Abstract
AIM
To investigate the role of heat shock protein (HSP)-glycoprotein (gp)96 in dendritic cells (DCs) and lymphocytes induction in gastric cancer (GC).
METHODS
Human GC cell lines KATOIII, MKN-28 and SGC-7901 were infected with adenovirus gp96 at a multiplicity of infection of 100. gp96-GC antigen peptide complexes were purified. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, lactate dehydrogenase (LDH) release assay and enzyme-linked immunosorbent assay were used to determine allo-reactive T cell stimulation, natural killer (NK) cell activity and expression of cytokines (such as interleukin (IL)-10, IL-12, interferon (IFN)-γ and tumor necrosis factor (TNF)-α), respectively. Effect of cytotoxic T lymphocyte (CTL) on DCs incubated with HSP-gp96 was also evaluated by LDH release. All assays were performed in triplicate and the average values were reported. Comparison between groups was conducted using Student’s t test.
RESULTS
T cells incubated with HSP-gp96 exhibited a marked increase in proliferation in a dose-dependent manner (P < 0.05). NK cell activity after gp96-GC peptide complex treatment was significantly higher than that after antigen peptide treatment (P < 0.05). The activity of CTLs incubated with DCs from three GC cells lines was obviously higher than that stimulated by GC antigen at ratios of 50: 1, 25: 1, 10: 1, and 5: 1 (P < 0.05). Furthermore, the secretion of TNF-α, IL-10, IL-12 (P70) and IFN-γ markedly increased after incubation with HSP-gp96 (P < 0.05).
CONCLUSION
HSP-gp96 promotes T cell response, enhances DC antigen presentation and induces cytokine secretion, as well. HSP-gp96 has potential as immunotherapy for elimination of residual GC cells.
Keywords: Gastric cancer, Heat shock protein gp96, T lymphocytes, Dendritic cells, Natural killer cells, Cytotoxic T lymphocytes, Immunization therapy
Core tip: Heat shock protein (HSP)- glycoprotein (gp)96 was studied to evaluate its capacity to stimulate immune cells. Compared with gastric cancer (GC)-derived peptide, purified gp96-GC peptide complex markedly increased proliferation of allogeneic normal T lymphocytes, and activity of natural killer cells and cytotoxic T lymphocytes. Therefore, HSP-gp96 derived from GC cells has immunotherapeutic potential for elimination of residual GC cells.
INTRODUCTION
Gastric cancer (GC) is the fourth most common type of cancer and its mortality rates rank second in the world[1]. Over 70% of newly developed GC cases and deaths occur in developing countries, especially in Eastern Asian countries, China, for instance[1]. One recent study indicated that 5-year overall survival of GC was 53% in Australia[2]. However, the 5-year survival rates for all stages are only 20%-25% in western countries, though with advanced interventions, the median survival time is only about 2 years[3].
Surgery and adjuvant chemoradiotherapy are traditional interventions of GC; however, prognosis is poor, which may due to incomplete removal of tumor cells. Therefore, how to eliminate residual GC cells has become the focus of GC treatment worldwide. Immunotherapy is emerging as a new approach to control residual disease, particularly as an alternative to conventional chemotherapy. To explore more effective immunotherapeutic strategies for GC patients, the tasks of identifying and applying other potent GC antigens shared among GC patients are critically important.
Heat shock proteins (HSPs), a group of highly conserved proteins, are induced by the perturbation or stress of various physical and chemical factors[4]. They participate in formation of various protein complexes, contributing to folding and extension as well as assembly. HSPs are defined as molecular chaperones regulating target protein transportation instead of protein synthesis[5]. HSPs are members of the most conserved proteins known in phylogeny, according to both structure and function[6,7]. Immunological significance of HSPs was realized from the observation that tumor cell-derived HSPs could immunize against tumors, which is attributed to HSP-chaperoned peptides derived from tumor antigens[8-10].
The HSP glycoprotein (gp)96 is named according to its molecular weight of 96 kDa and acts as a chaperone protein that localizes in the endoplasmic reticulum in eukaryotes[11-13]. Recent studies in solid tumors (i.e., melanoma and colon cancer) have shown that tumor-derived HSP-gp96 is immunogenic and able to stimulate tumor-specific cytotoxic T lymphocyte (CTL) generation[14,15]. It is noteworthy that HSP-gp96 is an effective treatment for fibrosarcoma melanoma, colon cancer, lung cancer, and spindle cell tumors induced by UV radiation in rat models[10,15,16]. In addition, a Phase III clinical trial of gp96-based vaccines treatment in melanoma and renal cell carcinoma is in progress[17,18]. So, whether HSP-gp96 has the capacity to stimulate CTLs and facilitate complete clearance of GC is also of great importance.
This study aimed to investigate whether GC cell-derived HSP-gp96 peptide complexes could be used as GC-associated antigens in cancer immunological treatment. The purified proteins were used to explore their ability to stimulate lymphocytes, facilitate transport of tumor antigen, enhance the transport of antigen and cytokine secretion in dendritic cells (DCs). Our results provide novel clues for HSP-gp96 as a potential immunotherapeutic target in GC and may provide hints for further studies in this concern.
MATERIALS AND METHODS
Reagents
KATOIII, MKN-28 and SGC-7901 GC cell lines were purchased from Shanghai Institute of Biochemistry and Cell Biology (China). Concanavalin A-Sepharose and ion exchange columns were purchased from Pharmacia (Kalamazoo, MI, United States). Markers for sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) assay and bovine serum albumin were purchased from Zhongshan Biological Technology (Beijing, China). Phytohemagglutinin, ammonium persulfate, and a-pyran-1-methyl mannose were purchased from Fluka (part of Sigma-Aldrich, St Louis, MO, United States). Monoclonal antibody against HSP-gp96 was purchased from Santa Cruz Biotechnology (Dallas, TX, United States). Glycine, acrylamide, and ammonium sulfate were purchased from Sigma-Aldrich. Cytokine kits for interleukin (IL)10, IL-12 (p70), interferon (IFN)-γ and tumor necrosis factor (TNF)-α were purchased from R&D Systems (Minneapolis, MN, United States). Flow cytometry reagents and antigens were purchased from BD Pharmingen (San Jose, CA, United States). Lactate dehydrogenase (LDH) release kit was purchased from Promega Biological Technology (Madison, WI, United States).
Construction of HSP-gp96 recombinant adenovirus (Ad-gp96)
Gene fragment of human HSP-gp96 was inserted into the pShuttle CMV shuttle vector, BJ5183-AD-1, which had been converted to facilitate homologous recombination in thallus (adenovirus, Ad) framework plasmid, pAdEasy-1, which had been pre-converted. Recombinant Ad plasmids containing target sequence were linearized for the transfection of AD293 cells. Virus produced in AD293 cells was concentrated using CsCl ultracentrifugation to produce high-titer recombinant Ad-gp96.
Isolation and characterization of gp96-GC antigen peptide complex
gp96-GC antigen peptide complexes were purified as previously described[19,20]. Human KATOIII, MKN-28 and SGC-7901 GC cell lines were infected with Ad-gp96 at a multiplicity of infection (MOI) of 100. One wk later, cells were lysed and centrifuged for 30 min at 2000 × g at 4 °C. After the supernatants were aspirated, samples were centrifuged for an additional 15 min at 15000 × g at 4 °C. After the supernatants were aspirated, the samples were precipitated using 50%, then 70% saturated ammonium sulfate. The resulting precipitate was resuspended in 5 × its volume in equilibrium liquid (20 mmol/L Tris-HCl, pH 7.4; 200 mmol/L NaCl; 2 mmol/L CaCl2, 2 mmol/L MgCl2), added to a concanavalin affinity column, and eluted using 10% a-pyran-1-methyl mannose. The eluant was further purified using a diethyl-aminoethyl ion-exchange column and eluted using a gradient of 300-1000 mmol/L NaCl. Fractions containing gp96-GC antigen peptide complex were identified using 10% SDS-PAGE and western blotting. Protein concentrations of the samples were determined using Bradford assays, and the protein purity achieved was 84%.
Preparation and identification of T cells and DCs
DCs were cultured according to previous report[21-24]. Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers and suspended in 5-mL culture flasks with RPMI 1640 containing 10% fetal calf serum. After cell concentration adjustment to 106 cells/mL, cells were incubated at 37 °C with 5% CO2 for 2 h. Cells that did not adhere were collected as T cells, and the adherent cells were treated with granulocyte-macrophage colony-stimulating factor (500 U/mL) and IL-4 (500 U/mL) before being divided into two separate DC populations. Both populations were cultured for 7 d, with half of the growth medium changed every 4 d. Cells were divided into two groups, one treated with purified gp96-GC peptide complex (1 μg/mL) and the other not; both groups were treated with 50 μg/mL TNF-α. On d 10, suspension cells were collected. Direct immunofluorescence labeling of the cells was performed to characterize the cell populations phenotypically. Cells were stained with CD3, CD14, CD19, CD83, CD1a, CD40, CD54, CD80, CD86, HLA-DR and HLA-ABC to identify DCs[25,26].
MTS assay
T cells (2 × 105/100 mL) were inoculated into 96-well U-bottom tissue culture plates prior to addition of purified gp96 peptide complex at three different concentrations (1, 2 and 5 μg/mL) in triplicate. After 5 d, the proportion of live cells was measured using a CellTiter 96 AQueous One Solution Cell Proliferation Assay kit (Promega). After 4 h incubation at 37 °C, the absorbance at 490 nm was measured using a Model 680 microplate reader.
Detection of NK cell activity
NK cells were cultured as previously described[27]. Target cells included KATOIII, MKN-28 and SGC-7901 cells at ratios of 20:1, 10:1 and 5:1 to NK cells stimulated with purified gp96 peptide complex at 1, 2 and 5 μg/mL with untreated NK cells as controls. LDH release was detected by colorimetry.
Detection of CTL activity
Conditions for the culture of CTLs have previously been described[27]. CTL activity was assayed for T cells and DCs incubated with purified gp96-GC peptide complex or tumor antigen peptide complex at 5 μg/mL for 7 d. KATOIII, MKN-28 and SGC-7901 cells were used as target:effector cell ratios of 50:1, 25:1, 10:1, 5:1 and 1:1. LDH release was detected by colorimetry.
Cytokine detection
A sandwich enzyme-linked immunosorbent assay (ELISA) method was adopted to detect expression levels of IL-10, IL-12 p70, IFN-γ and TNF-α in cell supernatants.
Statistical analysis
All assays were performed in triplicate and the average values were reported. Data are presented as average ± SD. SPSS version 10.0 was used for all analyses, with comparisons between groups made using Student’s t test. P < 0.05 was considered significant.
RESULTS
gp96-GC peptide complex isolation and its effect on allogeneic normal T lymphocytes
gp96-GC antigen peptide complex was purified from Ad-gp96-infected (MOI = 100) human GC cell lines, including KATOIII, MKN-28 and SGC-7901. The size and purity of isolated HSP-gp96-GC antigen peptide complex was confirmed by western blot (Figure 1). To investigate gp96-GC peptide complex function, T cells (2 × 105/100 mL) were incubated with purified gp96 peptide complex at three different gradients (1, 2 and 5 μg/mL), or with antigen peptide. After 5 d, MTS assay was applied to evaluate T cell proliferation. T cells incubated with gp96 peptide complexes exhibited a significant and concentration-dependent higher proliferation than that with antigen peptide, and greatest difference in proliferation was observed at the 5 μg/mL concentration (P < 0.05; Figure 2).
Figure 1.
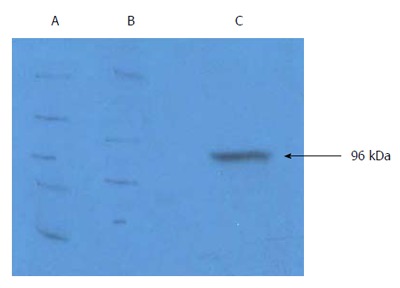
Characterization of purified gp96-GC antigen peptide complexes. A: Western blot of gp96-GC antigen peptide complexes from KATOIII cells; B: KATOIII GC cell proteins; C: Purified eluted fraction from KATOIII cells using diethyl-aminoethyl-Sepharose anion exchange chromatography.
Figure 2.
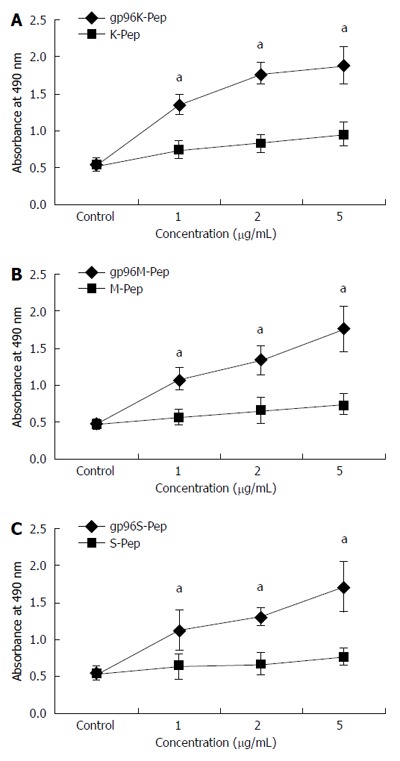
Proliferation assays of gastric cancer cell strains treated with purified gp96-peptide complexes. MTS assays were performed for: A: KATOIII source GP96-gastric cancer (GC) antigen peptide complexes (gp96K-Pep) vs KATOIII source antigen peptide (K-Pep); B: MKN-28 source GP96-GC antigen peptide complexes (gp96M-Pep) vs MKN-28 source antigen peptide (M-Pep); C: SGC-7901 source gp96-GC antigen peptide complexes (p96S-Pep) vs SGC-7901 source antigen peptide (S-Pep). aP < 0.05.
Effect of gp96-GC peptide complex on NK cells
To assess the effects of the gp96-GC peptide complex on NK cells, KATOIII, MKN-28 and SGC-7901 cells were incubated with NK cells at different ratios (20:1, 10:1 and 5:1, respectively). Co-cultured cells were stimulated with 1, 2 or 5 μg/mL purified gp96-GC peptide complex. NK cell activity after gp96-GC peptide complex treatment from three GC cells at different concentrations and target:effector ratios was significantly higher than that after antigen peptide treatment (P < 0.05; Figure 3).
Figure 3.
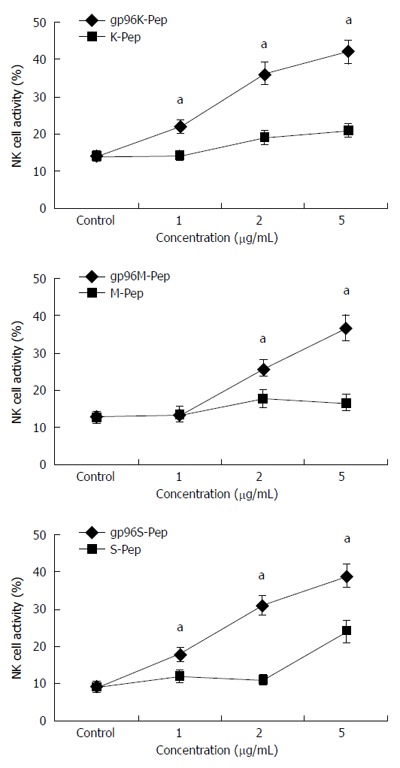
Activity of natural killer cells assayed in the presence of gp96-peptide complexes. At an effector to target cell ratio of 10:1, natural killer cells were incubated with three different gastric cancer (GC) cell strains in the presence of 1, 2 and 5 μg/mL gp96-peptide complexes or gp96 purified from Ad-gp96 expression. NK cell activity for (A) KATOIII, (B) MKN-28 and (C) SGC-7901 cells incubated with gp96-peptide complexes (gp96K-Pep, gp96M-Pep and gp96S-Pep) vs GC cell source antigen peptide (K-Pep, M-Pep and S-Pep) for each of the three cell lines, respectively. aP < 0.05.
Effect of gp96-GC peptide complex on CTL activity
On d 7, DCs expressed high levels of specific markers, including CD11c, CD80, CD86 and HLA-DR (data not shown). Cytotoxicity was evaluated in T cells and DCs incubated with purified gp96-GC peptide complex or tumor antigen peptide complex as control, at the concentration of 5 μg/mL for 7 d. KATOIII, MKN-28 and SGC-7901 cells were used as target cells in co-culture, at ratios of 50:1, 25:1, 10:1, 5:1 and 1:1 to effector cells. Compared with tumor antigen peptide, cells treated with gp96-GC peptide complex from SGC-7901 cells and tumor antigen did not show significant difference in cytotoxicity when the target:effector ratio was 5:1 (P > 0.05). The CTL activity was highly increased by the gp96 peptide complex at target:effector ratios of 50:1, 25:1, 10:1 and 5:1 (P < 0.05) (Figure 4). The CTL activity after incubation with DCs stimulated by gp96-GC peptide complexes at target:effector ratios of 50:1, 25:1, 10:1 and 5:1 was significantly higher than for the tumor antigen group (P < 0.05) (Figure 5).
Figure 4.
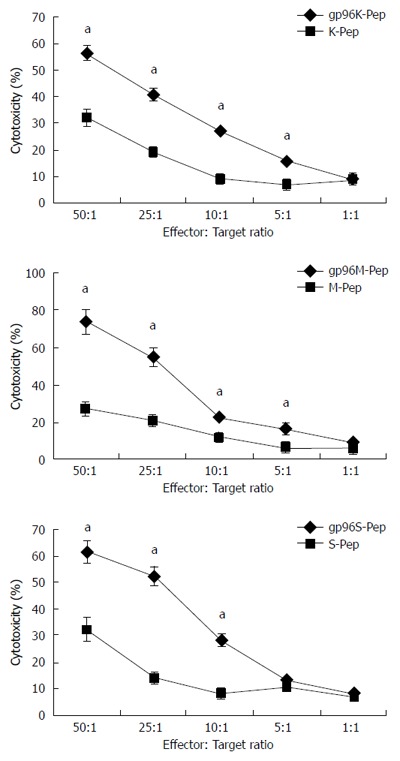
Cytotoxicity assays for T cells. Cytotoxic T lymphocytes (CTLs) were incubated with KATOIII, MKN-28 or SGC-7901 cells in the presence of gp96-peptide complexes (gp96K-Pep, gp96M-Pep and gp96S-Pep) vs gastric cancer cell source antigen peptide (K-Pep, M-Pep and S-Pep) at the target to effector cell ratios indicated. aP < 0.05.
Figure 5.
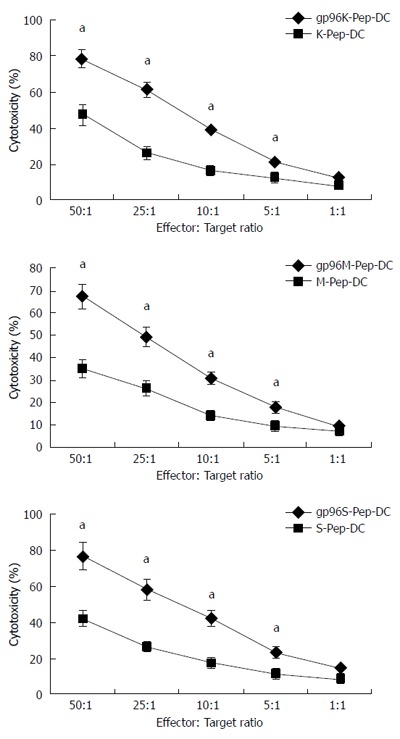
Cytotoxicity assays for dendritic cells. Dendritic cells (DCs) were incubated with KATOIII, MKN-28 and SGC-7901 cells in the presence of gp96-peptide complexes (gp96K-Pep-DC, gp96M-Pep-DC and gp96S-Pep-DC) vs gastric cancer cell source antigen peptide (K-Pep-DC, M-Pep-DC and S-Pep-DC) at the target to effector cell ratios indicated. aP < 0.05.
Cytokine expression by PBMCs and DCs after gp96-GC peptide complex treatment
Sandwich ELISA was used to detect IL-10, IL-12 p70, IFN-γ and TNF-α levels in supernatants of PBMCs and DCs, after co-culture with KATOIII, MKN-28 and SGC-7901 cells expressing gp96-GC peptide complexes, or without as control. Compared with untreated PBMCs and DCs, secretion of TNF-α, IL-10, IL-12 p70 and IFN-γ was markedly improved in the presence of 5 μg/mL gp96-GC peptide complex stimulation (P < 0.05; Figure 6) in both PBMCs and DCs.
Figure 6.
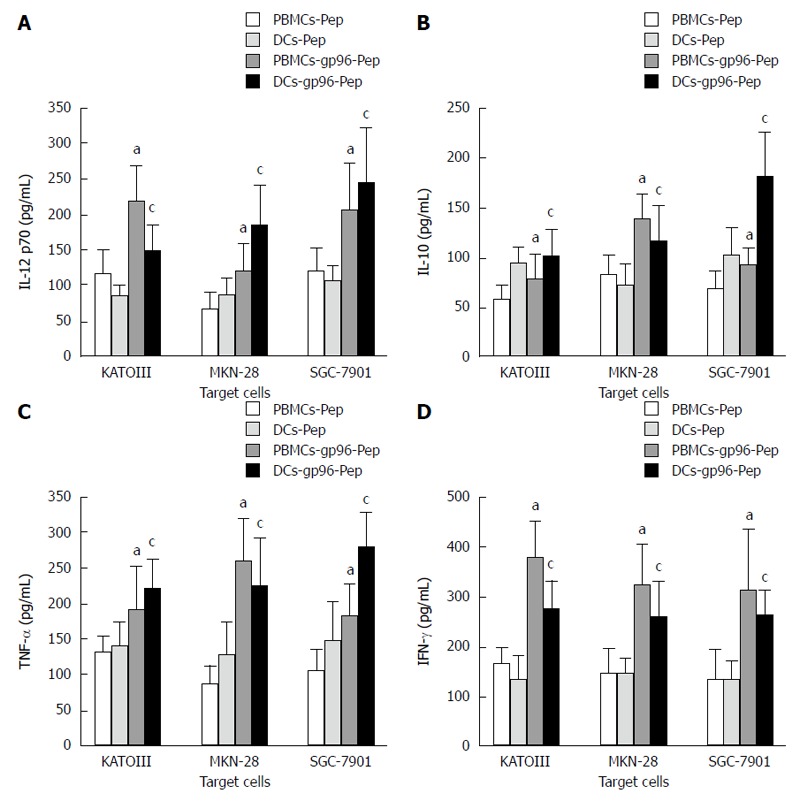
Cytokine-specific sandwich enzyme-linked immunosorbent assays. Cytokine expression by peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs) in the presence or absence of gp96-peptide complexes purified from KATOIII, MKN-28 and SGC-7901 cells. A: IL-12 p70; B: IL-10; C: TNF-α; D: IFN-γ. PBMCs cultured with gp96-GC peptide complexes vs PBMCs cultured with gastric cancer (GC) peptide, aP < 0.05; DCs cultured with gp96-GC peptide complexes vs DCs cultured with GC peptide, cP < 0.05.
DISCUSSION
Similar to other types of cancer, full recovery from GC is difficult due to residual cells that survive after aggressive postoperative chemotherapy or radiotherapy. Although immunotherapy combined with high-dose chemotherapy holds great promise for GC treatment, clinical studies have not yet achieved expected results. Other than optimizing immunotherapy, there is urgent need for novel GC antigen development that can be used as universal antigens to stimulate anti-GC CTL responses in most patients. In the present study, we chose HSP-gp96 as our target in GC cells, because previous studies in solid tumors[28,29] have shown that tumor-derived HSP-gp96 had high efficacy in generating tumor-specific immune responses. However, there are no reports in GC yet. Considering the heterogeneity of different tumors, preclinical studies are needed to explore the feasibility of GC-derived HSP-gp96as immunotherapy agent in GC.
Thus, HSP-gp96 was adopted for its capacity for stimulating immune cells. Compared with GC-derived peptide, purified gp96-GC peptide complex markedly improved allogeneic normal T lymphocyte proliferation, and increased the activity of NK cells and CTLs. HSP-based vaccines are personalized vaccines carrying the fingerprint of a given tumor, which can circumvent the need to identify tumor antigens for each patient. It is still unknown, but very likely that HSP chaperone peptides originate from endogenously degraded proteins, consisting of normal cell proteins and tumor-associated antigens[30,31]. Therefore, the CTLs were not reactive to normal cells, such as blood cells, including DCs, B cells and PBMCs[32]. CTLs can recognize antigens localized in the cytoplasm of target cells through formation of peptide complexes with MHC class I molecules[33]. HSP-gp96 localizes at the surface of antigen presenting cells (APCs), and the protein level, though in nanomolar concentrations, is still sufficient to induce a CTL response. Correspondingly, immunizing doses of gp96-peptide complexes are low[34]. HSP-gp96 is a vaccine that does not require adjuvant, thereby eliminating additional risks for toxicity[34,35].
Recent studies have shown that APCs, such as DCs and monocytes, can internalize HSPs spontaneously through receptor (such as CD91)-mediated endocytosis and then direct chaperoned proteins/peptides into the intracellular pathway for MHC class I-restricted presentation to CD8+ T cells, concomitant with induction of DC maturation and cytokine secretion[36,37]. Our results also showed that HSP-gp96 alone was able to activate immature DCs (data not shown). However, most mature or activated DCs were obtained after gp96 treatment and further maturation induced by TNF-α[32]. In our study, these DCs were used to generate gp96-specific CTLs.
The ability of HSPs to facilitate cross-presentation of MHC class I-restricted epitopes and to prime CD8+ cell effector responses is well established[31]. Recent studies have also revealed that HSP-peptide complexes could lead to antigen presentation on MHC class II molecules, and then to activated CD4+ T cells[38,39]. In line with these findings, we might have induced a CD4+ T cell response specific for MHC class II-restricted peptides that were chaperoned by gp96 in our experiment. This is supported by the fact that IL-10, IL-12, IFN-γ and TNF-α were increased after PBMCs were stimulated by the gp96-peptide complex. It is possible that HSP-gp96 peptide complexes’ uptake led to antigen presentation of both MHC class I and II molecules on mature DCs, thereby activating CD8+ CTLs and CD4+ T cells as well. However, the evidence in support of this claim was not comprehensive. Hence, further studies are still needed, especially concerning the role and mechanism of MHC class II molecules and peptides in activating specific CD4+ T cells.
In conclusion, our study shows that GC-derived gp96 peptide complex plays an important role in activating lymphocytes and promoting tumor antigen presentation by activating CTLs. These CTLs might be promising effector cells in GC immunotherapy because they are potent and cancer cell-specific, able to target and kill GC cells, rather than normal cells. Thus, our study provides strong and direct evidence supporting application of the HSP-gp96-based immunotherapy targeting GC, especially for residual GC cells. Another advantage of the HSP-gp96 peptide complex is that it can be isolated from the patient’s own tumor tissue, to avoid the need for virus-mediated therapies and transforming DNA as well, which needs further clinical exploration[40].
COMMENTS
Background
Gastric cancer (GC) is the fourth most common type of cancer worldwide and has poor prognosis. Immunotherapy is a new approach to provide an alternative treatment to conventional chemotherapy for patients with GC. Heat shock protein (HSP)-glycoprotein (gp)96 is immunogenic and potent in stimulating the generation of tumor-specific cytotoxic T lymphocytes (CTLs), and may offer immunotherapy for elimination of residual GC cells.
Research frontiers
Previous studies in solid tumors, such as lung cancer, colon adenocarcinoma, fibrosarcoma melanoma and spindle cell tumors, have shown high efficacy of tumor-derived HSP-gp96 in generating tumor-specific immune responses. However, there are no such reports for GC.
Innovations and breakthroughs
In this study, purified gp96-GC peptide complex was shown to markedly improve the proliferation of allogeneic normal T lymphocytes, and increase the activity of both natural killer cells and CTLs.
Applications
The results from this study provide novel clues for further investigation of HSP-gp96 as a potential target in an immunotherapy approach for GC.
Terminology
MTS assay is a colorimetric assay for assessing cell metabolic activity. NAD(P)H-dependent cellular oxidoreductase enzymes may, under defined conditions, reflect the number of viable cells present. Other closely related tetrazolium dyes, including XTT, MTT and the WSTs, are used in conjunction with the intermediate electron acceptor, 1-methoxy phenazine methosulfate.
Peer-review
The fact that HSP can be used as a vaccination tool in gastric cancer, opens the possibility to another immune-based approach in this disease. This study has established that the GC-derived gp96-peptide complex played an important role in activating lymphocytes and promoted the presentation of tumor antigens by activated CTLs. The authors need more data of HSP-gp96 in animal tests.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Institutional Ethnics Committee of the First Affiliated Hospital of Zhejiang University, Hangzhou, China.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Zhejiang University, Hangzhou, China.
Conflict-of-interest statement: The authors declare that no conflict of interest exists in this study.
Data sharing statement: The anonymized dataset is available from the corresponding author (hongzhang1013@163.com) and will be provided on request after obtaining all authors’ agreement.
Peer-review started: November 15, 2016
First decision: January 10, 2017
Article in press: April 12, 2017
P- Reviewer: Martin-Villa JM S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
References
- 1.Levin D, Constant S, Pasqualini T, Flavell R, Bottomly K. Role of dendritic cells in the priming of CD4+ T lymphocytes to peptide antigen in vivo. J Immunol. 1993;151:6742–6750. [PubMed] [Google Scholar]
- 2.Thomson IG, Gotley DC, Barbour AP, Martin I, Jayasuria N, Thomas J, Smithers BM. Treatment results of curative gastric resection from a specialist Australian unit: low volume with satisfactory outcomes. Gastric Cancer. 2014;17:152–160. doi: 10.1007/s10120-013-0240-3. [DOI] [PubMed] [Google Scholar]
- 3.Meyer HJ, Wilke H. Treatment strategies in gastric cancer. Dtsch Arztebl Int. 2011;108:698–705; quiz 706. doi: 10.3238/arztebl.2011.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 5.Csermely P, Schnaider T, Soti C, Prohászka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 6.Beliakoff J, Whitesell L. Hsp90: an emerging target for breast cancer therapy. Anticancer Drugs. 2004;15:651–662. doi: 10.1097/01.cad.0000136876.11928.be. [DOI] [PubMed] [Google Scholar]
- 7.Ciocca DR, Oesterreich S, Chamness GC, McGuire WL, Fuqua SA. Biological and clinical implications of heat shock protein 27,000 (Hsp27): a review. J Natl Cancer Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava PK, Udono H. Heat shock protein-peptide complexes in cancer immunotherapy. Curr Opin Immunol. 1994;6:728–732. doi: 10.1016/0952-7915(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 9.Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci USA. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 11.Qu D, Mazzarella RA, Green M. Analysis of the structure and synthesis of GRP94, an abundant stress protein of the endoplasmic reticulum. DNA Cell Biol. 1994;13:117–124. doi: 10.1089/dna.1994.13.117. [DOI] [PubMed] [Google Scholar]
- 12.Linderoth NA, Popowicz A, Sastry S. Identification of the peptide-binding site in the heat shock chaperone/tumor rejection antigen gp96 (Grp94) J Biol Chem. 2000;275:5472–5477. doi: 10.1074/jbc.275.8.5472. [DOI] [PubMed] [Google Scholar]
- 13.Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol. 2001;167:6731–6735. doi: 10.4049/jimmunol.167.12.6731. [DOI] [PubMed] [Google Scholar]
- 14.Janetzki S, Blachere NE, Srivastava PK. Generation of tumor-specific cytotoxic T lymphocytes and memory T cells by immunization with tumor-derived heat shock protein gp96. J Immunother. 1998;21:269–276. doi: 10.1097/00002371-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Rivoltini L, Castelli C, Carrabba M, Mazzaferro V, Pilla L, Huber V, Coppa J, Gallino G, Scheibenbogen C, Squarcina P, et al. Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinoma-specific T cells. J Immunol. 2003;171:3467–3474. doi: 10.4049/jimmunol.171.7.3467. [DOI] [PubMed] [Google Scholar]
- 16.Hoos A, Levey DL. Vaccination with heat shock protein-peptide complexes: from basic science to clinical applications. Expert Rev Vaccines. 2003;2:369–379. doi: 10.1586/14760584.2.3.369. [DOI] [PubMed] [Google Scholar]
- 17.Castelli C, Rivoltini L, Rini F, Belli F, Testori A, Maio M, Mazzaferro V, Coppa J, Srivastava PK, Parmiani G. Heat shock proteins: biological functions and clinical application as personalized vaccines for human cancer. Cancer Immunol Immunother. 2004;53:227–233. doi: 10.1007/s00262-003-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer. 2000;88:232–238. doi: 10.1002/1097-0215(20001015)88:2<232::aid-ijc14>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Meng SD, Song J, Rao Z, Tien P, Gao GF. Three-step purification of gp96 from human liver tumor tissues suitable for isolation of gp96-bound peptides. J Immunol Methods. 2002;264:29–35. doi: 10.1016/s0022-1759(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 20.Dai J, Liu B, Caudill MM, Zheng H, Qiao Y, Podack ER, Li Z. Cell surface expression of heat shock protein gp96 enhances cross-presentation of cellular antigens and the generation of tumor-specific T cell memory. Cancer Immun. 2003;3:1. [PubMed] [Google Scholar]
- 21.Zhu KQ, Zhang SJ. Study of Loss of Heterozygosity on Chromosome 9p21-22 in Sporadic Gliomas. Zhejiang Daxue Xuebao. 2001;30:87–89. [Google Scholar]
- 22.Tong XM, Jin J, Xue YQ, Wang YG. The study of dendritic cells derived from acute myeloid leukemia cells. Zhonghua Neike Zazhi. 2004;43:45–48. [PubMed] [Google Scholar]
- 23.Tong XM, Jin J, Qian WB, Meng HT, Xue YQ. Biological features of dendritic cells derived from chronic myeloid leukemia cells in vitro. Zhejiang Daxue Xuebao. 2005;34:348–352, 357. doi: 10.3785/j.issn.1008-9292.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Tong XM, Yao HP, Qian WB, Zhu LF, Fu ZH, Huang ZL, Jin J. The biological characteristics of dendritic cells derived in vitro from myelogeneous leukemia cells and healthy donor cells. Int J Lab Hematol. 2008;30:372–381. doi: 10.1111/j.1751-553X.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 25.Romani N, Reider D, Heuer M, Ebner S, Kämpgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinagawa N, Yamazaki K, Tamura Y, Imai A, Kikuchi E, Yokouchi H, Hommura F, Oizumi S, Nishimura M. Immunotherapy with dendritic cells pulsed with tumor-derived gp96 against murine lung cancer is effective through immune response of CD8+ cytotoxic T lymphocytes and natural killer cells. Cancer Immunol Immunother. 2008;57:165–174. doi: 10.1007/s00262-007-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Li X, Qiu L, Zhang X, Chen L, Cao S, Wang F, Meng S. Treg suppress CTL responses upon immunization with HSP gp96. Eur J Immunol. 2009;39:3110–3120. doi: 10.1002/eji.200939593. [DOI] [PubMed] [Google Scholar]
- 29.Randazzo M, Terness P, Opelz G, Kleist C. Active-specific immunotherapy of human cancers with the heat shock protein Gp96-revisited. Int J Cancer. 2012;130:2219–2231. doi: 10.1002/ijc.27332. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 32.Qian J, Wang S, Yang J, Xie J, Lin P, Freeman ME, Yi Q. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clin Cancer Res. 2005;11:8808–8815. doi: 10.1158/1078-0432.CCR-05-1553. [DOI] [PubMed] [Google Scholar]
- 33.Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004;5:685–692. doi: 10.1038/ni1088. [DOI] [PubMed] [Google Scholar]
- 34.Heike M, Weinmann A, Bethke K, Galle PR. Stress protein/peptide complexes derived from autologous tumor tissue as tumor vaccines. Biochem Pharmacol. 1999;58:1381–1387. doi: 10.1016/s0006-2952(99)00178-1. [DOI] [PubMed] [Google Scholar]
- 35.Caudill MM, Li Z. HSPPC-96: a personalised cancer vaccine. Expert Opin Biol Ther. 2001;1:539–547. doi: 10.1517/14712598.1.3.539. [DOI] [PubMed] [Google Scholar]
- 36.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 37.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 38.Doody AD, Kovalchin JT, Mihalyo MA, Hagymasi AT, Drake CG, Adler AJ. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J Immunol. 2004;172:6087–6092. doi: 10.4049/jimmunol.172.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SenGupta D, Norris PJ, Suscovich TJ, Hassan-Zahraee M, Moffett HF, Trocha A, Draenert R, Goulder PJ, Binder RJ, Levey DL, et al. Heat shock protein-mediated cross-presentation of exogenous HIV antigen on HLA class I and class II. J Immunol. 2004;173:1987–1993. doi: 10.4049/jimmunol.173.3.1987. [DOI] [PubMed] [Google Scholar]
- 40.Wierecky J, Müller MR, Wirths S, Halder-Oehler E, Dörfel D, Schmidt SM, Häntschel M, Brugger W, Schröder S, Horger MS, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66:5910–5918. doi: 10.1158/0008-5472.CAN-05-3905. [DOI] [PubMed] [Google Scholar]


