Abstract
AIM
To investigate the effects of Ground Cherry (Physalis angulata L.) standardized supercritical CO2 extract in trinitrobenzenesulphonic acid (TNBS) model of rat intestinal inflammation.
METHODS
The animals were divided into groups that received vehicle or P. angulata extract (PACO2) orally at the doses 25, 50 and 100 mg/kg daily by 5 d before TNBS damage. Protective effects of PACO2 were assessed by macroscopic analysis, biochemical determinations of the levels of myeloperoxidase (MPO), alkaline phosphatase (ALP), glutathione and cytokines (such as INF-γ, IL-1β, IL-6, IL-10 and TNF-α), gene expression evaluation (including Hsp70, heparanase, NF-κB, mitogen-activated protein kinases (Mapk) 1, 3, 6 and 9, and the mucins genes Muc 1, 2, 3 and 4) and histopathological studies using optical, and electronic (transmission and scanning) microscopy.
RESULTS
PACO2 extract promoted a significant reduction in MPO and ALP activities, reducing oxidative stress and neutrophil infiltration. These effects were accompanied by significant reduction of colonic levels of IFN-γ and IL-6 and down-regulation of heparanase, Hsp70, Mapk3, Mapk9, Muc1 and Muc2 genes expression when compared with TNBS-control animals. In addition, protective effects were also evidenced by reduced neutrophil infiltration, recovery of cell architecture and replacement of mucin by histopathological and ultrastructural analysis.
CONCLUSION
Physalis angulata supercritical CO2 extract is an intestinal anti-inflammatory product that modulates oxidative stress, immune response and expression of inflammatory mediators, with potentially utility for treating inflammatory bowel disease.
Keywords: Intestinal disease, Inflammatory bowel disease, Physalis angulata L. trinitrobenzenesulphonic acid
Core tip: We report, at the first time, the protective effects of a supercritical CO2 plant extract from aerial parts of Ground Cherry (Physalis angulata L.) in a model of intestinal inflammation induced by trinitrobenzenesulphonic acid in rats. The effects were related to presence of plant steroids, compounds chemically related to glucocorticoids, reference drugs used to treat human inflammatory bowel disease (IBD). The intestinal anti-inflammatory activity of Physalis angulata plant extract was related to its capacity to modulate oxidative stress, immune response and gene expression of inflammatory mediators. This way, the standardized plant extract of Ground Cherry enriched with phytosterols has potential for use to treat IBD.
INTRODUCTION
Inflammatory bowel diseases (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), are diseases of modern society associated with a modern and urbanized lifestyle and caused by an increase in stress, bad diet habits and sedentariness[1,2]. CD and UC are chronic inflammatory disorders affecting the gastrointestinal tract and are characterized by periods of exacerbation followed by prolonged intervals of remission of symptoms[3,4]. The etiopathogenesis of these diseases has not been fully elucidated, but is presumed to result from a complex interplay among genetic, environmental, microbial and immune factors[5]. An exaggerated and inappropriate mucosal immune response mediated by mucosal T cells triggers intense synthesis and release of several pro-inflammatory mediators, including reactive species of oxygen and nitrogen, and a multitude of pro- and anti-inflammatory cytokines[6,7].
The available drugs, such as 5-aminosalicylate derivatives, glucocorticoids, immunosuppressive and monoclonal antibodies, have exhibited beneficial effects in the treatment of IBD, but do not represent a definitive cure. Indeed, these drugs have several side effects, and many patients do not respond to these treatments[3,8,9]. Dissatisfaction with the current pharmacological treatments has resulted in an increased interest to use complementary medicine approaches, including medicinal plant extracts or natural active compounds[10]. Based on this, our research group has been interested in studying the pharmacological activities of natural products against intestinal inflammation, focusing on tropical medicinal plants, of which Ground Cherry (Physalis angulata L.) was selected based upon its ethnopharmacological, pharmacological and chemical data.
Ground cherry is a native Brazilian weed from the Solanaceae family, which grows especially in the Brazilian Amazon Forest and other tropical countries of Africa, America and Asia, where it has been used traditionally as an anti-inflammatory herbal product and to treat several health disorders, such as cold, cough, fever, pain, malaria and nervous diseases[11,12]. Fruits from the Ground Cherry are used as food in several countries, particularly as a sophisticated additive to salads. Reputed pharmacological effects, such as antinociceptive, immunosuppressive, anti-protozoal, antineoplastic and anti-inflammatory, have been associated with the constituent presence of sitosterol, stigmasterol and other phytosterols, representing the major components of the plant extract[13-16].
Based on the ethnopharmacological data and pharmacological and chemical studies, a standardized CO2 supercritical preparation from aerial parts of P. angulata containing 10%-18% of phytosterols was patented by our research group, claiming corticoid-like effects evidenced by reduction of TNF-α, IL-6, IL1-β and COX-2 levels in non-stimulated and stimulated human fibroblasts and keratinocytes[17]. In the study presented herein, we evaluated this standardized plant extract from P. angulata in a model of intestinal inflammation induced by trinitrobenzenesulphonic acid (TNBS) in rats.
MATERIALS AND METHODS
Plant material and extract standardization
Physalis angulata L. was cultivated using organic agricultural methods and submitted to taxonomic identification at herbarium Irina Gemtchujinikov (Department of Botany, Institute of Biosciences, Universidade Estadual Paulista - UNESP/SP/BR), where a voucher specimen was deposited. The aerial parts were collected, dehydrated in hothouse with air circulation and renewal, and pulverized in an industrial mill.
A supercritical extraction system (Parker Autoclave Engineers, Erie, PA, United States), under the conditions of 300 bar, 40 °C and CO2 flux of 5 L/min during 150 min, was used to generate a supercritical CO2 extract enriched with phytosterols, including sitosterol, stigmasterol and physalins, which are the main constituents of Physalis plants. The enrichment of supercritical CO2 extract was compared with water:butileneglycol 1:1 extract and evaluated by Liebermann-Burchard reaction and ultraviolet spectrometry at 621 nm (Hewlett-Packard, Palo Alto, CA, United States). P. angulata L. supercritical CO2 extract (PACO2) was standardized in 10%-18% of total phytosterols and provided by Chemyunion Química Ltda (Sorocaba, Brazil).
Animals
Male Wistar rats (180-200 g) obtained from ANILAB - Animais de Laboratório, Paulínia, São Paulo (Brazil), were housed in standard environmental conditions (21 °C, 60%-70% humidity) with 12-h light/dark cycle and air filtration. Animals had free access to water and food (Biobase). Experimental protocols met the ‘‘Guidelines of Animal Experimentation’’ approved by the Ethical Committee for Animal Research (Protocol number 042/04-CEEA), Institute of Biosciences, Universidade Estadual Paulista (UNESP).
Induction of colitis and assessment of the inflammatory process
Colitis was induced using the method originally described by Morris et al[18]. Briefly, animals were fasted overnight and then anaesthetized. Under anesthesia, they were inoculated with 10 mg of TNBS dissolved in 0.25 mL 50% ethanol (v/v), by means of a Teflon cannula inserted 8 cm into the anus. During and after the TNBS administration, the rats remained in a head-down position until they recovered from the anesthesia.
Rats received 25, 50 or 100 mg/kg of the PACO2 extract orally at 96, 72, 48, 24 and 2 h before colitis induction, using an esophageal catheter (volume: 10 mL/kg). Two additional groups were included for reference: a non-colitis group and a colitic group (TNBS-control group) that received vehicle (10 mL/kg methylcellulose) orally. The animal body weights, the occurrence of diarrhea (as detected by perianal fur soiling) and the total food intakes for each group were recorded daily. Animals from all groups (n = 7) were killed at 48 h after colitis induction.
The colonic segments were obtained after laparotomy and the adhesions eventual occurrence between the colon and adjacent organs were noted. These colonic segments were placed on an ice-cold plate, cleaned of fat and mesentery, blotted on filter paper, and then the colon was weighed and its length measured under a constant load (2 g). The colon was opened longitudinally and scored for macroscopically visible damage on a 0-10 scale, according to Bell et al[19]. Subsequently, the colon was longitudinally divided into different pieces to be used for the following determinations: myeloperoxidase (MPO), and alkaline phosphatase (ALP) activity, total glutathione (GSH) content, IL-1β, IL-6, IL-10, TNF-α and INF-γ levels, and gene expression analysis.
Biochemical assays in colonic specimens
MPO activity was measured according to the technique described by Krawisz et al[20] and the results were expressed as MPO units per g of wet tissue. Total GSH content was quantified with the recycling assay[21] and the results were expressed as nmol per g of wet tissue. ALP activity was measured spectrophotometrically, as previously described, and the results were expressed as mU per mg of protein[22,23]. Quantification of cytokines (TNF-α, IL-1β, IL-6, IL-10 and INF-γ) was made on colonic samples previously weighed, homogenized, minced on an ice-cold plate and resuspended in a centrifugation tube containing 10 mmol/L phosphate-buffered saline (pH 7.4; 1:5 w/v). The tubes were placed in a shaker submerged in a 37 °C water bath for 20 min and then centrifuged at 9000 g for 30 s at 4 °C. The supernatants were frozen at -80 °C until assayed. The TNF-α, IL-1β, IL-6, IL-10 and INF-γ levels were quantified by a DuoSet ELISA Kit (R&D Systems, Inc., Minneapolis, MN, United States) to measure the concentration of the natural and recombinant rat enzyme according to the manufacturer’s instructions. The results were expressed as pg per mL.
Gene expression analysis
Colon samples (100 mg) were collected and stored in -80 °C until use for analyses of genes: GAPDH, β-actin, HPRT, HSP70, heparanase, NF-κB, mitogen-activated protein kinase (MAP)K1, MAPK3, MAPK6, MAPK9, MUC1, MUC2, MUC3, and MUC4. For the homogenization, we used 1 mL of Trizol® (Invitrogen-Life Technologies, Carlsbad, CA, United States) and a Polytron homogenizer. The total RNA extraction was made according to the Trizol® manufacturer’s protocol. The purity was determined by A260/A280 ratio using a Nanodrop 2000 (Thermo Scientific, Waltham, MA, United States). After that, 1 μg of the total RNA of colon tissue samples was incubated with DNAse I (1 U/mg RNA; Invitrogen), and then reverse transcribed with SuperScript® III (200 U/mL; Life Technologies) and oligo-d (T) primer. Primers for targets and reference genes were designed based on the rat sequences and using the IDT primer quest software (http://www.idtdna.com/primerquest/Home/Index; Invitrogen). Relative real-time RT-PCR analysis was performed with a StepOne Plus™ (Applied Biosystems Inc., Foster City, CA, United States) using Power SYBR Green PCR Master Mix® (Life Technologies) for all the genes. Amplification efficiencies for target and reference genes were similar. The primer sequences, fragment size, annealing temperature, primer concentration, NCBI reference sequence and sample concentration for each gene are shown in Table 1.
Table 1.
Details of primers used for real-time PCR analysis
| Target | Sequence | Annealing, °C | Oligo concentration, nmol/L | Fragment size, bp | NCBI reference sequence | cDNA concentration, μL |
| GAPDH | F TGACTCTACCCACGGCAAGTTCAA | 60 | 200 | 141 | NM_017008.3 | 1.000 |
| R ACGACATACTCAGCACCAGCATCA | ||||||
| β-actin | F TTGCTGACAGGATGCAGAAGGAGA | 60 | 100 | 159 | NM_031144.2 | 1.000 |
| R ACTCCTGCTTGCTGATCCACATCT | ||||||
| HPRT | F AGGGAAGTGACAATCTACCTGACG | 60 | 100 | 81 | AA900579.1 | 0.125 |
| R GAAATGTCTGTTGCTGCGTCCCTT | ||||||
| HSP70 | F ACTCCTTCGTTCGGTCTGCAATCA | 60 | 200 | 92 | NM_031971.2 | 0.125 |
| R CTGGGAATGCAAAGCACACGTGAA | ||||||
| Heparanase | F TGTCAAGAGTGAAAGGCCCAGACA | 60 | 200 | 141 | NM_022605.1 | 0.125 |
| R GCAGCTTCAAGTGCTTGGTGACAT | ||||||
| NF-κB | F AAACCAAAGCCCTGAAAGGCCATC | 60 | 200 | 120 | XM_342346.4 | 0.125 |
| R TCGGAAGGCCTCGAATGACATCAA | ||||||
| MAPK1 | F AACAGGTTGTTCCCAAACGCTGAC | 60 | 200 | 187 | NM_053842.1 | 0.500 |
| R AGTCGTCCAGCTCCATGTCAAACT | ||||||
| MAPK3 | F TACCTGGACCAGCTCAACCACATT | 60 | 200 | 173 | NM_017347.2 | 0.500 |
| R AGCAGGTCAAGAGCTTTGGAGTCA | ||||||
| MAPK6 | F AACTGAGCCAGTGGAAGAAGGGAA | 60 | 200 | 164 | NM_031622.2 | 1.000 |
| R TTAACGTGGCCTGGATGGACTTGA | ||||||
| MAPK9 | F TCATGGGAGAGCTGGTGAAAGGTT | 60 | 200 | 106 | NM_017322.2 | 1.000 |
| R ATGAACTCTGCGGATGGTGTTCCT | NM_001270544.1 | |||||
| NM_001270545.1 | ||||||
| MUC1 | F CCGCTACTACCAAGAACTGAAG | 60 | 200 | 102 | NM_012602.1 | 0.500 |
| R GAGCCTGACCTGAACTTGATAG | ||||||
| MUC2 | F GGCTCTGCTCTCTGTGTTATAG | 60 | 200 | 123 | U68172.1 | 1.000 |
| R CAGTTTGGGAAGAAGGTAGGG | ||||||
| MUC3 | F GGGAAATAGACCCTGCAGTTAG | 60 | 200 | 107 | U76551.1 | 0.125 |
| R GATCATCGCTTGCCGTCATA | ||||||
| MUC4 | F ATGTGGAGGTGGGAGAAATG | 60 | 200 | 122 | AF240632.1 | 0.500 |
| R CCCTGGAACTGGAATTAGAGAC |
Reactions were optimized to provide maximum amplification efficiency for each gene. PCR was performed in 25 μL reaction volumes in duplicate, in a MicroAmp® Fast Optical 96-Well Reaction Plate, 0.1 mL (Applied Biosystems Inc.), and the specificity of each PCR product was determined by melting curve analysis. Negative controls (water replacing cDNA) were run in every plate.
The relative expression of each target gene was calculated using the ∆∆Ct method with efficiency correction[24]; the control was a cDNA sample from each cell type analyzed. To select the most stable reference gene for detailed analyses, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-actin and HPRT amplification profiles were compared using the RefFinder software (http://www.leonxie.com/referencegene.php?type=reference). All gene expression analysis was performed with β-actin as the reference gene for colon tissue.
Histological evaluation and ultrastructure analysis
A representative colon fragment located 1 cm above the lesion was collected for histological slide preparation and stained with hematoxylin and eosin (HE) for analysis of the microscopic damage. Images were acquired using Zeiss Imager Axio Vision 4.8.2.0 software. Samples of the colon were fixed in 2.5% glutaraldehyde and 4% paraformaldehyde solutions in 0.1 mol/L phosphate buffer (pH 7.3) for transmission electron microscopy (TEM) analysis. Afterwards, the material was post-fixed in a 1% osmium tetroxide solution in 0.1 mol/L phosphate buffer (pH 7.3) at room temperature for 2 h, dehydrated through a graded series of acetone, and embedded in Araldite® resin. Ultra-thin sections (70 ηm) were double-stained with uranyl acetate and lead citrate. All samples were analyzed and images were acquired using a Tecnai Spirit TEM from Fei Company, 80 kV. For scanning electron microscopy (SEM) analysis, small fragments of the colon were fixed in a 2.5% glutaraldehyde solution in 0.1 mol/L phosphate buffer (pH 7.3) and post-fixed in 1% osmium tetroxide in 0.1 mol/L phosphate buffer (pH 7.3) at room temperature for 2 h. Samples were dehydrated through the serial application of increasingly concentrated ethanol, critical point-dried with liquid CO2, and sputter-coated with gold (10 ηm). All samples were analyzed and images were acquired using a QUANTA 200 SEM from Fei Company, 80 kV.
Statistical analysis
The parametric results are expressed as the mean ± SE of the mean, and the differences between means were tested for statistical significance using one-way analysis of variance followed by Dunnett’s post-test. Nonparametric data (score) are expressed as the median (range) and were analyzed with the Kruskal-Wallis test, followed by Dunn post-test. Differences between proportions were analyzed by Fisher’s exact test. Statistical significance was set at P ≤ 0.05.
RESULTS
TNBS instillation resulted in colonic inflammation, which was evidenced after 48 h by severe necrosis of the mucosa (extending for 2 to 3 cm along the colon), hyperemia, significant increase in the colonic weight/length ratio, incidence of diarrhea and adherence to adjacent organs (Table 2). These effects were related to significant reduction in both body weight and food intake (data not shown).
Table 2.
Macroscopic effects of the PACO2 standardized extract (25, 50 or 100 mg/kg) in acute phase response of intestinal inflammation induced by trinitrobenzenesulphonic acid
| Group | Damage score, 0-101 | Extension of lesion, cm2 | Colonic weight, mg/cm2 | Diarrhea3 | Adherence3 |
| Non-colitic | 0a | 0a | 82.7 ± 2.10a | 0%a | 0%a |
| TNBS-control | 7 (2-8) | 2.55 ± 0.28 | 136.0 ± 5.87 | 87.50% | 81.25% |
| PACO2 - 25 mg/kg | 7 (5-8) | 2.44 ± 0.18 | 135.0 ± 6.86 | 100.00% | 71.42% |
| PACO2 - 50 mg/kg | 7 (2-9) | 2.11 ± 0.34 | 124.0 ± 7.72 | 85.71% | 71.42% |
| PACO2 - 100 mg/kg | 7 (4-9) | 2.93 ± 0.20 | 162.0 ± 5.04 | 100.00% | 57.14% |
Score data are expressed as the median (range);
Extension of lesion and colonic weight data are expressed as the mean ± SE of the mean;
Diarrhea and adherence were analyzed by Fisher's exact test.
P ≤ 0.01.
Biochemically, the colonic damage induced by TNBS was characterized by increased activities of MPO (13-fold) and ALP (4-fold) and high levels of colonic TNF-α, IL-1β, IL-6, IFN-γ and IL-10 compared with healthy animals. Furthermore, significant colonic GSH depletion took place in the inflamed colon.
Administration of P. angulata extract (PACO2) at the doses of 50 and 100 mg/kg decreased ALP activity relative to TNBS-control group. Reduction of INF-γ and IL-6 colonic levels, and MPO activity were observed after treatment with 50 and 100 mg/kg, respectively. The 25 mg/kg dose of PACO2 did not alter any cytokine analyzed (Tables 3 and 4).
Table 3.
Effects of the PACO2 standardized extract (25, 50 or 100 mg/kg) on glutathione content, myeloperoxidase and alkaline phosphatase activities in acute phase response of intestinal inflammation induced by trinitrobenzenesulphonic acid
| Group | GSH, nmol/g tissue | MPO, U/g tissue | ALP, mU/mg protein |
| Non-colitic | 1687 ± 54.2a | 104 ± 13.7a | 8.91 ± 2.17a |
| TNBS control | 1295 ± 60 | 1703 ± 390 | 28.9 ± 3.07 |
| PACO2, 25 mg/kg | 1208 ± 33.8 | 1360 ± 286 | 21.0 ± 2.44 |
| PACO2, 50 mg/kg | 1350 ± 85.1 | 921 ± 181 | 18.0 ± 1.62c |
| PACO2, 100 mg/kg | 1428 ± 50.6 | 831 ± 108c | 19.5 ± 1.50c |
Data are expressed as mean ± standard error of the mean.
P ≤ 0.05 and
P ≤ 0.01. GSH: Glutathione; MPO: Myeloperoxidase; ALP: Alkaline phosphatase.
Table 4.
Effects of the PACO2 standardized extract (25, 50 or 100 mg/kg) on cytokine levels in acute phase response of intestinal inflammation induced by trinitrobenzenesulphonic acid
| Group | IFN-γ | TNF-α | IL1-β | IL-6 | IL-10 |
| Non-colitic | 78.2 ± 7.9c | 121.2 ± 7.0a | 1395.0 ± 136.9a | 421.1 ± 45.3a | 467.1 ± 31.1a |
| TNBS control | 125 ± 20.4 | 240.3 ± 34.3 | 2205.0 ± 147.4 | 893.8 ± 105.5 | 675.5 ± 38.6 |
| PACO2, 25 mg/kg | 92.1 ± 8.6 | 246.6 ± 34.3 | 1867.0 ± 221.4 | 891.9 ± 87.09 | 674.5 ± 40.4 |
| PACO2, 50 mg/kg | 67 ± 11.7c | 167.9 ± 23.6 | 2106.0 ± 128.9 | 563.4 ± 55.5c | 626.9 ± 66.5 |
| PACO2, 100 mg/kg | 76.4 ± 12.7c | 205.5 ± 34.5 | 2323.0 ± 154.1 | 649.5 ± 86.9 | 537.4 ± 41.1 |
Data are expressed as mean ± SE of the mean.
P ≤ 0.05 and
P ≤ 0.01.
Expression of genes involved in the inflammatory process (Hpse, Hsp70, Mapk1, Mapk3, Mapk6, Mapk9, and NF-κB) and genes involved in the protective functions (Muc1, Muc2, Muc3, and Muc4) were differentially affected by the plant extract treatment. PACO2 was able to diminish the expression of Hsp70, Mapk3, Mapk9, Muc1, and Muc2 at doses of 25, 50 and 100 mg/kg and Hpse at doses of 25 and 100 mg/kg, as compared with the TNBS-control group (Figure 1).
Figure 1.
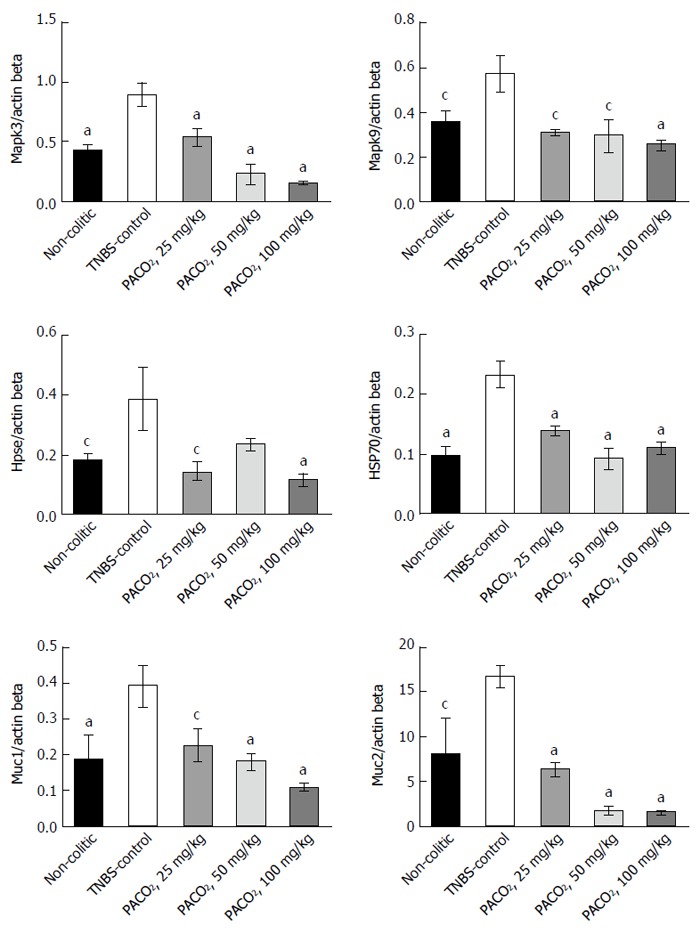
Effects of the PACO2 standardized extract (25, 50 or 100 mg/kg) in the gene expression of Mapk3, Mapk9, heparanase, Hsp70, Muc1 and Muc2 in acute phase response of intestinal inflammation induced by trinitrobenzenesulphonic acid. Data are expressed as mean ± standard error of the mean. cP ≤ 0.05, aP ≤ 0.01 vs TNBS control group.
Histopathological evaluation demonstrated that treatments with PACO2 led to improved colon cytoarchitecture, including the restoration of tubular glands containing goblet cells, especially at the doses of 50 mg/kg and 100 mg/kg; moreover, edema was significantly reduced, as compared to the TNBS-control group (Figure 2). In SEM, PACO2 at a dose of 25 mg/kg showed a slight recovery of the polygonal shape of absorptive cells; however, the number of goblet cells remained elevated. At a dose of 50 mg/kg, a greater recovery of polygonal appearance and increase of the main was observed compared to the TNBS-control group. PACO2 at a dose of 100 mg/kg exhibited a good recovery of mucosal architecture, with goblet cells similar to healthy animals, including crypts and mucin (Figure 3). In transmission ultrastructure analysis, reduced mucin granules were observed in colonic goblet cells at a dose of 25 mg/kg, but the intercellular edema was lower. Already at dose of 50 mg/kg, the colonocytes are juxtaposed with nucleus in basal position and preserved microvilli. The 100 mg/kg-treated group showed goblet cells with mucus granules similar to healthy animals, the nucleus in basal position, mild intercellular edema and atypical microvilli (Figure 4).
Figure 2.
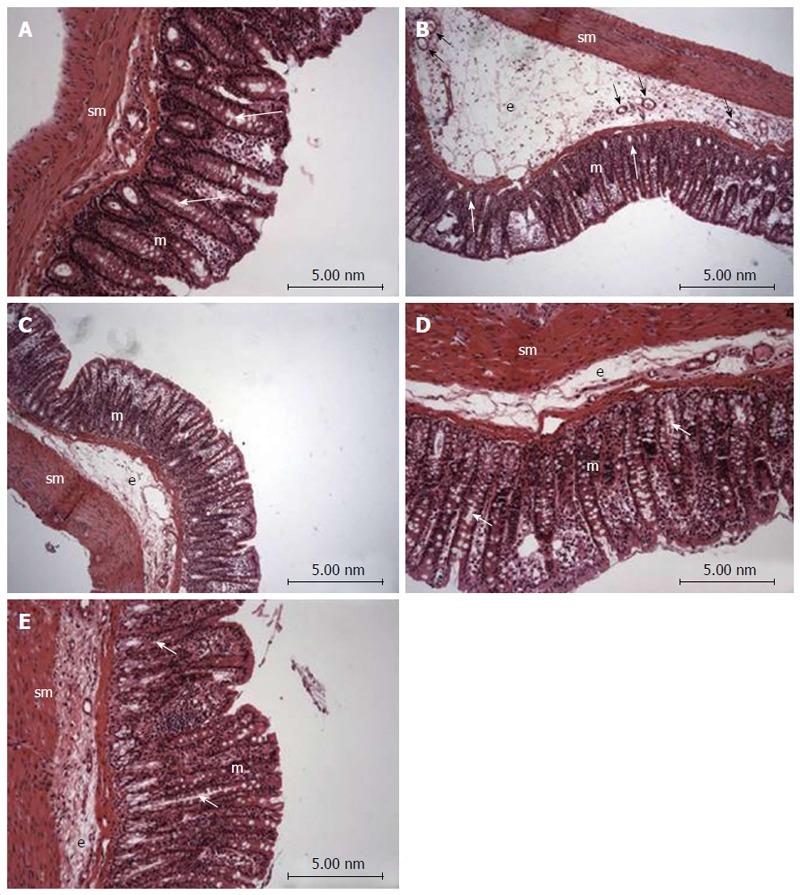
Photomicrography (hematoxylin and eosin) of colon samples from different experimental groups. Mucosa (m), tubular glands (white arrows), blood vessels (black arrow), submucosa (sm) and edema (e). In A (non-colitic group): the mucosa (m) contains numerous straight tubular glands (white arrows) with many lightly stained goblet cells; the crypt (black arrow), submucosa (sm) and luminal epithelium was intact with a typical morphology; In B (TNBS control group): tubular glands (white arrows) were reduced and the goblet cells were atypical; a disruption with extensive edema (e) and blood vessels (black arrow) can be observed. In PACO2-treated groups, colon cytoarchitecture was recovering and included the restoration of tubular glands (white arrows) containing goblet cells, especially at the doses of 50 mg/kg (D) and 100 mg/kg (E); edema (e) was significantly reduced in animals treated with at doses of 50 mg/kg (D) and 100 mg/kg (E) and in a lower proportion in animals treated with doses of 25 mg/kg (C), when compared to the TNBS control group (B).
Figure 3.
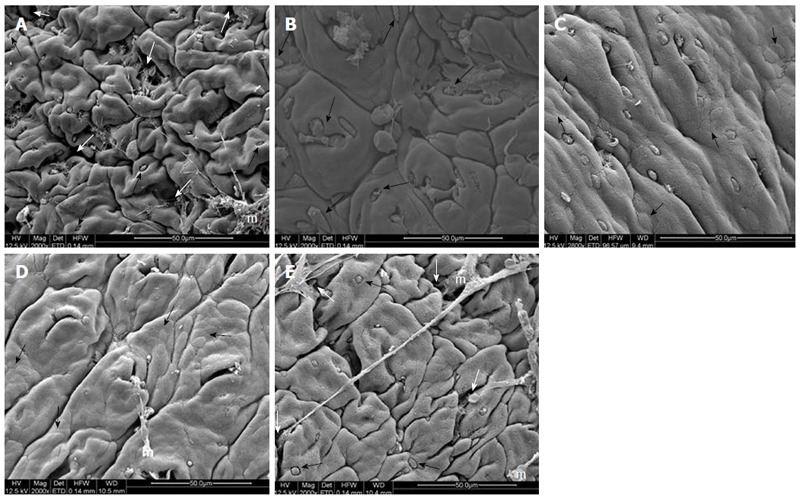
Electromicrographs (scanning electron microscopy) of colon samples from different experimental groups. Crypts (white arrows), goblet cells (black arrows) and mucin (m). In A (non-colitic group): regular mucosal architecture with polygonal units as structural subunits, regular microvilli giving a smooth velvety appearance, crypts (white arrows), goblet cells (black arrows) and mucin (m) extruded; In B (TNBS control group): complete loss of the smooth velvety appearance and polygonal shape of absorptive cells, hyperplasia of goblet cells (white arrows), absence of mucin extrusion and crypts, characteristics of ulcerative colitis and Crohn’s disease; In C (PACO2 25 mg/kg-treated group): slight recovery of polygonal shape (black arrows) of absorptive cells, however the number of goblet cells remained elevated; In D (PACO2 50 mg/kg-treated group): smaller number of goblet cells, greater recovery of polygonal appearance (black arrows) and increased mucin (m) compared to TNBS control group; In E (PACO2 100mg/kg-treated group): good recovery of mucosa architecture, with goblet cells (black arrows) similar to healthy animals including crypts (white arrows) and mucin (m) for protection.
Figure 4.
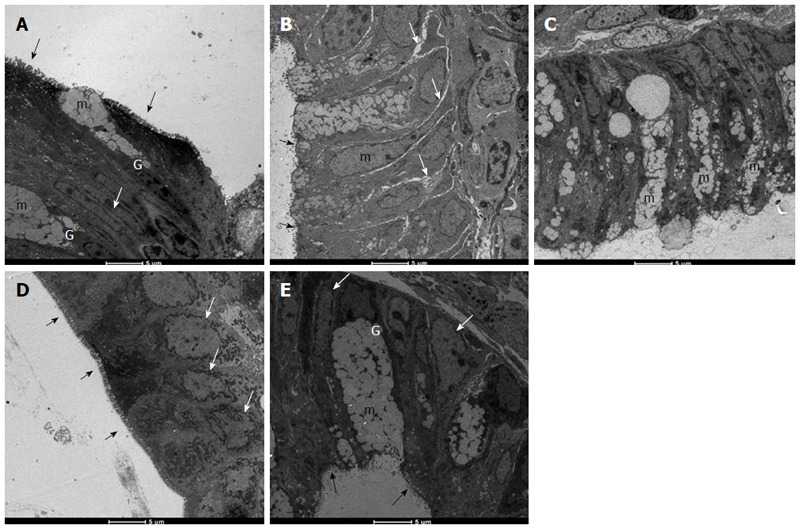
Electromicrographs (transmission electron microscopy) of colon samples from different experimental groups. Goblet cells (G), mucus granules (m); Microvilli (black arrows); nucleus in a basal position (white arrow); edema (grey arrows). In A (non-colitic group): colonic goblet cells (G) showing the mucus granules (m); absorptive epithelial cells with a well-developed brush border containing numerous regular microvilli (black arrows) typical of enterocytes and its nucleus in a basal position (n) shows chromatin (white arrow); In B (TNBS control group): mucin granules in colonic goblet cells are reduced and disarranged (m); surface cells have no distinct brush border with atypical microvilli (black arrow); the intercellular space was enhanced as a signal of edema (white arrows); In C (PACO2 25 mg/kg-treated group): mucin granules in colonic goblet cells are reduced and disarranged (m), but intercellular edema is reduced (white arrows); In D (PACO2 50 mg/kg-treated group): juxtaposed colonocytes with nucleus in basal position (white arrows) and preserved microvilli (black arrows); In E (PACO2 100 mg/kg-treated group): goblet cells (G) showing the mucus granules (m) similar to healthy animals; nucleus in basal position (yellow arrows); mild intercellular edema and atypical microvilli (black arrows).
DISCUSSION
The combination of products that improve the anti-inflammatory activity of drugs would be an important approach for IBD treatment, since the current pharmacological treatments for IBD, including aminosalicylates, corticosteroids, biological agents and immunosuppressant, result in serious side effects and most patients do not respond to these therapies[3,8,9]. The results of this study showed that a phytosterol standardized extract obtained from aerial parts of P. angulata improved the response of animals throughout the duration of intestinal damage induced by TNBS, modulating oxidative stress, immune response and inflammatory genes expression.
The biochemical changes that occur after administration of TNBS were similar to what occurs in humans, mainly excessive production of reactive oxygen and nitrogen metabolites[25]. Neutrophils are responsible for tissue injury in inflammatory diseases, producing free radicals and hypochlorous acid that increase the local oxidative stress and cause damage[26]. In this process, MPO plays a central role, generating reactive intermediates (primarily hypochlorous acid) and leading to oxidative damage of lipids and proteins, thereby acting as an enzyme strongly linked to both inflammation and oxidative stress[27]. Indeed, MPO acts as an inflammatory marker, so that activity reduction of this enzyme is also related to lower cellular infiltration into the colon.
ALP is also a sensitive inflammatory process biochemical marker, which is increased as a consequence of tissue-non-specific isoform and associated with an influx of inflammatory cells[28,29]. However, studies suggest that ALP release occurs as a consequence of lipid peroxidation of membranes[30]. Although oxidative stress can also be measured by GSH levels (the first line of defense against the toxic products of oxygen and other hydroperoxides[31,32]), PACO2 at the tested dose was ineffective for avoiding the GSH depletion induced by inflammatory process. Nevertheless, the inhibitory effects on the MPO and ALP activity can be interpreted either by antioxidant or anti-inflammatory properties of the PACO2.
The focus on the adaptive immune response in IBD led to a consensus that the mucosa is dominated by CD4+ (Th1) lymphocyte phenotypes, characterized by the production of INF-γ[33]. Immune response in the colon after TNBS administration is also characterized by increased production of IFN-γ[34]. The increased synthesis of IFN-γ has been associated with the aggravation of disease in patients with IBD[35]. IL-6 plays a central role in diverse inflammatory responses via recruiting CD4 (Th17) lymphocyte phenotypes, so it is evident that neutralization of IL-6 has a beneficial effect on intestinal inflammation, acting in tissue remodeling and neutrophil infiltration[35-37]. Our results showed that PACO2 anti-inflammatory properties were also related to down-regulation of colonic IFN-γ and IL-6 levels; but, at the tested doses, no effects were observed on other cytokines, such as TNF-α, IL-1β and IL-10.
The genetic profiling identified a number of genes or genetic loci that have been associated with IBD conditions, and many of these genes are directly related to signaling pathways that regulate the innate and adaptive immune systems, triggering complex intracellular cascades[38,39]. One of these ways of controlling signaling are MAPKs, an evolutionarily conserved family of serine/threonine kinases involved in a wide range of biological processes, such as cell growth, proliferation, differentiation, migration and apoptosis[40,41]. Studies show that Mapk1 and Mapk3 were significantly expressed in animals from the TNBS-control group, indicating that these genes play a significant role in the inflammation caused by TNBS, and are expressed in the active phases of IBD[38,42]. Indeed, evidence has shown that Mapk3 activation plays an essential role in the signaling mechanisms that lead to increased neutrophil adhesion[43]. Our data showed that PACO2 decreased expression of Mapk3 genes, probably contributing to the reduced neutrophil infiltration evidenced by histopathological analysis. In fact, reduced neutrophil infiltration promoted by PACO2 can be closely associated to inhibition of MPO activity and reduction of IL-6 colonic levels.
Another kinase that has the ability to phosphorylate other protein targets is Mapk9, also known as JNK2[38]. In our research, we found increased Mapk9 gene expression after TNBS administration, indicating a participation of this MAPK in the development of inflammation. Mapk9 has been implicated as an important regulator of cytokine release and in the response of the neutrophils to release inflammatory stimuli[44]. Indeed, a specific inhibitor of Mapk9 in the murine IBD model reduced production of inflammatory cytokines, including IFN-γ and IL-6[45]. This way, it is possible to suggest that modulatory effects of PACO2 on cytokine production was dependent of down-regulation of Mapk9 gene expression.
A complex network involving heparanase and other inflammatory pathways, such as hsp70 and NF-κB, has been proposed to explain the immunomodulatory response in inflammatory conditions[46,47]. Heparanase has an important role in body physiology and inflammatory responses, where its expression is an important mechanism underlying chronic colonic inflammation[48]. In fact, it has been hypothesized that heparanase activates macrophages, increasing NF-κB signaling and releasing several cytokines and reactive oxygen species, as well as heparanase, via TNF-α-dependent mechanisms, promoting disruption of the cell membrane[46-49].
Recently, the relationship among heparanase, hsp70, NF-κB and cytokine profile was identified by our research group in the TNBS-model of intestinal inflammation, demonstrating the pharmacological importance of the heparanase modulation as a target of new drugs[50]. Indeed, in the complex inflammatory response, heat shock proteins have been implicated in the pathogenesis of IBD, mainly Hsp70 that confers resistance to various tissues and are synthesized rapidly after exposure to the offending agent, being useful in maintaining homeostasis, facilitating the repair of damaged areas and providing protection against injuries[51,52]. According to results, the anti-inflammatory properties of PACO2 were closely related to inhibitory gene expression of both heparanase and Hsp70, leading to a lower colonic damage as observed by histopathological analysis.
Another important factor related to gut homeostasis is mucus production, responsible for formation of gel layers covering the gastrointestinal tract and providing a semi-permeable barrier between the lumen and the epithelium[53]. The mucus gel barrier, formed by building blocks of mucins, which determine the thickness and properties of mucus, contributes to maintaining the integrity of the epithelium in the colon and plays a key role in IBD[53,54]. In small and large intestine, Muc2 is the major secretory mucin synthesized and secreted by goblet cells, whereas goblet and absorptive cells express the membrane-bound mucins Muc1, Muc3, Muc4 and Muc13 in the apical membrane[53-55]. Although the hypothesis that mucins and mucus barrier are protective factors in IBD is difficult to prove in humans, animal models, including the TNBS model, permit detailed analysis of the role of mucins in intestinal inflammation. In fact, our results showed that TNBS increased colonic Muc1 and Muc2 expression, probably as a response against inflammatory process and to maintain the mucosal barrier integrity[56]. Although PACO2 reversed these effects, an increased production of mucus was observed by histopathological analysis and was clearly evidenced in the TEM and SEM studies.
The main structural damage induced by TNBS was detected by histopathological analysis, and included membrane disruption, reduction in mucus production and increase of inflammatory cells. These effects were clearly related to reduction in MPO activity and modulation of IL-6 and INF-γ, whereas membrane integrity was affected by heparanase and Hsp70 up-regulation. These damaged effects were completely reversed by treatment with different doses of PACO2, which acted as an anti-inflammatory agent modulating oxidative stress, immune response and inflammatory gene expression.
All protective effects produced by PACO2 can be partially attributed to presence of phytosterols in the standardized plant extract, natural compounds with several pharmacological activities able to regulate the balance of Th1 and Th2 response, and inhibition of nitric oxide production, TNF-α and IFN-γ[9,57]. The main group of sterols in this plant are the seco-sterols (named physalins) and molecules derived from ergostane[9,58], which were identified as active compounds from P. angulata, reducing vascular permeability, neutrophil infiltration[59], inflammatory cytokine production such as of IFN-γ and IL-6, and modulating the expression of Mapks[60], as observed in our experiments. Indeed, administration of seco-sterols of P. angulata has been useful to treat autoimmune diseases, allergies or in cases of transplant rejection with lower toxicity when compared to glucocorticoids[59]. These studies showed that phytosterols of P. angulata are promising compounds for treatment of inflammatory diseases, suggesting that the search for new natural compounds similar to steroids such as glucocorticoids is a promising field of research to obtain new drugs with efficacy and safety.
In conclusion, the standardized extract of P. angulata L.-PACO2-exerts intestinal anti-inflammatory effect by modulating a series of pathways and mediators of the inflammatory response, mainly related to oxidative stress and immune response. These effects were related to the presence of phytosterols, natural compounds structurally related to steroids, which are promising substances for the treatment of inflammatory diseases. In this way, PACO2 from Physalis angulata is an innovative active ingredient and a phytopharmaceutical preparation with potential clinical applications in the control of intestinal inflammation.
ACKNOWLEDGMENTS
The authors acknowledge technical support provided by Aline W Fantinati, Alexandre S Chagas, Alexandre Tanimoto, Adriana Del Ben, Tainan FS Curimbaba, Juliana Severi e Juliana R Ribeiro. Chemyunion Chemistry provided us with the standard extract of Physalis angulata (PACO2).
COMMENTS
Background
Inflammatory bowel disease (IBD) is a chronic inflammatory process of the gut, including two different disorders: ulcerative colitis and Crohn’s disease. Nowadays, the search for new drugs to treat IBD is based in immunomodulators, modulators of intestinal microbiota and antioxidative natural and synthetic compounds. This manuscript describes a study of a potential product for use as complementary therapy based on a phytopharmaceutical preparation with Ground Cherry (Physalis angulata), a rich phytosterols medicinal plant.
Research frontiers
The study demonstrated that a CO2 supercritical herbal preparation containing steroidal natural compounds produces a potent intestinal anti-inflammatory activity. These data open a new perspective for use of supercritical extraction method as well as for the study of phytosterols as active components with intestinal anti-inflammatory effects.
Innovations and breakthroughs
Several medicinal plants have been studied as immunomodulators in several chronic diseases, but this research is the first to investigate Ground Cherry using a supercritical carbon dioxide extraction and fractionation for its effects on chemically-induced intestinal inflammation.
Applications
The main application of these data is their potential application as a phytopharmaceutical preparation complementary to current drugs to treat IBD.
Peer-review
This is very well done study, very supported, results are excellent, and observations do support the conclusion. The article is good work, perfectly analyzed, presented the results and discussed them perfectly.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: Approved by the Ethical Committee for Animal Research (Protocol number 042/04-CEEA), Institute of Biosciences, Universidade Estadual Paulista (UNESP).
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Peer-review started: December 14, 2016
First decision: February 9, 2017
Article in press: April 12, 2017
P- Reviewer: Al-Rejaie SS, Prasad S S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang FF
References
- 1.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 2.Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: a pathologic and clinical entity. 1932. Mt Sinai J Med. 2000;67:263–268. [PubMed] [Google Scholar]
- 3.Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD) Pharmacol Rep. 2011;63:629–642. doi: 10.1016/s1734-1140(11)70575-8. [DOI] [PubMed] [Google Scholar]
- 4.Gitnick G. Inflammatory bowel disease: a new assessment. Scand J Gastroenterol Suppl. 1996;220:83–86. doi: 10.3109/00365529609094756. [DOI] [PubMed] [Google Scholar]
- 5.Witaicenis A, Luchini AC, Hiruma-Lima CA, Felisbino SL, Garrido-Mesa N, Utrilla P, Gálvez J, Di Stasi LC. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: comparison with prednisolone and sulphasalazine. Chem Biol Interact. 2012;195:76–85. doi: 10.1016/j.cbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Tang C, Chen S, Qian H, Huang W. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112–124. doi: 10.1111/j.1365-2567.2011.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 2012;18:5848–5861. doi: 10.3748/wjg.v18.i41.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto T, Nakahigashi M, Saniabadi AR. Review article: diet and inflammatory bowel disease--epidemiology and treatment. Aliment Pharmacol Ther. 2009;30:99–112. doi: 10.1111/j.1365-2036.2009.04035.x. [DOI] [PubMed] [Google Scholar]
- 9.Morrison G, Headon B, Gibson P. Update in inflammatory bowel disease. Aust Fam Physician. 2009;38:956–961. [PubMed] [Google Scholar]
- 10.Kong SC, Hurlstone DP, Pocock CY, Walkington LA, Farquharson NR, Bramble MG, McAlindon ME, Sanders DS. The Incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseases. J Clin Gastroenterol. 2005;39:138–141. [PubMed] [Google Scholar]
- 11.Di Stasi LC, Hiruma CA, Guimarães EM, Santos CM. Medicinal Plants popularly used in brazilian amazon. Fitoterapia. 1994;65:529–540. [Google Scholar]
- 12.Richter RK, Carlson TJ. Reporting biological assay results on tropical medicinal plants to host country collaborators. J Ethnopharmacol. 1998;62:85–88. doi: 10.1016/s0378-8741(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 13.Jin Z, Mashuta MS, Stolowich NJ, Vaisberg AJ, Stivers NS, Bates PJ, Lewis WH, Hammond GB. Physangulidines A, B, and C: three new antiproliferative withanolides from Physalis angulata L. Org Lett. 2012;14:1230–1233. doi: 10.1021/ol203498a. [DOI] [PubMed] [Google Scholar]
- 14.Lima Mda S, Evangelista AF, Santos GG, Ribeiro IM, Tomassini TC, Pereira Soares MB, Villarreal CF. Antinociceptive properties of physalins from Physalis angulata. J Nat Prod. 2014;77:2397–2403. doi: 10.1021/np5003093. [DOI] [PubMed] [Google Scholar]
- 15.Wu SY, Leu YL, Chang YL, Wu TS, Kuo PC, Liao YR, Teng CM, Pan SL. Physalin F induces cell apoptosis in human renal carcinoma cells by targeting NF-kappaB and generating reactive oxygen species. PLoS One. 2012;7:e40727. doi: 10.1371/journal.pone.0040727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Sun L, Ma L, Li J, Hu L, Liu J. Investigation of the immunosuppressive activity of Physalin H on T lymphocytes. Int Immunopharmacol. 2010;10:290–297. doi: 10.1016/j.intimp.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Velasquez Pereda MC, Nogueira C, de Campos Diemant G, Berlin S, Mussi L, De Tarso Vieira e Rosa P, Polezel MA. inventors. Brazil: 2009. [Google Scholar]
- 18.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 19.Bell CJ, Gall DG, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol. 1995;268:G622–G630. doi: 10.1152/ajpgi.1995.268.4.G622. [DOI] [PubMed] [Google Scholar]
- 20.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 21.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 22.Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J Biol Chem. 1946;164:321–329. [PubMed] [Google Scholar]
- 23.Smith GP, Harris H, Peters TJ. Studies of the biochemical and immunological properties of human neutrophil alkaline phosphatase with comparison to the established alkaline phosphatase isoenzymes. Clin Chim Acta. 1984;142:221–230. doi: 10.1016/0009-8981(84)90380-2. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruidenier L, Verspaget HW. Antioxidants and mucosa protectives: realistic therapeutic options in inflammatory bowel disease? Mediators Inflamm. 1998;7:157–162. doi: 10.1080/09629359891081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhiman M, Estrada-Franco JG, Pando JM, Ramirez-Aguilar FJ, Spratt H, Vazquez-Corzo S, Perez-Molina G, Gallegos-Sandoval R, Moreno R, Garg NJ. Increased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas’ disease. Clin Vaccine Immunol. 2009;16:660–666. doi: 10.1128/CVI.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karakas M, Koenig W. Myeloperoxidase production by macrophage and risk of atherosclerosis. Curr Atheroscler Rep. 2012;14:277–283. doi: 10.1007/s11883-012-0242-3. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez de Medina F, Vera B, Gálvez J, Zarzuelo A. Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 2002;70:3097–3108. doi: 10.1016/s0024-3205(02)01568-0. [DOI] [PubMed] [Google Scholar]
- 29.Tuin A, Poelstra K, de Jager-Krikken A, Bok L, Raaben W, Velders MP, Dijkstra G. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58:379–387. doi: 10.1136/gut.2007.128868. [DOI] [PubMed] [Google Scholar]
- 30.Dalaklioglu S, Genc GE, Aksoy NH, Akcit F, Gumuslu S. Resveratrol ameliorates methotrexate-induced hepatotoxicity in rats via inhibition of lipid peroxidation. Hum Exp Toxicol. 2013;32:662–671. doi: 10.1177/0960327112468178. [DOI] [PubMed] [Google Scholar]
- 31.DeLeve LD, Kaplowitz N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol Ther. 1991;52:287–305. doi: 10.1016/0163-7258(91)90029-l. [DOI] [PubMed] [Google Scholar]
- 32.Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Corridoni D, Arseneau KO, Cominelli F. Inflammatory bowel disease. Immunol Lett. 2014;161:231–235. doi: 10.1016/j.imlet.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen W, Durum SK. Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochem Res. 2010;35:940–946. doi: 10.1007/s11064-009-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broom OJ, Widjaya B, Troelsen J, Olsen J, Nielsen OH. Mitogen activated protein kinases: a role in inflammatory bowel disease? Clin Exp Immunol. 2009;158:272–280. doi: 10.1111/j.1365-2249.2009.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 40.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 41.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 42.Quaglio AE, Castilho AC, Di Stasi LC. Experimental evidence of MAP kinase gene expression on the response of intestinal anti-inflammatory drugs. Life Sci. 2015;136:60–66. doi: 10.1016/j.lfs.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 43.József L, Khreiss T, Fournier A, Chan JS, Filep JG. Extracellular signal-regulated kinase plays an essential role in endothelin-1-induced homotypic adhesion of human neutrophil granulocytes. Br J Pharmacol. 2002;135:1167–1174. doi: 10.1038/sj.bjp.0704561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitsuyama K, Suzuki A, Tomiyasu N, Tsuruta O, Kitazaki S, Takeda T, Satoh Y, Bennett BL, Toyonaga A, Sata M. Pro-inflammatory signaling by Jun-N-terminal kinase in inflammatory bowel disease. Int J Mol Med. 2006;17:449–455. [PubMed] [Google Scholar]
- 45.Assi K, Pillai R, Gómez-Muñoz A, Owen D, Salh B. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology. 2006;118:112–121. doi: 10.1111/j.1365-2567.2006.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hermano E, Lerner I, Elkin M. Heparanase enzyme in chronic inflammatory bowel disease and colon cancer. Cell Mol Life Sci. 2012;69:2501–2513. doi: 10.1007/s00018-012-0930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldberg R, Meirovitz A, Hirshoren N, Bulvik R, Binder A, Rubinstein AM, Elkin M. Versatile role of heparanase in inflammation. Matrix Biol. 2013;32:234–240. doi: 10.1016/j.matbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vlodavsky I, Beckhove P, Lerner I, Pisano C, Meirovitz A, Ilan N, Elkin M. Significance of Heparanase in Cancer and Inflammation. Cancer Microenvironment. 2011;5:115–132. doi: 10.1007/s12307-011-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edovitsky E, Lerner I, Zcharia E, Peretz T, Vlodavsky I, Elkin M. Role of endothelial heparanase in delayed-type hypersensitivity. Blood. 2006;107:3609–3616. doi: 10.1182/blood-2005-08-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quaglio AE, Castilho AC, Di Stasi LC. Experimental evidence of heparanase, Hsp70 and NF-κB gene expression on the response of anti-inflammatory drugs in TNBS-induced colonic inflammation. Life Sci. 2015;141:179–187. doi: 10.1016/j.lfs.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 51.Kaufmann SH. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 52.Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 53.Einerhand AW, Renes IB, Makkink MK, van der Sluis M, Büller HA, Dekker J. Role of mucins in inflammatory bowel disease: important lessons from experimental models. Eur J Gastroenterol Hepatol. 2002;14:757–765. doi: 10.1097/00042737-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Buisine MP, Desreumaux P, Debailleul V, Gambiez L, Geboes K, Ectors N, Delescaut MP, Degand P, Aubert JP, Colombel JF, et al. Abnormalities in mucin gene expression in Crohn’s disease. Inflamm Bowel Dis. 1999;5:24–32. doi: 10.1097/00054725-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoebler C, Gaudier E, De Coppet P, Rival M, Cherbut C. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig Dis Sci. 2006;51:381–389. doi: 10.1007/s10620-006-3142-y. [DOI] [PubMed] [Google Scholar]
- 57.Sun L, Liu J, Liu P, Yu Y, Ma L, Hu L. Immunosuppression effect of Withangulatin A from Physalis angulata via heme oxygenase 1-dependent pathways. Process Biochemistry. 2011;46:482–488. [Google Scholar]
- 58.Bastos GN, Santos AR, Ferreira VM, Costa AM, Bispo CI, Silveira AJ, Do Nascimento JL. Antinociceptive effect of the aqueous extract obtained from roots of Physalis angulata L. on mice. J Ethnopharmacol. 2006;103:241–245. doi: 10.1016/j.jep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Vieira AT, Pinho V, Lepsch LB, Scavone C, Ribeiro IM, Tomassini T, Ribeiro-dos-Santos R, Soares MB, Teixeira MM, Souza DG. Mechanisms of the anti-inflammatory effects of the natural secosteroids physalins in a model of intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2005;146:244–251. doi: 10.1038/sj.bjp.0706321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun L, Liu J, Cui D, Li J, Yu Y, Ma L, Hu L. Anti-inflammatory function of Withangulatin A by targeted inhibiting COX-2 expression via MAPK and NF-kappaB pathways. J Cell Biochem. 2010;109:532–541. doi: 10.1002/jcb.22430. [DOI] [PubMed] [Google Scholar]


