Abstract
Peripartum hormones and sensory cues from young modify the maternal brain in ways that can render females either at risk for, or resilient to, elevated anxiety and depression. The neurochemical systems underlying these aspects of maternal emotional and mood states include the inhibitory neurotransmitter GABA and the neuropeptide oxytocin (OXT). Data from laboratory rodents indicate that increased activity at the GABAA receptor contributes to the postpartum suppression of anxiety-related behaviour that is mediated by physical contact with offspring, whereas dysregulation in GABAergic signalling results in deficits in maternal care, as well as anxiety- and depression-like behaviours during the postpartum period. Similarly, activation of the brain OXT system accompanied by increased OXT release within numerous brain sites in response to reproductive stimuli also reduces postpartum anxiety- and depression-like behaviours. Studies of peripartum women are consistent with these findings in rodents. Given the similar consequences of elevated central GABA and OXT activity on maternal anxiety and depression, balanced and partly reciprocal interactions between these two systems may be essential for their effects on maternal emotional and mood states, in addition to other aspects of postpartum behaviour and physiology.
Keywords: anxiety, depression, GABA, maternal, oxytocin
Introduction
Some of the most dramatic behavioural adaptations seen in adult mammals occur across the transition from late pregnancy to the early postpartum period. For example, by the time females give birth, any aversion toward neonates is replaced by avidity and relative passivity is supplanted by pugnaciousness (1–6). A critical influence on these and probably most other peripartum behavioural adaptations is the mother's emotional and mood states. It has long been thought that emotional hyper-reactivity in laboratory animals, including high anxiety-related behaviours, can interfere with maternal caregiving and aggression (1,7). More recently, some attention has been paid to the possibility that emotional hyporeactivity, involving abnormally low anxiety or possibly high depression-like behaviours, may be equally detrimental to rodent mothering (8,9). In humans, emotional and mood states that are neither too high (e.g. anxiety, mania), nor too low (e.g. lack of concern, depression) are similarly optimal for mothers to best attend to the needs of their infants (10,11).
A plethora of neurochemicals including steroid hormones, neuropeptides and classic neurotransmitters, fluctuates across late pregnancy and the early postpartum period to regulate maternal physiology, caregiving behaviours, cognition, emotions and mood (12–18). Our purpose here is not to exhaustively review aspects of these literatures instead, to focus on the studies explicating how maternal anxiety- and depression-related behaviours in laboratory rodents are influenced by central nervous system activity of the inhibitory neurotransmitter, GABA, and the neuropeptide oxytocin (OXT). We also highlight some of the many compelling studies implicating these neurochemical systems in regulating anxiety and depression in human mothers. Because it will be clear that elevated GABA and OXT signalling can each reduce symptoms of anxiety and/or depression in both nonhuman animals and humans, we conclude by proposing that the balanced activity of these two systems, and perhaps reciprocal interactions between them, may be essential for their positive effects on maternal emotions and mood.
Anxiety and depression across pregnancy and the early postpartum period
Humans
Anxiety
There are considerable individual differences in the trajectory of anxiety across pregnancy and the postpartum period in women because it is strongly influenced by social and experiential factors. Nevertheless, for many women, these times of the reproductive cycle fortunately involve either little change or even a reduction in their pre-existing anxiety symptoms (19–21). A variety of hormones and other neuro-chemicals released at parturition and when human mothers receive suckling or other tactile inputs from their infants, including GABA and OXT, are presumed to help protect against elevated anxiety (13,22–24). Unfortunately, not all postpartum women are so well protected. A recent review of the literature revealed that rates of diagnoses for obsessive–compulsive disorder [OCD; characterised by obsessive or intrusive thoughts and compulsive behaviours that interfere with everyday life; Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV] (25) and generalised anxiety disorder (GAD; characterised by excessive and irrational worry for longer than 6 months) (25) are higher in pregnant or postpartum women compared to the general population (rates of OCD = 3–4% versus 1–2%; GAD = 4–8% versus 3–4%) (26). These increases partly reflect both the higher susceptibility for relapse in women who are particularly at-risk for anxiety disorders due to their history of high anxiety (19,21,27) and cases of new onset of OCD during the peripartum period (26). Elevation in these anxiety disorders is of particular concern because they produce tremendously detrimental effects on mothers' general well-being, their ability to provide sensitive caregiving, and numerous aspects of infant development (28,29). Importantly, the lack of regular screening for anxiety in the peripartum population and an emphasis of public mental health campaigns on peripartum depression lead to significant underreporting of women's anxiety symptoms (30). Consequently, the problem of high peripartum anxiety is often considered to be much greater than it may at first appear, with subclinical and clinical elevations perhaps affecting up to 20–30% of women (26,27,31,32).
Depression
Depressive symptoms are also influenced by reproductive events in women. Postpartum ‘blues’, a transient and mild condition characterised by mood disturbances beginning a few days after parturition and lasting less than 2 weeks (therefore not fulfilling the time criterion for major depression), is extremely common, with prevalence estimates suggesting that it affects up to 84% of childbearing women (33,34). Research on postpartum depression, however, is particularly hampered by inconsistency of its definition with regard to the time criterion of the maximum interval between parturition and onset of the major or minor depressive episode. The definitions have ranged from 4 weeks (DSM-IV, specifier: depressive episode ‘with postpartum onset’) (25), to over 6 weeks [International Classification of Diseases (ICD)-10, depression specifier: ‘as associated with the puerperium’] (35), and up to 12 months (‘perinatal depression’) (36). Given this heterogeneity in postpartum depression criteria, the multitude of instruments used to assess it, and the lack of differentiation between point-, 1-month or other period prevalences, it is probably not surprising that reported rates of peripartum depression vary quite widely across studies.
Nonetheless, meta-analyses indicate that the point prevalence of depression is highest in the third month postpartum (but based on large confidence intervals of the estimates) and that the incidence of depression (i.e. the percentage of women with depressive episodes that begin during pregnancy or postpartum) is comparable between pregnancy and the first 3 months postpartum. This notably underscores the relevance of the postpartum period as a time of increased risk for depression onset because the duration of pregnancy is approximately 300% longer than the first 3 months postpartum (36,37). Additionally, when directly comparing samples of childbear-ing women from conception until 12 months postpartum with mothers who had not recently given birth to their children, there were no substantial differences in depression prevalence and incidence estimates, with the exception of one study indicating an increased risk of newly developing depression during the first 5 weeks postpartum (36,38). Initially, this contradictory picture may be explained not only by mere inconsistencies between studies, but also by a longer history of depression in the nonrecent mothers, whose depression onset was triggered by their longer-ago deliveries (38). Similar to anxiety, women with a pre-existing mood disorder (depression or bipolar disorder) are especially at risk for relapse of symptoms during the first few weeks after giving birth (39–41). Also similar to anxiety, depression is not regularly screened for and surely under-reported in the peripartum population, so even though the risk for depressive episodes during the postpartum period does not appear to be considerably increased compared to nonchildbearing times, postpartum depression is without doubt a common condition.
A neuroendocrine basis for postpartum depression involving the withdrawal of hormones at parturition has often been presumed, so it is perhaps surprising that adoptive mothers of infants or toddlers also show higher depressive symptoms soon after becoming a parent (42,43). Depression is also common for new fathers (44). Endocrine flux can occur in nonparturient humans when they become parents and interact with infants (45), although the changes are certainly less dramatic than those occurring in recently parturient women. Thus, both non-endocrine factors (e.g. environmental and personality factors) and endocrine factors even unrelated to reproductive state are relevant for depression in new human parents.
Laboratory rodents
Anxiety-like behaviours
The temporal progression of anxiety-related behaviour in most reproductive female laboratory rats is generally characterised by an increase during some points of pregnancy followed by a postpartum decrease to levels that fall even below those seen in cycling virgins. Specifically during pregnancy, anxiety is higher during the second week and again a few days before parturition compared to before pregnancy or during very early pregnancy (46–50). There are a few reports, however, showing no difference between late-pregnant and nulliparous rats in their anxiety-related behaviour (51,52) or a decrease in anxiety on some days of the last week of pregnancy (53,54). There may also be species differences in anxiety profiles across pregnancy because both early and late pregnancy are associated with reduced responses to novelty in ewes (55).
Regardless of the reported changes in anxiety during pregnancy, most studies find that, soon after giving birth, most female rodents and sheep show lower indices of anxiety compared to nulliparous females (12,13,52). The anxiolytic effect of current maternal state in primiparous rats appears to last only through the first postpartum week and requires recent physical contact with the pups (either suckling or nonsuckling) (56). Importantly, not every mother rat responds to offspring touch with a reduction in anxiety because dams with the lowest trait anxiety are more anxious after having spent time with their litter, indicating important individual differences in the factors influencing postpartum anxiety, which can be overlooked when examining mothers as a whole (9). Importantly, unlike recently parturient female rats that as a whole show relatively low anxiety, pseudopregnant rats that are ovariectomised and virgins given a regimen of pregnancy-like ovarian hormones that is rapidly terminated show either increased (57), decreased (58) or unchanged (59) anxiety-related behaviour. Such results make the obvious point that these experimental models do not accurately mimic the endocrine, sensory and neural events occurring with natural pregnancy and parturition.
Depression-like behaviours
A burgeoning but still relatively small animal literature indicates that depression-like behaviours in female rodents (most often tested in the forced swim and sucrose preference tests) are particularly low during the beginning of the third week pregnancy, but that these behaviours mostly do not differ among females tested at the very end of pregnancy, during the early postpartum period, or when nulliparous (47,50,60–65). These studies can be somewhat difficult to interpret because the depression paradigms used do not take into consideration the pregnancy-specific physiological and behavioural alterations, including increased body surface and body fat relevant for interpreting behaviour in the forced swim test or altered food demands relevant for the sucrose preference test, and so they might not provide a uncomplicated reflection of depression-like behaviour. Studies using tests of saccharin rather than sucrose preference may avoid the metabolic issues and, indeed, pregnant and lactating rats have been observed to have a lower preference for saccharin than do virgins (66). This is consistent with the findings that late pregnant and early postpartum female rats also have reduced interest in other nonpup rewards, such as cocaine (67,68).
Rodent models attempting to mimic the endocrine flux of parturition by using ovariectomised nulliparous females treated with progesterone and/or oestradiol followed by their abrupt withdrawal show increased or occasionally decreased depression-like behaviours (59,69–73). Valuable insight can be gained by the fact that the depression-like behaviours (and anxiety-like behaviours; see above) observed in most steroid-hormone withdrawal studies are dissimilar to that found in naturally postpartum animals. It is likely that other neurochemicals changing during the very early postpartum period in response to interactions with neonates (including GABA, OXT and prolactin) prevent any increase in anxiety- and depression-like behaviours in parous females after physiologically occurring steroid withdrawal (14,74,75). Furthermore, the rapid withdrawal of progesterone in most pharmacological steroid studies is temporally dissimilar to the less-abrupt progesterone decline normally occurring at parturition. This is relevant because slow progesterone withdrawal elicits less dramatic changes in females' anxiety-related behaviours compared to what is found after the hormone's sudden absence (76,77) and the same may be true for depression-like behaviors.
Modification of central GABA release during the peripartum period
GABA is the primary inhibitory neurotransmitter in the brain and there is a vast scientific literature on its involvement in anxiety in humans and other animals (78,79). GABA acts on at least three distinct transmembrane receptors, the ionotropic GABAA receptor (GABAAR) that is comprised of five of up to 19 receptor subunits, a metabotropic GABAB receptor existing as hetero- or homodimers comprised of two receptor subunits, and the relatively poorly studied ionotropic GABAC receptor that is similar to the GABAAR in that it is also made from five subunits. Activity of GABAARs is traditionally considered to be of the utmost importance for modulating anxiety, although there is emerging evidence for a role of the GABAB receptor (80,81). Expectedly, the density of GABAARs and expression of its receptor subunits most relevant for anxiety are particularly high in brain regions underlying anxiety (82,83).
Humans
It is intuitive that changes in central GABA release would be related to changes in anxiety across the peripartum period in humans and nonhumans animals, although there is a paucity of work on this, especially in women. Women's cerebrospinal fluid (CSF) levels of GABA have been seen to drop during late pregnancy (24) and increase during labour (84), although the importance of such changes for women's anxiety is questionable because the relationship between CSF GABA and general anxiety in humans is often not significant (85–87). Determining GABA concentrations and their effects on GABAARs in specific brain regions would probably be more fruitful for understanding peripartum changes in human anxiety. The only study to do so used proton magnetic resonance spectroscopy (1H-MRS) and found that GABA levels in the occipital cortex were lower in postpartum women (up to 6 months after parturition) compared to women who had not recently given birth and examined during the follicular phase of the menstrual cycle (88). The postpartum women who were studied closest to parturition tended to have the lowest occipital GABA levels. The occipital cortex is not typically implicated in depression, and at no time point were the women's occipital GABA levels related to the presence of postpartum depression (anxiety was not assessed), so the relevance of this finding for peripartum mood or emotional regulation is unknown. Nonetheless, this study demonstrates that reproductive state affects cortical GABA in women and it will be important in future studies to examine cortical and subcortical (especially limbic) regions more closely associated with anxiety or depression.
Laboratory rodents
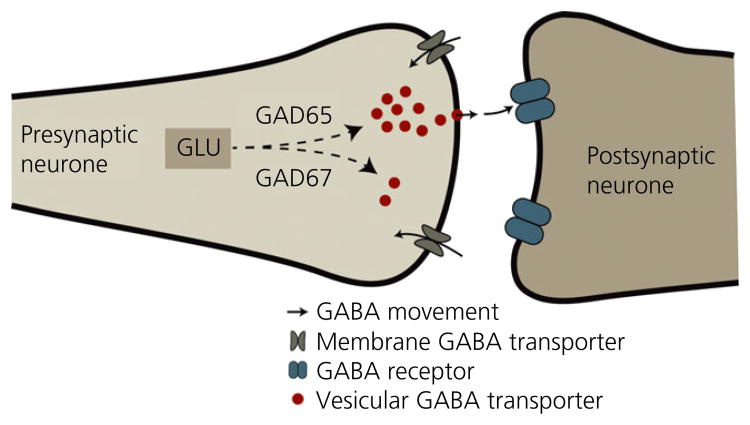
In laboratory rats, CSF concentrations of GABA are high in lactating rats that interact with pups, but are almost nondetectable in dams whose pups have been removed for as little as 6 h. The high GABA levels are restored after mothers and pups are reunited (89). Because anxiety-related behaviours in postpartum rats are not affected by ovariectomy, hypophysectomy or inhibiting steroidogenesis (56,90,91), the postpartum increase in GABA levels is presumably not mediated by the mother's current endocrine state but is instead is the result of rapid GABA release when mothers interact with offspring. Pregnancy and later interaction with offspring influence GABA synthesis and release in numerous specific forebrain sites involved in postpartum behaviour. In ewes, parturition and subsequent interaction with lambs increases maternal GABA release in the olfactory bulbs, medial preoptic area and bed nucleus of the stria terminalis (92,93). Altered GABA synthesis, as indicated by expression of glutamate decarboxylase (GAD), is also found in the late-pregnant and early postpartum rat olfactory system (94,95) and in the mouse rostral lateral septum (96). Baseline GABA release is lower in the basolateral amygdala of pregnant rats compared to cycling rats, which may disinhibit amygdalar output neurones and result in the increased anxiety observed during some points of pregnancy (97). In the cerebral cortex, late pregnancy and the early postpartum period in mice is associated with reduced GABA turnover compared to nulliparous females (98), but postpartum laboratory rats have higher basal GABA release and turnover in the medial frontal cortex compared to virgins (99,100). The latter finding is consistent with recent data indicating that the medial frontal cortex of postpartum rats has higher expression of both the 65-kDa molecular weight isoform of GAD (GAD65) and the vesicular GABA transporter compared to dioestrous virgins, suggesting greater potential for cortical GABA synthesis and release in mothers (101) (Fig. 1).
Fig. 1.
Highly schematic representation of neuronal GABA synthesis and release. GABA is synthesised from glutamate (GLU) by two isoforms of glutamate decarboxylate (GAD). GAD67 is found throughout the cytoplasm and produces the pool of GABA necessary for intracellular functions, whereas GAD65 is more localised to neuronal terminals and synthesises the majority of the GABA that will be packaged by vesicular GABA transporters into synaptic vesicles for release into the synapse.
Modification of central GABAA receptors during the peripartum period
Concomitant with the above-mentioned changes in central GABA synthesis and release across pregnancy and the early postpartum period, many studies in laboratory rodents have demonstrated dramatic plasticity of GABAARs and their capacity to mediate GABAergic inhibition. There is a significant increase in the affinity of total forebrain GABAARs for GABA in mid-to-late pregnant rats compared to cycling females, with a further increase in postpartum females despite the fact that they have reduced receptor density (102). The cortex must not drive this change in affinity because its GABAARs have been reported to have decreased affinity for their ligand during late pregnancy (103). Brain sites contributing to the postpartum reduction in total forebrain GABAAR density are also unknown because [3H]flunitrazepam and [3H]muscimol binding (indicating the densities of benzodiazepine and GABAA receptor binding sites, respectively) in numerous forebrain regions involved in anxiety-related behaviours do not differ among virgin, pregnant and early postpartum female laboratory rats (104,105).
As noted above, GABAARs are comprised of five of up to 19 known potential receptor subunits, and the expression of many of these subunits is affected by female reproductive state (106,107). Peripartum changes in the expression of specific GABAAR subunits reflect a homeostatic mechanism that maintains ideal levels of inhibition in the face of elevated neurosteroids. Given that neurosteroids can act as positive allosteric modulators on GABAARs to enhance GABAergic inhibition and, at high levels, even directly gate these receptors, increased neurosteroid levels such as that occurring during pregnancy have the potential to dramatically alter GABAergic signalling. Remarkably, plasma and cerebral progesterone levels increase by almost 200-fold during pregnancy and this is accompanied by elevations in its metabolites allopregnanolone and tetrahydrodeoxycorticosterone (108). These neurosteroid concentrations during pregnancy can be sufficient to cause sedation in nonpregnant animals (109). Thus, GABAAR plasticity during pregnancy is necessary to offset these incredibly elevated neurosteroid concentrations. Considerable attention has been given in this context to expression of the GABAAR δ subunit, which is uniquely sensitive to neurosteroid modulation (110,111). δ subunit expression is down-regulated in the hippocampus during late pregnancy, and rebounds upon restoration of ovarian hormone/neurosteroid levels the early postpartum period (64,112–114). In addition to maintaining normal GABAAR-mediated inhibition in the hippocampus in the face of elevated neurosteroids during pregnancy, plasticity of the δ subunit also occurs through the first postpartum week, when neurosteroid levels are quite low (103). During this time, we found that δ subunit expression is also up-regulated in the midbrain periaqueductal gray (PAG; considered to be a ‘final common pathway’ for anxiety and fearful behaviours and is also highly sensitive to tactile inputs from offspring) (115) in the face of what appears to be decreased GABA synthesis and release (101). Together, these changes may modify tonic inhibition of GABAergic interneurones in the PAG to help blunt anxiety- or fear-related behaviours in new mothers (116).
There are only a few studies of peripartum changes in GABAB receptor signalling. GABAB subunit expression in the hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei does not significantly differ between virgin and lactating rats (117), although examining other sites could still be an interesting endeavour given this receptor's emerging role in anxiety and depression (80,81) and that GABAB receptor signalling affects other postpartum processes in rats such as maternal behaviour and suckling-induced milk letdown (118,119).
GABAergic signalling and maternal anxiety- and depression-like behaviours
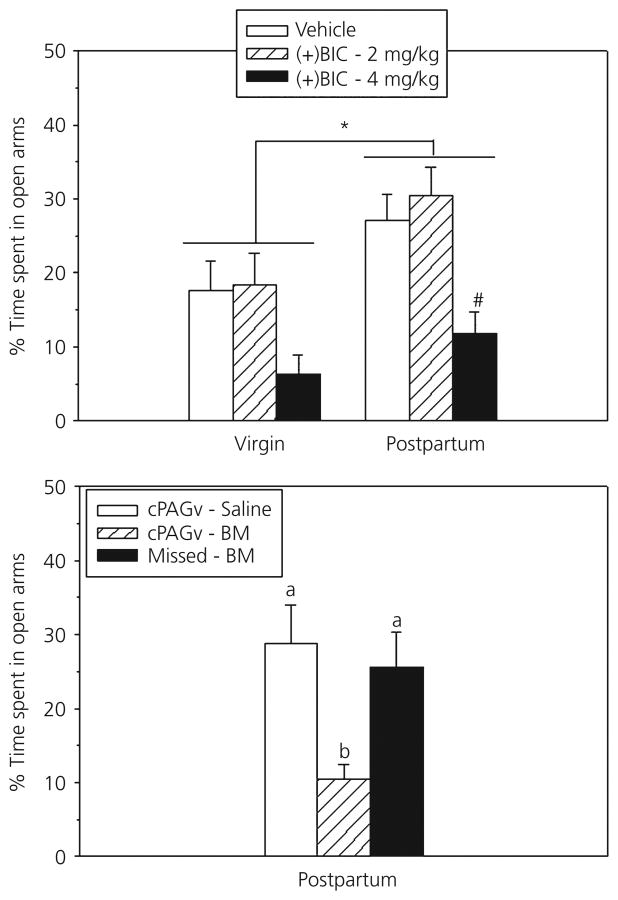
The homeostatic plasticity described above would require fine-tuned alterations in GABA release, GABAAR activation, GABAAR subunit expression, and GABAAR affinity and density. Disruptions of this balanced regulatory process has been proposed to contribute to abnormal anxiety- and depression-like behaviours during the postpartum period (107,113). In support, treating postpartum rats with GABAAR antagonists, including bicuculline and pentylenetetrazol, prevents their normal anxiolytic phenotype (120-122). Because nulliparous females are often at or near a ceiling for anxiety behaviours, these antagonists have considerably less of an effect on them (121,122). The specific brain sites mediating the anxiogenic effects of GABAAR antagonism in dams include the ventrocaudal periaqueductal gray (cPAGv), where bicuculline infusion increases anxiety in mothers to levels typical of nulliparous rats (121) (Fig. 2). By contrast, the ventromedial hypothalamus and amygdala do not appear to be sites where GABAAR activity is involved in postpartum anxiolysis (123).
Fig. 2.
Top: Peripheral injection of a higher dose of the GABAA receptor antagonist bicuculline (+BIC) reduces the percentage of time female rats spend in the open arms of the elevated plus maze, with a greater effect in postpartum rats, as indicated by the hash symbol (#). This peripheral effect of bicuculline in dams is reproduced by site-selectively infusing bicuculline methiodide (BM) into the ventrocaudal periaqueductal gray (cPAGv), but not if the infusions missed the cPAGv. *Main effect of reproductive state. Adapted from Miller et al. (121).
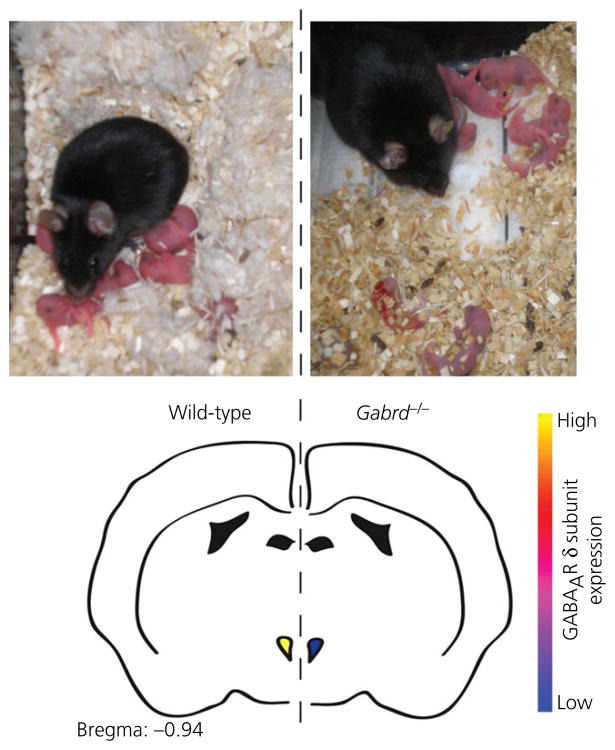
Failure to properly regulate central GABAAR subunit expression during pregnancy and postpartum has been implicated in the pathophysiology of postpartum emotional and mood dysregulation. Mice deficient in the GABAAR δ subunit (Gabrd-/- mice) exhibit increased depression-like behaviours during the first few days postpartum, as indicated by increased passive coping in the forced-swim test and a lower preference for sucrose over water compared to wild-type females. They also exhibit more behaviours indicative of anxiety (digging, burrowing and circling) when exposed to a novel environment (64). Importantly, such differences are not seen between virgin Gabrd-/- and wild-type females or males (64,124), and so they may be specific to sex and reproductive state. Gabrd-/- dams are also inadequate mothers and fail to build nests or retrieve scattered pups (Fig. 3). As a result, pups born to Gabrd-/- mothers are more likely to die as a result of cannibalism and/or neglect. Cross-fostering experiments confirm that the increased mortality rate is a result of the abnormal maternal behaviours of Gabrd-/- mice rather than the characteristics of the pups or an interaction between the two factors (64). Abnormal mothering is also seen in heterozygous Gabrd-/- mice and can be rescued by selective agonism of GABAARs that contain δ subunits (64).
Fig. 3.
Top: Gabrd-/- mice exhibit deficits in maternal care, including failure to build a nest and keep the pups in close proximity (right) compared to wild-type dams (left). Bottom: schematic representation of the loss of the GABAAR δ subunit in corticotrophin-releasing hormone neurones in the paraventricular nucleus of Gabrd-/- mice, which is associated with elevated stress reactivity, depression-like behaviours and the deficits in maternal care Adapted from Maguire and Mody (64) and Sakar et al. (226), as well as J Maguire and I. Mody, unpublished data
These data suggest that the inability to regulate δ subunit-containing GABAARs results in increased anxiety- and depression-like behaviours and abnormal mothering restricted to the postpartum period. Because interactions with pups reduce anxiety- and depression-like behaviours in rats (56,74), it could be possible that the reduced maternal behaviour in the Gabrd-/- or Gabrd-/- mice contributes to their increased anxiety- and depression-like behaviours. In addition, Gabrd-/- mice exhibit higher hypothalamic-pituitary-adrenal (HPA) axis reactivity to stress as a result of the loss of GABAergic control of corticotrophin-releasing hormone (CRH) cells in the PVN (Fig. 3) (125). Dysregulation of the HPA axis has been implicated in the pathophysiology of depression in nonpostpartum (126,127) and postpartum humans (14,128). Indeed, blocking CRH signalling with antalarmin from days 14-21 of pregnancy in Gabrd-/-mice decreases their depression-like behaviours postpartum and increases the survival rate of their pups (J. Maguire and I. Mody, unpublished data).
Modifications of central OXT during the peripartum period
Humans
One of the hallmarks of parturition and lactation is elevated OXT release. This occurs from the neurohypophysis into the general circulation, which facilitates parturition and milk ejection, and also is thought to occur in all mammals centrally within hypothalamic and imbic brain regions that influence maternal behaviour, anxiety- and depression-related behaviours, as well as stress coping (12,15). Comparable neurobiological mechanisms are assumed to underlie human and rodent maternal adaptations, although our information regardng changes in the OXT system in human mothers comes only from assessments of OXT in blood or CSF. Plasma OXT levels may gradually ncrease during the course of pregnancy, although some reports are inconsistent with this observation (129,130). This may be because single-point assessments of plasma OXT concentrations greatly vary between subjects as a result of rhythmic or episodic OXT release superimposed on their tonic OXT release, leading to short-term OXT fluctuations consistent with the short half-life of OXT in the peripheral circulation (129). In a study assessing OXT concentrations immediately following normal vaginal delivery, OXT levels were elevated at 15, 30 and 45 min after delivery, and returned to prepartum levels by 60 min (131). During the postpartum period, plasma OXT concentrations are mostly driven by suckling-induced bursts of OXT released during breast-feeding, with no baseline differences between breastfeeding and nonbreastfeeding women (132). With regard to the CSF, it has been suggested that its OXT may reflect centrally released OXT from dendrites and perikarya within the hypothalamus or from axon terminals in other limbic regions (15,133). In pregnant women, CSF OXT concentrations do not appear to be higher than in nonpregnant women, although there is some evidence for a rise in CSF OXT levels during labour (24,134,135).
Laboratory rodents
The much larger literature from laboratory rodents reveals that OXT synthesis is elevated within the SON and PVN (the primary neuronal sources of OXT) during the peripartum period beginning with late pregnancy (136,137). Local release of OXT within the SON and PVN, septum, preoptic area, bed nucleus of the stria terminalis and olfactory bulb is strongly stimulated by acute pelvic and other sensory stimuli received during parturition, and later by the suckling and other tactile inputs that mothers receive from their offspring (93,138,139). Such contemporaneous release of central and peripheral OXT helps ensure coordination among maternal physiological and behavioural functions requisite for offspring survival (14,140). These reproduction-related events also stimulate other neurochemical systems such as GABA and prolactin, which are up-regulated during the peripartum period (141,142). Thus, OXT is likely to act in concert with these systems to ensure not only birth, milk ejection and maternal care, but also the finely-tuned peripartum changes in anxiety- and depression-related behaviours.
In addition to OXT release, OXT receptor (OXTR) mRNA in rodents is elevated in the lateral septum, amygdala and medial preoptic area during pregnancy, and within the olfactory bulb, BNST and ventromedial hypothalamus during parturition (143,144). Consistent with these increases in mRNA expression, elevated OXTR binding is found in most of these brain regions (145). Dramatic alterations in circulating ovarian steroids, including progesterone and oestrogens, before parturition underlie the changes in OXT and OXTR expression in some brain regions (58).
OXTR-mediated signalling and maternal anxiety-related behaviour
Humans
There has been considerable interest in elucidating the role of OXT for the aetiology of anxiety disorders and other mental processes in humans (146,147). Advancing knowledge in this field is hampered by the fact that OXT concentrations in human plasma may or may not reflect brain OXT activity and therefore have to be interpreted with caution (15,147,148). Similarly, in human studies, OXT is often administered intranasally, although ultimate evidence regarding its uptake into the brain is lacking (149) but see in rats and mice (150). There are also sex differences in the relationships between OXT signalling and emotional processing in humans (151–153), emphasising that caution is warranted when extrapolating data obtained in men to women of any reproductive state. With these caveats in mind, there is a negative association between plasma OXT and aspects of anxiety in peripartum women (154,155), which can be associated with breastfeeding and physical contact with the infant (23). Fascinatingly, an association between OXT and anxiety was not found in a large sample of women who were not recently parturient (156), and so the relationship between OXT and anxiety even differs across reproductive state within sex.
Laboratory rodents
Similar to humans, the anxiolytic properties of OXT in laboratory rats and mice depends on sex and reproductive state. Acute i.c.v. administration of synthetic OXT produces inconsistent effects on anxiety-related behaviour, probably because it depends on the basal stress level of the animal. Thus, in male mice that received an i.c.v. infusion of OXT within hours after stereotaxic surgery, an anxiolytic effect is found compared to vehicle-treated surgery-stressed mice (157), whereas no such effect is found in unstressed rats tested 5 days after implantation of a guide cannula (158,159). Importantly, local infusions of OXT into either the central amygdala (140,160,161) or PVN (159,162) consistently produce anxiolytic effects in male and virgin female rats or mice. Interestingly, an involvement of endogenous brain OXT on anxiety is only found under conditions of elevated brain OXT system activity. Such a condition is a given during the peripartum period when there is high availability of OXT and its receptor in the brain (52,159). Thus, blockade of OXTR-mediated effects by i.c.v. infusion of an OXTR antagonist has anxiolytic effects in pregnant and lactating rats but not in virgin females (52). Similarly, in males, such anxiolytic properties of brain OXT are only found after mating-induced stimulation of central OXT release (163). Furthermore, chronically infusing synthetic OXT using osmotic minipumps to increase brain OXT availability in virgin, ovariectomised steroid-primed rats attenuates their emotional and neuronal responses to an acute noise stress (164,165). In mothers, one of the many sites other than the amygdala and PVN where brain OXT acts to produce its anxiolytic effect is the midbrain cPAGv. Antagonism of OXTRs in the cPAGv increases dams' anxiety-related behaviours on the elevated plus maze, whereas infusion of OXT restores low anxiety in mothers that have not recently been in physical contact with their pups (166).
The OXTR-mediated intraneuronal signalling cascades underlying the anxiolytic effect of OXT acting at least within the PVN include the mitogen-activated protein (MAP) kinase pathway. This pathway is activated within 5–10 min after OXT administration to male and virgin female rats, as indicated by increased phosphorylation of various kinases, including MAP kinase kinase (MEK). Pharmacological blockade of the MAP kinase (MAPK) cascade (i.e. blockade of MEK kinase activity) prevented the anxiolytic effect of OXT administered into the PVN of male and virgin female rats, demonstrating that OXTR-mediated activation of the MAP kinase pathway, specifically phosphorylation (p) of extracellular signal-regulated kinase (ERK) (males) (162) and MEK1/2 (females) (159) is essential for acute OXT effects on anxiety. Importantly, and in agreement with the increased presence of OXT in the local extracellular fluid as a result of pup-related stimuli and increased intra-PVN release during lactation, there is a general up-regulation of the MAPK pathway in the postpartum PVN: cytosolic pMEK1/2 levels are approximately 25% higher on postpartum day 8 compared to what is found in virgins. These high pMEK levels and subsequent nuclear translocation of pERK1 are necessary for the anxiolytic phenotype typically observed during lactation (159) because blockade of the MAPK pathway results in increased anxiety-related behaviours in dams but not virgins. Another specific peripartum adaptation in this system is that that further elevation of OXT availability by local infusion of synthetic OXT bilaterally into the PVN of postpartum rats does not result in further increase in MEK1/2 phosphorylation and/or produce the anxiolytic effect seen in male and virgin female rats (159,162). Thus, the MAPK pathway is an important intracellular mechanism activated by high extracellular OXT in the PVN and probably elsewhere in the brain to mediate postpartum anxiolysis. In this context, it is worth noting that this pathway, which is also activated by OXT in the hippocampus during lactation, has been related to improved spatial memory in postpartum mice (167).
OXTR-mediated signalling and maternal depression-related behaviour
Humans
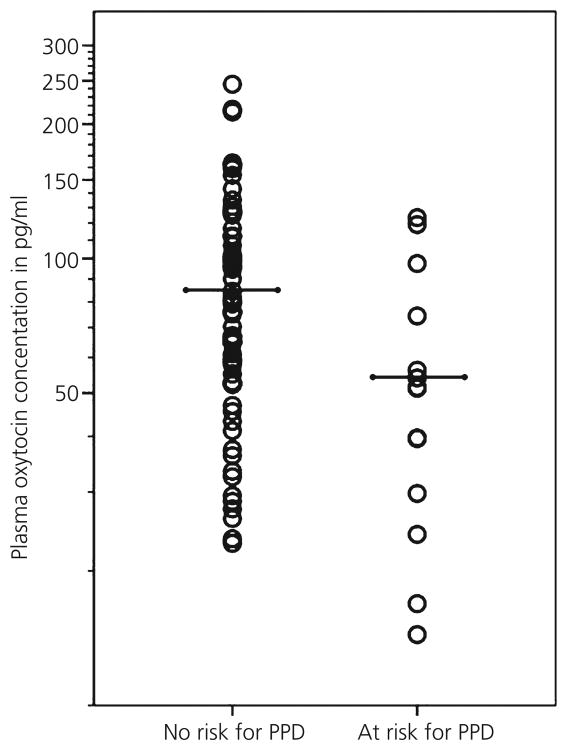
Lower plasma OXT is associated with more depressive symptoms during the third trimester of pregnancy and at 8 weeks postpartum in women (but not at 2 weeks postpartum) (168). As evidence for a link between pre- and postpartum psychobiological processes, there is an inverse association between plasma OXT during pregnancy and postpartum depressive symptoms, even after controlling for depressive symptoms during pregnancy (Fig. 4) (148). This finding highlights the potential of plasma OXT levels during pregnancy as prepartum predictors of risk for developing postpartum depression, and it may then be speculated that the window of opportunity to modify OXT activity to reduce postpartum depression may be before parturition. Moreover, it provides evidence that, somewhat similar to the infant HPA axis response to stress, the relationship of the OXT system with maternal depression may be subject to ‘peripartum programming’ in that there is a link between maternal prepartum neuroendocrine activity and postpartum behavioural or mental processes (169,170). In line with the above-mentioned finding, OXT during pregnancy predicts maternal bonding behaviours (i.e. positive affect and gaze during interactions with infant) and cognitive attachment representations of the child, both of which are vulnerable to postpartum depression (131). On the other hand, it has been found that intranasal OXT does not make mothers with postnatal depression happier (instead it makes them sadder), even though their perception of the relationship with their baby improves (171). However, the latter finding should be interpreted with caution because the size of the sample of that study was small and individual variation in sensitivity to OXT (e.g. as a result of early experiences) may modulate the biobehavioural effects of intranasally applied OXT (171–174).
Fig. 4.
Individual oxytocin (OXT) concentrations, as well as group means, in a group of women at risk and a group of women without risk for developing postpartum depression (PPD). OXT values are shown on a logarithmic scale. Women at risk have lower OXT concentrations. Adapted from Skrundz et al. (148).
The neurobiological mechanisms underlying OXT-related mood adaptations during the peripartum period in humans are mostly unknown. Some insight can be garnered from studies of nonperi-partum humans, in which intranasal OXT application is related to mostly increased amygdala responses to negatively-valenced and other stimuli in women, whereas decreased amygdala responses are found in men (151,175–180). However, nulliparous and postpartum women differ in how OXT affects their amygdala responses to negative pictures, with nulliparous women displaying greater sensitivity to the attenuation of amygdala responses by OXT, bringing them down to levels comparable to those observed in a group of post-partum breastfeeding women (181). These differences are presumably a result of the differences between these groups in endogenous OXT levels, which are elevated in postpartum women (182). Hence, peripartum changes in depressive symptoms may be a consequence of changes in the release of and sensitivity to OXT, driven by changes in the hormonal milieu during the peripartum period (11).
Laboratory rodents
Additional insight into the neurobiological mechanisms underlying OXT-related mood adaptations during the peripartum period in humans can also be garnered from rodent studies. There is growing evidence, at least in male rats and mice, for antidepressant-like effects of acute or repeated systemic administration of OXT in the forced swim test (183,184), in the learned helpless test (185) and the tail suspension test (157). Antidepressant-like effects of OXT have also been seen after intracerebral administration in male mice (157). These results suggest that systemic or central administration of OXT has antidepressant-like properties (186). However, these potential antidepressant-like effects of OXT could not be confirmed in a psychopathologic animal model (male or female rats selectively bred for high innate anxiety and comorbid depression-related behaviour), as neither acute nor chronic i.c.v. OXT infusion their immobility in the forced swim test (186).
To our knowledge, there is no direct evidence for the effects of high OXT availability on depression-related behaviour in pregnant or lactating rodents. However, there are several symptoms of depression that are likely to be the result of a dysregulated OXT system. For example, an important symptom of depression in humans is impaired social interaction, especially with the child, and general social withdrawal. OXT has been shown to promote naturally occurring social preference, as well as maternal behaviour (187-189). Therefore, we suggest that imbalanced OXT system activity may contribute to social withdrawal and the lack of strong mother-infant bonding in postpartum depression. Furthermore, OXT itself was shown to have rewarding properties, given that both centrally applied OXT as well as drugs of abuse increase dopamine release in the nucleus accumbens as part of the brain's reward circuitry (190,191). Interestingly, in lactating dams, suckling increases the functional magnetic resonance imaging activity in regions of the reward circuitry, including the accumbens-prefrontal cortical pathway (11,192). Because this activation was prevented by pre-administration of an OXTR antagonist, it may be that suckling is rewarding for the mother via central OXT release, encouraging her to continue engaging in nursing behaviour. This finding is also consistent with the observation that, during early lactation, dams find pups more rewarding than cocaine (193). Taken together, elevated OXT either via exogenous application or endogenous release in response to specific stimuli results in a positive hedonic state. Consequently, impaired OXT system activation may underlie the disrupted mother-infant bonding characteristic of postpartum depression as a result of the lack of stimulation of the reward circuitry during mother-infant interactions.
OXT likely modulates maternal depression by regulating the CRH system. As noted above, the depression-like behaviours and poor mothering of postpartum mice with null mutation for the delta subunit of the GABA receptor (Gabrd-/- mice) are associated with high HPA axis responsiveness, and their phenotype can be rescued by CRH receptor antagonism (J. Maguire and I. Mody, unpublished data). There are similar interactions between OXT and components of the stress system that can influence depression-like behaviours in peripartum laboratory rats. OXT is a robust inhibitor of the HPA axis (52), particularly the brain CRH system (165). Given that CRH triggers depression-like symptoms in nulliparous rodents, and is a putative causal factor for major depression (194,195), up-regulation of neuronal OXT (and prolactin) (196) activity during the peripartum period likely dampens the CRH system and its depressive actions. Interestingly, chronic stress during pregnancy can prevent this peri-partum adaptation in the OXT system. In a chronic psychosocial stress paradigm for pregnant rats that combined restraint stress (twice daily for 1 h) and overcrowding with unknown conspecifics between pregnancy days 4–16, stressed dams did not show the rise in OXT mRNA expression within the PVN that is typical of the peri-partum period (197). Moreover, pregnancy stress prevented the anxiolytic effect of motherhood and resulted in an abnormally high frequency of arched-back nursing by the stressed dams (197). These effects of pregnancy stress on emotional responses and maternal behaviours may not both be mediated by stress-induced glucocorticoid release because, although chronic administration of corticosterone to pregnant or postpartum rats does increase postpartum depression-like behaviours, it reduces maternal behaviour (198,199). In humans, both impaired and increased maternal care and infant attachment have been reported after chronic stress. In this context, it would be also of interest to study the effects of pregnancy stress on other relevant neurochemicals systems, such as vasopressin, which not only promotes maternal care and aggression toward potentially harmful intruders to the nest, but also exerts anxiogenic effects postpartum (200).
Moreover, the dramatic physiological (in particular neuroendocrine) changes occurring during the peripartum period also affect hippocampal plasticity. Specifically, the continuous high levels of circulating glucocorticoids are responsible for reduced cell proliferation, neurogenesis and dendritic architecture during the postpartum period (18,201,202). Reduced hippocampal neurogenesis has often been related to major depression and other stress-related psychopathologies (203,204) and may contribute to mothers' increased risk of developing psychiatric disorders, including postpartum depression. However, it has been found that stress during pregnancy reverses the postpartum reduction in hippocampal plasticity (202). In this context, it is also of interest to note that OXT contributes to hippocampal cell proliferation and neurogenesis (205), but to what extent this neuropeptide is involved in these hippocampal processes during the peripartum period remains to be determined.
Interactions between central GABA and OXT systems
The similar positive consequences of elevated GABA and OXT on peripartum anxiety and depression might suggest redundancy between these neurochemical systems to ensure an optimal maternal emotional state. One can instead conjecture that such similar consequences suggest functional interactions (if not interdependency) between these systems. The literature on interactions between central GABA and OXT systems is quite small, although interactions between them are already known to be critical for other peripartum adaptations of the brain. A particularly salient example involves the large population of OXT-synthesising cells of the hypothalamic SON. At the end of pregnancy, there is a decrease in the ratio of α1 : α2 GABAAR subunit expression on SON OXT cells, which alleviates neurosteroid-potentiated GABAergic inhibition and permits increased activity of these OXT cells necessary for the bolus release of OXT characteristic of parturition and milk letdown (206). Dramatic remodelling of glial morphology and neurone-to-neurone communication among OXT-synthesising cells occurs peri-partum (207), which results in elevated heterosynaptic inhibition of local GABA transmission (208). This increased excitability of OXTergic cells facilitates synchronisation of cell firing during times of high peripheral and central OXT release. In turn, increased local OXT release within the SON rapidly elicits the formation of new GABAergic synaptic contacts and up-regulates GABAergic inhibitory input onto these OXT cells, which may be necessary for their quiescence during times of low OXT release and characteristic bursts of firing at other times (209,210).
Outside the SON and presumably in brains of mostly male laboratory rodents, it has been observed that OXT increases GABA release and GABAAR-mediated inhibition in brain sites as divergent in function as the lateral hypothalamus (211), hippocampus (212,213), cortex (214) and hypoglossal nucleus (215). Probably relevant to maternal anxiety and depression is the finding that stimulating endogenous OXT release or otherwise activating OXT receptors in the central amygdala of ovariectomised or cycling female rats suppresses the display of emotion-related behaviours (160,161). This results from OXT-sensitive GABAergic cells in the lateral central amygdala inhibiting neural firing in the medial central amygdala, an effect that can be blocked by the GABAA receptor antagonist bicuculline (216,217). Some other central effects of OXT can also be prevented by bicuculline (218,219). Furthermore, OXT synergises with benzodiazepines to inhibit activity in the medial central amygdala (220) and these cells have been found to project to the cPAGv (221). If such findings extend to peripartum females, which would not be surprising because their GABA and OXT systems are already up-regulated and highly sensitive compared to males or nulliparous females, it would demonstrate an important limbic-midbrain pathway through which GABA and OXT could interact to affect mother's emotional and mood-related behaviours.
Conclusions
Appropriately balanced adaptations of the neurochemical systems regulating peripartum anxiety- and depression-like behaviours are essential for overall maternal well-being and caregiving abilities, and thus the normal development (if not survival) of their offspring. It is clear from the review above that, although adaptations in both the GABA and OXT systems are reasonably well-studied for roles in regulating the anxiety and depressive behaviours of peri-partum rodents, knowledge of peripartum changes in the central GABA system in women and its influence on their peripartum anxiety and depression is almost completely lacking. Furthermore, the work on OXT in peripartum rodents focuses only on anxiety whereas in peripartum women, it focuses more on depression. Filling these gaps will tremendously enhance the translational value of the animal research and broaden our perspective on which systems would be best targeted to improve both mental health concerns of peripartum women. Nonetheless, one can conclude that high cerebral GABA and OXT signalling are likely crucial for optimising maternal emotions and mood during the peripartum period, and that these neurochemicals may depend on each other for their success. Interactions between GABA and OXT refine neural network activity by altering signal-to-noise ratios (213). If the same is true for brain sites where OXT and GABAA receptor activity produce similar effects on anxiety- and depressive-like behaviours in postpartum rats (which for anxiety includes the cPAGv) (166,121), OXT released in response to interactions with offspring could alter already elevated local GABAergic activity to refine how output neurones respond to emotion-and mood-relevant stimuli. Of course, GABA and OXT are not the only neurochemical systems involved in this important function. As noted above, there are some interesting interactions between GABA and OXT with the CRH system. Prolactin is also very well-known for regulating peripartum emotional and caregiving behaviours (142,222,223) and interacts with both GABA and OXT (224,225). Thus, dysregulation of the normally finely-tuned adaptations of the maternal brain GABA, OXT, CRH and prolactin systems likely underlies the high incidence of emotional and mood disorders during the peripartum period, with adverse consequences for maternal caregiving behaviours and the development of offspring.
Acknowledgments
The authors declare that they have no conflicts of interest.
References
- 1.Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: emotionality differences between nulliparous and parturient females. Physiol Behav. 1981;27:863–868. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- 2.Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- 3.Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behav Cogn Neurosci Rev. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- 4.Numan M, Fleming AS, Levy F. Maternal behavior. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd. New York, NY: Elsevier; 2006. pp. 1921–1993. [Google Scholar]
- 5.Bosch OJ. Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Philos Trans R Soc Lond B Biol Sci. 2013;368:20130085. doi: 10.1098/rstb.2013.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonstein JS, Pereira M, Marler CA, Morrell JI. Parental Behavior. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction. 4th. New York, NY: Elsevier; (in press) [Google Scholar]
- 7.Hard E, Hansen S. Reduced fearfulness in the lactating rat. Physiol Behav. 1985;35:641–643. doi: 10.1016/0031-9384(85)90155-6. [DOI] [PubMed] [Google Scholar]
- 8.Neumann ID, Krömer SA, Bosch OJ. Effects of psycho-social stress during pregnancy on neuroendocrine and behavioral parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology. 2005;30:791–806. doi: 10.1016/j.psyneuen.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Ragan CM, Lonstein JS. Differential postpartum sensitivity to the anxiety-modulating effects of offspring contact is associated with innate anxiety and brainstem levels of dopamine beta-hydroxylase in female laboratory rats. Neuroscience. 2014;256:433–444. doi: 10.1016/j.neuroscience.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming AS, Steiner M, Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm Behav. 1997;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- 11.Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE. In search of neural endophenotypes of postpartum psychopathology and disrupted maternal. J Neuroendocrinol. 2014;26:665–684. doi: 10.1111/jne.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann ID. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depress Anxiety. 2003;17:111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- 13.Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–141. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Workman JL, Barha CK, Galea LA. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci. 2012;126:54–72. doi: 10.1037/a0025538. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Soeken TA, Cromer SJ, Martinez SR, Hardy LR, Strathearn L. Oxytocin and postpartum depression: delivering on what's known and what's not. Brain Res. 2014 doi: 10.1016/j.brainres.2013.11.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galea LA, Leuner B, Slattery D. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26:641–648. doi: 10.1111/jne.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heron J, O'Connor TG, Evans J, Golding J, Glover V, ALSPAC Study Team The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80:65–73. doi: 10.1016/j.jad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 20.van Bussel JC, Spitz B, Demyttenaere K. Women's mental health before, during, and after pregnancy: a population-based controlled cohort study. Birth. 2006;33:297–302. doi: 10.1111/j.1523-536X.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- 21.Dipietro JA, Costigan KA, Sipsma HL. Continuity in self-report measures of maternal anxiety, stress, and depressive symptoms from pregnancy through two years postpartum. J Psychosom Obstet Gynaecol. 2008;29:115–124. doi: 10.1080/01674820701701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog Brain Res. 2001;133:241–249. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- 23.Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- 24.Altemus M, Fong J, Yang R, Damast S, Luine V, Ferguson D. Changes in cerebrospinal fluid neurochemistry during pregnancy. Biol Psychiatry. 2004;56:386–392. doi: 10.1016/j.biopsych.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Task Force on DSM-IV Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 26.Ross LE, McLean LM. Anxiety disorders during pregnancy and the postpartum period: a systematic review. J Clin Psychiatry. 2006;67:1285–1298. doi: 10.4088/jcp.v67n0818. [DOI] [PubMed] [Google Scholar]
- 27.Britton JR. Maternal anxiety: course and antecedents during the early postpartum period. Depress Anxiety. 2008;25:793–800. doi: 10.1002/da.20325. [DOI] [PubMed] [Google Scholar]
- 28.Glasheen C, Richardson GA, Fabio A. A systematic review of the effects of postnatal maternal anxiety on children. Arch Womens Ment Health. 2010;13:61–74. doi: 10.1007/s00737-009-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson J, Redshaw M. Anxiety in the perinatal period: antenatal and postnatal influences and women's experience of care. J Reprod Infant Psychol. 2013;31:465–478. [Google Scholar]
- 30.Woolhouse H, Brow S, Krastev A, Perlen S, Gunn J. Seeking help for anxiety and depression after childbirth: results of the Maternal Health Study. Arch Womens Ment Health. 2009;12:75–83. doi: 10.1007/s00737-009-0049-6. [DOI] [PubMed] [Google Scholar]
- 31.Matthey S, Barnett B, Howie P, Kavanagh DJ. Diagnosing postpartum depression in mothers and fathers: whatever happened to anxiety? J Affect Disord. 2003;74:139–147. doi: 10.1016/s0165-0327(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 32.Britton JR. Pre-discharge anxiety among mothers of well newborns: prevalence and correlates. Acta Paediatr. 2005;94:1771–1776. doi: 10.1111/j.1651-2227.2005.tb01852.x. [DOI] [PubMed] [Google Scholar]
- 33.Gale S, Harlow BL. Postpartum mood disorders: a review of clinical and epidemiological factors. J Psychosom Obstet Gynaecol. 2003;24:257–266. doi: 10.3109/01674820309074690. [DOI] [PubMed] [Google Scholar]
- 34.Henshaw C, Foreman D, Cox J. Postnatal blues: a risk factor for postnatal depression. J Psychosom Obstet Gynaecol. 2004;25:267–272. doi: 10.1080/01674820400024414. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. ICD-10: international statistical classification of diseases and related health problems. Geneva: World Health Organization; 1992. [Google Scholar]
- 36.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 37.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 38.Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. Br J Psychiatry. 1993;16:327–331. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- 39.Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202:5–14. doi: 10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viguera AC, Tondo L, Koukopoulos AE, Reginaldi D, Lepri B, Baldessarini RJ. Episodes of mood disorders in 2,252 pregnancies and postpartum periods. Am J Psychiatry. 2011;168:1179–1185. doi: 10.1176/appi.ajp.2011.11010148. [DOI] [PubMed] [Google Scholar]
- 41.Di Florio A, Forty L, Gordon-Smith K, Heron J, Jones L, Craddock N, Jones I. Perinatal episodes across the mood disorder spectrum. JAMA Psychiatry. 2013;70:168–175. doi: 10.1001/jamapsychiatry.2013.279. [DOI] [PubMed] [Google Scholar]
- 42.Fields ES, Meuchel JM, Jaffe CJ, Jha M, Payne JL. Post adoption depression. Arch Womens Ment Health. 2010;13:147–151. doi: 10.1007/s00737-009-0137-7. [DOI] [PubMed] [Google Scholar]
- 43.Mott SL, Schiller CE, Richards JG, O'Hara MW, Stuart S. Depression and anxiety among postpartum and adoptive mothers. Arch Womens Ment Health. 2011;14:335–343. doi: 10.1007/s00737-011-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wee KY, Skouteris H, Pier C, Richardson B, Milgrom J. Correlates of ante- and postnatal depression in fathers: a systematic review. J Affect Disord. 2011;130:358–377. doi: 10.1016/j.jad.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Hum Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- 46.Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumann ID, Wigger A, Liebsch G, Holsboer F, Landgraf R. Increased basal activity of the hypothalamo-pituitary-adrenal axis during pregnancy in rats bred for high anxiety-related behavior. Psychoneuroendocrinology. 1998;23:449–463. doi: 10.1016/s0306-4530(98)00023-7. [DOI] [PubMed] [Google Scholar]
- 48.Roy V, Lointier L, Chapillon P. The emotional reactivity increase at midpregnancy is attenuated in female rats handled during their infancy. Behav Brain Res. 2003;145:23–30. doi: 10.1016/s0166-4328(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 49.Macbeth AH, Gautreaux C, Luine VN. Pregnant rats show enhanced spatial memory, decreased anxiety, and altered levels of monoaminergic neurotransmitters. Brain Res. 2008;1241:136–147. doi: 10.1016/j.brainres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pawluski JL, van den Hove DL, Rayen I, Prickaerts J, Steinbusch HW. Stress and the pregnant female: impact on hippocampal cell proliferation, but not affective-like behaviors. Horm Behav. 2011;59:572–580. doi: 10.1016/j.yhbeh.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Bitran D, Hilvers RJ, Kellogg CK. Ovarian endocrine status modulates the anxiolytic potency of diazepam and the efficacy of gamma-aminobutyric acid-benzodiazepine receptor-mediated chloride ion transport. Behav Neurosci. 1991;105:653–662. doi: 10.1037//0735-7044.105.5.653. [DOI] [PubMed] [Google Scholar]
- 52.Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behavior in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 53.Picazo O, Fernandez-Guasti A. Changes in experimental anxiety during pregnancy and lactation. Physiol Behav. 1993;54:295–299. doi: 10.1016/0031-9384(93)90114-u. [DOI] [PubMed] [Google Scholar]
- 54.Zuluaga MJ, Agrati D, Pereira M, Uriarte N, Fernandez-Guasti A, Ferreira A. Experimental anxiety in the black and white model in cycling pregnant and lactating rats. Physiol Behav. 2005;84:279–286. doi: 10.1016/j.physbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Vierin M, Bouissou MF. Pregnancy is associated with low fear reactions in ewes. Physiol Behav. 2001;72:579–587. doi: 10.1016/s0031-9384(01)00416-4. [DOI] [PubMed] [Google Scholar]
- 56.Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Horm Behav. 2005;47:241–255. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. J Neurosci. 1998;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Windle RJ, Gamble LE, Kershaw YM, Wood SA, Lightman SL, Ingram CD. Gonadal steroid modulation of stress-induced hypothalamo-pituitary-adrenal activity and anxiety behavior: role of central oxytocin. Endocrinology. 2006;147:2423–2431. doi: 10.1210/en.2005-1079. [DOI] [PubMed] [Google Scholar]
- 59.Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced ‘depression’ in female rats. Physiol Behav. 2004;83:505–513. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 60.Molina-Hernández M, Contreras CM, Téllez-Alcántara P. Antidepressant-like effects of pregnancy and progesterone in Wistar rats as measured in the differential reinforcement of the low-rate 72 s task. Psychopharmacology. 2000;151:306–311. doi: 10.1007/s002130000496. [DOI] [PubMed] [Google Scholar]
- 61.Molina-Hernandez M, Tellez-Alcantara NP. Antidepressant-like actions of pregnancy, and progesterone in Wistar rats forced to swim. Psycho-neuroendocrinology. 2001;26:479–491. doi: 10.1016/s0306-4530(01)00007-5. [DOI] [PubMed] [Google Scholar]
- 62.Frye CA, Walf AA. Hippocampal 3alpha,5alpha-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78:531–540. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 63.Lavi-Avnon Y, Shayit M, Yadid G, Overstreet HD, Weller A. Immobility in the swim test and observations of maternal behavior in lactating Flinders sensitive line rats. Behav Brain Res. 2005;161:155–163. doi: 10.1016/j.bbr.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Maguire J, Mody I. GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Craft RM, Kostick ML, Rogers JA, White CL. Tsutsui KT Forced swim test behavior in postpartum rats. Pharmacol Biochem Behav. 2010;96:402–412. doi: 10.1016/j.pbb.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wade GN, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J Comp Physiol Psychol. 1969;69:291–300. doi: 10.1037/h0028208. [DOI] [PubMed] [Google Scholar]
- 67.Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35:136–145. [PubMed] [Google Scholar]
- 68.Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- 69.Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- 70.Beckley EH, Finn DA. Inhibition of progesterone metabolism mimics the effect of progesterone withdrawal on forced swim test immobility. Pharmacol Biochem Behav. 2007;87:412–419. doi: 10.1016/j.pbb.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suda S, Segi-Nishida E, Newton SS, Duman RS. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry. 2008;64:311–319. doi: 10.1016/j.biopsych.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Green AD, Barr AM, Galea LA. Role of estradiol withdrawal in ‘anhedonic’ sucrose consumption: a model of postpartum depression. Physiol Behav. 2009;97:259–265. doi: 10.1016/j.physbeh.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 73.Schiller CE, O'Hara MW, Rubinow DR, Johnson AK. Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiol Behav. 2013;119:137–144. doi: 10.1016/j.physbeh.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pawluski JL, Lieblich SE, Galea LA. Offspring-exposure reduces depressive-like behavior in the parturient female rat. Behav Brain Res. 2009;197:55–61. doi: 10.1016/j.bbr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Navarre BM, Laggart JD, Craft RM. Anhedonia in postpartum rats. Physiol Behav. 2010;99:59–66. doi: 10.1016/j.physbeh.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saavedra M, Contreras CM, Azamar-Arizmendi G, Hernandez-Lozano M. Differential progesterone effects on defensive burying and forced swimming tests depending upon a gradual decrease or an abrupt suppression schedules. Pharmacol Biochem Behav. 2006;83:130–135. doi: 10.1016/j.pbb.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 77.Doornbos B, Fokkema DS, Molhoek M, Tanke MA, Postema F, Korf J. Abrupt rather than gradual hormonal changes induce postpartum blues-like behavior in rats. Life Sci. 2009;84:69–74. doi: 10.1016/j.lfs.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 78.Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 79.Smith KS, Rudolph U. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABAA receptor subtypes. Neuropharmacology. 2012;62:54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cryan JF, Kaupmann K. Don't worry ‘B’ happy!: a role for GABAB receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Kumar K, Sharma S, Kumar P, Deshmukh R. Therapeutic potential of GABAB receptor ligands in drug addiction, anxiety, depression and other CNS disorders. Pharmacol Biochem Behav. 2013;110:174–184. doi: 10.1016/j.pbb.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- 83.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sethuraman R, Lee TL, Chui JW, Tachibana S. Changes in amino acids and nitric oxide concentration in cerebrospinal fluid during labor pain. Neurochem Res. 2006;31:1127–1133. doi: 10.1007/s11064-006-9133-8. [DOI] [PubMed] [Google Scholar]
- 85.Honig A, Bartlett JR, Bouras N, Bridges PK. Amino acid levels in depression: a preliminary investigation. J Psychiatr Res. 1988;22:159–164. doi: 10.1016/0022-3956(88)90001-5. [DOI] [PubMed] [Google Scholar]
- 86.Rimon R, Lepola U, Jolkkonen J, Halonen T, Riekkinen P. Cerebrospina fluid gamma-aminobutyric acid in patients with panic disorder. Biol Psychiatry. 1995;38:737–741. doi: 10.1016/0006-3223(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 87.Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OA, Charney DS, Krystal JH. Reductions in occipital cortex GABA levels in panic disorder detected with 1 h-magnetic resonance spectroscopy. Arch Gen Psychiatry. 2001;58:556–561. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- 88.Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krysta JH, Rothman DL, Mason GF. Preliminary evidence of reduced occipita GABA concentrations in puerperal women: a 1H-MRS study. Psycho-pharmacology. 2006;186:425–433. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- 89.Qureshi GA, Hansen S, Sodersten P. Offspring control of cerebrospina fluid GABA concentrations in lactating rats. Neurosci Lett. 1987;75:85–88. doi: 10.1016/0304-3940(87)90080-2. [DOI] [PubMed] [Google Scholar]
- 90.Hansen S. Mechanisms involved in the control of punished responding in mother rats. Horm Behav. 1990;24:186–197. doi: 10.1016/0018-506x(90)90004-h. [DOI] [PubMed] [Google Scholar]
- 91.Kellogg CK, Barrett KA. Reduced progesterone metabolites are not critical for plus-maze performance of lactating female rats. Pharmacol Biochem Behav. 1999;63:441–448. doi: 10.1016/s0091-3057(99)00041-6. [DOI] [PubMed] [Google Scholar]
- 92.Kendrick KM, Keverne EB, Chapman C, Baldwin BA. Microdialysis measurement of oxytocin, aspartate, gamma-aminobutyric acid and glutamate release from the olfactory bulb of the sheep during vagi-nocervical stimulation. Brain Res. 1988;442:171–174. doi: 10.1016/0006-8993(88)91447-3. [DOI] [PubMed] [Google Scholar]
- 93.Kendrick KM, Keverne EB, Hinton MR, Goode JA. Oxytocin, amino acid and monoamine release in the region of the medial preoptic area and bed nucleus of the stria terminalis of the sheep during parturition and suckling. Brain Res. 1992;569:199–209. doi: 10.1016/0006-8993(92)90631-i. [DOI] [PubMed] [Google Scholar]
- 94.Munaro NI. Maternal behavior: glutamic acid decarboxylase activity in the olfactory bulb of the rat. Pharmacol Biochem Behav. 1990;36:81–84. doi: 10.1016/0091-3057(90)90129-6. [DOI] [PubMed] [Google Scholar]
- 95.Rodríguez C, Guillamón A, Pinos H, Collado P. Postpartum changes in the GABAergic system in the bed nucleus of the accessory olfactory tract. Neurochem Int. 2004;44:179–183. doi: 10.1016/s0197-0186(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 96.Zhao C, Driessen T, Gammie SC. Glutamic acid decarboxylase 65 and 67 expression in the lateral septum is up-regulated in association with the postpartum period in mice. Brain Res. 2012;1470:35–44. doi: 10.1016/j.brainres.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Young BJ, Cook CJ. Reduced basal GABA concentrations in the ratamygdala during pregnancy. Physiol Behav. 2006;87:817–820. doi: 10.1016/j.physbeh.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Smolen A, Smolen TN, Han PC. Alterations in regional brain GABA concentration and turnover during pregnancy. Pharmacol Biochem Behav. 1993;44:63–69. doi: 10.1016/0091-3057(93)90281-w. [DOI] [PubMed] [Google Scholar]
- 99.Kornblatt JJ, Grattan DR. Lactation alters gamma-aminobutyric acid neuronal activity in the hypothalamus and cerebral cortex in the rat. Neuroendocrinology. 2001;73:175–184. doi: 10.1159/000054634. [DOI] [PubMed] [Google Scholar]
- 100.Arriaga-Avila V, Martínez-Abundis E, Cárdenas-Morales B, Mercado-Gomez O, Aburto-Arciniega E, Miranda-Martínez A, Kendrick KM, Guevara-Guzman R. Lactation reduces stress-caused dopaminergic activity and enhances GABAergic activity in the rat medial prefrontal cortex. J Mol Neurosci. 2014;52:515–524. doi: 10.1007/s12031-013-0104-7. [DOI] [PubMed] [Google Scholar]
- 101.Ahmed EA, Lonstein JS. Vesicular GABA transporter and glutamic acid decarboxylase expression in the neural anxiety network of postpartum and virgin female rats. Society for Neuroscience Abstracts. 2012;483:10. RR13. [Google Scholar]
- 102.Majewska MD, Fordrice F, Falkay G. Pregnancy-induced alterations of GABAA receptor sensitivity in maternal brain - an antecedent of post-partum blues. Brain Res. 1989;482:397–401. doi: 10.1016/0006-8993(89)91208-0. [DOI] [PubMed] [Google Scholar]
- 103.Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiologica modulation of GABAA receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–235. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- 104.Ferreira A, Hansen S, Nielsen M, Archer T, Minor BG. Behavior of mother rats in conflict tests sensitive to antianxiety agents. Behav Neurosci. 1989;103:193–201. doi: 10.1037//0735-7044.103.1.193. [DOI] [PubMed] [Google Scholar]
- 105.Miller SM, Lonstein JS. Autoradiographic analysis of GABAA receptor binding in the neural anxiety network of postpartum and non-postpartum laboratory rats. Brain Res Bull. 2011;86:60–64. doi: 10.1016/j.brainresbull.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Biggio G, Cristina MM, Follesa P, Concas A, Sanna E. GABAA receptor function and gene expression during pregnancy and postpartum. Int Rev Neurobiol. 2009;85:73–94. doi: 10.1016/S0074-7742(09)85006-X. [DOI] [PubMed] [Google Scholar]
- 107.Mackenzie G, Maguire J. The role of ovarian hormone-derived neurosteroids on the regulation of GABAA receptors in affective disorders. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-013-3423-z. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sundstrom I, Backstrom T, Wang M, Olsson T, Seippel L, Bixo M. Premenstrual syndrome, neuroactive steroids and the brain. Gynecol Endocrinol. 1999;13:206–220. doi: 10.3109/09513599909167557. [DOI] [PubMed] [Google Scholar]
- 110.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Griffiths J, Lovick T. Withdrawal from progesterone increases expression of alpha4, beta1, and delta GABAA receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J Comp Neurol. 2005;486:89–97. doi: 10.1002/cne.20540. [DOI] [PubMed] [Google Scholar]
- 113.Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci. 2009;29:9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu X, Gangisetty O, Carver CM, Reddy DS. Estrous cycle regulation of extrasynaptic delta-containing GABAA receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther. 2013;346:146–160. doi: 10.1124/jpet.113.203653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. J Neurosci. 1997;17:3364–3378. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lovick TA. Plasticity of GABAA receptor subunit expression during the oestrous cycle of the rat: implications for premenstrual syndrome in women. Exp Physiol. 2006;91:655–660. doi: 10.1113/expphysiol.2005.032342. [DOI] [PubMed] [Google Scholar]
- 117.Richards DS, Villalba RM, Alvarez FJ, Stern JE. Expression of GABAB receptors in magnocellular neurosecretory cells of male, virgin female and lactating rats. J Neuroendocrinol. 2005;17:413–423. doi: 10.1111/j.1365-2826.2005.01324.x. [DOI] [PubMed] [Google Scholar]
- 118.Voisin DL, Herbison AE, Chapman C, Poulain DA. Effects of central GABAB receptor modulation upon the milk ejection reflex in the rat. Neuroendocrinology. 1996;63:368–376. doi: 10.1159/000126977. [DOI] [PubMed] [Google Scholar]
- 119.Arrati PG, Carmona C, Dominguez G, Beyer C, Rosenblatt JS. GABA receptor agonists in the medial preoptic area and maternal behavior in lactating rats. Physiol Behav. 2006;87:51–65. doi: 10.1016/j.physbeh.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 120.Hansen S, Ferreira A, Selart ME. Behavioral similarities between mother rats and benzodiazepine-treated non-maternal animals. Psychopharmacology. 1985;86:344–347. doi: 10.1007/BF00432226. [DOI] [PubMed] [Google Scholar]
- 121.Miller SM, Piasecki CC, Peabody MF, Lonstein JS. GABAA receptor antagonism in the ventrocaudal periaqueductal gray increases anxiety in the anxiety-resistant postpartum rat. Pharmacol Biochem Behav. 2010;95:457–465. doi: 10.1016/j.pbb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 122.Miller SM, Piasecki CC, Lonstein JS. Use of the light-dark box to compare the anxiety-related behavior of virgin and postpartum female rats. Pharmacol Biochem Behav. 2011;100:130–137. doi: 10.1016/j.pbb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hansen S, Ferreira A. Effects of bicuculline infusions in the ventromedial hypothalamus and amygdaloid complex on food intake and affective behavior in mother rats. Behav Neurosci. 1986;100:410–415. doi: 10.1037//0735-7044.100.3.410. [DOI] [PubMed] [Google Scholar]
- 124.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mody I, Maguire J. The reciprocal regulation of stress hormones and GABA(A) receptors. Front Cell Neurosci. 2012;6:4. doi: 10.3389/fncel.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW. Gestational stress induces post-partum depression-like behavior and alters maternal care in rats. Psychoneuroendocrinology. 2004;29:227–244. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 127.Carroll BJ, Iranmanesh A, Keenan DM, Cassidy F, Wilson WH, Veldhuis JD. Pathophysiology of hypercortisolism in depression: pituitary and adrenal responses to low glucocorticoid feedback. Acta Psychiatr Scand. 2012;125:478–491. doi: 10.1111/j.1600-0447.2011.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- 129.de Geest K, Thiery M, Piron-Possuyt G, Vanden Driessche R. Plasma oxytocin in human pregnancy and parturition. J Perinat Med. 1985;13:3–13. doi: 10.1515/jpme.1985.13.1.3. [DOI] [PubMed] [Google Scholar]
- 130.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 131.Nissen E, Lilja G, Widstrom AM, Uvnas-Moberg K. Elevation of oxytocin levels early post partum in women. Acta Obstet Gynecol Scand. 1995;74:530–533. doi: 10.3109/00016349509024384. [DOI] [PubMed] [Google Scholar]
- 132.Johnston JM, Amico JA. A prospective longitudinal study of the release of oxytocin and prolactin in response to infant suckling in long term lactation. J Clin Endocrinol Metab. 1986;62:653–657. doi: 10.1210/jcem-62-4-653. [DOI] [PubMed] [Google Scholar]
- 133.Veening JG, de Jong T, Barendregt HP. Oxytocin-messages via the cerebrospinal fluid: behavioral effects; a review. Physiol Behav. 2010;101:193–210. doi: 10.1016/j.physbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 134.Takagi T, Tanizawa O, Otsuki Y, Sugita N, Haruta M, Yamaji K. Oxytocin in the cerebrospinal fluid and plasma of pregnant and nonpregnant subjects. Horm Metab Res. 1985;17:308–310. doi: 10.1055/s-2007-1013526. [DOI] [PubMed] [Google Scholar]
- 135.Takeda S, Kuwabara Y, Mizuno M. Effects of pregnancy and labor on oxytocin levels in human plasma and cerebrospinal fluid. Endocrinol Jpn. 1985;32:875–880. doi: 10.1507/endocrj1954.32.875. [DOI] [PubMed] [Google Scholar]
- 136.Broad KD, Kendrick KM, Sirinathsinghji DJ, Keverne EB. Changes in oxytocin immunoreactivity and mRNA expression in the sheep brain during pregnancy, parturition and lactation and in response to oestrogen and progesterone. J Neuroendocrinol. 1993;5:435–444. doi: 10.1111/j.1365-2826.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 137.Zingg HH, Rozen F, Breton C, Larcher A, Neculcea J, Chu K, Russo C, Arslan A. Gonadal steroid regulation of oxytocin and oxytocin receptor gene expression. Adv Exp Med Biol. 1995;395:395–404. [PubMed] [Google Scholar]
- 138.Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76:593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- 139.Neumann ID, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 140.Neumann ID. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog Brain Res. 2001;133:143–152. doi: 10.1016/s0079-6123(01)33011-x. [DOI] [PubMed] [Google Scholar]
- 141.Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID. Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J Neurosci. 2001;21:3207–3214. doi: 10.1523/JNEUROSCI.21-09-03207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5:249–257. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- 143.Young LJ, Muns S, Wang Z, Insel TR. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J Neuroendocrinol. 1997;9:859–865. doi: 10.1046/j.1365-2826.1997.00654.x. [DOI] [PubMed] [Google Scholar]
- 144.Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW, Douglas AJ. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology. 2007;148:5095–5104. doi: 10.1210/en.2007-0615. [DOI] [PubMed] [Google Scholar]
- 145.Insel TR. Regional changes in brain oxytocin receptors post-partum: time-course and relationship to maternal behavior. J Neuroendocrinol. 1990;2:539–545. doi: 10.1111/j.1365-2826.1990.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 146.Slattery DA, Neumann ID. Oxytocin and major depressive disorder: experimental and clinical evidence for links to aetiology and possible treatment. Pharmaceuticals. 2010;3:702–724. doi: 10.3390/ph3030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translationa medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 148.Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36:1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Evans SL, Monte OD, Noble P, Averbeck BB. Intranasal oxytocin effects on social cognition: a critique. Brain Res. 2014 doi: 10.1016/j.brainres.2013.11.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 151.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 152.Chen FS, Johnson SC. An oxytocin receptor gene variant predicts attachment anxiety in females and autism-spectrum traits in males. Soc Psychol Personal Sci. 2012;3:93–99. [Google Scholar]
- 153.Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Uvnäas-Moberg K, Widströom AM, Nissen E, Bjöorvell H. Personality traits in women 4 days postpartum and their correlation with plasma levels of oxytocin and prolactin. J Psychosom Obstet Gynaecol. 1990;11:261–273. [Google Scholar]
- 155.Nissen E, Gustavsson P, Widströom AM, Uvnäas-Moberg K. Oxytocin, prolactin, milk production and their relationship with personality traits in women after vaginal delivery or Cesarean section. J Psychosom Obstet Gynaecol. 1998;19:49–58. doi: 10.3109/01674829809044221. [DOI] [PubMed] [Google Scholar]
- 156.Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology. 2013;38:694–701. doi: 10.1016/j.psyneuen.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 157.Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 158.Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jurek B, Slattery DA, Maloumby R, Hillerer K, Koszinowski S, Neumann ID, van den Burg EH. Differential contribution of hypothalamic MAPK activity to anxiety-like behavior in virgin and lactating rats. PLoS One. 2012;7:e37060. doi: 10.1371/journal.pone.0037060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 162.Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via Erk1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- 163.Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 165.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Figueira RJ, Peabody MF, Lonstein JS. Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behav Neurosci. 2008;122:618–628. doi: 10.1037/0735-7044.122.3.618. [DOI] [PubMed] [Google Scholar]
- 167.Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- 168.Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J Womens Health (Larchmt) 2013;22:352–361. doi: 10.1089/jwh.2012.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Terenzi MG, Jiang QB, Cree SJ, Wakerley JB, Ingram CD. Effect of gonadal steroids on the oxytocin-induced excitation of neurons in the bed nuclei of the stria terminalis at parturition in the rat. Neuroscience. 1999;91:1117–1127. doi: 10.1016/s0306-4522(98)00687-3. [DOI] [PubMed] [Google Scholar]
- 170.Meinlschmidt G, Martin C, Neumann ID, Heinrichs M. Maternal cortisol in late pregnancy and hypothalamic-pituitary-adrenal reactivity to psychosocial stress postpartum in women. Stress. 2010;13:163–171. doi: 10.3109/10253890903128632. [DOI] [PubMed] [Google Scholar]
- 171.Mah BL, Van Ijzendoorn MH, Smith R, Bakermans-Kranenburg MJ. Oxytocin in postnatally depressed mothers: its influence on mood and expressed emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:267–272. doi: 10.1016/j.pnpbp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 172.Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol Psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 173.Bakermans-Kranenburg MJ, Van IJzendoorn MH, Riem MM, Tops M, Alink LR. Oxytocin decreases handgrip force in reaction to infant crying in females without harsh parenting experiences. Soc Cogn Affect Neurosci. 2011;6:294–300. doi: 10.1093/scan/nsr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011;6:556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 177.Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 179.Striepens N, Scheele D, Kendrick KM, Becker B, Schafer L, Schwalba K, Reul J, Maier W, Hurlemann R. Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci USA. 2012;109:18144–18149. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bertsch K, Gamer M, Schmidt B, Schmidinger I, Walther S, Kastel T, Schnell K, Buchel C, Domes G, Herpertz SC. Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am J Psychiatry. 2013;170:1169–1177. doi: 10.1176/appi.ajp.2013.13020263. [DOI] [PubMed] [Google Scholar]
- 181.Rupp HA, James TW, Ketterson ED, Sengelaub DR, Ditzen B, Heiman JR. Amygdala response to negative images in postpartum vs nulliparous women and intranasal oxytocin. Soc Cogn Affect Neurosci. 2014;9:48–54. doi: 10.1093/scan/nss100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kanat M, Heinrichs M, Domes G. Oxytocin and the social brain: neural mechanisms and perspectives in human research. Brain Res. 2014 doi: 10.1016/j.brainres.2013.11.003. in press. [DOI] [PubMed] [Google Scholar]
- 183.Arletti R, Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987;41:1725–1730. doi: 10.1016/0024-3205(87)90600-x. [DOI] [PubMed] [Google Scholar]
- 184.Arletti R, Benelli A, Poggioli R, Luppi P, Menozzi B, Bertolini A. Aged rats are still responsive to the antidepressant and memory-improving effects of oxytocin. Neuropeptides. 1995;29:177–182. doi: 10.1016/0143-4179(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 185.Nowakowska E, Kus K, Bobkiewicz-Kozlowska T, Hertmanowska H. Role of neuropeptides in antidepressant and memory improving effects of venlafaxine. Pol J Pharmacol. 2002;54:605–613. [PubMed] [Google Scholar]
- 186.Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology. 2010;58:56–6. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 187.Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebro-ventricular administration of oxytocin. Proc Natl Acad Sci USA. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Neumann ID. The advantage of living social: brain neuropeptides mediate the beneficial consequences of sex and motherhood. Front Neuroendocrinol. 2009;30:483–489. doi: 10.1016/j.yfrne.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 189.Lukas M, Neumann ID. Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav Brain Res. 2013;251:85–94. doi: 10.1016/j.bbr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 190.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 191.Melis MR, Succu S, Sanna F, Boi A, Argiolas A. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur J Neurosci. 2009;30:1349–1357. doi: 10.1111/j.1460-9568.2009.06912.x. [DOI] [PubMed] [Google Scholar]
- 192.Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology. 2007;194:309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Keck ME, Holsboer F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides. 2001;22:835–844. doi: 10.1016/s0196-9781(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 195.Keck ME. Corticotropin-releasing factor, vasopressin and receptor systems in depression and anxiety. Amino Acids. 2006;31:241–250. doi: 10.1007/s00726-006-0333-y. [DOI] [PubMed] [Google Scholar]
- 196.Donner N, Bredewold R, Maloumby R, Neumann ID. Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur J Neurosci. 2007;25:1804–1814. doi: 10.1111/j.1460-9568.2007.05416.x. [DOI] [PubMed] [Google Scholar]
- 197.Hillerer KM, Reber SO, Neumann ID, Slattery DA. Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: implications for postpartum anxiety and mood disorders. Endocrinology. 2011;152:3930–3940. doi: 10.1210/en.2011-1091. [DOI] [PubMed] [Google Scholar]
- 198.Brummelte S, Pawluski JL, Galea LA. High post-partum levels of corti-costerone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: a model of post-partum stress and possible depression. Horm Behav. 2006;50:370–382. doi: 10.1016/j.yhbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 199.Brummelte S, Galea LA. Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav. 2010;58:769–779. doi: 10.1016/j.yhbeh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 200.Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. Proc Natl Acad Sci USA. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leuner B, Mirescu C, Noiman L, Gould E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus. 2007;17:434–442. doi: 10.1002/hipo.20278. [DOI] [PubMed] [Google Scholar]
- 202.Hillerer KM, Neumann ID, Couillard-Despres S, Aigner L, Slattery DA. Lactation-induced reduction in hippocampal neurogenesis is reversed by repeated stress exposure. Hippocampus. 2014;24:673–683. doi: 10.1002/hipo.22258. [DOI] [PubMed] [Google Scholar]
- 203.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weiss-taub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;30:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 204.Fuchs E. Neurogenesis in the adult brain: is there an association with mental disorders? Eur Arch Psychiatry Clin Neurosci. 2007;257:247–249. doi: 10.1007/s00406-007-0741-3. [DOI] [PubMed] [Google Scholar]
- 205.Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22:861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABAA-receptor function in the adult brain: insights from the oxytocin neurone. Trends Neurosci. 2000;23:190–195. doi: 10.1016/s0166-2236(99)01540-4. [DOI] [PubMed] [Google Scholar]
- 207.Theodosis DT, Montagnese C, Rodriguez F, Vincent JD, Poulain DA. Oxytocin induces morphological plasticity in the adult hypothalamo-neuro-hypophysial system. Nature. 1986;322:738–740. doi: 10.1038/322738a0. [DOI] [PubMed] [Google Scholar]
- 208.Piet R, Bonhomme R, Theodosis DT, Poulain DA, Oliet SH. Modulation of GABAergic transmission by endogenous glutamate in the rat supraoptic nucleus. Eur J Neurosci. 2003;17:1777–1785. doi: 10.1046/j.1460-9568.2003.02611.x. [DOI] [PubMed] [Google Scholar]
- 209.Theodosis DT, Koksma JJ, Trailin A, Langle SL, Piet R, Lodder JC, Timmerman J, Mansvelder H, Poulain DA, Oliet SH, Brussaard AB. Oxytocin and estrogen promote rapid formation of functional GABA synapses in the adult supraoptic nucleus. Mol Cell Neurosci. 2006;31:785–794. doi: 10.1016/j.mcn.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 210.Israel JM, Poulain DA, Oliet SH. Oxytocin-induced postinhibitory ebound firing facilitates bursting activity in oxytocin neurons. J Neurosci. 2008;28:385–394. doi: 10.1523/JNEUROSCI.5198-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yao Y, Fu LY, Zhang X, van den Pol AN. Vasopressin and oxytocin excite MCH neurons, but not other lateral hypothalamic GABA neurons. Am J Physiol Regul Integr Comp Physiol. 2012;302:R815–R824. doi: 10.1152/ajpregu.00452.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Muhlethaler M, Charpak S, Dreifuss JJ. Contrasting effects of neurohy-pophysial peptides on pyramidal and non-pyramidal neurones in the rat hippocampus. Brain Res. 1984;308:97–107. doi: 10.1016/0006-8993(84)90921-1. [DOI] [PubMed] [Google Scholar]
- 213.Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, Song M, Wu CF. Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addict Biol. 2012;17:758–769. doi: 10.1111/j.1369-1600.2012.00439.x. [DOI] [PubMed] [Google Scholar]
- 215.Wrobel LJ, Reymond-Marron I, Dupre A, Raggenbass M. Oxytocin and vasopressin enhance synaptic transmission in the hypoglossal motor nucleus of young rats by acting on distinct receptor types. Neuroscience. 2010;165:723–735. doi: 10.1016/j.neuroscience.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 216.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 217.Terenzi MG, Ingram CD. Oxytocin-induced excitation of neurones in the rat central and medial amygdaloid nuclei. Neuroscience. 2005;134:345–354. doi: 10.1016/j.neuroscience.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 218.Condés-Lara M, Rojas-Piloni G, Martínez-Lorenzana G, López-Hidalgo M, Rodríguez-Jimenez J. Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A-delta and C fiber primary afferent excitation of spinal cord cells. Brain Res. 2009;1247:38–49. doi: 10.1016/j.brainres.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 219.Bulbul M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T. Hypothalamic oxytocin attenuates CRF expression via GABA(A) receptors in rats. Brain Res. 2011;1387:39–45. doi: 10.1016/j.brainres.2011.02.091. [DOI] [PubMed] [Google Scholar]
- 220.Viviani D, Terrettaz T, Magara F, Stoop R. Oxytocin enhances the inhibitory effects of diazepam in the rat central medial amygdala. Neuropharmacology. 2010;58:62–68. doi: 10.1016/j.neuropharm.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 221.Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 222.Mann PE, Bridges RS. Lactogenic hormone regulation of materna behavior. Prog Brain Res. 2001;133:251–262. doi: 10.1016/s0079-6123(01)33019-4. [DOI] [PubMed] [Google Scholar]
- 223.Groer M, Davis M, Casey K, Short B, Smith K, Groer S. Neuroendocrine and immune relationships in postpartum fatigue. MCN Am J Matern Child Nurs. 2005;30:133–138. doi: 10.1097/00005721-200503000-00012. [DOI] [PubMed] [Google Scholar]
- 224.Donner N, Neumann ID. Effects of chronic intracerebral prolactin on the oxytocinergic and vasopressinergic system of virgin ovariectomized rats. Neuroendocrinology. 2009;90:315–322. doi: 10.1159/000225986. [DOI] [PubMed] [Google Scholar]
- 225.Kokay IC, Petersen SL, Grattan DR. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology. 2011;152:526–535. doi: 10.1210/en.2010-0668. [DOI] [PubMed] [Google Scholar]
- 226.Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011;31:18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]