Abstract
AIM
To investigate the acute toxicity, phytochemical profile, antidiarrheal activity and mechanisms of action of Maytenus erythroxylon (M. erythroxylon) ethanol extract.
METHODS
A castor oil-induced diarrhea model was used to evaluate antidiarrheal activity. Intestinal transit and gastric emptying protocols were used to evaluate a possible antimotility effect. KATP channels, nitric oxide, presynaptic α2-adrenergic and tissue adrenergic receptors were investigated to uncover antimotility mechanisms of action and castor oil-induced enteropooling to elucidate antisecretory mechanisms.
RESULTS
All tested doses of the extract (62.5, 125, 250 and 500 mg/kg) possessed antidiarrheal activity, with a significant decrease of the evacuation index. This activity is possibly related to a reduced gastric emptying (125, 250 and 500 mg/kg) and to a decreased percentage of intestinal transit for all tested doses. That last effect seems to be modulated by nitric oxide, KATP channels and tissue adrenergic receptors. Besides, the extract also presented antisecretory effect due to a decrease of intestinal fluid accumulation.
CONCLUSION
The antidiarrheal effect of M. erythroxylon found in this study involves antimotility and antisecretory mechanisms that may be attributed to the chemical compounds found in this species: saponins, flavonoids, tannins, triterpenes and steroids.
Keywords: Medicinal plants, Celastraceae, Maytenus erythroxylon, Diarrhea, Antidiarrheal activity
Core tip: Maytenus erythroxylon Reissek, known as “casca grossa” and “bom-nome” in Brazil, is a species with indication to treat gastrointestinal disorders, like ulcers and diarrhea. Diarrhea is a pathological condition characterized by an increase in three or more defecations in 24 h, being of multiple origin, whether infectious or not. There is a search for new therapeutic alternatives for the treatment of diarrhea, since the current drugs on the market present serious undesirable effects. Species of Maytenus genus appear in this scenario as andiarrheics, due to their ethnopharmacological support and promising results from research.
INTRODUCTION
Maytenus erythroxylon Reissek (Celastraceae), popularly known as “bom nome”[1] and “casca-grossa”, is a small shrubby tree, measuring about 3.8 m high[2] and used traditionally to treat diseases of the gastrointestinal tract.
Studies with Maytenus genus have presented promising results for treatment of gastrointestinal disorders, like peptic ulcers[3-7] and diarrhea[6,8]. Besides, a lot of Maytenus species possess popular indication for treatment of diarrhea, such as M. rigida Mart[6]. and M. senegalensis Lam. Exell[9]. Most of their biological activities are attributed to the presence of phenolic compounds, particularly flavonoids, tannins, glycosides, terpenes, steroids and alkaloids[10], which have already been referenced in pharmacologic studies as antidiarrheal agents[11-14].
Diarrhea is a debilitating gastrointestinal condition[15] that involves an increase of unformed stools and also of the defecation frequency (three times or more in a day)[16]. The etiology of diarrheal disorders is multifactorial, attributed to factors such as infectious agents, microorganisms and their toxins, increased fluid secretion, malabsorption of biliary salts[17], food allergies[18] and some medications, like antibiotics[19]. Diarrhea is responsible for up to 5 million deaths each year[20], especially of children of less than 5 years, corresponding to 500000 deaths annually in developing countries[21] and associated with factors such as poor home environments, undernutrition and lack of access to essential services[22].
Available drugs used in diarrhea pharmacotherapy are related to contraindications and undesirable effects, like bronchospasm, vomiting and fever[16]. In this context, the World Health Organization (WHO) created a Diarrheal Disease Control Program that stimulates studies with natural products, especially traditional medicinal plants, for the management of diarrhea worldwide[23].
From this perspective, the present study aimed to present the phytochemical profile, acute toxicity, antidiarrheal activity and mechanisms of action of the ethanol extract obtained from the aerial parts from Maytenus erythroxylon (EtOHE-Me).
MATERIALS AND METHODS
Reagents
The drugs and reagents were prepared immediately before use. The following drugs were used: carboxymethylcellulose (Formula Brasil®, Brazil); castor oil (Tayuyna Lab Ltda®, Brazil); loperamide hydrochloride (2 mg; Janssen Cilag Farmacêutica Ltda®, Brazil); activated charcoal meal (Proquímios®, Brazil); and glibenclamide, L-NG-nitroarginine methyl ester (L-NAME), propranolol and yohimbine (all from Sigma-Aldrich®, United States).
Plant materials
Plant samples used in the antidiarrheal activity evaluation in mice were obtained from the leaves of M. erythroxylon Reissek. Plants were collected in the city of Mamanguape, Paraíba state, Brazil and identified by Dr Zelma Glebya Maciel Quirino, botanist from Centro de Ciências Aplicadas e Educação/Federal University of Paraiba (UFPB; Paraíba, Brazil). A voucher number 6051 (JPB) was deposited in the Herbarium Lauro Pires Xavier of the Department of Botany of UFPB. The aerial parts (665 g) of M. erythroxylon were air-dried at 40 °C for 4 d, powdered and macerated with 96% ethanol for 3 d. The solution was filtered and evaporated to dryness under reduced pressure at 40 °C. The yield (w/w) of the crude ethanol extract of Maytenus erythroxylon (EtOHE-Me) was 55.5 g (8%).
Animals
Swiss adult male and female mice (Mus musculus), weighing between 25-35 g, were obtained from the Central Animal House of Instituto de Pesquisa em Fármacos e Medicamentos (IPeFarM) of the UFPB. They were kept at temperatures between 23-25 °C, with a 12-h light/dark cycle in the animal house, fed with Purina® chow and water ad libitum for 2 wk prior to experimentation. Intragastric gavage administration was carried out with conscious animals, using straight gavage needles appropriate for the animal size. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
Phytochemical screening of EtOHE-Me
EtOHE-Me was subjected to preliminary phytochemical screening[24] for the detection of the presence of various phytoconstituents (alkaloids, saponins, steroids, triterpenoids, flavonoids and tannins). Alkaloids were detected using the Dragendorff’s reagent, resulting in the appearance of a precipitate at the bottom of the test tube. Flavonoids were considered present when a yellow color appeared upon AlCl3 reagent addition and tannins when a green or black color was produced with FeCl3. For the detection of sterols and triterpenes, petroleum ether was used and extracted with CHCl3. Sterols were detected when a green to pink color appeared and pink to purple color for terpenes, following treatment of the CHCl3 layer with acetic anhydride and concentrated HCl. Saponins were detected when persistent froth appeared after vigorous shaking of diluted samples.
The metabolic fingerprinting assessment of EtOHE-Me was also performed by 1H-nuclear magnetic resonance (NMR) and 13C-NMR spectroscopy. The 1H-NMR and 13C-NMR spectra were obtained by Varian Mercury NMR spectrometer (UNICAL) operating at 200 MHz (1H) and 50 MHz (13C). The sample was prepared for analysis by dissolving an amount of EtOHE-Me in deuterated chloroform (CDCl3; Cambridge Isotope Laboratories, United States). Chemical shifts (δ) were expressed in parts per million (ppm), and for 1H-NMR they were referenced to the characteristic peaks of protons belonging to non-deuterated fractions of the solvent (δH 7.24). For 13C-NMR, the same parameters were utilized (δC 77.0).
Toxicological evaluation
Investigation of the acute toxicity of EtOHE-Me in mice: The toxicological research was conducted in order to assess behavioral parameters and to determine LD50, according to the model described by Almeida et al[25] and Anvisa[26]. Male and female mice (n = 7) were fasted for 12 h and treated with EtOHE-Me orally in a single dose (2000 mg/kg, solubilized in saline solution 0.9%) for two groups (male and female mice). Simultaneously, two other groups (male and female) were treated with NaCl 0.9% (10 mL/kg). Then, a behavioral screening was carried out and signs and symptoms of acute toxicity were observed and noted for 72 h. For 14 d, the animals were evaluated with respect to water and food consumption and body weight gain, and to observe if there were deaths. At the end of the experiment, the animals were euthanized for macroscopic analysis of organs (heart, spleen, liver and kidneys).
Pharmacological assays
Effect of EtOHE-Me on castor oil-induced diarrhea in mice: The antidiarrheal activity was evaluated according to the model described by Awouters et al[27]. Male mice were divided into six groups (n = 7) and pretreated orally with NaCl 0.9% (10 mL/kg), loperamide 5 mg/kg and EtOHE-Me (62.5, 125, 250 and 500 mg/kg). After 1 h, 10 mL/kg of castor oil was administered orally to each animal in order to induce diarrhea. Feces were counted for 4 h and classified according to their consistency in solids, semisolids or liquids. Then, the Evacuation Index (EI), Percentual of Wet feces (%) and Diarrheal Inhibition (%) were calculated.
EI = ∑ (solid stools × 1) + (semisolid stools × 2) + (liquid 3 × 3)
% DI = (Mean of saline group - mean of treated group)/Mean of saline group × 100
Effects of EtOHE-Me on gastric emptying: Alterations in gastric emptying were assessed according to the model described by Scarpignato et al[28]. After 1 h of pretreatment as described above, 0.4 mL of semisolid colored marker (phenol red 0.05% in 1.5% carboxymethylcellulose) was administered to the non-treated control group (the zero-time control group) and the mice were euthanized immediately. The treated groups received this marker and euthanized 30 min after administration. The abdominal cavity was opened for stomach removal, with necessity of ligation of the pyloric and lower esophageal sphincters to avoid loss of the stomach contents. The gastric content was collected in Falcon® tubes, solubilized in 7 mL of distilled water and centrifuged at 3000 rpm for 15 min. Then, 1 mL of the supernatant was mixed with 1 mL of 0.025 N NaOH and stirred using a vortex. From this material, 150 μL were pipetted into duplicate microplates and the spectrophotometric reading was made for wavelength equal to 570 nm. The results were expressed as percentage of gastric emptying in relation to the control (zero-time group).
% gastric emptying = (100 - mean absorbance of sample)/Mean absorbance of zero-time control group × 100
Effects of EtOHE-Me on normal intestinal transit: Alterations in normal intestinal transit were evaluated according to the model described by Stickney and Northup[29]. After 60 min of the pretreatment, 10 mL/kg (p.o.) Black marker (5% charcoal suspension in 5% Arabic gum) was administered. After 30 min, the animals were euthanized for removal of the small intestine (pylorus to the ileocecal junction). Using a ruler, the total length of the small intestine and the distance traveled by the black marker (last portion comprising at least one continuous score) were measured to calculate the percentage of the charcoal meal route depending on the total length of the intestine.
% intestinal transit = length traveled by charcoal meal/Total intestinal length × 100
Antimotility mechanisms of action of EtOHE-Me
The antimotility mechanisms of action were evaluated according to the model described by Santos and Rao[30]. Male mice were fasted for 24 h and subsequently treated orally with NaCl 0.9% (10 mL/kg) and EtOHE-Me at its best dose (500 mg/kg). To obtain information about the mechanism of action, different drugs acting via a well-known mechanism were administered either alone and in association with EtOHE-Me, such as glibenclamide (1 mg/kg i.p.), a blocker of KATP channels, L-NAME (1 mg/kg i.p.), an inhibitor of nitric oxide synthase (NOs), propranolol (1 mg/kg i.p.), a non-selective adrenergic antagonist, and yohimbine (1 mg/kg i.p.), a presynaptic α2-adrenergic antagonist. These drugs were dissolved in NaCl 0.9% and given 30 min before extract administration. After 60 min, 10 mL/kg (p.o.) of the black marker (5% charcoal suspension in 5% Arabic gum) was administered and 30 min later, the animals were euthanized for removal of the small intestine to calculate the percentage of intestinal transit.
Antisecretory mechanisms of action of EtOHE-Me
The antisecretory mechanism of action was evaluated according to Ezeja and Anaga[31] using the castor oil-induced enteropooling model. The animals were fasted for 24 h and treated orally with NaCl 0.9% (10 mL/kg), loperamide 5 mg/kg and EtOHE-Me at its best dose (500 mg/kg). After 1 h, 10 mL/kg of castor oil was administered to animals orally. Then, 1 h later, the animals were euthanized for removal of the small intestine, after which the intestinal content was measured with the aid of a graduated cylinder.
Ethical consideration
All protocols performed in the present study were in accordance with international principles for research with laboratory animals[32].
Animal care and use statement
All experimental procedures were approved by the Institutional Committee for Ethics in Animal Use from UFPB (No. 0105/14).
Statistical analysis
Parametric data were expressed as mean ± SD and non-parametric data as median (minimum-maximum values). The data were subjected to t-test to compare two groups (control and treated group) and variance analysis (one-way ANOVA) to compare more than two groups, followed by a Dunnett and Tukey test (parametric) or Kruskal-Wallis followed by Dunn test (non-parametric). P < 0.05 was considered as statistically significant. GraphPad Software© 5.0 (United States) was used for data processing.
RESULTS
Phytochemical screening of EtOHE-Me
In the present study, the results demonstrated the presence of saponins, flavonoids, tannins, steroids and triterpenes in EtOHE-Me (Table 1).
Table 1.
Preliminary phytochemical screening of EtOHE-Me
| Test | Result |
| Alkaloids | - |
| Flavonoids | + |
| Tannins | + |
| Steroids and triterpenoids | + |
| Saponins | + |
(+) Present, (-) Absent. EtOHE-Me: Ethanol extract of Maytenus erythroxylon.
The NMR spectrum 1 of 13C (50 MHz, CDCl3) showed the presence of signals relating to quaternary, metinic, methylene and methyl carbons, suggesting the presence of terpenes. It was observed in the regions δC 124.15 and δC 145.06 of the spectrum signals that suggest the presence of olefinic carbons referring to pentacyclic triterpenes.
There was also present a signal in δC 80.69 referring to carbinolic carbon. The signal δC 173.86 suggested the presence of carbonyl of an acid or esters of triterpene. The chemical shifts at the 6.68 δC region are characteristic of methyl carbons of friedelan pentacyclic triterpenes, indicating the presence of ketone compounds at C-3.
The NMR spectrum 2 of 1H (200 MHz, CDCl3) showed an envelope of signals in the region between 2.22 to 0.78 ppm, characteristic of protons from terpenes. The chemical shifts in the region of δH 5.28 and δH 5.03 are characteristic of olefinic hydrogens. The spectra showed no signals in the aromatic region (δH 6.5 and δH 8.0).
Investigation of the acute toxicity of EtOHE-Me in mice
The results showed low toxicity after the single-dose administration (2000 mg/kg) of EtOHE-Me, evidenced by lack of death during 14 d of the experiment and no apparent behavioral changes. Furthermore, there were no changes in body weight (Table 2) or organ weights of treated animals (Table 3), and no changes in the consumption of water and food, when compared to the group treated only with NaCl 0.9% (Table 4).
Table 2.
Effect of the oral administration of EtOH extract obtained from the leaves of Maytenus erythroxylon over the weight gain of male and female mice for 14 d
| Sex | Weight gain1 | Vehicle | EtOHE-Me |
| (NaCl 0.9%) | (2000 mg/kg) | ||
| Female | Inicial | 30.78 ± 2.30 | 28.09 ± 2.53NS |
| Final | 35.51 ± 2.00 | 34.65 ± 3.37NS | |
| Male | Inicial | 31.41 ± 2.00 | 30.71 ± 2.26NS |
| Final | 39.06 ± 1.32 | 38.93 ± 1.83NS |
Data are presented in g and expressed as mean ± SD.
No significant differences (P > 0.05) between treated (EtOHE-Me) vs non-treated (NaCl 0.9%) mice. EtOHE-Me: Ethanol extract of Maytenus erythroxylon.
Table 3.
Effect of the oral administration of EtOH extract obtained from the leaves of Maytenus erythroxylon on the organ index of male and female mice for 14 d
| Sex | Organ index1 | Vehicle | EtOHE-Me |
| (NaCl 0.9%) | (2000 mg/kg) | ||
| Female | Liver | 52.66 ± 6.68 | 53.59 ± 41.61NS |
| Heart | 4.27 ± 0.74 | 3.86 ± 0.56NS | |
| Kidneys | 11.40 ± 0.81 | 10.81 ± 2.31NS | |
| Spleen | 5.53 ± 0.83 | 5.16 ± 1.03NS | |
| Male | Liver | 51.59 ± 2.57 | 52.33 ± 4.16NS |
| Heart | 4.71 ± 0.75 | 4.24 ± 0.45NS | |
| Kidneys | 12.18 ± 1.31 | 13.16 ± 0.97NS | |
| Spleen | 5.53 ± 0.94 | 4.78 ± 0.37NS |
Data are presented in mg/g and expressed as mean ± SD.
No significant differences (P > 0.05) between treated (EtOHE-Me) vs non-treated (NaCl 0.9%) mice. EtOHE-Me: Ethanol extract of Maytenus erythroxylon.
Table 4.
Effect of the oral administration of EtOH extract obtained from the leaves of Maytenus erythroxylon on the consumption of water and food of male and female mice for 14 d
| Intake | Vehicle | EtOHE-Me |
| (NaCl 0.9%) | (2000 mg/kg) | |
| Water consumption (mL) | ||
| Female | 30.78 ± 2.30 | 28.09 ± 2.53NS |
| Male | 35.51 ± 2.00 | 34.65 ± 3.37NS |
| Food consumption (g) | ||
| Female | 31.41 ± 2.00 | 30.71 ± 2.26NS |
| Male | 39.06 ± 1.32 | 38.93 ± 1.83NS |
1Data are presented in g and expressed as mean ± SD.
No significant differences (P > 0.05) between treated (EtOHE-Me) vs non-treated (NaCl 0.9%) mice. EtOHE-Me: Ethanol extract of Maytenus erythroxylon.
Effect of EtOHE-Me on castor oil-induced diarrhea in mice
In the present study, mice in the control group treated only with vehicle (NaCl 0.9%) showed intense signs of diarrhea, with respective evacuation index of 21 (19-25) and 47% wet feces. Pretreatment with EtOHE-Me at all doses (62.5, 125, 250 and 500 mg/kg) decreased the evacuation index of 8 (5-11) with 62% showing diarrhea inhibition (P < 0.05), 7 (6-8) showing 66% (P < 0.05), 6.5 (3-7) showing 69% (P < 0.05) and 4 (3-5) showing 80% (P < 0.001) respectively, when compared with the NaCl 0.9% control group. The standard antidiarrheal drug loperamide (5 mg/kg) produced a significant inhibition of all parameters evaluated (Table 5).
Table 5.
Effect of oral administration of EtOHE-Me and loperamide on castor oil induced-diarrhea in mice
| Treatment, as p.o. | Dose in mg/kg | Evacuation index | Wet feces | Inhibition of diarrhea |
| NaCl 0.9% | - | 21 (19-25) | 47% | - |
| Loperamide | 5 | 0 (0-4)2 | 0% | 100% |
| EtOHE-Me | 62.5 | 8 (5-11)1 | 4% | 62% |
| 125 | 7 (6-8)1 | 2% | 66% | |
| 250 | 6.5 (3-7)1 | 0% | 69% | |
| 500 | 4 (3-5)13 | 0% | 81% |
Significant differences between treated groups vs NaCl 0.9% control group (P < 0.05);
Significant differences between loperamide group vs NaCl 0.9% control group (P < 0.001);
Significant differences between EtOHE-Me 250 mg/kg vs EtOHE-Me 500 mg/kg (P < 0.05). Data are expressed as median (minimum-maximum). EtOHE-Me: Ethanol extract of Maytenus erythroxylon.
Effects of EtOHE-Me on gastric emptying of mice
The animals treated with NaCl 0.9% showed 79% of gastric emptying and and the treatment with EtOHE-Me (125, 250 or 500 mg/kg) and loperamide significantly reduced gastric emptying in 66% (P < 0.05), 45% (P < 0.001), 47% (P < 0.001) and 53% (P < 0.001) respectively, when compared to the NaCl 0.9% control group (Figure 1).
Figure 1.
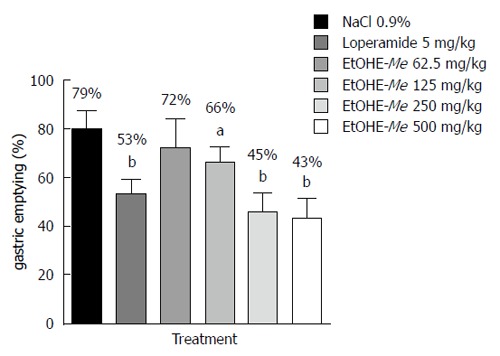
Effect of oral administration of EtOHE-Me and loperamide on gastric emptying of mice. Data are presented as mean ± SD. aP < 0.05, bP < 0.001, vs NaCl 0.9% group. EtOHE-Me: Ethanol extract of Maytenus erythroxylon.
Effects of EtOHE-Me on intestinal transit of mice
The distance travelled by charcoal in terms of percent of the total length of intestine was 76% in the NaCl 0.9% control group. The treatment with loperamide and EtOHE-Me in all doses produced significant (P < 0.001) reduction in the percentage of intestinal transit in 25%, 57%, 49%, 41% and 35% respectively, when compared to the control group (Figure 2).
Figure 2.
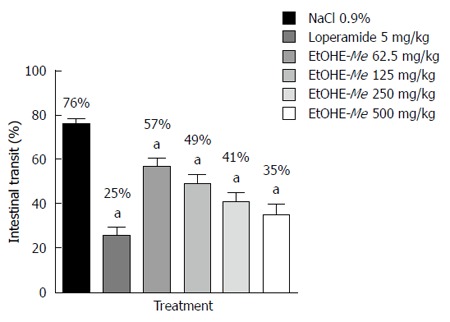
Effect of oral administration of EtOHE-Me and loperamide on intestinal transit of mice. Data are presented as mean ± SD. aP < 0.001 vs NaCl 0.9% group. EtOHE-Me: Ethanol extract of Maytenus erythroxylon.
Antimotility mechanisms of action of EtOHE-Me
The distance travelled by charcoal meal was 78% in the NaCl 0.9% control group. The treatment with EtOHE-Me at its best dose (500 mg/kg) produced significant (P < 0.001) reduction in the percentage of intestinal transit (36%), when compared to the NaCl 0.9% group. Although, when EtOHE-Me was associated with the standard drugs L-NAME, glibenclamide and propranolol, an increase in the intestinal transit was observed to 78%, 70% and 74% respectively. The same effect was not reproduced when EtOHE-Me was administrated along with yohimbine (36% of intestinal transit) (Figure 3).
Figure 3.
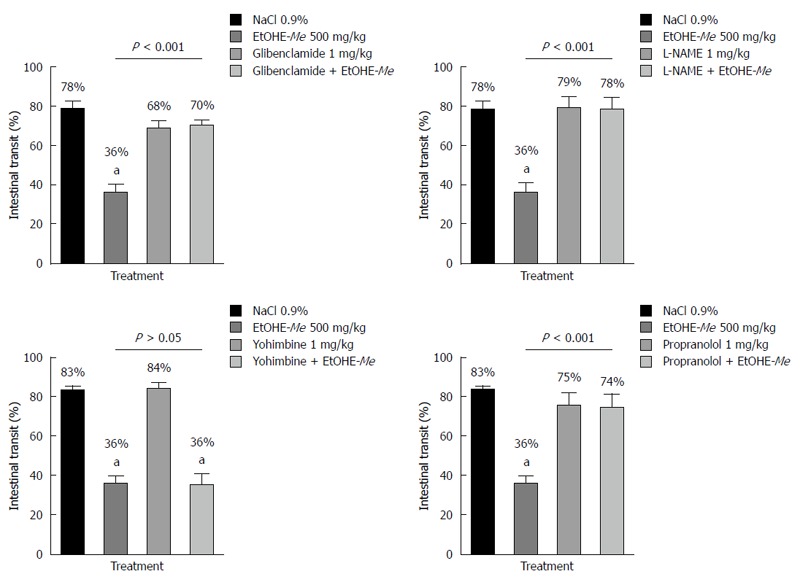
Effect of oral administration of Ethanol extract of Maytenus erythroxylon Glibenclamide, L-NG-nitroarginine methyl ester, propranolol and yohimbine on intestinal transit of mice. Data are presented as mean ± standard deviation. aP < 0.05 vs NaCl 0.9% group. EtOHE-Me: Ethanol extract of Maytenus erythroxylon; L-NAME: L-NG-nitroarginine methyl ester.
Antisecretory mechanisms of action of EtOHE-Me
EtOHE-Me at its best dose (500 mg/kg) reduced intestinal fluid (0.6429 ± 0.1272), with 51% of fluid inhibition (P < 0.001), when compared to the NaCl 0.9% control group (1.325 ± 0.2053) (Figure 4).
Figure 4.
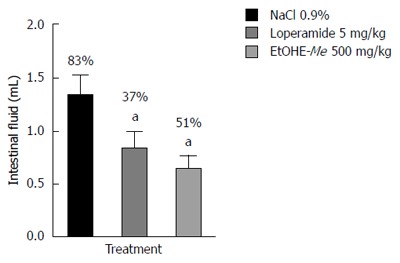
Effect of oral administration of Ethanol extract of Maytenus erythroxylon and loperamide in castor oil-induced enteropooling in mice. Data are presented as mean ± SD. aP < 0.001 vs NaCl 0.9% group. EtOHE-Me: Ethanol extract of Maytenus erythroxylon.
DISCUSSION
Phytochemical screening showed the presence of saponins, flavonoids, tannins, triterpenes and steroids in EtOHE-Me. Therefore, the absence of signals in the aromatic region along with the previous isolated fridelane terpene from Maytenus erythroxylon, 3β-friedelinol[33] corroborate that the signals presented in the 1H and 13C NMR spectra of the extract sample evaluated are from terpenes. The compounds found in the extract are mostly liked to increase water and electrolyte absorption in the colon, decrease intestinal irritability, and reduce intestinal propulsion and spasmolitic effect[11-14]. Considering those findings, they might be responsible for the biological activities evidenced in the present study.
The studies of acute toxicity are important to determine the LD50 and set doses to be used in later experimental models[26]. The single-dose administration of EtOHE-Me did not alter any parameter evaluated and showed no deaths, with LD50 considered over 2000 mg/kg (p.o.) and the extract considered safe for pharmacological studies.
Then, it was investigated whether Maytenus erythroxylon ethanol extract possessed antidiarrheal effect. For that, the castor oil-induced diarrhea model in mice was used. Castor oil is a potent laxative agent and induces diarrhea through its active compound, the ricinoleic acid[34], which acts in the upper small intestine where castor oil is hydrolyzed. It produces cytotoxicity of epithelial cells[35], decreases absorption[36], increases water flux[37], increases fluid and electrolyte accumulation[38], enhances intestinal motility and alters the gastric contractions[39], representing effects similar to physiopathologic conditions that cause diarrhea in humans. Castor oil produces its laxative effect in association with the release of platelet activating factor, nitric oxide (NO), tachykinins (TKs), cAMP[26,40] and prostaglandins via EP3 and EP4 receptors’ binding[41].
EtOHE-Me presented antidiarrheal activity, decreasing the evacuation index at all doses, with crescent percentiles of diarrhea inhibition, along with the standard drug loperamide. These results corroborate a study by Santos et al[6] with Maytenus rigida Mart. ethanolic extract, which was shown to be able to reduce the total number of fecal output and the diarrheic feces for all tested doses.
In order to evaluate if EtOHE-Me affected gastrointestinal motility, gastric emptying and intestinal transit protocols were performed. The findings suggested an antimotility activity mediated by EtOHE-Me, since it was efficient in decreasing gastric emptying and intestinal transit. Similar results were found for a flavonoid-rich fraction of Maytenus ilicifolia Reissek, which was able to inhibit the intestinal transit in a more potent way than the gastric emptying[8]. Those results suggested the presence of different mechanisms of action in the different segments of the gastrointestinal system, and likely not liked to gastric dysfunction, since Maytenus species are well known for enhancing the protective effects of the stomach preserving its normal physiology[3-7].
The control of gastrointestinal motility is very complex and involves multiple signaling pathways, such as NO, gastrin, opioids, 5-hydroxytryptamine, dopamine, catecholamines and acetylcholine[42]. Thus, the mechanistic studies targeting nitrergic and adrenergic pathways were assessed, as well as, the participation of KATP channels involved in the antimotility effect previously evaluated. For that matter, we used drugs with well-known mechanisms for blocking these pathways, including glibenclamide, a KATP channels blocker, L-NAME, an inhibitor of NOs, propranolol, a non-selective adrenergic antagonist, and yohimbine, a presynaptic α2-adrenergic antagonist.
The results from this experiment suggested the participation of NO and KATP channels, that might involve the NO-cGMP-KATP pathway, as well as of tissue adrenergic receptors in the antimotility activity, due to the effect reversal when EtOHE-Me was administered along with the respective blockers. It is also possible to suggest that this effect does not involve presynaptic α2-adrenergic receptors, since EtOHE-Me still decreased intestinal transit in the presence of yohimbine, a blocker of this pathway.
In order to determine if antidiarrheal activity of EtOHE-Me was also associated with a reduction in fluid accumulation, the castor oil induced-enteropooling model was used. It is possible to suggest through the present results that EtOHE-Me ability to reduce diarrhea may also be due to its antisecretory effect and that this mechanism of action might be related to inhibition of secretion, reducing intraluminal fluid accumulation and/or enhancing water and ion absorption. Species such as Psidium guajava and Anacardium occidentale, largely used in traditional medicine as antidiarrheics[24], have already demonstrated a decrease of fluid accumulation, underlining their antidiarrheal properties[43,44].
Thus, this work showed, for the very first time, that the ethanol extract of Maytenus erythroxylon potently reduced diarrheal episodes, due to inhibition of gastrointestinal motility via nitrergic pathways and KATP channels, through tissue adrenergic receptors modulation, and by its antisecretory activity. Those results must be closely related to the secondary metabolites found in the extract: saponins, flavonoids, tannins, triterpenes and steroids. These effects, accompanied by the safety of its administration, validate the popular utilization of Maytenus erythroxylon.
ACKNOWLEDGMENTS
We are grateful to José Crispim Duarte and the members of the Laboratório de Farmacologia do Trato Gastrintestinal of the Programa de Pós-Graduação em Produtos Naturais e Sintéticos Bioativos for technical support. The authors also thank Cody Lyman for his careful language assistance.
COMMENTS
Background
A variety of herbal medicines from the Maytenus genus, such as M. rigida and M. ilicifolia, have been shown to produce results in the treatment of diarrhea in folk medicine, and this activity has already been validated by pharmacological studies. M. erythroxylon, the species selected for this study, popularly known as “bom-nome” and “casca grossa”, in folk medicine is used to treat gastrointestinal disorders. Given the need for new antidiarrheal therapies, this study aimed to evaluate, for the first time, the antidiarrheal activity of this species, as well as its mechanisms of action, the acute toxicity and phytochemical profile, validating its popular use and contributing to the search for new therapies for diarrhea.
Research frontiers
Maytenus genus presents a variety of species with promising results in pharmacological trials, including the ones evaluating biological activities in the gastrointestinal tract, as gastroprotective, antiinflammatory and antidiarrheic effects. Maytenus erythroxylon is a species with folk use to treat ulcers and diarrhea, but with no toxicological, pharmacological and phytochemical studies in the literature. Thus, this species was selected for the present study in order to contribute to its validation and promote new therapies for the treatment of diarrhea.
Innovations and breakthroughs
This study evaluated, for the first time, the antidiarrheal effect promoted by the species M. erythroxylon Reissek in animal models, as well as its acute toxicity and phytochemical profile.
Applications
This study validated the popular use of M. erythroxylon Reissek and contributes to the search for new therapies for diarrhea.
Terminology
The antidiarrheal activity of ethanol extract (EtOHE) obtained from the leaves of M. erythroxylon (EtOHE-Me) was assessed in the present study. In addition, the lethal dose 50% (LD50) was evaluated, along with behavioral alterations and the phytochemical profile of this extract by means of colorimetric reactions and nuclear magnetic resonance spectroscopy.
Peer-review
The authors demonstrated that EtOHE-Me displayed an antidiarrheal effect in the castor oil-induced diarrhea mouse model and showed that this activity is related to a decrease in gastric emptying and intestinal transit, with this last result being related to nitric oxide, KATP and tissue adrenergic receptors. It was also shown that the antidiarrheal activity is associated with antisecretory mechanisms.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Federal University of Paraíba (UFPB).
Institutional animal care and use committee statement: The experimental protocols were approved by the Committee for Ethics in Animal Experimentation (CEUA/UFPB) under number 0105/14.
Conflict-of-interest statement: The authors declare that no conflict of interest exists.
Data sharing statement: No additional data are available.
Peer-review started: December 1, 2016
First decision: February 9, 2017
Article in press: March 15, 2017
P- Reviewer: Kamiya T S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH
References
- 1.Cestaro LA, Soares JJ. Variações florística e estrutural e relações fitogeográficas de um fragmento de floresta decídua no Rio Grande do Norte, Brasil. Acta Bot Bras. 2004;18:203–218. [Google Scholar]
- 2.Castro ASF, Moro MF and Menezes MOT. O Complexo Vegetacional da Zona Litorânea no Ceará: Pecém, São Gonçalo do Amarante. Acta Bot Bras. 2012;26:108–124. [Google Scholar]
- 3.Souza-Formigoni ML, Oliveira MG, Monteiro MG, da Silveira-Filho NG, Braz S, Carlini EA. Antiulcerogenic effects of two Maytenus species in laboratory animals. J Ethnopharmacol. 1991;34:21–27. doi: 10.1016/0378-8741(91)90185-g. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez FG, Portela TY, Stipp EJ, Di Stasi LC. Antiulcerogenic and analgesic effects of Maytenus aquifolium, Sorocea bomplandii and Zolernia ilicifolia. J Ethnopharmacol. 2001;77:41–47. doi: 10.1016/s0378-8741(01)00268-9. [DOI] [PubMed] [Google Scholar]
- 5.de Andrade SF, Lemos M, Comunello E, Noldin VF, Filho VC, Niero R. Evaluation of the antiulcerogenic activity of Maytenus robusta (Celastraceae) in different experimental ulcer models. J Ethnopharmacol. 2007;113:252–257. doi: 10.1016/j.jep.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Santos VL, Costa VBM, Agra MF, Silva BA, Batista LM. Pharmacological studies of ethanolic extracts of Maytenus rigida Mart (Celastraceae) in animal models. Braz J of Pharmacogn. 2007;17:336–342. [Google Scholar]
- 7.Mota KSL, Pita JCLR, Estevam EC. Evaluation of the toxicity and antiulcerogenic activity of the ethanol extract of Maytenus obtusifolia Mart. leaves. Braz J of Pharmacogn. 2008;18:441–446. [Google Scholar]
- 8.Baggio CH, Freitas CS, Mayer B, Dos Santos AC, Twardowschy A, Potrich FB, Cipriani TR, de Souza LM, Sassaki GL, Iacomini M, et al. Muscarinic-dependent inhibition of gastric emptying and intestinal motility by fractions of Maytenus ilicifolia Mart ex. Reissek. J Ethnopharmacol. 2009;123:385–391. doi: 10.1016/j.jep.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Koné WM, Atindehou KK. Ethnobotanical inventory of medicinal plants used in traditional veterinary medicine in Northern Côte d’Ivoire (West Africa) S African J of Bot. 2008;74:76–84. [Google Scholar]
- 10.Niero R, de Andrade SF, Cechinel Filho V. A review of the ethnopharmacology, phytochemistry and pharmacology of plants of the Maytenus genus. Curr Pharm Des. 2011;17:1851–1871. doi: 10.2174/138161211796391029. [DOI] [PubMed] [Google Scholar]
- 11.Di Carlo G, Autore G, Izzo AA, Maiolino P, Mascolo N, Viola P, Diurno MV, Capasso F. Inhibition of intestinal motility and secretion by flavonoids in mice and rats: structure-activity relationships. J Pharm Pharmacol. 1993;45:1054–1059. doi: 10.1111/j.2042-7158.1993.tb07180.x. [DOI] [PubMed] [Google Scholar]
- 12.Villaseñor IM, Canlas AP, Faustino KM, Plana KG. Evaluation of the bioactivity of triterpene mixture isolated from Carmona retusa (Vahl.) Masam leaves. J Ethnopharmacol. 2004;92:53–56. doi: 10.1016/j.jep.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Giliani AU, Ghayur MN, Khalid A, Zaheer-ul-Haq MI. Presence of antispasmodic, antidiarrheal, antisecretory, calcium antagonist and acetylcholinesterase inhibitory steroidal alkaloids in Sarcococca saligna. Planta Med. 2005;71:120–125. doi: 10.1055/s-2005-837777. [DOI] [PubMed] [Google Scholar]
- 14.Sainia NK, Singhala M, Awasthib A, Mishra G. Total Tannin Content and Antidiarrheal Activity of Tecomaria capensis Leaves Extract. Nat Prod J. 2013;3:218–223. [Google Scholar]
- 15.Prichard W, Fick L. When Diarrhea Can Become Deadly: Legionnaires’ Disease Complicated by Bowel Obstruction. Case Rep Gastroenterol. 2016;10:781–786. doi: 10.1159/000453657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tadesse WT, Hailu AE, Gurmu AE, Mechesso AF. Experimental assessment of antidiarrheal and antisecretory activity of 80% methanolic leaf extract of Zehneria scabra in mice. BMC Complement Altern Med. 2014;14:460. doi: 10.1186/1472-6882-14-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surawicz CM. Mechanisms of diarrhea. Curr Gastroenterol Rep. 2010;12:236–241. doi: 10.1007/s11894-010-0113-4. [DOI] [PubMed] [Google Scholar]
- 18.Berin MC, Mayer L. Immunophysiology of experimental food allergy. Mucosal Immunol. 2009;2:24–32. doi: 10.1038/mi.2008.72. [DOI] [PubMed] [Google Scholar]
- 19.Philpott HL, Nandurkar S, Lubel J, Gibson PR. Drug-induced gastrointestinal disorders. Postgrad Med J. 2014;90:411–419. doi: 10.1136/postgradmedj-2013-100316rep. [DOI] [PubMed] [Google Scholar]
- 20.Palla AH, Khan NA, Bashir S, Ur-Rehman N, Iqbal J, Gilani AH. Pharmacological basis for the medicinal use of Linum usitatissimum (Flaxseed) in infectious and non-infectious diarrhea. J Ethnopharmacol. 2015;160:61–68. doi: 10.1016/j.jep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 21.Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2016;12:CD005436. doi: 10.1002/14651858.CD005436.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet. 2010;375:870–872. doi: 10.1016/S0140-6736(09)61798-0. [DOI] [PubMed] [Google Scholar]
- 23.Sanger GJ, Alpers DH. Development of drugs for gastrointestinal motor disorders: translating science to clinical need. Neurogastroenterol Motil. 2008;20:177–184. doi: 10.1111/j.1365-2982.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 24.Agra MF, Silva KN, Basílio IJLD, Freitas PF, Barbosa-Filho JM. Survey of medicinal plants used in the region Northeast of Brazil. Rev Bras Farmacogn. 2008;18:472–508. [Google Scholar]
- 25.Almeida RN, Falcão ACGM, Diniz RST, Quintanas-Júnior LJ, Polari RM, Barbosa-Filho JM, Agra MF, Duarte JC, Ferreira CD, Antoniolli AR, et al. Metodologia para avaliação de plantas com atividade no Sistema Nervoso Central e alguns dados experimentais. Rev Bras Farm. 1999;80:72–76. [Google Scholar]
- 26.Anvisa. Guia para condução de estudos não-clínicos de toxicologia e segurança farmacológica necessários ao desenvolvimento de medicamentos. Brasília: Gerência de Avaliação de Segurança e Eficácia - GESEF; 2013. [Google Scholar]
- 27.Awouters F, Niemegeers CJ, Lenaerts FM, Janssen PA. Delay of castor oil diarrhoea in rats: a new way to evaluate inhibitors of prostaglandin biosynthesis. J Pharm Pharmacol. 1978;30:41–45. doi: 10.1111/j.2042-7158.1978.tb13150.x. [DOI] [PubMed] [Google Scholar]
- 28.Scarpignato C, Capovilla T, Bertaccini G. Action of caerulein on gastric emptying of the conscious rat. Arch Int Pharmacodyn Ther. 1980;246:286–294. [PubMed] [Google Scholar]
- 29.STICKNEY JC, NORTHUP DW. Effect of gastric emptying upon propulsive motility of small intestine of rats. Proc Soc Exp Biol Med. 1959;101:582–583. doi: 10.3181/00379727-101-25024. [DOI] [PubMed] [Google Scholar]
- 30.Santos FA, Rao VS. Quinine-induced inhibition of gastrointestinal transit in mice: possible involvement of endogenous opioids. Eur J Pharmacol. 1999;364:193–197. doi: 10.1016/s0014-2999(98)00842-5. [DOI] [PubMed] [Google Scholar]
- 31.Adeyemi OO, Akindele AJ, Ogunleye EA. Evaluation of the antidiarrhoeal effect of Sanseviera liberica Gerome & amp; Labroy (Agavaceae) root extract. J Ethnopharmacol. 2009;123:459–463. doi: 10.1016/j.jep.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 33.Duarte MC, Costa VCO, Oliveira SL, Sousa DF, Silva Filho RN, Tavares JF, Silva MS. Triterpeno Friedelano isolado das folhas de. Maytenus erythroxylon. 33rd Annual Meeting of the Brazilian Chemical Society.Águas de Lindóia, SP, Brasil: Brazilian Society of Chemistry. 2009 [Google Scholar]
- 34.Gaginella TS, Stewart JJ, Olsen WA, Bass P. Actions of ricinoleic acid and structurally related fatty acids on the gastrointestinal tract. II. Effects on water and electrolyte absorption in vitro. J Pharmacol Exp Ther. 1975;195:355–361. [PubMed] [Google Scholar]
- 35.Gadacz TR, Gaginella TS, Phillips SF. Inhibition of water absorption by ricinoleic acid. Evidence against hormonal mediation of the effect. Am J Dig Dis. 1976;21:859–862. doi: 10.1007/BF01072077. [DOI] [PubMed] [Google Scholar]
- 36.Beubler E, Juan H. Effect of ricinoleic acid and other laxatives on net water flux and prostaglandin E release by the rat colon. J Pharm Pharmacol. 1979;31:681–685. doi: 10.1111/j.2042-7158.1979.tb13628.x. [DOI] [PubMed] [Google Scholar]
- 37.Racusen LC, Binder HJ. Ricinoleic acid stimulation of active anion secretion in colonic mucosa of the rat. J Clin Invest. 1979;63:743–749. doi: 10.1172/JCI109358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart JJ, Bass P. Effect of intravenous C-terminal octapeptide of cholecystokinin and intraduodenal ricinoleic acid on contractile activity of the dog intestine. Proc Soc Exp Biol Med. 1976;152:213–217. doi: 10.3181/00379727-152-39363. [DOI] [PubMed] [Google Scholar]
- 39.Izzo AA, Capasso R, Pinto L, Di Carlo G, Mascolo N, Capasso F. Effect of vanilloid drugs on gastrointestinal transit in mice. Br J Pharmacol. 2001;132:1411–1416. doi: 10.1038/sj.bjp.0703975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tunaru S, Althoff TF, Nüsing RM, Diener M, Offermanns S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc Natl Acad Sci U S A. 2012;109:9179–9184. doi: 10.1073/pnas.1201627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almeida CE, Karnikowski MG, Foleto R, Baldisserotto B. Analysis of antidiarrhoeic effect of plants used in popular medicine. Rev Saude Publica. 1995;29:428–433. doi: 10.1590/s0034-89101995000600002. [DOI] [PubMed] [Google Scholar]
- 42.Berridge MJ. Cell Signalling Biology - Module 7. Available from: http://www.cellsignallingbiology.org.
- 43.Ojewole JA, Awe EO, Chiwororo WD. Antidiarrhoeal activity of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract in rodents. J Smooth Muscle Res. 2008;44:195–207. doi: 10.1540/jsmr.44.195. [DOI] [PubMed] [Google Scholar]
- 44.Araújo TS, Costa DS, Sousa NA, Souza LK, de Araújo S, Oliveira AP, Sousa FB, Silva DA, Barbosa AL, Leite JR, et al. Antidiarrheal activity of cashew GUM, a complex heteropolysaccharide extracted from exudate of Anacardium occidentale L. in rodents. J Ethnopharmacol. 2015;174:299–307. doi: 10.1016/j.jep.2015.08.020. [DOI] [PubMed] [Google Scholar]


