ABSTRACT
Venezuelan equine encephalitis virus (VEEV) is a mosquito-borne RNA virus that causes low mortality but high morbidity rates in humans. In addition to natural outbreaks, there is the potential for exposure to VEEV via aerosolized virus particles. There are currently no FDA-licensed vaccines or antiviral therapies for VEEV. Passive immunotherapy is an approved method used to protect individuals against several pathogens and toxins. Human polyclonal antibodies (PAbs) are ideal, but this is dependent upon serum from convalescent human donors, which is in limited supply. Non-human-derived PAbs can have serious immunoreactivity complications, and when “humanized,” these antibodies may exhibit reduced neutralization efficiency. To address these issues, transchromosomic (Tc) bovines have been created, which can produce potent neutralizing human antibodies in response to hyperimmunization. In these studies, we have immunized these bovines with different VEEV immunogens and evaluated the protective efficacy of purified preparations of the resultant human polyclonal antisera against low- and high-dose VEEV challenges. These studies demonstrate that prophylactic or therapeutic administration of the polyclonal antibody preparations (TcPAbs) can protect mice against lethal subcutaneous or aerosol challenge with VEEV. Furthermore, significant protection against unrelated coinfecting viral pathogens can be conferred by combining individual virus-specific TcPAb preparations.
IMPORTANCE With the globalization and spread or potential aerosol release of emerging infectious diseases, it will be critical to develop platforms that are able to produce therapeutics in a short time frame. By using a transchromosomic (Tc) bovine platform, it is theoretically possible to produce antigen-specific highly neutralizing therapeutic polyclonal human antibody (TcPAb) preparations in 6 months or less. In this study, we demonstrate that Tc bovine-derived Venezuelan equine encephalitis virus (VEEV)-specific TcPAbs are highly effective against VEEV infection that mimics not only the natural route of infection but also infection via aerosol exposure. Additionally, we show that combinatorial TcPAb preparations can be used to treat coinfections with divergent pathogens, demonstrating that the Tc bovine platform could be beneficial in areas where multiple infectious diseases occur contemporaneously or in the case of multipathogen release.
KEYWORDS: alphavirus, transchromosomic bovine, Venezuelan equine encephalitis virus, passive immunotherapy
INTRODUCTION
Venezuelan equine encephalitis virus (VEEV) is an enveloped, single-stranded, positive-sense RNA virus that causes epizootic and epidemic outbreaks in equines and humans. In equines, VEEV causes >80% mortality (1); in humans, VEEV is rarely fatal (<1%), causing neurological disease that can lead to permanent neurological sequelae in 4 to 14% of symptomatic individuals (2, 3). VEEV is not only infectious from the natural route of infection but also highly infectious when aerosolized. Currently, there are no FDA-licensed vaccines or antiviral therapies available to combat VEEV infection. Due to the lack of prevention and treatment strategies, the development of new vaccines and antiviral therapies is of high importance.
Passive immunotherapy is a potentially promising approach for the treatment of acute infections such as those cause by VEEV. This approach has been approved for multiple pathogens, including Ebola virus, influenza virus, and anthrax (reviewed in references 4 and 5). Three types of products are currently available for passive immunotherapy: (i) human polyclonal antibodies (PAbs) derived from convalescent-phase serum, (ii) PAbs derived from serum of experimentally immunized animals, and (iii) monoclonal antibodies (MAbs) produced in vitro or via recombinant techniques (reviewed in references 6 and 7). Human PAbs recognize multiple epitopes and are broadly neutralizing, but the requirement for convalescent-phase serum makes acquiring large quantities in a short period of time difficult. Compared to human PAbs, animal PAbs and MAbs can be generated rapidly in large quantities, but the risk of serious adverse events due to anti-animal PAb immune responses is high. Animal PAbs can be processed into Fab fragments to reduce adverse events, while animal MAbs can undergo a “humanization” process in which a variety of methods can be used, including complementarity-determining region (CDR) graphing and variable-domain resurfacing; however, all methods used to reduce adverse events can lead to a reduced efficacy of the antibodies (Abs) (reviewed in references 6 and 8). Finally, unlike PAbs, which recognize multiple epitopes, MAbs recognize only a single epitope, raising the possibility of rapid neutralization escape by the infecting microorganism.
A new approach to overcoming the limitations of these strategies is the transchromosomic (Tc) bovine. Tc bovines have been engineered to possess a human artificial chromosome containing the human antibody heavy chain and kappa chain. In addition, these animals have a triple deletion of bovine heavy chain genes and lambda cluster light chain genes (IGHM−/− IGHML1−/− IGL−/−) (9–11). Tc bovines produce three kinds of IgG antibody: human IgG (hIgG), chimeric IgG (containing human heavy chain and bovine kappa chain), and trans-class-switched bovine IgG. The majority of the antibody produced is fully human IgG (70 to 80%), while a smaller portion is chimeric IgG and bovine IgG. Chimeric IgG is produced because the gene of the bovine Igκ light chain has not been deleted from Tc bovines. During the purification process, chimeric and bovine IgGs can be separated from the fully human IgG, resulting in a genetically human polyclonal antibody preparation. Tc bovines produce as much as 300 g of human IgG/animal/month, allowing the production of highly concentrated antibody preparations in a very short time frame, with no further treatment being required for therapeutic use. The Tc bovine platform has been used to make antigen-specific human antibodies to diverse viral pathogens such as Ebola virus (12, 13), anthrax (10), Middle East respiratory syndrome coronavirus (MERS-CoV) (14), and hantavirus (15).
In these studies, we compared optimized VEEV immunogens (inactivated virus, expression plasmid DNA, and a commercial inactivated alphavirus trivalent veterinary vaccine) for the stimulation of neutralizing and protective PAb responses in Tc bovines. Subcutaneous (s.c.) and aerosol challenge experiments demonstrated that purified TcPAb preparations protected mice from mortality at relatively low doses (5 mg/kg of body weight) when used either prophylactically or therapeutically, and the protective effect was confirmed by in vivo imaging of challenge virus replication. In addition, the inactivated virus preparation derived from wild-type VEEV elicited the greatest neutralizing and most protective TcPAb responses. Finally, we show for the first time that that the Tc bovine platform can provide significant protection against lethal aerosol coinfection with multiple pathogens (VEEV and influenza virus) to mice when TcPAb preparations are combined.
RESULTS
Tc bovines hyperimmunized with VEEV antigens produce VEEV-specific antibodies.
Tc bovines were immunized up to five times with VEEV-specific antigens (Table 1) in a 12-week period, and serum was collected for analysis of VEEV-specific antibodies. In order to determine if the choice of antigen used to generate the VEEV-specific human PAbs resulted in PAbs of various efficacies, Tc bovines were immunized with one of three different VEEV antigens: (i) the commercial livestock vaccine (CLV), a trivalent vaccine containing formalin-inactivated VEEV, eastern equine encephalitis virus (EEEV), and western equine encephalitis virus (WEEV); (ii) plasmid DNA (pDNA694) containing codon-optimized structural genes with a 4-amino-acid (aa) deletion in the capsid; and (iii) the V3000 nt3A virus inactivated by treatment with the psoralen derivative 4′-aminomethyltrioxsalen (AMT) coupled with short-duration UV irradiation.
TABLE 1.
Serum collected from transchromosomic bovines hyperimmunized with VEEV antigen
| Antigen | Bovine | Serum collectiona |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| V1D21 | V2D10 | V2D21 | V3D9/10 | V3D21 | V4D10 | V4D21 | V5D10 | ||
| CLV | 2221 | x | |||||||
| 2180 | x | ||||||||
| pDNA694 | 2184 | x | x | x | x | x | |||
| 2186 | x | x | x | x | x | x | |||
| V3000 nt3A AMT | 2178 | x | x | x | x | x | x | x | x |
| 2183 | x | x | x | x | x | x | x | x | |
V, immunization; D, day; x, day when serum was collected postimmunization.
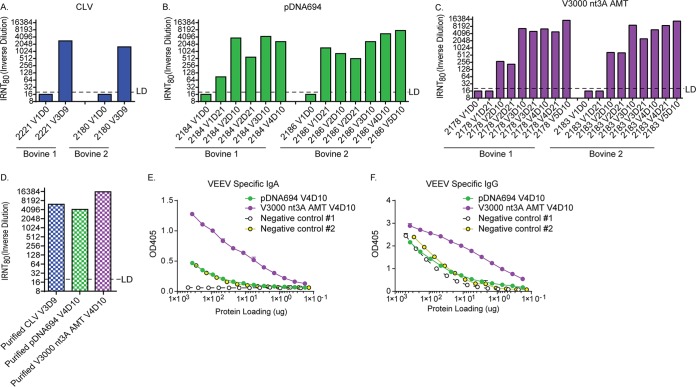
First, serum was tested for VEEV-specific neutralizing antibodies (Fig. 1A to C) by using an infectivity reduction neutralization test (IRNT). VEEV-specific neutralization activity was detected in vaccination 3 day 9 (V3D9) serum of Tc bovines hyperimmunized with CLV antigen (∼1:2,800) (Fig. 1A). A more extensive time course determining when VEEV-specific neutralization could be detected was performed with Tc bovines hyperimmunized with pDNA694 (Fig. 1B) or V3000 nt3A AMT (Fig. 1C). Neutralization activity was present after the first immunization with pDNA694 but not with V3000 nt3A AMT; however, further immunizations with V3000 nt3A AMT resulted in higher levels of VEEV-specific neutralization activity than in serum from Tc bovines hyperimmunized with pDNA694 (∼1:14,000 and ∼1:7,500, respectively). It is worth noting that the CLV immunizations likely contained much more virus protein, even if only one-third was VEEV, than the V3000 nt3A AMT immunizations, which, at ∼1 × 108 PFU, were below the level of detection of a Bradford protein assay (16).
FIG 1.
VEEV-specific antibody responses generated by hyperimmunization of Tc bovines. (A to C) Neutralizing antibody in serum from Tc bovines hyperimmunized with CLV (A), pDNA694 (B), or V3000 nt3A AMT (C) at various times postimmunization. (D to F) Sera from two Tc bovines hyperimmunized with CLV (V3D9), pDNA694 (V4D10), or V3000 nt3A AMT (V4D10) were pooled and purified for human antibodies as described in Materials and Methods. Purified antibodies were then tested for neutralizing antibody (D), VEEV-specific human IgA (E), or VEEV-specific human IgG (F). LD, limit of detection.
After human antibody purification from Tc bovine serum, CLV V3D9, pDNA694 V4D10, and V3000 nt3A AMT V4D10 preparations were tested for VEEV-specific neutralization activity and human IgG and IgA levels (Fig. 1D to F). All three purified TcPAb preparations retained high VEEV-specific neutralization capacities (Fig. 1D). In a VEEV-specific enzyme-linked immunosorbent assay (ELISA), very high background levels were observed in the purified TcPAb preimmunization negative-control preparations, and there were large differences in background levels between the two negative controls. The first negative control consistently exhibited lower background levels of both IgA and IgG, while the second negative control produced ELISA reactivity similar to that of the pDNA694-generated VEEV TcPAb preparation (Fig. 1E and F). It is possible that the Tc bovines were exposed to an ELISA cross-reactive, nonneutralizing immunogen prior to the initial VEEV immunization, resulting in high background levels in the VEEV-specific ELISA. Regardless, the purified TcPAb from V3000 nt3A AMT-hyperimmunized bovines had consistently higher levels of both VEEV-specific IgA (Fig. 1E) and IgG (Fig. 1F) than both negative controls, CLV- and pDNA694-hyperimmunized bovines.
Purified VEEV TcPAbs can protect against subcutaneous challenge.
The ability of purified VEEV TcPAbs to protect against subcutaneous challenge with single-dose intraperitoneal (i.p.) prophylactic or therapeutic treatment was tested initially (Fig. 2). All mice that received the negative-control TcPAbs succumbed to infection by 6 days postinfection (dpi) and had high levels of replication in the head region at 5 dpi, as determined by an in vivo imaging system (IVIS) (Fig. 2A). Virtually all mice treated prophylactically survived challenge (Fig. 2B to D); exhibited no clinical signs of disease, including weight loss; and did not have detectable replication in the head (as determined by IVIS analysis). One mouse treated with the CLV TcPAb (Fig. 2D) had weight loss beginning at 6 dpi but had no detectable signs of replication in the head at 5 dpi, as determined by IVIS analysis (Fig. 2H). All mice treated therapeutically with VEEV TcPAbs survived (Fig. 2E to G) but exhibited clinical signs of disease, including weight loss, and virus was detectable at 5 dpi in the heads of all mice imaged (Fig. 2I). The level of IVIS signals in mice treated with anti-VEEV TcPAbs therapeutically was significantly lower (P < 0.001) than that in the negative-control TcPAb group (Fig. 2I). When the different anti-VEEV TcPAb therapeutic groups were compared, V3000 nt3A-derived TcPAb-treated mice had less IVIS signal in the head than did mice in the pDNA694 TcPAb-treated group (P < 0.05) but not mice treated with CLV-derived TcPAbs (Fig. 2I). These data indicate that all three VEEV TcPAbs could prevent measureable disease in nearly all mice after a lethal s.c. VEEV challenge with prophylactic treatment; however, therapeutic treatment prevented death but could not protect from disease.
FIG 2.
Efficacy of anti-VEEV TcPAb treatment against subcutaneous challenge. Six-week-old BALB/c mice were either untreated or treated once intraperitoneally with 100 μg of anti-VEEV TcPAb prophylactically, 12 h before (B to D), or therapeutically, 12 h after (E to G), subcutaneous challenge in the rear footpad with 1,000 PFU of V3000 nluc TaV. Three mice from the control, prophylactic, or therapeutic groups were imaged by IVIS analysis at 5 days postchallenge, and photon flux was quantitated for the head (H and I, respectively). One mouse representing the fewest clinical infection signs and one mouse representing the greatest number and severity of clinical infection signs are shown in IVIS images from the control (A), prophylactic (B to D), and therapeutic (E to G) groups. All IVIS images are set to the same scale. Daily weights are shown for mice that received the negative-control TcPAb (black lines) (B to G), prophylactic (colored lines) (B to D), or therapeutic (colored lines) (E to G) treatments. For panels H and I, statistical significance for IVIS imaging was determined by Student's t test. *, P < 0.05 for pDNA694 TcPAb versus V3000 nt3A AMT TcPAb; ***, P < 0.001 for the negative control versus VEEV TcPAb; *****, P < 0.0001 for the negative control versus VEEV TcPAb; ns, not significant.
Purified VEEV TcPAbs can protect against aerosol challenge.
Aerosol exposure is more likely to occur in the context of bioweapon use and represents a more stringent route of challenge with VEEV than s.c. infection (17–19). Therefore, this route was used to determine if there was a difference in efficacy between the different anti-VEEV TcPAb preparations. Considering the difficulty in protection from VEEV aerosol exposure (17–19), we decided to treat the mice with TcPAb by two different routes: i.p. or both intraperitoneal and intranasal (i.n.). The additional Ab treatment route has been demonstrated to increase the efficacy of protection against aerosol alphavirus exposure (19).
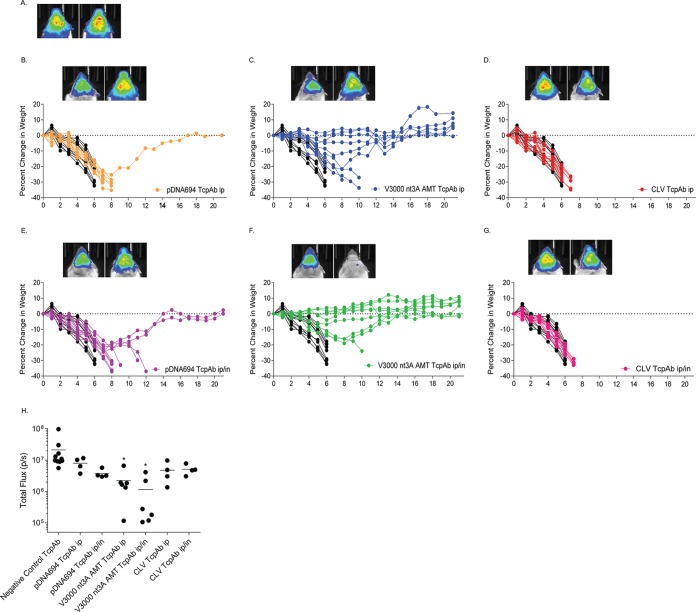
With standard-dose aerosol exposure (50 to 100 50% lethal doses [LD50]), which causes uniform mortality in 6 to 9 days, CLV-derived TcPAbs (Fig. 3D) demonstrated less efficacy, especially in i.p. prophylactic treatment, than did pDNA694 (Fig. 3B)- or V3000 nt3A AMT (Fig. 3C)-derived TcPAbs, both of which produced significantly more survivors and extended average survival times (ASTs) (P < 0.05) (Table 2). Additionally, only mice treated prophylactically i.p./i.n. with V3000 nt3A AMT-derived TcPAb had significantly less IVIS signal in the head than did mice treated with the negative-control TcPAb, while all of the other prophylactically anti-VEEV TcPAb-treated groups had at least one mouse exhibiting levels of IVIS signal in the head similar to those of mice treated with the negative-control TcPAb (Fig. 3H). Interestingly, the CLV-derived TcPAb was more effective when mice were treated therapeutically i.p./i.n. than prophylactically i.p./i.n. (P = 0.01) (Table 2 and Fig. 3G versus 4G). However, there was not a significant difference between mice that received the negative-control TcPAb therapeutically and mice that received the anti-VEEV TcPAbs because at least one mouse had levels of IVIS signal in the head that were similar to those of the control mice (Fig. 4H). There was no significant difference in percent mortality or survival time for the pDNA694- or V3000 nt3A AMT-derived TcPAbs (Table 2) between prophylactic treatment (Fig. 3) and therapeutic treatment (Fig. 4).
FIG 3.
Efficacy of prophylactic anti-VEEV TcPAb treatment against standard-dose aerosol challenge. Six-week-old BALB/c mice were treated once i.p. or i.p./i.n. with 100 μg of either the control TcPAb or the indicated anti-VEEV TcPAb 12 h before aerosol challenge with a standard-dose aerosol (50 to 100 LD50) of V3000 nluc TaV. (H) Four mice from the control or prophylactic groups were imaged by IVIS analysis at 5 days postchallenge, and photon flux was quantitated for the head. (A to G) One mouse representing the fewest clinical infection signs and one mouse representing the greatest number and severity of clinical infection signs are shown in IVIS images from the control (A) or prophylactic (B to G) groups. All IVIS images are set to the same scale. Daily weights are shown for mice that received the negative-control TcPAb (black lines) (B to G) or prophylactic (colored lines) (B to G) treatments. (H) Statistical significance for IVIS imaging was determined by Student's t test. *, P < 0.05 compared to the negative-control TcPAb.
TABLE 2.
Efficacy of human VEEV-specific TcPAb in protection against standard-dose aerosol challengec
| TcPAb | Route | Timinga | % survival | AST (days) ± SDb |
|---|---|---|---|---|
| Negative control | Prophylactic | 0 | 7.6 ± 1.1 | |
| Therapeutic | 0 | 8.6 ± 0.5 | ||
| CLV | i.p. | Prophylactic | 20 | 6.9 ± 0.2 |
| i.p./i.n. | 60 | 7.0 ± 0.0 | ||
| i.p. | Therapeutic | 50 | 7.8 ± 0.4 | |
| i.p./i.n. | 100 | NA | ||
| pDNA694 | i.p. | Prophylactic | 80* | 9.3 ± 1.3# |
| i.p./i.n. | 80 | 8.0 ± 0.0 | ||
| i.p. | Therapeutic | 90 | 8.5 ± 0.0 | |
| i.p./i.n. | 90 | 8.0 ± 0.0 | ||
| V3000 nt3A AMT | i.p. | Prophylactic | 90* | 9.0 ± 0.0# |
| i.p./i.n. | 100 | NA | ||
| i.p. | Therapeutic | 80 | 7.8 ± 0.3 | |
| i.p./i.n. | 70 | 8.3 ± 0.8 |
Prophylactic treatment 12 h before challenge; therapeutic treatment at 12 h postchallenge.
NA, not applicable.
*, P < 0.05 by a chi-square test comparing CLV with pDNA694 and V3000 nt3A AMT; #, P < 0.05 by a Mantel-Cox log rank test comparing CLV with pDNA694 and V3000 nt3A AMT.
FIG 4.
Efficacy of therapeutic anti-VEEV TcPAb treatment against standard-dose aerosol challenge. Six-week-old BALB/c mice were treated once i.p. or i.p./i.n. with 100 μg of either the control TcPAb or the indicated anti-VEEV TcPAb 12 h after aerosol challenge with a standard-dose aerosol (50 to 100 LD50) of V3000 nluc TaV. (H) Four mice from the control or therapeutic groups were imaged by IVIS analysis at 5 days postchallenge, and photon flux was quantitated for the head. (A to G) One mouse representing the fewest clinical infection signs and one mouse representing the greatest number and severity of clinical infection signs are shown in IVIS images from the control (A) or therapeutic (B to G) groups. All IVIS images are set to the same scale. Daily weights are shown for mice that received the negative-control TcPAb (black lines) (B to G) or therapeutic (colored lines) (B to G) treatments. (H) None of the treatments were significantly different from the control by Student's t test (P > 0.05).
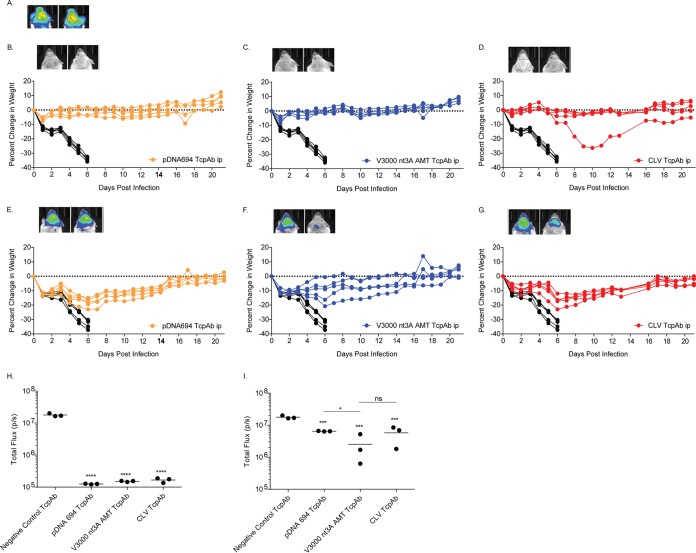
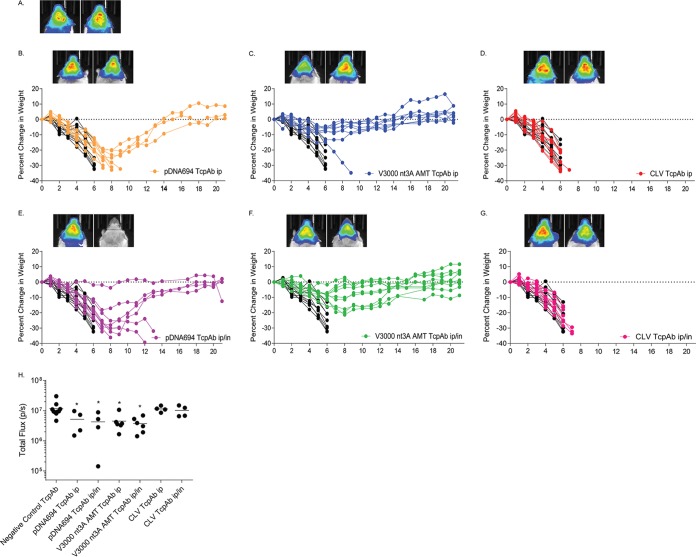
To further explore efficacy differences between the different VEEV TcPAb preparations, mice were exposed to high-dose aerosol challenge (>100 LD50) (Table 3). Increasing the aerosol dose dramatically decreased the efficacies of all three preparations when the mice received only one dose of the TcPAb; however, efficacy differences, as measured by survival, were apparent (Table 3). CLV TcPAb treatment no longer provided protection either prophylactically or therapeutically against VEEV aerosol challenge and yielded ASTs similar to those for mice treated with the negative-control TcPAbs (Table 3). Although pDNA694 TcPAbs offered little protection (10 to 20%), mice had a significant increase in ASTs compared to those for CLV (Table 3) and negative-control (Table 3) TcPAb-treated mice. Interestingly, mice treated with the V3000 nt3A AMT TcPAbs exhibited significantly increased survival (40 to 50%) and an extended AST compared to those for both CLV and pDNA694 TcPAb-treated mice. These data suggest that the repertoire of PAbs generated from Tc bovines hyperimmunized with V3000 nt3A AMT is more effective at protecting mice both prophylactically and therapeutically against a high-dose aerosol challenge.
TABLE 3.
Efficacy of human VEEV-specific TcPAb in protection against high-dose aerosol challengec
| TcPAb | 1 dosea |
2 dosesb |
||||||
|---|---|---|---|---|---|---|---|---|
| Route | Timing | % survival | AST (days) ± SD | Route | Timing | % survival | AST (days) ± SD | |
| Negative control | Prophylactic | 0 | 6.0 ± 0.0 | Prophylactic | 0 | 6.3 ± 0.6 | ||
| Therapeutic | 0 | 6.5 ± 0.5 | Therapeutic | 0 | 6.4 ± 0.8 | |||
| CLV | i.p. | Prophylactic | 0 | 6.5 ± 0.5 | i.p. | Prophylactic | 0 | 6.4 ± 0.5 |
| i.p./i.n. | 0 | 6.5 ± 0.5 | i.p./i.n. | 0 | 6.3 ± 0.5 | |||
| i.p. | Therapeutic | 0 | 6.4 ± 0.5 | i.p. | Therapeutic | 0 | 6.6 ± 0.5 | |
| i.p./i.n. | 0 | 6.5 ± 0.5 | i.p./i.n. | 0 | 6.8 ± 0.4 | |||
| pDNA694 | i.p. | Prophylactic | 0 | 7.7 ± 0.8# | i.p. | Prophylactic | 10 | 7.8 ± 0.5# |
| i.p./i.n. | 20 | 7.8 ± 0.5# | i.p./i.n. | 20 | 9.0 ± 1.6# | |||
| i.p. | Therapeutic | 10 | 7.9 ± 0.9# | i.p. | Therapeutic | 30 | 8.4 ± 1.6# | |
| i.p./i.n. | 0 | 7.5 ± 0.5# | i.p./i.n. | 40*** | 8.7 ± 2.1# | |||
| V3000 nt3A AMT | i.p. | Prophylactic | 40*,** | 8.4 ± 0.8#,## | i.p. | Prophylactic | 66*,** | 9.7 ± 1.2# |
| i.p./i.n. | 40* | 10.0 ± 1.0#,## | i.p./i.n. | 88*,** | 10.5 ± 0.0#,## | |||
| i.p. | Therapeutic | 50*,** | 8.3 ± 0.4#,## | i.p. | Therapeutic | 88*,** | 9.0 ± 0.0# | |
| i.p./i.n. | 50*,** | 9.5 ± 2.3#,## | i.p./i.n. | 100*,** | NA# | |||
Prophylactic treatment 12 h before challenge; therapeutic treatment at 12 h postchallenge.
Prophylactic treatment 12 h before challenge and again at 48 h postchallenge; therapeutic treatment at 12 h postchallenge and again at 48 h postchallenge. NA, not applicable.
*, P < 0.05 by a chi-square test comparing CLV and V3000 nt3A AMT; **, P < 0.05 by a chi-square test comparing pDNA694 and V3000 nt3A AMT; ***, P < 0.05 by a chi-square test comparing CLV and pDNA694; #, P < 0.05 by a Mantel-Cox log rank test comparing CLV, V3000 nt3A AMT, and pDNA694; ##, P < 0.05 by a Mantel-Cox log rank test comparing CLV with V3000 nt3A AMT and pDNA694 with V3000 nt3A AMT.
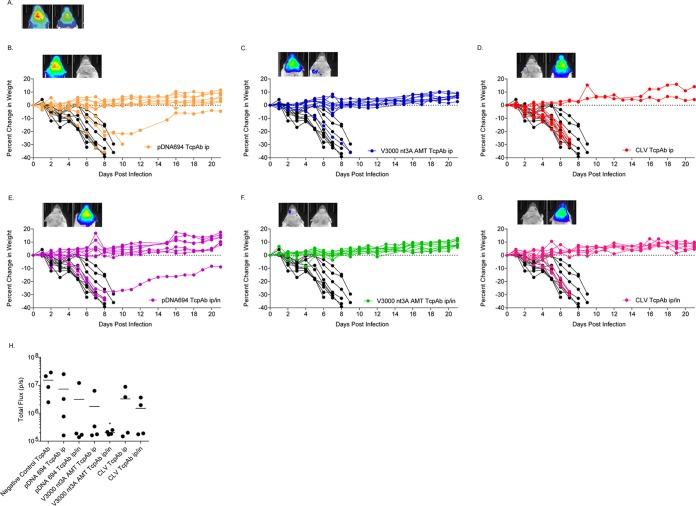
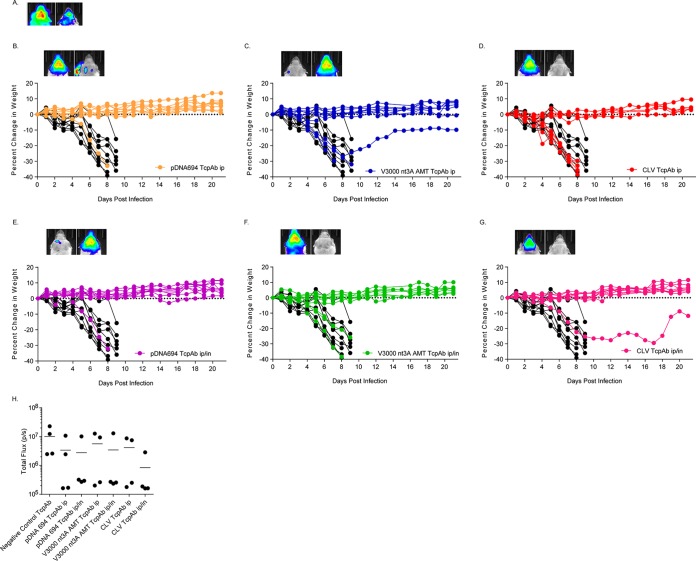
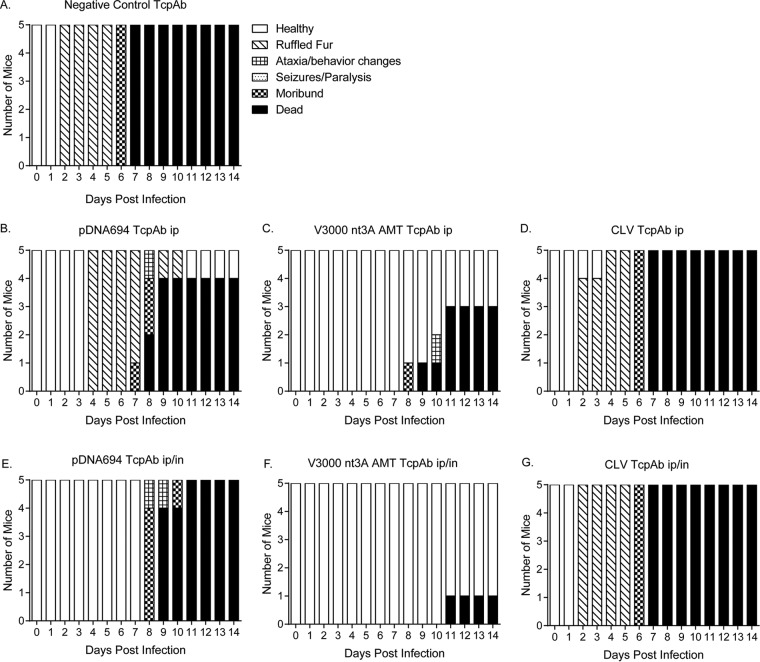
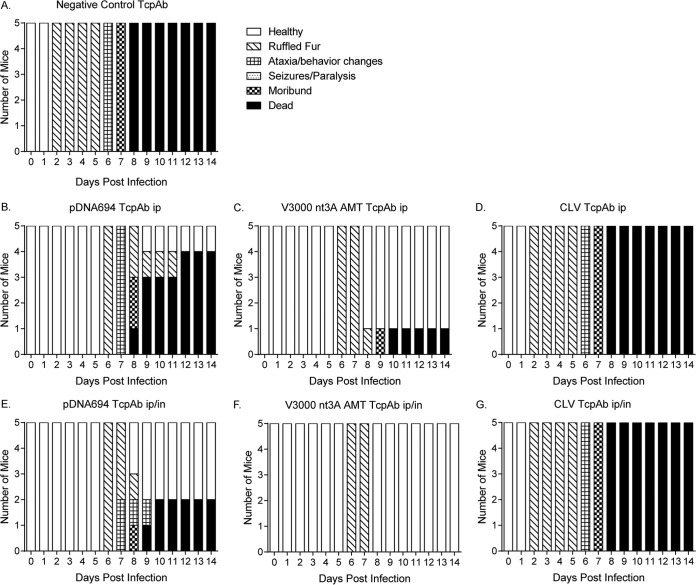
Finally, we determined if the dosing multiplicity affected the efficacy of the anti-VEEV TcPAb preparations against a high-dose aerosol challenge, considering that multiple doses are likely to be given in the context of human therapeutic treatment. Mice were given two doses of TcPAbs (Table 3) using either prophylactic (Fig. 5 and 6) or therapeutic (Fig. 7 and 8) treatment strategies. The administration of two doses of CLV-derived TcPAbs to either the prophylactic group (Fig. 5D and G) or the therapeutic group (Fig. 7D and G) did not increase survival or extend ASTs (Table 3) compared to mice that received the negative-control TcPAbs. Additionally, clinical signs were similar between mice that received the negative-control TcPAb (Fig. 6A and 8A) and mice that received the CLV TcPAb using either strategy (Fig. 6D and G and 8D and G). Similarly, in the prophylactic group, two doses of pDNA694-derived TcPAbs did not alter survival or extend the AST compared to a single dose (Table 1). However, in the therapeutic i.p./i.n. group, two doses of pDNA694-derived TcPAbs significantly increased the number of survivors compared to a single dose (P = 0.03). Furthermore, prophylactic doses of pDNA694-derived TcPAbs given i.p. produced a 2-day delay in the onset of clinical signs of disease compared to mice that received the negative-control TcPAbs (Fig. 6A and B). Mice that received two doses of V3000 nt3A AMT TcPAbs had twice the number of survivors compared to those that received one dose, both prophylactically i.p./i.n. (40% to 88% survival; P = 0.03) and therapeutically i.p./i.n. (50% to 100% survival; P = 0.01). Unlike other groups, not all mice that received the V3000 nt3A AMT TcPAbs prophylactically exhibited clinical signs of disease (Fig. 6F). Additionally, when mice were treated prophylactically, only the V3000 nt3A AMT TcPAb groups exhibited significantly less IVIS signal in the head than did the negative-control TcPAb group (Fig. 5H).
FIG 5.
Efficacy of prophylactic anti-VEEV TcPAb treatment against high-dose aerosol challenge. Six-week-old BALB/c mice were treated with 100 μg of either the control TcPAb or the indicated anti-VEEV TcPAb followed by a high-dose aerosol (>100 LD50) challenge with V3000 nluc TaV. Two doses of TcPAb were administered either i.p. (B to D) or i.p./i.n. (E to G), with the first dose 12 h before challenge and the second dose 48 h after challenge. Four to eight mice from the control or prophylactic groups were imaged by IVIS analysis at 5 days postchallenge, and photon flux was quantitated for the head (H). One mouse exhibiting the fewest clinical infection signs and one mouse exhibiting the greatest number and severity of clinical infection signs are shown in IVIS images from the control (A) or prophylactic (B to G) groups. All IVIS images are set to the same scale. Daily weights are shown for mice that received the negative-control TcPAb (black lines) (B to G) or prophylactic (colored lines) (B to G) treatments. *, P < 0.05 compared to the negative-control TcPAb as determined by Student's t test.
FIG 6.
Efficacy of prophylactic anti-VEEV TcPAb treatment against clinical signs of disease after high-dose aerosol challenge. Six-week-old BALB/c mice were treated with 100 μg of either the control TcPAb or the indicated anti-VEEV TcPAb followed by a high-dose aerosol (>100 LD50) challenge with V3000 nluc TaV. Two doses of TcPAb were administered either i.p. (B to D) or i.p./i.n. (E to G), with the first dose 12 h before challenge and the second dose 48 h after challenge. Mice were monitored daily for clinical signs of disease.
FIG 7.
Efficacy of therapeutic anti-VEEV TcPAb treatment against high-dose aerosol challenge. Six-week-old BALB/c mice were treated with 100 μg of either the control TcPAb or the indicated anti-VEEV TcPAb followed by a high-dose aerosol (>100 LD50) challenge with V3000 nluc TaV. Two doses of TcPAb were administered either i.p. (B to D) or i.p./i.n. (E to G), with the first dose 12 h after challenge and the second dose 48 h after challenge. Four to eight mice from the control or therapeutic groups were imaged by IVIS analysis at 5 days postchallenge, and photon flux was quantitated for the head (H). One mouse representing the fewest clinical infection signs and one mouse representing the greatest number and severity of clinical infection signs are shown in IVIS images from the control (A) or therapeutic (B to G) groups. All IVIS images are set to the same scale. Daily weights are shown for mice that received the negative-control TcPAb (black lines) (B to G) or therapeutic (colored lines) (B to G) treatments. *, P < 0.05 compared to the negative-control TcPAb as determined by Student's t test.
FIG 8.
Efficacy of therapeutic anti-VEEV TcPAb treatment against clinical signs of disease after high-dose aerosol challenge. Six-week-old BALB/c mice were treated with 100 μg of either the control TcPAb or the indicated anti-VEEV TcPAb followed by a high-dose aerosol (>100 LD50) challenge with V3000 nluc TaV. Two doses of TcPAb were administered either i.p. (B to D) or i.p./i.n. (E to G), with the first dose 12 h after challenge and the second dose 48 h after challenge. Mice were monitored daily for clinical signs of disease.
When comparing the pDNA694 TcPAb and V3000 nt3A AMT TcPAb groups only, the V3000 nt3A AMT TcPAb treatments produced significantly higher rates of survival (Fig. 5A, C, D, and F and 7A, C, D, and F). Furthermore, although all mice that received TcPAbs therapeutically exhibited clinical signs of disease (Fig. 8), mice that received V3000 nt3A AMT TcPAbs exhibited clinical signs for fewer days (Fig. 8C and F) than did surviving mice that received pDNA694 TcPAbs (Fig. 8B and E). Overall, the hierarchy of efficacy of the different VEEV TcPAbs remained when mice received two doses of anti-VEEV TcPAbs, with CLV TcPAbs exhibiting the lowest efficacy and V3000 nt3A AMT TcPAbs exhibiting the highest efficacy. The superior protective efficacy of V3000 nt3A AMT-derived TcPAb preparations is consistent with the higher IRNT80 and IgG/IgA levels measured in the in vitro assays.
Virus replication and weight loss 5 days after challenge.
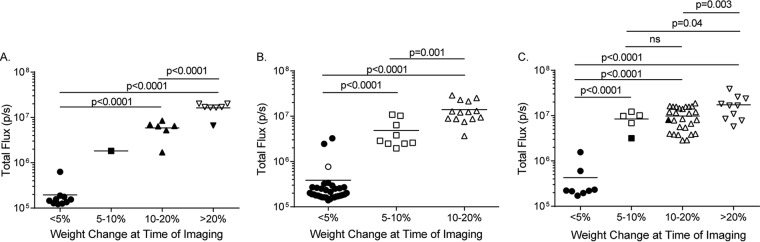
Assessment of the disease and virus replication characteristics of mice challenged by different routes may be informative regarding the establishment of TcPAb treatment regimens. To study this, we analyzed the quantitated IVIS signals in comparison with weight loss and mortality data for all VEEV-infected mice (negative-control TcPAb and anti-VEEV TcPAb treatments). In these studies, all mice given negative-control TcPAb succumbed to infection and exhibited high levels of virus replication in the head, as measured by IVIS signals. IVIS confirmed that for all challenge types, mice exhibiting little/no weight loss also had little/no replication in the head compared to mice with weight loss (Fig. 9). Furthermore, the data suggest that the different challenge routes resulted in different disease profiles for the mice after TcPAb treatment. With s.c. challenge, surviving mice, all of which were treated with anti-VEEV TcPAb, exhibited substantial virus replication in the head and weight loss (Fig. 9A). However, with both aerosol doses, the manifestation of either of these disease characteristics was nearly always associated with mortality (Fig. 9B and C). Furthermore, while significant differences in replication measured by IVIS signals were associated with increased weight loss (e.g., 5 to 10% versus 10 to 20% weight loss with standard-dose aerosol and 5 to 10% versus >20% with high-dose aerosol) (Fig. 9B and C), they were not associated with differences in mortality. This finding suggests that complete protection from disease and replication will be required for the aerosol challenge route. Therefore, additional, higher-concentration doses of TcPAb may be required.
FIG 9.
Relationship of quantitated IVIS signals with weight loss for infected mice. Shown are data plotting the IVIS signal versus weight loss for subcutaneous (A), low-dose aerosol (B), or high-dose aerosol (C) challenge of negative-control TcAb- and anti-VEEV TcAb-treated mice. Closed symbols are mice that survived, and open symbols are mice that succumbed to challenge. All negative-control TcAb-treated mice succumbed to infection.
TcPAbs can protect against simultaneous infection with two pathogens.
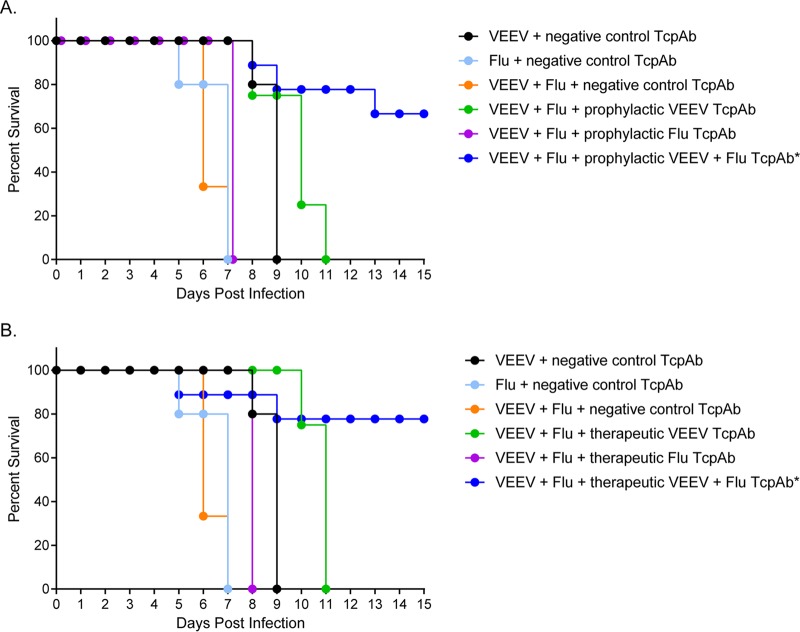
A significant advantage of the TcPAb platform would be the ability to protect against or treat infections with multiple pathogens, either before the time when an infecting organism is identified or in the case of multiorganism release. Therefore, the development of a multiagent-specific antiviral therapeutic is of high priority. To test the potential use of the TcPAb preparations in this context, influenza virus-sensitive DBA2 mice were treated either prophylactically or therapeutically with an equal mixture of VEEV TcPAbs (V3000 nt3A AMT TcPAbs) and influenza virus TcPAbs or given negative-control TcPAbs and then exposed to a lethal aerosol dose of a combination of VEEV and H1N1 influenza virus. As a baseline measure of disease manifestation, negative-control-treated mice were also exposed to each virus individually. In control TcPAb-treated animals, the combination of the two viruses significantly (P < 0.001) decreased the AST compared to those of individual infections (Fig. 10), suggesting an increase in disease severity with coinfection. As an additional control, coinfected mice were treated with the individual TcPAbs (VEEV TcPAb or influenza virus TcPAb), and all mice succumbed to infection (Fig. 10), further demonstrating that the coinfected mice received a lethal dose of both viruses. In contrast, coinfected mice treated either prophylactically (67% survival; P = 0.0013) (Fig. 10A) or therapeutically (78% survival; P = 0.0004) (Fig. 10B) with the two TcPAbs exhibited significantly higher survival rates than did mice that were given the negative-control TcPAbs (0%) (Fig. 10). These data suggest that the TcPAb platform can be useful in the treatment of infections with multiple coinfecting pathogens.
FIG 10.
Efficacy of combined TcPAb treatment against aerosol coinfection with influenza virus and VEEV. Six-week-old DBA2 mice were treated with the negative-control TcPAb or treated either prophylactically or therapeutically with anti-VEEV TcPAb (V3000 nt3A AMT) or anti-influenza virus TcPAb (H1N1-H3N2) before lethal aerosol challenge with VEEV and influenza virus (H1N1). (A) Mice treated prophylactically received two intraperitoneal doses of either 100 μg anti-VEEV, 200 μg anti-influenza virus TcPAb, or a combination of both TcPAbs, with the first dose 12 h before challenge and the second dose at 48 h postchallenge. (B) Mice treated therapeutically received two intraperitoneal doses of 100 μg anti-VEEV, 200 μg anti-influenza virus TcPAb, or a combination of both TcPAbs, with the first dose 12 h after challenge and the second dose 48 h after challenge. Statistical significance was determined by a Mantel-Cox log rank test. *, P < 0.001 versus the negative-control TcPAb. In negative-control groups, the survival time for each virus singly was significantly longer than that for combined infection (P < 0.01) (n = 9 for all groups except for mice that received only the single anti-VEEV or anti-influenza virus TcPAb, where n = 4).
DISCUSSION
Passive immunotherapy is a powerful tool for treating various diseases (reviewed in references4, 5, and 20). Although broadly neutralizing anti-VEEV MAbs from mice have been shown to protect against different clades of VEEV (21) even after having been humanized (22), PAbs are able to recognize multiple epitopes, thus decreasing the risk of escape mutants. Furthermore, PAb preparations can be generated very rapidly after virus emergence or in the context of battlefield use, with little analysis of epitope recognition or virus escape potential being required. In this study, Tc bovines that produce genetically human PAbs were hyperimmunized with either a commercial trivalent alphavirus vaccine, pDNA expressing VEEV TrD wild-type (WT) structural proteins, or inactivated cDNA clone-derived TrD virus. These approaches were used since live-attenuated viruses are not permitted to be used to immunize food livestock. Rigorous safety testing must occur to demonstrate the complete inactivation of biosafety level 3 (BSL-3) pathogens; therefore, we utilized the attenuated nt3A mutant of VEEV for inactivation and stringent interferon receptor-null mouse safety testing for validation of the inactivation of the preparations. We then compared the three immunogens to determine (i) the antibody stimulation provided by each immunogen, (ii) the protective efficacy of TcPAbs against s.c. and aerosol challenges with WT VEEV TrD, and (iii) if the antigen used to hyperimmunize Tc bovines resulted in different efficacies of the resulting TcPAb preparations.
All three antigens used to hyperimmunize the Tc bovines produced VEEV-specific neutralizing Abs that were similarly protective in vivo against subcutaneous challenge. However, differences in efficacy between the TcPAbs were observed during aerosol challenge. Previous work with both mouse PAbs (19) and MAbs (17, 18) demonstrated that protection of mice from aerosol challenge is more difficult than protection against s.c. challenge. Furthermore, clade IA/B viruses like TrD are the most difficult to protect against in an aerosol challenge (21, 22). These studies are the first to demonstrate that antigen-specific TcPAbs can protect against aerosol exposure. Additionally, when comparing the efficacies of the two TcPAbs generated from inactivated virus, it is not unexpected that the VEEV-specific TcPAbs generated from V3000 nt3A AMT inactivation were more effective than were those generated from the CLV antigen for two reasons. First, CLV is created by formalin inactivation of TC83, the unlicensed vaccine strain of VEEV. Formalin has been shown to alter immunogenic epitopes by cross-linking adjacent proteins on the virion (23–25). Second, the majority of the neutralizing antibodies are raised against the E2/E1 glycoprotein. TC83 has mutations in the E2/E1 glycoprotein compared to TrD, potentially altering the efficacy of the antibodies versus the WT. However, it must also be noted that due to timing and TcPAb processing constraints, only three bovine immunizations of the CLV were performed, compared with four for pDNA694 and V3000 nt3A AMT.
Our studies with VEEV-specific TcPAbs demonstrate for the first time that the type of antigen used to hyperimmunize Tc bovines influences the efficacy of treatment in vivo against an aerosol challenge. Differences in efficacy following a high-dose aerosol challenge between the pDNA694-derived TcPAbs and V3000 nt3A AMT-derived TcPAbs mirror the differences observed in vitro with higher neutralization and higher levels of V3000-derived nt3A AMT TcPAbs by the VEEV-specific IgA and IgG ELISAs. Similar results were observed when TcPAbs against MERS-CoV were generated: killed whole virus generated TcPAbs with superior neutralization results in vitro compared with those from recombinant spike protein nanoparticles; however, the two MERS-CoV TcPAbs were not tested for differences in efficacy in vivo (14).
Most importantly, we have demonstrated the efficacy of anti-VEEV TcPAbs in protection of mice from mortality after either s.c. or aerosol challenge with WT TrD. As described above, the V3000 nt3A AMT-derived TcPAbs were most protective in virtually all cases, with a single low dose of Ab (5 mg/kg) delivered i.p. protecting challenged mice against lethality in both prophylactic and therapeutic treatments and completely preventing disease when given prophylactically. Prophylactic treatment with a single low dose given i.p. also protected mice from lethality and disease after a standard aerosol challenge and was partially protective against mortality with therapeutic treatment. In a more realistic human treatment scenario, two doses given i.p. and i.n. significantly reduced disease signs and protected most mice from lethality even after a high-dose aerosol challenge. In other studies, pathogen-specific TcPAbs have required the use of a dose of 25 mg/kg TcPAbs or higher for protection (12, 13). A MERS-CoV TcPAb was demonstrated to reduce virus replication at 5 mg/kg; however, the mouse model used is not a lethal model of infection (26), and protection from lethal infection could not be ascertained. Furthermore, as seen with previous studies using mouse Abs (19), supplementation with intranasal administration of TcPAbs increased the efficacy of VEEV TcPAbs against aerosol challenge. Considering the stringency of the high-dose TrD aerosol challenge model in mice (100% mortality within 6 days), this suggests a significant potential for efficacy in humans, who only rarely succumb to VEEV infection. However, additional studies will be required to determine the full pre- and postchallenge treatment window in which TcAb preparations have disease- or lethality-protective efficacy. Additionally, we are pursuing the efficacy of the TcPAbs in nonhuman primates, in which the disease is similar to that in humans, where they rarely succumb to infection (27, 28).
Coinfections commonly occur both during respiratory infections (reviewed in references 29 and 30) and also in individuals who are immunocompromised (reviewed in reference 31) or as a result of coinfection of arthropod vectors with cocirculating arboviruses, such as the causative agents of the emerging diseases chikungunya and Zika fever (reviewed in reference 32). Additionally, it is conceivable that multiple pathogens could be combined for aerosol release. In our studies, significant protection from mortality and reduced clinical signs were observed after prophylactic or therapeutic treatment combined with a lethal dual-aerosol challenge of VEEV and influenza viruses. This suggests that that the Tc bovine PAb platform is an effective tool for treating coinfections in addition to individual pathogen exposures.
In summary, we have investigated the feasibility of using anti-VEEV TcPAbs to prevent or treat disease. This platform provides the ability to generate pathogen-specific antibodies on a large scale in a short period of time, as would be needed after the release of a bioterrorism agent or during an epidemic/pandemic outbreak. Furthermore, the demonstration that TcPAbs are able to protect mice both prophylactically and therapeutically against coinfection with VEEV and H1N1 influenza virus indicates that the system is highly flexible and adaptable to many possible viral threats. Given that we and others have now extensively tested hyperimmunogen formulations and immunization protocols with Tc bovines, we estimate that it would require only approximately 6 months or less from the first isolation of an emerging virus to the production of commercial-quality human therapeutics with this system. Thus, Tc bovines represent a significant resource for combating natural and aerosol infections with many viral pathogens.
MATERIALS AND METHODS
Cultured cells.
BHK-21 cells (ATCC CCL-10) were maintained in RPMI 1640 medium supplemented with 10% donor bovine serum (DBS) and 10% tryptose phosphate broth (TPB). Vero cells (ATCC CCL-81) and Huh7 cells, generously provided by Charles Rice (Rockefeller University, New York, NY), were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). C7/10 mosquito cells, generously provided by Ilya Frolov (University of Alabama at Birmingham, Birmingham, AL), were maintained in DMEM supplemented with 10% FBS and 10% TPB. All media for cell lines were also supplemented with penicillin (100 U/ml), streptomycin (0.05 mg/ml), and l-glutamine (0.05 mg/ml).
Viruses and replicons.
The construction of the VEEV Trinidad donkey strain (TrD) cDNA clone (V3000) was previously described (33). V3000 nt3A was constructed by creating a G-to-A mutation at nucleotide 3 as previously described (34), using QuikChange XL site-directed mutagenesis. The VEEV nanoluciferase (nluc) reporter virus (V3000 nluc TaV) was constructed (as described previously [35]) by inserting a cleavable in-frame fusion of nluc between the capsid and E3 followed by the Thosea asigna virus (TaV) 2A-like protease. This virus has virulence similar to that of unmodified parental viruses in mice (28) (not shown).
Virus stocks were generated from cDNA clones by in vitro RNA synthesis (IVT) from linearized cDNA plasmid templates as previously described (35). Briefly, IVT (mMessage mMachine; Ambion) was used to generate infectious, capped viral RNA genomes that were electroporated into BHK-21 or C7/10 cells. The supernatant was clarified by centrifugation at 18 to 24 h postelectroporation, and single-use aliquots were stored at −80°C. Virus stock titers were determined by a standard plaque assay on BHK-21 cells.
Influenza virus H1N1 A/California/04/09 was obtained from BEI (catalog number NR-13658) to seed MDCK cells at a multiplicity of infection (MOI) of 1, and cells were harvested at 72 h postinfection. The supernatant was clarified by centrifugation, and single-use aliquots were stored at −80°C. Virus stock titers were determined by a standard plaque assay on MDCK cells. The construction and packaging of VEEV (TrD)-based enhanced green fluorescent protein (eGFP) replicons, generously provided by Robert Johnston (University of North Carolina, Chapel Hill, NC), were previously described (36, 37). Briefly, RNA from the replicon genome along with the capsid and glycoprotein helpers were generated by IVT from linearized cDNA plasmids and coelectroporated into BHK-21 cells. Packaged replicon particles were harvested at 18 to 24 h postelectroporation, clarified by centrifugation followed by concentration over 20% sucrose, and resuspended in Opti-MEM. For each preparation, 10% of the total volume was evaluated by serial passage on BHK-21 cells for the presence of cytopathic effects (CPE) to ensure that no propagation-competent viral recombinants or contaminants were present (38).
Transchromosomic bovines.
Tc bovines were produced as previously described (9–11). Briefly, the Tc bovines used in this study are homozygous for triple knockouts of the endogenous bovine immunoglobulin genes (IGHM−/− IGHML1−/− IGL−/−) and carry a human artificial chromosome (HAC) vector labeled isKcHACD (9–11). This HAC vector consists of two human chromosome fragments: a human chromosome 14 fragment contains the entire human immunoglobulin heavy chain locus, except that the IGHM constant region remains bovine and the key regulatory sequences were bovinized, and a human chromosome 2 fragment contains the entire human immunoglobulin κ light chain locus (9–11).
Antigen preparation. (i) Plasmid DNA.
The DNA plasmid antigen (pDNA694) was created based on previous work by Dupuy et al. (39), and the structural genes of VEEV clone IA/B strain Trinidad donkey (GenBank accession number L01442) were codon optimized by using GeneOptimizer (Life Technologies) and synthesized by DNA2.0, Inc., with EcoRI and NheI restriction sites for placement into the pCAGGS expression vector in either the wild-type sequence or a sequence containing a 4-amino-acid deletion in the capsid (aa 64 to 68) that limits capsid protein-mediated host transcription shutoff (40, 41). Bulk preparation of plasmid DNA for bovine immunization was performed by DNA 2.0, and the expression of the antigen was confirmed by Western blotting (not shown).
(ii) Inactivated virus (V3000 nt3A AMT).
VEEV V3000 nt3A was generated by electroporation of the C7/10 mosquito cell line as described above and then purified by using a discontinuous sucrose gradient (as described previously [42]). Virus was inactivated by using 10 μg/ml of the psoralen derivative AMT (Sigma) with 10 min of UV inactivation (≥13 J/cm2). Virus was considered inactivated if no plaques were detected by a plaque assay, no cytopathic effect was observed after inactivated material was cocultured with BHK cells, and no morbidity or mortality was observed after intracranial injection of undiluted material into interferon alpha/beta/gamma receptor-deficient mice.
Immunization of transchromosomic bovines. (i) Commercial livestock vaccine.
Two Tc bovines (bovines 2180 and 2221) were immunized with a licensed animal vaccine containing inactivated VEEV, EEEV, and WEEV (encephalomyelitis vaccine; Intervet) at two times the recommended equine doses (2 ml) for vaccination 1 (V1) and V2 and at four times the recommended dose (4 ml) for V3. To enhance immune responses, an SAB Biotherapeutics proprietary adjuvant formulation (SAB-adj-2) was multiply injected adjacent to the licensed animal vaccine vaccination sites.
(ii) Plasmid DNA.
Two Tc bovines (bovines 2184 and 2186) were immunized with the VEEV pDNA694 DNA vaccine at 10 mg per animal per vaccination by intramuscular electroporation using the TriGrid delivery system (TDS; Ichor Medical Systems) as previously described (9–11). One milliliter of SAB-adj-1 was multiply injected adjacent to the DNA vaccination sites by using a needle and syringe. The Tc bovines in both groups were vaccinated 5 times (V1 to V5) at 3-week intervals.
(iii) Inactivated VEEV nt3A.
Two Tc bovines (bovines 2178 and 2183) were immunized with inactivated V3000 nt3A AMT at 1 × 108 PFU/dose formulated with SAB-adj-2. The Tc bovines in both groups were vaccinated 5 times (V1 to V5) at 3-week intervals.
(iv) Inactivated influenza virus.
Two Tc bovines (bovines 2186 and 635) were immunized with a licensed 2012-2013 trivalent seasonal influenza vaccine [Fluzone TIV (Sanofi-Pasteur) containing A/California/07/2009 (H1N1), A/Victoria/361/2011 (H3N2), and B/Texas/6/2011 (B/Wisconsin/1/2010-like)] at a total of 1 mg of hemagglutinin (HA)/dose formulated with SAB-adj-2. The Tc bovines were vaccinated 4 times (V1 to V4) at 3-week intervals.
Plasma collection and human immunoglobulin production.
Prior to the first immunization (V1), plasma was collected from each Tc bovine as the negative control. Hyperimmune plasma (up to 2.1% of the body weight of each cow) was collected from immunized Tc bovines 10 days after each vaccination starting from the second immunization (V2) to the fifth immunization (V5). Plasma was collected by using an automated plasmapheresis system (Autopheresis C model 200; Baxter Healthcare). Plasma samples were stored frozen at −80°C until purifications were performed. The frozen Tc plasma bags were thawed at room temperature (RT) overnight, and equal volumes of plasma from each Tc bovine within a group at the selected time point were pooled. The pH of the samples was then adjusted to 4.80 with the dropwise addition of 20% acetic acid, fractionated by caprylic acid at a caprylic acid/total protein ratio of 1.0 for 30 min at RT, and then clarified by centrifugation at 10,000 × g for 20 min at RT. The supernatant containing IgG was then neutralized to pH 7.50, filtered through a 0.22-μm filter, and affinity purified by using a KappaSelect anti-human IgG light chain-specific column (GE Healthcare Life Sciences), which does not exclude other isotypes of human antibody (e.g., IgA). Residual bovine IgG in the KappaSelect-purified IgG sample was then removed by passage through a Capto HC15 anti-bovine IgG heavy chain-specific affinity column (GE Healthcare Life Sciences). The Capto HC15 column flowthrough that contains fully human IgG was then formulated by a Millipore Labscale tangential flow filtration (TFF) system. The final purified fully human IgG was placed in a buffer at pH 5.5 consisting of 10 mM glutamic acid monosodium salt, 262 mM d-sorbitol, and 0.05 mg/ml of Tween 80 and contained 1.5 to 2.8% fully human IgA. Purified hIgG was sterile filtered with a 0.22-μm filter. Analysis of the purified IgG product by high-performance liquid chromatography (HPLC) size exclusion chromatography indicated that there were no IgG aggregates or IgG dimers (not shown).
Infectivity reduction neutralization test.
V3000 eGFP propagation-incompetent replicons were diluted in virus diluent (phosphate-buffered saline [PBS]–1% donor bovine serum [DBS]) and reacted with bovine or mouse serum for 1 h at 37°C before being used to infect 24-well plates of Vero cells for 1 h at 37°C. Culture medium was added to the cells, and the cells were incubated for 24 h before being fixed with 4% paraformaldehyde (PFA). GFP-expressing (infected) cells were then quantified on an Olympus CKX41 inverted fluorescence microscope. The dilution of serum that resulted in an 80% reduction in GFP cells was used to calculate the IRNT80.
ELISA.
Polysorp ELISA plates were coated with 50 μl/well of 2 μg/ml of antigen (either inactivated V3000 nt3A or chimeric Sindbis virus [SINV]-VEEV [C. Sun, C. L. Gardner, and W. B. Klimstra, unpublished data]) diluted in sodium bicarbonate (pH 9.0) overnight at 4°C. Plates were blocked with PBS with 0.1% Tween 20 and 3% bovine serum albumin (BSA) overnight at 4°C. Bovine serum was diluted in PBS with 0.1% Tween 20 and 3% BSA and added to the plates in duplicate overnight at 4°C. A 1:2,000 dilution of human IgG-horseradish peroxidase (HRP) (KPL) was added for 1 h at room temperature. 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was incubated with IgG-HRP for 30 min to 1 h at room temperature, and the reaction was stopped with 1% SDS. The plates were read at an optical density at 405 nm (OD405) (Spectra Max; Molecular Devices).
Mice.
All mice were housed under specific-pathogen-free conditions, and all experiments were conducted at ABSL-3 in accordance with AAALAC-approved institutional guidelines for animal care and use approved by the University of Pittsburgh IACUC committee. The study protocols were approved by the IACUCs at the University of Pittsburgh, SAB Biotherapeutics, and the Walter Reed Army Institute of Research/Naval Medical Research Center in compliance with all applicable Federal regulations governing the protection of animals in research.
Protection conferred by TcPAb.
Six-week-old BALB/c mice (Charles River Laboratories) received 100 μg TcPAbs intraperitoneally or 100 μg intraperitoneally and 100 μg intranasally either prophylactically or therapeutically as described in the figure legends. Mice were challenged either subcutaneously with 1,000 PFU of V3000-derived virus in the rear footpad or via aerosol with either 50 to 100 LD50 or >100 LD50 of V3000 nluc TaV. Aerosol exposures were performed as previously described, using the AeroMP exposure system (Biaera Technologies, Hagerstown, MD), inside a class III biological safety cabinet (43). For coinfection aerosol exposure, 6-week-old DBA2 (Charles River) mice were exposed to a lethal dose of V3000 and A/California/07/2009 (H1N1) and treated intraperitoneally with the combined anti-VEEV/anti-Flu TcPAb preparation either prophylactically or therapeutically as described in the figure legends. All mice were weighed daily and monitored for clinical signs of disease, which was increased to twice daily upon the onset of clinical signs of disease.
In vivo imaging.
At 5 days postchallenge, mice were injected with 10 μg the Nano-Glo substrate (Promega) either s.c. or intravenously (i.v.) and imaged by using the IVIS Spectrum CT instrument (PerkinElmer) for either 2 min (i.v.) or 4 min (s.c.) post-substrate injection on the autoexposure setting. Images of representative animals from each group were chosen based on the animals exhibiting the greatest or least weight loss to illustrate the range of disease detected by IVIS analysis. The total flux (photons per second) in the head region, taken as a measure of brain replication, was calculated for 3 to 4 animals in each treatment group based on the radiance (photons per second per square centimeter per steradian) and was quantified by using Living Image software (PerkinElmer). We demonstrated previously (28) that the nluc reporter virus signal as measured by IVIS analysis gives results for virus replication similar to those of titration of PFU from tissues of infected mice. In the present studies, the dynamic range of the IVIS imager signal from the heads of uninfected mice to the heads of highly infected mice is approximately 100-fold (∼1 × 105 to 2 × 105 photons/s to ∼1 × 107 to 2 × 107 photons/s, respectively).
Statistical analysis.
A Mantel-Cox log rank test (GraphPad PRISM Software) was used to determine statistically significant differences in survival times, while a chi-square test (GraphPad PRISM Software) was used to determine statistically significant differences in percent survival. One-tailed Student's t test (Microsoft Excel) was used to determine statistical significance for all other experiments.
ACKNOWLEDGMENTS
We thank Theron Gilliland, Jr., Chelsea Maksin, Katherine O'Malley, Nicolas Garcia, and Matthew Dunn for their excellent technical assistance.
This study was supported by Defense Threat Reduction Agency grants HDTRA1411340 (K.R.), N62645-14-C-4013-NMLC (W.B.K.), and N62645-14-C-4012-NMLC (E.S.) and NIH shared equipment grant S10OD01636801 for the IVIS imager (K.D.R.).
REFERENCES
- 1.Weaver SC, Powers AM, Brault AC, Barrett AD. 1999. Molecular epidemiological studies of veterinary arboviral encephalitides. Vet J 157:123–138. doi: 10.1053/tvjl.1998.0289. [DOI] [PubMed] [Google Scholar]
- 2.Weaver SC, Ferro C, Barrera R, Boshell J, Navarro JC. 2004. Venezuelan equine encephalitis. Annu Rev Entomol 49:141–174. doi: 10.1146/annurev.ento.49.061802.123422. [DOI] [PubMed] [Google Scholar]
- 3.Johnson KM, Martin DH. 1974. Venezuelan equine encephalitis. Adv Vet Sci Comp Med 18:79–116. [PubMed] [Google Scholar]
- 4.Casadevall A, Pirofski LA. 2005. The potential of antibody-mediated immunity in the defence against biological weapons. Expert Opin Biol Ther 5:1359–1372. doi: 10.1517/14712598.5.10.1359. [DOI] [PubMed] [Google Scholar]
- 5.Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. 2010. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med 38:e66–. doi: 10.1097/CCM.0b013e3181d44c1e. [DOI] [PubMed] [Google Scholar]
- 6.Dixit R, Herz J, Dalton R, Booy R. 2016. Benefits of using heterologous polyclonal antibodies and potential applications to new and undertreated infectious pathogens. Vaccine 34:1152–1161. doi: 10.1016/j.vaccine.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall A, Dadachova E, Pirofski LA. 2004. Passive antibody therapy for infectious diseases. Nat Rev Microbiol 2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadzadeh V, Farajnia S, Feizi MA, Nejad RA. 2014. Antibody humanization methods for development of therapeutic applications. Monoclon Antib Immunodiagn Immunother 33:67–73. doi: 10.1089/mab.2013.0080. [DOI] [PubMed] [Google Scholar]
- 9.Sano A, Matsushita H, Wu H, Jiao JA, Kasinathan P, Sullivan EJ, Wang Z, Kuroiwa Y. 2013. Physiological level production of antigen-specific human immunoglobulin in cloned transchromosomic cattle. PLoS One 8:e78119. doi: 10.1371/journal.pone.0078119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroiwa Y, Kasinathan P, Sathiyaseelan T, Jiao JA, Matsushita H, Sathiyaseelan J, Wu H, Mellquist J, Hammitt M, Koster J, Kamoda S, Tachibana K, Ishida I, Robl JM. 2009. Antigen-specific human polyclonal antibodies from hyperimmunized cattle. Nat Biotechnol 27:173–181. doi: 10.1038/nbt.1521. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita H, Sano A, Wu H, Jiao JA, Kasinathan P, Sullivan EJ, Wang Z, Kuroiwa Y. 2014. Triple immunoglobulin gene knockout transchromosomic cattle: bovine lambda cluster deletion and its effect on fully human polyclonal antibody production. PLoS One 9:e90383. doi: 10.1371/journal.pone.0090383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dye JM, Wu H, Hooper JW, Khurana S, Kuehne AI, Coyle EM, Ortiz RA, Fuentes S, Herbert AS, Golding H, Bakken RA, Brannan JM, Kwilas SA, Sullivan EJ, Luke TC, Smith G, Glenn G, Li W, Ye L, Yang C, Compans RW, Tripp RA, Jiao JA. 2016. Production of potent fully human polyclonal antibodies against Ebola Zaire virus in transchromosomal cattle. Sci Rep 6:24897. doi: 10.1038/srep24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bounds CE, Kwilas SA, Kuehne AI, Brannan JM, Bakken RR, Dye JM, Hooper JW, Dupuy LC, Ellefsen B, Hannaman D, Wu H, Jiao JA, Sullivan EJ, Schmaljohn CS. 2015. Human polyclonal antibodies produced through DNA vaccination of transchromosomal cattle provide mice with post-exposure protection against lethal Zaire and Sudan ebolaviruses. PLoS One 10:e0137786. doi: 10.1371/journal.pone.0137786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luke T, Wu H, Zhao J, Channappanavar R, Coleman CM, Jiao JA, Matsushita H, Liu Y, Postnikova EN, Ork BL, Glenn G, Flyer D, Defang G, Raviprakash K, Kochel T, Wang J, Nie W, Smith G, Hensley LE, Olinger GG, Kuhn JH, Holbrook MR, Johnson RF, Perlman S, Sullivan E, Frieman MB. 2016. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci Transl Med 8:326ra21. doi: 10.1126/scitranslmed.aaf1061. [DOI] [PubMed] [Google Scholar]
- 15.Hooper JW, Brocato RL, Kwilas SA, Hammerbeck CD, Josleyn MD, Royals M, Ballantyne J, Wu H, Jiao JA, Matsushita H, Sullivan EJ. 2014. DNA vaccine-derived human IgG produced in transchromosomal bovines protect in lethal models of hantavirus pulmonary syndrome. Sci Transl Med 6:264ra162. doi: 10.1126/scitranslmed.3010082. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Hunt AR, Bowen RA, Frederickson S, Maruyama T, Roehrig JT, Blair CD. 2011. Treatment of mice with human monoclonal antibody 24h after lethal aerosol challenge with virulent Venezuelan equine encephalitis virus prevents disease but not infection. Virology 414:146–152. doi: 10.1016/j.virol.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodchild SA, O'Brien LM, Steven J, Muller MR, Lanning OJ, Logue CH, D'Elia RV, Phillpotts RJ, Perkins SD. 2011. A humanised murine monoclonal antibody with broad serogroup specificity protects mice from challenge with Venezuelan equine encephalitis virus. Antiviral Res 90:1–8. doi: 10.1016/j.antiviral.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Elvin SJ, Bennett AM, Phillpotts RJ. 2002. Role for mucosal immune responses and cell-mediated immune functions in protection from airborne challenge with Venezuelan equine encephalitis virus. J Med Virol 67:384–393. doi: 10.1002/jmv.10086. [DOI] [PubMed] [Google Scholar]
- 20.Kaveri SV. 2012. Intravenous immunoglobulin: exploiting the potential of natural antibodies. Autoimmun Rev 11:792–794. doi: 10.1016/j.autrev.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Phillpotts RJ. 2006. Venezuelan equine encephalitis virus complex-specific monoclonal antibody provides broad protection, in murine models, against airborne challenge with viruses from serogroups I, II and III. Virus Res 120:107–112. doi: 10.1016/j.virusres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien LM, Goodchild SA, Phillpotts RJ, Perkins SD. 2012. A humanised murine monoclonal antibody protects mice from Venezuelan equine encephalitis virus, Everglades virus and Mucambo virus when administered up to 48 h after airborne challenge. Virology 426:100–105. doi: 10.1016/j.virol.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 23.Fraenkel-Conrat H, Olcott HS. 1948. The reaction of formaldehyde with proteins; cross-linking between amino and primary amide or guanidyl groups. J Am Chem Soc 70:2673–2684. doi: 10.1021/ja01188a018. [DOI] [PubMed] [Google Scholar]
- 24.Fraenkel-Conrat H, Olcott HS. 1948. Reaction of formaldehyde with proteins; cross-linking of amino groups with phenol, imidazole, or indole groups. J Biol Chem 174:827–843. [PubMed] [Google Scholar]
- 25.Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, Ranganathan K. 2012. Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol 16:400–405. doi: 10.4103/0973-029X.102496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, Gale MJ Jr, Baric RS, Enjuanes L, Gallagher T, McCray PB Jr, Perlman S. 2014. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A 111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pratt WD, Gibbs P, Pitt ML, Schmaljohn AL. 1998. Use of telemetry to assess vaccine-induced protection against parenteral and aerosol infections of Venezuelan equine encephalitis virus in non-human primates. Vaccine 16:1056–1064. doi: 10.1016/S0264-410X(97)00192-8. [DOI] [PubMed] [Google Scholar]
- 28.Reed DS, Lind CM, Sullivan LJ, Pratt WD, Parker MD. 2004. Aerosol infection of cynomolgus macaques with enzootic strains of Venezuelan equine encephalitis viruses. J Infect Dis 189:1013–1017. doi: 10.1086/382281. [DOI] [PubMed] [Google Scholar]
- 29.Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh YH, Dugas A. 2016. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses 10:394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim FJ, de Klerk N, Blyth CC, Fathima P, Moore HC. 2016. Systematic review and meta-analysis of respiratory viral coinfections in children. Respirology 21:648–655. doi: 10.1111/resp.12741. [DOI] [PubMed] [Google Scholar]
- 31.Munawwar A, Singh S. 2016. Human herpesviruses as copathogens of HIV infection, their role in HIV transmission, and disease progression. J Lab Physicians 8:5–18. doi: 10.4103/0974-2727.176228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coffey LL, Failloux AB, Weaver SC. 2014. Chikungunya virus-vector interactions. Viruses 6:4628–4663. doi: 10.3390/v6114628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis NL, Willis LV, Smith JF, Johnston RE. 1989. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: analysis of a viable deletion mutant. Virology 171:189–204. doi: 10.1016/0042-6822(89)90526-6. [DOI] [PubMed] [Google Scholar]
- 34.White LJ, Wang JG, Davis NL, Johnston RE. 2001. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. J Virol 75:3706–3718. doi: 10.1128/JVI.75.8.3706-3718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun C, Gardner CL, Watson AM, Ryman KD, Klimstra WB. 2014. Stable, high-level expression of reporter proteins from improved alphavirus expression vectors to track replication and dissemination during encephalitic and arthritogenic disease. J Virol 88:2035–2046. doi: 10.1128/JVI.02990-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacDonald GH, Johnston RE. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J Virol 74:914–922. doi: 10.1128/JVI.74.2.914-922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 38.Gardner CL, Burke CW, Tesfay MZ, Glass PJ, Klimstra WB, Ryman KD. 2008. Eastern and Venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: impact of altered cell tropism on pathogenesis. J Virol 82:10634–10646. doi: 10.1128/JVI.01323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupuy LC, Richards MJ, Ellefsen B, Chau L, Luxembourg A, Hannaman D, Livingston BD, Schmaljohn CS. 2011. A DNA vaccine for Venezuelan equine encephalitis virus delivered by intramuscular electroporation elicits high levels of neutralizing antibodies in multiple animal models and provides protective immunity to mice and nonhuman primates. Clin Vaccine Immunol 18:707–716. doi: 10.1128/CVI.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garmashova N, Atasheva S, Kang W, Weaver SC, Frolova E, Frolov I. 2007. Analysis of Venezuelan equine encephalitis virus capsid protein function in the inhibition of cellular transcription. J Virol 81:13552–13565. doi: 10.1128/JVI.01576-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garmashova N, Gorchakov R, Volkova E, Paessler S, Frolova E, Frolov I. 2007. The Old World and New World alphaviruses use different virus-specific proteins for induction of transcriptional shutoff. J Virol 81:2472–2484. doi: 10.1128/JVI.02073-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. 2003. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J Virol 77:12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed DS, Xhillari D, Weiss AL, Jaeger RJ. 2016. Aerosol exposure to pathogenic bacteria and virus particles: standard operating procedure, p 445–459. In Salem H, Katz SA (ed), Aerobiology: the toxicology of airborne pathogens and toxins. The Royal Society of Chemistry, London, United Kingdom. [Google Scholar]