Abstract
Microglia have a variety of functions in the brain, including synaptic pruning, CNS repair and mediating the immune response against peripheral infection. Microglia rapidly become activated in response to CNS damage. Depending on the nature of the stimulus, microglia can take a number of activation states, which correspond to altered microglia morphology, gene expression and function. It has been reported that early microglia activation following traumatic brain injury (TBI) may contribute to the restoration of homeostasis in the brain. On the other hand, if they remain chronically activated, such cells display a classically activated phenotype, releasing pro-inflammatory molecules, resulting in further tissue damage and contributing potentially to neurodegeneration. However, new evidence suggests that this classification is over-simplistic and the balance of activation states can vary at different points. In this article, we review the role of microglia in TBI, analyzing their distribution, morphology and functional phenotype over time in animal models and in humans. Animal studies have allowed genetic and pharmacological manipulations of microglia activation, in order to define their role. In addition, we describe investigations on the in vivo imaging of microglia using translocator protein (TSPO) PET and autoradiography, showing that microglial activation can occur in regions far remote from sites of focal injuries, in humans and animal models of TBI. Finally, we outline some novel potential therapeutic approaches that prime microglia/macrophages toward the beneficial restorative microglial phenotype after TBI.
Keywords: traumatic brain injury, CCI, microglia, neuroinflammation, TSPO, polarization states
Introduction
Traumatic brain injury (TBI) is the biggest cause of death and disability in the under 40s in the developed world (Langlois et al., 2006; Hyder et al., 2007), both in the civilian and military context. The overall majority of TBIs is a single event; however, repeated injuries among soldiers and athletes are associated with the development of Chronic Traumatic Encephalopathy (CTE) although the pathology of CTE can also be seen after single TBI and its causation remains uncertain (Omalu et al., 2011; Goldstein et al., 2012). Survivors often suffer from debilitating cognitive, emotional and physical impairments. This is further aggravated by the constant failure of clinical trials (reviewed in Loane and Faden, 2010; Gruenbaum et al., 2016), leaving patients without any treatment.
Brain injury can trigger neurodegeneration, which is a major determinant of long-term outcome. Head injury is a major risk factor for the development of dementia, suggested by the presence of amyloid-β (Aβ) plaques in around 30% of post-mortem brain tissue from TBI patients (Roberts et al., 1994). Additionally, amyloid plaques have been observed in surgically removed tissue surrounding contusions in brains of survivors of TBI (Ikonomovic et al., 2004; DeKosky et al., 2007). Recently, it has been shown by Positron Emission Tomography (PET) imaging that the distribution of amyloid plaque in TBI survivors overlaps with that in patients with Alzheimer's disease (AD) but also involves the cerebellum, an area of the brain not typically involved in AD (Scott et al., 2016). Other pathological features of AD are also present in TBI, including increased phosphorylated tau and acetylcholine deficiency (Jordan, 2000; Tran et al., 2011; Goldstein et al., 2012; McKee and Daneshvar, 2015; Shin and Dixon, 2015). In particular, repetitive mild TBI leading to CTE is characterized by perivascular tau pathology, which is also irregularly distributed in the depths of cortical sulci. Another AD susceptibility factor is the Apolipoprotein E isoform 4 (ApoE4), which has been linked with exiguous neurological outcome after TBI (Verghese et al., 2011). This has been associated with alterations in the integrity of the blood brain barrier (BBB) in individuals with ApoE4 genotype (Methia et al., 2001; Nishitsuji et al., 2011).
Additionally, TBI has been linked with Parkinson's disease (Jafari et al., 2013), various psychiatric disorders leading to increased risk of suicide, an overall increase in mortality (McMillan et al., 2011) and peripheral immune suppression (Hazeldine et al., 2015). There are currently no neuroprotective drug treatments available and a major challenge is to understand how brain trauma causes neurodegeneration and other long-term impairments.
A common feature of the neurological pathologies developing as a consequence of TBI is that they initiate and are potentiated by an inflammatory response. Microglial activation occurs early after experimental (Chiu et al., 2016) and human TBI (Ramlackhansingh et al., 2011; Johnson et al., 2013) and can persist for years, detectable both in vivo and post-mortem. Sites of activation often coincide with neuronal degeneration and axonal abnormality (Maxwell et al., 2010; Giunta et al., 2012). It was hypothesized that the inflammatory response to TBI therefore could be associated with the subsequent development of neurodegenerative disorders. However, new evidence indicates that glial activation may have also reparative/restorative effects. Thus, it is critical to investigate what causes this inflammatory response, what are the consequences for neuronal degeneration and survival and whether this can be modified with anti-inflammatory therapeutic approaches.
In this review, we analyse the role of microglia in TBI patients and animal models, from imaging studies used to visualize changes in microglia activation in vivo and ex vivo to studies in post-mortem brains of TBI patients and the prospects of therapies targeting microglia activation in TBI.
Microglia activation states in TBI
TBI occurs when brain structure and physiology are disrupted due to an extrinsic biomechanical insult to the cranium, resulting in neuronal, axonal and vascular damage. In response to TBI, the brain orchestrates a complex immunological tissue reaction similar to ischemic reperfusion injury (Werner and Engelhard, 2007). It was suggested that, as macrophages, microglia can migrate to the site of the injury, in order to establish a protective environment mitigating deleterious consequences of the injury (Faden et al., 2016). The acute function of microglia in response to TBI is to eradicate cellular and molecular debris. Microglial removal of damaged cells is a very important step in the restoration of the normal brain environment. Damaged cells release Danger-associated molecular patterns (DAMPs), which can become potent inflammatory stimuli, resulting in further tissue damage (Solito and Sastre, 2012; Zhang et al., 2012). Moreover, activated microglia are also capable of releasing noxious substances such as pro-inflammatory cytokines, reactive oxygen species (ROS), nitrogen species and excitatory neurotransmitters i.e., glutamate, which exacerbate damage (Kreutzberg, 1996). While pro-inflammatory cytokines are directly deleterious, they also stimulate the release of glutamate from microglia in an autocrine/paracrine fashion. Therefore, depending on the released amount, this can lead to direct neurotoxic effects on neurons, synapses and dendrites, through ionotropic glutamate receptors and interfere with the glutamate buffering ability of astrocytes by inhibiting astrocytic glutamate transporters (Takaki et al., 2012).
It was initially hypothesized that there is a temporary transition in function of the inflammatory milieu, which in the latter phases favors a protracted inflammatory profile associated with chronic microglial activation, precipitating neurological manifestations, although this view has been found to be far too simplistic.
As in other CNS injuries, it seems that microglia activation in TBI results in different phenotypes, corresponding to neurotoxic or neuroprotective priming states. Depending on the stage of the disease and the chronicity, microglia are stimulated differentially and this leads to particular activation states, which correspond to altered microglia morphology, gene expression and function. Microglia are activated in-situ by pro-inflammatory cytokines such as IFN-γ, IL-1α, IL-6, and TNF-α (Ziebell and Morganti-Kossmann, 2010) and become primed. Microglia morphology can switch from a “normal,” ramified shape to a hypertrophic, “bushy” morphology. In response to extensive tissue damage or pathogen invasion, microglia can change into an amoeboid morphology, primarily acting in a phagocytic/macrophage fashion and being difficult to differentiate from infiltrating macrophages (Figure 1). In general, these activation states are classified along a spectrum with M1 or “classically activated” at one end and M2 or “alternatively activated” and the opposite end, similar to macrophages. The proinflammatory M1 phenotype favors the production and release of cytokines that can exacerbate neural injury (Hu et al., 2015). In contrast, the M2 is associated with the release of neurotrophic factors that promote repair and a phagocytic role. However, in recent years, transcriptomic analysis has revealed that microglia and macrophages display a much broader transcriptional repertoire than M1 and M2, depending on the different environmental signals received (Hickman et al., 2013; Xue et al., 2014). Therefore, it is rare to find clear M1 or M2 microglial phenotypes in chronic diseases, since these states are transitory and eventually include mixed activation states. This seems particularly important in TBI as indicated by the many animal studies showing a mixed expression of different markers associated with both M1 and M2 phenotype (Table 1), also described as transitory state (Mtrans) by some authors (Kumar et al., 2016a). Additionally, the inevitable recruitment of bone marrow-derived monocytes into the injured brain parenchyma following experimental TBI and the subsequent differentiation into macrophages complicates the interpretation of the findings, especially with regard to the ability of these cells to change phenotype.
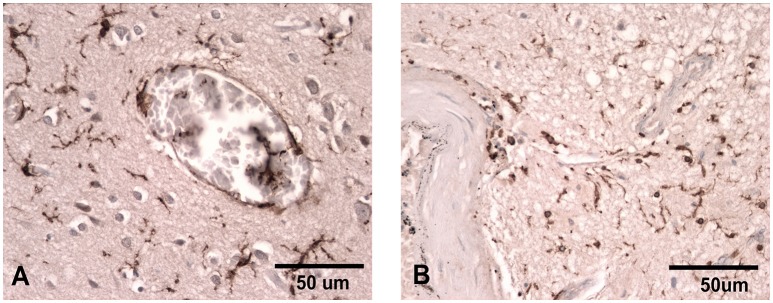
Figure 1.
Iba-1 staining illustrating the ramified microglial morphology associated with normal surveillance activity (A) compared to that seen in patients where there has been damage to the vasculature and the microglia take on a more rounded phagocytic appearance (B). Scale bar corresponds to 50 μM.
Table 1.
Microglia/macrophage polarization in experimental models of Traumatic Brain Injury.
| References | Species, strain, sex | Model, severity and injury details | Time post injury | Key markers | Simplified key results of M1/M2-like marker analysis |
|---|---|---|---|---|---|
| Jin et al., 2012 | Mouse C57B6/J ♂ |
CCI, moderate 3 mm | dpi: 1, 3, 7 14, and 28 |
M1: CD86 M2: CD206 |
M1: ▴ 7 + 28 dpi M2: ▴ 1 dpi, Sham levels 28 dpi |
| Bedi et al., 2013 | Mouse C57B6 ♂ |
CCI, moderate ns | dpi: 1 |
M1: FcγRII/III (CD16a/b) M2: CD206 |
M1/2 ratio: ▴ at 24 hpi |
| Walker et al., 2012 | Mouse C57B6 ♂ |
CCI, moderate ns | hpi: 24, 48, 72, 120 | M2/M1 ratio of CD206/CD86 | M1: ▴ 24–72 hpi |
| Hsieh et al., 2013 | Mouse C57BL/6 ♂, YARG/Yet40 knock-in |
CCI, moderate 2 mm flat | dpi: 1, 4, 7, 14 |
M1: IL-12p40 M2: Arg-1 |
M1: ▴ macrophages 1–14 dpi M2: ▴ macrophages 1 dpi Mixed M1/M2 expression |
| Tchantchou and Zhang, 2013 | Mouse C57B6 ♂ |
CCI, moderate 3 mm flat | dpi: 3,7, 21 |
M1: iNOS M2: Arg-1 |
M1: ▴ 3 + 7 dpi, M2: no Arg-1 |
| Wang et al., 2013 | Mouse C57B6 ♂ |
CCI, moderate 3 mm flat | dpi: 1, 3, 5, 7, 14 |
M1: CD16/32, iNOS, CD11b, CD86 M2: CD206, IL-10, Ym1/2, TGF-β, Arg-1, CCL22 |
M1: ▴ 5–14 dpi M2: ▴ 3–5 dpi |
| Wang et al., 2015 | Mouse C57B6 ♂ |
CCI, moderate 3 mm flat | dpi: 7, 35 |
M1: CD16, iNOS M2: CD206, IL-10 |
M1: ▴ 7 dpi M2: ▴ 7 dpi |
| Kumar et al., 2013 | Mouse C57B6 ♂, |
CCI, severe 3.5 mm flat | dpi: 1, 7 |
M1: IL-1β, TNF-α, CD86, iNOS, CCL2, CCL3 M2a: Arg-1, Ym1, CD206/MRC, Fizz-1 M2c: IL-4Rα, SOCS3, TGF-β |
Mixed M1/M2 expression M1: aged > young M2a: marker specific ▴▾ M2c: young > aged Aged animals ▴ Ym1 on highly activated microglia/macrophages Young animals ▴Ym1 on ramified cells |
| Loane et al., 2014a | Mouse C57Bl/6 ♂ |
CCI, severe 3.5 mm flat | wpi: 1, 5, 12, 52 |
M1: MHC II M2: Ym-1 |
M1/MHC II: ▴ 1–52 wpi M2: ▴ 1–5 wpi, absent 12–54 wpi |
| Loane et al., 2014b | Mouse C57Bl/6 ♂ |
CCI, severe 3.5 mm flat | dpi: 28 |
M1: iNOS M2: Arg-1 |
M1: ▴ iNOS/CD11b M2: ▾Arg-1/CD11b |
| Kumar et al., 2016a | Mouse C57Bl/6 ♂ |
CCI, severe 3.5 mm flat | hpi: 1, 6, 24 dpi: 7 |
mRNA: M1: IL-1β, IL-12, TNF-α, IL-6, iNOS M2a: Arg-1, Ym1, CD206, Fizz-1, IL-1rn M2c: IL-4Rα, SOCS3, TGF-β Flow cytometry: M1: iNOS, IL-12 M2: TGF-β, Ym1, CD206, Arg-1 Histology: M1: CD16/32, iNOS Mtrans: CD16/32+TGF-β, iNOS+, Arg-1 M2: TGF-β, Arg-1 |
Mixed expression of M1/M2 mRNA and protein M1/trans dominate contusion and peri-contusional tissue at 7 dpi |
| Kumar et al., 2016b | Mouse C57Bl/6 ♂ NOX2 −/− |
CCI, severe 3.5 mm flat | dpi: 1, 3, 5, 7, 21, 28 |
M1: IL-1β, NOS2, TNF-α, IL-6, IL-12b M2: Arg-1, Ym1, SOCS3, Fizz-1, IL1rn, IL-4Rα M2c: IL-4Rα, SOCS3, TGF-β |
Mixed expression of M1/2 up to 7 dpi |
| Morganti et al., 2015 | Mouse Dbl-Het C57Bl/6 ♂/♀ |
CCI, moderate 3 mm convex | hpi: 3, 6, 12 dpi: 1, 2, 7, 14, 28 |
M1: CD68, CD45, MHC II, MARCO, NOS2, TNF-α, CCL2, IL-1β, IL-6, INF-γ M2a: Arg-1, Ym1, Fizz1, CD206, IL-4, IL-13 M2c: CD36, CD163, TGF-β, IL-10, IL-4Rα, IL-1Ra |
Mixed expression of M1 and M2a/c marker mRNA in leucocytes M1: ▴ 3 hpi – 14 dpi M2a: ▴12 hpi – 7dpi M2c: ▴ 6 hpi – 7dpi |
| Morganti et al., 2016 | Mouse C57Bl6/J ♂ |
CCI, moderate 3 mm convex | dpi: 1, 2, 7 |
M1: CCL2, CCL3, CCL4, CCL6, CCL7, CCL9, CCL12, CCL19, CCL25, CD14, CXCL1, CXCL2, CXCL3, CXCL9, CXCL10, CXCL16, DUSP1, IL-1β, IL-15, IL-16, PTX3, TLR2, TNF-α M2: Arg-1, CD36, CX3CL1, IL4R, IL25, TGF-βm TGM2, TLR1, TLR8 |
Mixed expression of M1 and M2 marker mRNA M1: ▴ 3–7 dpi M2: ▴ 3–7 dpi Lower fold changes in M2 expression compared to M1 |
| Zanier et al., 2014 | Mouse C57Bl/6 ♂ |
CCI, moderate 3 mm | dpi: 3, 7 |
M1: CD11b, TNF-α, CD68, CD86, IL-1β M2a/c: Ym1, Arg-1, CD206, SOCS3, IL-10 |
Mixed expression of M1 and M2 marker mRNA M1: ▴ at 3 + 7 dpi M2: ▴ Ym1, Arg-1, SOCS3 at 3 dpi |
| Febinger et al., 2015 | Mouse CX3CR1−/− CX3CR1+/+ ♂ |
CCI, mild 3 mm | dpi: 7, 15, 30 |
M1: IL-1β, CD86, iNOS, MARCO M2: TGF-β, Arg-1, CD206 |
M2a: ▴ Ym1, CD206 in CX3CR1−/− only at 7 dpi ▴ TGF-β in CX3CR1−/− mRNA M1: ▾ iNOS, IL-1β 30 dpi: ▴ M1: Marco, CD68 |
| Zanier et al., 2016 | Mouse CX3CR1−/− CX3CR1+/+ ♂ |
CCI, Moderate 3 mm | dpi: 1, 2, 4, 7 wpi: 5 |
M1: CD11b, CD68, iNOS, IL-1β,TNF-α M2: Ym1 |
CX3CR1−/−, 4 dpi: ▴ sensorimotor performance ▾TUNEL positive cells ▾ M1, CD68, iNOS CX3CR1−/− 5 wpi: ▾ sensorimotor performance ▴TUNEL positive cells ▴ M1 iNOS |
| Desai et al., 2016 | Mouse C57BL6/N ♂ |
CCI, moderate 3 mm flat | hpi: 4 dpi: 1, 4 |
M1: CD16, CD32 M2: CD-206, Arg-1, Ym1 |
M1: ▴ CD16, CD32 at 1–4 dpi M2: ▴ CD206, Arg-1, Ym1 at 1–4 dpi |
| Braun et al., 2017 | Mouse C57BL/6 CD-1 C3H/OuJ C3H/HeJ CX3CR1 ♂ |
CCI, severe 3 mm convex | hpi: 24, 72 wpi: 3 |
M1: TNF-α, IL-12 M2: TGF-β, IL-10 |
M1/M2 ratio: ▴ 24 + 72 hpi, 3 wpi |
| Ansari, 2015 | Rat Sprague-Dawley ♂ |
CCI, severe 5 mm flat | hpi: 2, 4, 6, 10, 24 |
M1: TNF-α, IL-1β, IL-6, IFN-γ M2: IL-4, IL-10, IL-13, Arg-1, Ym1, Fizz1, MRC-1 |
Mixed response M1: ▴ TNF-α, IL-1β, IL-6 mRNA/protein at 2-10 hpi M2: ▴ Arg-1: 6-24 hpi; Ym1: 6 hpi, Fizz1: 4-24 hpi |
| Turtzo et al., 2014 | Rat Wistar ♀ |
CCI, severe 5 mm flat | dpi: 1, 3, 5, 7, 14, 30 wpi: 8 |
M1: CD40, CD68, TNF-α, CD86, CD80, NOS2 M2: CD163, CD206 |
Mixed expression of M1 and M2 protein expression and mRNA M1: ▴CD86 at 5, 7 dpi, TNF-α 1, 3 dpi M2: ▴ CD163 at 5, 7 dpi, CD206 at 7 dpi |
| Barrett et al., 2017 | Mouse C57Bl/6 ♂ NOX2 −/− |
CCI, severe 3.5 mm flat | dpi: 72 | M2: IL-4Rα, SOCS3, TGF-β, SHIP1, Arg-1, Ym1, IL-10 | NOX2 −/− mice show a robust increase in M2 markers ▴ IL-4Rα, SOCS3, TGF-β, SHIP1, Arg-1, Ym1, IL-10 at 72 hpi vs WT |
| Cao et al., 2012 | Rat Sprague-Dawley ♂ |
mFPI, moderate 2 atm 4.8 mm craniotomy | dpi: 7, 28 |
M1: TNF-α, CD45 M2a: Arg-1 M2c: TGF-βI, TGF-βRII |
M1 and M2c marker expression Cortex: M1 and M2c: ▴ nonsignificant CD45, TGF-βI, TGF-βRII at 7 dpi, no change in M2a (Arg-1) Thalamus: M1 and M2c: π CD45, TGF-βI, TGF-βRII at 7 and 28 dpi, no change in M2a (Arg-1) |
| Bachstetter et al., 2013 | Mouse p38α MAPK −/− C57BL/6J ♂/♀ |
mFPI, moderate 1.2 atm 3 mm craniotomy | hpi: 3, 9, 24 dpi 7 |
M1: IL-1β, IL-6, TNF-α, CCL2, CCL5, CXCL1, CD45, CD68, MHC M2: Arg-1, Ym1 |
Mixed expression of M1 and M2 markers mRNA and cytokines/chemokines M1: ▴ IL-6, TNF-α, CCL3 3, 9 hpi, elevated at 7 dpi M2: ▴ Arg-1, Ym1 9 hpi, ▾ below sham levels at 7 dpi, Rod-like microglia p38α MAPK −/−: ▴ M1 and M2 response (mRNA/protein) at 6 hpi ▴ motor performance ▾ Synaptic loss, microglia activation |
| Fenn et al., 2014 | Mouse BALB/c ♂ |
mFPI, moderate 1.2 -1.5 atm 3 mm craniotomy | hpi: 4, 72 |
M1: IL-1β, CD14, TNF-α, iNOS, IFN-γ, CCL2 M2: Arg-1, IL-4Rα, IGF-1, IL-10, IL4 |
Mixed mRNA expression in ipsilateral cortex and hippocampus, M2 markers less expressed at 72 hpi Cortex, 4 hpi: M1: ▴ IL-1β, CD14, TNF-α, CCL2; M2: ▴ Arg-1, IGF-1 72 hpi: M1: ▴ IFN-γ, CCL2, iNOS; M2: ▴ IL4 Hippocampus, 4 hpi: M1: ▴ IL-1β, CD14, TNF-α, CCL2; M2: ▴ IL-4, IL-10 72 hpi: M1: ▴ IL-1β, CD14, TNF-α, iNOS; M2 ▴ IL-10 ▴ Primed microglia |
| Fenn et al., 2015 | Mouse BALB/c ♂ |
mFPI, moderate 1.2 -1.5 atm 3 mm craniotomy | dpi: 1 |
M1: IL-1β, CD14, TNF-α, iNOS M2: Arg-1, IL-10 |
Mixed expression at 1 dpi Hippocampus: M1: ▴ IL-1β, CD14, TNF-α, CCL2; M2: ▴ Arg-1 |
|
Whole brain CD11b positive cells: M1: ▴ IL-1β, CD14, TNF-α, CCL2, iNOS; M2: ▴ Arg-1 |
|||||
| Truettner et al., 2016 | Rat Sprague-Dawley ♂ |
mFPI, moderate 1.8–2.2 atm 4.8 mm craniotomy | hpi: 4, 24 |
M1: iNOS, IL-1β, IL-1α, TNF-α, IL-6. IL-12, CCL2 M2: Arg-1, CD163, CD206, IL-10, Ym1, TGF- β M1/M2: iNOS/Arg-1 ratio |
Mixed expression of M1 and M2 mRNA at 4 and 24 hpi, M1 more pronounced ▴ M1 ratio at 4 h, similar M1/M2 ratio at 24 h in microglia mRNA 4 h: M1: ▴ all; M2: ▴ (lower relative expression) mRNA 24 h: M1: ▴ TNF-α ▴ CD206, TGF-β |
| Chhor et al., 2016 | Mouse OF-1 Postnatal day 7 ♂/♀ |
WD, closed 2 mm footplate 10 g weight dropped 10 cm | hpi: 2, 6, 14, 24 dpi: 5 |
M1 (Cytotoxic): IL-1 α, IL-1β, IL-3, IL-6, IL-9, IL-12p40, IL-12p70, IL-17, INF-γ, TNF-α, CXCL1, CCL3, CCL4, CCL5, Cox-2, iNOS, CD32, CD86 M2a (Reparative/regenerative): IL4, IL-13, G-CSF, CCL2, Arg-1, CD206, IGF-1, Gal-3 M2c (Immunomodulatory): IL-5, IL-10, SphK1, IL-1rn, SOCS3, IL-4ra |
Moderate mixed increase in M1 and M2a/c mRNA Ipsilateral hemisphere: M1: ▴ all markers except TNF-α and IL-12(p70) between 6, 14 and 24 hpi M2a/c: ▴ all markers except TNF-α and IL-12(p70) between 6, 14 and 24 hpi CD11b+ isolated microglia/macrophages: M1: ▴CD32/CD86 up to 1 dpi M2a/c: ▴Arg-1, CD206, SOCS3, IL-1rn, Gal3 up to 1 dpi, IGF-1 at 5 dpi Pronounced contralateral effects |
| Semple et al., 2010 | Mouse CCL2 −/− C57Bl/6 ♂ |
WD, closed 333 g weight dropped 2 cm, silicone tip | hpi: 2, 4, 12, 24 dpi: 4, 7, 14, 28 |
M1: IL-1α, IL-1β, IL-6, IL-12p40, IL-12p70, CCL3, CXCL1, CXCL2, CCL5, TNF-α, INF-γ, M2a: G-CSF, IL-10 |
Primarily M1 driven response M1: ▴ IL-1α, IL-6, IL-12p40, CCL5 at 12 hpi M2: ▴ G-CSF1, IL-10 only at 12 hpi in CCL2 −/− CCL2 −/−: ▾lesion volume, macrophage accumulation and astrocyte activation at 14, 28 dpi |
Levels of severity for CCI: Mild: 0.5 mm, Moderate: 0.95–1.5 mm; Severe: >2.0 mm.
CCI, Controlled Cortical Impact; mFPI, midline Fluid Percussion Injury, WD, Weight drop.
Dpi, Days post injury; hpi, hours post injury; wpi, weeks post injury.
Additional complications arise from the clinical observation that outcome after TBI can be different between male and female adults and post-pubescent adolescents in some but not all studies (Coimbra et al., 2003), with females having lower mortality and less complications (Phelan et al., 2007; Berry et al., 2009; Ley et al., 2013). This can be attributed, at least partially, to astrocytes and microglia, as recently reviewed (Caplan et al., 2017). Gonadal steroid hormones such as progesterone and estradiol are directly involved in the glial response under injury conditions in and during development (Lenz and McCarthy, 2015). Moreover, as progesterone can shift macrophage polarization from M1 to M2 phenotype (Menzies et al., 2011), is seems possible that similar effects could occur in microglia as well, whether the concept of microglia polarization is accepted or not.
The polarization states of microglia could therefore be relevant in the progression of TBI into other neurological disorders such as AD and could interfere with the recovery of the patients and the effectiveness of particular anti-inflammatory treatment.
In addition, the role of infiltrating macrophages and other immune cells are also relevant in the context of microglia dynamics; injury type and parameters may play an important role, with focal models of TBI generally eliciting a more pronounced and localized inflammatory response due to significant tissue damage and pronounced infiltration of peripheral immune cells through the compromised BBB. In line with this, factors that have been implicated in BBB breakdown after injury, such as ApoE (mentioned above) and the extracellular enzymes matrix metalloproteinases (MMPs) have been found to contribute to contusion expansion and vasogenic edema after TBI (Guilfoyle et al., 2015).
Microglial activation in animal models of TBI
In the past five decades, several animal models have been developed to characterize the biomechanical and pathophysiological patterns of TBI and to provide reliably ways to test treatment strategies. These models almost exclusively employ a single-hit injury and only in the past decade repeated injury models emerged that try to mimic the effect of repetitive injuries. The different animal models address distinct pathophysiological aspects of TBI and have different etiological and construct validity (O'Connor et al., 2011). The most widely applied models of TBI are open-head models, that induce an injury via the intact dura mater upon the underlying cortical tissue (reviewed in Xiong et al., 2013), with focal, diffuse and/or mixed injury patterns, depending on the type of injury. In order to do so, rigid impactors (Controlled Cortical Impact, CCI; weight drop, WD), fluidic pressure (Fluid Percussion Injury, FPI) and blast waves are employed. Additionally, high-velocity probes or projectiles are used to model penetrating brain injury. The CCI, FPI and the various WD models have a long history and were extensively characterized in the past, including assessment of long-term outcome (Kochanek et al., 2002; Immonen et al., 2009).
With the recognition that multiple head injuries, even of sub-threshold severity, are connected to chronic sequel, such as CTE (McKee et al., 2016; Vile and Atkinson, 2017), new models were developed to account for those findings. A growing number of studies in repeated injury rodent models (Shitaka et al., 2011; Klemenhagen et al., 2013; Mannix et al., 2014; Semple et al., 2016; Robinson et al., 2017) indicate that the glial response, in particular increases in microglia numbers and changes in morphology, shares an overall similar pattern with the traditional single-injury models. Interestingly, a few studies showed that repeated injuries can exacerbate the glial response, e.g., microglia or astrocyte cell density, as compared to a single injury (Ojo et al., 2013; Petraglia et al., 2014; Xu et al., 2016; Gao et al., 2017; Tyburski et al., 2017). Recent findings in human post-mortem tissue support the overall notion that repeated injuries result in a stronger glial response by showing that repeated head injury was a predictor of CD68 cell density and phosphorylated tau (Cherry et al., 2016).
The involvement of microglia in the complex pathophysiological cascade following brain injury has long been recognized. Shortly after the description of microglia by Del Rio-Hortega, accumulation of “microglia-like” cells were reported in the tissue around autologous blood injections and a cortical stab wound (Carmichael, 1929; Dunning and Stevenson, 1934) in the rabbit. Similar to reactive astrocytes, microglia are versatile cells, which is exemplified by their heterogeneous morphology (Boche et al., 2013). Perivascular microglia respond to pressure on the thinned mouse skull (Roth et al., 2014) by changing into jellyfish morphology; CCI causes some microglia to align with axon initial segment, the site where action potentials are generated (Baalman et al., 2015) and midline FPI yields several rod-like microglia (Ziebell et al., 2012).
Depending on the employed markers and methods, significantly increasing microglia activation in animal models of TBI is usually apparent from day 1 to 3 post-injury (p.i.) (Bye et al., 2007; Elliott et al., 2011) and can persist chronically beyond 28 days p.i., a usual cut-off time-point for many animal studies. A few studies have elegantly emphasized the long-term nature of microglia activation after experimental TBI, primarily with immunohistochemical analysis showing that major histocompatibility complex (MHC)-II positive cells were still present in the ipsilateral hemisphere at 3 months after WD injury (Holmin and Mathiesen, 1999). Recently, a significant loss of ramified, but increase of hypertrophic microglia in the injured rodent cortex at 1 year after moderate CCI in mice was demonstrated (Loane et al., 2014a). This suggests that, although microglial activation occurs early after TBI, it can change the phenotype and function over time (see Table 1).
Manipulation of microglial activation in animal models of TBI
Several studies have investigated the effects of microglial elimination in brain injury models, in order to understand their function and the potential as therapeutic approach. The use of the transgenic CD11b-HSVTK mice in response to ganciclovir (GCV) treatment allowed the study of the depletion of proliferating microglia in models of brain or peripheral nerve injury. These transgenic mice express a mutant form of the gene herpes simplex virus 1 thymidine kinase (HSV-1 TKmt-30) driven by the myeloid specific promoter CD11b. HSV-1 TK is capable of phosphorylating specific nucleoside analogs such as GCV, which lead to inhibition of DNA synthesis and cell death during cell proliferation (Cheng et al., 1983; Faulds and Heel, 1990; Black et al., 1996).
Unexpectedly, eliminating proliferative CD11b positive cells and therefore microglia and potentially macrophages in a model of hypoglossal nerve axotomy did not result in pronounced changes in motor neuron loss (Gowing et al., 2006). In another study, employing a repeated closed-head injury in the CD11b-TK mice, low dose of GCV reduced the microglial population after TBI but did not alter the extent of axonal injury as visualized by silver staining. Additionally, higher doses were found to be toxic and aggravated the TBI induced damage (Bennett and Brody, 2014).
Rapid elimination of ~95% of all microglia can be achieved by administration of the colony stimulating factor 1 receptor (CSF1R) inhibitor PLX3397. This approach was used in a model of neuronal injury (Rice et al., 2015) consisting of a diphtheria toxin-induced neuronal lesion in transgenic mice carrying a transgene for the diphtheria toxin A-chain. The diphtheria toxin was activated in transgenic mice by feeding them food without doxycycline, triggering neurotoxicity. The treatment with PLX3397 had different outcomes depending on whether microglia elimination took place in the recovery period only or was administered during the lesion as well as the recovery period. Elimination of microglia after the lesion resulted in improved functional recovery, while microglia depletion during the insult led to greater neuronal loss (Rice et al., 2015). Therefore, in models of CNS damage, it seems that chronically activated microglia display a pro-inflammatory phenotype that may contribute to further synaptic loss. In addition, using the same approach of treatment with the CSF1R inhibitor, it was recently shown that microglia are involved in changes in neuronal network activity and spreading depolarization after excitotoxic lesions and ischemia. Depletion of microglia led to dysregulated neuronal calcium responses, calcium overload and increased neuronal death and infarct size (Szalay et al., 2016), with similar reports in a mouse model of transient focal cerebral ischemia and reperfusion (Jin et al., 2017).
From these findings, it seems obvious that general ablation of microglia is not a productive strategy and perhaps it would be more effective to suppress a specific phenotype at a particular time-point. One approach would be to characterize the different microglial functions based on morphology and phenotype markers in order to find the right time for a microglia-targeting therapeutic strategy.
Polarization of microglia in TBI models
The polarization of microglia is a relatively new concept, not seen without controversy (Ransohoff, 2016), which has been already investigated in a number of studies, primarily in the CCI model in mice. Only a few studies employed rats or other established TBI models, such as FPI and WD. Data from clinically relevant repeated and penetrating models is lacking even for rodents. To the knowledge of the authors, no study has investigated microglia polarization in gyrencephalic models such as ferrets, sheep or pigs, even though these species had previously been employed in CCI, FPI, penetrating, and blast models.
In the established rodent models (see Table 1), data indicates that microglia and infiltrating macrophages, responding to injury, do not lean toward an extreme end of the spectrum, as shown in several studies through the expression of both M1 and M2-like markers in the “acute” phase. However, it seems that in the subsequent “subacute” and “chronic” phase, the expression of M2-like markers is reduced, while M1-like markers are still expressed, indicating that the pro-inflammatory action of microglia/macrophages exacerbates the pathology. In contrast, studies in non-human primates suggest a trophic role for chronically activated microglia after TBI, indicating a restorative phenotype in the chronic phase (Nagamoto-Combs et al., 2007, 2010).
Another issue is the apparent differences in the expression of murine and human markers of potential microglia polarization, which have been found for macrophages (reviewed in Murray and Wynn, 2011). However, the employed cellular markers of M1-like and the different M2-like microglia/macrophages are not always clearly indicative of the presumed phenotype, which calls for new and more specific markers in murine models (Jablonski et al., 2015). Additionally, mRNA extraction of brain homogenates does not always yield reliable information on the M1/2-like spectrum. Ideally, microglia and macrophages are to be separated by flow cytometry and then assayed for their activation spectrum using transcriptomic analysis (Hickman et al., 2013; Xue et al., 2014).
The studies that employed different therapeutic strategies show that an increase in M2-like markers is often associated with a better cognitive and histopathological outcome (Table 2). It seems therefore imperative for future studies targeting microglia polarization as therapeutic strategies, to assess several markers in the target cells within a sufficient temporal window in order to show a long-term positive outcome.
Table 2.
Treatments in experimental models of TBI targeting inflammation and microglia.
| References | Species, Strain, sex | Treatment | Injury | Simplified treatment outcomes |
|---|---|---|---|---|
| Thal et al., 2011 | Mouse C57BL6/CrlN ♂ |
Pioglitazone, rosiglitazone, PPARγ agonists | CCI |
Pioglitazone: Lesion volume at 24 hpi M1 Lesion volume at 24 hpi M1
 : iNOS, TNF-α, IL-1β mRNA at 24 hpi No beneficial effect of rosiglitazone : iNOS, TNF-α, IL-1β mRNA at 24 hpi No beneficial effect of rosiglitazone |
| Besson et al., 2005 | Rat Sprague-Dawley ♂ |
Fenofibrate, PPARα agonist | FPI |
24 hpi: Neurologic deficits Neurologic deficits Oedema Oedema7 dpi:  Neurologic deficits Neurologic deficits |
| Bye et al., 2007 | Mouse C57BL6 ♂ |
Minocycline | Closed-head WD |
4 hpi: IL-1 β IL-1 β1 dpi:  Lesion volume Lesion volume Activated microglia accumulation (F4/80) Activated microglia accumulation (F4/80) Motor performance Motor performance4 dpi: Beneficial effects on lesion size and motor function loss No effect on apoptotic cell death, neutrophil infiltration |
| Homsi et al., 2010 | Mouse Swiss ♂ |
Minocycline | Closed-head WD |
1 dpi: Lesion volume Lesion volume Microglia/macrophages (CD11b) Microglia/macrophages (CD11b)2 dpi -12 wpi:  Hyper-locomotor activity Hyper-locomotor activity Body weight Body weight |
| Kovesdi et al., 2012 | Rat Sprague-Dawley ♂ |
Minocycline | Blast injury, mild |
 Serum biomarkers of inflammation, vascular and neuronal injury at 51 dpi Serum biomarkers of inflammation, vascular and neuronal injury at 51 dpi Brain biomarkers in multiple brain regions at 51 dpi Brain biomarkers in multiple brain regions at 51 dpi Locomotor activity, spatial memory Locomotor activity, spatial memory Anxiety Anxiety |
| Hanlon et al., 2016 | Rat PD 11 Sprague-Dawley ♂/♀ |
Minocycline | Closed-head CCI, repeated |
 Microglia/macrophages (ED1) accumulation at 3 dpi, Effect lost at 7 dpi, trend toward increase at 21 dpi Microglia/macrophages (ED1) accumulation at 3 dpi, Effect lost at 7 dpi, trend toward increase at 21 dpi Spatial memory vs vehicle No effect: Iba1 cells, axonal injury, white matter loss and neurodegeneration Spatial memory vs vehicle No effect: Iba1 cells, axonal injury, white matter loss and neurodegeneration |
| Hanlon et al., 2017 | Rat PD 11 Sprague-Dawley ♂/♀ |
Minocycline | Closed-head CCI |
Short-term treatment (3 days): Microglia reactivity in cortex, hippocampus and white matter at 3 but not 7 dpi Microglia reactivity in cortex, hippocampus and white matter at 3 but not 7 dpi Neurodegeneration at 3 but not 7 dpi Neurodegeneration at 3 but not 7 dpiLong-term treatment (9 days):  Microglial reactivity and neurodegeneration up to 15 dpi in cortex and hippocampus Microglial reactivity and neurodegeneration up to 15 dpi in cortex and hippocampus Spatial memory vs vehicle No effects of sex Spatial memory vs vehicle No effects of sex |
| Chhor et al., 2016 | Mouse OF-1 Postnatal day 7 ♂/♀ |
Minocycline | Closed-head WD |
24 hpi: M1:  IL-1β, IL-6 IL-1β, IL-6M2a/c:  IGF-1, IL-1rn IGF-1, IL-1rn Ventricular volume Ventricular volume Cleaved caspase 3 positive cells in cortex, hippocampus and striatum Cleaved caspase 3 positive cells in cortex, hippocampus and striatum Iba1 positive cells in cortex but not hippocampus/striatum Iba1 positive cells in cortex but not hippocampus/striatumNo beneficial effects at 5 dpi |
| Lloyd et al., 2008 | Mouse CD-1 ♂ |
Minozac | Closed-head CCI |
12 hpi: M1  : cytokines levels, IL-1β, IL-6, TNF-α, CCL2 in hippocampus and cortex : cytokines levels, IL-1β, IL-6, TNF-α, CCL2 in hippocampus and cortex28 dpi:  Astrogliosis Astrogliosis Exploratory deficits Exploratory deficits |
| Tchantchou and Zhang, 2013 | Mouse C57B6 ♂ |
WWL70, alpha/beta hydrolase domain 6 inhibitor | CCI |
 iNOS iNOS ARG-1 staining ARG-1 staining BBB disruption, lesion size, neurodegeneration BBB disruption, lesion size, neurodegeneration Motor/working memory performance Motor/working memory performance |
| Loane et al., 2014b | Mouse C57Bl/6 ♂ |
VU0360172, mGlu5 positive allosteric modulator | CCI |
M1:  iNOS/CD11b iNOS/CD11bM2:  Arg-1/CD11b Arg-1/CD11b motor performance motor performance lesion size, neurodegeneration lesion size, neurodegeneration |
| Wang et al., 2015 | Mouse C57B6 ♂ |
Scriptaid, class I/II histone deacetylase inhibitor | CCI |
M1:  CD16 cells CD16 cells CD16+iNOS mRNA CD16+iNOS mRNAM2:  CD206 cells CD206 cells  CD206 mRNA CD206 mRNA Sensorimotor performance Sensorimotor performance White matter integrity White matter integrity |
| Morganti et al., 2015 | Mouse Dbl-Het C57Bl/6 ♂/♀ |
CCX872, CCR2 selective antagonist | CCI |
Acute phase: M1  : CD68, CD45, CCL2, IL-1β, IL-6 M2a ▾: Arg-1, Fizz1 : CD68, CD45, CCL2, IL-1β, IL-6 M2a ▾: Arg-1, Fizz1M2c ▾: TGF-β and IL-10 Chronic phase: M1  : CD68, CD45, CCL2, IL-1β, TNF-α : CD68, CD45, CCL2, IL-1β, TNF-αM2a ▾: Fizz1  Spatial learning/memory Spatial learning/memory Macrophage invasion Macrophage invasion |
| Kumar et al., 2016a,b | Mouse C57Bl/6 NOX −/−♂ | gp91ds-tat, selective NOX2 inhibitor | CCI |
M1:  CD16/32 CD16/32M2:  TGF-β TGF-βM1:  Nox2 Nox2M2:  Ym1, Arg-1 Ym1, Arg-1 lesion size, neurodegeneration lesion size, neurodegeneration motor/spatial memory performance motor/spatial memory performance |
| Cao et al., 2012 | Rat Sprague-Dawley ♂ |
Ibuprofen | FPI |
 TGF-βI TGF-βInonsignificant  in TSPO, CD45 at 7 dpi in the thalamus in TSPO, CD45 at 7 dpi in the thalamus |
| Chio et al., 2013; Cheong et al., 2013 | Rat Sprague-Dawley ♂ |
Etanercept (TNF-α antagonist) | FPI |
3/7 dpi: Neurological severity score Neurological severity score Iba1/TNF-α positive cells in multiple brain regions Iba1/TNF-α positive cells in multiple brain regions TNF-α protein levels TNF-α protein levels Oedema Oedema Neurogenesis Neurogenesis |
CCI, Controlled Cortical Impact; FPI, Fluid percussion injury, WD, Weight drop.
Dpi, Days post injury, hpi, hours post injury; wpi, weeks post injury;
Green color indicates beneficial, while red indicates detrimental actions of the treatment.
Imaging of microglia activation in TBI
Measurement of microglial activation in vivo has become possible using PET and Single Photon Emission Computed Tomography (SPECT) due to the development of radioligands that bind to the 18-kDa translocator protein (TSPO). TSPO is a five-transmembrane domain protein localized in the outer mitochondrial membrane (Jaremko et al., 2014). The best studied putative function of TSPO relates to its role in transporting cholesterol into the mitochondrial inner membrane space, the rate-limiting step of steroid and neurosteroid biosynthesis (Papadopoulos et al., 2006). However, this function has been lately questioned due to the findings from knockout mice that did not show abnormal steroidogenesis (Morohaku et al., 2014; Tu et al., 2014). Despite almost 40 years of study, the precise functional role of TSPO is far from clear (Selvaraj and Stocco, 2015).
Imaging of activated microglia after experimental TBI
Autoradiographic studies and PET using TSPO ligands in experimental TBI have informed our understanding of the time-course and spatial distribution of microglial activation following brain trauma. The first TSPO ligand, PK11195, was synthesized in the 1980s. Autoradiographic studies localized binding of radiolabelled PK11195 to activated microglia (Banati et al., 1997) and, as a result, [11C]PK11195 was adopted as a PET radioligand to image neuroinflammation in vivo (Liu et al., 2014). Increased TSPO-specific radioligand binding was detected as early as 6 h after CCI in rats, with significantly elevated radioligand binding in the ipsilateral and contralateral cortices at 24 h and delayed thalamic upregulation at 28 days p.i. (Donat et al., 2016). Additionally, we previously observed a small increase of [3H]PK11195 binding in several brain regions of newborn piglets subjected to FPI at 6 h p.i. (Donat et al., 2014). Similarly, increased TSPO up-regulation indicative of microglia activation was reported in models including FPI (Yu et al., 2010; Cao et al., 2012), CCI (Venneti et al., 2007; Folkersma et al., 2011b; Wang et al., 2014), penetrating brain injury (Miyazawa et al., 1995; Grossman et al., 2012), closed-head injury (CHI) (Grossman et al., 2003) and dynamic cortical deformation (Soustiel et al., 2008).
In vivo studies employing [11C]PK11195 PET in rats following CCI 1 day after injury showed no differences in whole brain uptake compared to baseline values or sham-treated controls, whereas scans at 10 days showed significantly increased uptake (Folkersma et al., 2011b). In another CCI rat model, uptake of [18F]DPA-714 (a second-generation TSPO ligand) was observed on day 2 after injury, peaking on day 6, and remaining elevated to day 16 (Wang et al., 2014). Similar findings were seen in a weight-drop mouse model, no differences were observed in [18F]DPA-714 binding at day 1, but increased uptake in focal brain lesions on repeated scans at days 7 and 16 (Israel et al., 2016).
Several experimental studies have also shown that TSPO expression increases not only in the vicinity of lesioned areas but also at locations remote from sites of focal damage. Following CCI in rats, the ipsilateral thalamus and hippocampus show increased [3H]PK11195 binding between 3 and 14 days after injury (Raghavendra Rao et al., 2000). In the FPI model, increased uptake of [18F]-fluoroethyl-DAA1106 (a second-generation TSPO ligand) appears in the ipsilateral striatum up to 4 weeks' post-injury (Yu et al., 2010). In our own study, using a CCI model in rats, we found increased binding of [123I]CLINDE (a second-generation TSPO ligand) in contralateral motor cortex early after injury (24 h), whereas increased binding in the ipsilateral thalamus was observed first at 28 days post-injury (Donat et al., 2016).
However, TSPO up-regulation does not appear to relay any information on potential polarization pattern (Kim and Yu, 2015). In a mouse model of intracranial hemorrhage, microglia exclusively expressed TSPO and in these cells CD16/32 (M1-like) or CD206 (M2-like) were evenly distributed (Bonsack et al., 2016).
While TSPO seems a more general biomarker of glia activation, likely showing the mitochondrial changes due to the increased metabolic demand resulting from glia activation and cell proliferation (Liu et al., 2014), other targets expressed by microglia (e.g., cannabinoid type 2 receptor, P2X7 and P2Y12 receptors) could be more indicative of microglial polarization, although less tracers are available for these targets.
Imaging microglia in humans
In humans, [11C]PK11195 PET has demonstrated increased TSPO expression in a variety of neuroinflammatory conditions (Owen and Matthews, 2011), including herpes encephalitis (Cagnin et al., 2001b), Alzheimer's disease (Cagnin et al., 2001a), multiple sclerosis (Banati et al., 2000), and stroke (Gerhard et al., 2005). In the normal human brain, [11C]PK11195 uptake is generally low, but with relatively high binding in subcortical structures, including midbrain, thalamus and putamen (Kumar et al., 2012; Kobayashi et al., 2017). The relatively increased TSPO ligand binding observed in midbrain, thalamus and basal ganglia (Kumar et al., 2012) is in keeping with the high density of microglia found in these regions in the mouse (Lawson et al., 1990) and human brain (Mittelbronn et al., 2001) in comparison to the cerebral cortex. However, increased TSPO ligand binding may reflect an increase in TSPO density rather than the number of cells expressing TSPO. These regions are highly connected through white matter to widespread brain regions. Owing to their dense connectivity, it is possible that even subtle neuronal injury, as may arise in normal aging, may result in the accumulation of secondary microglial activation in these regions, and therefore increased TSPO binding.
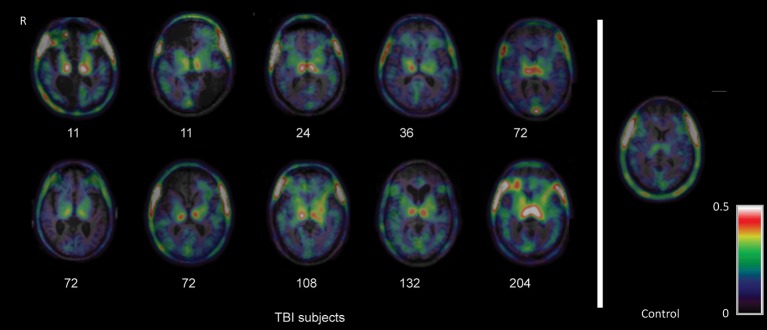
PET imaging with TSPO ligands provides a method to visualize microglial activity following TBI in vivo, although the specificity of TSPO for microglia still remains controversial. In an early study, we used [11C]PK11195 PET to investigate a group of 10 patients in the chronic phase 11 months to 17 years after single moderate-severe TBI (Figure 2; Ramlackhansingh et al., 2011). [11C]PK11195 binding potential was significantly increased in subcortical structures, particularly the thalami, putamen, and parts of the white matter. In keeping with animal work, this chronic microglial activation was seen in regions remote from focal damage and was also reduced around areas of focal injury. High [11C]PK11195 binding in the thalamus was associated with more severe cognitive impairment, suggesting either that microglial activation might be directly contributing to cognitive impairment or that this was a response to underlying brain injury that caused the cognitive impairment.
Figure 2.
Imaging of chronic microglial activation after TBI. Images of [11C]PK11195 PET images are shown superimposed on the T1 MRI scan at the level of the thalamus for 10 TBI patients, 11 months to 17 years after injury, and a representative control participant. Numbers indicate time since injury (months). R right. The figure has been reproduced with permission of the copyright holder (Ramlackhansingh et al., 2011).
These initial findings have been replicated and extended in further studies. A second study by another group also used [11C]PK11195 PET following TBI (Folkersma et al., 2011a). They again showed increased binding in subcortical regions remote from the focal traumatic pathology, which included the thalamus, putamen, pons, and hippocampus. Increased microglial activation has also been observed in sportsmen exposed to repetitive TBI (Coughlin et al., 2015, 2017). A group of 14 active or recently retired National Football League players with a history of concussions were studied using the second generation TSPO ligand [11C]DPA-713. Increased binding was seen predominantly in medial temporal lobe regions in sportsmen who also showed subtle evidence of white matter damage on diffusion MRI. In our follow-up study, we used the second generation TSPO ligand [11C]PBR28 (Scott et al., 2015). A similar distribution of subcortical microglial activation was again seen years after single moderate/severe TBI. Prominent white matter microglial activation in areas of white matter damage was measured using magnetic resonance imaging (MRI). In addition, areas with high microglial activation showed high levels of brain atrophy over the next 6 months. This suggests that chronically activated microglia are seen in areas of persistent white matter damage, which are progressively degenerating even many years after injury.
These studies consistently show microglial activation in regions far removed from sites of focal injuries. Whilst at first glance surprising, the observation is in keeping with the evolution of microglial activation after experimental TBI (Donat et al., 2016) and is likely to reflect slowly progressive changes within damaged white matter, in particular Wallerian degeneration (Figure 3). In our initial study, the level of thalamic microglial activation after TBI showed a strong correlation with the level of traumatic axonal injury in thalamo-cortical projections. This suggests that traumatic axonal injury might be playing a causative role in increasing chronic microglial activation (Scott et al., 2015). The effect is not specific to TBI and a similar progression of microglial activity is seen following lacunar infarcts. This suggests that as the after effects of axonal injury develop and Wallerian degeneration progresses, microglial activation might be observed in cortical and subcortical regions remote from the initial injury. A number of factors might explain the thalamic rather than cortical preponderance of microglial activation after TBI. Firstly, there is a high density of projection neurons converging in the thalamus, which might lead to a regional amplification of widespread, but sub-threshold cortical pathology (Cagnin et al., 2003). Secondly, as cortico-thalamic projections are 10 times more numerous that thalamo-cortical projections, a more intense microglial reaction to anterograde neurodegeneration might contribute to the persistent microglial reaction in the thalamus (Figure 3).
Figure 3.
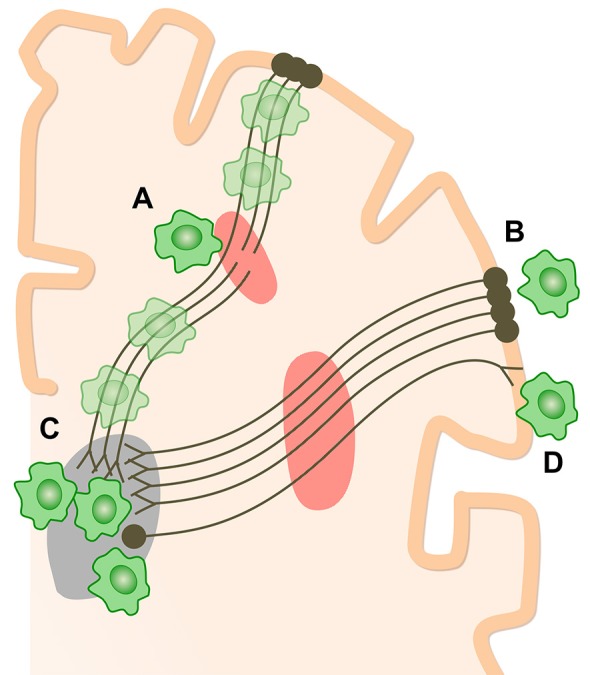
How chronic microglial activation and axonal injury may be linked after TBI. Microglial activation (green cells) and traumatic axonal injury in thalamo-cortical white matter tracts (red areas) have been demonstrated after TBI. Sites of chronic microglial activation can co-localize with axonal abnormality (A) as well as along the entire axonal tract affected by injury. Remote from sites of primary axonal injury, microglia may be observed both in retrograde projection areas, toward the cell bodies of damaged neurons (B), and in anterograde areas (C,D). The thalamus is a highly-connected structure. Thalamic microglial activation may be observed after TBI because of the high density of connections to damaged axons. The number of cortico-thalamic projections far exceeds thalamo-cortical projections. If microglial activation preferentially favors anterograde involvement, then relatively increased activation would be expected in the thalamus (C) compared to corresponding cortical areas (B).
Anti-inflammatory treatments targeting microglia activation in TBI
The relevance of microglia in the general pathophysiological response to TBI is recognized as a potential therapeutic avenue (reviewed in Chio et al., 2015) and has prompted several studies to investigate the effects of certain drugs on microglial polarization in brain injury models. Although studies in animal models indicate that microglia/macrophages respond to TBI with a transient M2 phenotype, followed by a shift to M1, and that the number of M1 cells is strongly correlated with the severity of white matter injury (Koh and DiPietro, 2011), this may differ in non-human and human primates. Therefore, therapies that prime microglia/macrophages toward the beneficial M2 phenotype after TBI may offer new anti-inflammatory strategies (Table 2). Several of these drugs, including minocycline, minozac, etanercept and the PPAR agonists fenofibrate and pioglitazone are FDA approved (Garrido-Mesa et al., 2013; Kim et al., 2014) confirming their safety and tolerability.
Progranulin
In response to TBI, microglia/macrophages and astrocytes release inflammatory mediators with dual effects on secondary brain damage progression. The neurotrophic and anti-inflammatory glycoprotein progranulin (PGRN) attenuates neuronal damage and microglia/macrophage activation in brain injury but mechanisms are still elusive. Intracerebroventricular administration of recombinant PGRN in mice immediately before trauma reduced brain damage and neurological deficits, and restored normal levels of cytokine transcription, axonal injury and astrogliosis 5 days after TBI in granulin knockout mice (Menzel et al., 2017).
Minocycline
Minocycline is a tetracycline derivative with anti-inflammatory and neuroprotective properties. Some of the proposed mechanisms for the anti-inflammatory properties for minocycline include the inhibitory effects on the activities of key enzymes, like iNOS, MMPs and PLA2 (Garrido-Mesa et al., 2013). In particular, it has been shown that it is able to inhibit M1 polarization state of microglia (Kobayashi et al., 2013), through the inhibition of NFκB and the interference with MAPK pathways. Minocycline significantly inhibited retinal neuroinflammation in an ischemia reperfusion (IR) model, characterized by inflammatory gene expression, leukocyte adhesion and invasion, and vascular permeability, however it failed to block neurodegeneration (Abcouwer et al., 2013). The effects of minocycline treatment in an animal model of TBI (closed head CCI in the neonate rat) revealed that the protective effects could be detected short term after injury (3 days after minocycline treatment) but not in chronic treatment (for 9 days after injury), in which microglial reactivity and neurodegeneration in all regions examined were exacerbated in minocycline-treated brain-injured animals (Hanlon et al., 2016, 2017). However, in other TBI models, such as blast injury, minocycline appears to prevent the development of neurobehavioral abnormalities (Kovesdi et al., 2012).
Minozac
Minozac (Mzc) is an anti-inflammatory molecule that selectively reduces excessive pro-inflammatory cytokine production by activated glia toward basal levels. There is evidence that administration of Mzc in a mouse closed-head, CCI at 3 and 9 h following TBI attenuates the acute increase in proinflammatory cytokine and chemokine levels and reduces the longer-term astrocyte activation, neurologic injury and neurobehavioral deficits observed over a 28-day recovery period (Lloyd et al., 2008)
Pharmacological inhibition of TNF-α
Pharmacological inhibition of TNF-α using etanercept has been shown to reduce the expression of microglial TNF-α in rodents subjected to FPI (Cheong et al., 2013; Chio et al., 2013) and improve the neurological outcome after stroke and TBI in humans (Tobinick et al., 2012). Etanercept also seems to be able to stimulate neurogenesis in rats (Cheong et al., 2013).
Modulation of glutamate receptors
Although glutamate released by microglia may be related to a neurotoxic effect, it was shown that activation of metabotropic glutamate receptor 5 (mGluR5) on microglia is a novel mechanism to attenuate M1-like microglial activation and associated microglial-mediated neurotoxicity, suggesting a self-regulatory mechanism. Positive allosteric activation of mGluR5 has powerful neuroprotective effects in experimental models of CNS injury (Loane et al., 2014b).
PPAR agonists
In traumatic brain injury, the PPARα agonist fenofibrate appears to represent a highly promising new anti-inflammatory compound. Besson et al. (2005) assessed the pharmacological role of fenofibrate in the FPI model in adult male Sprague-Dawley rats. The study revealed that the administration of fenofibrate during a clinically relevant therapeutic “time window of opportunity” at 1 h after trauma mediated a significant post-traumatic neuroprotection. This was demonstrated by improved neurological scores in the fenofibrate group at 24 h and 7 days after trauma, compared to vehicle-treated animals (Besson et al., 2005). In the case of PPARγ agonists, both pioglitazone and rosiglitazone seem to have protective effects, in particular pioglitazone reduced the histological damage and inflammation in the CCI model of TBI (Thal et al., 2011).
HDAC inhibition
HDAC inhibitors have been found to have anti-inflammatory and neuroprotective effect in models of TBI. Scriptaid, a novel inhibitor of class I/II HDACs, was found to facilitate and enhance recovery of motor functions after CCI and protected white matter up to 35 day after TBI, as shown by reductions in abnormally dephosphorylated neurofilament protein, increases in myelin basic protein, anatomic preservation of myelinated axons, and improved nerve conduction (Wang et al., 2015). Furthermore, Scriptaid shifted microglia/macrophage polarization toward the protective M2 phenotype and mitigated inflammation. In primary co-cultures of microglia and oligodendrocytes, Scriptaid increased expression of microglial glycogen synthase kinase 3 beta (GSK3β), which phosphorylated and inactivated phosphatase and tensin homolog (PTEN), thereby enhancing phosphatidylinositide 3-kinases (PI3K)/Akt signaling and polarizing microglia toward M2. The increase in GSK3β in microglia and their phenotypic switch to M2 was associated with increased preservation of neighboring oligodendrocytes.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitors were able to alter M1-/M2-like balance in favor of the anti-inflammatory M2-like phenotype in a CCI model of TBI (Kumar et al., 2016a,b).
Conclusions
In recent years, there has been a growing interest in investigating the activation of microglia in TBI, because of the potential role in the progression of those patients to neurodegenerative and psychiatric diseases. The studies of microglia in animal models of TBI have allowed the manipulation of microglial numbers (by genetic or pharmacological ablation), indicating their important role in neuroprotection especially at early times post-injury, although this sequence seems to be model and species-specific. In addition, this has provided insights into the time course of the different profile of microglial activation phenotypes in the injury site and the spreading of inflammation to other areas of the brain, such as the thalamus.
In vivo molecular imaging of TSPO potentially provides an extremely useful biomarker of microglial activation and the effect of immunomodulatory drugs. In TBI patients, it has allowed the observation of chronically activated microglia located in areas of persistent white matter damage, even long time post injury. However, it currently only provides a one-dimensional measure, i.e., the amount of microglial activation within a particular brain region. It is clearly too simplistic to describe microglial activation in vivo along a single dimension (i.e., from “low activity” to “high activity”). Crucially, on the basis of current evidence, TSPO molecular imaging cannot discriminate activation phenotype, but probably reflects the (potentially uneven) summation of activity from microglia across all functional states.
In addition, there has been some debate regarding the possibility that certain TSPO ligands also recognize astrocytes or other immune cells in the brains of TBI patients. Other limitations of the application of second-generation ligands include that the binding affinities are influenced by a common polymorphism (rs6971) in the TSPO gene which causes a single amino acid substitution (A147T) in the protein (Owen et al., 2012). Because 147T TSPO binds ligands with lower affinity than 147A, this produces three classes of binding affinity across a population, which studies must therefore control for. Even after accounting for TSPO genotype, however, many second-generation TSPO ligands show high between-subject variability in uptake when using analysis methods which rely on measurement of the ligand in arterial blood (Guo et al., 2012). As for [11C]PK11195, high and variable plasma protein binding may be a factor (Lockhart et al., 2003). Methods of analysis such as the simplified reference tissue model (SRTM) have been developed that do not require arterial blood data, but rather use the PET imaging data from a reference region (or reference tissue) instead (Gunn et al., 1997).
To improve the interpretation of the TSPO signal, a detailed characterization is needed of how TSPO expression in humans varies with the diversity of microglial phenotypes seen in vivo. Future TBI work needs to provide a description of the time-course of microglial phenotype change after TBI and its relationship to TSPO expression. Novel PET ligands showing specificity for distinct functional subtypes of microglial activation would be of great utility, with cannabinoid type 2 and purinergic receptors, such as P2Y12 and P2X7, possibly providing suitable targets in the future. Another option is to combine TSPO PET imaging with different biomarkers that disambiguate the TSPO signal in a particular context, in particular neurofilament light (NFL). NFL levels in CSF have been proposed as a valid biomarker to accurately assess the level of trauma and predict the clinical outcome of the patient (Zhang et al., 2016).
Recent investigations have indicated that simple suppression of microglia activation can exert only limited beneficial effect and the inhibition of M1-like responses might be detrimental similar to a simple stimulation of M2-like phenotypes, as indicated by increased fibrosis which seems modulated by Arginase-1 in peripheral and central infection (Hesse et al., 2001; Aldrich and Kielian, 2011). In addition, pharmacological treatment with the anti-inflammatory drugs tested in several models may not have similar effects when administered in TBI patients, as seen in clinical trials for AD (Lleo et al., 2007), limiting the potential therapeutic impact. It seems therefore imperative for future studies that target microglia polarization as therapeutic strategies, to assess several markers in the target cells within a sufficient temporal window in order to show a long-term positive outcome.
Author contributions
MS wrote most of the introduction, the manipulation of microglia in animal models and the treatments section; CD wrote most of the section on animal models of TBI and preclinical TSPO imaging in animal models, made the tables, the references and wrote part of the conclusions; GS wrote most of the part of the imaging with TSPO, made two figures and part of the conclusions; SG contributed with the image of microglia (Figure 1) and edited the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All appropriate permissions have been obtained from the copyright holders of any work that has been reproduced in this manuscript.
Acknowledgments
We thank Prof. David J. Sharp (Imperial College London) for critical reading of the manuscript.
Footnotes
Funding. ISSF Networks of Excellence award 2015 (reference 105603/Z/14/Z, Wellcome Trust).
References
- Abcouwer S. F., Lin C. M., Shanmugam S., Muthusamy A., Barber A. J., Antonetti D. A. (2013). Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J. Neuroinflammat. 10:149. 10.1186/1742-2094-10-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich A., Kielian T. (2011). Central nervous system fibrosis is associated with fibrocyte-like infiltrates. Am. J. Pathol. 179, 2952–2962. 10.1016/j.ajpath.2011.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. A. (2015). Temporal profile of M1 and M2 responses in the hippocampus following early 24 h of neurotrauma. J. Neurol. Sci. 357, 41–49. 10.1016/j.jns.2015.06.062 [DOI] [PubMed] [Google Scholar]
- Baalman K., Marin M. A., Ho T. S., Godoy M., Cherian L., Robertson C., et al. (2015). Axon initial segment-associated microglia. J. Neurosci. 35, 2283–2292. 10.1523/JNEUROSCI.3751-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter A. D., Rowe R. K., Kaneko M., Goulding D., Lifshitz J., Van Eldik L. J. (2013). The p38alpha MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J. Neurosci. 33, 6143–6153. 10.1523/JNEUROSCI.5399-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati R. B., Myers R., Kreutzberg G. W. (1997). PK (‘peripheral benzodiazepine’)--binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J. Neurocytol. 26, 77–82. 10.1023/A:1018567510105 [DOI] [PubMed] [Google Scholar]
- Banati R. B., Newcombe J., Gunn R. N., Cagnin A., Turkheimer F., Heppner F., et al. (2000). The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 123 (Pt 11), 2321–2337. 10.1093/brain/123.11.2321 [DOI] [PubMed] [Google Scholar]
- Barrett J. P., Henry R. J., Villapol S., Stoica B. A., Kumar A., Burns M. P., et al. (2017). NOX2 deficiency alters macrophage phenotype through an IL-10/STAT3 dependent mechanism: implications for traumatic brain injury. J. Neuroinflammat. 14, 65. 10.1186/s12974-017-0843-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi S. S., Smith P., Hetz R. A., Xue H., Cox C. S. (2013). Immunomagnetic enrichment and flow cytometric characterization of mouse microglia. J. Neurosci. Methods 219, 176–182. 10.1016/j.jneumeth.2013.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. E., Brody D. L. (2014). Acute reduction of microglia does not alter axonal injury in a mouse model of repetitive concussive traumatic brain injury. J. Neurotrauma. 31, 1647–1663. 10.1089/neu.2013.3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C., Ley E. J., Tillou A., Cryer G., Margulies D. R., Salim A. (2009). The effect of gender on patients with moderate to severe head injuries. J. Trauma 67, 950–953. 10.1097/TA.0b013e3181ba3354 [DOI] [PubMed] [Google Scholar]
- Besson V. C., Chen X. R., Plotkine M., Marchand-Verrecchia C. (2005). Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci. Lett. 388, 7–12. 10.1016/j.neulet.2005.06.019 [DOI] [PubMed] [Google Scholar]
- Black M. E., Newcomb T. G., Wilson H. M., Loeb L. A. (1996). Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy. Proc. Natl. Acad. Sci. U.S.A. 93, 3525–3529. 10.1073/pnas.93.8.3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D., Perry V. H., Nicoll J. A. (2013). Review: activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 39, 3–18. 10.1111/nan.12011 [DOI] [PubMed] [Google Scholar]
- Bonsack F., IV, Alleyne C. H., Jr., Sukumari-Ramesh S. (2016). Augmented expression of TSPO after intracerebral hemorrhage: a role in inflammation? J. Neuroinflammat. 13:151. 10.1186/s12974-016-0619-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M., Vaibhav K., Saad N., Fatima S., Brann D. W., Vender J. R., et al. (2017). Activation of Myeloid TLR4 Mediates T Lymphocyte polarization after Traumatic Brain Injury. J. Immunol. 198, 3615–3626. 10.4049/jimmunol.1601948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye N., Habgood M. D., Callaway J. K., Malakooti N., Potter A., Kossmann T., et al. (2007). Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp. Neurol. 204, 220–233. 10.1016/j.expneurol.2006.10.013 [DOI] [PubMed] [Google Scholar]
- Cagnin A., Brooks D. J., Kennedy A. M., Gunn R. N., Myers R., Turkheimer F. E., et al. (2001a). In-vivo measurement of activated microglia in dementia. Lancet 358, 461–467. 10.1016/S0140-6736(01)05625-2 [DOI] [PubMed] [Google Scholar]
- Cagnin A., Gerhard A., Banati R. B. (2003). In vivo Imaging of Neuroinflammation in Neurodegenerative Diseases. (Neuroinflammation: Mechanisms and Management). Totowa, NJ: Humana Press Inc. [Google Scholar]
- Cagnin A., Myers R., Gunn R. N., Lawrence A. D., Stevens T., Kreutzberg G. W., et al. (2001b). In vivo visualization of activated glia by [11C] (R)-PK11195-PET following herpes encephalitis reveals projected neuronal damage beyond the primary focal lesion. Brain 124(Pt 10), 2014–2027. 10.1093/brain/124.10.2014 [DOI] [PubMed] [Google Scholar]
- Cao T., Thomas T. C., Ziebell J. M., Pauly J. R., Lifshitz J. (2012). Morphological and genetic activation of microglia after diffuse traumatic brain injury in the rat. Neuroscience 225, 65–75. 10.1016/j.neuroscience.2012.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan H. W., Cox C. S., Bedi S. S. (2017). Do microglia play a role in sex differences in TBI? J. Neurosci. Res. 95, 509–517. 10.1002/jnr.23854 [DOI] [PubMed] [Google Scholar]
- Carmichael E. A. (1929). Microglia: an experimental study in rabbits after intracerebral injection of blood. J. Neurol. Psychopathol. 9, 209–216. 10.1136/jnnp.s1-9.35.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., et al. (1983). Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc. Natl. Acad. Sci. U.S.A. 80, 2767–2770. 10.1073/pnas.80.9.2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong C. U., Chang C. P., Chao C. M., Cheng B. C., Yang C. Z., Chio C. C. (2013). Etanercept attenuates traumatic brain injury in rats by reducing brain TNF- alpha contents and by stimulating newly formed neurogenesis. Mediat. Inflamm. 2013:620837. 10.1155/2013/620837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. D., Tripodis Y., Alvarez V. E., Huber B., Kiernan P. T., Daneshvar D. H., et al. (2016). Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 4, 112. 10.1186/s40478-016-0382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhor V., Moretti R., Le Charpentier T., Sigaut S., Lebon S., Schwendimann L., et al. (2016). Role of microglia in a mouse model of paediatric traumatic brain injury. Brain Behav. Immun. 63, 197–209. 10.1016/j.bbi.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio C. C., Chang C. H., Wang C. C., Cheong C. U., Chao C. M., Cheng B. C., et al. (2013). Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-alpha. BMC Neurosci. 14:33. 10.1186/1471-2202-14-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio C. C., Lin M. T., Chang C. P. (2015). Microglial activation as a compelling target for treating acute traumatic brain injury. Curr. Med. Chem. 22, 759–770. 10.2174/0929867321666141106124657 [DOI] [PubMed] [Google Scholar]
- Chiu C. C., Liao Y. E., Yang L. Y., Wang J. Y., Tweedie D., Karnati H. K., et al. (2016). Neuroinflammation in animal models of traumatic brain injury. J. Neurosci. Methods 272, 38–49. 10.1016/j.jneumeth.2016.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra R., Hoyt D. B., Potenza B. M., Fortlage D., Hollingsworth-Fridlund P. (2003). Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! J. Trauma. 54, 689–700. 10.1097/01.TA.0000058314.31655.5F [DOI] [PubMed] [Google Scholar]
- Coughlin J. M., Wang Y., Minn I., Bienko N., Ambinder E. B., Xu X., et al. (2017). Imaging of glial cell activation and white matter integrity in brains of active and recently retired national football league players. JAMA Neurol. 74, 67–74. 10.1001/jamaneurol.2016.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin J. M., Wang Y., Munro C. A., Ma S., Yue C., Chen S., et al. (2015). Neuroinflammation and brain atrophy in former NFL players: an in vivo multimodal imaging pilot study. Neurobiol. Dis. 74, 58–65. 10.1016/j.nbd.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky S. T., Abrahamson E. E., Ciallella J. R., Paljug W. R., Wisniewski S. R., Clark R. S., et al. (2007). Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch. Neurol. 64, 541–544. 10.1001/archneur.64.4.541 [DOI] [PubMed] [Google Scholar]
- Desai A., Park T., Barnes J., Kevala K., Chen H., Kim H. Y. (2016). Reduced acute neuroinflammation and improved functional recovery after traumatic brain injury by alpha-linolenic acid supplementation in mice. J. Neuroinflammat. 13:253. 10.1186/s12974-016-0714-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donat C. K., Fischer F., Walter B., Deuther-Conrad W., Brodhun M., Bauer R., et al. (2014). Early increase of cannabinoid receptor density after experimental traumatic brain injury in the newborn piglet. Acta Neurobiol. Exp. (Wars) 74, 197–210. [DOI] [PubMed] [Google Scholar]
- Donat C. K., Gaber K., Meixensberger J., Brust P., Pinborg L. H., Hansen H. H., et al. (2016). Changes in binding of [(123)I]CLINDE, a high-affinity translocator protein 18 kDa (TSPO) selective radioligand in a rat model of traumatic brain injury. Neuromol. Med. 18, 158–169. 10.1007/s12017-016-8385-y [DOI] [PubMed] [Google Scholar]
- Dunning H. S., Stevenson L. (1934). Microglia-like cells and their reaction following injury to the liver, spleen and kidney. Am. J. Pathol. 10, 343–348.7 [PMC free article] [PubMed] [Google Scholar]
- Elliott M. B., Tuma R. F., Amenta P. S., Barbe M. F., Jallo J. I. (2011). Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J. Neurotrauma 28, 973–981. 10.1089/neu.2010.1672 [DOI] [PubMed] [Google Scholar]
- Faden A. I., Wu J., Stoica B. A., Loane D. J. (2016). Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br. J. Pharmacol. 173, 681–691. 10.1111/bph.13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulds D., Heel R. C. (1990). Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 39, 597–638. 10.2165/00003495-199039040-00008 [DOI] [PubMed] [Google Scholar]
- Febinger H. Y., Thomasy H. E., Pavlova M. N., Ringgold K. M., Barf P. R., George A. M., et al. (2015). Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury. J. Neuroinflammat. 12:154. 10.1186/s12974-015-0386-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn A. M., Gensel J. C., Huang Y., Popovich P. G., Lifshitz J., Godbout J. P. (2014). Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol. Psychiatry 76, 575–584. 10.1016/j.biopsych.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn A. M., Skendelas J. P., Moussa D. N., Muccigrosso M. M., Popovich P. G., Lifshitz J., et al. (2015). Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J. Neurotrauma. 32, 127–138. 10.1089/neu.2014.3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkersma H., Boellaard R., Yaqub M., Kloet R. W., Windhorst A. D., Lammertsma A. A., et al. (2011a). Widespread and prolonged increase in (R)-11C-PK11195 binding after traumatic brain injury. J. Nucl. Med. 52, 1235–1239. 10.2967/jnumed.110.084061 [DOI] [PubMed] [Google Scholar]
- Folkersma H., Foster Dingley J. C., van Berckel B. N., Rozemuller A., Boellaard R., Huisman M. C., et al. (2011b). Increased cerebral (R)-[11C]-PK11195 uptake and glutamate release in a rat model of traumatic brain injury: a longitudinal pilot study. J. Neuroinflammat. 8:67. 10.1186/1742-2094-8-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Han Z., Bai R., Huang S., Ge X., Chen F., et al. (2017). The accumulation of brain injury leads to severe neuropathological and neurobehavioral changes after repetitive mild traumatic brain injury. Brain Res. 1657, 1–8. 10.1016/j.brainres.2016.11.028 [DOI] [PubMed] [Google Scholar]
- Garrido-Mesa N., Zarzuelo A., Galvez J. (2013). Minocycline: far beyond an antibiotic. Br. J. Pharmacol., 169, 337–352. 10.1111/bph.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A., Schwarz J., Myers R., Wise R., Banati R. B. (2005). Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage 24, 591–595. 10.1016/j.neuroimage.2004.09.034 [DOI] [PubMed] [Google Scholar]
- Giunta B., Obregon D., Velisetty R., Sanberg P. R., Borlongan C. V., Tan J. (2012). The immunology of traumatic brain injury: a prime target for Alzheimer's disease prevention. J. Neuroinflammat. 9:185. 10.1186/1742-2094-9-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. E., Fisher A. M., Tagge C. A., Zhang X. L., Velisek L., Sullivan J. A., et al. (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 134ra160. 10.1126/scitranslmed.3003716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing G., Vallieres L., Julien J. P. (2006). Mouse model for ablation of proliferating microglia in acute CNS injuries. Glia 53, 331–337. 10.1002/glia.20288 [DOI] [PubMed] [Google Scholar]
- Grossman R., Paden C. M., Fry P. A., Rhodes R. S., Biegon A. (2012). Persistent region-dependent neuroinflammation, NMDA receptor loss and atrophy in an animal model of penetrating brain injury. Fut. Neurol. 7, 329–339. 10.2217/fnl.12.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman R., Shohami E., Alexandrovich A., Yatsiv I., Kloog Y., Biegon A. (2003). Increase in peripheral benzodiazepine receptors and loss of glutamate NMDA receptors in a mouse model of closed head injury: a quantitative autoradiographic study. Neuroimage 20, 1971–1981. 10.1016/j.neuroimage.2003.06.003 [DOI] [PubMed] [Google Scholar]
- Gruenbaum S. E., Zlotnik A., Gruenbaum B. F., Hersey D., Bilotta F. (2016). Pharmacologic neuroprotection for functional outcomes after traumatic Brain injury: a systematic review of the clinical literature. CNS Drugs 30, 791–806. 10.1007/s40263-016-0355-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle M. R., Carpenter K. L., Helmy A., Pickard J. D., Menon D. K., Hutchinson P. J. (2015). Matrix metalloproteinase expression in contusional traumatic brain injury: a paired microdialysis study. J. Neurotrauma 32, 1553–1559. 10.1089/neu.2014.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn R. N., Lammertsma A. A., Hume S. P., Cunningham V. J. (1997). Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 6, 279–287. 10.1006/nimg.1997.0303 [DOI] [PubMed] [Google Scholar]
- Guo Q., Owen D. R., Rabiner E. A., Turkheimer F. E., Gunn R. N. (2012). Identifying improved TSPO PET imaging probes through biomathematics: the impact of multiple TSPO binding sites in vivo. Neuroimage 60, 902–910. 10.1016/j.neuroimage.2011.12.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon L. A., Huh J. W., Raghupathi R. (2016). Minocycline transiently reduces microglia/macrophage activation but exacerbates cognitive deficits following repetitive traumatic brain injury in the neonatal rat. J. Neuropathol. Exp. Neurol. 75, 214–226. 10.1093/jnen/nlv021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon L. A., Raghupathi R., Huh J. W. (2017). Differential effects of minocycline on microglial activation and neurodegeneration following closed head injury in the neonate rat. Exp. Neurol. 290, 1–14. 10.1016/j.expneurol.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeldine J., Lord J. M., Belli A. (2015). Traumatic brain injury and peripheral immune suppression: primer and prospectus. Front. Neurol. 6:235. 10.3389/fneur.2015.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M., Modolell M., La Flamme A. C., Schito M., Fuentes J. M., Cheever A. W., et al. (2001). Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533–6544. 10.4049/jimmunol.167.11.6533 [DOI] [PubMed] [Google Scholar]
- Hickman S. E., Kingery N. D., Ohsumi T. K., Borowsky M. L., Wang L. C., Means T. K., et al. (2013). The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905. 10.1038/nn.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmin S., Mathiesen T. (1999). Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport 10, 1889–1891. 10.1097/00001756-199906230-00017 [DOI] [PubMed] [Google Scholar]
- Homsi S., Piaggio T., Croci N., Noble F., Plotkine M., Marchand-Leroux C., et al. (2010). Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. J. Neurotrauma 27, 911–921. 10.1089/neu.2009.1223 [DOI] [PubMed] [Google Scholar]
- Hsieh C. L., Kim C. C., Ryba B. E., Niemi E. C., Bando J. K., Locksley R. M., et al. (2013). Traumatic brain injury induces macrophage subsets in the brain. Eur. J. Immunol. 43, 2010–2022. 10.1002/eji.201243084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Leak R. K., Shi Y., Suenaga J., Gao Y., Zheng P., et al. (2015). Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurol. 11, 56–64. 10.1038/nrneurol.2014.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder A. A., Wunderlich C. A., Puvanachandra P., Gururaj G., Kobusingye O. C. (2007). The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22, 341–353. [PubMed] [Google Scholar]
- Ikonomovic M. D., Uryu K., Abrahamson E. E., Ciallella J. R., Trojanowski J. Q., Lee V. M., et al. (2004). Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 190, 192–203. 10.1016/j.expneurol.2004.06.011 [DOI] [PubMed] [Google Scholar]
- Immonen R. J., Kharatishvili I., Grohn H., Pitkanen A., Grohn O. H. (2009). Quantitative MRI predicts long-term structural and functional outcome after experimental traumatic brain injury. Neuroimage 45, 1–9. 10.1016/j.neuroimage.2008.11.022 [DOI] [PubMed] [Google Scholar]
- Israel I., Ohsiek A., Al-Momani E., Albert-Weissenberger C., Stetter C., Mencl S., et al. (2016). Combined [(18)F]DPA-714 micro-positron emission tomography and autoradiography imaging of microglia activation after closed head injury in mice. J. Neuroinflammat. 13:140. 10.1186/s12974-016-0604-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski K. A., Amici S. A., Webb L. M., Ruiz-Rosado Jde D., Popovich P. G., Partida-Sanchez S., et al. (2015). Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 10:e0145342. 10.1371/journal.pone.0145342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S., Etminan M., Aminzadeh F., Samii A. (2013). Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov. Disord. 28, 1222–1229. 10.1002/mds.25458 [DOI] [PubMed] [Google Scholar]
- Jaremko L., Jaremko M., Giller K., Becker S., Zweckstetter M. (2014). Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 343, 1363–1366. 10.1126/science.1248725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W. N., Shi S. X., Li Z., Li M., Wood K., Gonzales R. J., et al. (2017). Depletion of microglia exacerbates postischemic inflammation and brain injury. J. Cereb. Blood Flow Metab. 37, 2224–2236. 10.1177/0271678x17694185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Ishii H., Bai Z., Itokazu T., Yamashita T. (2012). Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS ONE 7:e41892. 10.1371/journal.pone.0041892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. E., Stewart J. E., Begbie F. D., Trojanowski J. Q., Smith D. H., Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136(Pt 1), 28–42. 10.1093/brain/aws322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. D. (2000). Chronic traumatic brain injury associated with boxing. Semin. Neurol. 20, 179–185. 10.1055/s-2000-9826 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Jenrow K. A., Brown S. L. (2014). Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 32, 103–115. 10.3857/roj.2014.32.3.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. J., Yu S. W. (2015). Translocator protein 18 kDa (TSPO): old dogma, new mice, new structure, and new questions for neuroprotection. Neural Regen. Res. 10, 878–880. 10.4103/1673-5374.158338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenhagen K. C., O'Brien S. P., Brody D. L. (2013). Repetitive concussive traumatic brain injury interacts with post-injury foot shock stress to worsen social and depression-like behavior in mice. PLoS ONE 8:e74510. 10.1371/journal.pone.0074510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Imagama S., Ohgomori T., Hirano K., Uchimura K., Sakamoto K., et al. (2013). Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 4:e525. 10.1038/cddis.2013.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Jiang T., Telu S., Zoghbi S. S., Gunn R. N., Rabiner E. A., et al. (2017). 11C-DPA-713 has much greater specific binding to translocator protein 18 kDa (TSPO) in human brain than 11C-(R)-PK11195. J. Cereb. Blood Flow Metab. 271678x17699223. 10.1177/0271678x17699223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek P. M., Hendrich K. S., Dixon C. E., Schiding J. K., Williams D. S., Ho C. (2002). Cerebral blood flow at one year after controlled cortical impact in rats: assessment by magnetic resonance imaging. J. Neurotrauma 19, 1029–1037. 10.1089/089771502760341947 [DOI] [PubMed] [Google Scholar]
- Koh T. J., DiPietro L. A. (2011). Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med. 13:e23. 10.1017/s1462399411001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovesdi E., Kamnaksh A., Wingo D., Ahmed F., Grunberg N. E., Long J. B., et al. (2012). Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front. Neurol. 3:111. 10.3389/fneur.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg G. W. (1996). Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318. 10.1016/0166-2236(96)10049-7 [DOI] [PubMed] [Google Scholar]
- Kumar A., Alvarez-Croda D. M., Stoica B. A., Faden A. I., Loane D. J. (2016a). Microglial/Macrophage polarization dynamics following traumatic brain injury. J. Neurotrauma 33, 1732–1750. 10.1089/neu.2015.4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Barrett J. P., Alvarez-Croda D. M., Stoica B. A., Faden A. I., Loane D. J. (2016b). NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain Behav. Immun. 58, 291–309. 10.1016/j.bbi.2016.07.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Muzik O., Shandal V., Chugani D., Chakraborty P., Chugani H. T. (2012). Evaluation of age-related changes in translocator protein (TSPO) in human brain using (11)C-[R]-PK11195 PET. J. Neuroinflammat. 9:232. 10.1186/1742-2094-9-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Stoica B. A., Sabirzhanov B., Burns M. P., Faden A. I., Loane D. J. (2013). Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging 34, 1397–1411. 10.1016/j.neurobiolaging.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois J. A., Rutland-Brown W., Wald M. M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil, 21, 375–378. 10.1097/00001199-200609000-00001 [DOI] [PubMed] [Google Scholar]
- Lawson L. J., Perry V. H., Dri P., Gordon S. (1990). Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170. 10.1016/0306-4522(90)90229-W [DOI] [PubMed] [Google Scholar]
- Lenz K. M., McCarthy M. M. (2015). A starring role for microglia in brain sex differences. Neuroscientist 21, 306–321. 10.1177/1073858414536468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley E. J., Short S. S., Liou D. Z., Singer M. B., Mirocha J., Melo N., et al. (2013). Gender impacts mortality after traumatic brain injury in teenagers. J. Trauma Acute Care Surg. 75, 682–686. 10.1097/TA.0b013e31829d024f [DOI] [PubMed] [Google Scholar]
- Liu G. J., Middleton R. J., Hatty C. R., Kam W. W., Chan R., Pham T., et al. (2014). The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol. 24, 631–653. 10.1111/bpa.12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A., Galea E., Sastre M. (2007). Molecular targets of non-steroidal anti-inflammatory drugs in neurodegenerative diseases. Cell Mol. Life Sci. 64, 1403–118. 10.1007/s00018-007-6516-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd E., Somera-Molina K., Van Eldik L. J., Watterson D. M., Wainwright M. S. (2008). Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J. Neuroinflammat. 5:28. 10.1186/1742-2094-5-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane D. J., Faden A. I. (2010). Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 31, 596–604. 10.1016/j.tips.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane D. J., Kumar A., Stoica B. A., Cabatbat R., Faden A. I. (2014a). Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 73, 14–29. 10.1097/NEN.0000000000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane D. J., Stoica B. A., Tchantchou F., Kumar A., Barrett J. P., Akintola T., et al. (2014b). Novel mGluR5 positive allosteric modulator improves functional recovery, attenuates neurodegeneration, and alters microglial polarization after experimental traumatic brain injury. Neurotherapeutics 11, 857–869. 10.1007/s13311-014-0298-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart A., Davis B., Matthews J. C., Rahmoune H., Hong G., Gee A., et al. (2003). The peripheral benzodiazepine receptor ligand PK11195 binds with high affinity to the acute phase reactant alpha1-acid glycoprotein: implications for the use of the ligand as a CNS inflammatory marker. Nucl. Med. Biol. 30, 199–206. 10.1016/S0969-8051(02)00410-9 [DOI] [PubMed] [Google Scholar]
- Mannix R., Berglass J., Berkner J., Moleus P., Qiu J., Andrews N., et al. (2014). Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. J. Neurosurg. 121, 1342–1350. 10.3171/2014.7.JNS14272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell W. L., MacKinnon M. A., Stewart J. E., Graham D. I. (2010). Stereology of cerebral cortex after traumatic brain injury matched to the Glasgow outcome score. Brain 133(Pt 1), 139–160. 10.1093/brain/awp264 [DOI] [PubMed] [Google Scholar]
- McKee A. C., Alosco M. L., Huber B. R. (2016). Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg. Clin. N. Am. 27, 529–535. 10.1016/j.nec.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A. C., Daneshvar D. H. (2015). The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 127, 45–66. 10.1016/B978-0-444-52892-6.00004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan T. M., Teasdale G. M., Weir C. J., Stewart E. (2011). Death after head injury: the 13 year outcome of a case control study. J. Neurol. Neurosurg. Psychiatr. 82, 931–935. 10.1136/jnnp.2010.222232 [DOI] [PubMed] [Google Scholar]
- Menzel L., Kleber L., Friedrich C., Hummel R., Dangel L., Winter J., et al. (2017). Progranulin protects against exaggerated axonal injury and astrogliosis following traumatic brain injury. Glia 65, 278–292. 10.1002/glia.23091 [DOI] [PubMed] [Google Scholar]
- Menzies F. M., Henriquez F. L., Alexander J., Roberts C. W. (2011). Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology 134, 281–291. 10.1111/j.1365-2567.2011.03488.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methia N., Andre P., Hafezi-Moghadam A., Economopoulos M., Thomas K. L., Wagner D. D. (2001). ApoE deficiency compromises the blood brain barrier especially after injury. Mol. Med. 7, 810–815. [PMC free article] [PubMed] [Google Scholar]
- Mittelbronn M., Dietz K., Schluesener H. J., Meyermann R. (2001). Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 101, 249–255. [DOI] [PubMed] [Google Scholar]
- Miyazawa N., Diksic M., Yamamoto Y. (1995). Chronological study of peripheral benzodiazepine binding sites in the rat brain stab wounds using [3H] PK-11195 as a marker for gliosis. Acta Neurochir. (Wien). 137, 207–216. 10.1007/BF02187195 [DOI] [PubMed] [Google Scholar]
- Morganti J. M., Jopson T. D., Liu S., Riparip L. K., Guandique C. K., Gupta N., et al. (2015). CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J. Neurosci. 35, 748–760. 10.1523/JNEUROSCI.2405-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti J. M., Riparip L. K., Rosi S. (2016). Call Off the Dog(ma): M1/M2 polarization is concurrent following traumatic brain injury. PLoS ONE 11:e0148001. 10.1371/journal.pone.0148001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohaku K., Pelton S. H., Daugherty D. J., Butler W. R., Deng W., Selvaraj V. (2014). Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 155, 89–97. 10.1210/en.2013-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J., Wynn T. A. (2011). Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 89, 557–563. 10.1189/jlb.0710409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto-Combs K., McNeal D. W., Morecraft R. J., Combs C. K. (2007). Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J. Neurotrauma 24, 1719–1742. 10.1089/neu.2007.0377 [DOI] [PubMed] [Google Scholar]
- Nagamoto-Combs K., Morecraft R. J., Darling W. G., Combs C. K. (2010). Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury. J. Neurotrauma 27, 565–585. 10.1089/neu.2009.0966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitsuji K., Hosono T., Nakamura T., Bu G., Michikawa M. (2011). Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 286, 17536–17542. 10.1074/jbc.M111.225532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor W. T., Smyth A., Gilchrist M. D. (2011). Animal models of traumatic brain injury: a critical evaluation. Pharmacol. Ther. 130, 106–113. 10.1016/j.pharmthera.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Ojo J. O., Mouzon B., Greenberg M. B., Bachmeier C., Mullan M., Crawford F. (2013). Repetitive mild traumatic brain injury augments tau pathology and glial activation in aged hTau mice. J. Neuropathol. Exp. Neurol. 72, 137–151. 10.1097/NEN.0b013e3182814cdf [DOI] [PubMed] [Google Scholar]
- Omalu B., Bailes J., Hamilton R. L., Kamboh M. I., Hammers J., Case M., et al. (2011). Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery 69, 173–183; discussion 183. 10.1227/neu.0b013e318212bc7b [DOI] [PubMed] [Google Scholar]
- Owen D. R., Matthews P. M. (2011). Imaging brain microglial activation using positron emission tomography and translocator protein-specific radioligands. Int. Rev. Neurobiol. 101, 19–39. 10.1016/B978-0-12-387718-5.00002-X [DOI] [PubMed] [Google Scholar]
- Owen D. R., Yeo A. J., Gunn R. N., Song K., Wadsworth G., Lewis A., et al. (2012). An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow Metab. 32, 1–5. 10.1038/jcbfm.2011.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V., Baraldi M., Guilarte T. R., Knudsen T. B., Lacapere J. J., Lindemann P., et al. (2006). Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 27, 402–409. 10.1016/j.tips.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Petraglia A. L., Plog B. A., Dayawansa S., Dashnaw M. L., Czerniecka K., Walker C. T., et al. (2014). The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg. Neurol. Int. 5:184. 10.4103/2152-7806.147566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan H. A., Shafi S., Parks J., Maxson R. T., Ahmad N., Murphy J. T., et al. (2007). Use of a pediatric cohort to examine gender and sex hormone influences on outcome after trauma. J. Trauma 63, 1127–1131. 10.1097/TA.0b013e318154c1b8 [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao V. L., Dogan A., Bowen K. K., Dempsey R. J. (2000). Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp. Neurol. 161, 102–114. 10.1006/exnr.1999.7269 [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh A. F., Brooks D. J., Greenwood R. J., Bose S. K., Turkheimer F. E., Kinnunen K. M., et al. (2011). Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol. 70, 374–383. 10.1002/ana.22455 [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M. (2016). A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 19, 987–991. 10.1038/nn.4338 [DOI] [PubMed] [Google Scholar]
- Rice R. A., Spangenberg E. E., Yamate-Morgan H., Lee R. J., Arora R. P., Hernandez M. X., et al. (2015). Elimination of microglia improves functional outcomes following extensive neuronal loss in the hippocampus. J. Neurosci. 35, 9977–9989. 10.1523/JNEUROSCI.0336-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. W., Gentleman S. M., Lynch A., Murray L., Landon M., Graham D. I. (1994). Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J. Neurol. Neurosurg. Psychiatr. 57, 419–425. 10.1136/jnnp.57.4.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S., Berglass J. B., Denson J. L., Berkner J., Anstine C. V., Winer J. L., et al. (2017). Microstructural and microglial changes after repetitive mild traumatic brain injury in mice. J. Neurosci. Res. 95, 1025–1035. 10.1002/jnr.23848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T. L., Nayak D., Atanasijevic T., Koretsky A. P., Latour L. L., McGavern D. B. (2014). Transcranial amelioration of inflammation and cell death after brain injury. Nature 505, 223–228. 10.1038/nature12808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G., Hellyer P. J., Ramlackhansingh A. F., Brooks D. J., Matthews P. M., Sharp D. J. (2015). Thalamic inflammation after brain trauma is associated with thalamo-cortical white matter damage. J. Neuroinflammat. 12:224. 10.1186/s12974-015-0445-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G., Ramlackhansingh A. F., Edison P., Hellyer P., Cole J., Veronese M., et al. (2016). Amyloid pathology and axonal injury after brain trauma. Neurology 86, 821–828. 10.1212/WNL.0000000000002413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj V., Stocco D. M. (2015). The changing landscape in translocator protein (TSPO) function. Trends Endocrinol. Metab. 26, 341–348. 10.1016/j.tem.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B. D., Bye N., Rancan M., Ziebell J. M., Morganti-Kossmann M. C. (2010). Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J. Cereb. Blood Flow Metab. 30, 769–782. 10.1038/jcbfm.2009.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B. D., Sadjadi R., Carlson J., Chen Y., Xu D., Ferriero D. M., et al. (2016). Long-term anesthetic-dependent hypoactivity after repetitive mild traumatic brain injuries in adolescent mice. Dev. Neurosci. 38, 220–238. 10.1159/000448089 [DOI] [PubMed] [Google Scholar]
- Shin S. S., Dixon C. E. (2015). Alterations in cholinergic pathways and therapeutic strategies targeting cholinergic system after Traumatic Brain injury. J. Neurotrauma 32, 1429–1440. 10.1089/neu.2014.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitaka Y., Tran H. T., Bennett R. E., Sanchez L., Levy M. A., Dikranian K., et al. (2011). Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exp. Neurol. 70, 551–567. 10.1097/NEN.0b013e31821f891f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solito E., Sastre M. (2012). Microglia function in Alzheimer's disease. Front. Pharmacol. 3:14. 10.3389/fphar.2012.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soustiel J. F., Palzur E., Vlodavsky E., Veenman L., Gavish M. (2008). The effect of oxygenation level on cerebral post-traumatic apoptotsis is modulated by the 18-kDa translocator protein (also known as peripheral-type benzodiazepine receptor) in a rat model of cortical contusion. Neuropathol. Appl. Neurobiol. 34, 412–423. 10.1111/j.1365-2990.2007.00906.x [DOI] [PubMed] [Google Scholar]
- Szalay G., Martinecz B., Lenart N., Kornyei Z., Orsolits B., Judak L., et al. (2016). Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 7:11499. 10.1038/ncomms11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki J., Fujimori K., Miura M., Suzuki T., Sekino Y., Sato K. (2012). L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: the ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J. Neuroinflammat. 9:275. 10.1186/1742-2094-9-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchantchou F., Zhang Y. (2013). Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J. Neurotrauma 30, 565–579. 10.1089/neu.2012.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal S. C., Heinemann M., Luh C., Pieter D., Werner C., Engelhard K. (2011). Pioglitazone reduces secondary brain damage after experimental brain trauma by PPAR-gamma-independent mechanisms. J. Neurotrauma 28, 983–993. 10.1089/neu.2010.1685 [DOI] [PubMed] [Google Scholar]
- Tobinick E., Kim N. M., Reyzin G., Rodriguez-Romanacce H., DePuy V. (2012). Selective TNF inhibition for chronic stroke and traumatic brain injury: an observational study involving 629 consecutive patients treated with perispinal etanercept. CNS Drugs 26, 1051–1070. 10.1007/s40263-012-0013-2 [DOI] [PubMed] [Google Scholar]
- Tran H. T., LaFerla F. M., Holtzman D. M., Brody D. L. (2011). Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J. Neurosci. 31, 9513–9525. 10.1523/JNEUROSCI.0858-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truettner J. S., Bramlett H. M., Dietrich W. D. (2016). Posttraumatic therapeutic hypothermia alters microglial and macrophage polarization toward a beneficial phenotype. J. Cereb. Blood Flow Metab. 271678X16680003. 10.1177/0271678x16680003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L. N., Morohaku K., Manna P. R., Pelton S. H., Butler W. R., Stocco D. M., et al. (2014). Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J. Biol. Chem. 289, 27444–27454. 10.1074/jbc.M114.578286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtzo L. C., Lescher J., Janes L., Dean D. D., Budde M. D., Frank J. A. (2014). Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J. Neuroinflammation 11:82. 10.1186/1742-2094-11-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburski A. L., Cheng L., Assari S., Darvish K., Elliott M. B. (2017). Frequent mild head injury promotes trigeminal sensitivity concomitant with microglial proliferation, astrocytosis, and increased neuropeptide levels in the trigeminal pain system. J. Headache Pain 18:16. 10.1186/s10194-017-0726-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S., Wagner A. K., Wang G., Slagel S. L., Chen X., Lopresti B. J., et al. (2007). The high affinity peripheral benzodiazepine receptor ligand DAA1106 binds specifically to microglia in a rat model of traumatic brain injury: implications for PET imaging. Exp. Neurol. 207, 118–127. 10.1016/j.expneurol.2007.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese P. B., Castellano J. M., Holtzman D. M. (2011). Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol., 10, 241–252. 10.1016/S1474-4422(10)70325-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile A. R., Atkinson L. (2017). Chronic traumatic encephalopathy: the cellular sequela to repetitive brain injury. J. Clin. Neurosci. 41, 24–29. 10.1016/j.jocn.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Walker P. A., Bedi S. S., Shah S. K., Jimenez F., Xue H., Hamilton J. A., et al. (2012). Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population. J. Neuroinflammat. 9:228. 10.1186/1742-2094-9-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Shi Y., Jiang X., Leak R. K., Hu X., Wu Y., et al. (2015). HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc. Natl. Acad. Sci. U.S.A. 112, 2853–2858. 10.1073/pnas.1501441112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zhang J., Hu X., Zhang L., Mao L., Jiang X., et al. (2013). Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 1864–1874. 10.1038/jcbfm.2013.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yue X., Kiesewetter D. O., Niu G., Teng G., Chen X. (2014). PET imaging of neuroinflammation in a rat traumatic brain injury model with radiolabeled TSPO ligand DPA-714. Eur. J. Nucl. Med. Mol. Imaging 41, 1440–1449. 10.1007/s00259-014-2727-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C., Engelhard K. (2007). Pathophysiology of traumatic brain injury. Br. J. Anaesth. 99, 4–9. 10.1093/bja/aem131 [DOI] [PubMed] [Google Scholar]
- Xiong Y., Mahmood A., Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142. 10.1038/nrn3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Nguyen J. V., Lehar M., Menon A., Rha E., Arena J., et al. (2016). Repetitive mild traumatic brain injury with impact acceleration in the mouse: multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp Neurol 275(Pt 3), 436–449. 10.1016/j.expneurol.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Xue J., Schmidt S. V., Sander J., Draffehn A., Krebs W., Quester I., et al. (2014). Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288. 10.1016/j.immuni.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I., Inaji M., Maeda J., Okauchi T., Nariai T., Ohno K., et al. (2010). Glial cell-mediated deterioration and repair of the nervous system after traumatic brain injury in a rat model as assessed by positron emission tomography. J. Neurotrauma 27, 1463–1475. 10.1089/neu.2009.1196 [DOI] [PubMed] [Google Scholar]
- Zanier E. R., Marchesi F., Ortolano F., Perego C., Arabian M., Zoerle T., et al. (2016). Fractalkine receptor deficiency is associated with early protection but late worsening of outcome following Brain Trauma in mice. J. Neurotrauma 33, 1060–1072. 10.1089/neu.2015.4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanier E. R., Pischiutta F., Riganti L., Marchesi F., Turola E., Fumagalli S., et al. (2014). Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma. Neurotherapeutics 11, 679–695. 10.1007/s13311-014-0277-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Puvenna V., Janigro D. (2016). Frontiers in neuroscience: biomarkers of traumatic brain injury and their relationship to pathology, in Translational Research in Traumatic Brain Injury, eds Laskowitz D., Grant G. (Boca Raton, FL: CRC Press; /Taylor and Francis Group; ), Chapter 12, 1–13. [PubMed] [Google Scholar]
- Zhang Z., Zhang Z. Y., Wu Y., Schluesener H. J. (2012). Immunolocalization of Toll-like receptors 2 and 4 as well as their endogenous ligand, heat shock protein 70, in rat traumatic brain injury. Neuroimmunomodulation 19, 10–19. 10.1159/000326771 [DOI] [PubMed] [Google Scholar]
- Ziebell J. M., Morganti-Kossmann M. C. (2010). Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7, 22–30. 10.1016/j.nurt.2009.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell J. M., Taylor S. E., Cao T., Harrison J. L., Lifshitz J. (2012). Rod microglia: elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. J. Neuroinflammat. 9:247. 10.1186/1742-2094-9-247 [DOI] [PMC free article] [PubMed] [Google Scholar]