pyrazole moiety in the phytosphingosine chain (4) give rise to a Th2-biased cytokine profile27, 28. These examples demonstrate that alterations of the sphingosine chain are tolerated and may afford analogues with interesting cytokine profiles.
Up to now, the mechanism of cytokine polarization is poorly understood, although there is ample of evidence suggesting that the stability of the CD1d/glycolipid/TCR-complex is an important contributing factor29. The potent Th1-response observed for α-GalCer derivatives featuring aromatic moieties in the acyl chain, is attributed to the formation of additional Van der Waals interactions with amino acid residues lining the A’-pocket30. Consequently these glycolipids exhibit an enhanced affinity for CD1d resulting in the observed cytokine bias. In analogy, the potent Th1-polarizing sugar-modified galactosylceramides previously synthesized in our lab, also feature a greater CD1d-affinity due to the formation of an extra binding pocket in CD1d via induced-fit31.
A study by McCarthy et al. indicates that although both the acyl chain and phytosphingosine contribute to the stability of the glycolipid/CD1d-complex, it is the phytosphingosine chain that controls TCR-binding and iNKT-cell activation, since ligand-induced conformational changes of the F’-pocket allosterically influence the CD1d/glycolipid footprint32. The aforementioned arguments excited us to explore a synthetic strategy for the preparation of phytosphingosine modified α-GalCer analogues, allowing us to introduce variations in the later stages of the synthesis via amide coupling. Molecular modeling studies indicate that the amide moiety, when placed at the right position, can potentially form a hydrogen bond with Tyr73 in the CD1d-binding groove and may consequently enhance the glycolipid/CD1d-complex stability (Fig. 2). The selected amide substituents are aimed at reinforcing the interactions (e.g., by π-π-stacking) with aromatic residues lining the F’-pocket.
Figure 2.

Model of the binding of 5a to mouse CD1d. The 5a carbons are depicted in green, while the Tyr73.A carbons are colored in yellow. The possible hydrogen bond with the amide oxygen atom is highlighted. Ribbons are colored as skyblue for the CD1d (chain A) and the mouse NKT TCR chains are colored in grey.
To ensure acceptable solubility of the envisioned analogues, we opted to equip these initially with an octanoyl moiety, which was shown to retain most of the antigenic properties when replacing the N-hexacosanoyl group of α-GalCer33.
Results and Discussion
Chemistry
An overview of the synthesized α-galactosylsphingamides is shown in Table 1.
Table 1.
Overview of the synthesized α-galactosylsphingamides.
| R1 | R1 = C7H15 | R1 = C11H23 | R1 = C15H31 | R1 = C19H39 | R1 = C25H51 |
|---|---|---|---|---|---|
| R2 | |||||
| C9H19 | 5a | ||||
| (CH2)2Ph | 5b | ||||
| (CH2)4Ph | 5c | ||||
| (CH2)6Ph | 5d | 6d | 7d | 8d | 9d |
| (CH2)8Ph | 5e | ||||
| Ph-m-C5H11 | 5f | 9f | |||
| Ph-p-C5H11 | 5g | ||||
| ((CH2)2O)2C2H5 | 5h |
As shown in the retrosynthetic analysis (Fig. 3), the α-galactosylsphingamides (5a-h, 6–9d and 9f) can be accessed from methyl ester 10, which is obtained through Lewis acid catalyzed glycosylation of glycosyl acceptor 12. The latter would be obtained by conversion of alcohol 13 to an azide with an inversion of stereochemistry at the carbon centre, followed by selective benzyl ether cleavage to afford the primary alcohol. The 2-deoxygalactose intermediate 14, prepared from commercially available tri-O-acetyl-d-galactal (16), would serve as a substrate for a Wittig olefination with phosphonium ylid 15.
Figure 3.

Retrosynthesis of the target α-galactosylsphingamides.
The synthetic route towards glycosyl acceptor 12 is outlined in Fig. 4. It was decided to start from commercially available tri-O-acetyl-d-galactal (16), which was readily converted into the 3,4,6-tri-O-benzyl-protected derivative 17 34. Hydrolysis of the enol ether was achieved upon treatment with 4 m sulfuric acid, furnishing 2-deoxygalactose intermediate 14 35. Next, the Wittig reaction on 14 with methyl(triphenylphosphoranylidene)acetate was investigated. When the reaction was carried out in THF, α,β-unsaturated ester 18 was obtained exclusively as the E-isomer in moderate yield (64%). Performing the reaction in refluxing toluene increased the yield to 79%, while also providing solely the E-isomer. To avoid Michael type side reactions and to eliminate the possibility for a 1,3-dipolar cycloaddition upon introduction of the azide, saturation of the double bond was accomplished by conjugate reduction with nickel(II) chloride and sodium borohydride. Mitsunobu reaction with diphenyl phosphoryl azide (DPPA) converts the C2-OH to an azido group with the appropriate stereochemistry (19). Next, selective deprotection of the primary hydroxyl was achieved by treatment of 19 with zinc chloride in acetic anhydride followed by Zemplén deacetylation of the intermediate acetate to furnish acceptor 12.
Figure 4.

Reagents and conditions: (a) (i) Et3N, H2O, MeOH, RT, 4d; (ii) NaH, BnBr, DMF, RT, overnight, 94% (over 2 steps); (b) 4 m H2SO4, DMF, 0 °C → RT, overnight, 85%; (c) methyl (triphenylphosphoranylidene)acetate, toluene, 85 °C, 6 h, 79%; (d) NiCl2∙6 H2O, NaBH4, MeOH/THF, 0 °C, 1 h, 94%; (e) PPh3, diethylazodicarboxylate, diphenylphosphorylazide, THF, −20 °C → RT, 7 h, 95%; (f) (i) ZnCl2, AcOH/Ac2O, 3.5 h, RT; (ii) MeOH, NaOMe, pH 10, RT, overnight, 67% over 2 steps; (g) TMSOTf, THF, −30 °C, 2 h, 67%; (h) AlMe3, amine, CH2Cl2, reflux, overnight, 56%-85%; (i) (i) PMe3, H2O, THF, RT, 7 h; (ii) EDC, octanoic acid, CH2Cl2, RT, overnight, 51%–69% over 2 steps; (j) H2, Pd black, MeOH, CHCl3, RT, overnight, 37%–58%.
Next, TMSOTf-promoted glycosylation of acceptor 12 with galactosyl trichloroacetimidate 11 36 afforded α-galactoside 10 in good yield and without notable formation of the β-glycoside. The ability to diversify the methyl ester after glycosylation is convenient as it reduces the number of linear steps towards the target α-GalCer analogues. A Lewis acid-catalyzed amidation with the appropriate amines gave intermediates 20a-h. Next, the azido group was subjected to Staudinger reduction with trimethylphosphine and the resulting amine was coupled with octanoic acid using EDC. Finally, catalytic hydrogenolysis afforded the desired compounds 5a-h. Analogues 6-9d and 9f with alternative acyl moieties were synthesized according to similar procedures.
Biological evaluation
To assess the biological activity of the α-galactosylsphingamides, we analyzed the IFN-γ- and IL-4-levels after intraperitoneal injection of 5 µg of the galactosylsphingamides 5a-h in mice (Fig. 5).
Figure 5.

IL-4 and IFN-γ secretion, measured at respectively 4 h and 16 h, after intraperitoneal injection of 5 µg of the glycolipids in mice. Data for one individual experiment using 8 mice for each glycolipid.
The marginal cytokine release induced by this series indicates that the introduction of an amide moiety in the phytosphingosine chain compromises the antigenic potency.
In an effort to explain the poor antigenicity of 5a-h, we next tested the binding of the Vα14Vβ8.2 TCR of the murine iNKT-cell hybridoma 2C12 to mCD1d-presenting ligands 5d,e using surface plasmon resonance (SPR) and compared it with the binding of PBS-2533, an α-GalCer analogue that also features an octanoic acid residue. It should be noted that in our hands, PBS-25 was inactive in the in vivo cytokine secretion assay (Supporting Figure S1). To further evaluate whether the low antigenicity of 5d is resulting from the amide functionality in the phytosphingosine chain, we synthesized glycolipid 23 (Fig. 6), which is the amide-deleted counterpart of α-galactosphingamides 5d and 5e. Surprisingly, all ligands were bound with similarly high TCR affinities (KD = 38–50 nM) (Fig. 7). Therefore, the TCR binding kinetics cannot explain the inability of the galactosylsphingamides to induce robust cytokine production in vivo. In addition, the maximal response (RU 120–220) observed for the individual sensorgrams of the α-galactosylsphingamides are comparable between the individual CD1d-ligand complexes, which were immobilized at similar levels, suggesting a similar CD1d ligand binding efficiency in vitro.
Figure 6.

Structures of reference compounds PBS-25 (22) and 23.
Figure 7.

Real-time TCR-binding kinetics to mCD1d-presented ligands. Each curve represents the TCR binding sensorgram to CD1d-glycolipid complexes at a different TCR concentration after reference substraction (binding response to CD1d without added glycolipid). Sensorgrams (top) indicate similar lipid loading levels for 5d-e. Single cycle TCR kinetics were measured for PBS-25 and 23. Here, TCR with increasing concentrations (3-fold) was sequentially added to CD1d-glycolipid with a final dissociation step of 10 min. Colored curves represent actual binding responses and black curves the fitted data. Kinetic values derived from the sensorgrams are listed below.
Since the current galactosylsphingamides are based on a C8 fatty acid, the short acyl chain will not fully occupy the A’ pocket of CD1d. Likely similar to PBS-2537, free fatty acid will be recruited to fill the remainder of the pocket. To assess whether these spacer lipids can potentially influence the ligand potency, we synthesized ligands based on 5d that contained longer acyl chains of 12, 16, 20, and 26 carbon atoms (6d-9d). Since the acyl chain is introduced at the final stages of the synthesis, the elongated fatty acid chains could easily be introduced, demonstrating the versatility of our synthetic approach. We next analyzed antigenicity by measuring IFN-γ- and IL-4-levels after intraperitoneal injection of 5 µg of the acyl-elongated galactosylsphingamides 6-9d in mice (Fig. 8). Surprisingly, cytokine levels are still inferior to those observed for α-GalCer but increase slightly upon elongating the acyl chain. Overall, the introduction of an amide functionality in the phytosphingosine chain appears to result in poor iNKT-cell stimulation. The recruitment of spacer lipids occupying the A’ pocket was observed for 5a-d, but the spacer lipid had no impact on the antigenic potency of the galactosylsphingamides since the antigenic potency of 8d and 9d, which have no space to fit a spacer lipid, also perform poorly.
Figure 8.

IFN-γ and IL-4 secretion, measured at 16 h and 4 h respectively, after intraperitoneal injection of 5 µg of the glycolipids in mice. Data for one individual experiment using 8 mice for each glycolipid.
Structural data
To gain insights into the poor antigenic potency of the glycolipids but high affinity TCR binding, we determined the crystal structure of the CD1d/5d-complex as well as that of the complex of CD1d with compound 23, which resembles 5d but lacks the amide group. As expected, 23 gives rise to well-defined electron density and is rigidly presented using the conserved H-bond network involving CD1d-residues Asp80 and the 3’- and 4’-OH of phytosphingosine, as well as CD1d Thr156 and the glycosidic oxygen, and most importantly, CD1d-residue Asp153 and the 2”-, and 3”-OH of the galactose (Fig. 9A–D). Surprisingly, galactosylsphingamide 5d is presented less well ordered by CD1d, as judged by the less contoured electron density (Fig. 9A,B). This slight disorder of the galactose presentation correlates well with the increase in the H-bond distance between CD1d and 5d. Especially the 2”-OH interaction with CD1d Asp153 is increased compared to 23 (3.0 vs. 2.6 Å, Fig. 9C,D), while the 3’-OH of phytosphingosine is also less intimately contacted by CD1d Asp80 (3.1 vs. 2.5 Å). Notewhorthy, the computationally modelled hydrogen bond between the amide oxygen and Tyr73 was not observed in the glycolipid/CD1d crystal structure. When looking down into the binding groove of CD1d (TCR view), we noticed lack of the F’-roof closure by 5d, while 23 induced the F’-roof formation (Fig. 9E,F). This is likely the result of the amide group perturbing the hydrophobic nature of the F’-pocket in general. Based on our previous work on microbial glycolipids, we postulated that the TCR-dissociation rate is increased for glycolipids that do not close the F’-roof before TCR-binding38. However, since we have not observed any differences in the binding kinetics, especially the dissociation rate, we speculate that a compensatory mechanism will stabilize the TCR-interaction and this could likely be driven by the amide group and a novel interaction within the F’-pocket of CD1d. This will have to be investigated in the future using TCR-co-crystal structures.
Figure 9.

(A,B) Electron density map of 5d and 23 with CD1d. Spacer lipid (palmitic acid) is depicted in orange. (C,D) H-bond interactions between 5d and 23 (yellow) with CD1d (grey). (E,F) Glycolipid ligand presentation shown in “TCR view” from top with molecular surface of CD1d in grey and ligands as yellow sticks.
We next compared the precise presentation of both ligand headgroups by CD1d. As suggested by the changes in hydrogen bond distances, the galactose of 5d is slightly elevated compared to 23, correlating with the increased distance in H-bonding interactions with CD1d (Fig. 10).
Figure 10.

Comparison between 5d (green) and 23 (grey)presented by CD1d.
This slightly flexible presentation of 5d is reminiscent to the presentation of the microbial glycosphingolipid GalA-GSL, which was also slightly shifted in the binding groove of CD1d compared to 22 but had comparable TCR association rates39. Therefore, the fine presentation of the galactose is likely not the reason for the biological inactivity of these galactosylsphingamides.
Since the only difference between both glycolipids is the presence (5d) or absence (23) of the amide group, this indicates that the amide is indeed responsible for the slight elevation of the galactose but more importantly for the failure in closure of the F’ roof in the binary complex, the latter of which is likely the main reason for the low antigenicity of the α-galactosylsphingamides (Fig. 10).
Conclusion
In summary, a series of α-GalCer analogues featuring an amide bond in the phytosphingosine were synthesized. The presented synthesis allows to alter an advanced methyl ester precursor after glycosylation, thereby reducing the number of linear steps towards the aimed α-GalCer analogues. Furthermore, the ability to diversify the acyl moiety in the penultimate step of the synthesis adds to the versatility of the presented synthesis route. Also, methyl ester 10 is an interesting intermediate for alternative derivatization of the phytosphingosine chain.
Biological evaluation revealed that the novel α-galactosylsphingamides are weak iNKT-cell antigens and that the main compromising factor is the implemented amide functionality. While extending the acyl chain offered partial restoration of the antigenic effect, overall iNKT-cell stimulation remained poor.
Crystal structure analysis revealed a slightly flexible presentation of the α-galactosylsphingamides to CD1d, where the sugar head group is slightly shifted in the CD1d binding groove. A similar binding has been observed for the microbial glycosphingolipid GalA-GSL, but never for known α-GalCer analogues39. These crystal structures support the hypothesis that although both the acyl chain and phytosphingosine contribute to the stability of the CD1d/glycolipid complex, it is the phytosphingosine chain that controls the CD1d/glycolipid footprint. How the amide group affects the biological activity of these compounds and how its presence compensates for the lack of the pre-formed F’ roof formation upon TCR binding is currently unknown and will have to be characterized by determining CD1d-galactosylsphingamide-TCR structures.
Experimental part
General
Precoated Macherey-Nagel SIL G/UV254 plates were used for TLC, and spots were examined under UV light at 254 nm and further visualized by sulfuric acid-anisaldehyde spray or by spraying with a solution of (NH4)6Mo7O24∙4 H2O (25 g/L) and (NH4)4Ce(SO4)4∙2 H2O (10 g/L) in H2SO4 (10%) followed by charring. Column chromatography was performed on Biosolve silica gel (32–63 µm, 60 Å). NMR spectra were obtained with a Varian Mercury 300 Spectrometer. Chemical shifts are given in ppm (δ) relative to the residual solvent signals, in the case of CDCl3: δ = 7.26 ppm for 1H and δ = 77.4 ppm for 13C and in the case of pyridine-d5: δ = 8.74, 7.58 and 7.22 ppm for 1H and δ = 149.9, 135.5 and 123.5 ppm for 13C. Exact mass measurements were performed on a Waters LCT Premier XE TOF equipped with an electrospray ionization interface and coupled to a Waters Alliance HPLC system. Samples were infused in a in a CH3CN/HCOOH (1000:1) mixture at 100 µL/min.
3,4,6-Tri-O-benzyl-D-galactal (17)
3,4,6-Tri-O-acetyl-d-galactal (22.05 g, 81 mmol) was dissolved in MeOH (250 mL), Et3N (67 mL) and H2O (8 mL) and the reaction mixture was stirred for 5 days at room temperature. After completion of the reaction the solvent was removed under reduced pressure. The crude product was dried by making azeotropic mixture with toluene to afford pure d-galactal in quantitative yield. This material was dissolved in dry DMF (250 mL) and cooled to 0 °C. Sodium hydride (60% dispersion, 13 g, 324 mmol) was added portionwise over a period of 15 min. After 30 minutes benzyl bromide (38.8 mL, 324 mmol) was added dropwise and the reaction mixture was stirred overnight, allowing the temperature to rise to room temperature. Upon completion of the reaction, the reaction mixture was quenched with MeOH at 0 °C. The mixture was extracted with EtOAc (4 × 200 mL) and the combined organic layers were washed with brine, dried over anhydrous Na2SO4 and the solvents were evaporated under reduced pressure. The crude was purified by silica gel chromatography (hexane/EtOAc: 9/1) furnishing 3,4,6-tri-O-benzyl-d-galactal (31.67 g, 94%) as a white solid.
1H-NMR (300 MHz, CDCl3): δ 3.65 (dd, J = 10.1 and 5.1 Hz, 1 H, Ha-6) 3.78 (dd, J = 10.1 and 7.2 Hz, 1 H, Hb-6) 3.92–3.96 (m, 1 H, H-4) 4.15–4.21 (m, 2 H, H-3 and H-5) 4.41 (d, J = 12.2, 1 H, CH 2Ph) 4.50 (d, J = 12.2, 1 H, CH 2Ph) 4.57–4.68 (m, 3 H, H-2, CH 2Ph) 4.82–4.90 (m, 2 H, H-2, CH 2Ph) 6.36 (d, J = 6.4 Hz, 1 H, H-1) 7.24–7.36 (m, 15 H, arom. H).
13C-NMR (75 MHz, CDCl3): δ 68.38, 70.70, 70.82, 71.21, 73.26, 73.35, 75.63, 99.90, 127.37, 127.48, 127.62, 127.83, 128.08, 128.24, 128.32, 137.94, 138.30, 138.44, 144.12.
Exact mass (ESI-MS) for C27H28NaO4 [M + Na]+ found, 439.1878; calcd, 439.1880.
2-Deoxy-3,4,6-tri-O-benzyl-D-galactose (14)
To a solution of 3,4,6-tri-O-benzyl-d-galactal (31.7 g, 76 mmol) in DMF (250 mL) maintained at 0 °C, H2SO4 (80 mL, 4.0 M) was added dropwise. The reaction mixture was allowed to rise to room temperature and was stirred for 16 h. Upon completion of the reaction, the mixture was neutralized with NaHCO3 (400 mL, sat. aq.) and extracted with Et2O (3 × 200 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered and evaporated. The crude residue was purified by silica gel chromatography (hexane/EtOAc: 85/15) furnishing 14 (28.13 g, 85%) as a white solid.
1H-NMR (α-anomer) (300 MHz, CDCl3): δ 1.90–2.09 (m, 1 H, Ha-2) 2.23 (td, J = 12.4, 3.6 Hz, 1 H, Hb-2) 3.41–3.73 (m, 2 H, CH2-6) 3.73–3.92 (m, 1 H, H-5) 4.00 (ddd, J = 12.0, 4.6 and 2.5 Hz, 1 H, H-3) 4.14 (t, J = 6.5 Hz, 1 H, H-4) 4.44 (d, J = 12.0 Hz, 1 H, CH 2Ph) 4.52 (d, J = 12.0 Hz, 1 H, CH 2Ph) 4.59–4.66 (m, 3 H, CH 2Ph) 4.94 (d, J = 11.8 Hz, 1 H, CH 2Ph) 5.47 (d, J = 3.1 Hz, 1 H, H-1) 7.20–7.43 (m, 15 H, arom. H).
13C-NMR (75 MHz, CDCl3): δ 30.92, 34.24, 69.0, 69.79, 70.08, 70.31, 71.65, 72.97, 73.29, 73.99, 74.19, 92.39, 94.54, 127.16, 127.40, 127.44, 127.53, 127.61, 128.02, 128.08, 128.22, 128.27, 137.67, 138.36, 138.45.
Exact mass (ESI-MS) for C27H30NaO5 [M + Na]+ found, 457.1987; calcd, 457.1985.
Data consistent with literature40.
(5R,6S,7R,E)-methyl 5,6,8- tri-O-benzyl-7-hydroxyoct-2-enoate (18)
2-Deoxy-3,4,6-tri-O-benzyl-d-galactose 14 (3.7 g, 8.52 mmol) and methyl(triphenylphosphoranylidene)acetate (5.68 g, 17.04 mmol) were dissolved in anhydrous toluene (150 mL) and stirred for 6 h at 85 °C. The reaction mixture was evaporated under reduced pressure, and the residue was purified by silica gel chromatography (hexane/EtOAc: 8/2) to give 18 (3.29 g, 79%) as a slightly yellow oil.
1H-NMR (300 MHz, CDCl3): δ 2.58 (ddd, J = 7.2, 5.7 and 1.3 Hz, 2 H, H-4) 3.48–3.60 (m, 2 H, H-8) 3.62–3.70 (m, 1 H, H-6) 3.73 (s, 3 H, OCH3) 3.76–3.84 (m, 1 H, H-5) 3.96–4.05 (m, 1 H, H-7) 4.49–4.52 (m, 2 H, CH 2Ph) 4.55–4.57 (m, 3 H, CH 2Ph) 4.67 (d, J = 11.4 Hz, 1 H, CH 2Ph) 5.88 (dt, J = 15.7 and 1.3 Hz, 1 H, H-2) 7.01 (dt, J = 15.7 and 7.4 Hz, 1 H, H-3) 7.23–7.40 (m, 15 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 33.81, 51.44, 69.66, 71.08, 72.56, 73.44, 74.06,78.48, 79.00, 123.21, 127.77, 127.86, 127.90, 127.94, 127.99, 128.07, 128.42, 128.45, 137.68, 137.75, 137.87, 145.57, 166.64.
Exact mass (ESI-MS) for C30H35O6 [M + H]+ found, 491.2427; Calcd., 491.2428.
(5R,6S,7R)-methyl 5,6,8- tri-O-benzyl -7-hydroxyoctanoate (13)
NiCl2.6 H2O (800 mg, 3.36 mmol) and NaBH4 (508 mg, 13.42 mmol) were added to a stirred solution of unsaturated ester 18 (3.29 g, 6.71 mmol) in MeOH (37 mL) and THF (3.7 mL) at 0 °C. After 1 h, the reaction mixture was filtered through Celite and the filter cake rinsed with Et2O. Evaporation of the filtrate yielded 13 (3.09 g, 94% yield) as a yellow oil which could be used in the next step without further purification.
1H-NMR (300 MHz CDCl3) δ 1.52–1.88 (m, 4 H, H-2 and H-4) 2.16–2.50 (m, 2 H, H-3) 3.01 (br. s, 1 H, -OH) 3.55 (d, J = 5.8 Hz, 2 H, H-8) 3.60–3.73 (m, 5 H, OCH3, H-5 and H-6) 3.98–4.05 (m, 1 H, H-7) 4.51–4.54 (m, 3 H, CH 2Ph) 4.56–4.58 (m, 1 H, CH 2Ph) 4.66 (d, J = 11.4 Hz, 1 H, CH 2Ph) 4.72 (d, J = 11.4 Hz, 1 H, CH 2Ph) 7.25–7.41 (m, 15 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 20.99, 30.27, 33.94, 51.48, 69.86, 71.06, 72.71, 73.42, 73.78, 78.97, 79.41, 127.71, 127.74, 127.85, 127.89, 127.96, 128.09, 128.38, 128.41, 138.03, 138.08, 173.83.
Exact mass (ESI-MS) for C30H36KO6 [M + K]+ found, 531.2151; Calcd., 531.2143.
(5R,6S,7S)-methyl 7-azido-5,6,8- tri-O-benzyl-octanoate (19)
To a solution of compound 13 (3.09 g, 6.27 mmol) in anhydrous THF (100 mL) were added PPh3 (3.29 g, 12.54 mmol), diethylazodicarboxylate (2.18 g, 5.7 mL, 12.54 mmol), and diphenylphosphorylazide (3.45 g, 2.71 mL, 12.54 mmol) at −20 °C. After addition, the reaction mixture was allowed to warm to room temperature and stirred for 7 h. The solvent was evaporated under reduced pressure to give an orange residue. Purification by silica gel chromatography (hexane/EtOAc: 9/1) afforded 19 (3.19 g, 95% yield) as a yellow oil.
1H-NMR (300 MHz CDCl3) δ 1.52–1.86 (m, 4 H, H-2 and H-4) 2.18–2.39 (m, 2 H, H-3) 3.55–3.83 (m, 8 H, OCH3, H-5, H-6, H-7 and H-8) 4.57–4.62 (m, 3 H, CH 2Ph) 4.65 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.68 (d, J = 11.3 Hz, 1 H, CH 2Ph) 4.78 (d, J = 11.3 Hz, 1 H, CH 2Ph) 7.18–7.46 (m, 15 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 20.72, 29.07, 33.94, 51.47, 61.93, 70.08, 71.95, 73.34, 73.83, 78.65, 78.74, 120.18, 120.24, 126.09, 127.66, 127.69, 127.71, 127.77, 127.91, 128.00, 128.37, 128.38, 128.41, 130.03, 130.05, 137.81, 137.91, 138.09, 173.84.
Exact mass (ESI-MS) for C30H35KN3O5 [M + K]+ found, 556.2210; Calcd., 556.2208.
(5R,6S,7S)-methyl 7-azido-5,6-di-O-benzyl-8-hydroxyoctanoate (12)
Azide 19 (1.4 g, 2.7 mmol) and ZnCl2 (7.38 g, 54 mmol) were dissolved in Ac2O/AcOH (2:1, 21 mL). The reaction was allowed to stir for 3.5 h at room temperature. Next H2O (10 mL) was added and the reaction mixture was extracted with CH2Cl2. The combined organic layers were washed with H2O, NaHCO3 (sat. aq.) and brine. Then the organic phase was dried over Na2SO4, filtered, and evaporated to afford 598 mg of crude acetate. The crude acetate was dissolved in MeOH (20 mL) and NaOMe (5.4 M solution in MeOH) was added till pH 10. After stirring overnight, Amberlite 120R (H+ form) was added to neutralize the reaction mixture. The mixture was diluted with MeOH (20 mL) and the exchange resin was filtered off and rinsed with MeOH. Evaporation of the filtrate under reduced pressure yielded a solid residue that was purified by silica gel chromatography (hexane/EtOAc: 7/3) to afford 12 (771 mg, 67% yield) as a colorless oil.
1H-NMR (300 MHz CDCl3) δ 1.56–1.87 (m, 4 H, H-5 and H-7) 2.25–2.34 (m, 2 H, H-6) 2.38 (t, J = 6.3 Hz, 1 H, OH) 3.58–3.96 (m, 8 H, OCH3, H-1, H-2, H-3 and H-4) 4.58 (dd, J = 20.1 and 11.4 Hz, 2 H, CH 2Ph) 4.68 (dd, J = 17.8 and 11.2 Hz, 2 H, CH 2Ph) 7.24–7.41 (m, 10 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 20.79, 29.38, 33.82, 51.51, 62.31, 63.11, 72.38, 73.71, 78.60, 79.84, 127.88, 128.00, 128.02, 128.11, 128.46, 128.52, 137.53, 137.75, 173.77.
Exact mass (ESI-MS) for C23H30N3O5 [M + H]+ found, 428.2177; Calcd., 428.2180.
(5R,6S,7S)-methyl-7-azido-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-octanoate (10)
To a mixture of 11 (670 mg, 1.13 mmol) in THF (9 mL), a solution of 12 (323 mg, 0.76 mmol) in THF (6 mL) was added. The reaction mixture was cooled to −30 °C and TMSOTf (0.3 mL, 0.11 mmol) was added dropwise. After stirring for 2 h. at −30 °C, the reaction mixture was neutralized with Et3N and evaporated to dryness. The residue was purified by silica gel chromatography (hexane/EtOAc: 8/2 + 1% v/v Et3N) to afford 10 (438 mg, 67% yield) as colorless liquid which solidified upon storage.
1H-NMR (300 MHz CDCl3) δ 1.46–1.79 (m, 4 H, CH2) 2.20 (t, J = 6.6 Hz, 2 H, CH2) 3.52 (br s., 1 H, H-4”) 3.56–3.71 (m, 7 H, H-2, H-2”, H-4, Hb-6”, OCH3) 3.84 (dd, J = 12.5 and 1.5 Hz, 1 H, H-3”) 3.93 (dd, J = 9.8 and 2.9 Hz, 1 H, Ha-1) 3.97–4.06 (m, 3 H, Hb-1, H-3 and Hb-6”) 4.13 (dd, J = 3.2 and 1.0 Hz, 1 H, H-5”) 4.43 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.50–4.63 (m, 4 H, CH 2Ph) 4.69 (d, J = 12.3 Hz, 1 H, CH 2Ph) 4.76 (d, J = 12.5 Hz, 1 H, CH 2Ph) 4.80 (d, J = 11.8 Hz, 1 H, CH 2Ph) 4.92 (d, J = 3.2 Hz, 1 H, H-1”) 5.41 (s, 1 H, H-8”) 7.14–7.36 (m, 23 H, arom. H) 7.44–7.48 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 20.83, 29.23, 33.89, 51.47, 61.65, 63.00, 68.31, 69.31, 71.95, 72.00, 73.54, 73.84, 74.59, 75.41, 77.42, 78.42, 78.95, 99.14, 101.05, 126.33, 127.46, 127.50, 127.65, 127.69, 127.79, 127.83, 127.92, 128.09, 128.22, 128.26, 128.39, 128.85, 137.82, 137.91, 138.11, 138.73, 138.75, 173.80.
Exact mass (ESI-MS) for C50H56N3O10 [M + H]+ found, 858.3948; Calcd., 858.3960.
General procedure for AlMe3 assisted amide coupling
To a solution of glycoside 10 (500 mg, 0.58 mmol) in anhydrous CH2Cl2 (10 mL) was added the appropriate amine (5 eq.) and AlMe3 (2 M in heptanes) (2.5 eq.). The reaction mixture was stirred overnight at reflux temperature. Next the reaction mixture was cooled to 0 °C and water was added. The mixture was extracted with CH2Cl2 and the combined organic phase was washed with H2O and brine, dried over Na2SO4, filtered, and evaporated. Purification by column chromatography gave the desired amides 20a (61%), 20b (64%), 20c (64%), 20d (52%), 20e (51%), 20 f (68%), 20 g (81%), 20 h (60%).
(5R,6S,7S)-7-azido-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-N-nonyloctanamide (20a)
1H-NMR (300 MHz CDCl3) δ 0.89 (t, J = 6.5 Hz, 3 H, terminal CH3) 1.23–1.35 (m, 12 H, CH2) 1.45 (dt, J = 13.4 and 7.8 Hz, 2 H, CH2) 1.53–1.81 (m, 4 H, CH2) 2.09 (t, J = 6.6 Hz, 2 H, CH2) 3.14–3.24 (m, 2 H, NHCH 2) 3.59–3.81 (m, 5 H, H-2, H-3, H-4, H-5” and Ha-6”) 3.93 (dd, J = 12.6 and 1.5 Hz, 1 H, Ha-1) 3.98–4.04 (m, 1 H, Hb-6”) 4.06 (d, J = 3.2 Hz, 1 H, H-3”) 4.08–4.14 (m, 2 H, Hb-1 and H-2”) 4.20 (dd, J = 3.5 and 1.2 Hz, 1 H, H-4”) 4.49 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.59–4.72 (m, 4 H, CH 2Ph) 4.75 (d, J = 12.3 Hz, 1 H, CH 2Ph) 4.82 (d, J = 12.3 Hz, 1 H, CH 2Ph) 4.88 (d, J = 12.0 Hz, 1 H, CH 2Ph) 4.98 (d, J = 3.3 Hz, 1 H, H-1”) 5.32 (t, J = 5.1 Hz, 1 H, (CO)NH) 5.48 (s, 1 H, H-8”) 7.22–7.43 (m, 23 H, arom. H) 7.51–7.56 (m, 2 H, arom. H). 13C NMR (75 MHz, CDCl3) δ 14.10, 21.81, 22.65, 26.93, 29.25, 29.29, 29.50, 29.67, 31.84, 36.57, 39.50, 61.55, 63.03, 68.35, 69.33, 71.96, 72.02, 73.59, 73.87, 74.58, 75.38, 75.84, 78.35, 79.20, 99.19, 101.06, 126.34, 127.47, 127.52, 127.68, 127.71, 127.75, 127.78, 127.89, 127.98, 128.11, 128.23, 128.27, 128.40, 128.43, 128.49, 128.87, 137.83, 137.98, 138.14, 138.74, 172.41.
Exact mass (ESI-MS) for C58H76N5O9 [M + NH4]+ found, 986.5641; Calcd., 986.5638.
(5R,6S,7S)-7-azido-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-N-2-phenylethyl)octanamide (20b)
1H-NMR (300 MHz CDCl3) δ 1.52–1.76 (m, 4 H, CH2) 2.02–2.07 (m, 2 H, (CO)CH2) 2.73 (t, J = 6.9 Hz, 2 H, H-11) 3.44 (q, J = 6.8 Hz, 2 H, NHCH 2) 3.56 (app. s, 1 H, H-2) 3.57–3.63 (m, 2 H, H-4 and H-3) 3.66–3.75 (m, 2 H, H-5” and Ha-6”) 3.88 (dd, J = 12.3 and 1.3 Hz, 1 H, H-3”) 3.95 (d, J = 3.3 Hz, 1 H, Ha-1) 3.97–4.06 (m, 2 H, H-2” and Hb-6”) 4.07–4.10 (m, 1 H, Hb-1) 4.15 (d, J = 2.9 Hz, 1 H, H-4”) 4.43 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.54–4.66 (m, 4 H, CH 2Ph) 4.71 (d, J = 12.4 Hz, 1 H, CH 2Ph) 4.78 (d, J = 12.4 Hz, 1 H, CH 2Ph) 4.83 (d, J = 11.8 Hz, 1 H, CH 2Ph) 4.93 (d, J = 3.2 Hz, 1 H, H-1”) 5.29 (t, J = 5.6 Hz, 1 H, (CO)NH) 5.43 (s, 1 H, H-8”) 7.12–7.38 (m, 28 H, arom. H) 7.48 (dd, J = 7.3 and 2.3 Hz, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 21.71, 29.25, 35.70, 36.49, 40.49, 61.55, 63.03, 68.34, 69.32, 71.94, 72.01, 73.57, 73.85, 74.56, 75.37, 75.83, 77.86, 78.28, 78.35, 78.70, 79.08, 79.63, 99.17, 101.04, 126.32, 126.49, 127.46, 127.50, 127.66, 127.69, 127.76, 127.87, 127.95, 128.09, 128.21, 128.26, 128.38, 128.40, 128.61, 128.72, 128.85, 128.94, 128.97, 129.15, 129.23, 129.37, 129.49, 129.54, 129.68, 129.89, 130.00, 137.81, 137.96, 138.12, 138.72, 138.87, 140.00, 172.47.
Exact mass (ESI-MS) for C57H62N4NaO9 [M + Na]+ found, 969.4413; Calcd., 969.4409.
(5R,6S,7S)-7-azido-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-N-(4-phenylbutyl)octanamide (20c)
1H-NMR (300 MHz CDCl3) δ 1.43–1.78 (m, 8 H, CH2) 2.07 (t, J = 6.8 Hz, 2 H, CH2) 2.62 (t, J = 7.5 Hz, 2 H, CH2) 3.21 (dd, J = 13.0 and 7.3 Hz, 2 H, NHCH 2) 3.56 (app. s, 1 H, H-2) 3.60–3.67 (m, 2 H, H-4 and Ha-6”) 3.69–3.79 (m, 2 H, H-5” and H-3) 3.91 (dd, J = 13.0 and 2.0 Hz, 1 H, Ha-1) 3.97–4.02 (m, 1 H, Hb-6”) 4.04 (d, J = 2.9 Hz, 1 H, H-3”) 4.07–4.10 (m, 2 H, H-2” and Hb-1) 4.18 (d, J = 2.9 Hz, 1 H, H-4”) 4.47 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.57–4.69 (m, 4 H, CH 2Ph) 4.74 (d, J = 12.6 Hz, 1 H, CH 2Ph) 4.81 (d, J = 12.6 Hz, 1 H, CH 2Ph) 4.86 (d, J = 11.9 Hz, 1 H, CH 2Ph) 4.98 (d, J = 3.2 Hz, 1 H, H-1”) 5.29 (t, J = 5.2 Hz, 1 H, (CO)NH) 5.47 (s, 1 H, H-8”) 7.14–7.41 (m, 28 H, arom. H) 7.49–7.53 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 21.79, 28.64, 29.22, 35.44, 36.54, 39.27, 61.55, 63.03, 68.35, 69.33, 71.95, 72.02, 73.59, 73.87, 74.57, 75.38, 75.84, 77.21, 78.32, 79.18, 99.18, 101.05, 125.82, 126.33, 127.47, 127.52, 127.67, 127.70, 127.75, 127.77, 127.87, 127.98, 128.10, 128.22, 128.27, 128.33, 128.37, 128.39, 128.42, 128.86, 137.81, 137.98, 138.11, 138.72, 142.05, 172.45.
Exact mass (ESI-MS) for C59H67N4O9 [M + H]+ found, 975.4905; Calcd., 975.4903.
(5R,6S,7S)-7-azido-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-N-(6-phenylhexyl)octanamide (20d)
1H-NMR (300 MHz CDCl3) δ 1.26–1.48 (m, 6 H, CH2) 1.52–1.78 (m, 6 H, CH2) 2.08 (t, J = 6.7 Hz, 2 H, CH2) 2.59 (t, J = 7.5 Hz, 2 H, CH2) 3.18 (dd, J = 13.4 and 7.2 Hz, 2 H, NHCH 2) 3.59 (app. s, 1 H, H-2) 3.61–3.67 (m, 2 H, H-3 and H-4) 3.68–3.79 (m, 2 H, H-5” and Ha-6”) 3.91 (dd, J = 12.7 and 1.1 Hz, 1 H, Ha-1) 3.98–4.02 (m, 1 H, Hb-6”) 4.04 (d, J = 3.5 Hz, 1 H, H-3”) 4.07–4.12 (m, 2 H, Hb-1 and H-2”) 4.19 (d, J = 2.7 Hz, 1 H, H-4”) 4.47 (d, J = 11.7 Hz, 1 H, CH 2Ph) 4.57–4.72 (m, 4 H, CH 2Ph) 4.75 (d, J = 12.4 Hz, 1 H, CH 2Ph) 4.81 (d, J = 12.4 Hz, 1 H, CH 2Ph) 4.86 (d, J = 12.1 Hz, 1 H, CH 2Ph) 4.97 (d, J = 3.5 Hz, 1 H, H-1”) 5.30 (t, J = 5.4 Hz, 1 H, (CO)NH) 5.47 (s, 1 H, H-8”) 7.15–7.42 (m, 28 H, arom. H) 7.50–7.54 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 21.80, 26.77, 28.89, 29.23, 29.58, 31.31, 35.84, 36.55, 39.43, 61.54, 63.02, 68.34, 69.32, 71.95, 72.02, 73.57, 73.86, 74.56, 75.37, 75.83, 77.20, 78.33, 79.19, 99.17, 101.04, 125.62, 126.32, 127.46, 127.51, 127.67, 127.70, 127.74, 127.76, 127.87, 127.96, 128.09, 128.22, 128.24, 128.26, 128.35, 128.38, 128.42, 128.86, 137.81, 137.97, 138.11, 138.71, 142.59, 172.42.
Exact mass (ESI-MS) for C61H71N4O9 [M + H]+ found, 1003.5232; Calcd., 1003.5216.
(5R,6S,7S)-7-azido-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-N-(8-phenyloctyl)octanamide (20e)
1H-NMR (300 MHz CDCl3) δ 1.23–1.49 (m, 10 H, CH2) 1.51–1.78 (m, 6 H, CH2) 2.08 (t, J = 6.0 Hz, 2 H, CH2) 2.59 (t, J = 7.5 Hz, 2 H, CH2) 3.18 (dd, J = 13.0 and 6.5 Hz, 2 H, NHCH 2) 3.59 (app. s, 1 H, H-2) 3.61–3.68 (m, 2 H, H-3 and H-4) 3.68–3.80 (m, 2 H, H-5” and Ha-6”) 3.91 (dd, J = 12.4 and 1.4 Hz, 1 H, Ha-1) 4.98–4.03 (m, 1 H, Hb-6”) 4.04 (d, J = 3.2 Hz, 1 H, H-3”) 4.06–4.13 (m, 2 H, H-2” and Hb-1) 4.19 (dd, J = 3.1 and 0.9 Hz, 1 H, H-4”) 4.47 (d, J = 11.4 Hz, 1 H, CH 2Ph) 4.56–4.71 (m, 4 H, CH 2Ph) 4.75 (d, J = 12.4 Hz, 1 H, CH 2Ph) 4.82 (d, J = 12.4 Hz, 1 H, CH 2Ph) 4.86 (d, J = 11.8 Hz, 1 H, CH 2Ph) 4.98 (d, J = 3.2 Hz, 1 H, H-1”) 5.31 (t, J = 5.1 Hz, 1 H, (CO)NH) 5.47 (s, 1 H, H-8”) 7.14–7.43 (m, 28 H, arom. H) 7.49–7.54 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 21.82, 26.90, 29.22, 29.39, 29.66, 31.46, 35.95, 36.58, 39.48, 61.55, 63.03, 68.36, 69.34, 71.96, 72.04, 73.58, 73.87, 74.59, 75.38, 75.84, 77.21, 78.34, 79.21, 99.20, 101.06, 110.00, 125.57, 126.34, 127.48, 127.53, 127.68, 127.71, 127.76, 127.79, 127.89, 127.98, 128.11, 128.23, 128.27, 128.40, 128.43, 128.87, 137.83, 137.98, 138.14, 138.73, 142.82, 172.42.
Exact mass (ESI-MS) for C63H75N4O9 [M + H]+ found, 1031.5575; Calcd., 1031.5529.
(5R,6S,7S)-7-azido-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-N-(3-pentylphenyl)octanamide (20f)
1H-NMR (300 MHz CDCl3) δ 0.88 (t, J = 6.7 Hz, 3 H, terminal CH3) 1.26–1.36 (m, 4 H, CH2) 1.54–1.65 (m, 4 H, CH2) 1.70–1.87 (m, 2 H, CH2) 2.26 (t, J = 6.9 Hz, 2 H, CH2) 2.56 (t, J = 8.0 Hz, 2 H, CH2) 3.54–3.59 (m, 2 H, H-2 and H-3) 3.62–3.69 (m, 2 H, H-4 and Ha-6”) 3.75 (dd, J = 6.7 and 3.6 Hz, 1 H, H-5”) 3.85 (dd, J = 12.4 and 1.2 Hz, 1 H, Ha-1) 3.93–4.07 (m, 4 H, H-2”, H-3”, Hb-1 and Hb-6”) 4.13 (d, J = 2.7 Hz, 1 H, H-4”) 4.43 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.64–4.52 (m, 4 H, CH 2Ph) 4.68 (d, J = 12.5 Hz, 1 H, CH 2Ph) 4.75 (d, J = 12.5 Hz, 1 H, CH 2Ph) 4.81 (d, J = 11.8 Hz, 1 H, CH 2Ph) 4.91 (d, J = 3.2 Hz, 1 H, H-1”) 5.40 (s, 1 H, H-8”) 6.85 (d, J = 7.2 Hz, 1 H, arom. H) 6.99 (s, 1 H, (CO)NH) 7.08–7.37 (m, 26 H, arom. H) 7.46 (dd, J = 7.3 and 2.2 Hz, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 14.03, 21.78, 22.52, 28.95, 31.09, 31.52, 35.93, 37.45, 61.49, 63.04, 68.27, 69.32, 71.91, 72.06, 73.61, 73.95, 74.54, 75.38, 75.84, 77.21, 78.19, 79.37, 99.17, 101.05, 116.85, 116.95, 119.63, 124.29, 126.33, 127.48, 127.52, 127.67, 127.71, 127.79, 127.85, 127.94, 128.10, 128.23, 128.27, 128.39, 128.50, 128.75, 128.86, 137.78, 137.82, 137.91, 138.01, 138.72, 143.97, 170.72.
Exact mass (ESI-MS) for C60H68N4NaO9 [M + Na]+ found, 1011.4886; Calcd., 1011.4879.
(5R,6S,7S)-7-azido-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-N-(4-pentylphenyl)octanamide (20g)
1H-NMR (300 MHz CDCl3) δ 0.88 (t, J = 6.8 Hz, 3 H, terminal CH3) 1.29–1.31 (m, 3 H, CH2) 1.55–1.67 (m, 5 H, CH2) 1.71–1.89 (m, 2 H, CH2) 2.26 (t, J = 6.8 Hz, 2 H, CH2) 2.57 (t, J = 7.6 Hz, 2 H, CH2) 3.59–3.67 (m, 2 H, H-2 and H-5”) 3.67–3.77 (m, 2 H, H-4 and Ha-6”) 3.82 (dd, J = 6.8 and 3.6 Hz, 1 H, H-3) 3.92 (dd, J = 12.5 and 1.5 Hz, 1 H, Ha-1) 3.98–4.14 (m, 4 H, Hb-1, H-2”, H-3” and Hb-6”) 4.20 (d, J = 2.5 Hz, 1 H, H-4”) 4.50 (d, J = 11.4 Hz, 1 H, CH 2Ph) 4.58–4.72 (m, 4 H, CH 2Ph) 4.75 (d, J = 12.3 Hz, 1 H, CH 2Ph) 4.82 (d, J = 12.3 Hz, 1 H, CH 2Ph) 4.88 (d, J = 11.7 Hz, 1 H, CH 2Ph) 4.98 (d, J = 3.2 Hz, 1 H, H-1”) 5.47 (s, 1 H, H-8”) 7.04–7.14 (m, 3 H, arom. H) 7.22–7.43 (m, 25 H, (CO)NH and arom. H) 7.51–7.56 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 14.02, 21.77, 22.52, 28.96, 31.18, 31.41, 35.30, 37.36, 61.50, 63.03, 68.26, 69.32, 71.91, 72.05, 73.60, 73.93, 74.54, 75.38, 75.83, 77.20, 78.21, 79.30, 99.16, 101.04, 119.77, 126.33, 127.48, 127.51, 127.67, 127.71, 127.78, 127.84, 127.94, 128.10, 128.22, 128.26, 128.39, 128.49, 128.81, 128.86, 135.43, 137.82, 137.91, 138.01, 138.71, 138.91, 170.66.
Exact mass (ESI-MS) for C60H68N4NaO9 [M + Na]+ found, 1011.4874; Calcd., 1011.4879.
(5R,6S,7S)-7-azido-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-N-(2-(2-ethoxyethoxy)ethyl)octanamide (20 h)
1H-NMR (300 MHz CDCl3) δ 1.17 (t, J = 7.0 Hz, 3 H, terminal CH3) 1.46–1.75 (m, 4 H, CH2) 1.99–2.08 (m, 2 H, CH2) 3.39 (q, 2 H, J = 5.2 Hz, NHCH 2) 3.43–3.62 (m, 11 H, CH2, H-2, H-3 and H-4) 3.63–3.73 (m, 2 H, H-5” and Ha-6”) 3.85 (dd, J = 12.6 and 1.8 Hz, 1 H, Ha-1) 3.91–3.97 (m, 1 H, Hb-6”) 3.99 (d, J = 3.0 Hz, 1 H, H-3”) 4.00–4.07 (m, 2 H, Hb-1 and H-2”) 4.13 (d, J = 3.0 Hz, 1 H, H-4”) 4.43 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.54 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.59–4.64 (m, 3 H, CH 2Ph) 4.69 (d, J = 12.6 Hz, 1 H, CH 2Ph) 4.76 (d, J = 12.6 Hz, 1 H, CH 2Ph) 4.81 (d, J = 11.8 Hz, 1 H, CH 2Ph) 4.92 (d, J = 3.2 Hz, 1 H, H-1”) 5.41 (s, 1 H, H-8”) 5.86 (t, J = 5.5 Hz, 1 H, (CO)NH) 7.16–7.37 (m, 23 H, arom. H) 7.44–7.48 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 15.60, 22.06, 29.82, 36.89, 39.50, 62.02, 63.43, 67.07, 68.78, 69.75, 70.14, 70.29, 70.71, 72.39, 72.45, 73.97, 74.26, 75.01, 75.82, 76.24, 77.63, 78.87, 79.50, 99.57, 101.47, 126.76, 127.88, 127.93, 128.10, 128.12, 128.19, 128.33, 128.52, 128.64, 128.69, 128.81, 128.82, 129.27, 138.25, 138.40, 138.58, 139.16, 172.98.
Exact mass (ESI-MS) for C55H67N4O11 [M + H]+ found, 959.4799; Calcd., 959.4801.
Representative procedure for Staudinger reduction and acylation with EDC
To a solution of azide 20c (350 mg, 0.36 mmol, 1eq.) in THF (5 mL) at room temperature, PMe3 (1 M solution of in THF, 5.38 mL, 5.38 mmol, 15 eq.) was added dropwise. After stirring for 3 h at room temperature, H2O (2 mL) was added and the reaction mixture was allowed to stir overnight at room temperature. Then the solvent was removed under reduced pressure. The crude product was dried by making azeotropic mixture with toluene to afford the crude amine. A mixture of the crude amine, EDC (112 mg, 0.72 mmol, 2 eq.) and octanoic acid (78 mg, 0.09 mL, 0.54 mmol, 1.5 eq.) in CH2Cl2 (10 mL) was stirred for 24 h at room temperature. The reaction mixture was diluted with CH2Cl2, washed with H2O (2 × 10 mL) and brine (1 × 10 mL), dried over Na2SO4, filtered, and evaporated to dryness. Purification by silica gel chromatography gave the desired the desired amides. 21a (74%), 21b (76%), 21c (82%), 21d (80%), 21e (81%), 21 f (87%), 21 g (87%), 21 h (70%).
(5R,6S,7S)-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-7-octanamido-N-nonyloctanamide (21a)
1H-NMR (300 MHz CDCl3) δ 0.85 (app. td, J = 6.7 and 2.8 Hz, 6 H, 2 x terminal CH3) 1.19–1.35 (m, 18 H, CH2) 1.39–1.54 (m, 4 H, CH2) 1.60–1.83 (m, 6 H, CH2) 1.90–1.97 (m, 2 H, CH2) 2.02–2.10 (m, 2 H, CH2) 3.17 (dd, J = 12.9 and 6.9 Hz, 2 H, NHCH 2)3.44 (m, 1 H, H-4) 3.52 (app. s, 1 H, H-5”) 3.74 (dd, J = 7.0 and 2.0 Hz, 1 H, H-3) 3.82 (d, J = 4.8 Hz, 2 H, H-1) 3.89–3.96 (m, 2 H, H-4” and Ha-6”) 4.04 (d, J = 3.6 Hz, 1 H, H-2”) 4.07–4.14 (m, 2 H, H-2 and Hb-6”) 4.18 (d, J = 3.2 Hz, 1 H, H-3”) 4.39 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.47 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.55 (d, J = 11.8 Hz, 1 H, CH 2Ph) 4.62 (d, J = 11.8 Hz, 1 H, CH 2Ph) 4.72–4.77 (m, 3 H, CH 2Ph) 4.84 (d, J = 11.8 Hz, 1 H, CH 2Ph) 4.93 (d, J = 3.4 Hz, 1 H, H-1”) 5.45 (s, 1 H, H-8”) 5.98 (d, J = 8.4 Hz, 1 H, NH(CO)) 6.05 (t, J = 5.7 Hz, 1 H, (CO)NH) 7.19–7.40 (m, 23 H, arom. H) 7.47–7.52(m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 14.09, 21.84, 22.64, 25.65, 26.99, 28.82, 29.07, 29.26, 29.33, 29.52, 29.60, 31.76, 31.84, 36.03, 36.65, 39.48, 50.02, 63.00, 68.08, 69.36, 71.52, 71.72, 73.28, 73.98, 74.18, 75.61, 76.10, 78.88, 99.60, 101.01, 126.27, 127.57, 127.63, 127.70, 127.75, 127.86, 127.89, 127.92, 128.11, 128.31, 128.38, 128.41, 128.88, 137.73, 138.23, 138.26, 138.39, 138.55, 172.81, 173.01.
Exact mass (ESI-MS) for C66H89N2O10 [M + H]+ found, 1069.6512; Calcd., 1069.6517.
(5R,6S,7S)-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-7-octanamido-N-(2-phenylethyl)octanamide (21b)
1H-NMR (300 MHz CDCl3) δ 0.89 (t, J = 6.6 Hz, 3 H, terminal CH3) 1.20–1.33 (m, 7 H, CH2) 1.44–1.82 (m, 7 H, CH2) 1.88–1.98 (m, 2 H, CH2) 2.02–2.07 (m, 2 H, CH2) 2.78 (t, J = 7.3 Hz, 2 H, CH2) 3.40–3.54 (m, 4 H, H-4, H-5” and CH2) 3.75 (dd, J = 7.0 and 2.4 Hz, 1 H, H-3) 3.79–3.94 (m, 3 H, H-1 and Ha-6”) 3.96 (d, J = 3.3 Hz, 1 H, H-4”) 4.07 (d, J = 3.3 Hz, 1 H, H-2”) 4.08–4.16 (m, 2 H, H-2 and Hb-6”) 4.18 (d, J = 3.2 Hz, 1 H, H-3”) 4.41 (d, J = 11.7 Hz, 1 H, CH 2Ph) 4.50 (d, J = 11.7 Hz, 1 H, CH 2Ph) 4.57 (d, J = 11.7 Hz, 1 H, CH 2Ph) 4.64 (d, J = 11.4 Hz, 1 H, CH 2Ph) 4.74–4.79 (m, 3 H, CH 2Ph) 4.86 (d, J = 11.3 Hz, 1 H, CH 2Ph) 4.96 (d, J = 3.6 Hz, 1 H, H-1”) 5.46 (s, 1 H, H-8”) 5.98 (d, J = 8.5 Hz, 1 H, NH(CO)) 6.14 (t, J = 5.6 Hz, 1 H, (CO)NH) 7.15–7.42 (m, 28 H, arom. H) 7.50–7.54 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 14.09, 21.77, 22.63, 25.67, 28.83, 29.08, 29.32, 31.77, 35.70, 36.00, 36.66, 40.60, 50.03, 62.99, 68.06, 69.35, 71.50, 71.73, 73.31, 74.02, 74.14, 75.64, 76.09, 76.57, 77.20, 77.43, 78.85, 78.88, 99.56, 101.01, 126.27, 126.33, 127.59, 127.64, 127.71, 127.79, 127.85, 127.91, 127.93, 128.11, 128.33, 128.36, 128.39, 128.42, 128.50, 128.71, 128.89, 137.73, 138.24, 138.27, 138.36, 138.54, 139.09, 172.92, 173.08.
Exact mass (ESI-MS) for C65H79N2O10 [M + H]+ found, 1047.5768; Calcd., 1047.5729.
(5R,6S,7S)-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-7-octanamido-N-(4-phenylbutyl)octanamide (21c)
1H-NMR (300 MHz CDCl3) δ 0.86 (t, J = 6.6 Hz, 3 H, terminal CH3) 1.17–1.28 (m, 9 H, CH2) 1.40–1.51 (m, 4 H, CH2) 1.53–1.81 (m, 7 H, CH2) 1.82–2.08 (m, 2 H, CH2) 2.57 (t, J = 7.5 Hz, 2 H, CH2) 3.19 (q, J = 6.6 Hz, 2 H, NHCH 2) 3.43–3.51 (m, 2 H, H-4 and H-5”) 3.72 (dd, J = 6.8 and 1.6 Hz, 1 H, H-3) 3.80 (d, J = 4.8 Hz, 2 H, H-1) 3.87–3.95 (m, 2 H, H-4” and Ha-6”) 4.04 (d, J = 3.2 Hz, 1 H, H-2”) 4.06–4.14 (m, 2 H, H-2 and Hb-6”) 4.17 (d, J = 3.0 Hz, 1 H, H-3”) 4.38 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.46 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.54 (d, J = 11.7 Hz, 1 H, CH 2Ph) 4.61 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.71–4.77 (m, 3 H, CH 2Ph) 4.84 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.92 (d, J = 3.4 Hz, 1 H, H-1”) 5.44 (s, 1 H, H-8”) 5.91 (d, J = 8.5 Hz, 1 H, NH(CO)) 6.08 (t, J = 5.4 Hz, 1 H, (CO)NH) 7.09–7.40 (m, 28 H, arom. H) 7.46–7.52 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 14.08, 21.80, 22.63, 25.65, 28.70, 28.77, 29.07, 29.18, 29.31, 31.76, 35.49, 35.96, 36.65, 39.24, 50.03, 63.00, 68.10, 69.36, 71.51, 71.74, 73.30, 73.99, 74.16, 75.61, 76.10, 77.20, 78.86, 99.64, 101.01, 125.72, 126.27, 127.56, 127.63, 127.72, 127.76, 127.88, 127.89, 127.91, 128.11, 128.27, 128.32, 128.34, 128.39, 128.42, 128.89, 129.96, 137.72, 138.22, 138.24, 138.39, 138.55, 142.18, 172.94, 173.06.
Exact mass (ESI-MS) for C67H83N2O10 [M + H]+ found, 1075.6052; Calcd., 1075.6042.
(5R,6S,7S)-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-7-octanamido-N-(6-phenylhexyl)octanamide (21d)
1H-NMR (300 MHz CDCl3) δ 0.86 (t, J = 6.6 Hz, 3 H, terminal CH3) 1.16–1.37 (m, 12 H, CH2) 1.38–1.83 (m, 10 H, CH2) 1.87–2.11 (m, 4 H, CH2) 2.56 (t, J = 7.5 Hz, 2 H, CH2) 3.16 (q, J = 7.5 Hz, 2 H, NHCH 2) 3.44–3.56 (m, 2 H, H-4 and H-5”) 3.74 (dd, J = 6.8 and 2.4 Hz, 1 H, H-3) 3.82 (d, J = 4.6 Hz, 2 H, H-1) 3.88–3.99 (m, 2 H, H-4” and Ha-6”) 4.05 (d, J = 3.3 Hz, 1 H, H-2”) 4.07–4.15 (m, 2 H, H-2 and Hb-6”) 4.18 (d, J = 3.2 Hz, 1 H, H-3”) 4.39 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.48 (d, J = 11.4 Hz, 1 H, CH 2Ph) 4.55 (d, J = 11.4 Hz, 1 H, CH 2Ph) 4.62 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.72–4.79 (m, 3 H, CH 2Ph) 4.84 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.93 (d, J = 3.5 Hz, 1 H, H-1”) 5.45 (s, 1 H, H-8”) 5.93 (d, J = 8.4 Hz, 1 H, NH(CO)) 6.04 (t, J = 5.7 Hz, 1 H, (CO)NH) 7.11–7.43 (m, 28 H, arom. H) 7.48–7.52 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 14.08, 21.83, 22.63, 25.65, 26.82, 28.83, 28.94, 29.07, 29.31, 29.53, 31.35, 31.75, 35.85, 36.03, 36.66, 39.42, 50.04, 63.00, 68.14, 69.36, 71.53, 71.75, 73.28, 73.96, 74.19, 75.61, 76.11, 77.21, 78.90, 99.66, 101.01, 125.58, 126.27, 127.55, 127.62, 127.71, 127.74, 127.88, 127.91, 128.11, 128.21, 128.32, 128.34, 128.39, 128.42, 128.89, 137.73, 138.23, 138.25, 138.42, 138.56, 142.64, 172.82, 173.00.
Exact mass (ESI-MS) for C69H86N2NaO10 [M + Na]+ found, 1125.6173; Calcd., 1125.6175.
(5R,6S,7S)-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-7-octanamido-N-(8-phenyloctyl)octanamide (21e)
1H-NMR (300 MHz CDCl3) δ 0.87 (t, J = 6.9 Hz, 3 H, terminal CH3) 1.17–1.35 (m, 14 H, CH2) 1.37–1.84 (m, 12 H, CH2) 1.87–2.00 (m, 2 H, CH2) 2.02–2.11 (m, 2 H, CH2) 2.58 (t, J = 7.6 Hz, 2 H, CH2) 3.16 (dd, J = 12.8 and 7.5 Hz, 2 H, NH-CH2) 3.45–3.51 (m, 1 H, H-4) 3.53 (br. s, 1 H, H-5”) 3.75 (dd, J = 6.8 and 2.3 Hz, 1 H, H-3) 3.84 (d, J = 4.9 Hz, 2 H, H-1) 3.88–3.97 (m, 2 H, H-4” and Ha-6”) 4.05 (d, J = 3.4 Hz, 1 H, H-2”) 4.07–4.17 (m, 2 H, H-2 and Hb-6”) 4.19 (d, J = 2.9 Hz, 1 H, H-3”) 4.42 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.54 (dd, J = 22.3 and 11.4 Hz, 2 H, CH 2Ph) 4.65 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.75–4.80 (m, 3 H, CH 2Ph) 4.87 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.96 (d, J = 3.5 Hz, 1 H, H-1”) 5.48 (s, 1 H, H-8”) 5.93 (d, J = 8.5 Hz, 1 H, NH(CO)) 6.04 (t, J = 5.6 Hz, 1 H, (CO)NH) 7.14–7.21 (m, 2 H, arom. H) 7.21–7.42 (m, 26 H, arom. H) 7.49–7.55 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 14.08, 21.85, 22.63, 25.65, 26.96, 28.82, 29.08, 29.25, 29.31, 29.42, 29.60, 31.46, 31.77, 35.94, 36.04, 36.65, 39.46, 50.03, 63.00, 68.07, 69.37, 71.52, 71.73, 73.31, 73.99, 74.18, 75.61, 76.10, 77.20, 78.88, 78.91, 99.61, 101.03, 125.54, 126.28, 127.59, 127.63, 127.71, 127.77, 127.86, 127.91, 128.12, 128.20, 128.32, 128.35, 128.40, 128.43, 128.90, 137.74, 138.24, 138.38, 138.56, 142.82, 172.82, 173.02.
Exact mass (ESI-MS) for C71H90N2NaO10 [M + Na]+ found, 1153.6510; Calcd., 1153.6488.
(5R,6S,7S)-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-7-octanamido-N-(3-pentylphenyl)octanamide (21f)
1H-NMR (300 MHz CDCl3) δ 0.83–0.88 (m, 6 H, 2 x terminal CH3) 1.22–1.33 (m, 12 H, CH2) 1.47–1.83 (m, 7 H, CH2) 1.85–2.03 (m, 3 H, CH2) 2.11–2.25 (m, 2 H, CH2) 2.54 (t, J = 7.4 Hz, 2 H, CH2) 3.46–3.53 (m, 2 H, H-4 and H-5”) 3.73 (dd, J = 7.7 and 1.5 Hz, 1 H, H-3) 3.80 (d, J = 4.9 Hz, 2 H, H-1) 3.85–3.93 (m, 1 H, Ha-6”) 3.95 (d, J = 3.3 Hz, 1 H, H-4”) 4.05 (d, J = 3.5 Hz, 1 H, H-2”) 4.07–4.14 (m, 2 H, H-2 and Hb-6”) 4.17 (d, J = 3.3 Hz, 1 H, H-3”) 4.38 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.48 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.57 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.62 (d, J = 11.5 Hz, 1 H, CH 2Ph) 4.74–4.80 (m, 3 H, CH 2Ph) 4.86 (d, J = 11.4 Hz, 1 H, CH 2Ph) 4.94 (d, J = 3.6 Hz, 1 H, H-1”) 5.44 (s, 1 H, H-8”) 6.00 (d, J = 8.6 Hz, 1 H, NH(CO)) 6.86 (d, J = 7.6 Hz, 1 H, arom. H) 7.12 (t, J = 7.9 Hz, 1 H, arom. H) 7.21–7.41 (m, 24 H, arom. H) 7.44–7.53 (m, 3 H, arom. H) 8.36 (s, 1 H, (CO)NH).
13C NMR (75 MHz, CDCl3) δ 14.03, 21.67, 22.53, 22.63, 25.70, 29.09, 29.33, 31.10, 31.54, 31.78, 36.01, 36.72, 50.00, 63.04, 67.99, 69.32, 71.49, 71.70, 73.26, 74.02, 74.13, 75.59, 76.10, 78.63, 99.55, 101.01, 116.86, 119.69, 123.80, 126.27, 127.56, 127.65, 127.73, 127.78, 127.82, 127.90, 127.93, 128.00, 128.12, 128.34, 128.37, 128.41, 128.45, 128.90, 137.74, 138.10, 138.14, 138.44, 138.56, 143.74, 171.59, 173.24.
Exact mass (ESI-MS) for C68H84KN2O10 [M + K]+ found, 1127.5773; Calcd., 1127.5758.
(5R,6S,7S)-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-7-octanamido-N-(4-pentylphenyl)octanamide (21g)
1H-NMR (300 MHz CDCl3) δ 0.86–0.93 (m, 6 H, 2 x terminal CH3) 1.22–1.38 (m, 12 H, CH2) 1.50–1.84 (m, 6 H, CH2) 1.87–2.07 (m, 3 H, CH2) 2.09–2.28 (m, 2 H, CH2) 2.54 (t, J = 7.1 Hz, 2 H, CH2) 3.46–3.54 (m, 2 H, H-4 and H-5”) 3.75 (dd, J = 8.0, 1.2 Hz, 1 H, H-3) 3.79–3.83 (m, 2 H, H-1) 3.89 (dd, J = 12.5 and 1.3 Hz, 1 H, Ha-6”) 3.95 (dd, J = 10.0 and 3.5 Hz, 1 H, H-4”) 4.08 (d, J = 3.5 Hz, 1 H, H-2”) 4.09–4.16 (m, 2 H, H-2 and Hb-6”) 4.19 (d, J = 2.8 Hz, 1 H, H-3”) 4.39 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.49 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.59 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.64 (d, J = 11.3 Hz, 1 H, CH 2Ph) 4.76–4.83 (m, 3 H, CH 2Ph) 4.88 (d, J = 11.3 Hz, 1 H, CH 2Ph) 4.95 (d, J = 3.5 Hz, 1 H, H-1”) 5.46 (s, 1 H, H-8”) 6.16 (d, J = 8.7 Hz, 1 H, NH(CO)) 7.06 (d, J = 8.5 Hz, 2 H, arom. H) 7.22–7.37(m, 22 H, arom. H) 7.38–7.46 (m, 3 H, arom. H) 7.49–7.5(m, 2 H, arom. H) 8.49 (s, 1 H, (CO)NH).
13C NMR (75 MHz, CDCl3) δ 14.02, 14.04, 14.08, 21.68, 22.52, 22.58, 22.63, 24.75, 25.69, 28.11, 28.90, 29.03, 29.09, 29.34, 31.23, 31.43, 31.62, 31.78, 33.64, 35.34, 36.51, 36.70, 49.97, 63.01, 67.87, 69.31, 71.50, 71.66, 73.27, 74.07, 74.12, 75.60, 76.07, 77.20, 78.55, 99.40, 101.01, 119.67, 126.27, 127.59, 127.68, 127.71, 127.82, 127.90, 127.94, 127.98, 128.12, 128.35, 128.37, 128.41, 128.44, 128.59, 128.90, 136.28, 137.72, 138.09, 138.14, 138.28, 138.35, 138.53, 171.57, 173.39.
Exact mass (ESI-MS) for C68H85N2O10 [M + H]+ found, 1089.6204; Calcd., 1089.6199.
(5R,6S,7S)-5,6-di-O-benzyl-8-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-D-galactopyranosyl)-7-octanamido-N-(2-(2-ethoxyethoxy)ethyl)octanamide (21 h)
1H-NMR (300 MHz CDCl3) δ 0.89 (t, J = 6.8 Hz, 3 H, terminal CH3) 1.18–1.35 (m, 12 H, CH2 and terminal CH3) 1.45–1.56 (m, 2 H, CH2) 1.60–1.88 (m, 3 H, CH2) 1.89–1.97 (m, 2 H, CH2) 2.10 (t, J = 6.2 Hz, 2 H, CH2) 3.40–3.48 (m, 2 H, CH2) 3.50–3.62 (m, 10 H, H-4, H-5” and CH2) 3.78 (dd, J = 6.1 and 2.4 Hz, 1 H, H-3) 3.80–3.99 (m, 4 H, CH2-1, H-2 and Ha-6”) 4.07 (d, J = 3.3 Hz, 1 H, H-4”) 4.10–4.18 (m, 1 H, Hb-6”) 4.19–4.25 (m, 2 H, H-2” and H-3”) 4.46 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.51 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.59 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.65 (d, J = 11.6 Hz, 1 H, CH 2Ph) 4.73–4.78 (m, 3 H, CH 2Ph) 4.87 (d, J = 11.4 Hz, 1 H, CH 2Ph) 4.96 (d, J = 3.4 Hz, 1 H, H-1”) 5.48 (s, 1 H, H-8”) 5.89 (d, J = 8.4 Hz, 1 H, NH(CO)) 6.26 (t, J = 5.3 Hz, 1 H, (CO)NH) 7.22–7.44 (m, 23 H, arom. H) 7.51–7.55 (m, 2 H, arom. H).
13C NMR (75 MHz, CDCl3) δ 14.08, 15.16, 21.82, 22.63, 25.67, 29.06, 29.16, 29.31, 31.75, 36.13, 36.68, 39.04, 50.12, 62.96, 66.61, 68.11, 69.38, 69.70, 69.81, 70.22, 71.57, 71.78, 73.30, 73.90, 74.23, 75.64, 76.12, 77.20, 78.94, 79.16, 99.58, 101.00, 126.28, 127.56, 127.60, 127.67, 127.70, 127.72, 127.88, 127.90, 128.09, 128.30, 128.33, 128.38, 128.41, 128.85, 137.77, 138.28, 138.30, 138.45, 138.60, 172.91, 173.00.
Exact mass (ESI-MS) for C63H83N2O12 [M + H]+ found, 1059.5945; Calcd., 1059.5941.
General procedure for debenzylation
A solution of the protected amide (0.06 mmol) in CHCl3 (3 mL) and EtOH (9 mL) was hydrogenated under atmospheric pressure in the presence of palladium black (35 mg). Upon reaction completion, the mixture was filtered through celite. The filter cake was rinsed with CHCl3 and EtOH and the filtrate was evaporated to dryness. After purification by silica gel chromatography (10% → 18% MeOH in CH2Cl2), final compounds 5a (43%), 5b (30%), 5c (42%), 5d (56%), 5e (56%), 5f (78%), 5g (71%), 5h (53%) were obtained as white powders.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-octanamido-N-nonyloctanamide (5a)
1H-NMR (300 MHz, pyridine-d5) δ 0.77–0.91 (m, 6 H, 2 x terminal CH3) 1.08–1.38 (m, 20 H, CH2) 1.59 (dt, J = 14.8 and 7.6 Hz, 2 H, CH2) 1.77 (dt, J = 14.8 and 7.6 Hz, 2 H, CH2) 1.92–2.04 (m, 1 H, CH2) 2.17–2.29 (m, 1 H, CH2) 2.34–2.60 (m, 6 H, CH2) 3.46 (dd, J = 13.0 and 6.8 Hz, 2 H, NHCH 2) 4.26–4.37 (m, 3 H, Ha-1, H-3 and H-4) 4.37–4.45 (m, 3 H, H-4” and H-6”) 4.48 (q, J = 6.2 Hz, 1 H, H-3”) 4.55 (d, J = 2.9 Hz, 1 H, H-5”) 4.60–4.69 (m, 2 H, Hb-1 and H-2”) 5.19–5.28 (m, 1 H, H-2) 5.56 (d, J = 3.8 Hz, 1 H, H-1”) 6.36 (br. s, 6 H, OH) 8.30 (t, J = 5.2 Hz, 1 H, NH(CO)) 8.46 (d, J = 8.6 Hz, 1 H, (CO)NH).
13C NMR (75 MHz, pyridine-d5) δ 14.60, 14.65, 23.23, 23.28, 23.47, 26.71, 27.78, 29.73, 29.88, 30.01, 30.18, 30.38, 30.66, 32.27, 32.43, 34.38, 37.14, 37.35, 40.11, 51.78, 63.03, 68.98, 70.68, 71.37, 71.96, 72.61, 73.42, 77.19, 101.92, 173.65.
Exact mass (ESI-MS) for C31H61N2O10 [M + H]+ found, 621.4347; Calcd., 621.4326; m.p.: 95–97 °C.
(5R,6 S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-octanamido-N-(2-phenylethyl)octanamide (5b)
1H-NMR (300 MHz, pyridine-d5) δ 0.80 (t, J = 6.2 Hz, 3 H, terminal CH3) 1.11–1.35 (m, 8 H, CH2) 1.77 (dt, J = 14.6 and 7.3 Hz, 2 H, CH2) 1.89–2.04 (m, 1 H, CH2) 2.11–2.26 (m, 1 H, CH2) 2.31–2.55 (m, 6 H, CH2) 2.93 (t, J = 7.2 Hz, 2 H, CH 2Ph) 3.68 (q, J = 6.8 Hz, 2 H, NHCH 2) 4.26–4.32 (m, 1 H, H-3) 4.32–4.54 (m, 6 H, H-4, Ha-1, H-4”, H-5” and CH2-6”) 4.56 (d, J = 2.5 Hz, 1 H, H-3”) 4.61–4.69 (m, 2 H, H-2” and Hb-1) 5.20–5.30 (m, 1 H, H-2) 5.57 (d, J = 3.4 Hz, 1 H, H-1”) 7.24–7.35 (m, 3 H, arom. H) 8.43–8.54 (m, 2 H, arom. H).
13C NMR (75 MHz, pyridine-d5) δ 29.03, 37.66, 37.83, 41.15, 44.16, 44.44, 46.70, 48.82, 51.21, 51.58, 51.69, 56.18, 66.16, 77.46, 83.33, 85.09, 85.81, 86.38, 87.06, 87.83, 91.60, 116.30, 141.31, 143.62, 144.10, 155.13, 188.15, 188.27.
Exact mass (ESI-MS) for C30H51N2O10 [M + H]+ found, 599.3541; Calcd., 599.3538; m.p. 56–58 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-octanamido-N-(4-phenylbutyl)octanamide (5c)
1H-NMR (300 MHz, pyridine-d5) δ 0.80 (t, J = 6.6 Hz, 3 H, terminal CH3) 1.07–1.42 (m, 10 H, CH2) 1.54–1.67 (m, 4 H, CH2) 1.76 (dt, J = 14.9 and 7.5 Hz, 2 H, CH2) 1.90–2.04 (m, 1 H, CH2) 2.12–2.27 (m, 1 H, CH2) 2.30–2.58 (m, 6 H, CH2) 3.44 (q, J = 5.8 Hz, 2 H, NHCH 2) 4.25–4.44 (m, 6 H, Ha-1, H-3, H-4, H-4” and CH2-6”) 4.48 (q, J = 5.7 Hz, 1 H, H-3”) 4.55 (d, J = 2.6 Hz, 1 H, H-5”) 4.60–4.68 (m, 2 H, Hb-1 and H-2”) 5.19–5.28 (m, 1 H, H-2) 5.56 (d, J = 3.8 Hz, 1 H, H-1”) 6.47 (br. s, 6 H, OH) 7.17–7.25 (m, 3 H, arom. H) 7.28–7.35 (m, 2 H, arom. H) 8.28 (t, J = 5.3 Hz, 1 H, (CO)NH) 8.46 (d, J = 8.6 Hz, 1 H, NH(CO)).
13C NMR (75 MHz, pyridine-d5) δ 14.60, 23.23, 23.46, 26.72, 29.58, 29.72, 30.01, 30.21, 30.39, 32.27, 34.41, 36.07, 37.14, 37.33, 39.79, 51.78, 63.04, 68.98, 70.68, 71.38, 71.97, 72.62, 73.43, 77.21, 101.92, 126.46, 129.09, 129.23, 136.58, 143.22, 173.66, 173.67.
Exact mass (ESI-MS) for C32H55N2O10 [M + H]+ found, 627.3860; Calcd., 627.3851; m.p. 57–59 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-octanamido-N-(6-phenylhexyl)octanamide(5d)
1H-NMR (300 MHz, pyridine-d5) δ 0.77–0.87 (m, 3 H, terminal CH3) 1.09–1.43 (m, 13 H, CH2) 1.54 (dt, J = 12.6 and 6.3 Hz, 3 H, CH2) 1.77 (dt, J = 14.4 and 7.1 Hz, 2 H, CH2) 1.91–2.05 (m, 1 H, CH2) 2.16–2.31 (m, 1 H, CH2) 2.33–2.60 (m, 6 H, CH2) 3.03 (q, J = 7.0 Hz, 2 H, CH2) 3.43 (d, J = 5.8 Hz, 2 H, NHCH 2) 4.27–4.46 (m, 6 H, Ha-1, H-3, H-4, H-4” and CH2-6”) 4.46–4.53 (m, 1 H, H-5”) 4.56 (app. s, 1 H, H-3”) 4.60–4.69 (m, 2 H, Hb-1 and H-2”) 5.19–5.29 (m, 1 H, H-2) 5.56 (d, J = 2.4 Hz, 1 H, H-1”) 6.40 (br. s, 6 H, OH) 7.19–7.29 (m, 3 H, arom. H) 7.30–7.39 (m, 2 H, arom. H) 8.29 (t, J = 5.7, 1 H, (CO)NH) 8.49 (d, J = 8.2 Hz, 1 H, NH(CO)).
13C NMR (75 MHz, pyridine-d5) δ 8.91, 14.59, 23.22, 23.45, 26.70, 27.58, 29.58, 29.71, 29.99, 30.35, 30.53, 32.08, 32.26, 34.37, 36.40, 37.13, 37.33, 40.05, 46.18, 51.78, 63.00, 68.92, 70.68, 71.34, 71.95, 72.56, 73.39, 77.17, 101.89, 126.41, 129.07, 129.21, 143.51, 173.66, 173.68.
Exact mass (ESI-MS) for C34H59N2O10 [M + H]+ found, 655.4161; Calcd., 655.4164; m.p. 135–137 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-octanamido-N-(8-phenyloctyl)octanamide (5e)
1H-NMR (300 MHz, pyridine-d5) δ 0.80 (t, J = 6.3 Hz, 3 H, terminal CH3) 1.09–1.36 (m, 16 H, CH2) 1.47–1.64 (m, 4 H, CH2) 1.69–1.83 (m, 2 H, CH2) 1.91–2.05 (m, 1 H, CH2) 2.14–2.30 (m, 1 H, CH2) 2.34–2.60 (m, 8 H, CH2) 3.45 (dd, J = 13.1 and 6.8 Hz, 2 H, NHCH 2) 4.26–4.45 (m, 6 H, Ha-1, H-3, H-4, H-4” and CH2-6”) 4.49 (q, J = 6.0 Hz, 1 H, H-5”) 4.56 (d, J = 3.0 Hz, 1 H, H-3”) 4.61–4.69 (m, 2 H, Hb-1 and H-2”) 5.19–5.29 (m, 1 H, H-2) 5.57 (d, J = 3.5 Hz, 1 H, H-1”) 6.56 (br. s, 6 H, OH) 7.21–7.29 (m, 3 H, arom. H) 7.33–7.40 (m, 2 H, arom. H) 8.30 (t, J = 5.4, 1 H, (CO)NH) 8.48 (d, J = 8.5 Hz, 1 H, NH(CO)).
13C NMR (75 MHz, pyridine-d5) δ 8.91, 14.58, 23.21, 23.45, 26.69, 27.73, 29.71, 29.80, 29.89, 29.99, 30.03, 30.61, 32.15, 32.26, 34.37, 36.47, 37.12, 37.33, 40.08, 46.18, 51.78, 63.00, 68.93, 70.67, 71.34, 71.95, 72.58, 73.40, 77.17, 101.88, 126.42, 129.09, 129.24, 143.59, 173.66.
Exact mass (ESI-MS) for C36H63N2O10 [M + H]+ found, 683.4477; Calcd., 683.4477; m.p. 128–130 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-octanamido-N-(3-pentylphenyl)octanamide (5f)
1H-NMR (300 MHz, pyridine-d5) δ 0.81 (t, J = 6.5 Hz, 6 H, 2 x terminal CH3) 1.11–1.34 (m, 14 H, CH2) 1.55 (dt, J = 14.8 and 7.4 Hz, 2 H, CH2) 1.77 (dt, J = 14.8 and 7.4 Hz, 2 H, CH2) 1.90–2.03 (m, 1 H, CH2) 2.17–2.31 (m, 1 H, CH2) 2.41 (t, J = 7.6 Hz, 2 H, CH2) 2.55 (t, J = 7.6 Hz, 2 H, CH2) 2.68 (dd, J = 14.2 and 7.0 Hz, 2 H, CH2) 4.24–4.31 (m, 1 H, H-3) 4.31–4.45 (m, 5 H, H-4, Ha-1, H-4” and H-6”) 4.48 (q, J = 5.4 Hz, 1 H, H-3”) 4.55 (d, J = 2.4 Hz, 1 H, H-5”) 4.60–4.69 (m, 2 H, H-2” and Hb-1) 5.20–5.29 (m, 1 H, H-2) 5.57 (d, J = 3.4 Hz, 1 H, H-1”) 6.48 (br. s., 6 H, OH) 6.99 (d, J = 7.5 Hz, 1 H, arom. H) 7.32 (t, J = 7.7 Hz, 1 H, arom. H) 7.88 (d, J = 8.0 Hz, 1 H, arom. H) 7.98 (s, 1 H, arom. H) 8.47 (d, J = 8.7 Hz, 1 H, NH(CO)) 10.60 (s, 1 H, (CO)NH).
13C NMR (75 MHz, pyridine-d5) δ 14.51, 14.57, 23.10, 23.21, 23.41, 26.70, 29.70, 29.98, 31.76, 31.98, 32.25, 34.30, 36.57, 37.11, 38.21, 51.65, 63.02, 68.80, 70.63, 71.37, 71.93, 72.61, 73.40, 77.15, 101.82, 117.89, 120.54, 124.09, 129.41, 141.00, 144.22, 172.70, 173.69.
Exact mass (ESI-MS) for C33H57N2O10 [M + H]+ found, 641.4001; Calcd., 641.4008; m.p. 165–167 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-octanamido-N-(4-pentylphenyl)octanamide (5g)
1H-NMR (300 MHz, pyridine-d5) δ 0.81 (dt, J = 9.1 and 7.0 Hz, 6 H, 2 x terminal CH3) 1.12–1.34 (m, 14 H, CH2) 1.55 (dt, J = 14.8 and 7.4 Hz, 2 H, CH2) 1.77 (dt, J = 14.9 and 7.5 Hz, 2 H, CH2) 1.89–2.05 (m, 1 H, CH2) 2.17–2.33 (m, 1 H, CH2) 2.42 (t, J = 7.8 Hz, 2 H, CH2) 2.53 (t, J = 7.8 Hz, 2 H, CH2) 2.61–2.71 (m, 2 H, CH2) 4.24–4.45 (m, 6 H, H-3, H-4, H-4”, H-6” and Ha-1) 4.49 (q, J = 5.9 Hz, 1 H, H-3”) 4.56 (d, J = 2.9 Hz, 1 H, H-5”) 4.61–4.69 (m, 2 H, Hb-1 and H-2”) 5.20–5.29 (m, 1 H, H-2) 5.57 (d, J = 3.7 Hz, 1 H, H-1”) 6.41 (br. s, 6 H, OH) 7.23 (app. s, 2 H, arom. H) 8.01 (d, J = 8.4 Hz, 2 H, arom. H) 8.48 (d, J = 8.7 Hz, 1 H, NH(CO)) 10.63 (s, 1 H, (CO)NH).
13C NMR (75 MHz, pyridine-d5) δ 14.55, 14.58, 23.14, 23.22, 23.42, 26.70, 29.71, 29.99, 31.95, 32.26, 34.31, 35.86, 37.13, 38.14, 51.66, 63.04, 68.81, 70.66, 71.38, 71.95, 72.62, 73.42, 77.18, 101.85, 120.56, 129.43, 138.26, 138.75, 172.58, 173.68.
Exact mass (ESI-MS) for C33H57N2O10 [M + H]+ found, 641.4011; Calcd., 641.4008; m.p. 147–149 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-octanamido-N-(2-(2-ethoxyethoxy)ethyl)octanamide (5h)
1H-NMR (300 MHz, pyridine-d5) δ 0.80 (t, J = 6.5 Hz, 3 H, terminal CH3) 1.08–1.22 (m, 8 H, terminal CH3 and CH2) 1.23–1.41 (m, 5 H, CH2) 1.76 (dt, J = 14.8 and 7.4 Hz, 2 H, CH2) 1.87–2.01 (m, 1 H, CH2) 2.11–2.24 (m, 1 H, CH2) 2.31–2.47 (m, 4 H, CH2) 2.47–2.58 (m, 2 H, CH2) 3.41 (q, J = 7.0 Hz, 2 H, CH2) 3.49–3.54 (m, 2 H, CH2) 3.56–3.61 (m, 2 H, CH2) 3.62–3.70 (m, 2 H, CH2) 4.22–4.44 (m, 6 H, Ha-1, H-3, H-4, H-4” and CH2-6”) 4.48 (q, J = 5.8 Hz, 1 H, H-5”) 4.55 (d, J = 2.7 Hz, 1 H, H-3”) 4.60–4.69 (m, 2 H, Hb-1 an H-2”d) 5.19–5.29 (m, 1 H, H-2) 5.56 (d, J = 3.7 Hz, 1 H, H-1”) 6.43 (br. s, 6 H, OH) 8.43 (d, J = 8.4 Hz, 1 H, NH(CO)) 8.47–8.52 (m, 1 H, (CO)NH).
13C NMR (75 MHz, pyridine-d5) δ 14.59, 15.84, 23.23, 23.39, 26.71, 29.72, 30.00, 30.37, 32.27, 34.46, 37.14, 37.21, 40.14, 51.74, 63.03, 66.84, 68.93, 70.50, 70.67, 70.85, 71.01, 71.38, 71.96, 72.64, 73.42, 77.21, 101.90, 173.64, 173.86.
Exact mass (ESI-MS) for C28H55N2O12 [M + H]+ found, 611.3750; Calcd., 611.3750; m.p. could not be determined due to hygroscopy.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-undecanamino-N-(6-phenylhexyl)octanamide (6d)
1H-NMR (300 MHz, pyridine-d5) δ 0.87 (t, J = 6.6 Hz, 3 H, terminal CH3) 1.13–1.41 (m, 20 H, CH2) 1.45–1.61 (m, 4 H, CH2) 1.73–1.87 (m, 2 H, CH2) 1.91–2.05 (m, 1 H, CH2) 2.18–2.60 (m, 9 H, CH2) 3.43 (q, J = 6.8 Hz, 2 H, NHCH 2) 4.26–4.53 (m, 7 H, Ha-1, H-3, H-4, H-4”, H-5” and CH2-6”) 4.56 (d, J = 3.1 Hz, 1 H, H-3”) 4.61–4.71 (m, 2 H, Hb-1 and H-2”) 5.21–5.31 (m, 1 H, H-2) 5.57 (d, J = 3.8 Hz, 1 H, H-1”) 5.96 (br. s, 6 H, OH) 7.17–7.27 (m, 3 H, arom. H) 7.31–7.38 (m, 2 H, arom. H) 8.27 (t, J = 5.8 Hz, 1 H, (CO)NH) 8.48 (d, J = 8.7 Hz, 1 H, NH(CO)).
13C NMR (75 MHz, pyridine-d5) δ 14.69, 23.33, 23.47, 26.77, 27.59, 29.62, 29.99, 30.14, 30.20, 30.26, 30.32, 30.55, 32.11, 32.51, 34.41, 36.43, 37.18, 37.34, 40.08, 51.77, 63.05, 68.96, 70.70, 71.40, 71.98, 72.62, 73.45, 77.22, 101.93, 126.45, 129.10, 129.24, 143.53, 173.68, 173.72.
Exact mass (ESI-MS) for C38H67N2O10 [M + H]+ found, 711.4781; Calcd., 711.4790; m.p. 91–93 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-pentadecanamino-N-(6-phenylhexyl)octanamide (7d)
1H-NMR (300 MHz, pyridine-d5) δ 0.88 (t, J = 6.7 Hz, 3 H, terminal CH3) 1.16–1.41 (m, 28 H, CH2) 1.45–1.61 (m, 4 H, CH2) 1.74–1.87 (m, 2 H, CH2) 1.91–2.04 (m, 1 H, CH2) 2.16–2.60 (m, 9 H, CH2) 3.43 (q, J = 6.7 Hz, 2 H, NHCH 2) 4.26–4.53 (m, 7 H, Ha-1, H-3, H-4, H-4”, H-5” and CH2-6”) 4.56 (d, J = 3.2 Hz, 1 H, H-3”) 4.61–4.71 (m, 2 H, Hb-1 and H-2”) 5.21–5.30 (m, 1 H, H-2) 5.57 (d, J = 3.9 Hz, 1 H, H-1”) 5.93 (br. s, 6 H, OH) 7.19–7.30 (m, 3 H, arom. H) 7.30–7.38 (m, 2 H, arom. H) 8.27 (t, J = 5.6 Hz, 1 H, (CO)NH) 8.47 (d, J = 8.6 Hz, 1 H, NH(CO)).
13C NMR (75 MHz, pyridine-d5) δ 14.69, 23.35, 23.47, 26.77, 27.59, 29.60, 30.02, 30.15, 30.18, 30.25, 30.34, 30.40, 30.57, 32.11, 32.52, 34.43, 36.43, 37.18, 37.34, 40.06, 51.77, 63.07, 68.99, 70.70, 71.40, 71.98, 72.64, 73.46, 77.25, 101.95, 126.43, 129.10, 129.24, 143.53, 173.61, 173.68.
Exact mass (ESI-MS) for C42H75N2O10 [M + H]+ found, 767.5412; Calcd., 767.5416; m.p. 148–150 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-nonadecanamino-N-(6-phenylhexyl)octanamide (8d)
1H-NMR (300 MHz, pyridine-d5) δ 0.88 (t, J = 6.6 Hz, 3 H, terminal CH3) 1.14–1.40 (m, 36 H, CH2) 1.45–1.61 (m, 4 H, CH2) 1.70–1.88 (m, 2 H, CH2) 1.90–2.05 (m, 1 H, CH2) 2.18–2.58 (m, 9 H, CH2) 3.44 (q, J = 6.7 Hz, 2 H, NHCH 2) 4.23–4.54 (m, 7 H, Ha-1, H-3, H-4, H-4”, H-5” and CH2-6”) 4.57 (d, J = 3.1 Hz, 1 H, H-3”) 4.61–4.72 (m, 2 H, Hb-1 and H-2”) 5.21–5.31 (m, 1 H, H-2) 5.57 (d, J = 3.8 Hz, 1 H, H-1”) 5.83 (br. s, 6 H, OH) 7.20–7.27 (m, 3 H, arom. H) 7.31–7.38 (m, 2 H, arom. H) 8.28 (t, J = 5.6 Hz, 1 H, (CO)NH) 8.49 (d, J = 9.0 Hz, 1 H, NH(CO)).
13C NMR (75 MHz, pyridine-d5) δ 14.69, 23.35, 23.47, 26.78, 27.61, 29.62, 30.02, 30.20, 30.26, 30.32, 30.43, 30.55, 32.11, 32.52, 34.41, 36.43, 37.19, 37.34, 40.08, 51.77, 63.07, 68.96, 70.68, 71.40, 71.97, 72.64, 73.45, 77.22, 101.93, 126.43, 129.10, 129.24, 143.53, 173.66, 173.71.
Exact mass (ESI-MS) for C46H83N2O10 [M + H]+ found, 823.6036; Calcd., 823.6042; m.p. 136–138 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-hexacosylamino-N-(6-phenylhexyl)octanamide (9d)
1H-NMR (300 MHz, pyridine-d5) δ 0.88 (t, J = 6.4 Hz, 3 H, terminal CH3) 1.11–1.43 (m, 42 H, CH2) 1.43–1.62 (m, 6 H, CH2) 1.81 (quint, J = 7.3 Hz, 2 H, CH2) 1.91–2.07 (m, 2 H, CH2) 2.12–2.30 (m, 2 H, CH2) 2.34–2.61 (m, 8 H, CH2) 3.03 (q, J = 7.4 Hz, 2 H, CH2) 3.43 (q, J = 6.6 Hz, 2 H, NHCH 2) 4.26–4.45 (m, 6 H, Ha-1, H-3, H-4, H-4” and CH2-6”) 4.49 (q, J = 5.9 Hz, 1 H, H-5”) 4.56 (d, J = 2.9 Hz, 1 H, H-3”) 4.60–4.70 (m, 2 H, Hb-1 and H-2”) 5.19–5.30 (m, 1 H, H-2) 5.57 (d, J = 3.8 Hz, 1 H, H-1”) 6.33 (br. s, 6 H, OH) 7.17–7.27 (m, 3 H, arom. H) 7.30–7.39 (m, 2 H, arom. H) 8.29 (t, J = 5.4 Hz, 1 H, (CO)NH) 8.49 (d, J = 8.5 Hz, 1 H, NH(CO)).
13C NMR (75 MHz, pyridine-d5) δ 8.89, 14.66, 23.32, 23.45, 26.75, 27.57, 29.59, 29.99, 30.14, 30.17, 30.25, 30.29, 30.37, 30.41, 30.52, 32.08, 32.49, 34.37, 36.40, 37.15, 37.31, 40.05, 46.15, 51.78, 63.00, 68.91, 70.67, 71.32, 71.95, 72.55, 73.40, 77.17, 101.87, 126.40, 129.07, 129.21, 143.50, 173.66, 173.69.
Exact mass (ESI-MS) for C52H95N2O10 [M + H]+ found, 907.6976; Calcd., 907.6981; m.p. 116–118 °C.
(5R,6S,7S)-5,6-dihydroxy-8-O-(α-D-galactopyranosyl)-7-hexacosylamino-N-(3-pentylphenyl)octanamide (9f)
1H-NMR (300 MHz, pyridine-d5) δ 0.80 (t, J = 6.9 Hz, 3 H, terminal CH3) 0.88 (t, J = 6.5 Hz, 3 H, terminal CH3) 1.14–1.47 (m, 48 H, CH2) 1.47–1.64 (m, 2 H, CH2) 1.74–1.89 (m, 2 H, CH2) 1.89–2.16 (m, 1 H, CH2) 2.16–2.33 (m, 1 H, CH2) 2.33–2.51 (m, 4 H, CH2) 2.51–2.60 (m, 2 H, CH2) 2.60–2.74 (m, 2 H, CH2) 4.23–4.46 (m, 6 H, Ha-1, H-3”, H-4, H-4” and CH2-6”) 4.46–4.53 (m, 1 H, H-3) 4.56 (br. s, 1 H, H-5”) 4.60–4.71 (m, 2 H, Hb-1 and H-2”) 5.20–5.31 (m, 1 H, H-2) 5.57 (d, J = 3.8 Hz, 1 H, H-1”) 6.27 (d, J = 6.1 Hz, 1 H, OH) 6.32 (d, J = 3.7 Hz, 1 H, OH) 6.46–6.58 (m, 2 H, OH) 6.72 (br. s, 1 H, OH) 6.97–7.08 (m, 2 H, arom. H and OH) 7.33 (t, J = 7.8 Hz, 1 H, arom. H) 7.88 (d, J = 7.9 Hz, 1 H, arom. H) 7.97 (s, 1 H, arom. H) 8.46 (d, J = 8.5 Hz, 1 H, NH(CO)) 10.59 (s, 1 H, (CO)NH).
13C NMR (75 MHz, pyridine-d5) δ 14.54, 14.69, 23.13, 23.35, 23.44, 26.78, 30.02, 30.17, 30.20, 30.26, 30.32, 30.40, 30.44, 31.80, 32.02, 32.52, 34.35, 36.61, 37.18, 38.24, 51.68, 63.07, 68.87, 70.68, 71.42, 71.98, 72.65, 73.46, 77.22, 101.88, 117.91, 120.55, 124.11, 129.45, 141.05, 144.26, 172.71, 173.68.
Exact mass (ESI-MS) for C51H93N2O10 [M + H]+ found, 893.6830; Calcd., 893.6825; m.p. 127–129 °C.
N-((2S,3S,4R)-1-O-(α-D-galactopyranosyl)-3,4-dihydroxy-16-phenylhexadecan-2-yl)octanamide(23)
Compound 23 was synthesized in an analogous way as described in scheme 2 and 3. A Wittig olefination between 14 and triphenyl(10-decylphenyl)phosphonium bromide was followed by selective reduction of the double bond. The azide moiety on C2 was installed using a Mitsunobu inversion with hydrazoic acid (HN3). Next, the primary hydroxyl group was selectively deprotected to give the corresponding acceptor, which was glycosylated with trichloroacetimidate donor 11. Staudinger reduction of the azide, followed by amide formation with octanoic acid, yielded, after overall deprotection, compound 23.
1H-NMR (300 MHz, pyridine-d5) δ 0.81 (t, J = 6.3 Hz, 3 H, terminal CH3) 1.11–2.01 (m, 32 H, CH2) 2.44 (t, J = 7.6 Hz, 2 H, CH2) 2.60 (t, J = 7.6 Hz, 2 H, CH2) 4.31–4.35 (m, 2 H, H-2” and H3) 4.38–4.46 (m, 3 H, Hb1 and CH2-6”) 4.47 (m, 1 H, H-4) 4.53 (m, 1 H, H-4”) 4.57 (m, 1 H, H-5”) 4.63–4.72 (m, 2 H, Ha1 and H-3”) 5.24–5.34 (m, 1 H, H-2) 5.60 (d, J = 3.7 Hz, 1 H, H-1”) 5.94 (br. s, 6 H, OH) 7.22–7.29 (m, 3 H, arom. H) 7.31–7.39 (m, 2 H, arom. H) 8.49 (d, J = 8.6 Hz, 1 H, NH).
13C NMR (75 MHz, pyridine-d5) δ 14.23, 22.89, 26.37, 26.50, 29.38, 29.56, 29.66, 29.82, 29.98, 30.01, 30.14, 30.38, 31.89, 34.42, 36.11, 36.77, 51.64, 62.68, 68.65, 70.30, 71.03, 71.67, 71.71, 72.50, 73.05, 76.72, 101.45, 128.73, 128.88.
Recombinant proteins, SPR studies and crystallography
Soluble mCD1-β2 M protein was expressed and purified from insect cells as described41. Glycolipid loading and SPR studies were performed as previously reported27. In brief, increasing concentrations of TCR (two-fold dilutions from 500 nM to 15.625 nM) were passed over streptavidin immobilized CD1d using a CAP chip on a Biacore T200. A reference substraction included the TCR binding response to CD1d to which no lipid has been added. In addition, single cycle kinetics were carried out with 3-fold dilutions of TCR (900nM-11nM), without reference substraction. Running buffer contained no detergent in an attempt to not extract these short-acyl chain ligands during the course of the experiment. CD1d-ligand-TCR complex formation, purification, crystallization and structure determination was performed as reported previously19, 25. Data collection and refinement statistics are shown in Table 2. Structure of mCD1d-5d and mCD1d-23 have been deposited in the Protein Data Bank (http://www.rcsb.org/) under accession codes 5TW2 and 5TW5, respectively.
Table 2.
Data collection and refinement statistics of crystal structures of the CD1d-23 and CD1d-5d complexes.
| Data collection statistics | CD1d-23 | CD1d-5d |
|---|---|---|
| Space group | P212121 | P212121 |
| Cell dimension | ||
| a, b, c, (Å) | 42.2, 106.2, 106.9 | 42.2, 107.5, 109.6 |
| a, b, g (°) | 90, 90, 90 | 90, 90, 90 |
| Resolution range (Å) [outer shell] | 45–1.85 [1.92–1.85] | 40–1.75 [1.81–1.75] |
| No. reflections | 40,100 | 51,068 |
| Rmeas (%) | 13.3 [97.9] | 6.9 [58.1] |
| Rpim (%) | 5.5 [41.3] | 3.3 [27.5] |
| Multiplicity | 5.4 [5.2] | 4.3 [4.3] |
| Average I/sI | 17.1 [2.5] | 35.8 [3.1] |
| Completeness (%) | 95.2 [98.6] | 99.7 [100.0] |
| Refinement statistics | ||
| No. atoms | 3,435 | 3,385 |
| Protein | 2,992 | 2,929 |
| Ligand (spacer/antigen) | 18/46 | 18/46 |
| Carbohydrate | 56 | 52 |
| Water | 323 | 340 |
| R/Rfree (%) | 21.4/24.3 | 20.3/22.1 |
| Ramachandran plot (%) | ||
| Favored | 97.5 | 98.3 |
| Allowed | 100 | 100 |
| R.m.s. deviations | ||
| Bonds (Å) | 0.008 | 0.008 |
| Angles (°) | 1.37 | 1.33 |
| B-factors (Å2) | ||
| Protein | 24.4 | 31.8 |
| Spacer/Lipid | 40.8/29.4 | 52.5/60.7 |
| Carbohydrate | 44.7 | 46.8 |
| Water | 31.0 | 39.7 |
In vivo cytokine secretion assay
Experiments were approved by and conducted according to the guidelines of the Ethical Committee of Laboratory Animal Welfare of Ghent University.
The different glycolipids were dissolved in DMSO at a concentration of 1 mg/mL, heated for 20 minutes at 80 °C and subsequently sonicated for 10 minutes at the same temperature. Next, 11 µL of the prepared solution was diluted with PBS in order to obtain a 10 µg/mL solution and the resulting solution was again warmed to 80 °C for 20 minutes followed by sonication for 10 minutes at this temperature. Then, 500 µL (5 µg) was injected i.p. in 8 different C57BL/6 mice and blood was collected 4 h (IL-4) and 16 h (IFN-γ) post injection. The levels of IL-4 and INF-γ were determined by ELISA (eBioscience). KRN7000 was employed as control.
Electronic supplementary material
Acknowledgements
Joren Guillaume is a fellow of the Agency for Innovation by Science and Technology (IWT) of Flanders. S.V.C. and D.E. received support of the Belgian Stichting tegen Kanker and the FWO Flanders. We would like to thank the support staff at the SSRL for access to remote data collection. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393) and the National Center for Research Resources (P41RR001209).
Author Contributions
J.G., J.J., M.R. conceived and designed the synthetic experiments. J.G. and M.R. carried out the chemical synthesis. J.W. and D.M.Z. conceived and designed the structural chemistry experiments. S.G.R. carried out the crystallography experiments. T.D. carried out the in vivo cytokine determination under the supervision of D.E., M.F. performed the modeling studies. J.G., D.Z. and S.V.C wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Dirk M. Zajonc and Serge Van Calenbergh contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04461-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Borg NA, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 3.Natori T, Morita M, Akimoto K, Koezuka Y. Agelasphins, novel antitumor and immunostimulatory cerebrosides from the marine sponge agelas-mauritianus. Tetrahedron. 1994;50:2771–2784. doi: 10.1016/S0040-4020(01)86991-X. [DOI] [Google Scholar]
- 4.Morita M, et al. Structure-activity relationship of alpha-galactosylceramides against b16-bearing mice. J. Med. Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 5.Koch M, et al. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat. Immunol. 2005;6:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 6.Zajonc DM, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat. Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg NA, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 8.Smyth MJ, et al. NKT cells - conductors of tumor immunity? Curr. Opin. Immunol. 2002;14:165–171. doi: 10.1016/S0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- 9.Sköld M, Behar SM. Role of CD1d-restricted NKT cells in microbial immunity. Infect. Immun. 2003;71:5447–5455. doi: 10.1128/IAI.71.10.5447-5455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak J, Lehuen A. Mechanism of regulation of autoimmunity by iNKT cells. Cytokine. 2011;53:263–270. doi: 10.1016/j.cyto.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Giaccone G, et al. A phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Canc. Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 12.Chang Y-J, et al. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc. Natl. Acad. Sci. USA. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang P-H, et al. Quantitative microarray analysis of intact glycolipid-CD1d interaction and correlation with cell-based cytokine production. J. Am. Chem. Soc. 2008;130:12348–12354. doi: 10.1021/ja8012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raju R, et al. Synthesis and evaluation of 3”- and 4”-deoxy and -fluoro analogs of the immunostimulatory glycolipid, KRN7000. Bioorg. Med. Chem. Lett. 2009;19:4122–4125. doi: 10.1016/j.bmcl.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou XT, et al. Synthesis and NKT cell stimulating properties of fluorophore- and biotin-appended 6”-amino-6”-deoxy-galactosylceramides. Org. Lett. 2002;4:1267–1270. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]
- 16.Trappeniers M, et al. 6′-Derivatised alpha-GalCer Analogues Capable of Inducing Strong CD1d-Mediated Th1-Biased NKT Cell Responses in Mice. J. Am. Chem. Soc. 2008;130:16468–16469. doi: 10.1021/ja8064182. [DOI] [PubMed] [Google Scholar]
- 17.Tashiro T, et al. RCAI-61, the 6′-O-methylated analog of KRN7000: its synthesis and potent bioactivity for mouse lymphocytes to produce interferon-gamma in vivo. Tetrahedron Lett. 2008;49:6827–6830. doi: 10.1016/j.tetlet.2008.09.074. [DOI] [Google Scholar]
- 18.Li X, Chen G, Garcia-Navarro R, Franck RW, Tsuji M. Identification of C-glycoside analogues that display a potent biological activity against murine and human invariant natural killer T cells. Immunology. 2009;127:216–225. doi: 10.1111/j.1365-2567.2008.02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Byun HS, Bittman R. Synthesis of Immunostimulatory α-C-Galactosylceramide Glycolipids via Sonogashira Coupling, Asymmetric Epoxidation, and Trichloroacetimidate-Mediated Epoxide Opening. Org. Lett. 2010;12:2974–2977. doi: 10.1021/ol1009976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Chien M, Tsuji M, Franck RW. E and Z alpha-C-galactosylceramides by Julia-Lythgoe-Kocienski chemistry: a test of the receptor-binding model for glycolipid immunostimulants. ChemBioChem. 2006;7:1017–1022. doi: 10.1002/cbic.200500386. [DOI] [PubMed] [Google Scholar]
- 21.Jukes J-P, et al. Non-glycosidic compounds can stimulate both human and mouse iNKT cells. Eur. J. Immunol. 2016;46:1224–1234. doi: 10.1002/eji.201546114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J-J, et al. Syntheses and biological activities of KRN7000 analogues having aromatic residues in the acyl and backbone chains with varying stereochemistry. Bioorg. Med. Chem. Lett. 2010;20:814–818. doi: 10.1016/j.bmcl.2009.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 24.Goff RD, et al. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J. Am. Chem. Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 25.Chang Y-J, et al. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc. Natl. Acad. Sci. USA. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toba T, et al. Synthesis and biological evaluation of truncated α-galactosylceramide derivatives focusing on cytokine induction profile. Bioorg. Med. Chem. 2012;20:2850–2859. doi: 10.1016/j.bmc.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Kim J, Oh K, Lee D-S, Park SB. Heteroaromatic Moieties in the Sphingosine Backbone of alpha-Galactosylceramides for Noncovalent Interactions with CD1d. ACS Med. Chem. Lett. 2012;3:151–154. doi: 10.1021/ml200278u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Oh K, Song H, Lee D-S, Park SB. Synthesis and Biological Evaluation of alpha-Galactosylceramide Analogues with Heteroaromatic Rings and Varying Positions of a Phenyl Group in the Sphingosine Backbone. J. Med. Chem. 2013;56:7100–7109. doi: 10.1021/jm400949h. [DOI] [PubMed] [Google Scholar]
- 29.Berkers CR, Ovaa H. Immunotherapeutic potential for ceramide-based activators of iNKT cells. Trends Pharmacol. Sci. 2005;26:252–257. doi: 10.1016/j.tips.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Schiefner A, Fujio M, Wu D, Wong CH, Wilson IA. Structural evaluation of potent NKT cell agonists: implications for design of novel stimulatory ligands. J. Mol. Biol. 2009;394:71–82. doi: 10.1016/j.jmb.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aspeslagh S, et al. Galactose-modified iNKT cell agonists stabilized by an induced fit of CD1d prevent tumour metastasis. EMBO J. 2011;30:2294–2305. doi: 10.1038/emboj.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy C, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J. Exp. Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goff RD, et al. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J. Am. Chem. Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 34.Maiti DK, et al. Synthesis of glycal-based chiral benzimidazoles by VO(acac)2-CeCl3 combo catalyst and their self-aggregated nanostructured materials. J. Org. Chem. 2009;74:8086–8097. doi: 10.1021/jo901458k. [DOI] [PubMed] [Google Scholar]
- 35.Wild R, Schmidt RR. Synthesis of sphingosines, 11. Convenient synthesis of phytosphingosine and sphinganine from D-galactal and D-arabitol. Liebigs Annalen. 1995;5:755–764. doi: 10.1002/jlac.1995199505111. [DOI] [Google Scholar]
- 36.Figueroa-Perez S, Schmidt RR. Total synthesis of alpha-galactosyl cerebroside. Carbohydr. Res. 2000;328:95–102. doi: 10.1016/S0008-6215(00)00092-6. [DOI] [PubMed] [Google Scholar]
- 37.Zajonc DM, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat. Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, et al. The V alpha 14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J. Exp. Med. 2010;207:2383–2393. doi: 10.1084/jem.20101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu D, et al. Design of natural killer T cell activators: structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc. Natl. Acad. Sci. USA. 2006;103:3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costantino V, Fattorusso E, Imperatore C, Mangoni A. Immunomodulating glycosphingolipids: an efficient synthesis of a 2′-deoxy-α-galactosyl-GSL. Tetrahedron. 2002;58:369–375. doi: 10.1016/S0040-4020(01)01163-2. [DOI] [Google Scholar]
- 41.Wang J, et al. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc. Natl. Acad. Sci. USA. 2010;107:1535–40. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


