Abstract
Obstructive sleep apnea (OSA) is a common breathing disorder, and continuous positive airway pressure (CPAP) therapy together with its alternatives has been developed to treat this disease. This network meta-analysis (NMA) was aimed to compare the efficacy of treatments for OSA. Cochrane Library, MEDLINE, and Embase were searched for eligible studies. A conventional and NMA was carried out to compare all therapies. Sleeping characteristics, including Apnea–Hypopnea Index (AHI), Epworth Sleepiness Scale (ESS), arterial oxygen saturation, and arousal index (AI), and changes of blood pressure were selected as outcomes. A total of 84 studies were finally included after rigorous screenings. For the primary outcomes of AHI and ESS, the value of auto-adjusting positive airway pressure (APAP), CPAP, and oral appliance (OA) all showed statistically reduction compared with inactive control (IC). Similar observation was obtained in AI, with treatments of the three active interventions. A lower effect of IC in SaO2 was exhibited when compared with APAP, CPAP, and OA. Similar statistically significant results were presented in 24 h systolic blood pressure and 24 h DBP when comparing with CPAP. Our NMA identified CPAP as the most efficacious treatment for OSA patients after the evaluation of sleeping characteristics and blood pressures. In addition, more clinical trials are needed for further investigation due to the existence of inconsistency observed in this study.
Keywords: obstructive sleep apnea, Apnea–Hypopnea Index, Epworth Sleepiness Scale, blood pressures, network meta-analysis
Introduction
Obstructive sleep apnea (OSA) is a common breathing disorder which is identified by repetitive air flow reduction or cessation during sleep (1). The prevalence of OSA is estimated between 2 and 4%, varying with obesity status, gender, and age of populations (2, 3), usually caused by repetitive pharynx dysfunction which leads to apnea and hypopnea that result in the down regulation of blood oxygen levels (4). Oxygen desaturation triggered by chronic hypoxia further causes repetitive arousals and significant changes in both transmural and intra-thoracic pressure. This mechanism can increase the sympathetic activity and oxidative stress on the heart and intra-thoracic vessels, eventually resulting in vascular damages (5).
The severity of OSA can be classified by the Apnea–Hypopnea Index (AHI), evaluating the episode frequency of apnea/hypopnea in 1 h (4), which can be used to predict the relative risk of OSA. For instance, a 10% weight loss is predicted to be correlated with a 26% decrease in the AHI (6). OSA can also be evaluated by using the Epworth Sleepiness Scale (ESS), which considers both daytime sleepiness and the average sleep propensity (2, 3).
Continuous positive airway pressure (CPAP) therapy is a highly effective treatment option for OSA patients (7). Massive evidence suggests that CPAP therapy not only improves the AHI of OSA patients but also stabilizes their blood pressure levels (8, 9). Besides that, CPAP therapy is able to provide OSA patients with additional reduction in both systolic blood pressure (SBP) and diastolic blood pressure (DBP) (2, 3). One major challenge of CPAP therapy is to identify a specifically effective pressure for individual OSA patient before CPAP therapy can be continuously applied to patients. This is usually achieved through standard manual titration and it is a time-consuming task (10). The above issue may be overcome by the auto-adjusting positive airway pressure (APAP) that only applies the lowest effective pressure to patients, and the corresponding pressure delivered is continuously adjusted depending on the residual symptoms detected on patients (11). CPAP also causes discomfort or nasal problems, and thus, it is not tolerable for all OSA patients (12). As a result, oral appliance (OA) therapy has been developed as an alternative to CPAP therapy for preventing airway collapse. APAP was reported to have equivalent performance in improving sleepiness compared to CPAP therapy (13). Although OA therapy is able to improve the AHI in OSA patients, several indexes of the OA therapy are inferior to those of CPAP therapy (14). Moreover, using mandibular advancement devices (an OA therapy) was associated with a reduction in SBP and DBP among OSA patients. However, such a benefit was not observed in OSA patients with CPAP therapy (2, 3).
Evidence in the current literature mainly comes from meta-analysis which was designed to answer the above questions. However, some conflicting results and conclusions appeared to be a major issue and this may arise from variations in study design, size, and participants (6, 15, 16). Thus, a network meta-analysis (NMA) with a large scale should be designed to integrate current MAs and clinical trials, increase the level of evidence and the credibility of individual studies as well as to provide clinicians with genuine consensus for the purpose of compensating the lack of head-to-head comparison.
Materials and Methods
Identification of Trials
We comprehensively investigated the databases of the Cochrane Library, MEDLINE, and Embase. Key words and subject terms included “obstructive sleep apnea,” “physical therapy modality,” “continuous positive airway pressure,” “auto-adjusting positive airway pressure,” and “oral appliance.” Controlled trials were identified using the Cochrane Highly Sensitive Search Strategy (sensitivity-maximizing and precision-maximizing version). The references of any related records were also screened in order to include additional qualified trials. Moreover, all articles screened and reviewed were published in English.
Inclusion Criteria
Trials were supposed to meet the following standards: (1) controlled trials were preferred in our study selection. Other types of studies were also included if their research topics are relevant; (2) trials or studies must recruit patients who were older than 18 years and diagnosed with OSA. (3) OSA was specifically defined as AHI > 5/h; (4) At least two of the following treatments were compared: CPAP, APAP, OA, and inactive control (IC, such as sham CPAP and placebo). (4) The outcomes of each study should include at least one of sleeping characteristics or blood pressure, including AHI, ESS, arterial oxygen saturation (SaO2), arousal index (AI), SBP, and DBP. AHI and ESS were assessed as primary outcomes, while the other outcomes were used as secondary outcomes.
Data Extraction
We recorded basic characteristics of trials, including information of publications (author, year, and country), design of trials (RCT or non-RCT, and blinding), and follow-up durations. The changes of indexed, which were related to the quality of sleep and blood pressure, were seen as the most significant part of the trials. All the endpoints were continuous valuables, so the weighted mean difference (WMD) considering the trial size between different therapies was computed as well as corresponding sample SD.
Statistical Methods
STATA 12.0 software (Stata Corp, College Station, TX, USA) was used to perform traditional MA and WMD with corresponding 95% confidence interval (CI) were computed. We assessed heterogeneity among the included studies by Cochran’s Q test (17) and the I2 test (18). If P > 0.1 and I2 < 50%, it suggested no significant heterogeneity existed and fixed-effects model was used. Otherwise, the random-effects model was applied if there was significant heterogeneity.
We combined direct and indirect evidence by NMA, a Bayesian framework based on Markov chain Monte Carlo method. STATA 12.0 software (Stata Corp, College Station, TX, USA) and WinBUGS software (MRC Bio-statistics Unit, Cambridge, UK) were applied as computational tools, which presented WMD with the corresponding 95% credible intervals (CrIs). In order to rank these therapies with respect to each clinical outcome, the surface of cumulative ranking curve area (SUCRA) was presented and generated a simulated ranking based on SUCRA values. For each comparison, the “design-by-treatment” interaction model was used to evaluate consistency between direct and indirect evidence. In the presence of significant inconsistency, the P-value of the “design-by-treatment” interaction model would be less than 0.05 and the result was displayed graphically in the node-splitting plots and net heat plots.
Risk of Bias Assessment
We assessed the risk of bias by using the Cochrane Collaboration’s criteria. Each study was assessed with respect to several types of bias (performance, detection, selection, attrition, and reporting bias) and classified as being at low, unclear, or high risk of bias for each potential source of bias. A “comparison adjusted” funnel plot was exhibited in order to illustrate publication bias, and the degree of symmetry in the funnel plot indicated whether the small-study effect was significant or not.
Results
Characteristics of Trials and Patients
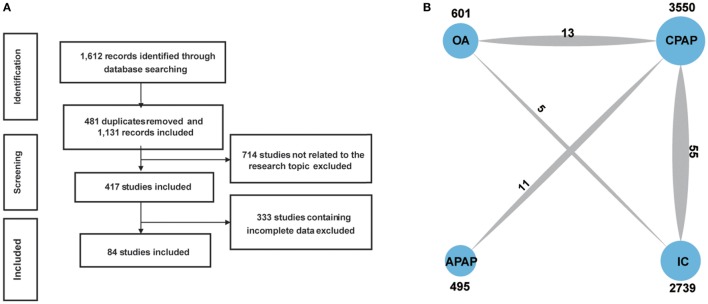
We retrieved and screened literature in the process showed in Figure 1A. A total of 1,612 records were identified through database searching and 481 were removed as duplicates. We excluded 714 records by reviewing their topics or abstracts. Another 333 records were removed after full-text reading since they contained incomplete data. A total of 84 studies were finally included and RCTs (9, 11, 13, 19–99). The pattern of evidence provided by studies was displayed in the network plot (Figure 1B). The size of nodes represents the sample size, and the thickness of lines indicates the number of trials comparing two therapies. CPAP was investigated in most trials. The baseline characteristics of studies were recorded in Table 1. Trials collected in our study were conducted around the world, 41 in Europe, 20 in North America, 6 in Brazil, 5 in China, 8 in Australia, and 1 in New Zealand, Japan, India, and Pakistan, respectively.
Figure 1.
Flowchart (A) and network plot (B). The network plot show direct comparison of different treatments, with node size corresponding to the sample size. The number of included studies for specific direct comparison decides the thickness of solid lines. Abbreviations: CPAP, continuous positive airway pressure; APAP, auto-adjusting positive airway pressure; IC, inactive control; OA, oral appliance.
Table 1.
Main characteristics of included studies.
| Reference | Country | Design | Blinding | Follow-up | Treatment 1 |
Treatment 2 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1 | n | Age | Male (%) | BMI | AHI (h−1) | ESS | t2 | n | Age | Male (%) | BMI | AHI (h−1) | ESS | |||||
| Salord et al. (19) | Spain | RCT | Double | – | CPAP | 42 | 48.5 ± 8.6 | 26 | 45.7 ± 5 | 68.3 ± 11 | 7.9 ± 4.5 | IC | 38 | 44.6 ± 9.4 | 29 | 45.7 ± 5 | 68.3 ± 11 | 7.9 ± 4.5 |
| Pepin et al. (11) | France | RCT | Double | 4 months | CPAP | 133 | 58 ± 3.5 | 70.8 | 37.7 ± 6.5 | 9 ± 2 | APAP | 143 | 58 ± 3.2 | 69.6 | – | 37.7 ± 6.5 | 9 ± 2 | |
| Paz et al. (20) | USA | RCT | Double | 1 months | IC | 74 | 51.0 ± 11.7 | 52.7 | 37.3 ± 8.3 | 20.6 ± 6.2 | 10 ± 1.2 | CPAP | 75 | 51.7 ± 11.8 | 54.7 | 37.3 ± 8.3 | 20.6 ± 6.2 | 10 ± 1.2 |
| Pamidi et al. (21) | Canada | RCT | Open-label | 2 months | CPAP | 26 | 53.8 ± 6.2 | 62 | 36.8 ± 7.8 | 34.2 ± 24.5 | 10 ± 5.9 | OA | 13 | 55.2 ± 8.4 | 77 | 36.8 ± 7.8 | 34.2 ± 24.5 | 10 ± 5.9 |
| Muxfeldt et al. (22) | Brazil | RCT | Open-label | 6 months | IC | 60 | 60.2 ± 8.4 | 41.7 | 33.8 ± 5.8 | 39 ± 18 | 12 ± 6 | CPAP | 57 | 60.8 ± 8.0 | 37.9 | 33.8 ± 5.8 | 39 ± 18 | 12 ± 6 |
| Martinez-Garcia et al. (23) | Spain | RCT | Open-label | – | CPAP | 115 | 75.5 ± 3.8 | 63.5 | 33 ± 7.3 | 53.5 ± 15.6 | 9.6 ± 4 | IC | 109 | 75.6 ± 4.0 | 73.4 | 33 ± 7.3 | 53.5 ± 15.6 | 9.6 ± 4 |
| Huang et al. (24) | China | RCT | Single | 6 months | IC | 37 | 62.7 ± 6.7 | 86.5 | 27.5 ± 2.6 | 28.7 ± 12.4 | 8.3 ± 3.4 | CPAP | 36 | 62.0 ± 6.8 | 77.8 | 27.5 ± 2.6 | 28.7 ± 12.4 | 8.3 ± 3.4 |
| Dalmases et al. (25) | Spain | RCT | Open-label | 3 months | CPAP | 17 | 70.8 ± 5.1 | 64.7 | 32.8 ± 3.9 | 61.2 ± 17.9 | 7.94 ± 2.99 | IC | 16 | 71.9 ± 6.0 | 75 | 32.8 ± 3.9 | 61.16 ± 17.86 | 7.94 ± 2.99 |
| Woodson et al. (26) | USA | RCT | Open-label | 12 months | CPAP | 23 | 57.1 ± 10 | 95.6 | 28.4 ± 2.4 | 31.3 ± 12.3 | 11.2 ± 5.3 | IC | 23 | 52.7 ± 10.4 | 82.6 | 28.4 ± 2.4 | 31.3 ± 12.3 | 11.2 ± 5.3 |
| Neikrug et al. (27) | USA | RCT | Open-label | 6 weeks | CPAP | 19 | 66.7 ± 8.5 | 63.2 | 27.8 ± 4.7 | 21.1 ± 14.9 | – | IC | 19 | 67.7 ± 10.0 | 73.7 | 27.8 ± 4.7 | 21.1 ± 14.9 | – |
| Lloberes et al. (28) | Spain | RCT | – | – | CPAP | 36 | 58.3 ± 9.4 | 70.7 | 31.4 ± 4.9 | 50.1 ± 21.6 | 6.76 ± 3.7 | IC | 42 | 58.3 ± 9.4 | 70.7 | 31.4 ± 4.9 | 50.1 ± 21.6 | 6.76 ± 3.7 |
| Gottlieb et al. (29) | USA | RCT | – | 3 months | CPAP | 90 | 63.5 ± 7.0 | 76 | 33 ± 5 | 25.4 ± 8.7 | 8 ± 3.8 | IC | 97 | 63.1 ± 7.7 | 78 | 33 ± 5 | 25.4 ± 8.7 | 8 ± 3.8 |
| Chasens et al. (30) | USA | RCT | Double | 1 month | CPAP | 12 | – | 58 | 50.2 ± 30.9 | 11.42 ± 4.62 | IC | 11 | – | 64 | – | 50.2 ± 30.9 | 11.42 ± 4.62 | |
| Berry and Sriram (13) | USA | RCT | – | – | APAP | 78 | 57.7 ± 12.1 | 93.5 | 34.2 ± 5.8 | – | 15.2 ± 4.4 | CPAP | 70 | 59.7 ± 12.6 | 92.3 | 34.2 ± 5.8 | – | 15.2 ± 4.4 |
| Schutz et al. (31) | Brazil | RCT | – | 2 months | CPAP | 9 | 38.62 ± 8.15 | – | 25.9 ± 5.31 | 25.1 ± 10.5 | – | OA | 9 | 42.33 ± 6.20 | – | 25.9 ± 5.31 | 25.1 ± 10.5 | – |
| Phillips et al. (32) | Australia | RCT | Open-label | 1 month | CPAP | 56 | 49.5 ± 11.2 | 80.9 | 29.5 ± 5.5 | 25.6 ± 12.3 | 9.1 ± 4.2 | OA | 52 | 49.5 ± 11.2 | 80.9 | 29.5 ± 5.5 | 25.6 ± 12.3 | 9.1 ± 4.2 |
| Pedrosa et al. (33) | Brazil | RCT | Open-label | 6 months | CPAP | 19 | 57.0 ± 2.1 | 74 | 36 ± 2.5 | 36 ± 6.5 | 12 ± 4 | IC | 16 | 55 ± 2 | 81 | 36 ± 2.5 | 36 ± 6.5 | 12 ± 4 |
| Martinez-Garcia et al. (9) | Spain | RCT | Single | 3 months | CPAP | 98 | 57.8 ± 9.5 | 72.4 | 34.3 ± 5.7 | 41.3 ± 18.7 | 8.9 ± 4 | IC | 96 | 58.2 ± 9.6 | 64.6 | 34.3 ± 5.7 | 41.3 ± 18.7 | 8.9 ± 4 |
| Diaferia et al. (34) | Brazil | RCT | – | 3 months | IC | 24 | 48.1 ± 11.2 | 100 | 27.4 ± 4.9 | 27.8 ± 20.3 | 12.7 ± 3 | CPAP | 27 | 48.1 ± 11.2 | 100 | 27.4 ± 4.9 | 27.8 ± 20.3 | 12.7 ± 3 |
| Andren et al. (35) | Sweden | RCT | Single | 3 months | OA | 36 | 57.0 ± 8.0 | 83 | 30 ± 4 | 23 ± 16 | 11 ± 5.4 | IC | 36 | 59 ± 9 | 75 | 30 ± 4 | 23 ± 16 | 11 ± 5.4 |
| Sivam et al. (36) | USA | RCT | Single | 2 months | CPAP | 27 | 47.0 ± 13.0 | 96.3 | 31.3 ± 3.8 | 37.2 ± 24.7 | 10 ± 4.8 | IC | 27 | 47 ± 13 | 96.3 | 31.3 ± 3.8 | 37.2 ± 24.7 | 10 ± 4.8 |
| Lee et al. (37) | USA | RCT | Double | 1 month | CPAP | 26 | 48.3 ± 9.3 | 84.6 | 29.8 ± 4.6 | 36.7 ± 21.8 | – | IC | 30 | 48.2 ± 9.0 | 83.3 | 29.8 ± 4.6 | 36.7 ± 21.8 | – |
| Kushida et al. (38) | USA | RCT | Double | 6 months | CPAP | 453 | 52.2 ± 12.2 | 65.3 | 32.4 ± 7.3 | 39.7 ± 24.9 | 10.07 ± 4.26 | IC | 422 | 50.8 ± 12.2 | 65.7 | 32.4 ± 7.3 | 39.7 ± 24.9 | 10.07 ± 4.26 |
| Hoyos et al. (39) | Australia | RCT | Double | 3 months | CPAP | 34 | 51.0 ± 12.3 | – | 31.6 ± 5.3 | 38.5 ± 14.7 | 10 ± 4 | IC | 31 | 46.4 ± 10.4 | – | 31.6 ± 5.3 | 38.5 ± 14.7 | 10 ± 4 |
| Sharma et al. (40) | India | RCT | Double | 3 months | CPAP | 86 | 45.1 ± 8.0 | 84 | 33.8 ± 4.7 | 47.9 ± 19.6 | 14.8 ± 3.7 | IC | 86 | 45 ± 8 | 95 | 33.8 ± 4.7 | 47.9 ± 19.6 | 14.8 ± 3.7 |
| Ryan et al. (41) | Canada | RCT | Open-label | 6 months | IC | 22 | 60.7 ± 10.3 | 86.4 | 27.3 ± 5.8 | 33.3 ± 16.4 | 4.5 ± 2.1 | CPAP | 22 | 62.8 ± 12.8 | 72.7 | 27.3 ± 5.8 | 33.3 ± 16.4 | 4.5 ± 2.1 |
| Phillips et al. (42) | Australia | RCT | Single | 2 months | CPAP | 37 | 49.4 ± 13.2 | 92.1 | 32.1 ± 4.3 | 41.2 ± 23.9 | 11.2 ± 4.9 | IC | 37 | 49 ± 13 | 92.1 | 32.1 ± 4.3 | 41.2 ± 23.9 | 11.2 ± 4.9 |
| Kohler et al. (43) | Swiss | RCT | Single | 1 month | CPAP | 20 | 63.6 ± 5.1 | 95 | 32.9 ± 6.5 | 36 ± 17.3 | 13.8 ± 2.6 | IC | 21 | 61.8 ± 7.5 | 100 | 32.9 ± 6.5 | 36 ± 17.3 | 13.8 ± 2.6 |
| Drager et al. (44) | Brazil | RCT | – | 3 months | IC | 18 | 44.1 ± 7.0 | – | 29 ± 2.6 | 58 ± 23 | 11 ± 5 | CPAP | 18 | 43 ± 7 | – | 29 ± 2.6 | 58 ± 23 | 11 ± 5 |
| Aarab et al. (45) | Netherlands | RCT | Single | 12 months | OA | 21 | 50.4 ± 8.9 | 80.9 | 27.1 ± 3.1 | 21.4 ± 11 | – | CPAP | 22 | 54.9 ± 10.1 | 68.2 | 27.1 ± 3.1 | 21.4 ± 11 | – |
| Nguyen et al. (46) | USA | RCT | Double | 3 months | CPAP | 10 | 52.9 ± 11.6 | 80 | 30.1 ± 4.7 | 38.8 ± 21.4 | – | IC | 10 | 53.9 ± 10.8 | 100 | 30.1 ± 4.7 | 38.8 ± 21.38 | – |
| Lozano et al. (47) | Spain | RCT | 3 months | IC | 35 | 59.2 ± 10.9 | 62.9 | 31.5 ± 5.6 | 46.8 ± 21.4 | – | CPAP | 29 | 59.2 ± 8.7 | 75.9 | 31.5 ± 5.6 | 46.78 ± 21.43 | – | |
| Lam et al. (48) | China | RCT | Double | 1 week | CPAP | 31 | 46.5 ± 10.8 | 27.8 ± 3.7 | 27.8 ± 3.8 | 33.4 ± 9.5 | 10.3 ± 4.9 | IC | 30 | 46.5 ± 10.8 | 27.2 ± 3.7 | 27.8 ± 3.8 | 33.4 ± 9.5 | 10.3 ± 4.9 |
| Durán-Cantolla et al. (49) | Spain | RCT | Double | 12 weeks | CPAP | 169 | 53.2 ± 10.2 | 79 | 31.9 ± 5.7 | 39.8 ± 22.7 | 10.3 ± 4.2 | IC | 171 | 51.7 ± 10.8 | 84 | 31.9 ± 5.7 | 39.8 ± 22.7 | 10.3 ± 4.2 |
| Barbe et al. (50) | Spain | RCT | Open-label | 12 months | CPAP | 179 | 55 ± 10 | 82 | 32 ± 5 | 43 ± 19 | 6.4 ± 2.4 | IC | 177 | 56 ± 10 | 85 | 32 ± 5 | 43 ± 19 | 6.4 ± 2.4 |
| Galetke et al. (51) | Germany | RCT | – | 6 weeks | CPAP | 19 | 51.7 ± 10.4 | 94.7 | 32.1 ± 5.7 | 40.5 ± 21.5 | 11.3 ± 4.7 | APAP | 15 | 52.1 ± 9.2 | 86.7 | 32.1 ± 5.7 | 40.5 ± 21.5 | 11.3 ± 4.7 |
| Gagnadoux et al. (52) | France | RCT | – | 2 months | CPAP | 59 | 50.3 ± 9.1 | – | 26.7 ± 3.5 | 34.2 ± 13 | 10.6 ± 4.5 | OA | 59 | 50.3 ± 9.1 | – | 26.7 ± 3.5 | 34.2 ± 13 | 10.6 ± 4.5 |
| Damjanovic et al. (53) | Germany | RCT | – | 9 months | APAP | 50 | 57.6 ± 2.1 | 74 | 31.8 ± 8.5 | 41.8 ± 24.7 | 8.5 ± 5.6 | CPAP | 50 | 59.4 ± 2.1 | 82 | 31.8 ± 8.5 | 41.8 ± 24.7 | 8.5 ± 5.6 |
| Siccoli et al. (54) | UK | RCT | – | 1 month | IC | 51 | 48.7 ± 10.6 | – | 34.5 ± 5 | – | 15.2 ± 4 | CPAP | 51 | 48.1 ± 9.5 | – | 34.5 ± 5 | – | 15.2 ± 4 |
| Ruttanaumpawan et al. (55) | Canada | RCT | – | 1 month | IC | 14 | 60.5 ± 10.3 | 85.7 | 32.3 ± 8.6 | 51.3 ± 15.6 | – | CPAP | 19 | 59.0 ± 7.8 | 94.7 | 32.3 ± 8.6 | 51.3 ± 15.6 | – |
| Petri et al. (56) | Denmark | RCT | Double | 1 month | OA | 27 | 50 ± 11 | 85.2 | 30.7 ± 5.2 | 39.1 ± 23.8 | 11.7 ± 4.3 | IC | 29 | 49 ± 10 | 79.3 | 30.7 ± 5.2 | 39.1 ± 23.8 | 11.7 ± 4.3 |
| Kohler et al. (57) | UK | RCT | Double | 1 month | IC | 49 | 48.7 ± 10.6 | – | 34.5 ± 5 | – | 15.2 ± 4 | CPAP | 50 | 48.1 ± 9.5 | – | 34.5 ± 5 | – | 15.2 ± 4 |
| Hoekema et al. (58) | Neherlands | RCT | Open-label | 3 months | OA | 47 | – | – | – | 39.4 ± 30.8 | – | CPAP | 47 | – | – | – | 39.4 ± 30.8 | – |
| Galetke et al. (59) | Germany | RCT | Single | 2 months | CPAP | 20 | – | – | – | 32.9 ± 19.1 | 10.3 ± 5.7 | APAP | 20 | – | – | – | 32.9 ± 19.1 | 10.3 ± 5.7 |
| Egea et al. (60) | Spain | RCT | – | 3 months | CPAP | 28 | 64 ± 0.9 | 96 | 31.7 ± 12.7 | 43 ± 23.3 | 8 ± 3.7 | IC | 32 | 63 ± 1.6 | 91 | 31.7 ± 12.7 | 43 ± 23.3 | 8 ± 3.7 |
| Cross et al. (61) | UK | RCT | Double | 6 weeks | IC | 29 | 48 ± 2 | 95.6 | 37 ± 5.4 | 63 ± 26.9 | – | CPAP | 29 | – | – | 37 ± 5.4 | 63 ± 26.9 | – |
| West et al. (62) | UK | RCT | Double | 3 months | CPAP | 19 | 57.8 ± 10.4 | 36.6 ± 4.9 | – | 14.7 ± 3.5 | IC | 21 | 54.5 ± 9.4 | – | 36.6 ± 4.9 | – | 14.7 ± 3.5 | |
| Smith et al. (63) | UK | RCT | Double | 6 weeks | IC | 26 | 61 ± 8 | 88.5 | 31 ± 4 | 36 ± 23 | 10 ± 5 | CPAP | 26 | 61 ± 8 | 88.5 | 31 ± 4 | 36 ± 23 | 10 ± 5 |
| Patruno et al. (64) | Italy | RCT | – | 3 months | APAP | 15 | 46.7 ± 11.6 | 80 | 36.4 ± 7.1 | 47.3 ± 14.7 | 15.8 ± 3.5 | CPAP | 16 | 47 ± 10.7 | 81.2 | 36.4 ± 7.1 | 47.3 ± 14.7 | 15.8 ± 3.5 |
| Martinez-Garcia et al. (65) | Spain | – | – | 3 months | CPAP | 10 | 68.1 ± 7.8 | 52.2 | 35.1 ± 5.2 | 40 ± 19.7 | 7.4 ± 5.1 | IC | 23 | 72.2 ± 3.2 | 50 | 35.1 ± 5.2 | 40 ± 19.7 | 7.4 ± 5.1 |
| Lam et al. (66) | China | RCT | – | 10 weeks | CPAP | 34 | 45 ± 1 | 79 | 27.6 ± 3.5 | 23.8 ± 11.1 | 12 ± 5.8 | OA | 34 | 45 ± 2 | 76 | 27.6 ± 3.5 | 23.8 ± 11.1 | 12 ± 5.8 |
| Haensel et al. (67) | Netherlands | RCT | Double | 2 weeks | CPAP | 25 | 48.2 ± 10.2 | 80 | 33.1 ± 8.2 | 63.6 ± 29.1 | – | IC | 25 | 49.0 ± 10.6 | 80 | 33.1 ± 8.2 | 63.6 ± 29.1 | – |
| Fietze et al. (68) | Germany | RCT | – | 6 weeks | APAP | 10 | 56.9 ± 9.3 | 100 | 32.6 ± 6.6 | 43.3 ± 30.2 | – | CPAP | 11 | 51.8 ± 13.5 | 90.9 | 32.6 ± 6.6 | 43.3 ± 30.2 | – |
| Drager et al. (69) | Brazil | RCT | Double | 4 months | IC | 12 | 47 ± 6 | – | 29.7 ± 2.9 | 62 ± 22 | 13 ± 5 | CPAP | 12 | 44 ± 7 | – | 29.7 ± 2.9 | 62 ± 22 | 13 ± 5 |
| Coughlin et al. (70) | UK | RCT | Single | 6 weeks | CPAP | 34 | 49.0 ± 8.3 | – | 36.1 ± 7.6 | – | 13.8 ± 4.9 | IC | 34 | 49.0 ± 8.3 | – | 36.1 ± 7.6 | – | 13.8 ± 4.9 |
| Robinson et al. (71) | UK | RCT | Open-label | 1 months | IC | 16 | 54 ± 8 | 86.1 | 33.2 ± 5.3 | – | 5.3 ± 1 | CPAP | 16 | 54 ± 8 | 86.1 | 33.2 ± 5.3 | – | 5.3 ± 1 |
| Hui et al. (72) | China | RCT | Single | 3 months | CPAP | 28 | 50.3 ± 1.6 | 78.6 | 27.5 ± 3.2 | 32.9 ± 16.9 | 10.7 ± 5.3 | IC | 28 | 51.2 ± 1.8 | 75 | 27.5 ± 3.2 | 32.9 ± 16.9 | 10.7 ± 5.3 |
| Campos-Rodriguez et al. (73) | Spain | RCT | Double | 1 months | CPAP | 34 | 55.3 ± 9.6 | 55.8 | 35.7 ± 5.6 | 58.3 ± 24.6 | 15 ± 3.9 | IC | 34 | 58.0 ± 7.0 | 64.7 | 35.7 ± 5.6 | 58.3 ± 24.6 | 15 ± 3.9 |
| Usui et al. (74) | Canada | RCT | – | 1 months | OA | 9 | 52.2 ± 4.1 | 77.8 | 31.3 ± 4.8 | – | – | CPAP | 8 | 55.0 ± 2.0 | 100 | 31.3 ± 4.8 | – | – |
| Marshall et al. (75) | New Zealand | RCT | Double | 3 weeks | IC | 29 | 45 ± 9.8 | 75.9 | 32.3 ± 3.5 | – | 12.5 ± 4.3 | CPAP | 29 | 45 ± 9.8 | 75.9 | 32.3 ± 3.5 | – | 12.5 ± 4.3 |
| Blanco et al. (76) | Spain | RCT | – | 2 weeks | OA | 8 | 55.6 ± 11.8 | 86.7 | 27.9 ± 4.3 | 33.8 ± 14.7 | – | IC | 7 | 55.6 ± 11.8 | 86.7 | 27.9 ± 4.3 | 33.8 ± 14.7 | – |
| Arias et al. (77) | Spain | RCT | Double | 3 months | IC | 25 | 52 ± 13 | 100 | 30.5 ± 4 | 44 ± 27.5 | – | CPAP | 25 | 52 ± 13 | 100 | 30.5 ± 4 | 44 ± 27.5 | – |
| Masa et al. (78) | Spain | RCT | Single | 3 months | CPAP | 126 | 51.0 ± 9.1 | 86.9 | 33.6 ± 8.4 | 61.8 ± 22 | 15.9 ± 3.5 | APAP | 119 | 52.2 ± 10.4 | 89.6 | 33.6 ± 8.4 | 61.8 ± 22 | 15.9 ± 3.5 |
| Mansfield et al. (79) | Australia | RCT | Single | 3 months | IC | 21 | 57.5 ± 1.6 | 88.9 | 33.3 ± 5.5 | 26.6 ± 20.6 | 8.8 ± 4.1 | CPAP | 19 | 57.2 ± 1.7 | 100 | 33.3 ± 5.5 | 26.6 ± 20.6 | 8.8 ± 4.1 |
| Lloberes et al. (80) | Spain | – | – | 3 months | APAP | 27 | 53.9 ± 7.7 | 81.5 | 32 ± 5.8 | 55.2 ± 24.2 | 13.3 ± 5.1 | CPAP | 30 | 58.6 ± 8.7 | 76.7 | 32 ± 5.8 | 55.2 ± 24.2 | 13.3 ± 5.1 |
| Ip et al. (81) | China | RCT | Single | 3 months | CPAP | 14 | 44.4 ± 6.9 | 100 | 29.6 ± 5.7 | 47.7 ± 15.3 | – | IC | 13 | 40.9 ± 11.1 | 100 | 29.6 ± 5.7 | 47.7 ± 15.3 | – |
| Hussain et al. (82) | Pakistan | RCT | Single | 1 week | APAP | 10 | 44.9 ± 9.7 | 90 | 35.9 ± 12.9 | 47.2 ± 35.6 | 11.1 ± 6.4 | CPAP | 10 | 44.9 ± 9.7 | 90 | 35.9 ± 12.9 | 47.2 ± 35.6 | 11.1 ± 6.4 |
| Gotsopoulos et al. (83) | Australia | RCT | Double | 1 month | IC | 33 | 48 ± 11 | 79.1 | 29.2 ± 27.6 | 27 ± 86.2 | – | OA | 31 | 48 ± 11 | 79.1 | 29.2 ± 27.6 | 27 ± 86.2 | – |
| Barnes et al. (84) | Australia | RCT | – | 3 months | CPAP | 97 | 47.0 ± 0.9 | 78.38 | 31 ± 5.9 | 21.3 ± 12.8 | 10.7 ± 3.9 | OA | 99 | 47.0 ± 0.9 | 78.38 | 31 ± 5.91 | 21.3 ± 12.8 | 10.7 ± 3.9 |
| Woodson et al. (85) | USA | RCT | Double | 6 months | IC | 30 | 46.0 ± 8.1 | 70 | 28.5 ± 4.2 | 15.4 ± 7.8 | 11.6 ± 3.5 | CPAP | 28 | 51.7 ± 8.6 | 75 | 28.5 ± 4.2 | 15.4 ± 7.8 | 11.6 ± 3.5 |
| Kaneko et al. (86) | Japan | RCT | Single | 1 month | IC | 12 | 55.2 ± 3.6 | 83.3 | 32.3 ± 8.7 | 45.2 ± 18.3 | 5.7 ± 3.1 | CPAP | 12 | 55.9 ± 2.5 | 91.7 | 32.3 ± 8.7 | 45.2 ± 18.3 | 5.7 ± 3.1 |
| Becker et al. (87) | Australia | RCT | Single | 3 months | CPAP | 16 | 54.4 ± 8.9 | 93.7 | 33.3 ± 5.1 | 62.5 ± 17.8 | 14.4 ± 2.5 | IC | 16 | 52.3 ± 8.4 | 87.5 | 33.3 ± 5.1 | 62.5 ± 17.8 | 14.4 ± 2.5 |
| Tan et al. (88) | UK | RCT | – | 2 months | CPAP | 10 | 50.9 ± 10.1 | 83.3 | 31.9 ± 6.8 | 22.2 ± 9.6 | 13.4 ± 4.6 | OA | 14 | 50.9 ± 10.1 | 83.3 | 31.9 ± 6.8 | 22.2 ± 9.6 | 13.4 ± 4.6 |
| Randerath et al. (89) | Germany | RCT | – | 6 weeks | CPAP | 20 | 56.5 ± 10.2 | 80 | 31.2 ± 6.4 | 17.5 ± 7.7 | – | OA | 20 | 56.5 ± 10.2 | 80 | 31.2 ± 6.4 | 17.5 ± 7.7 | – |
| Pepperell et al. (90) | UK | RCT | Double | 4 weeks | IC | 59 | 51.0 ± 9.8 | – | 35.3 ± 6 | – | 16 ± 3.1 | CPAP | 59 | 50.1 ± 10.4 | – | 35.3 ± 6 | – | 16 ± 3.1 |
| Gotsopoulos et al. (91) | Australia | RCT | Double | 3 months | OA | 73 | 48 ± 11 | 80.8 | 29 ± 4.5 | – | – | IC | 73 | 48 ± 11 | 80.8 | 29 ± 40.1 | – | – |
| Monasterio et al. (92) | Spain | RCT | Single | 6 months | IC | 59 | 54 ± 9 | 91 | 29.5 ± 3.3 | 21 ± 6 | 13.2 ± 4.3 | CPAP | 66 | 53 ± 9 | 81 | 29.5 ± 3.3 | 21 ± 6 | 13.2 ± 4.3 |
| Bardwell et al. (93) | USA | RCT | – | 1 week | IC | 16 | 48 ± 2.2 | – | 29.6 ± 5.2 | – | – | CPAP | 20 | 47 ± 1.9 | – | 29.6 ± 5.2 | – | – |
| Barbé et al. (94) | Spain | RCT | Single | 6 weeks | CPAP | 29 | 54 ± 2 | 89.6 | 29 ± 5.4 | 54 ± 16.1 | 7 ± 2.1 | IC | 25 | 52 ± 2 | 96 | 29 ± 5.4 | 54 ± 16.1 | 7 ± 2.1 |
| Ballester et al. (95) | Spain | RCT | – | 3 months | IC | 37 | 54 ± 1.5 | 86.5 | 34 ± 7.3 | 58 ± 18.2 | 11.4 ± 6.1 | CPAP | 68 | 53 ± 1.3 | 88.2 | 34 ± 7.3 | 58 ± 18.2 | 11.4 ± 6.1 |
| Redline et al. (96) | USA | RCT | – | 2 months | IC | 46 | 49.2 ± 10.5 | – | 32 ± 8.5 | – | 10.6 ± 5.6 | CPAP | 51 | 48.1 ± 9.2 | – | 32 ± 8.5 | – | 10.6 ± 5.6 |
| Ferguson et al. (97) | Canada | RCT | – | 3 months | OA | 24 | 44 ± 10.6 | – | 32 ± 8.2 | 25.3 ± 15.0 | – | CPAP | 24 | 44 ± 10.6 | – | 32 ± 8.2 | 25.3 ± 15 | – |
| Meurice et al. (98) | Canada | RCT | Double | 3 weeks | APAP | 8 | 54 ± 11 | 100 | 34.2 ± 5.7 | 40.5 ± 17.7 | 15.2 ± 4.2 | CPAP | 8 | 54 ± 11 | 100 | 34.2 ± 5.7 | 40.5 ± 17.7 | 15.2 ± 4.2 |
| Ferguson et al. (99) | Canada | RCT | – | 4 months | OA | 25 | 46.2 ± 10.9 | 88.9 | 30.4 ± 4.8 | 19.7 ± 13.8 | – | CPAP | 21 | 46.2 ± 10.9 | 88.9 | 30.4 ± 4.8 | 19.7 ± 13.8 | – |
Treatment: CPAP, continuous positive airway pressure; APAP, auto-adjusting positive airway pressure; IC, inactive control; OA, oral appliance.
Baseline: BMI, body mass index; AHI, Apnea–Hypopnea Index; ESS, Epworth Sleepiness Scale.
Meta-analysis Result
We summarized pair-wise comparisons from MA and details of available data were shown in Table 2. When compared with IC, CPAP showed better efficacy regarding all outcomes with WMD = 23.82, 95% CI: (18.49, 29.15) for AHI, WMD = 2.07, 95% CI: (1.51, 2.62) for ESS, WMD = 10.76, 95% CI: (7.84, 13.69) for AI, and WMD = −9.35, 95% CI: (−11.41, −7.29) for SaO2. In addition, CPAP could significantly reduce blood pressure than IC [WMD = 1.89, 95% CI: (0.86, 2.92) for 24 h SBP; WMD = 1.70, CI: (1.13, 2.27) for 24 h DBP; WMD = 3.09, 95% CI: (2.18, 4.01) for dSBP; WMD = 1.98, 95% CI: (1.25, 2.70) for dDBP; and WMD = 4.22, 95% CI: (2.14, 6.30) for nSBP; WMD = 1.97, 95% CI: (0.81, 3.13) for nDBP]. No statistical difference occurred in the comparison of CPAP versus APAP. However, CPAP presented greater reduction than OA in AHI [WMD = −8.77, 95% CI: (−16.05, −1.50)] and AI [WMD = −2.59, 95% CI: (−4.91, −0.27)], as well as blood pressure [WMD = −9.57, 95% CI: (−11.34, −7.81) for 24 h SBP; WMD = −7.11, 95% CI: (−8.06, −6.15) for 24 h DBP; WMD = −7.87, 95% CI: (−13.28, −2.46) for dSBP], and it led to an increase in SaO2 (WMD = 4.91, 95% CI: 2.85–6.97) versus OA. We found significant improvement with treatment of OA compared with IC, in the outcomes of AHI [WMD = 9.40, 95% CI: (5.57, 13.23)], ESS [WMD = 1.15, 95% CI: (0.43, 1.87)], AI [WMD = 9.16, 95% CI: (2.70, 15.61)], SaO2 [WMD = −2.57, 95% CI: (−3.53, −1.61)], 24 h SBP [WMD = 1.64, 95% CI: (0.02, 3.26)], and dDBP [WMD = 2.15, 95% CI: (0.02, 4.28)]. Generally, according to the MA results, CPAP and OA were proved to be more efficacious than IC, while there were no obvious difference in the effectiveness of CPAP and APAP. CPAP showed higher ability of reducing AHI, AI, and blood pressure than OA.
Table 2.
Direct pair-wise comparison results of obstructive sleep apnea.
| Outcomes | IC versus CPAP |
CPAP versus APAP |
CPAP versus OA |
IC versus OA |
||||
|---|---|---|---|---|---|---|---|---|
| WMD (95% CI) | I2 (%) | WMD (95% CI) | I2 (%) | WMD (95% CI) | I2 (%) | WMD (95% CI) | I2 (%) | |
| AHI | 23.82 (18.49, 29.15) | 93.2 | 0.10 (−3.15, 3.34) | 0.0 | −8.77 (−16.05, −1.50) | 96.2 | 9.40 (5.57, 13.23) | 32.7 |
| ESS | 2.07 (1.51, 2.62) | 88.0 | 0.08 (−0.68, 0.85) | 12.7 | −0.31 (−1.02, 0.39) | 0.0 | 1.15 (0.43, 1.87) | 0.0 |
| AI | 10.76 (7.84, 13.69) | 14.9 | 0.32 (−3.05, 3.69) | 0.0 | −2.59 (−4.91, −0.27) | 0.0 | 9.16 (2.70, 15.61) | 78.9 |
| SaO2 | −9.35 (−11.41, −7.29) | 56.8 | 2.71 (−0.88, 6.29) | 0.0 | 4.91 (2.85, 6.97) | 60.0 | −2.57 (−3.53, −1.61) | 0.0 |
| 24 h SBP | 1.89 (0.86, 2.92) | 23.3 | – | – | −9.57 (−11.34, −7.81) | 0.0 | 1.64 (0.02, 3.26) | 0.0 |
| 24 h DBP | 1.70 (1.13, 2.27) | 0.0 | – | – | −7.11 (−8.06, −6.15) | 0.0 | 1.18 (−0.02, 2.38) | 0.0 |
| dSBP | 3.09 (2.18, 4.01) | 6.8 | −1.70 (−5.11, 1.71) | – | −7.87 (−13.28, −2.46) | 56.5 | 2.81 (−0.13, 5.74) | 0.0 |
| dDBP | 1.98 (1.25, 2.70) | 14.4 | −1.30 (−3.47, 0.87) | – | −2.40 (−8.01, 3.26) | – | 2.15 (0.02, 4.28) | 0.0 |
| nSBP | 4.22 (2.14, 6.30) | 68.0 | −1.10 (−4.74, 2.54) | – | −3.90 (−12.88, 5.08) | – | 1.06 (−2.09, 4.21) | 0.0 |
| nDBP | 1.97 (0.81, 3.13) | 45.9 | −1.30 (−3.42, 0.82) | – | −2.10 (−7.80, 3.60) | – | 0.95 (−1.23, 3.14) | 0.0 |
Treatment: CPAP, continuous positive airway pressure; APAP, auto-adjusting positive airway pressure; IC, inactive control; OA, oral appliance.
Outcomes: AHI, Apnea–Hypopnea Index; ESS, Epworth Sleepiness Scale; SaO2, arterial oxygen saturation; AI, arousal index; SBP, systolic blood pressure; DBP, diastolic blood pressure; d, daytime; n, nighttime; WMD, weighted mean difference; CI, confidence interval.
Network Meta-analysis
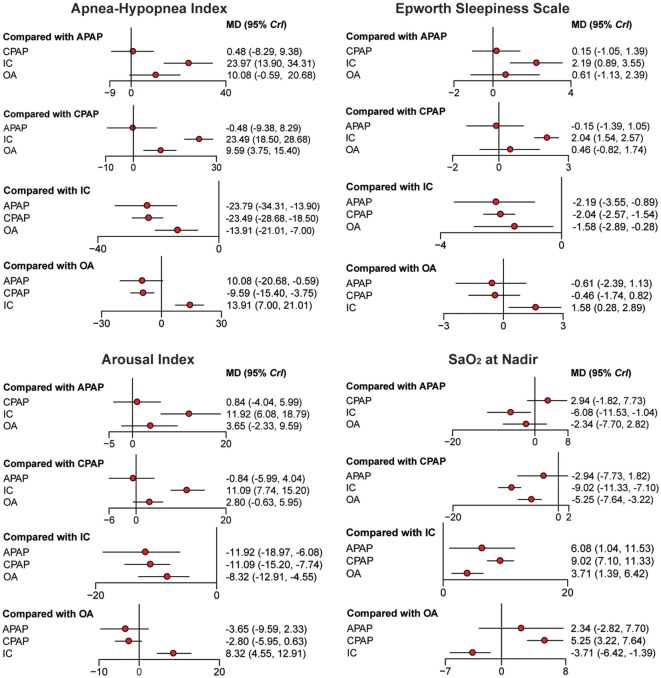
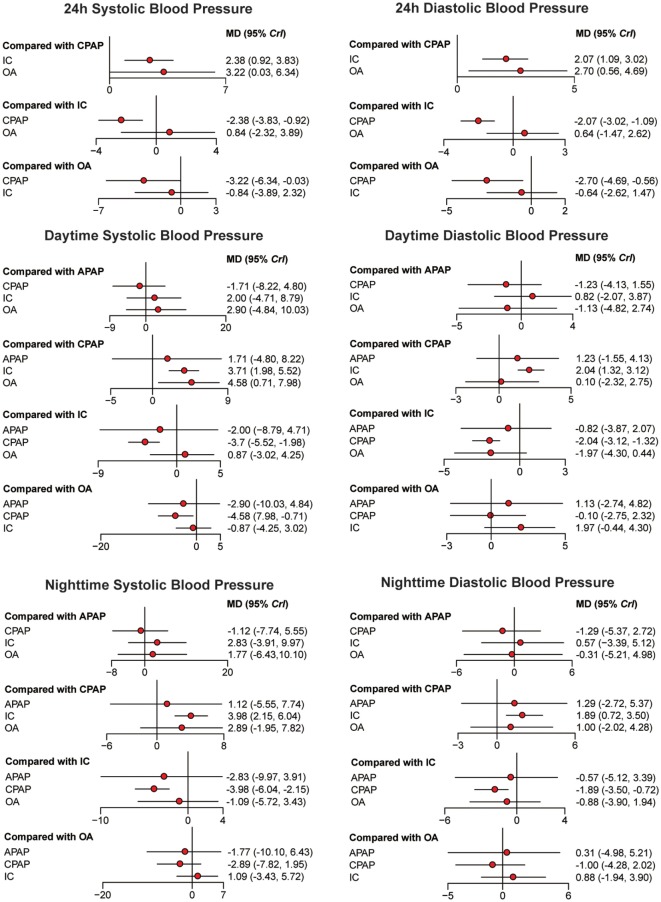
Bayesian models allowed for more refined estimates of efficacy when participants were treated with APAP, CPAP, OA, and IC. Available data was recorded in Table 3 and displayed graphically in the forest plots in Figure 2 for sleep characteristics and Figure 3 for blood pressure. For the primary outcomes of AHI, APAP, CPAP, and OA all showed statistically reduction versus IC [mean difference (MD) = −23.97, 95% CrI: (13.90, 34.31) for APAP; MD = −23.49, 95% CrI: (−28.68, −18.50) for CPAP; MD = −13.91, 95% CrI: (−21.01, −7.00) for OA]. Significant decrease of AHI occurred in comparison of CPAP versus OA [MD = −9.59, 95% CrI: (−15.40, −3.75) for CPAP]. Similarly, statistical significance was observed in ESS for APAP, CPAP, and OA compared with IC, with MD = 2.19, 95% CrI: (0.89, 3.55) for APAP; MD = −2.04, 95%CrI: (1.54, 2.57) for CPAP; and MD = −1.58 95% CrI: (−2.89, −0.28) for OA. Similar observation was obtained in AI, with treatments of the three active interventions [MD = 11.92, 95% CrI: (6.09, 18.97) for APAP versus IC; MD = 11.09, 95%CrI: (7.74, 15.20) for CPAP versus IC; MD = −8.32, 95% CrI: (−12.91, −4.55) for IC versus OA]. Increase in SaO2 was exhibited in compares with IC [MD = −6.08, 95% CrI: (−11.53, −1.04) for APAP versus IC; MD = −9.02, 95% CrI: (−11.33, −7.10) for CPAP versus IC; MD = 3.71, 95% CrI: (1.39, 6.42) for IC versus OA]. In addition, we found great difference between OA and CPAP concerning SaO2 [MD = −5.25, 95% CrI: (−7.64, −3.22)]. For the secondary outcomes of blood pressure, more negative results were obtained. Data in 24 h SBP and 24 h DBP was merely available among CPAP, OA, and IC. Similar statistically significant results were presented in 24 h SBP [MD = 2.38, 95% CrI: (0.92, 3.83) for IC; MD = 3.22, 95% CrI: (0.03, 6.34) for OA] and 24 h DBP [MD = 2.07, 95% CrI: (1.09, 3.02) for IC; MD = 2.70, 95% CrI: (0.56, 4.69) for OA] when comparing with CPAP. We got complete information of all the interventions in terms of SBP and DBP during daytime and nighttime. A high similarity among the four plots was founded. CPAP contributed to significant reduction in daytime SBP [MD = 3.70, 95% CrI: (1.98, 5.52) for IC versus CPAP], daytime DBP [MD = 2.04, 95% CrI: (1.32, 3.12) for IC versus CPAP], nighttime SBP [MD = 3.98, 95% CrI: (2.15, 6.04) for IC versus CPAP] and nighttime DBP [MD = −1.89, 95% CrI: (−3.50 to −0.72) versus IC]. A comparison of OA versus CPAP in daytime SBP was also noted for indicating significant difference [MD = 4.58, 95% CrI: (0.71, 7.98)]. In all, based on the network results of primary outcomes, namely, AHI and ESS, significant improvement of APAP, CPAP, and OA were observed compared with IC, outcomes were at least in favor of CPAP when compared with OA, and APAP and CPAP could be classified as identical.
Table 3.
Network meta-analysis results of obstructive sleep apnea.
| APAP | CPAP | IC | OA | |||
|---|---|---|---|---|---|---|
| ESS | APAP | 1 | 0.48 (−8.29, 9.38) | 23.97 (13.90, 34.31) | 10.08 (−0.59, 20.68) | AHI |
| CPAP | 0.15 (−1.05, 1.39) | 1 | 23.49 (18.50, 28.68) | 9.59 (3.75, 15.40) | ||
| IC | 2.19 (0.89, 3.55) | 2.04 (1.54, 2.57) | 1 | −13.91 (−21.01, −7.00) | ||
| OA | 0.61 (−1.13, 2.39) | 0.46 (−0.82, 1.74) | −1.58 (−2.89, −0.28) | 1 | ||
| SaO2 | APAP | 1 | 0.84 (−4.04, 5.99) | 11.92 (6.08, 18.79) | 3.65 (−2.33, 9.59) | AI |
| CPAP | 2.94 (−1.82, 7.73) | 1 | 11.09 (7.74, 15.2) | 2.80 (−0.63, 5.95) | ||
| IC | −6.08 (−11.53, −1.04) | −9.02 (−11.33, −7.10) | 1 | −8.32 (−12.91, −4.55) | ||
| OA | −2.34 (−7.70, 2.82) | −5.25 (−7.64, −3.22) | 3.71 (1.39, 6.42) | 1 | ||
| 24 h DBP | CPAP | – | 1 | 2.38 (0.92, 3.83) | 3.22 (0.03, 6.34) | 24 h SBP |
| IC | – | 2.07 (1.09, 3.02) | 1 | 0.84 (−2.32, 3.89) | ||
| OA | – | 2.7 (0.56, 4.69) | 0.64 (−1.47, 2.62) | 1 | ||
| Daytime DBP | APAP | 1 | −1.71 (−8.22, 4.80) | 2.00 (−4.71, 8.79) | 2.90 (−4.84, 10.03) | Daytime SBP |
| CPAP | −1.23 (−4.13, 1.55) | 1 | 3.71 (1.98, 5.52) | 4.58 (0.71, 7.98) | ||
| IC | 0.82 (−2.07, 3.87) | 2.04 (1.32, 3.12) | 1 | 0.87 (−3.02, 4.25) | ||
| OA | −1.13 (−4.82, 2.74) | 0.1 (−2.32, 2.75) | −1.97 (−4.30, 0.44) | 1 | ||
| Nighttime DBP | APAP | 1 | −1.12 (−7.74, 5.55) | 2.83 (−3.91, 9.97) | 1.77 (−6.43, 10.10) | Nighttime SBP |
| CPAP | −1.29 (−5.37, 2.72) | 1 | 3.98 (2.15, 6.04) | 2.89 (−1.95, 7.82) | ||
| IC | 0.57 (−3.39, 5.12) | 1.89 (0.72, 3.50) | 1 | −1.09 (−5.72, 3.43) | ||
| OA | −0.31 (−5.21, 4.98) | 1.00 (−2.02, 4.28) | −0.88 (−3.90, 1.94) | 1 | ||
Treatment: CPAP, continuous positive airway pressure; APAP, auto-adjusting positive airway pressure; IC, inactive control; OA, oral appliance.
Outcomes: AHI, Apnea–Hypopnea Index; ESS, Epworth Sleepiness Scale; SaO2, arterial oxygen saturation; AI, arousal index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Bold font indicates statistically significant difference.
Figure 2.
Forest plots regarding Apnea–Hypopnea Index, Epworth Sleepiness Scale, arousal index, and SaO2. Mean difference (MD) with 95% credible interval (CrIs) indicate the relative efficacy. Abbreviations: CPAP, continuous positive airway pressure; APAP, auto-adjusting positive airway pressure; IC, inactive control; OA, oral appliance.
Figure 3.
Forest plots regarding 24 h systolic blood pressure (SBP), 24 h diastolic blood pressure, daytime SBP, daytime SBP, nighttime SBP, and nighttime SBP. Mean difference (MD) with 95% credible interval (CrIs) indicate the relative efficacy. Abbreviations: CPAP, continuous positive airway pressure; APAP, auto-adjusting positive airway pressure; IC, inactive control; OA, oral appliance.
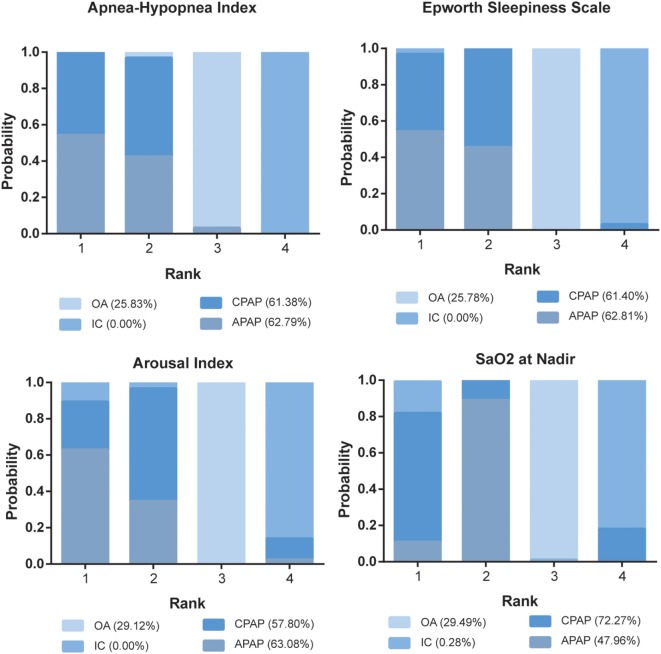
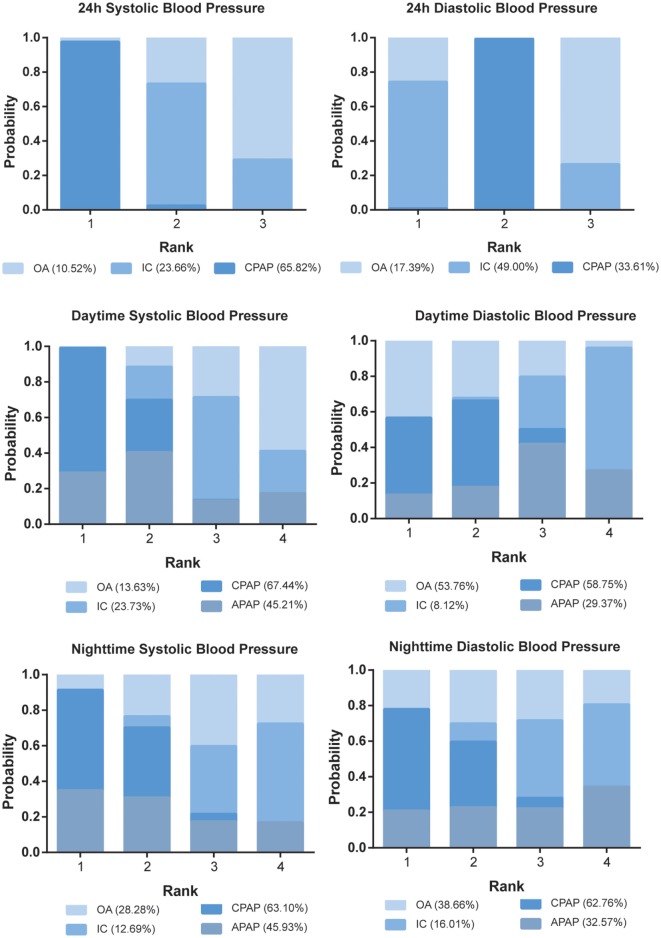
Ranking Scheme Based on SUCRA
The ranking probability of each treatment in terms of 10 outcomes was illustrated in Figures 4 and 5. CPAP and APAP were ranked top two in improving sleep characteristics, with similar ranking score in AHI (61.38% for CPAP and 62.79% for APAP) and ESS (61.40% for CPAP and 62.81% for APAP), best for APAP in AI (63.08%) and best for CPAP in SaO2 (72.27%). CPAP kept ranking as number one in reducing blood pressure and APAP became moderate. OA was regarded as a mild intervention, and IC was the last choice under all the circumstances. CPAP was recommended based on the ranking results.
Figure 4.
Stacked bar charts showing the rankings of four therapies for Apnea–Hypopnea Index, Epworth Sleepiness Scale, arousal index, and SaO2 at Nadir. The percentage number included in each pair of brackets indicates the cumulative ranking probability of the corresponding therapy. Abbreviations: CPAP, continuous positive airway pressure; APAP, auto-adjusting positive airway pressure; IC, inactive control; OA, oral appliance.
Figure 5.
Stacked bar charts showing the rankings of four therapies for 24 h systolic blood pressure (SBP), 24 h diastolic blood pressure, daytime SBP, daytime SBP, nighttime SBP, and nighttime SBP. The percentage number included in each pair of brackets indicates the cumulative ranking probability of the corresponding therapy. Abbreviations: CPAP, continuous positive airway pressure; APAP, auto-adjusting positive airway pressure; IC, inactive control; OA, oral appliance.
Risk of Bias and Consistency
Jadad scale of included studies was presented in Table S1 in Supplementary Material, which indicated medium–high quality and low risk of publication bias for all included studies. And the symmetry of the “comparison adjusted” funnel plots in Figures S1 and S2 in Supplementary Material had suggested that there was no remarkable publication bias. According to the node-splitting plots in Figure S3 in Supplementary Material, all the P-values were higher than 0.05, suggesting no significant inconsistency in terms of sleep characteristics. However, great inconsistency was obtained in the comparison of CPAP versus IC with respect to blood pressure, which indicated that indirect evidence had higher extent in reducing 24 h blood pressure, and the comparison of OA versus IC, in which indirect evidence provided adverse results (Figure S4 in Supplementary Material). Furthermore, the P-values showed that there was no consistency in the comparisons among CPAP, OA, and IC. The result of assessment for consistency was displayed in Figures S5 and S6 in Supplementary Material.
Discussion
In this study, NMA regarding AHI, ESS, AI, SaO2, and blood pressures was performed to evaluate the efficacy of CPAP, APAP, and OA in OSA patients. OSA is a detrimental disease since it results in not only sleepiness and snoring but also significant health problems such as atrial fibrillation (100).
As a first line therapy for OSA, CPAP was first recommended by the American College of Physicians (100), and Wright and White proposed in 2000 that the effect of CPAP on sleepiness is clinically significant since CPAP therapy is able to improve the life quality of OSA patients (101). Xu et al. confirmed that CPAP could also decrease the total cholesterol level, especially for younger and more obese patients who use CPAP in the long term, while the issue of lipid metabolism was not clinically significant (102).
Our NMA confirmed previous findings that CPAP is effective in improving AHI, ESS, AI, and SaO2. Ha et al. revealed that CPAP is superior to positional therapy in reducing the severity of sleep apnea (103). Previous studies have revealed that CPAP are capable of preventing upper airway collapse and arousals, as well as reducing oxidative stresses. Besides that, CPAP therapy can alleviate the corresponding symptoms of OSA such as excessive daytime sleepiness and snoring (104, 105). As Liu et al. concluded, CPAP was associated with significant reductions in SBP, DBP, and nocturnal DBP in patients with OSA and hypertension. Our research further revealed the positive effects of CPAP on nighttime DBP and daytime blood pressures (106). CPAP therapy significantly reduces BP in patients with OSA but the effect size may not be clinically significant. APAP presented a similar efficacy to CPAP on improving sleeping quality and reducing daytime sleepiness, since the comparisons between CPAP and APAP on AHI and ESS manifest neither statistical difference nor distinctive preferences. The above result is supported by Gao et al. who concluded that the effect of APAP on AHI improvements was identical to standard manual titration (10). Gao et al. also pointed out that the acceptance and compliance of automatic titrated method had the same performance as manual titration. Since APAP has the potential advantage in saving time and cost, automatic titration was also recommended as an alternative therapy in clinical practice (10). Despite we concluded that CPAP and APAP are identical in clinical outcomes, Xu et al. claimed that the use of APAP is slightly favored compared with fixed pressure CPAP in some aspects, such as compliance and patient preference, though the clinical relevance still requires further study (107). Despite the efficacy of APAP in sleeping quality, blood pressure outcomes are in favor of CPAP based on the SUCRA result, this preference might be the result of the relatively lower average pressure that APAP applies to OSA patients.
Similar to CPAP, OA exerts its function by relieving upper airway collapse during sleep through the modification of the position of mandible, tongue, and pharyngeal structures (58). Though inferior to CPAP, OA was also confirmed to be effective in improving symptoms and life qualities for patients with OSA. Okuno et al. supported our notion that compared with untreated patients, significant reduction in AHI and AI was found after OA therapy was applied (14). Due to its efficacy and cost saving features, OA has become increasingly popular and performed its clinical application as alternative therapy for CPAP (58). Yet, it should also be noted that the efficacy of OA is directly related to its type (18). Umemoto et al. found that fixed OAs are superior in treating OSA than twin-block appliances because of their ability to prevent mouth opening and reduce incisal overjet (108).
A high inconsistency between direct and indirect evidence was observed regarding the outcome of 24 h SBP, 24 h DBP, and daytime SBP, which concurs with Liu et al. that the beneficial effects of CPAP are inconsistent (106). Despite our conclusion that CPAP could significantly reduce all blood pressure outcomes, there existed studies that claimed non-significant decrease in BP was found (109, 110). This inconsistency might come from the discordance of OSA patient baseline characteristics and CPAP uses. Since studies reached agreement that the efficacy of CPAP increases with the severity of OSA, frequent apneic episodes may benefit the most from CPAP (111). Based on these conclusions, it is highly possible that results from different studies may vary due to the discordance in the baseline characteristics of different patients.
Our NMA originally combined conclusions on sleeping behavior and blood pressure; thus, a more overall efficacy of different therapy could be drawn. However, there also existed some limitations. Though an amount of 84 studies were included in our NMA, the sample size is still limited and resulted in the inconsistency discussed above. The included studies also presented deficient comparison between different therapy, such as APAP and OA. Moreover, the baseline of studies should be more unified to ensure the credibility and accuracy of our conclusions. Thus, larger comparison with better designed clinical trials is still required for a more comprehensive conclusion.
In all, CPAP, APAP, and OA are proved to be effective, which is supported by previous evidences. Based on primary outcomes, namely, AHI and ESS, significant improvement was observed compared with IC, outcomes are at least in favor of CPAP when compared with OA, and APAP and CPAP are classified as identical. Apart from ESS that represents reduction in daytime sleepiness, CPAP also presented significant improvements with respect to secondary outcomes like blood pressure. APAP tended to have slightly better performance than CPAP in AHI and ESS but are less promising in blood pressures on the basis of SUCRA. Our NMA identified CPAP as most efficacious treatment for OSA patients after synthetically evaluation on ESS, AHI, AI, SaO2, and blood pressures. Though inferior to CPAP and exerted no distinctive benefits on blood pressure, OA still manifested significant improvements in AHI and ESS compared with IC, indicating its feasibility as an alternative therapy for OSA patients. Larger clinical trials on the efficacy of CPAP on blood pressure for patients with OSA are needed for further investigation on the inconsistency observed.
Author Contributions
TL performed the research and designed the research study; WL analyzed and interpreted the data and wrote the paper; HZ drafted the manuscript; ZW made critical revision of the manuscript. All authors approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This research was supported by Science and Technology Project of Liaoning Province (No. 2014021022).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fneur.2017.00289/full#supplementary-material.
References
- 1.Mehta V, Vasu TS, Phillips B, Chung F. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med (2013) 9:271–9. 10.5664/jcsm.2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bratton DJ, Gaisl T, Schlatzer C, Kohler M. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med (2015) 3:869–78. 10.1016/S2213-2600(15)00416-6 [DOI] [PubMed] [Google Scholar]
- 3.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA (2015) 314:2280–93. 10.1001/jama.2015.16303 [DOI] [PubMed] [Google Scholar]
- 4.Knudsen TB, Laulund AS, Ingerslev J, Homoe P, Pinholt EM. Improved apnea-hypopnea index and lowest oxygen saturation after maxillomandibular advancement with or without counterclockwise rotation in patients with obstructive sleep apnea: a meta-analysis. J Oral Maxillofac Surg (2015) 73:719–26. 10.1016/j.joms.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: a systematic review and meta-analysis. Respirology (2015) 20:889–95. 10.1111/resp.12573 [DOI] [PubMed] [Google Scholar]
- 6.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax (2015) 70:258–64. 10.1136/thoraxjnl-2014-205361 [DOI] [PubMed] [Google Scholar]
- 7.Varounis C, Katsi V, Kallikazaros IE, Tousoulis D, Stefanadis C, Parissis J, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: a systematic review and meta-analysis. Int J Cardiol (2014) 175:195–8. 10.1016/j.ijcard.2014.04.240 [DOI] [PubMed] [Google Scholar]
- 8.Jaiswal M, Srivastava GN, Pratap CB, Sharma VK, Chaturvedi TP. Effect of oral appliance for snoring and obstructive sleep apnea. Int J Orthod Milwaukee (2015) 26:67–71. [PubMed] [Google Scholar]
- 9.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, Lloberes P, Diaz de Atauri MJ, Somoza M, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA (2013) 310:2407–15. 10.1001/jama.2013.281250 [DOI] [PubMed] [Google Scholar]
- 10.Gao W, Jin Y, Wang Y, Sun M, Chen B, Zhou N, et al. Is automatic CPAP titration as effective as manual CPAP titration in OSAHS patients? A meta-analysis. Sleep Breath (2012) 16:329–40. 10.1007/s11325-011-0495-z [DOI] [PubMed] [Google Scholar]
- 11.Pepin JL, Tamisier R, Baguet JP, Lepaulle B, Arbib F, Arnol N, et al. Fixed-pressure CPAP versus auto-adjusting CPAP: comparison of efficacy on blood pressure in obstructive sleep apnoea, a randomised clinical trial. Thorax (2016) 71:726–33. 10.1136/thoraxjnl-2015-207700 [DOI] [PubMed] [Google Scholar]
- 12.Morris LG, Setlur J, Burschtin OE, Steward DL, Jacobs JB, Lee KC. Acoustic rhinometry predicts tolerance of nasal continuous positive airway pressure: a pilot study. Am J Rhinol (2006) 20:133–7. [PubMed] [Google Scholar]
- 13.Berry RB, Sriram P. Auto-adjusting positive airway pressure treatment for sleep apnea diagnosed by home sleep testing. J Clin Sleep Med (2014) 10:1269–75. 10.5664/jcsm.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okuno K, Sato K, Arisaka T, Hosohama K, Gotoh M, Taga H, et al. The effect of oral appliances that advanced the mandible forward and limited mouth opening in patients with obstructive sleep apnea: a systematic review and meta-analysis of randomised controlled trials. J Oral Rehabil (2014) 41:542–54. 10.1111/joor.12162 [DOI] [PubMed] [Google Scholar]
- 15.Lin MT, Lin HH, Lee PL, Weng PH, Lee CC, Lai TC, et al. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. Sleep Breath (2015) 19:809–17. 10.1007/s11325-014-1082-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sukhal S, Khalid M, Tulaimat A. Effect of wakefulness-promoting agents on sleepiness in patients with sleep apnea treated with CPAP: a meta-analysis. J Clin Sleep Med (2015) 11:1179–86. 10.5664/jcsm.5096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lettieri CJ, Paolino N, Eliasson AH, Shah AA, Holley AB. Comparison of adjustable and fixed oral appliances for the treatment of obstructive sleep apnea. J Clin Sleep Med (2011) 7:439–45. 10.5664/jcsm.1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahrens A, McGrath C, Hagg U. A systematic review of the efficacy of oral appliance design in the management of obstructive sleep apnoea. Eur J Orthod (2011) 33:318–24. 10.1093/ejo/cjq079 [DOI] [PubMed] [Google Scholar]
- 19.Salord N, Fortuna AM, Monasterio C, Gasa M, Perez A, Bonsignore MR, et al. A randomized controlled trial of continuous positive airway pressure on glucose tolerance in obese patients with obstructive sleep apnea. Sleep (2016) 39:35–41. 10.5665/sleep.5312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paz YMHL, Hazen SL, Tracy RP, Strohl KP, Auckley D, Bena J, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest (2016) 150(1):80–90. 10.1016/j.chest.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pamidi S, Wroblewski K, Stepien M, Sharif-Sidi K, Kilkus J, Whitmore H, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. a randomized controlled trial. Am J Respir Crit Care Med (2015) 192:96–105. 10.1164/rccm.201408-1564OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muxfeldt ES, Margallo V, Costa LM, Guimaraes G, Cavalcante AH, Azevedo JC, et al. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension (2015) 65:736–42. 10.1161/hypertensionaha.114.04852 [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Garcia MA, Chiner E, Hernandez L, Cortes JP, Catalan P, Ponce S, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J (2015) 46:142–51. 10.1183/09031936.00064214 [DOI] [PubMed] [Google Scholar]
- 24.Huang Z, Liu Z, Luo Q, Zhao Q, Zhao Z, Ma X, et al. Long-term effects of continuous positive airway pressure on blood pressure and prognosis in hypertensive patients with coronary heart disease and obstructive sleep apnea: a randomized controlled trial. Am J Hypertens (2015) 28:300–6. 10.1093/ajh/hpu147 [DOI] [PubMed] [Google Scholar]
- 25.Dalmases M, Sole-Padulles C, Torres M, Embid C, Nunez MD, Martinez-Garcia MA, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest (2015) 148:1214–23. 10.1378/chest.15-0171 [DOI] [PubMed] [Google Scholar]
- 26.Woodson BT, Gillespie MB, Soose RJ, Maurer JT, de Vries N, Steward DL, et al. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg (2014) 151:880–7. 10.1177/0194599814544445 [DOI] [PubMed] [Google Scholar]
- 27.Neikrug AB, Liu L, Avanzino JA, Maglione JE, Natarajan L, Bradley L, et al. Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep (2014) 37:177–85. 10.5665/sleep.3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloberes P, Sampol G, Espinel E, Segarra A, Ramon MA, Romero O, et al. A randomized controlled study of CPAP effect on plasma aldosterone concentration in patients with resistant hypertension and obstructive sleep apnea. J Hypertens (2014) 32:1650–1657; discussion 1657. 10.1097/hjh.0000000000000238 [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med (2014) 370:2276–85. 10.1056/NEJMoa1306766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chasens ER, Korytkowski M, Sereika SM, Burke LE, Drumheller OJ, Strollo PJ, Jr. Improving activity in adults with diabetes and coexisting obstructive sleep apnea. West J Nurs Res (2014) 36:294–311. 10.1177/0193945913500567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schutz TC, Cunha TC, Moura-Guimaraes T, Luz GP, Ackel-D’Elia C, Alves Eda S, et al. Comparison of the effects of continuous positive airway pressure, oral appliance and exercise training in obstructive sleep apnea syndrome. Clinics (Sao Paulo) (2013) 68:1168–74. 10.6061/clinics/2013(08)17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med (2013) 187:879–87. 10.1164/rccm.201212-2223OC [DOI] [PubMed] [Google Scholar]
- 33.Pedrosa RP, Drager LF, de Paula LK, Amaro AC, Bortolotto LA, Lorenzi-Filho G. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest (2013) 144:1487–94. 10.1378/chest.13-0085 [DOI] [PubMed] [Google Scholar]
- 34.Diaferia G, Badke L, Santos-Silva R, Bommarito S, Tufik S, Bittencourt L. Effect of speech therapy as adjunct treatment to continuous positive airway pressure on the quality of life of patients with obstructive sleep apnea. Sleep Med (2013) 14:628–35. 10.1016/j.sleep.2013.03.016 [DOI] [PubMed] [Google Scholar]
- 35.Andren A, Hedberg P, Walker-Engstrom ML, Wahlen P, Tegelberg A. Effects of treatment with oral appliance on 24-h blood pressure in patients with obstructive sleep apnea and hypertension: a randomized clinical trial. Sleep Breath (2013) 17:705–12. 10.1007/s11325-012-0746-7 [DOI] [PubMed] [Google Scholar]
- 36.Sivam S, Phillips CL, Trenell MI, Yee BJ, Liu PY, Wong KK, et al. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J (2012) 40:913–8. 10.1183/09031936.00177011 [DOI] [PubMed] [Google Scholar]
- 37.Lee IS, Bardwell W, Ancoli-Israel S, Loredo JS, Dimsdale JE. Effect of three weeks of continuous positive airway pressure treatment on mood in patients with obstructive sleep apnoea: a randomized placebo-controlled study. Sleep Med (2012) 13:161–6. 10.1016/j.sleep.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the apnea positive pressure long-term efficacy study (APPLES). Sleep (2012) 35:1593–602. 10.5665/sleep.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax (2012) 67:1081–9. 10.1136/thoraxjnl-2011-201420 [DOI] [PubMed] [Google Scholar]
- 40.Sharma SK, Agrawal S, Damodaran D, Sreenivas V, Kadhiravan T, Lakshmy R, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med (2011) 365:2277–86. 10.1056/NEJMoa1103944 [DOI] [PubMed] [Google Scholar]
- 41.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke (2011) 42:1062–7. 10.1161/strokeaha.110.597468 [DOI] [PubMed] [Google Scholar]
- 42.Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med (2011) 184:355–61. 10.1164/rccm.201102-0316OC [DOI] [PubMed] [Google Scholar]
- 43.Kohler M, Stoewhas AC, Ayers L, Senn O, Bloch KE, Russi EW, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med (2011) 184:1192–9. 10.1164/rccm.201106-0964OC [DOI] [PubMed] [Google Scholar]
- 44.Drager LF, Pedrosa RP, Diniz PM, Diegues-Silva L, Marcondes B, Couto RB, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension (2011) 57:549–55. 10.1161/hypertensionaha.110.165969 [DOI] [PubMed] [Google Scholar]
- 45.Aarab G, Lobbezoo F, Heymans MW, Hamburger HL, Naeije M. Long-term follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration (2011) 82:162–8. 10.1159/000324580 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen PK, Katikireddy CK, McConnell MV, Kushida C, Yang PC. Nasal continuous positive airway pressure improves myocardial perfusion reserve and endothelial-dependent vasodilation in patients with obstructive sleep apnea. J Cardiovasc Magn Reson (2010) 12:50. 10.1186/1532-429x-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lozano L, Tovar JL, Sampol G, Romero O, Jurado MJ, Segarra A, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens (2010) 28:2161–8. 10.1097/HJH.0b013e32833b9c63 [DOI] [PubMed] [Google Scholar]
- 48.Lam JC, Lam B, Yao TJ, Lai AY, Ooi CG, Tam S, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J (2010) 35:138–45. 10.1183/09031936.00047709 [DOI] [PubMed] [Google Scholar]
- 49.Durán-Cantolla J, Aizpuru F, Montserrat JM, Ballester E, Terán-Santos J, Aguirregomoscorta JI, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ (2010) 341:1142. 10.1136/bmj.c5991 [DOI] [PubMed] [Google Scholar]
- 50.Barbe F, Duran-Cantolla J, Capote F, de la Pena M, Chiner E, Masa JF, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med (2010) 181:718–26. 10.1164/rccm.200901-0050OC [DOI] [PubMed] [Google Scholar]
- 51.Galetke W, Randerath WJ, Stieglitz S, Laumanns C, Anduleit N, Richter K, et al. Comparison of manual titration and automatic titration based on forced oscillation technique, flow and snoring in obstructive sleep apnea. Sleep Med (2009) 10:337–43. 10.1016/j.sleep.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 52.Gagnadoux F, Fleury B, Vielle B, Petelle B, Meslier N, N’Guyen XL, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J (2009) 34:914–20. 10.1183/09031936.00148208 [DOI] [PubMed] [Google Scholar]
- 53.Damjanovic D, Fluck A, Bremer H, Muller-Quernheim J, Idzko M, Sorichter S. Compliance in sleep apnoea therapy: influence of home care support and pressure mode. Eur Respir J (2009) 33:804–11. 10.1183/09031936.00023408 [DOI] [PubMed] [Google Scholar]
- 54.Siccoli MM, Pepperell JC, Kohler M, Craig SE, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on quality of life in patients with moderate to severe obstructive sleep apnea: data from a randomized controlled trial. Sleep (2008) 31:1551–8. 10.1093/sleep/31.11.1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruttanaumpawan P, Gilman MP, Usui K, Floras JS, Bradley TD. Sustained effect of continuous positive airway pressure on baroreflex sensitivity in congestive heart failure patients with obstructive sleep apnea. J Hypertens (2008) 26:1163–8. 10.1097/HJH.0b013e3282fb81ed [DOI] [PubMed] [Google Scholar]
- 56.Petri N, Svanholt P, Solow B, Wildschiodtz G, Winkel P. Mandibular advancement appliance for obstructive sleep apnoea: results of a randomised placebo controlled trial using parallel group design. J Sleep Res (2008) 17:221–9. 10.1111/j.1365-2869.2008.00645.x [DOI] [PubMed] [Google Scholar]
- 57.Kohler M, Pepperell JC, Casadei B, Craig S, Crosthwaite N, Stradling JR, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J (2008) 32:1488–96. 10.1183/09031936.00026608 [DOI] [PubMed] [Google Scholar]
- 58.Hoekema A, Voors AA, Wijkstra PJ, Stegenga B, van der Hoeven JH, Tol CG, et al. Effects of oral appliances and CPAP on the left ventricle and natriuretic peptides. Int J Cardiol (2008) 128:232–9. 10.1016/j.ijcard.2007.06.016 [DOI] [PubMed] [Google Scholar]
- 59.Galetke W, Anduleit N, Richter K, Stieglitz S, Randerath WJ. Comparison of automatic and continuous positive airway pressure in a night-by-night analysis: a randomized, crossover study. Respiration (2008) 75:163–9. 10.1159/000097767 [DOI] [PubMed] [Google Scholar]
- 60.Egea CJ, Aizpuru F, Pinto JA, Ayuela JM, Ballester E, Zamarron C, et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med (2008) 9:660–6. 10.1016/j.sleep.2007.06.018 [DOI] [PubMed] [Google Scholar]
- 61.Cross MD, Mills NL, Al-Abri M, Riha R, Vennelle M, Mackay TW, et al. Continuous positive airway pressure improves vascular function in obstructive sleep apnoea/hypopnoea syndrome: a randomised controlled trial. Thorax (2008) 63:578–83. 10.1136/thx.2007.081877 [DOI] [PubMed] [Google Scholar]
- 62.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax (2007) 62:969–74. 10.1136/thx.2006.074351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith LA, Vennelle M, Gardner RS, McDonagh TA, Denvir MA, Douglas NJ, et al. Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J (2007) 28:1221–7. 10.1093/eurheartj/ehm131 [DOI] [PubMed] [Google Scholar]
- 64.Patruno V, Aiolfi S, Costantino G, Murgia R, Selmi C, Malliani A, et al. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest (2007) 131:1393–9. 10.1378/chest.06-2192 [DOI] [PubMed] [Google Scholar]
- 65.Martinez-Garcia MA, Gomez-Aldaravi R, Soler-Cataluna JJ, Martinez TG, Bernacer-Alpera B, Roman-Sanchez P. Positive effect of CPAP treatment on the control of difficult-to-treat hypertension. Eur Respir J (2007) 29:951–7. 10.1183/09031936.00048606 [DOI] [PubMed] [Google Scholar]
- 66.Lam B, Sam K, Mok WY, Cheung MT, Fong DY, Lam JC, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax (2007) 62:354–9. 10.1136/thx.2006.063644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haensel A, Norman D, Natarajan L, Bardwell WA, Ancoli-Israel S, Dimsdale JE.Effect of a 2 week CPAP treatment on mood states in patients with obstructive sleep apnea: a double-blind trial. Sleep Breath (2007) 11:239–44. 10.1007/s11325-007-0115-0 [DOI] [PubMed] [Google Scholar]
- 68.Fietze I, Glos M, Moebus I, Witt C, Penzel T, Baumann G. Automatic pressure titration with APAP is as effective as manual titration with CPAP in patients with obstructive sleep apnea. Respiration (2007) 74:279–86. 10.1159/000100364 [DOI] [PubMed] [Google Scholar]
- 69.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med (2007) 176:706–12. 10.1164/rccm.200703-500OC [DOI] [PubMed] [Google Scholar]
- 70.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J (2007) 29:720–7. 10.1183/09031936.00043306 [DOI] [PubMed] [Google Scholar]
- 71.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J (2006) 27:1229–35. 10.1183/09031936.06.00062805 [DOI] [PubMed] [Google Scholar]
- 72.Hui DS, To KW, Ko FW, Fok JP, Chan MC, Ngai JC, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax (2006) 61:1083–90. 10.1136/thx.2006.064063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, Merino-Sanchez M, Gonzalez-Benitez MA, Beltran-Robles M, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest (2006) 129:1459–67. 10.1378/chest.129.6.1459 [DOI] [PubMed] [Google Scholar]
- 74.Usui K, Bradley TD, Spaak J, Ryan CM, Kubo T, Kaneko Y, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol (2005) 45:2008–11. 10.1016/j.jacc.2004.12.080 [DOI] [PubMed] [Google Scholar]
- 75.Marshall NS, Neill AM, Campbell AJ, Sheppard DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax (2005) 60:427–32. 10.1136/thx.2004.032078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blanco J, Zamarron C, Abeleira Pazos MT, Lamela C, Suarez Quintanilla D. Prospective evaluation of an oral appliance in the treatment of obstructive sleep apnea syndrome. Sleep Breath (2005) 9:20–5. 10.1007/s11325-005-0003-4 [DOI] [PubMed] [Google Scholar]
- 77.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation (2005) 112:375–83. 10.1161/circulationaha.104.501841 [DOI] [PubMed] [Google Scholar]
- 78.Masa JF, Jiménez A, Durán J, Capote F, Monasterio C, Mayos M, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med (2004) 170:1218–24. 10.1164/rccm.200312-1787OC [DOI] [PubMed] [Google Scholar]
- 79.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med (2004) 169:361–6. 10.1164/rccm.200306-752OC [DOI] [PubMed] [Google Scholar]
- 80.Lloberes P, Rodriguez B, Roca A, Sagales MT, de la Calzada MD, Gimenez S, et al. Comparison of conventional nighttime with automatic or manual daytime CPAP titration in unselected sleep apnea patients: study of the usefulness of daytime titration studies. Respir Med (2004) 98:619–25. 10.1016/j.rmed.2003.12.014 [DOI] [PubMed] [Google Scholar]
- 81.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med (2004) 169:348–53. 10.1164/rccm.200306-767OC [DOI] [PubMed] [Google Scholar]
- 82.Hussain SF, Love L, Burt H, Fleetham JA. A randomized trial of auto-titrating CPAP and fixed CPAP in the treatment of obstructive sleep apnea-hypopnea. Respir Med (2004) 98:330–3. 10.1016/j.rmed.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 83.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep (2004) 27:934–41. 10.1093/sleep/27.5.934 [DOI] [PubMed] [Google Scholar]
- 84.Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med (2004) 170:656–64. 10.1164/rccm.200311-1571OC [DOI] [PubMed] [Google Scholar]
- 85.Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg (2003) 128:848–61. 10.1016/S0194-5998(03)00461-3 [DOI] [PubMed] [Google Scholar]
- 86.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med (2003) 348:1233–41. 10.1056/NEJMoa022479 [DOI] [PubMed] [Google Scholar]
- 87.Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation (2003) 107:68–73. 10.1161/01.CIR.0000042706.47107.7A [DOI] [PubMed] [Google Scholar]
- 88.Tan YK, L’Estrange PR, Luo YM, Smith C, Grant HR, Simonds AK, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod (2002) 24:239–49. 10.1093/ejo/24.3.239 [DOI] [PubMed] [Google Scholar]
- 89.Randerath WJ, Heise M, Hinz R, Ruehle KH. An individually adjustable oral appliance vs continuous positive airway pressure in mild-to-moderate obstructive sleep apnea syndrome. Chest (2002) 122:569–75. 10.1378/chest.122.2.569 [DOI] [PubMed] [Google Scholar]
- 90.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling JR, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet (2002) 359:204–10. 10.1016/s0140-6736(02)07445-7 [DOI] [PubMed] [Google Scholar]
- 91.Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med (2002) 166:743–8. 10.1164/rccm.200203-208OC [DOI] [PubMed] [Google Scholar]
- 92.Monasterio C, Vidal S, Duran J, Ferrer M, Carmona C, Barbé F, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med (2001) 164:939–43. 10.1164/ajrccm.164.6.2008010 [DOI] [PubMed] [Google Scholar]
- 93.Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: a placebo-controlled study. Psychosom Med (2001) 63:579–84. 10.1097/00006842-200107000-00010 [DOI] [PubMed] [Google Scholar]
- 94.Barbé F, Mayoralas LR, Duran J, Masa JF, Maimó A, Montserrat JM, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness: a randomized, controlled trial. Ann Intern Med (2001) 134:1015–23. 10.7326/0003-4819-134-11-200106050-00007 [DOI] [PubMed] [Google Scholar]
- 95.Ballester E, Badia JR, Hernández L, Carrasco E, De Pablo J, Fornas C, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med (1999) 159:495–501. 10.1164/ajrccm.159.2.9804061 [DOI] [PubMed] [Google Scholar]
- 96.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med (1998) 157:858–65. 10.1164/ajrccm.157.3.9709042 [DOI] [PubMed] [Google Scholar]
- 97.Ferguson KA, Ono T, Lowe AA, al-Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax (1997) 52:362–8. 10.1136/thx.52.4.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meurice JC, Marc I, Series F. Efficacy of auto-CPAP in the treatment of obstructive sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med (1996) 153:794–8. 10.1164/ajrccm.153.2.8564134 [DOI] [PubMed] [Google Scholar]
- 99.Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest (1996) 109:1269–75. 10.1378/chest.109.5.1269 [DOI] [PubMed] [Google Scholar]
- 100.Qureshi WT, Nasir UB, Alqalyoobi S, O’Neal WT, Mawri S, Sabbagh S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol (2015) 116:1767–73. 10.1016/j.amjcard.2015.08.046 [DOI] [PubMed] [Google Scholar]
- 101.Wright J, White J. Continuous positive airways pressure for obstructive sleep apnoea. Cochrane Database Syst Rev (2000) (2):CD001106. 10.1002/14651858.CD001106 [DOI] [PubMed] [Google Scholar]
- 102.Xu H, Yi H, Guan J, Yin S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Atherosclerosis (2014) 234:446–53. 10.1016/j.atherosclerosis.2014.03.034 [DOI] [PubMed] [Google Scholar]
- 103.Ha SC, Hirai HW, Tsoi KK. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta-analysis of randomized trials. Sleep Med Rev (2014) 18:19–24. 10.1016/j.smrv.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 104.Gordon P, Sanders MH. Sleep.7: positive airway pressure therapy for obstructive sleep apnoea/hypopnoea syndrome. Thorax (2005) 60:68–75. 10.1136/thx.2003.007195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Strollo PJ, Jr, Rogers RM. Obstructive sleep apnea. N Engl J Med (1996) 334:99–104. 10.1056/NEJM199601113340207 [DOI] [PubMed] [Google Scholar]
- 106.Liu L, Cao Q, Guo Z, Dai Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) (2016) 18:153–8. 10.1111/jch.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu T, Li T, Wei D, Feng Y, Xian L, Wu H, et al. Effect of automatic versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: an up-to-date meta-analysis. Sleep Breath (2012) 16:1017–26. 10.1007/s11325-011-0626-6 [DOI] [PubMed] [Google Scholar]
- 108.Umemoto G, Toyoshima H, Yamaguchi Y, Aoyagi N, Yoshimura C, Funakoshi K. Therapeutic efficacy of twin-block and fixed oral appliances in patients with obstructive sleep apnea syndrome. J Prosthodont (2017). 10.1111/jopr.12619 [DOI] [PubMed] [Google Scholar]
- 109.Bratton DJ, Stradling JR, Barbe F, Kohler M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax (2014) 69:1128–35. 10.1136/thoraxjnl-2013-204993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alajmi M, Mulgrew AT, Fox J, Davidson W, Schulzer M, Mak E, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung (2007) 185:67–72. 10.1007/s00408-006-0117-x [DOI] [PubMed] [Google Scholar]
- 111.Fava C, Dorigoni S, Dalle Vedove F, Danese E, Montagnana M, Guidi GC, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest (2014) 145:762–71. 10.1378/chest.13-1115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.