Abstract
Cannabidiol (CBD) has been traditionally used in Cannabis-based preparation, however historically, it has received far less interest as a single drug than the other components of Cannabis. Currently, CBD generates considerable interest due to its beneficial neuroprotective, antiepileptic, anxiolytic, antipsychotic, and anti-inflammatory properties. Therefore, the CBD scaffold becomes of increasing interest for medicinal chemists. This review provides an overview of the chemical structure of natural and synthetic CBD derivatives including the molecular targets associated with these compounds. A clear identification of their biological targets has been shown to be still very challenging.
Keywords: cannabidiol, cannabidiol derivative, cannabinoid receptor, molecular target, therapeutic application
Introduction
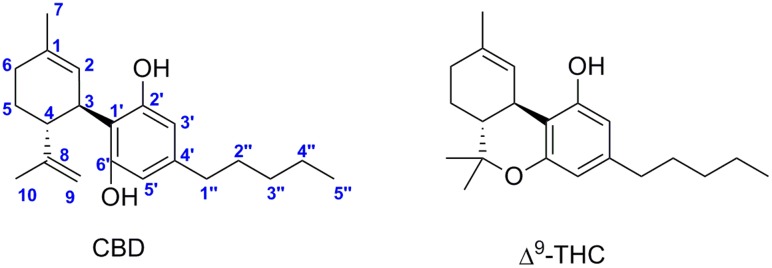
In the mid-seventies, major efforts were focused on the identification of new natural cannabinoids isolated from preparations of Cannabis sativa and of other subspecies and varieties, such as Cannabis indica and Cannabis ruderalis. The two most abundant and most therapeutically relevant components of the plants are (–)-trans-Δ9–tetrahydrocannabinol (Δ9–THC) and (-)-cannabidiol (CBD) (Figure 1). Over these last two decades, the endocannabinoid system (ECS) related to the effects of Cannabis sativa has being emerging as target of pharmacotherapy showing very considerable physiological significance (Mechoulam et al., 2014). This system includes two cannabinoid receptors (CB1 and CB2) and endogenous ligands named endocannabinoids (Matsuda et al., 1990; Munro et al., 1993). CB1 receptor is abundant in the brain, but to a less extend in peripheral tissues. CB2 receptor is mainly expressed in immune cells.
FIGURE 1.
Cannabidiol (CBD) and (–)-trans-Δ9–tetrahydrocannabinol (Δ9–THC).
Δ9–THC is responsible for the psychoactive effects of Cannabis sativa mediated by the activation of CB1 receptor in the brain, whereas CBD is considered non-psychotropic. Currently, CBD is clinically used in association with Δ9–THC in a cannabis-based preparation (Sativex®) that contains equimolar content of both for managing neuropathic symptoms associated with multiple sclerosis (Fernandez, 2016). CBD as a single drug is currently generating considerable interest due to its beneficial neuroprotective (Fernandez-Ruiz et al., 2013; Scuderi et al., 2014; Ibeas Bih et al., 2015), antiepileptic (Devinsky et al., 2015; Wright et al., 2015), hypoxia-ischemia (Lafuente et al., 2011; Mori et al., 2017), anxiolytic (Massi et al., 2013; Schier et al., 2014), antipsychotic (Bhattacharyya et al., 2010), analgesic (Maione et al., 2011), anti-inflammatory (Ruiz-Valdepeñas et al., 2011; Burstein, 2015), anti-asthmatic (Ribeiro et al., 2015; Vuolo et al., 2015), and antitumor properties (McAllister et al., 2011; Massi et al., 2013) among others(Mechoulam et al., 2007; Zhornitsky and Potvin, 2012; Renard et al., 2017; Watt and Karl, 2017). In 2016, GW pharmaceuticals reported the first positive results of CBD (Epidiolex®) in phase III clinical trials for treatment-resistant seizure disorders, including Lennox–Gastaut and Dravet syndromes. An overview of regulatory approvals and clinical trials of CBD has been recently published (Fasinu et al., 2016).
The molecular targets involved in the diverse therapeutic properties produced by CBD are still not very well-understood (Morales et al., 2017). Unlike Δ9–THC, CBD does not bind to the orthosteric binding site of the CB1 and CB2 cannabinoid receptors (McPartland et al., 2007). Despite this lack of orthosteric affinity, CBD has been shown to antagonize the effects of the CB1/CB2 agonists CP–55,940 and WIN55212 at the mouse CB1 and at the human CB2 receptors (Pertwee et al., 2002; Thomas et al., 2007). Therefore, allosteric activity of CBD at these receptors has been hypothesized. In a recent report, CBD was shown to be a negative allosteric modulator of Δ9–THC and the endogenous cannabinoid 2–AG providing a possible explanation for some in vivo CBD effects (Laprairie et al., 2015; Morales et al., 2016). CBD has also been shown to modulate endocannabinoid tone by inhibiting the cellular uptake of the endocannabinoid anandamide (Leweke et al., 2012). This effect has been attributed to the fact that CBD competes with anandamide for binding to fatty acid-binding proteins (FABPs) which are intracellular proteins involved in the transport of anandamide to its metabolic enzyme fatty acid amide hydrolase (FAAH) (Elmes et al., 2015). Other possible molecular targets of CBD have been explored. Modulation of the GPR55 receptor by CBD has been evaluated in different signaling pathway assays. CBD acts as an antagonist preventing [35S]GTPγS binding and Rho activation (Ryberg et al., 2007; Whyte et al., 2009; Ford et al., 2010), modulating Ca2+ mobilization (Lauckner et al., 2008) and β-arrestin recruitment (Yin et al., 2009). CBD has also been proposed as an antagonist of the GPR18 cannabinoid receptor (McHugh et al., 2012, 2014). Certain actions of CBD such as anti-inflammatory and immunosuppressive effects appear to be partially mediated through the serotonin and adenosine receptors that are not considered part of the ECS. For instance, CBD acts as a full 5-HT1A agonist, 5-HT2A weak partial agonist and a non-competitive 5HT3A antagonist (Russo et al., 2005; Yang et al., 2010; Rock et al., 2012). The ability of CBD to activate the A1A adenosine receptor has also been proposed (Gonca and Darıcı, 2014). Other molecular targets have also been studied, among them, the PPARγ nuclear receptors (O’Sullivan et al., 2009; Esposito et al., 2011; Scuderi et al., 2014), glycine receptors (Ahrens et al., 2009; Xiong et al., 2012), GABAA receptors (Bakas et al., 2016), and transient receptor potential (TRP) channels (De Petrocellis et al., 2011, 2012). Studies focused on the possible epigenetic regulation of skin differentiation genes by CBD revealed that CBD is a transcriptional repressor that can control cell proliferation and differentiation through DNA methylation (Pucci et al., 2013). Despite all of this data, the mechanistic bases for the effects of CBD remain complex.
Cannabidiol constitutes one of the most important components of therapeutic interest from Cannabis sativa. However, unlike the numerous synthesized cannabimimetics generated to provide a synthetic alternative to THC, CBD derivatives have only been superficially explored. The purpose of this review is to provide a structural overlook at natural and synthetic CBD derivatives. Due to the fact that diverse molecular targets are involved in the therapeutic properties produced by CBD, we associated CBD structures to their biological targets. Thus, this review is intended to be a useful tool especially for medicinal chemists.
The basic structure of the CBD derivatives described in this review consists of 5-alkyl resorcinols substituted in position 2 by a propenylcyclohexene. Structural modifications on the alkyl side-chain, on the propenylcyclohexene, and substitution of the phenolic hydroxyl groups are concerned. Quinone CBD analogs are also included in this classification as far as their structures are closely related to CBD.
Natural Cannabidiol Derivatives
In a recently published review dedicated to the diversity of cannabis phytocannabinoids, the authors updated the inventory of naturally occurring CBD derivatives (Hanuš et al., 2016). Herein, we are associating these structures to their possible molecular targets.
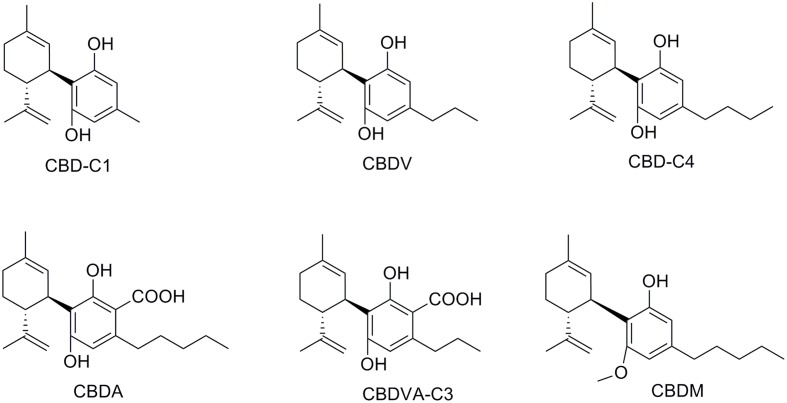
Of the over 100 natural cannabinoids identified in Cannabis Sativa, seven have been classified as CBD-type compounds including CBD (Figure 2) (ElSohly and Slade, 2005; ElSohly and Gul, 2014; Aizpurua-Olaizola et al., 2016). All of them have the same absolute configuration than CBD; they are 5′-methyl-2′-(prop-1-en-2-yl)-1′,2′,3′,4′-tetrahydro-[1,1′-biphenyl]-2,6-dioles retaining the trans-(1R,6R) configuration. Cannabidiolic acid (CBDA) and cannabidivarinic acid (CBDVA-C3) are C3′-carboxylic derivatives, whereas cannabidiorcol (CBD-C1), cannabidiol-C4 also named as nor-cannabidiol (CBD-C4), and cannabidivarin (CBDV) differ from CBD by the length of their C4′-side chain. Cannabidiol monomethyl ether (CBDM), the C6′-methoxy CBD analog, was also isolated from the plant. Despite the potential therapeutic interest of these naturally occurring CBD derivatives, only a few related pharmacological studies have been reported (Table 1). Like most non-steroidal anti-inflammatory drugs, CBDA is characterized by a carboxylic group resulting in a selective inhibition of cyclooxygenase-2 (Takeda et al., 2008). CBDA does not have effect on anandamide inactivation in FAAH assays (Inhibition of [14C]-anandamide uptake: IC50 > 50 μM) contrary to CBD (IC50 = 28 μM) (Bisogno et al., 2001; Ligresti, 2006). Other molecular targets proposed for CBDA include GPR55 (Anavi-Goffer et al., 2012) and TRPA1 with moderate activity (De Petrocellis et al., 2011). CBDA has been shown to be an inhibitor of cell migration in the highly aggressive human breast cancer MDA-MB-231 by alteration of Rho GTPase activity (Takeda et al., 2012). CBDV, the C4′-propyl analog of CBD, displays very weak affinity for CB1 and CB2 receptors (Hill et al., 2013; Rosenthaler et al., 2014), whereas it has been reported to inhibit the activity of the putative endogenous ligand LPI in hGPR55-HEK293 cells (Anavi-Goffer et al., 2012). CBDV also targets the human TRPA1 channel (De Petrocellis et al., 2011, 2012). In several animal seizures models, CBDV exerted notable anticonvulsant effects without affecting normal motor function (Hill et al., 2012). The mechanisms through which CBDV exerts its antiepileptic effects are uncertain (Jones and Whalley, 2015). CBDV is currently in Phase II clinical trials as an antiepileptic drug under the name GWP42006.1
FIGURE 2.
Natural phytocannabinoid CBD analogs.
Table 1.
CB1/CB2 cannabinoid receptor binding, molecular targets and therapeutic potential of CBD derivatives.
| Compounds | CB1R Ki [nM] | CB2R Ki [nM] | Reference | Other targets | Therapeutic potential | Reference |
|---|---|---|---|---|---|---|
| (-)-CBD | >10000 | >10000 | Bisogno et al., 2001 | NAM-CB1; FABPs; GPR55; GPR18; 5-HT1A; 5-HT2A; 5-HT3A; GlyR A1A; PPARγ; GABAA, TRPs | Neuroprotection; epilepsy; anxiety; psychosis; inflammation | Fasinu et al., 2016; Morales et al., 2017 |
| (-)-CBDA | COX-2; TRPA1; DAGLα | Inflammation; cancer; bacteria | Appendino et al., 2008; Takeda et al., 2008, 2012; De Petrocellis et al., 2011 | |||
| (-)-CBDV | 14711 ± 5734 | 574.2 ± 146 | Rosenthaler et al., 2014 | GPR55; TRPA1; CYP1A1 | Convulsion; epilepsy | De Petrocellis et al., 2011; Anavi-Goffer et al., 2012; Hill et al., 2012, 2013; Yamaori et al., 2013; Rosenthaler et al., 2014; Jones and Whalley, 2015 |
| Machaeridol B | - | - | - | Plasmodium falciparum; Leishmania donovani | Muhammad et al., 2003 | |
| Ferruginene C | NR | Weak | Seephonkai et al., 2011 | TRPA1 | Cancer | Seephonkai et al., 2011 |
| (-)-7-OH-CBD | >10000 | >10000 | Bisogno et al., 2001 | - | ||
| (+)-7-OH-CBD | 5.3 ± 0.5 | 101.0 ± 5.1 | Fride et al., 2004 | - | - | |
| (-)-7-COOH-CBD | >10000 | >10000 | Bisogno et al., 2001 | - | - | |
| (+)-7-COOH-CBD | 13.2 ± 0.4 | 312.8 ± 15.8 | Fride et al., 2004 | - | - | |
| CBE | - | - | CYP1A1 | - | Yamaori et al., 2013 | |
| (+)-CBD | 842 ± 36 | 203 ± 16 | Bisogno et al., 2001 | - | - | |
| H2-CBD | >1000 | - | Ben-Shabat et al., 2006 | - | Inflammation | Ben-Shabat et al., 2006 |
| H4-CBD | 145 | - | Ben-Shabat et al., 2006 | - | Inflammation | Ben-Shabat et al., 2006 |
| HU-444 | >10000 | >10000 | Haj et al., 2015 | - | Inflammation | Haj et al., 2015 |
| HU-446 | ∼1000 | >10000 | Kozela et al., 2015 | - | Inflammation | Haj et al., 2015 |
| HU-465 | 76.7 ± 58 | 12.1 ± 2.3 | Kozela et al., 2015 | - | Inflammation | Haj et al., 2015 |
| (-)-DMH-CBD | >10000 | >1000 | Bisogno et al., 2001 | - | Anxiety; pain; inflammation; cancer | Burstein, 2015; Juknat et al., 2016 |
| (+)-DMH-CBD | 17.4 ± 1.8 | 211 ± 23 | Bisogno et al., 2001 | - | - | |
| (-)-7-OH-DMH-CBD | >4000 | 671 ± 12 | Bisogno et al., 2001 | TRPV1, opioid, α2-AR | - | Fride et al., 2005; Pertwee et al., 2005 |
| (+)-7-OH-DMH-CBD | 2.5 ± 0.03 | 44.0 ± 3.1 | Bisogno et al., 2001 | - | - | |
| (-)-7-COOH-DMH-CBD (HU-320) | > 1000 | >4000 | Bisogno et al., 2001 | - | Inflammation, immunosuppression | Sumariwalla et al., 2004 |
| (+)-7-COOH-DMH-CBD | 5.8 ± 0.7 | 155.5 ± 5.3 | Fride et al., 2004 | - | - | |
| H2-DMH-CBD | 124 | - | Ben-Shabat et al., 2006 | - | ||
| H4-DMH-CBD | 17 | - | Ben-Shabat et al., 2006 | - | ||
| HU-308 | >10000 | 22.7 ± 3.9 | Hanus et al., 1999 | - | Inflammation; hepatic ischemia; osteoporosis; pain | Ofek et al., 2006; Rajesh et al., 2007a,b; Burstein, 2015 |
| (-)-CBDD | >10000 | >10000 | Hanus et al., 2005 | 15-LOX | Atherosclerosis, body weight regulation | Takeda et al., 2009, 2011, 2015 |
| (+)-CBDD | >10000 | >10000 | Hanus et al., 2005 | - | - | |
| (-)-DMH-CBDD | >10000 | >10000 | Hanus et al., 2005 | - | - | |
| (+)-DMH-CBDD | >10000 | <10000 | Hanus et al., 2005 | - | - | |
| KLS-13019 | - | - | Neuroprotection | Kinney et al., 2016 | ||
| HUF-101 | - | - | - | Anxiety; depression, psychosis; convulsion | Breuer et al., 2016 | |
| CBD-aldehyde-diacetate | - | - | - | Convulsion | Carlini et al., 1975 | |
| 6-Oxo-CBD-diacetate | - | - | - | Convulsion | Carlini et al., 1975 | |
| 6-OH-CBD-triacetate | - | - | - | Convulsion | Carlini et al., 1975 | |
| 9-OH-CBD-triacetate | - | - | - | Convulsion | Carlini et al., 1975 | |
| HU-331 | NR | NR | Kogan et al., 2004 | Topoisomerase IIα; Caspases; ROS; PARP | Cancer | Kogan et al., 2004, 2007; Petronzi et al., 2013; Regal et al., 2014 |
| CBD-Q (V) | - | - | PPARγ | Neuroprotection | Appendino et al., 2015 | |
| CBD-Q (VIII) | - | - | PPARγ | Neuroprotection | Appendino et al., 2015 | |
| Abn-CBD | - | - | GPR55; GPR18 | Vasodilatation; bacteria; diabetes; colitis | Johns et al., 2007; Ryberg et al., 2007; Appendino et al., 2008; Console-Bram et al., 2014; Krohn et al., 2016; McKillop et al., 2016 | |
| O-1602 | NR | NR | GPR55; GPR18 | Central nervous system; metabolism | Johns et al., 2007; Romero-Zerbo et al., 2011; Ashton, 2012; Console-Bram et al., 2014 | |
| CBG | 897 ± 596 | 153 ± 42 | Rosenthaler et al., 2014 | α2-AR; TRP; COX-1; COX-2; 5-HT1A | Bacteria; bowel inflammation; depression | Appendino et al., 2008; Cascio et al., 2010; El-Alfy et al., 2010; De Petrocellis et al., 2011; Ruhaak et al., 2011; Borrelli et al., 2013; Rosenthaler et al., 2014 |
| Cannabimovone | >10000 | >10000 | Taglialatela-Scafati et al., 2010 | TPRV1 | - | Taglialatela-Scafati et al., 2010 |
NR, no response.
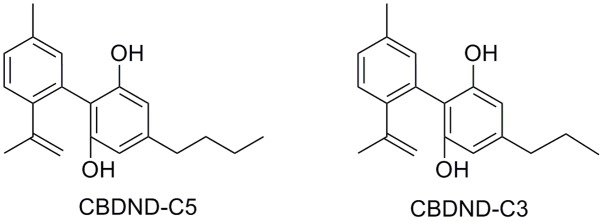
Two aromatic analogs of CBD have been isolated from Lebanese hashish (ElSohly and Slade, 2005): cannabinodiol (CBND-C5), and cannabinodivarin (CBND-C3) (Figure 3) whose structural elucidation required their total synthesis (Robert et al., 1977). CBND-C5 found in the plant’s flowers in low concentration, is considered a product of CBD photochemical conversion.
FIGURE 3.
Aromatic analogs of CBD.
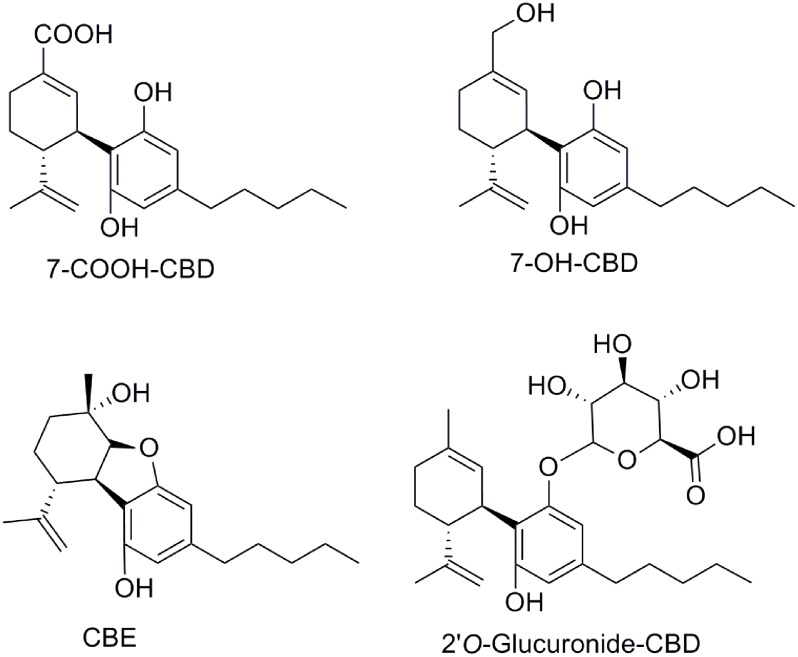
The conversion of CBD into human metabolites has been the subject of a recent interesting review (Ujváry and Hanuš, 2016). CBD biotransformation shows considerable species variability. The main biotransformation, including hydroxylation and oxidation, involves the CYP450 enzyme family. While 7-hydroxy-CBD (7-OH-CBD) derivatives are found in low concentration, the most abundant metabolites are hydroxylated 7-carboxylic acid derivatives of CBD (7-COOH-CBD, Figure 4). Glucuronidation of CBD seems to frequently occur at the phenolic oxygen (Figure 4). Another cannabinoid metabolite, the so called cannabielsoin (CBE), has been identified in plants as a product of photo-oxidation from CBD and CBDA (Shani and Mechoulam, 1974; Ujváry and Hanuš, 2016), or by biotransformation using tissue cultures under normal growth conditions (Hartsel et al., 1983; Yamamoto et al., 1991). CBE was also identified as a metabolite in guinea pigs, mice, rabbits, and rats (Yamamoto et al., 1991). Despite the fact that CBD metabolites have been the subject of many studies, few in vivo studies have been published. Therefore, their therapeutic benefits remain to be established.
FIGURE 4.
Selected metabolites of CBD.
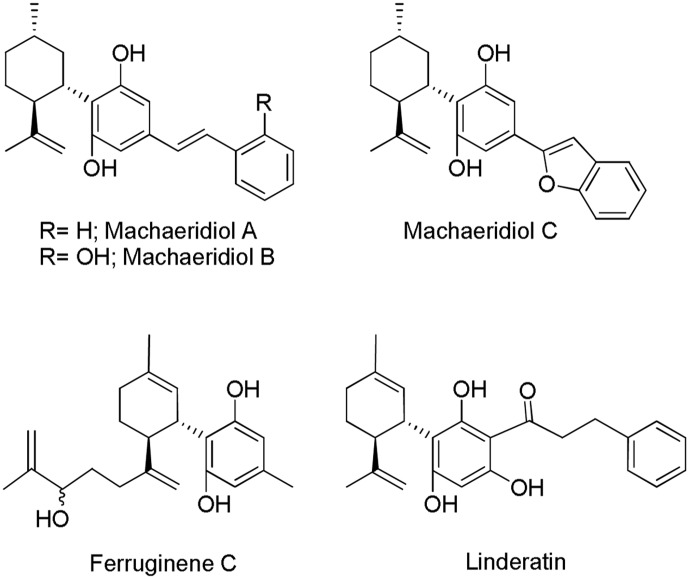
Beyond the Cannabis plant, other naturally occuring products have been reported to interact with the ECS (Gertsch et al., 2010). However, only few of them are CBD-based compounds. Isolation and characterization of (+)-trans-hexahydrodibenzopyrans from the stem bark of the Amazonian liana Machaerium multiflorum Spruce led to the identification of the CBD related structures machaeridiols A, B, and C (Figure 5) (Muhammad et al., 2003). The total synthesis of these compounds via an efficient highly regio- and stereoselective approach has also been described (Huang et al., 2007). Although their activity at the CB1 and CB2 cannabinoid receptors has not been reported, these compounds displayed antimicrobial, antifungal, and antiparasitic activity in diverse in vitro assays (Muhammad et al., 2003). Machaeridiol B stands out as the most potent inhibitor against Plasmodium falciparum [chloroquine-sensitive (D6) and chloroquine-resistant (W2) clones] and Leishmania donovani with IC50 values in the low micromolar range (Table 1).
FIGURE 5.
Cannabidiol (CBD)-related Machaerium multiflorum, Rhododendron ferrugineum L. and, Lindera umbellata Thunb. compounds.
Ferruginene C, a methylpentanol derivative of CBD (Figure 5), was recently isolated from the leaves of Rhododendron ferrugineum L. as a mixture of diastereoisomers (Seephonkai et al., 2011). Ferruginene C has been shown to be cytotoxic in the HL-60 cancer cell-line (IC50 13.7 μM) with selectivity toward non-cancerous cell-line. It binds weakly to CB2 and TPRV1 receptors, but it did not show significant affinity for CB1 and 5-HT1A receptors (Table 1).
Even though linderatin (Figure 5), isolated from fresh leaves of Lindera umbellata Thunb. (Tanaka et al., 1984), is not considered a phytocannabinoid (Hanuš et al., 2016), it is interesting to include in the present review since closely related to CBD. No biological data have been reported so far.
Synthetic Cbd Analogs
Due to the promising therapeutic effects of CBD in a wide variety of diseases, synthetic CBD derivatives have attracted the attention of drug discovery programs in both industry and academia with the aim to improve the potency, efficacy, or pharmacokinetic properties of this interesting phytocannabinoid.
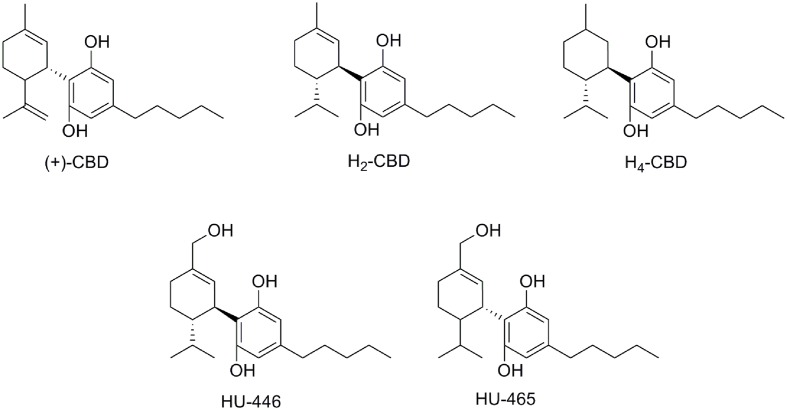
Synthetic approaches for different CBD metabolites such as 7-COOH-CBD or 7-OH-CBD (Figure 4) have been reported (Tchilibon and Mechoulam, 2000; Mechoulam and Hanuš, 2002). Moreover, structural modifications on different pharmacophoric positions such as the lipophilic side chain, the phenolic hydroxyl groups or the C7-methyl have been widely accomplished. In addition to the (-)-CBD enantiomers, the (+)-CBD derivatives [(+)-CBD depicted in Figure 6] have also been synthesized and pharmacologically evaluated (Bisogno et al., 2001; Fride et al., 2004; Hanus et al., 2005). Measurements of the binding affinities of these compounds for the CB1 and CB2 cannabinoid receptors yielded unexpected outcomes. Contrary to the naturally occurring (-)-CBD analogs, which showed no orthosteric affinity, most of the compounds in the (+)-CBD series bind to both receptors displaying selectivity toward CB1 (Table 1).
FIGURE 6.
(+)-CBD and hydrogenated CBD derivatives.
Hydrogenation of CBD yielded the dihydro- and tetrahydro-cannabidiol derivatives H2-CBD and H4-CBD (Figure 6) (Ben-Shabat et al., 2006). Their effects on the production of reactive oxygen intermediates, nitric oxide, and tumor necrosis factor showed their anti-inflammatory capacity. In contrast to CBD, H2-CBD, and H4-CBD have affinity for the cannabinoid CB1 receptor (Table 1). Additionally, the (-)- and (+)-dihydro-7-hydroxy-CBD enantiomers (HU-446 and HU-465, Figure 6) have recently been synthesized and biologically characterized in an inflammatory model of encephalitogenic T cells (Kozela et al., 2015). Both compounds showed anti-inflammatory potential in inflammatory and autoimmune diseases models. However, only the (+)-enantiomer (HU-465) displays affinity for the cannabinoid CB1 and CB2 receptors (Table 1).
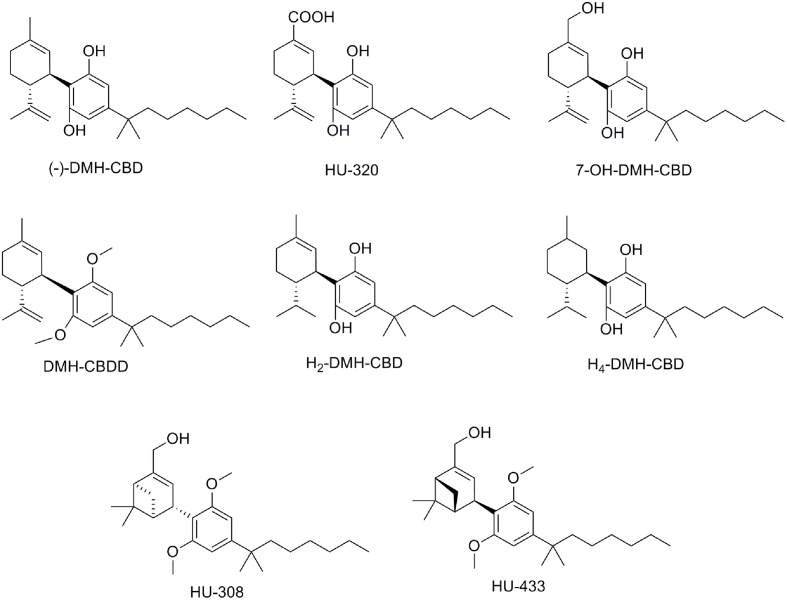
1′,1′ -Dimethylheptyl-CBD Derivatives
Taking into account that substitution of the pentyl chain of Δ9–THC by a 1′, 1′-dimethylheptyl (DMH) lipophilic alkyl chain resulted in more active compounds than natural Δ9–THC (Mechoulam et al., 1988), a similar approach was performed for the CBD scaffold (Mechoulam et al., 1990; Hanus et al., 2005) (Table 1). Thus, the synthesis of DMH-CBD derivatives, such as DMH-CBD, HU-320, DMH-CBDD, and 7-OH-DMH-CBD (Figure 7) have been reported by Mechoulam and coworkers (Leite et al., 1982; Hanus et al., 2005). Introduction of the DMH alkyl chain in the (-)-DMH-CBD series did not change the lack of CB1 and CB2 receptor affinity except for (-)-7-OH-DMH-CBD that moderately binds to CB2 (Table 1) (Bisogno et al., 2001). However, in the case of the (+)-DMH-CBD series, the presence of the DMH alkyl chain improved both CB1 receptor affinity compared to (+)-CBD (Table 1). (-)-DMH-CBD analogs have displayed anxiolytic, analgesic, anti-inflammatory, or antiproliferative effects in diverse assays (Burstein, 2015). For instance, (-)-DMH-CBD has shown anti-inflammatory and antiproliferative properties in human acute myeloid leukemia, microglial or encephalitogenic T cells (Juknat et al., 2016). The carboxylic acid HU-320 produced strong anti-inflammatory and immunosuppressive effects in an in vivo model of collagen-induced arthritis (Sumariwalla et al., 2004). Interestingly, (-)-7-OH-DMH-CBD exhibited potent inhibition of electrically evoked contractions of the mouse vas deferens that was not mediated through CB1, CB2, TRPV1, opioid, or α2-adrenergic receptors (Fride et al., 2005; Pertwee et al., 2005).
FIGURE 7.
Dimethylheptyl (DMH)-CBD derivatives.
As previously mentioned for the pentyl CBD derivatives, hydrogenation of DMH-CBD has been studied (Ben-Shabat et al., 2006). Partial hydrogenation gave H2-DMH-CBD (Figure 7) as the major epimer (hydrogenation at C8) with small amounts of the hydrogenated C1 epimer being obtained. Full hydrogenation allowed the formation of H4-DMH-CBD (Figure 7). These hydrogenated compounds, which bind to the CB1 receptor with affinity constants in the nanomolar range, displayed weak anti-inflammatory effects when compared to CBD or DMH-CBD.
The pinene dimethoxy-DMH-CBD derivative HU-308 (Figure 7) was identified decades ago as a potent peripheral CB2-selective agonist (Mechoulam et al., 1990; Hanus et al., 1999). HU-308 has shown very interesting properties such as anti-inflammatory, analgesic, neuroprotective or antitumor effects, and has been used as a pharmacological tool in numerous cannabinoid studies contributing to the progress in this field (e.g., Hanus et al., 1999; Ofek et al., 2006; Rajesh et al., 2007a,b; Burstein, 2015). More recently, the efficacy of HU-308 and HU-433, two enantiomers, has been tested in ovariectomy-induced bone loss and ear inflammation (Smoum et al., 2015) showing an inverse relationship between binding affinity and biological potency.
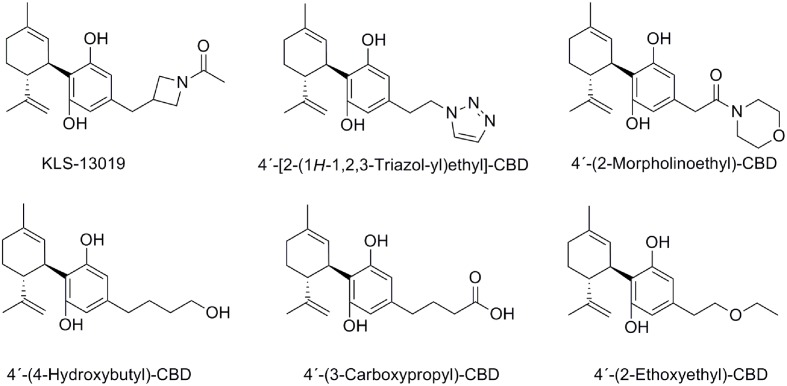
Other Modifications on the C4′-Alkyl Chain
In order to improve oral bioavailability and solubility issues, a novel series of CBD analogs have recently been synthesized (Kinney et al., 2016) (Figure 8). Structural modifications at the pharmacophoric lipophilic chain allowed fine-tuning the “drug-likeness” of this scaffold by variation of different physicochemical parameters such as the number of hydrogen bond donors, acceptors, and polar surface area. Among these new derivatives depicted in Figure 8, KLS-13019 stands out as being 50-fold more potent and more than 400-fold safer than CBD preventing damage to hippocampal neurons induced by ammonium acetate and ethanol with improved oral bioavailability compared to CBD (Kinney et al., 2016).
FIGURE 8.
Cannabidiol analogs modified on the C4′-alkyl chain.
Halogenated CBD Derivatives
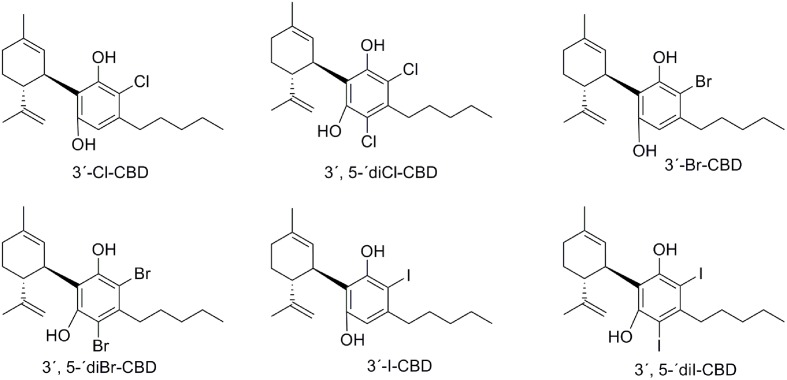
Structural modifications of CBD include halogenated substituents on the phenol ring. The first reported halogenations occurred at the 3′ and/or 5′ positions by chlorine, bromine or iodine substitution, allowing the preparation of 3′-Cl-CBD, 3′,5′-diCl-CBD, 3′-Br-CBD, 3′,5′-diBr-CBD, 3′-I-CBD, and 3′,5′-diI-CBD (Figure 9) (Usami et al., 1999). These halogenated compounds were evaluated in murine models of barbiturate-induced sleep prolongation, electroshock-induced seizures and locomotor activity resulting in activity similar to CBD for the monohalogenated analogs, whereas the dihalogenated derivatives displayed lower activity (Table 1).
FIGURE 9.
Chlorinated, brominated, and iodinated CBD derivatives.
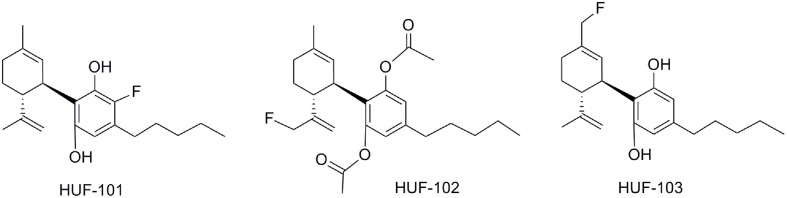
The synthesis and pharmacological evaluation of three new fluorine halogenated CBD derivatives have been reported (Breuer et al., 2016). Two of these were fluorinated at the cyclohexenyl ring substituent (Figure 10: HUF-102 and HUF-103), and the third one was fluorinated at the phenol ring (HUF-101). HUF-101 displayed the most promising results in four mice behavioral assays (elevated plus-maze, forced swimming test, prepulse inhibition, and marble burying test) that target anxiolytic, antidepressant, antipsychotic and anticompulsive activity respectively. HUF-101 may be an interesting prototype for further development since it showed higher potency than CBD in the animal assays cited above. In these tests, HUF-102 did not show activity at the doses tested (1–10 mg/kg), whereas HUF-103 showed moderate to low activity compared to HUF-101.
FIGURE 10.
Fluorinated CBD derivatives.
Modifications on the Hydroxyl Groups
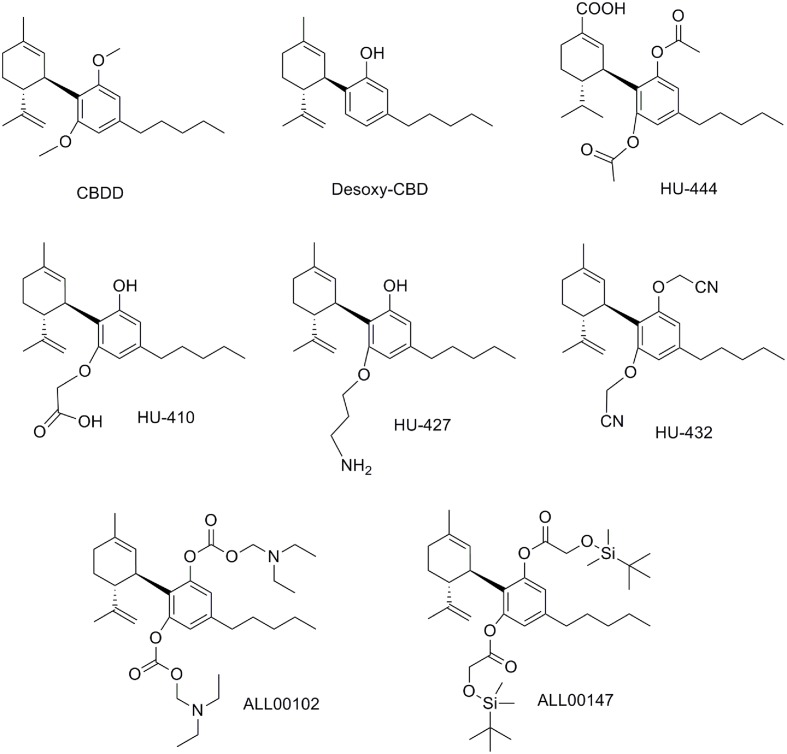
Modifications on the resorcinol hydroxyl groups have been explored. Computational studies suggested that the removal of one of the CBD hydroxyl groups may enable the ligand to reach the CB1 binding site (Reggio et al., 1995). Thus, desoxy-CBD represented in Figure 11 was synthesized and evaluated. Pharmacological data for desoxy-CBD corroborated the computational studies showing CB1 partial agonism in the mouse vas deferens assay.
FIGURE 11.
Cannabidiol derivatives modified at the hydroxyl groups.
Different research groups have developed acetylations and alkylations at one or both phenolic hydroxyls. For instance, the dimethylated CBD derivative named CBDD (Figure 11), as well as the monomethylated derivative (CBD-2′-monomethylether o O-methylcannabidiol) revealed higher potency and selectivity as 15-lipoxygenase inhibitors compared to CBD (Takeda et al., 2009, 2011). Consequently, the resorcinol moiety seems to be a determinant for the activity in this target. Further studies performed with CBDD suggest that this compound is not only a potential prototype for atherosclerosis treatment, but also a pharmacological tool to study the mechanisms of body weight regulation (Takeda et al., 2015). Other alkylations on the phenolic hydroxyl group have been reported such as O-propyl- and O-pentylcannabidiol that have been structurally characterized but no pharmacological data have been described so far (Hendricks et al., 1978).
Cannabidiol derivatives bearing one or both hydroxyl substitutions have been reported in the patent literature to be active as anti-inflammatory agents (Mechoulam et al., 2008). Selected examples disclosed in this patent (HU-410, HU-427, and HU-432) are depicted in Figure 11. It is interesting to highlight that some of these compounds present improved solubility, stability and bioavailability parameters when compared with CBD. Likewise, the non-CB1, non-CB2 ligand HU-444 has shown anti-inflammatory properties in vitro and in vivo in a murine model of collagen-induced arthritis (Haj et al., 2015).
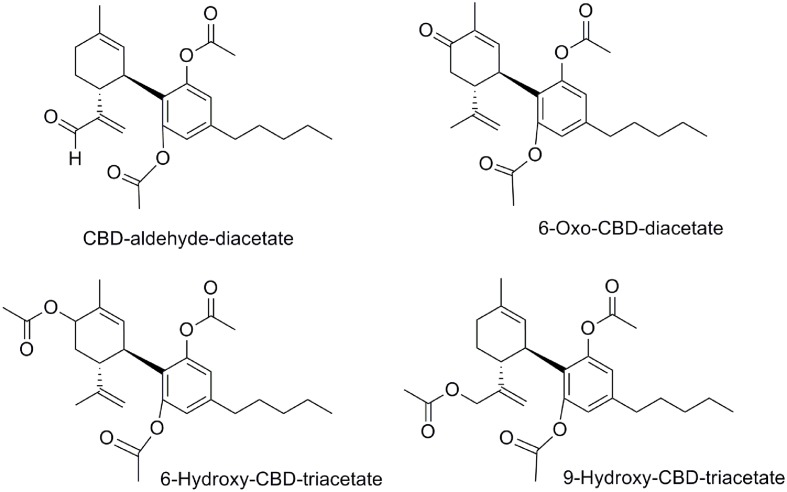
In addition, the in vivo anticonvulsant activity of four diacetylated-CBD analogs (CBD-aldehyde-diacetate, 6-oxo-CBD-diacetate, 6-hydroxy-CBD-triacetate, and 9-hydroxy-CBD-triacetate, Figure 12) was demonstrated in a mouse model (Carlini et al., 1975). Their effects against maximal electroshock convulsions, potentiation of pentobarbital sleeping-time and reduction of spontaneous motor activity were evaluated obtaining significant anticonvulsant effects at high doses. It is noteworthy that the safety, efficiency, and potency of these four compounds were lower than that of CBD in the same assays.
FIGURE 12.
Diacetylated-CBD analogs.
At that point it is interesting to mention that these diacetate CBD derivatives could have been considered as prodrugs. Considering that CBD is rapidly distributed in adipose tissues and it undergoes a CYP3A- and CYP2C- dependent first-pass metabolism to give 7-hydroxy-CBD (Fasinu et al., 2016), a prodrug concept could be very useful. Therefore, the phenyl acetate groups could be deacetylated to give CBD. The pharmaceutical company, AllTranz, now called Zyberba Pharmaceutics, developed transdermal solutions of CBD-esters and -carbonates among others. The dicarbonate All00102 and the diglicolate AL00147 shown in Figure 11 are two examples disclosed in a AllTranz’s patent (Stinchcomb et al., 2009). Another company, Kalytera Therapeutics is currently undertaking the preclinical stage of K-1012, a bi-phosphate derivative of CBD designed as a prodrug indicated for acute respiratory distress syndrome.2
Quinone Derivatives of CBD
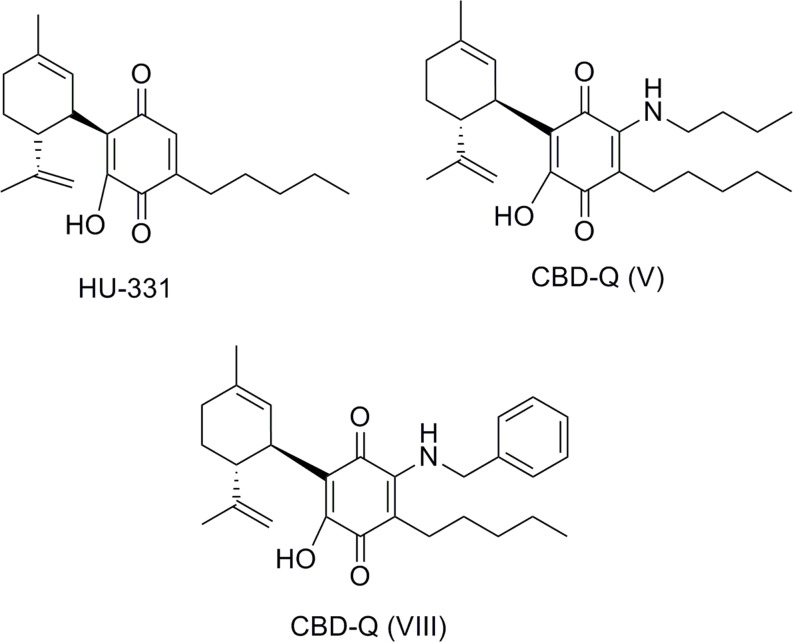
The quinone derivative of CBD, HU331, was first synthesized in Mechoulam et al. (1968) by oxidation of CBD. HU331 has been suggested to be a CBD metabolite having inhibitory effect on cytochrome P450 (Bornheim and Grillo, 1998). It was not until Kogan et al. (2004) that the antineoplastic activity of HU-331 was reported. HU-331 was very effective in reducing growth of human colon carcinoma HT-29 cells in nude mice. The mechanism by which HU-331 acts as an antitumor agent is independent of the CB1 and CB2 cannabinoid receptors. HU-331 does not promote cell death via cell cycle arrest, cell apoptosis, or caspase activation. Extensive studies have shown that HU-331 anticancer properties were due to selective inhibition of the ATPase function of human topoisomerase IIα (Kogan et al., 2007; Peters and Kogan, 2007; Regal et al., 2014). Thus, HU-331 with a selective topoisomerase inhibition is expected to have less off-target toxicity than doxorubicin which antitumor activity is mediated through numerous mechanisms, such as apoptosis, abrogation of the cell cycle, activation of caspases, generation of ROS, and inhibition of both topoisomerases among others.
Structural modifications realized on the substituents of HU-331 led to the benzoquinones having anti-proliferative activity against diverse cancer cell lines (Petronzi et al., 2013). Unlike HU-331, benzoquinone mechanism of action involves caspase activation, poly-(ADP-ribose)-polymerase (PARP) protein cleavage, and reactive oxygen species (ROS) production. These data show the influence of CBD structure compared to the quinone core on the processes producing anticancer effects.
A recent patent from VivaCell Technology discloses HU-331 analogs which act as PPARγ agonists showing a neuroprotective profile in different models (Appendino et al., 2015). The disclosed quinones are substituted in position 3′ by different amines or carboxylates that were synthesized by amination of CBD or esterification of CBDA respectively. Compounds CBD-Q (V) and CBD-Q (VIII) illustrated in Figure 13 are representative of the HU-331 analogs.
FIGURE 13.
Quinones related to CBD.
Miscellaneous CBD Derivatives
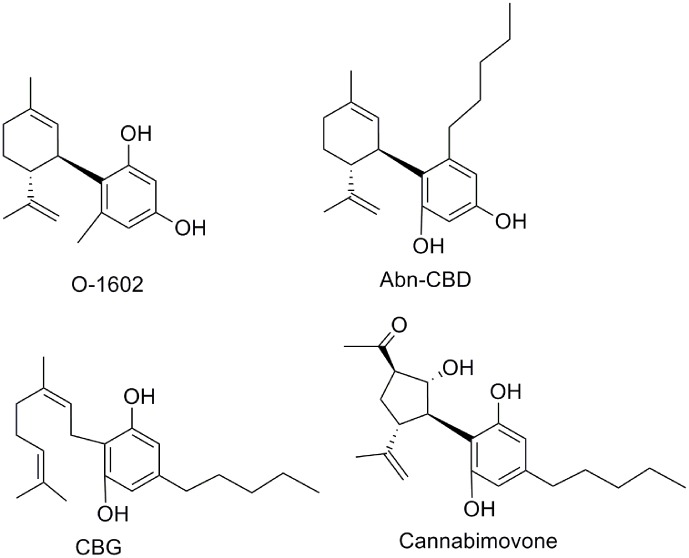
Abnormal cannabidiol (Abn-CBD) (Razdan et al., 1974), a non-psychoactive synthetic regioisomer of CBD (Figure 14), has been the subject of numerous studies that have shown Abn-CBD therapeutic potential as a vasodilator (Johns et al., 2007), antibacterial (Appendino et al., 2008), antidiabetic (McKillop et al., 2016), or anti-colitis agent (Krohn et al., 2016). Recently, two molecular targets, GPR55 and GPR18, have been identified for Abn-CBD (Johns et al., 2007; Ryberg et al., 2007; Console-Bram et al., 2014). Abn-CBD stimulated [35S]GTPγS binding at GPR55 (Oka et al., 2007) and increased calcium mobilization and ERK1/2 phosphorylation at GPR18 (Console-Bram et al., 2014).
FIGURE 14.
Miscellaneous CBD derivatives.
The synthetic cannabinoid O-1602 that does not bind significantly to CB1 or CB2 receptors, stimulates GTPγS activation in membranes from human recombinant GPR55-expressing cells (EC50 = 1.4 nM) (Johns et al., 2007; Console-Bram et al., 2014). In vivo, O-1602 showed anti-inflammatory activity in mice with cerulein-induced acute pancreatitis characterized by an increased expression of GPR55 receptor (Li et al., 2013). O-1602 has also been shown to increase levels of GPR18-mediated MAPK activity and calcium mobilization, but not β-arrestin signaling, thus supporting that O-1602 acts as a biased-agonist at GPR18 (Console-Bram et al., 2014). Data have been reported suggesting the therapeutic potential of O-1602 for diseases related to the central nervous system (Ashton, 2012), or to metabolic diseases (Romero-Zerbo et al., 2011).
Another minor component of Cannabis sativa is cannabigerol (CBG) (Gaoni and Mechoulam, 1971). Structurally, CBG can be considered the cyclohexenyl-opened analog of CBD. Different therapeutic applications have been proposed for CBG, more recently CBG has been shown to have antibacterial action (Appendino et al., 2008), antidepressant-like action (El-Alfy et al., 2010), and anti-inflammatory properties for bowel disease (Borrelli et al., 2013). Molecular targets of CBG include the α2 adrenergic receptor, TRP channels, cyclooxygenase (COX-1 and COX-2) enzymes, as well as the 5-HT1A and cannabinoid receptors (Cascio et al., 2010; De Petrocellis et al., 2011; Ruhaak et al., 2011). Cannabimovone is one of the latest natural phytocannabinoids that has been extracted from a cultivar of hemp rich in CBD (Taglialatela-Scafati et al., 2010). The terpenoid structure of cannabimovone replaces the cyclohexenyl ring of CBD by a functionalized cyclopentane including four contiguous stereocenters. Its total synthesis has been reported very recently (Carreras et al., 2016). Cannabimovone is devoid of CB1 and CB2 activity, whereas it is a weak TPRV1 agonist.
Conclusion
A significant amount of preclinical data has shown the high therapeutic potential of CBD especially in inflammatory mouse models. According to ClinicalTrials.gov records, CBD is currently tested in clinical phases for different inflammatory diseases. The results of the first clinical study of CDB for the treatment of inflammatory bowel have been published very recently. Unfortunately, the effects of CBD on Crohn’s disease were ineffective in a randomized placebo-controlled trial on 20 patients probably due to low used doses (Naftali et al., 2017). The potential antiepileptic effects of CBD in patients suffering seizures associated with Lennox–Gastaut syndrome and in children and young patients with Dravet syndrome are currently on-going. The research has tended to focus on CBD therapeutic applications. Less attention has been paid to the therapeutic utility of CBD derivatives.
Despite the identifications of CBD metabolites and naturally occurring CBD analogs, in general, their pharmacological properties have not been extensively studies. In what concerns synthetic CBD-based compounds, several of them have shown interesting pharmacological properties but none has been introduced into clinical trials yet. In a pharmacological point of view, whereas CBD does not have affinity for both classical CB1 and CB2 cannabinoid receptors, most of (+)-CBD derivatives do bind to CB1 and/or CB2 receptors. Others, such as Abn-CBD, O-1602, CBG, cannabimovone, ferruginene C, (-)-CBDV, and (-)-CBDA, have shown activity at other receptors including TPRV1, GPR35 and/or GPR18 receptors, or enzymes such as COX-2. A limitation of the development of CBD synthetic derivatives probably resides in the lack of a unique common molecular target.
In future therapeutic development of CBD derivatives, it will be prudent to take into account some structural considerations around the CBD scaffold. One of them is the possible atropisomerism around the phenyl–hexenyl bond. Ortho-substitution on the phenyl ring could have stereochemical consequences generating hindered rotation of the phenyl–hexenyl bond due to steric or electronic constraints, generating two isolable conformers in the case of slow interconversion (Berber et al., 2014; Flos et al., 2016). Thus, it is necessary to consider the implication of a possible atropoisomerism for new CBD analogs discovery (Clayden et al., 2009).
The complexity of the pharmacological processes of CBD and CBD analogs suggest that a better understanding of their mechanism of action is required to devise successful synthetic CBD-based drug therapies.
Author Contributions
PM, PHR, and NJ substantially contributed to the redaction of the manuscript. Then, they all approved the manuscript to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. Financial support was provided by Spanish Grants from Ministerio de Economía y Competitividad SAF2015-68580-C2-2-R (MINECO/FEDER) (NJ) and NIH grants RO1 DA003934 and KO5 DA021358 (PR).
ClinicalTrials.gov. A Study of GWP42006 in People With Focal Seizures. https://clinicaltrials.gov/ct2/show/NCT02369471 (accessed September 22, 2016).
https://kalytera.co/programs/preclinical/ (accessed June 8, 2017).
References
- Ahrens J., Demir R., Leuwer M., De La Roche J., Krampfl K., Foadi N., et al. (2009). The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha-1 and alpha-1-beta glycine receptor function. Pharmacology 83 217–222. 10.1159/000201556 [DOI] [PubMed] [Google Scholar]
- Aizpurua-Olaizola O., Soydaner U., Öztürk E., Schibano D., Simsir Y., Navarro P., et al. (2016). Evolution of the cannabinoid and terpene content during the growth of cannabis sativa plants from different chemotypes. J. Nat. Prod. 79 324–331. 10.1021/acs.jnatprod.5b00949 [DOI] [PubMed] [Google Scholar]
- Anavi-Goffer S., Baillie G., Irving A. J., Gertsch J., Greig I. R., Pertwee R. G., et al. (2012). Modulation of L-α-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J. Biol. Chem. 287 91–104. 10.1074/jbc.M111.296020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendino G., Cabello de Alba M. L. B., Munoz Blanco E. (2015). Preparation of novel cannabidiol quinone derivatives as PPARγ agonists. International Patent No WO2015/158381 Washington, DC. [Google Scholar]
- Appendino G., Gibbons S., Giana A., Pagani A., Grassi G., Stavri M., et al. (2008). Antibacterial Cannabinoids from Cannabis sativa : a structure-activity study. J. Nat. Prod. 71 1427–1430. 10.1021/np8002673 [DOI] [PubMed] [Google Scholar]
- Ashton J. C. (2012). The atypical cannabinoid O-1602: targets, actions, and the central nervous system. Cent. Nerv. Syst. Agents Med. Chem. 12 233–239. 10.2174/187152412802430156 [DOI] [PubMed] [Google Scholar]
- Bakas T., Devenish S., Van Nieuwenhuizen P., Arnold J., McGregor I., Collins M. (2016). “The actions of cannabidiol and 2-arachidonyl glicerol on GABA-A receptors,” in Proceedings of the 26th Annual Symposium on the Cannabinoids, International Cannabinoid Research Society, Bukovina, 28. [Google Scholar]
- Ben-Shabat S., Hanuš L. O., Katzavian G., Gallily R. (2006). New cannabidiol derivatives: synthesis, binding to cannabinoid receptor, and evaluation of their antiinflammatory activity. J. Med. Chem. 49 1113–1117. 10.1021/jm050709m [DOI] [PubMed] [Google Scholar]
- Berber H., Lameiras P., Denhez C., Antheaume C., Clayden J. (2014). Atropisomerism about aryl-csp3 bonds: the electronic and steric influence of ortho -substituents on conformational exchange in cannabidiol and linderatin derivatives. J. Org. Chem. 79 6015–6027. 10.1021/jo5006069 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S., Morrison P. D., Fusar-Poli P., Martin-Santos R., Borgwardt S., Winton-Brown T., et al. (2010). Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35 764–774. 10.1038/npp.2009.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D. E., Brandi I., et al. (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 134 845–852. 10.1038/sj.bjp.0704327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornheim L. M., Grillo M. P. (1998). Characterization of cytochrome P450 3A inactivation by cannabidiol-hydrozyquinone as a P450 inactivator. Chem. Res. Toxicol. 11 1209–1216. 10.1021/tx9800598 [DOI] [PubMed] [Google Scholar]
- Borrelli F., Fasolino I., Romano B., Capasso R., Maiello F., Coppola D., et al. (2013). Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 85 1306–1316. 10.1016/j.bcp.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Breuer A., Haj C. G., Fogaça M. V., Gomes F. V., Silva N. R., Pedrazzi J. F., et al. (2016). Fluorinated cannabidiol derivatives: enhancement of activity in mice models predictive of anxiolytic, antidepressant and antipsychotic effects. PLoS ONE 11:e0158779 10.1371/journal.pone.0158779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S. (2015). Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorgan. Med. Chem. 23 1377–1385. 10.1016/j.bmc.2015.01.059 [DOI] [PubMed] [Google Scholar]
- Carlini E. A., Mechoulam R., Lander N. (1975). Anticonvulsant activity of four oxygenated cannabidiol derivatives. Res. Commun. Chem. Pathol. Pharmacol. 12 1–15. [PubMed] [Google Scholar]
- Carreras J., Kirillova M. S., Echavarren A. M. (2016). Synthesis of (-)-cannabimovone and structural reassignment of anhydrocannabimovone through gold(I)-catalyzed cycloisomerization. Angew. Chemie - Int. Ed. 55 7121–7125. 10.1002/anie.201601834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio M. G., Gauson L. A., Stevenson L. A., Ross R. A., Pertwee R. G. (2010). Evidence that the plant cannabinoid cannabigerol is a highly potent α 2-adrenoceptor agonist and moderately potent 5HT 1A receptor antagonist. Br. J. Pharmacol. 159 129–141. 10.1111/j.1476-5381.2009.00515.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayden J., Moran W. J., Edwards P. J., Laplante S. R. (2009). The challenge of atropisomerism in drug discovery. Angew. Chemie - Int. Ed. 48 6398–6401. 10.1002/anie.200901719 [DOI] [PubMed] [Google Scholar]
- Console-Bram L., Brailoiu E., Brailoiu G. C., Sharir H., Abood M. E. (2014). Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br. J. Pharmacol. 171 3908–3917. 10.1111/bph.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L., Ligresti A., Moriello A. S., Allarà M., Bisogno T., Petrosino S., et al. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163 1479–1494. 10.1111/bph.2011.163.issue-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L., Orlando P., Moriello A. S., Aviello G., Stott C., Izzo A. A., et al. (2012). Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 204 255–266. 10.1111/j.1748-1716.2011.02338.x [DOI] [PubMed] [Google Scholar]
- Devinsky O., Marsh E., Friedman D., Thiele E., Laux L., Sullivan J., et al. (2015). Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 15 270–278. 10.1016/S1474-4422(15)00379-8 [DOI] [PubMed] [Google Scholar]
- El-Alfy A. T., Ivey K., Robinson K., Safwat A., Radwan M., Slade D., et al. (2010). Antidepressant-like effect of Δ9 -tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Biochem. Behav. 95 434–442. 10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmes M. W., Kaczocha M., Berger W. T., Leung K., Ralph B. P., Wang L., et al. (2015). Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 290 8711–8721. 10.1074/jbc.M114.618447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly M. A., Gul W. (2014). “Constituents of cannabis sativa,” in Handbook of Cannabis, ed. Pertwee R. G. (Oxford: Oxford University Press; ), 3–22. 10.1093/acprof [DOI] [Google Scholar]
- ElSohly M. A., Slade D. (2005). Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 78 539–548. 10.1016/j.lfs.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Esposito G., Scuderi C., Valenza M., Togna G. I., Latina V., de Filippis D., et al. (2011). Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 6:e28668 10.1371/journal.pone.0028668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasinu P. S., Phillips S., Elsohly M. A., Walker L. A. (2016). Current status and prospects for cannabidiol preparations as new therapeutic agents. Pharmacotherapy 36 781–796. 10.1111/j.1875-9114.2016.01780.x [DOI] [PubMed] [Google Scholar]
- Fernandez O. (2016). THC:CBD in daily practice: available data from UK, germany and spain. Eur. Neurol. 75 1–3. 10.1159/000444234 [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J., Sagredo O., Pazos M. R., Garcia C., Pertwee R., Mechoulam R., et al. (2013). Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 75 323–333. 10.1111/j.1365-2125.2012.04341.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flos M., Lameiras P., Denhez C., Mirand C., Berber H. (2016). Atropisomerism about Aryl-C(sp3) Bonds: conformational behavior of substituted phenylcyclohexanes in solution. J. Org. Chem. 81 2372–2382. 10.1021/acs.joc.5b02856 [DOI] [PubMed] [Google Scholar]
- Ford L. A., Roelofs A. J., Anavi-Goffer S., Mowat L., Simpson D. G., Irving A. J., et al. (2010). A role for L-alpha-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br. J. Pharmacol. 160 762–771. 10.1111/j.1476-5381.2010.00743.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fride E., Feigin C., Ponde D. E., Breuer A., Hanuš L., Arshavsky N., et al. (2004). (+)-Cannabidiol analogues which bind cannabinoid receptors but exert peripheral activity only. Eur. J. Pharmacol. 506 179–188. 10.1016/j.ejphar.2004.10.049 [DOI] [PubMed] [Google Scholar]
- Fride E., Ponde D., Breuer A., Hanuš L. (2005). Peripheral, but not central effects of cannabidiol derivatives: mediation by CB1 and unidentified receptors. Neuropharmacology 48 1117–1129. 10.1016/j.neuropharm.2005.01.023 [DOI] [PubMed] [Google Scholar]
- Gaoni Y., Mechoulam R. (1971). The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 93 217–224. 10.1021/ja00730a036 [DOI] [PubMed] [Google Scholar]
- Gertsch J., Pertwee R. G., Di Marzo V. (2010). Phytocannabinoids beyond the cannabis plant - do they exist? Br. J. Pharmacol. 160 523–529. 10.1111/j.1476-5381.2010.00745.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonca E., Darıcı F. (2014). The effect of cannabidiol on ischemia/reperfusion-induced ventricular arrhythmias: the role of adenosine A1 receptors. J. Cardiovasc. Pharmacol. Ther. 1:76 10.1177/1074248414532013 [DOI] [PubMed] [Google Scholar]
- Haj C. G., Sumariwalla P. F., Hanuš L., Kogan N. M., Yektin Z., Mechoulam R., et al. (2015). HU-444, a novel, potent anti-inflammatory, nonpsychotropic cannabinoid. J. Pharmacol. Exp. Ther. 355 66–75. 10.1124/jpet.115.226100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L., Breuer A., Tchilibon S., Shiloah S., Goldenberg D., Horowitz M., et al. (1999). HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. U.S.A. 96 14228–14233. 10.1073/pnas.96.25.14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanuš L. O., Meyer S. M., Muñoz E., Taglialatela-Scafati O., Appendino G. (2016). Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep. 33 1357–1392. 10.1039/c6np00074f [DOI] [PubMed] [Google Scholar]
- Hanus L. O., Tchilibon S., Ponde D. E., Breuer A., Fride E., Mechoulam R. (2005). Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org. Biomol. Chem. 3 1116–1123. 10.1039/b416943c [DOI] [PubMed] [Google Scholar]
- Hartsel S. C., Loh W. H., Robertson L. W. (1983). Biotransformation of cannabidiol to cannabielsoin by suspension cultures of Cannabis sativa and Saccharum officinarum. Planta Med. 48 17–19. 10.1055/s-2007-969870 [DOI] [PubMed] [Google Scholar]
- Hendricks H., Malingré T. M., Batterman S., Bos R. (1978). The essential oil of Cannabis sativa. Pharm. Weekbl. 113 413–424. [DOI] [PubMed] [Google Scholar]
- Hill A. J., Mercier M. S., Hill T. D. M., Glyn S. E., Jones N. A., Yamasaki Y., et al. (2012). Cannabidivarin is anticonvulsant in mouse and rat. Br. J. Pharmacol. 167 1629–1642. 10.1111/j.1476-5381.2012.02207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. D. M., Cascio M. G., Romano B., Duncan M., Pertwee R. G., Williams C. M., et al. (2013). Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br. J. Pharmacol. 170 679–692. 10.1111/bph.12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Wang Q., Zheng J., Zhang J., Pan X., She X. (2007). A general route to 5,6-seco-hexahydrodibenzopyrans and analogues: first total synthesis of (+)-Machaeridiol B and (+)-Machaeriol B. Tetrahedron 63 1014–1021. 10.1016/j.tet.2006.10.067 [DOI] [Google Scholar]
- Ibeas Bih C., Chen T., Nunn A. V. W., Bazelot M., Dallas M., Whalley B. J. (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 12 699–730. 10.1007/s13311-015-0377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns D. G., Behm D. J., Walker D. J., Ao Z., Shapland E. M., Daniels D. A., et al. (2007). The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br. J. Pharmacol. 152 825–831. 10.1038/sj.bjp.0707419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. A., Whalley B. J. (2015). Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res. 111 113–114. 10.1016/j.eplepsyres.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Juknat A., Kozela E., Kaushansky N., Mechoulam R., Vogel Z. (2016). Anti-inflammatory effects of the cannabidiol derivative dimethylheptyl-cannabidiol – studies in BV-2 microglia and encephalitogenic T cells. J. Basic Clin. Physiol. Pharmacol. 27 289–296. 10.1515/jbcpp-2015-0071 [DOI] [PubMed] [Google Scholar]
- Kinney W. A., McDonnell M. E., Zhong H. M., Liu C., Yang L., Ling W., et al. (2016). Discovery of KLS-13019, a cannabidiol-derived neuroprotective agent, with improved potency, safety, and permeability. ACS Med. Chem. Lett. 7 424–428. 10.1021/acsmedchemlett.6b00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan N. M., Rabinowitz R., Levi P., Gibson D., Sandor P., Schlesinger M., et al. (2004). Synthesis and antitumor activity of quinonoid derivatives of cannabinoids. J. Med. Chem. 47 3800–3806. 10.1021/jm040042o [DOI] [PubMed] [Google Scholar]
- Kogan N. M., Schlesinger M., Priel E., Rabinowitz R., Berenshtein E., Chevion M., et al. (2007). HU-331, a novel cannabinoid-based anticancer topoisomerase II inhibitor. Mol. Cancer Ther. 6 173–183. 10.1158/1535-7163.MCT-06-0039 [DOI] [PubMed] [Google Scholar]
- Kozela E., Haj C., Hanuš L., Chourasia M., Shurki A., Juknat A., et al. (2015). HU-446 and HU-465, derivatives of the non-psychoactive cannabinoid cannabidiol, decrease the activation of encephalitogenic T cells. Chem. Biol. Drug Des. 87 143–153. 10.1111/cbdd.12637 [DOI] [PubMed] [Google Scholar]
- Krohn R. M., Parsons S. A., Fichna J., Patel K. D., Yates R. M., Sharkey K. A., et al. (2016). Abnormal cannabidiol attenuates experimental colitis in mice, promotes wound healing and inhibits neutrophil recruitment. J. Inflamm. 13:21 10.1186/s12950-016-0129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente H., Alvarez F. J., Pazos M. R., Alvarez A., Rey-Santano M. C., Mielgo V., et al. (2011). Cannabidiol reduces brain damage and improves functional recovery after acute hypoxia-ischemia in newborn pigs. Pediatr. Res 70 272–277. 10.1203/PDR.0b013e3182276b11 [DOI] [PubMed] [Google Scholar]
- Laprairie R. B., Bagher A. M., Kelly M. E. M., Denovan-Wright E. M. (2015). Cannabidiol is a negative allosteric modulator of the type 1 cannabinoid receptor. Br. J. Pharmacol. 172 4790–4805. 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner J. E., Jensen J. B., Chen H.-Y., Lu H.-C., Hille B., Mackie K. (2008). GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. U.S.A. 105 2699–2704. 10.1073/pnas.0711278105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite J. R., Carlini E. A., Lander N., Mechoulam R. (1982). Anticonvulsant effects of the (-) and (+)isomers of cannabidiol and their dimethylheptyl homologs. Pharmacology 24 141–146. 10.1159/000137588 [DOI] [PubMed] [Google Scholar]
- Leweke F. M., Piomelli D., Pahlisch F., Muhl D., Gerth C. W., Hoyer C., et al. (2012). Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2:e94 10.1038/tp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Feng J., Li Y., Yuece B., Lin X., Yu L., et al. (2013). Anti-inflammatory role of cannabidiol and O-1602 in cerulein-induced acute pancreatitis in mice. Pancreas 42 123–129. 10.1097/MPA.0b013e318259f6f0 [DOI] [PubMed] [Google Scholar]
- Ligresti A. (2006). Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 318 1375–1387. 10.1124/jpet.106.105247 [DOI] [PubMed] [Google Scholar]
- Maione S., Piscitelli F., Gatta L., Vita D., De Petrocellis L., Palazzo E., et al. (2011). Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br. J. Pharmacol. 162 584–596. 10.1111/j.1476-5381.2010.01063.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P., Solinas M., Cinquina V., Parolaro D. (2013). Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 75 303–312. 10.1111/j.1365-2125.2012.04298.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346 561–564. 10.1038/346561a0 [DOI] [PubMed] [Google Scholar]
- McAllister S. D., Murase R., Christian R. T., Lau D., Zielinski A. J., Allison J., et al. (2011). Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 129 37–47. 10.1007/s10549-010-1177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D., Page J., Dunn E., Bradshaw H. B. (2012). Δ9-Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 165 2414–2424. 10.1111/j.1476-5381.2011.01497.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D., Roskowski D., Xie S., Bradshaw H. B. (2014). Δ9-THC and N-arachidonoyl glycine regulate BV-2 microglial morphology and cytokine release plasticity: implications for signaling at GPR18. Front. Pharmacol. 4:162 10.3389/fphar.2013.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop A. M., Moran B. M., Abdel-Wahab Y. H. A., Gormley N. M., Flatt P. R. (2016). Metabolic effects of orally administered small-molecule agonists of GPR55 and GPR119 in multiple low-dose streptozotocin-induced diabetic and incretin-receptor-knockout mice. Diabetologia 59 2674–2685. 10.1007/s00125-016-4108-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J. M., Glass M., Pertwee R. G. (2007). Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br. J. Pharmacol. 152 583–593. 10.1038/sj.bjp.0707399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R., Ben-Zvi Z., Gaoni Y. (1968). Hashish-XIII. On the nature of the beam test. Tetrahedron 24 5615–5624. 10.1016/0040-4020(68)88159-1 [DOI] [PubMed] [Google Scholar]
- Mechoulam R., Feigenbaum J. J., Lander N., Segal M., Hiltunen T. U. C., Consroe P. (1988). Enantiomeric cannabinoids: stereospecificity of psychotropic activity. Experientia 44 762–764. 10.1007/BF01959156 [DOI] [PubMed] [Google Scholar]
- Mechoulam R., Hanuš L. (2002). Cannabidiol: an overview of some chemical and pharmacological aspects, Part I: Chemical aspects. Chem. Phys. Lipids 121 35–43. 10.1016/S0009-3084(02)00144-5 [DOI] [PubMed] [Google Scholar]
- Mechoulam R., Hanuš L. O., Pertwee R., Howlett A. C. (2014). Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 15 757–764. 10.1038/nrn3811 [DOI] [PubMed] [Google Scholar]
- Mechoulam R., Kogan N. M., Gallily R., Breuer A. (2008). Novel Cannabidiol Derivatives and their use as anti-inflammatory agents. ternational Patent No WO2008/107879 Washington, DC. [Google Scholar]
- Mechoulam R., Lander N., Breuer A., Zahalka J. (1990). Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative. Tetrahedron Asymmetry 1 315–318. 10.1016/S0957-4166(00)86322-3 [DOI] [Google Scholar]
- Mechoulam R., Peters M., Murillo-Rodriguez E., Hanus L. O. (2007). Cannabidiol–recent advances. Chem. Biodivers. 4 1678–1692. 10.1002/cbdv.200790147 [DOI] [PubMed] [Google Scholar]
- Morales P., Goya P., Jagerovic N., Hernandez-Folgado L. (2016). Allosteric modulators of the CB1 cannabinoid receptor: a structural update review. Cannabis Cannabinoid Res. 1 22–30. 10.1089/can.2015.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales P., Hurst D. P., Reggio P. H. (2017). “Molecular targets of the phytocannabinoids: a complex picture,” in Progress in the Chemistry of Organic Natural Products: Phytocannabinoids, Unravelling the Complex Chemistry and Pharmacology of Cannabis sativa, eds Kinghorn A. D., Falk H., Gibbons S., Kobayashi J. (Berlin: Springer; ), 10.1007/978-3-319-45541-9_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M. A., Meyer E., Soares L. M., Milani H., Guimarães F. S., de Oliveira R. M. W. (2017). Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 75 94–105. 10.1016/j.pnpbp.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Muhammad I., Li X.-C., Jacob M. R., Tekwani B. L., Dunbar D. C., Ferreira D. (2003). Antimicrobial and antiparasitic (+)- trans -hexahydrodibenzopyrans and analogues from Machaerium multiflorum. J. Nat. Prod. 66 804–809. 10.1021/np030045o [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas K. L., Abu-Shaar M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature 365 61–65. 10.1038/365061a0 [DOI] [PubMed] [Google Scholar]
- Naftali T., Mechulam R., Marii A., Gabay G., Stein A., Bronshtain M., et al. (2017). Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig. Dis. Sci. 62 1–6. 10.1007/s10620-017-4540-z [DOI] [PubMed] [Google Scholar]
- Ofek O., Karsak M., Leclerc N., Fogel M., Frenkel B., Wright K., et al. (2006). Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Aacd. Sci. U.S.A. 103 696–701. 10.1021/jm4005626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S., Nakajima K., Yamashita A., Kishimoto S., Sugiura T. (2007). Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 362 928–934. 10.1016/j.bbrc.2007.08.078 [DOI] [PubMed] [Google Scholar]
- O’Sullivan S. E., Sun Y., Bennett A. J., Randall M. D., Kendall D. A. (2009). Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 612 61–68. 10.1016/j.ejphar.2009.03.010 [DOI] [PubMed] [Google Scholar]
- Pertwee R. G., Ross R. A., Craib S. J., Thomas A. (2002). (-)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur. J. Pharmacol. 456 99–106. 10.1016/S0014-2999(02)02624-9 [DOI] [PubMed] [Google Scholar]
- Pertwee R. G., Thomas A., Stevenson L. A., Maor Y., Mechoulam R. (2005). Evidence that (-)-7-hydroxy-4′-dimethylheptyl-cannabidiol activates a non-CB1, non-CB2, non-TRPV1 target in the mouse vas deferens. Neuropharmacology 48 1139–1146. 10.1016/j.neuropharm.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Peters M., Kogan N. M. (2007). HU-331: a cannabinoid quinone, with uncommon cytotoxic properties and low toxicity. Expert Opin. Investig. Drugs 16 1405–1413. 10.1517/13543784.16.9.1405 [DOI] [PubMed] [Google Scholar]
- Petronzi C., Festa M., Peduto A., Castellano M., Marinello J., Massa A., et al. (2013). Cyclohexa-2,5-diene-1,4-dione-based antiproliferative agents?: design, synthesis, and cytotoxic evaluation. J. Exp. Clin. Cancer Res. 32:24 10.1186/1756-9966-32-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M., Rapino C., Di Francesco A., Dainese E., D’Addario C., Maccarrone M. (2013). Epigenetic control of skin differentiation genes by phytocannabinoids. Br. J. Pharmacol. 170 581–591. 10.1111/bph.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M., Mukhopadhyay P., Batkai S., Hasko G., Liaudet L., Huffman J. W., et al. (2007a). CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am. J. Physiol. Heart Circ. Physiol. 293 H2210–H2218. 10.1152/ajpheart.00688.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M., Pan H., Mukhopadhyay P., Bátkai S., Osei-Hyiaman D., Haskó G., et al. (2007b). Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J. Leukoc. Biol. 82 1382–1389. 10.1189/jlb.0307180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razdan R. K., Dalzell H. C., Handrick G. R. (1974). Hashis. a simple one-step synthesis of (-)-delta1-tetrahydrocannabinol (THC) from p-mentha-2,8-dien-1-ol and olivetol. J. Am. Chem. Soc. 96 5860–5865. 10.1021/ja00825a026 [DOI] [PubMed] [Google Scholar]
- Regal K. M., Mercer S. L., Deweese J. E. (2014). HU-331 is a catalytic inhibitor of topoisomerase II α. Chem. Res. Toxicol. 27 2044–2051. 10.1021/tx500245m [DOI] [PubMed] [Google Scholar]
- Reggio P. H., Bramblett R. D., Yuknavich H., Seltzman H. H., Fleming D. N., Fernando S. R., et al. (1995). The design, synthesis and testing of desoxy-CBD: further evidence for a region of steric interference at the cannabinoid receptor. Life Sci. 56 2025–2032. 10.1016/0024-3205(95)00185-9 [DOI] [PubMed] [Google Scholar]
- Renard J., Norris C., Rushlow W., Laviolette S. R. (2017). Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci. Biobehav. Rev. 75 157–165. 10.1016/j.neubiorev.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Ribeiro A., Almeida V. I., Costola-de-Souza C., Ferraz-de-Paula V., Pinheiro M. L., Vitoretti L. B., et al. (2015). Cannabidiol improves lung function and inflammation in mice submitted to LPS-induced acute lung injury. Immunopharmacol. Immunotoxicol. 37 35–41. 10.3109/08923973.2014.976794 [DOI] [PubMed] [Google Scholar]
- Robert J. J., Ch L., Ludwig Bercht C. A., van Ooyen R., Spronck H. J. W. (1977). Cannabinodiol: conclusive identification and synthesis of a new cannabinoid from Cannabis sativa. Phytochemistry 16 595–597. 10.1016/0031-9422(77)80023-X [DOI] [Google Scholar]
- Rock E. M., Bolognini D., Limebeer C. L., Cascio M. G., Anavi-Goffer S., Fletcher P. J., et al. (2012). Cannabidiol, a nonpsychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT 1A somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 165 2620–2634. 10.1111/j.1476-5381.2011.01621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Zerbo S. Y., Rafacho A., Díaz-Arteaga A., Suárez J., Quesada I., Imbernon M., et al. (2011). Role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. J. Endocrinol. 211 177–185. 10.1530/JOE-11-0166 [DOI] [PubMed] [Google Scholar]
- Rosenthaler S., Pöhn B., Kolmanz C., Nguyen Huu C., Krewenka C., Huber A., et al. (2014). Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures. Neurotoxicol. Teratol. 46 49–56. 10.1016/j.ntt.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Ruhaak L. R., Felth J., Karlsson P. C., Rafter J. J., Verpoorte R., Bohlin L. (2011). Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol. Pharm. Bull. 34 774–778. 10.1248/bpb.34.774 [DOI] [PubMed] [Google Scholar]
- Ruiz-Valdepeñas L., Martínez-Orgado J. A., Benito C., Millán A., Tolón R. M., Romero J. (2011). Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: an intravital microscopy study. J. Neuroinflammation 8:5 10.1186/1742-2094-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E. B., Burnett A., Hall B., Parker K. K. (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30 1037–1043. 10.1007/s11064-005-6978-1 [DOI] [PubMed] [Google Scholar]
- Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.-O., Leonova J., et al. (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152 1092–1101. 10.1038/sj.bjp.0707460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier A., Ribeiro N., Coutinho D., Machado S., Arias-Carrion O., Crippa J., et al. (2014). Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS Neurol. Disord. - Drug Targets 13 953–960. 10.2174/1871527313666140612114838 [DOI] [PubMed] [Google Scholar]
- Scuderi C., Steardo L., Esposito G. (2014). Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARg involvement. Phyther. Res. 28 1007–1013. 10.1002/ptr.5095 [DOI] [PubMed] [Google Scholar]
- Seephonkai P., Popescu R., Zehl M., Krupitza G., Urban E., Kopp B. (2011). Ferruginenes A-C from rhododendron ferrugineum and their cytotoxic evaluation. J. Nat. Prod. 74 712–717. 10.1021/np100778k [DOI] [PubMed] [Google Scholar]
- Shani A., Mechoulam R. (1974). Cannabielsoic acids: isolation and synthesis by a novel oxidative cyclization. Tetrahedron 30 2437–2446. 10.1016/S0040-4020(01)97114-5 [DOI] [Google Scholar]
- Smoum R., Baraghithy S., Chourasia M., Breuer A., Mussai N., Attar-Namdar M., et al. (2015). CB2 cannabinoid receptor agonist enantiomers HU-433 and HU-308: an inverse relationship between binding affinity and biological potency. Proc. Natl. Acad. Sci. U.S.A. 112 8774–8779. 10.1073/pnas.1503395112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb A. L., Golinski M. J., Hammell D. C., Howard J. L., Banks S. L. (2009). Prodrugs of cannabidiol, compositions comprising prodrugs of cannabidiol, and methods of using the same. Patent No US2009036523A1 Washington, DC. [Google Scholar]
- Sumariwalla P. F., Gallily R., Tchilibon S., Fride E., Mechoulam R. (2004). A novel synthetic, nonpsychoactive cannabinoid acid ( HU-320 ) with antiinflammatory properties in murine collagen-induced arthritis. Arthitis Rheum 50 985–998. 10.1002/art.20050 [DOI] [PubMed] [Google Scholar]
- Taglialatela-Scafati O., Pagani A., Scala F., De Petrocellis L., Di Marzo V., Grassi G., et al. (2010). Cannabimovone, a cannabinoid with a rearranged terpenoid skeleton from hemp. Eur. J. Org. Chem 2067–2072. 10.1002/ejoc.200901464 [DOI] [Google Scholar]
- Takeda S., Hirayama A., Urata S., Mano N., Aramaki H. (2011). Cannabidiol-2’,6’-dimethyl ether as an effective protector of 15- lipoxygenase-mediated low-density lipoprotein oxidation in vitro. Biol. Pharm. Bull. 34 1252–1256. 10.1016/j.drudis.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Hirota R., Teradaira S., Takeda-imoto M. (2015). Cannabidiol-2’,6’-dimethyl ether stimulates body weight gain in apolipoprotein E-deficient BALB/c, KOR/Stm. J. Toxicol. Sci. 40 739–743. 10.2131/jts.40.739 [DOI] [PubMed] [Google Scholar]
- Takeda S., Misawa K., Yamamoto I., Watanabe K. (2008). Cannabidiolic acid as a selective cyclooxygenase-2 inhibitory component in cannabis. Drug Metab. Dispos. 36 1917–1921. 10.1124/dmd.108.020909 [DOI] [PubMed] [Google Scholar]
- Takeda S., Okajima S., Miyoshi H., Yoshida K., Okamoto Y., Okada T., et al. (2012). Cannabidiolic acid, a major cannabinoid in fiber-type cannabis, is an inhibitor of MDA-MB-231 breast cancer cell migration. Toxicol. Lett. 214 314–319. 10.1016/j.toxlet.2012.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Usami N., Yamamoto I., Watanabe K. (2009). Cannabidiol-2′,6′-dimethyl ether, a cannabidiol derivative, is a highly potent and selective 15-lipoxygenase inhibitor. Drug Metab. Dispos. 37 1733–1737. 10.1124/dmd.109.026930 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Ichino K., Ito K. (1984). A novel dihydrochalcone, linderatin from Lidera Umbellata Var, Lancea. Chem. Pharm. Bull. (Tokyo) 32 3747–3750. 10.1248/cpb.32.3747 [DOI] [Google Scholar]
- Tchilibon S., Mechoulam R. (2000). Synthesis of a primary metabolite of cannabidiol. Org. Lett. 2 3301–3303. 10.1021/ol006369a [DOI] [PubMed] [Google Scholar]
- Thomas A., Baillie G. L., Phillips A. M., Razdan R. K., Ross R. A., Pertwee R. G. (2007). Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 150 613–623. 10.1038/sj.bjp.0707133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujváry I., Hanuš L. (2016). Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res. 1 90–101. 10.1089/can.2015.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami N., Okuda T., Yoshida H., Kimura T., Watanabe K., Yoshimura H., et al. (1999). Synthesis and pharmacological evaluation in mice of halogenated cannabidiol derivatives. Chem. Pharm. Bull. 47 1641–1645. 10.1248/cpb.47.1641 [DOI] [PubMed] [Google Scholar]
- Vuolo F., Petronilho F., Sonai B., Ritter C., Hallak J. E. C., Zuardi A. W., et al. (2015). Evaluation of serum cytokines levels and the role of cannabidiol treatment in animal model of asthma. Mediators Inflamm. 2015:538670 10.1155/2015/538670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt G., Karl T. (2017). In vivo evidence for therapeutic properties of cannabidiol (CBD) for alzheimer’s disease. Front. Pharmacol. 8:20 10.3389/fphar.2017.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte L. S., Ryberg E., Sims N. A., Ridge S. A., Mackie K., Greasley P. J., et al. (2009). The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. U.S.A. 106 16511–16516. 10.1073/pnas.0902743106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S., Sommerville K., Jones N. A., Whalley B. J. (2015). Progress report on new antiepileptic drugs : a summary of the twelfth eilat conference (EILAT XII). Epilepsy Res. 111 111–113. 10.1016/j.eplepsyres.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Xiong W., Cui T., Cheng K., Yang F., Chen S. R., Willenbring D., et al. (2012). Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J. Exp. Med. 209 1121–1134. 10.1084/jem.20120242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I., Gohda H., Narimatsu S., Watanabe K., Yoshimura H. (1991). Cannabielsoin as a new metabolite of cannabidiol in mammals. Pharmacol. Biochem. Behav. 40 541–546. 10.1016/0091-3057(91)90360-E [DOI] [PubMed] [Google Scholar]
- Yamaori S., Okushima Y., Masuda K., Kushihara M., Katsu T., Narimatsu S., et al. (2013). Structural requirements for potent direct inhibition of human cytochrome P450 1A1 by cannabidiol: role of pentylresorcinol moiety. Biol. Pharm. Bull. 36 1197–1203. 10.1248/bpb.b13-00183 [DOI] [PubMed] [Google Scholar]
- Yang K.-H., Galadari S., Isaev D., Petroianu G., Shippenberg T. S., Oz M. (2010). The nonpsychoactive cannabinoid cannabidiol inhibits 5-hydroxytryptamine3A receptor-mediated currents in Xenopus laevis oocytes. J. Pharmacol. Exp. Ther. 333 547–554. 10.1124/jpet.109.162594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Chu A., Li W., Wang B., Shelton F., Otero F., et al. (2009). Lipid G protein-coupled receptor ligand identification using beta-arrestin Path Hunter assay. J. Biol. Chem. 284 12328–12338. 10.1074/jbc.M806516200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S., Potvin S. (2012). Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals 5 529–552. 10.3390/ph5050529 [DOI] [PMC free article] [PubMed] [Google Scholar]