Abstract
Impaired gene regulation lies at the heart of many disorders, including developmental diseases and cancer. Furthermore, the molecular pathways that control gene expression are often the target of cellular parasites, such as viruses. Gene expression is controlled through multiple mechanisms that are coordinated to ensure the proper and timely expression of each gene. Many of these mechanisms target the life cycle of the RNA molecule, from transcription to translation. Recently, another layer of regulation at the RNA level involving RNA modifications has gained renewed interest of the scientific community. The discovery that N6-methyladenosine (m6A), a modification present in mRNAs and long noncoding RNAs, can be removed by the activity of RNA demethylases, launched the field of epitranscriptomics; the study of how RNA function is regulated through the addition or removal of post-transcriptional modifications, similar to strategies used to regulate gene expression at the DNA and protein level. The abundance of RNA post-transcriptional modifications is determined by the activity of writer complexes (methylase) and eraser (RNA demethylase) proteins. Subsequently, the effects of RNA modifications materialize as changes in RNA structure and/or modulation of interactions between the modified RNA and RNA binding proteins or regulatory RNAs. Disruption of these pathways impairs gene expression and cellular function. This review focuses on the links between the RNA modification m6A and its implications in human diseases.
Keywords: N6-methyladenosine, Epitranscriptomics, Cancer, Viral replication, Metabolic disease
The N6-methyladenosine RNA modification
The modification N6-methyladenosine (m6A) is present in several classes of RNAs, including the poly(A) fraction of mRNA, where it is the most abundant internal RNA modification [1]. m6A was first identified among poly(A) RNAs in the 1970s [2], [3], [4], [5], [6], [7], [8], [9]. In the poly(A) fraction of RNA, the m6A modification is present in a well-defined RNA motif, RRACH (R can be either A or G, and H can be A, C or U). The frequency of the methylation consensus motif far exceeds the m6A content of mRNA [1], suggesting that the primary sequence is likely necessary, but not sufficient for the m6A modification to occur. Indeed, high-throughput sequencing experiments have demonstrated that the m6A modification is not randomly distributed in mature transcripts, but is enriched at specific transcript landmarks such as the last transcribed exon, near the 5′ end, or in long exons [10], [11], [12], [13]. Furthermore, a correlation between m6A modification and tandem alternative polyadenylation (APA) site usage has been described. In a restricted group of mRNAs, knockdown of the m6A methylation complex, whose structure and function will be detailed below, resulted in a switch from proximal to distal APA site usage [10]. In a different study, sequencing of intact transcripts after m6A immunoprecipitation, revealed a strong bias toward the presence of m6A in transcript isoforms with shorter 3′UTRs [14], lacking regulatory elements.
The presence of m6A in the poly(A) fraction of RNA is widely conserved among eukaryotes [1]. In the yeast (Saccharomyces cerevisiae), cell fate decisions in response to starvation, such as dividing under a pseudo-hyphal foraging program or undergoing meiosis to form protective spores, are dependent on m6A [15], [16]. In Arabidopsis thaliana, loss of components of the methylation complex, and consequent loss of m6A, results in embryonic lethality [17], [18]. Later in development, loss of m6A yields plants with altered growth patterns and reduced apical dominance, with flowers that show defects in their floral organ number, size, and identity [19]. In the fruit fly (Drosophila melanogaster), loss of components of the methylase complex, and consequent loss of m6A, results in impaired neuronal function, flightless animals, and a sex bias toward maleness [20], [21]. In zebrafish (Danio rerio), the protein YTH N6-methyladenosine RNA binding protein 2 (Ythdf2), an m6A reader, facilitates removal of maternal mRNAs during the maternal-to-zygotic transition (MZT), an important developmental step. Loss of ythdf2 decelerates the decay of m6A-modified maternal mRNAs and impedes zygotic genome activation [22]. Knockdown of components of the methylase complex in zebrafish embryos resulted in multiple developmental defects including smaller head and eyes, smaller brain ventricle, and curved notochord [23]. These observations suggest that in eukaryotes, m6A has crucial functions in development. In mice, genes encoding for methyltransferase like 3 (METTL3) and METTL14 are essential genes, and m6A is required for embryonic stem cells (ESC) to exit pluripotency [11], [24]. The landscape of m6A modification is very similar in mice and humans [11], [12]. Comparison of the m6A landscape of human, chimpanzee, and rhesus monkeys identified sets of m6A modification sites consistent with patterns of stabilizing or directional selection, with evolutionary gains of m6A signal correlating with evolution of higher mRNA levels [25].
Writing the m6A modification: the methylase complex
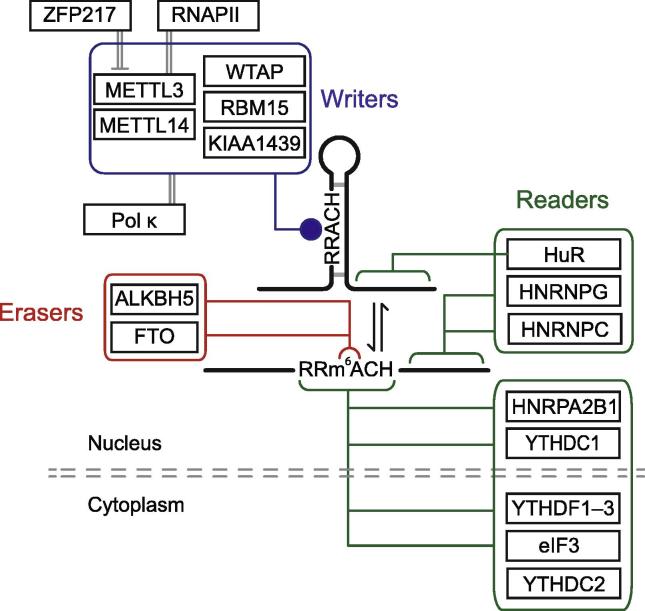
The addition of the methyl group to the position N6 of adenosine is catalyzed by a large protein complex [26] (Figure 1). The core of this complex is a stable heterodimer of METTL3 [27] and METTL14 [28]. Although both proteins have methyltransferase domains, crystal structure studies of the heterodimer demonstrate that only METTL3 has catalytic activity, while METTL14 stabilizes METTL3 and its interaction with the RNA molecule [29], [30], [31]. A physical interaction between METTL3 and RNA polymerase II (RNAPII) has been observed under conditions that induce slow progression or frequent pausing of RNAPII, suggesting that m6A deposition occurs co-transcriptionally. Attenuated transcription leads to increased levels of m6A, which is detrimental to translation [32]. UV-induced DNA damage leads to increased levels of m6A and localization of METTL3 and METTL14 to sites of UV-induced DNA damage [33]. Accumulation of m6A at sites of UV-induced DNA damage requires ADP-ribose polymerase (PARP) and is required for early recruitment of DNA polymerase kappa (Pol κ), an enzyme implicated in both nucleotide excision repair and trans-lesion synthesis [33]. WT1-associated protein (WTAP) can interact with the METTL3/METTL14 heterodimer and is required for proper localization of the methylase complex to specific loci in the nucleus and efficient m6A modification [23], [28], [34], [35]. Additional proteins identified as interactors of METTL3/METTL14 include KIAA1429 [35] and RNA binding motif protein 15 (RBM15) [36]. While the molecular function of KIAA1429 remains unknown, RBM15, which is required for the function of X inactive specific transcript (Xist), has been shown to target the methylation complex to specific RRACH motifs to promote methylation [36]. The activity of the methylase complex can be inhibited by sequestration of METTL3 by the transcription factor zinc finger protein 217 (ZFP217), which in mouse ESCs (mESCs) is enriched at the promoters of m6A-modified RNAs [37].
Figure 1.
The m6A regulatory pathway
Schematic representation of known components of the m6A regulatory pathways. The methylase complex (contained in the blue box) can modify adenosines in an RRACH context (R = G/A, H = G/A/C). The function of the methylase complex can be inhibited by ZNF217. The methylase complex interacts with RNAPII during transcription and recruits Pol κ to sites of DNA damage. Deposition of m6A can alter the RNA secondary structure. The demethylases (contained in the red box) can remove the methyl group. Two types of reader proteins (included in the green boxes) can interact with modified RNA through direct recognition of the methyl group or interaction with an RNA secondary structure induced by m6A modification. ALKBH5, alkB homolog 5; eIF3, eukaryotic initiation factor 3; FTO, fat mass and obesity-associated protein; HNRNPA2B1, heterogeneous nuclear ribonucleoprotein A2/B1; HuR, human antigen R; METTL3, methyltransferase like 3; Pol κ, DNA polymerase κ; PBM15, RNA binding motif protein 15; RNAPII, RNA polymerase II; WTAP, WT1-associated protein; YTHDC1, YTH domain containing 1; YTHDF, YTH N6-methyladenosine RNA binding protein; ZFP217, zinc finger protein 217.
m6A is a dynamic mRNA modification: the demethylases
Discovery of RNA m6A demethylases raised the possibility that m6A can be dynamically regulated in the cell. Two enzymes capable of removing m6A have been identified to date: fat mass and obesity-associated protein (FTO) [38] and alkB homolog 5 (ALKBH5) [39], enzymes of the non-heme Fe(II)- and α-ketoglutarate-dependent dioxygenase AlkB family of proteins (Figure 1). While m6A methylation is broadly conserved across eukaryotes, orthologues of FTO and ALKBH5 have not been identified in S. cerevisiae, suggesting that in some organisms demethylation is passive [40]. FTO was first identified in genome-wide association studies and was linked to increased body mass index (BMI) [41], [42], [43]. FTO localizes to the nucleus, and in mice, mRNA levels of Fto are higher in the brain, particularly in the hypothalamic nuclei governing energy balance. Furthermore, Fto mRNA levels in the arcuate nucleus are regulated by feeding and fasting [44]. Initially, 3-methylthymine in single-stranded DNA (ssDNA) was proposed as the substrate of FTO [44]. A later study demonstrated that FTO preferred 3-methyluracil in single-stranded RNA (ssRNA) over 3-methylthymine in ssDNA [45]. Preference for a single-stranded substrate was confirmed by protein structure analysis [46]. In 2011, He and colleagues reported that FTO preferred m6A in vitro, and manipulation of FTO in vivo led to changes in cellular m6A levels [38]. Oxidative demethylation of m6A in RNA by FTO leads to the formation of N6-hydroxymethyladenosine and N6-formyladenosine, further expanding the range of chemical modifications present in RNAs [47]. Recently, FTO was described to show higher affinity for the modification N6,2′-O-dimethyladenosine (m6Am) as a substrate. m6Am is found on the first nucleotide adjacent to the 7-methylguanosine cap. Transcripts with a m6Am modification are more stable due to resistance to the mRNA-decapping enzyme, decapping mRNA 2 (DCP2) [48]. Alkbh5 is expressed in most tissues, and is particularly abundant in the testes. While Alkbh5 null mice are viable and appear anatomically normal, they exhibit impaired fertility due to the apoptosis of meiotic metaphase-stage spermatocytes [39]. A study of human asthenozoospermia patients found a correlation between elevated METTL3 and m6A levels, and sperm motility [49]. ALKBH5 localizes to the nucleus, and loss of ALKBH5 impairs mRNA export, RNA metabolism, and assembly of mRNA processing factors in nuclear speckles [39]. Structural studies of ALKBH5 revealed a region that excludes binding of dsDNA [50], [51], [52], [53]. Although the RRACH motif is critical for m6A methylation, it is not required for demethylase activity [54]. Rather, the conformational changes in the RNA structure induced by m6A modification provide a platform for interactions between the demethylases and the target RNA [54].
The effect of m6A on RNA biology: the m6A readers
How the cell ‘perceives’ the presence of m6A on the RNA determines the downstream effect of this modification. The presence of m6A creates a binding site for proteins that preferentially recognize modified bases, and binding of m6A readers to target transcripts can determine multiple aspects of RNA metabolism. A number of proteins have been identified as direct m6A readers (Figure 1). Several proteins with a YTH domain were shown to bind m6A-modified RNAs. For instance, YTHDF2 selectively binds m6A-modified mRNAs, and recruits them to mRNA decaying sites, controlling mRNA stability [55]. YTHDF2 promotes RNA degradation through deadenylation, mediated by the CCR4-NOT complex [56]. YTHDF1, another m6A reader, interacts with the translation machinery and actively promotes translation of target mRNAs [57]. YTHDF3 cooperates with YTHDF1 to enhance translation, and impacts YTHDF2-mediated mRNA decay [58], [59]. Therefore, these three proteins, YTHDF1, 2, and 3, coordinate to provide spatiotemporal control over RNA metabolism. The protein YTH domain containing 1 (YTHDC1) is a nuclear m6A reader that regulates splicing and is required for the function of Xist, a long non-coding RNA (lncRNA) involved in X chromosome inactivation [36], [60]. The eukaryotic initiation factor 3 (eIF3) complex, through a multisubunit interface, interacts with m6A-modified 5′UTR to directly recruit the 40S preinitiation complex to the 5′UTR of mRNAs to stimulate translation initiation. This mechanism allows mRNA with m6A-modified 5′UTRs to be translated in a cap-independent way [61]. In the nucleus, heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1) binds m6A-modified transcripts and regulates splicing and microRNA (miRNA) maturation [62]. In addition, m6A has been shown to affect RNA secondary structure, modulating the ability of RNA binding proteins (RBPs) to bind to RNA in the neighborhood of m6A modification sites. This mechanism, termed “m6A-switch”, facilitates the binding of HNRNPC and HNRNPG to targets, to regulate mRNA abundance and splicing [63], [64], [65], [66], [67] (Figure 1). Binding of human antigen R (HuR) to RNA is constrained by distance to m6A modification sites, and is blocked by m6A on some transcripts [68].
m6A and cancer
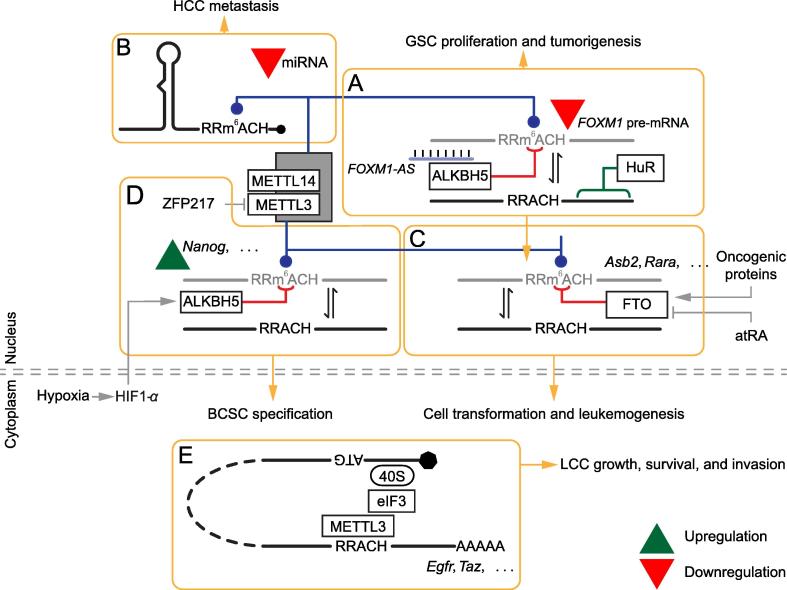
Studies on the role of METTL3 in ESCs uncovered several pluripotency transcription factors as targets of the m6A methylase complex, raising the possibility that pluripotency is regulated through m6A-dependent pathways. These studies demonstrated that loss of m6A leads to increased mRNA half-life of transcription factors such as NANOG, blocking the capacity of pluripotent cells to progress through differentiation [11], [24]. The phenotype observed on teratoma assays suggested that loss of m6A could also play a role in cancer progression [11], [24]. Indeed, multiple studies have implicated both m6A writers and erasers in cancer. Manipulation of m6A levels in glioblastoma stem-like cells (GSCs) severely impacted growth, self-renewal, and tumor development [69], [70]. Reduction of m6A levels, through knockdown of Mettl3 and/or Mettl14, resulted in enhanced in vitro growth and self-renewal of GSCs and promoted the ability of GSCs to form brain tumors in vivo. At the molecular level, knockdown of Mettl3 and/or Mettl14 resulted in the upregulation of several oncogenes, including the genes encoding ADAM metallopeptidase domain 19 (ADAM19), EPH receptor A3 (EPHA3), and Kruppel-like factor 4 (KLF4), and downregulation of tumor suppressors such as genes encoding cyclin dependent kinase inhibitor 2A (CDKN2A), breast cancer 2, early onset (BRCA2), and tumor protein (TP53l11). Knockdown of Adam19, an m6A modified transcript, dramatically reduced growth and self-renewal of GSCs [69]. Increased levels of m6A in glioblastoma cells, achieved through overexpression of Mettl3, or treatment of cells with the FTO inhibitor ethyl ester form of meclofenamic acid (MA2), inhibited tumor progression [69]. High expression of ALKBH5 in glioblastoma patients predicts poor prognosis [70]. ALKBH5 is highly expressed in cell lines or patient-derived primary glioblastoma cultures enriched for GSCs, and knockdown of ALKBH5 impairs GSC self-renewal in vitro and decreases proliferation and tumorigenesis of GSCs in vivo. Analysis of gene expression and m6A landscape of ALKBH5 knockdown cells uncovered forkhead box M1 (FOXM1) as both a target of ALKBH5 and potential regulator of genes dysregulated upon ALKBH5 knockdown. FOXM1 is a transcription factor with a critical role in the self-renewal and tumorigenesis of GSCs. Knockdown of ALKBH5 leads to a gain of m6A on FOXM1 nascent transcript and a decrease in association with HuR, resulting in a decrease of FOXM1 expression. Interestingly, ALKBH5 interaction with FOXM1 is promoted by FOXM1-AS, a lncRNA transcribed in the opposite orientation of FOXM1, with a 457-nt overlap at the 3′UTR (Figure 2A) [70]. These studies suggest that RNA demethylases are a potential target for anti-glioblastoma therapy [69], [70]. In hepatocellular carcinoma (HCC), downregulation of METTL14 results in lower m6A levels, and enhances the metastatic capacity of HCC cells [71]. The processing of the metastasis-associated miRNA miR126 requires METTL14 activity and is involved in HCC metastasis [71] (Figure 2B).
Figure 2.
Links between m6A regulation and cancer
Schematic representation of m6A pathway components and targets involved in cancer progression. Blue lines represent methylation, red lines represent demethylation and green lines indicate m6A reader proteins. A.FOXM1-AS promotes interaction of ALKBH5 with FOXM1. HuR interacts with demethylated FOXM1 pre-mRNA and enhances expression of FOXM1. B. Downregulation of METTL14 disrupts miRNA processing. C. Over-expression of FTO driven by leukemic oncogenic proteins leads to downregulation of target genes. D. Hypoxia leads to upregulation of ALKBH5 and ZFP217, resulting in loss of m6A levels in NANOG mRNA. E. METTL3 promotes translation of mRNA targets in the cytoplasm. ALKBH5, alkB homolog 5; ASB2, ankyrin repeat and SOCS box containing 2; atRA, all-trans-retinoic acid; BCSC, breast cancer stem cell; EGFR, epidermal growth factor receptor; eIF3, eukaryotic initiation factor 3; FOXM1, forkhead box M1; FOXM1-AS, FOXM1 antisense transcript; GSC, glioblastoma stem-like cell; HCC, hepatocellular carcinoma; HIF1α, hypoxia-inducible factor 1α; HuR, human antigen R; LCC, lung cancer cell; METTL3, methyltransferase like 3; RARA, retinoic acid receptor alpha; TAZ, tafazzin; ZFP217, zinc finger protein 217.
FTO was found to play an oncogenic role in acute myeloid leukemia (AML). FTO is expressed at higher levels in certain subtypes of AML, as it can be upregulated by leukemic oncogenes. Over-expression of FTO in two mixed-lineage leukemia (MLL)-rearranged AML cell lines promoted cell growth/proliferation and viability, while decreasing apoptosis and the global mRNA m6A level [72]. Notably, genes with m6A sites that are hypo-methylated upon overexpression of FTO tend to be downregulated in FTO-overexpressing AML cells. Overexpression of FTO leads to a reduction of m6A levels, downregulation of mRNA and protein levels in critical genes, such as those encoding ankyrin repeat and SOCS box containing 2 (ASB2) and retinoic acid receptor alpha (RARA). ASB2 and RARA are upregulated during normal hematopoiesis and are important regulators of all-trans-retinoic acid (atRA)-induced differentiation of leukemia cells. Demonstrating the importance of ASB2 and RARA as FTO targets, the over-expression of these genes can rescue the effects of FTO over-expression, while knockdown was sufficient to rescue the inhibitory effect of FTO knockdown on AML cell growth and viability. Importantly, this study demonstrates that the inhibition of ASB2 and RARA expression by FTO contributes to the response of AML cells to atRA treatment [72] (Figure 2C). Analysis of datasets from the Cancer Genome Atlas (TCGA) Research Network AML study revealed a strong association between mutations and/or copy number variation of m6A regulatory genes and TP53 in AML patients. Furthermore, alterations in m6A regulatory genes confer a worse survival in AML patients [73]. In a meta-analysis study, the FTO polymorphism rs9939609 was found to be significantly associated with pancreatic cancer, although no other significant association was found [74].
Hypoxia is an important feature of the tumor microenvironment, and multiple studies have connected hypoxia to m6A regulatory pathways. The m6A demethylase ALKBH5 is a direct target of hypoxia-inducible factor 1α (HIF-1 α) [75]. In breast cancer cells, hypoxia leads to upregulation of ALKBH5, which then results in decreased m6A levels [76]. Upregulation of ALKBH5 also induced the loss of m6A in NANOG mRNA, which in turn increased its stability and NANOG protein levels in breast cancer stem cells (BCSCs). Therefore, hypoxia-dependent ALKBH5 upregulation mediates NANOG expression and BCSC specification and/or maintenance [76]. Exposure of breast cancer cells to hypoxia also leads to the upregulation of ZNF217, a transcription factor known to interact with METTL3 and inhibit its function [77] (Figure 2D). In gynecological tumor cell lines, hypoxia leads to a reduction of YTHDC1 protein levels, through changes in splicing that generate mRNA isoforms targeted by nonsense-mediated decay [78]. The m6A reader YTHDC2 [79] has been shown to promote translation of HIF-1 α and contributes to colon tumor metastasis [80]. These studies reveal how the tumor microenvironment can impact gene expression through the epitranscriptome, with devastating outcomes.
METTL3 has also been proposed to promote growth, survival, and invasive capacity of lung and cervical cancer cells in a methylation-independent manner [81]. Lin and colleagues described how METTL3 promotes the translation of specific mRNAs, including those encoding epidermal growth factor receptor (EGFR) and tafazzin (TAZ), through interaction with RNA, the translation initiation machinery, and the ribosome [81]. In this study the authors show that although METTL3 knockdown has little effect on the steady state level of methylated mRNAs, the protein expression level of a specific group of genes was reduced. While knockdown of METTL3 resulted in the re-localization of the EGFR and TAZ mRNAs to sub-polysome fractions, tethering of METTL3 to target mRNAs robustly enhanced translation. The catalytic activity of METTL3 is dispensable, as tethering a catalytically-dead METTL3 also enhances translation of target mRNA (Figure 2E). This study, with results in line with those of other studies concerning viruses that replicate in the cytoplasm (see below), uncovers a function for METTL3 in the cytoplasm.
m6A and viral replication
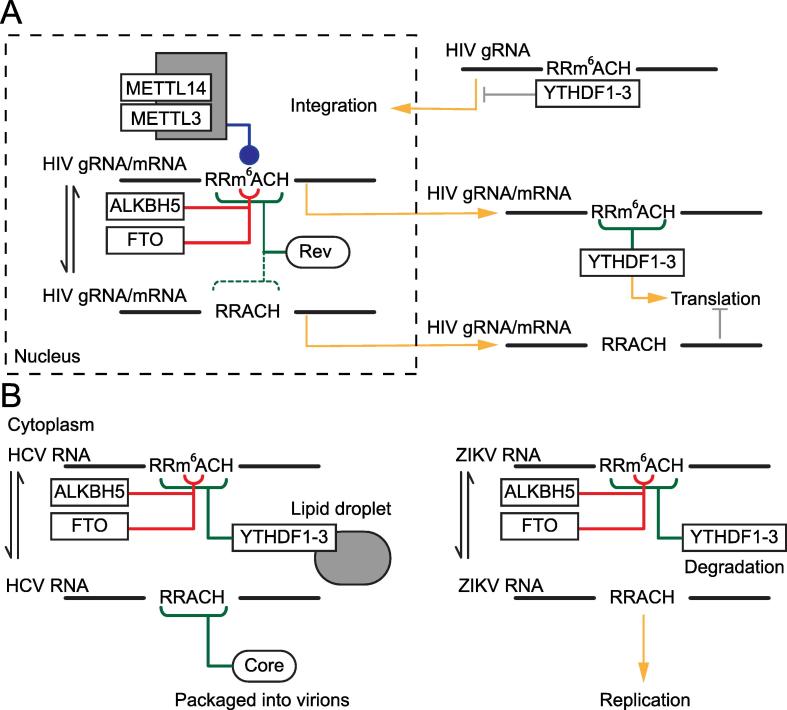
The presence of m6A, and in some cases the exact location of the m6A modification, was observed in viral transcripts as early as the 1970’s. Pioneering work revealed the presence of m6A in Rous sarcoma virus (RSV), adenovirus type 2, Simian virus 40 (SV40), and influenza RNAs [82], [83], [84], [85], [86], [87], [88]. While the presence of m6A in viral RNAs was well established, the role of the modification in viral biology remained unknown. In an early attempt to understand how m6A influences RSV biology, the Beemon Lab mutated the consensus motif around methylated adenosines. Although these studies demonstrated that mutations of the RRACH motif resulted in loss of methylation, no effect on viral RNA processing or viral life cycle was found when the virus was modified to prevent m6A methylation [89], [90]. A different study, in which the researchers used a competitive inhibitor of methionine transferase, cycloleucine, suggested that m6A is required for the formation of late SV40 RNAs in untransformed cells [91], however it is important to note that cycloleucine is not a m6A-specific inhibitor, and the results should be interpreted with caution. Recently, work from three separate groups demonstrated that m6A plays an important role in HIV-1 biology. Lichinchi and colleagues [92] showed that acute viral infection triggers a massive increase in host and viral levels of m6A, and identified 56 host genes with a role in viral infection that are specifically m6A modified upon infection. The authors also demonstrate that loss of m6A decreased viral replication, while elevation of m6A levels through knockdown of ALKBH5 resulted in increased replication of HIV. The authors focused on two m6A sites at the stem loop II (important structural motif) region of HIV-1 Rev response element (RRE), and demonstrated that one of the m6A sites, A7883 (in the loop region), is required to enhance binding of HIV-1 Rev protein to this structural RNA element, an interaction required for viral RNA to exit from the nucleus. Interestingly, A7883 is well conserved across isolates, suggesting the importance of this site. Further studies will be required to understand if Rev is a direct or indirect reader of m6A. Kennedy and colleagues also detected m6A, and showed that it is required for HIV-1 replication. The authors showed that the YTHDF1−3 proteins bind to and enhance expression of viral RNAs. Cullen and colleagues also demonstrated that four m6A-modified sites are conserved across isolates, again suggesting that maintaining m6A sites confers survival benefits [93]. Tirumuru and colleagues showed that the m6A reader proteins YTHDF1−3 bind HIV-1 RNA and inhibit HIV-1 post-entry infection by blocking viral reverse transcription and promoting degradation of the gag mRNA. At a later stage, m6A is required for production of Gag protein in virus producing cells, as m6A might promote translation of viral proteins in infected cells [94]. While there are some conflicting results between these studies, these papers demonstrate the importance of m6A on HIV-1 biology, and suggest that targeting this pathway might offer new tools to control viral infection (Figure 3A).
Figure 3.
m6A is involved in viral replication
Schematic representation of the intersection between m6A pathway and viral replication. Blue lines represent methylation, red lines represent demethylation and green lines indicate m6A reader proteins. A. Writers, erasers, and readers in the m6A pathway influence HIV RNA transport, stability, and translation, in both nucleus and cytoplasm. B. m6A pathway regulates viruses that replicate in the cytoplasm. ALKBH5, alkB homolog 5; FTO, fat mass and obesity-associated protein; HCV, hepatitis C virus; HIV, human immunodeficiency virus; METTL3, methyltransferase like 3; YTHDF, YTH N6-methyladenosine RNA binding protein; ZIKV, Zika virus.
Viruses of the Flaviviridae family, which replicate exclusively in the cytoplasm, have been shown to be m6A modified [95], [96]. Comparison of m6A profiles across multiple Flaviviridae viruses suggests that the m6A modification is potentially conserved in this family [96]. Furthermore, m6A is conserved across multiple isolates of Zika virus (ZIKV) [95]. Loss of m6A or impairment of m6A reader proteins of the YTH family result in higher viral particle production of both ZIKV and hepatitis C virus (HCV), while loss of demethylase activity negatively impacts viral particle production. As observed with HIV infection, ZIKV infection leads to changes in the m6A profile of the host cell. In the case of ZIKV infection, new m6A peaks in host transcripts are enriched at the 5′UTR and the CDS, while the lost m6A peaks tend to localize at the exon junction and at the 3′UTR [95]. HCV infection leads to a re-localization of YTH proteins to loci of HCV particle production. YTH proteins interact with the HCV RNA and suppress viral production [96]. Mutagenesis studies on m6A sites in the viral E1 gene of HCV suggest that the interaction of the viral RNA with host YTHDF proteins or viral core proteins is regulated by the presence of m6A on the viral gene [96] (Figure 3B).
m6A and metabolic diseases
In mammals, metabolism, as well as other features of physiology, is regulated in a temporal way by the circadian system. At the cellular level, the circadian clock is ‘paced’ by feedback loops at the transcriptional and translational levels that result in periodic gene expression. Modification of RNA with m6A, through its effects on RNA metabolism, directly impacts the length of the circadian period, with loss of m6A resulting in circadian period elongation [97]. Metabolism, at the organism level, has also been linked to RNA post-transcriptional modifications through FTO. FTO was first associated with obesity in a series of population studies that linked variations in the first intron of FTO to elevated BMI [41], [42], [43]. Although the link between the FTO polymorphism and BMI has been confirmed in multiple studies, how expression of FTO leads to obesity is not well understood. Several studies propose that the polymorphisms in the first intron of FTO control expression of other genes, such as those encoding RPGRIP1 like (RPGRIP1L), iroquois-related homeobox 3 (IRX3), and IRX5 [98], [99], [100], [101]. Studies in mouse models, on both loss of function and overexpression of Fto, revealed a role for FTO in energy homeostasis and body composition [102], [103], [104]. Global loss of Fto resulted in postnatal growth retardation and significant reduction in adipose tissue and lean body mass [102]. A dominant missense point mutation (I367F) in the Fto gene resulted in reduced fat mass, increased energy expenditure, and unchanged physical activity without postnatal growth retardation or perinatal lethality [103]. Ubiquitous overexpression of FTO results in a dose-dependent increase in body and fat mass of mice [104]. Furthermore, FTO plays a critical role in the regulation of adipogenesis through its effect on RNA splicing, particularly for the gene encoding the adipogenic regulatory factor RUNX1 translocation partner 1 (RUNX1T1) [105]. In skeletal muscle cells, FTO-dependent demethylation of mRNA m6A methylation is involved in the regulation of skeletal muscle lipid accumulation [106]. In humans, a missense mutation in the coding region of FTO (R316Q), inactivates enzymatic function and results in a rare autosomal-recessive lethal syndrome [107].
Concluding remarks
Organisms face innumerable challenges to their physiology, both during and after embryonic development. At the cellular level, the ability to execute developmental programs and respond to environmental challenges requires precise and coordinated control of gene expression. As a central player in gene expression, the RNA molecule is the target of several of these regulatory pathways, and disruption of any of these mechanisms can result in disease and have catastrophic consequences. This review focused on the link between pathways that rely on m6A to modulate gene expression and its implications in human disease. RNA post-transcriptional modifications, through their impact on transcription, splicing, mRNA stability, and rate of translation, regulate essential features of a cell. It is not surprising that dysregulation of these pathways is implicated in human disease. Although some details are still lacking, it is clear that the balance between methylation and demethylation at specific RNA transcripts plays a critical role in human health and represents an attractive target for therapy. Inhibition of the RNA demethylases ALKBH5 and FTO is a potential strategy to target cancer stem cells [69], [70], [76]. Furthermore, atRA treatment of AML cells demonstrates that m6A-related pathways can also be targeted as a potential strategy to enhance other available therapies [72]. Because m6A plays an important role in the biology of several viruses with considerable impact on human health, manipulating m6A regulation has also been explored as a potential anti-viral therapy. Use of 3-deazaadenosine (DAA), a drug that can block methylation, can inhibit replication of HIV-1 as well as RSV and influenza A virus [93], [108], [109], [110], [111]. Although these experiments do not conclusively show that m6A inhibition can be used as an antiviral therapy, since this drug is not an m6A specific inhibitor, several studies have demonstrated that targeting m6A pathway is a viable strategy. As m6A regulation is involved in multiple pathways, targeting m6A-dependent pathways can potentially lead to unwanted side effects. Therefore, development of drugs that inhibit these enzymes in a context-specific manner, or target the interaction between specific m6A targets and the interacting proteins will be critical for the success of m6A-targeted therapies.
Competing interests
The author declares no competing interests.
Acknowledgments
I thank Ryan A. Flynn, George Leiman, H. Efsun Arda, and John Karijolich for discussion and critical reading of the manuscript. I apologize to colleagues whose works are not discussed due to space limitation. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, United States of America.
Handled by Yun-Gui Yang
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
References
- 1.Bokar JA. The biosynthesis and functional roles of methylated nucleosides in eukaryotic mRNA. Fine-Tuning of RNA Functions by Modification and Editing. Heidelberg: Springer, Berlin Heidelberg; 2005, p. 141–77.
- 2.Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei C.M., Gershowitz A., Moss B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell. 1975;4:379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- 4.Lavi S., Shatkin A.J. Methylated Simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci U S A. 1975;72:2012–2016. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei C.M., Moss B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry (Mosc) 1977;16:1672–1676. doi: 10.1021/bi00627a023. [DOI] [PubMed] [Google Scholar]
- 6.Schibler U., Kelley D.E., Perry R.P. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J Mol Biol. 1977;115:695–714. doi: 10.1016/0022-2836(77)90110-3. [DOI] [PubMed] [Google Scholar]
- 7.Adams J.M., Cory S. Modified nucleosides and bizarre 5′-termini in mouse myeloma mRNA. Nature. 1975;255:28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- 8.Furuichi Y., Shatkin A.J., Stavnezer E., Bishop J.M. Blocked, methylated 5′-terminal sequence in avian sarcoma virus RNA. Nature. 1975;257:618–620. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- 9.Furuichi Y., Morgan M., Shatkin A.J., Jelinek W., Salditt-Georgieff M., Darnell J.E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975;72:1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke S., Alemu E.A., Mertens C., Gantman E.C., Fak J.J., Mele A. A majority of m6A residues are in the last exons, allowing the potential for 3′UTR regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batista P.J., Molinie B., Wang J., Qu K., Zhang J., Li L. M6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 13.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molinie B., Wang J., Lim K.S., Hillebrand R., Lu Z.X., Van Wittenberghe N. M6A -LAIC-seq reveals the census and complexity of the m6A epitranscriptome. Nat Methods. 2016;13:692–698. doi: 10.1038/nmeth.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwala S.D., Blitzblau H.G., Hochwagen A., Fink G.R. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 2012;8:e1002732. doi: 10.1371/journal.pgen.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clancy M.J., Shambaugh M.E., Timpte C.S., Bokar J.A. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 2002;30:4509–4518. doi: 10.1093/nar/gkf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong S., Li H., Bodi Z., Button J., Vespa L., Herzog M. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vespa L., Vachon G., Berger F., Perazza D., Faure J.D., Herzog M. The immunophilin-interacting protein AtFIP37 from Arabidopsis is essential for plant development and is involved in trichome endoreduplication. Plant Physiol. 2004;134:1283–1292. doi: 10.1104/pp.103.028050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodi Z., Zhong S., Mehra S., Song J., Graham N., Li H. Adenosine Methylation in Arabidopsis mRNA is associated with the 3′ end and reduced levels cause developmental defects. Front Plant Sci. 2012;3:48. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lence T., Akhtar J., Bayer M., Schmid K., Spindler L., Ho C.H. M6A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–247. doi: 10.1038/nature20568. [DOI] [PubMed] [Google Scholar]
- 21.Haussmann I.U., Bodi Z., Sanchez-Moran E., Mongan N.P., Archer N., Fray R.G. M6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 22.Zhao B.S., Wang X., Beadell A.V., Lu Z., Shi H., Kuuspalu A. M6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature. 2017;542:475–478. doi: 10.1038/nature21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ping X.L., Sun B.F., Wang L., Xiao W., Yang X., Wang W.J. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geula S., Moshitch-Moshkovitz S., Dominissini D., Mansour A.A., Kol N., Salmon-Divon M. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 25.Ma L., Zhao B., Chen K., Thomas A., Tuteja J.H., He X. Evolution of transcript modification by N6-methyladenosine in primates. Genome Res. 2017;27:385–392. doi: 10.1101/gr.212563.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bokar J.A., Rath-Shambaugh M.E., Ludwiczak R., Narayan P., Rottman F. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem. 1994;269:17697–17704. [PubMed] [Google Scholar]
- 27.Bokar J.A., Shambaugh M.E., Polayes D., Matera A.G., Rottman F.M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J., Yue Y., Han D., Wang X., Fu Y., Zhang L. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Feng J., Xue Y., Guan Z., Zhang D., Liu Z. Structural basis of N6-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 30.Wang P., Doxtader K.A., Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Śledź P., Jinek M. Structural insights into the molecular mechanism of the m6A writer complex. eLife. 2016;5:e18434. doi: 10.7554/eLife.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slobodin B., Han R., Calderone V., Vrielink J.A.F.O., Loayza-Puch F., Elkon R. Transcription Impacts the Efficiency of mRNA Translation via Co-transcriptional N6-adenosine Methylation. Cell. 2017;169:e12. doi: 10.1016/j.cell.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang Y., Laurent B., Hsu C.H., Nachtergaele S., Lu Z., Sheng W. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature. 2017;543:573–576. doi: 10.1038/nature21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horiuchi K., Kawamura T., Iwanari H., Ohashi R., Naito M., Kodama T. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288:33292–33302. doi: 10.1074/jbc.M113.500397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz S., Mumbach M.R., Jovanovic M., Wang T., Maciag K., Bushkin G.G. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patil D.P., Chen C.K., Pickering B.F., Chow A., Jackson C., Guttman M. M6A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguilo F., Zhang F., Sancho A., Fidalgo M., Di Cecilia S., Vashisht A. Coordination of m6A mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell. 2015;17:689–704. doi: 10.1016/j.stem.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.M., Li C.J. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz S., Agarwala S.D., Mumbach M.R., Jovanovic M., Mertins P., Shishkin A. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421. doi: 10.1016/j.cell.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scuteri A., Sanna S., Chen W.M., Uda M., Albai G., Strait J. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dina C., Meyre D., Gallina S., Durand E., Körner A., Jacobson P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 44.Gerken T., Girard C.A., Tung Y.C., Webby C.J., Saudek V., Hewitson K.S. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia G., Yang C.G., Yang S., Jian X., Yi C., Zhou Z. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 2008;582:3313–3319. doi: 10.1016/j.febslet.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han Z., Niu T., Chang J., Lei X., Zhao M., Wang Q. Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature. 2010;464:1205–1209. doi: 10.1038/nature08921. [DOI] [PubMed] [Google Scholar]
- 47.Fu Y., Jia G., Pang X., Wang R.N., Wang X., Li C.J. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mauer J., Luo X., Blanjoie A., Jiao X., Grozhik A.V., Patil D.P. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature. 2017;541:371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y., Huang W., Huang J.T., Shen F., Xiong J., Yuan E.F. Increased N6-methyladenosine in human sperm RNA as a risk factor for asthenozoospermia. Sci Rep. 2016;6:24345. doi: 10.1038/srep24345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu C., Liu K., Tempel W., Demetriades M., Aik W., Schofield C.J. Structures of human ALKBH5 demethylase reveal a unique binding mode for specific single-stranded N6-methyladenosine RNA demethylation. J Biol Chem. 2014;289:17299–17311. doi: 10.1074/jbc.M114.550350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aik W., Scotti J.S., Choi H., Gong L., Demetriades M., Schofield C.J. Structure of human RNA N6-methyladenine demethylase ALKBH5 provides insights into its mechanisms of nucleic acid recognition and demethylation. Nucleic Acids Res. 2014;42:4741–4754. doi: 10.1093/nar/gku085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng C., Liu Y., Wang G., Deng Z., Zhang Q., Wu W. Crystal structures of the human RNA demethylase Alkbh5 reveal basis for substrate recognition. J Biol Chem. 2014;289:11571–11583. doi: 10.1074/jbc.M113.546168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen W., Zhang L., Zheng G., Fu Y., Ji Q., Liu F. Crystal structure of the RNA demethylase ALKBH5 from zebrafish. FEBS Lett. 2014;588:892–898. doi: 10.1016/j.febslet.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou S., Toh J.D.W., Wong K.H.Q., Gao Y.G., Hong W., Woon E.C.Y. N6-Methyladenosine: a conformational marker that regulates the substrate specificity of human demethylases FTO and ALKBH5. Sci Rep. 2016;6:25677. doi: 10.1038/srep25677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du H., Zhao Y., He J., Zhang Y., Xi H., Liu M. YTHDF2 destabilizes m6A -containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X., Zhao B.S., Roundtree I.A., Lu Z., Han D., Ma H. N6-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi H., Wang X., Lu Z., Zhao B.S., Ma H., Hsu P.J. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27:315. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li A., Chen Y.S., Ping X.L., Yang X., Xiao W., Yang Y. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao W., Adhikari S., Dahal U., Chen Y.S., Hao Y.J., Sun B.F. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Meyer K.D., Patil D.P., Zhou J., Zinoviev A., Skabkin M.A., Elemento O. 5′UTR m6A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alarcón C.R., Goodarzi H., Lee H., Liu X., Tavazoie S., Tavazoie S.F. HNRNPA2B1 is a mediator of m6A -dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roost C., Lynch S.R., Batista P.J., Qu K., Chang H.Y., Kool E.T. Structure and thermodynamics of N6-methyladenosine in RNA: a spring-loaded base modification. J Am Chem Soc. 2015;137:2107–2115. doi: 10.1021/ja513080v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spitale R.C., Flynn R.A., Zhang Q.C., Crisalli P., Lee B., Jung J.W. Structural imprints in vivo decode RNA regulatory mechanisms. Nature. 2015;519:486–490. doi: 10.1038/nature14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou K.I., Parisien M., Dai Q., Liu N., Diatchenko L., Sachleben J.R. N6-methyladenosine modification in a long noncoding RNA hairpin predisposes its conformation to protein binding. J Mol Biol. 2016;428:822–833. doi: 10.1016/j.jmb.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu N., Dai Q., Zheng G., He C., Parisien M., Pan T. N6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu N., Zhou K.I., Parisien M., Dai Q., Diatchenko L., Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017 doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., Li Y., Toth J.I., Petroski M.D., Zhang Z., Zhao J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui Q., Shi H., Ye P., Li L., Qu Q., Sun G. M6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang S., Zhao B.S., Zhou A., Lin K., Zheng S., Lu Z. M6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma J.Z., Yang F., Zhou C.C., Liu F., Yuan J.H., Wang F. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N6-methyladenosine-dependent primary microRNA processing. Hepatology. 2017;65:529–543. doi: 10.1002/hep.28885. [DOI] [PubMed] [Google Scholar]
- 72.Li Z., Weng H., Su R., Weng X., Zuo Z., Li C. FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine rna demethylase. Cancer Cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwok C.T., Marshall A.D., Rasko J.E.J., Wong J.J.L. Genetic alterations of m6A regulators predict poorer survival in acute myeloid leukemia. J Hematol Oncol. 2017;10:39. doi: 10.1186/s13045-017-0410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li G., Chen Q., Wang L., Ke D., Yuan Z. Association between FTO gene polymorphism and cancer risk: evidence from 16,277 cases and 31,153 controls. Tumour Biol. 2012;33:1237–1243. doi: 10.1007/s13277-012-0372-9. [DOI] [PubMed] [Google Scholar]
- 75.Thalhammer A., Bencokova Z., Poole R., Loenarz C., Adam J., O’Flaherty L. Human alkB homologue 5 is a nuclear 2-oxoglutarate dependent oxygenase and a direct target of hypoxia-inducible factor 1α (HIF-1α) PLoS One. 2011;6:e16210. doi: 10.1371/journal.pone.0016210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C., Samanta D., Lu H., Bullen J.W., Zhang H., Chen I. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang C., Zhi W.I., Lu H., Samanta D., Chen I., Gabrielson E. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7:64527–64542. doi: 10.18632/oncotarget.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirschfeld M., Zhang B., Jaeger M., Stamm S., Erbes T., Mayer S. Hypoxia-dependent mRNA expression pattern of splicing factor YT521 and its impact on oncological important target gene expression. Mol Carcinog. 2014;53:883–892. doi: 10.1002/mc.22045. [DOI] [PubMed] [Google Scholar]
- 79.Xu C., Liu K., Ahmed H., Loppnau P., Schapira M., Min J. Structural basis for the discriminative recognition of N6-methyladenosine RNA by the human YT521-B homology domain family of proteins. J Biol Chem. 2015;290:24902–24913. doi: 10.1074/jbc.M115.680389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanabe A., Tanikawa K., Tsunetomi M., Takai K., Ikeda H., Konno J. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett. 2016;376:34–42. doi: 10.1016/j.canlet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 81.Lin S., Choe J., Du P., Triboulet R., Gregory R.I. The m6A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dimock K., Stoltzfus C.M. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry (Mosc) 1977;16:471–478. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- 83.Beemon K., Keith J. Localization of N6-methyladenosine in the Rous sarcoma virus genome. J Mol Biol. 1977;113:165–179. doi: 10.1016/0022-2836(77)90047-x. [DOI] [PubMed] [Google Scholar]
- 84.Sommer S., Salditt-Georgieff M., Bachenheimer S., Darnell J.E., Furuichi Y., Morgan M. The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res. 1976;3:749–765. doi: 10.1093/nar/3.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Canaani D., Kahana C., Lavi S., Groner Y. Identification and mapping of N6-methyladenosine containing sequences in Simian virus 40 RNA. Nucleic Acids Res. 1979;6:2879–2899. doi: 10.1093/nar/6.8.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Furuichi Y., LaFiandra A., Shatkin A.J. 5′-Terminal structure and mRNA stability. Nature. 1977;266:235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- 87.Krug R.M., Morgan M.A., Shatkin A.J. Influenza viral mRNA contains internal N6-methyladenosine and 5′-terminal 7-methylguanosine in cap structures. J Virol. 1976;20:45–53. doi: 10.1128/jvi.20.1.45-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hashimoto S.I., Green M. Multiple methylated cap sequences in adenovirus type 2 early mRNA. J Virol. 1976;20:425–435. doi: 10.1128/jvi.20.2.425-435.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kane S.E., Beemon K. Inhibition of methylation at two internal N6-methyladenosine sites caused by GAC to GAU mutations. J Biol Chem. 1987;262:3422–3427. [PubMed] [Google Scholar]
- 90.Csepany T., Lin A., Baldick C.J., Beemon K. Sequence specificity of mRNA N6-adenosine methyltransferase. J Biol Chem. 1990;265:20117–20122. [PubMed] [Google Scholar]
- 91.Finkel D., Groner Y. Methylations of adenosine residues (m6A) in pre-mRNA are important for formation of late Simian virus 40 mRNAs. Virology. 1983;131:409–425. doi: 10.1016/0042-6822(83)90508-1. [DOI] [PubMed] [Google Scholar]
- 92.Lichinchi G., Gao S., Saletore Y., Gonzalez G.M., Bansal V., Wang Y. Dynamics of the human and viral m6A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol. 2016;1:16011. doi: 10.1038/nmicrobiol.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kennedy E.M., Bogerd H.P., Kornepati A.V., Kang D., Ghoshal D., Marshall J.B. Posttranscriptional m6A editing of HIV-1 mRNAs enhances viral gene expression. Cell Host Microbe. 2016;19:675–685. doi: 10.1016/j.chom.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tirumuru N., Zhao B.S., Lu W., Lu Z., He C., Wu L. N6-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. eLife. 2016;5:e15528. doi: 10.7554/eLife.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lichinchi G., Zhao B.S., Wu Y., Lu Z., Qin Y., He C. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe. 2016;20:666–673. doi: 10.1016/j.chom.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gokhale N.S., McIntyre A.B., McFadden M.J., Roder A.E., Kennedy E.M., Gandara J.A. N6-methyladenosine in Flaviviridae viral RNA genomes regulates infection. Cell Host Microbe. 2016;20:654–665. doi: 10.1016/j.chom.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fustin J.M., Doi M., Yamaguchi Y., Hida H., Nishimura S., Yoshida M. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 98.Stratigopoulos G., LeDuc C.A., Cremona M.L., Chung W.K., Leibel R.L. Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J Biol Chem. 2011;286:2155–2170. doi: 10.1074/jbc.M110.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stratigopoulos G., Martin Carli J.F., O’Day D.R., Wang L., Leduc C.A., Lanzano P. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 2014;19:767–779. doi: 10.1016/j.cmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yeo G.S. The role of the FTO (Fat Mass and Obesity Related) locus in regulating body size and composition. Mol Cell Endocrinol. 2014;397:34–41. doi: 10.1016/j.mce.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Smemo S., Tena J.J., Kim K.H., Gamazon E.R., Sakabe N.J., Gómez-Marín C. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507:371–375. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fischer J., Koch L., Emmerling C., Vierkotten J., Peters T., Brüning J.C. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 103.Church C., Lee S., Bagg E.A., McTaggart J.S., Deacon R., Gerken T. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet. 2009;5:e1000599. doi: 10.1371/journal.pgen.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Church C., Moir L., McMurray F., Girard C., Banks G.T., Teboul L. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42:1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao X., Yang Y., Sun B.F., Shi Y., Yang X., Xiao W. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu W., Feng J., Jiang D., Zhou X., Jiang Q., Cai M. AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N6-methyladenosine. Sci Rep. 2017;7:41606. doi: 10.1038/srep41606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boissel S., Reish O., Proulx K., Kawagoe-Takaki H., Sedgwick B., Yeo G.S. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet. 2009;85:106–111. doi: 10.1016/j.ajhg.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chiang P.K. Biological effects of inhibitors of S-adenosylhomocysteine hydrolase. Pharmacol Ther. 1998;77:115–134. doi: 10.1016/s0163-7258(97)00089-2. [DOI] [PubMed] [Google Scholar]
- 109.Flexner C.W., Hildreth J.E., Kuncl R.W., Drachman D.B. 3-Deaza-adenosine and inhibition of HIV. Lancet Lond Engl. 1992;339:438. doi: 10.1016/0140-6736(92)90133-n. [DOI] [PubMed] [Google Scholar]
- 110.Fischer A.A., Müller K., Scholtissek C. Specific inhibition of the synthesis of influenza virus late proteins and stimulation of early, M2, and NS2 protein synthesis by 3-deazaadenosine. Virology. 1990;177:523–531. doi: 10.1016/0042-6822(90)90517-u. [DOI] [PubMed] [Google Scholar]
- 111.Bader J.P., Brown N.R., Chiang P.K., Cantoni G.L. 3-Deazaadenosine, an inhibitor of adenosylhomocysteine hydrolase, inhibits reproduction of Rous sarcoma virus and transformation of chick embryo cells. Virology. 1978;89:494–505. doi: 10.1016/0042-6822(78)90191-5. [DOI] [PubMed] [Google Scholar]