ABSTRACT
Early sensing of viral components or infection-induced tissue damage is a prerequisite for the successful control of pathogenic viruses by the host innate immune system. Recent results from our laboratory show how immune cells use the DNA-sensing machinery to detect intracellular damage generated early during infection by an RNA virus, namely, dengue virus (DENV). Conversely, we found that DENV can efficiently dismantle this sensing mechanism by targeting the cyclic GMP-AMP synthase (cGAS) and the stimulator of interferon (IFN) genes (STING), two crucial host factors involved in DNA detection and type I IFN production. These findings highlight the relevance of the DNA-sensing mechanism in the detection and control of infections by RNA viruses. In this review, we discuss how DENV modulates the innate immune DNA-sensing pathway, activated in the context of cellular damage during infection.
KEYWORDS: innate immunity, dengue virus, interferon, cGAS, STING, mitochondria, damage, DNA sensing, mtDNA
GLOBAL BURDEN OF ARBOVIRAL INFECTIONS
Despite significant advances in the development of vaccines and antivirals over the last century, pathogenic viruses still represent one of the most substantial threats for humans. For instance, the global spread of Aedes mosquitos, which now circulate in areas where almost half of the world's human population lives, has dramatically increased the incidence of some arboviral infections with high impact on health, namely, yellow fever, dengue, Japanese encephalitis, chikungunya, and, more recently, Zika (1). These facts highlight the need for effective therapeutic approaches to contain the global burden that these viruses represent.
INNATE IMMUNE EVASION STRATEGIES BY (+)ssRNA VIRUSES: HIDING VERSUS SPECIFIC INHIBITION
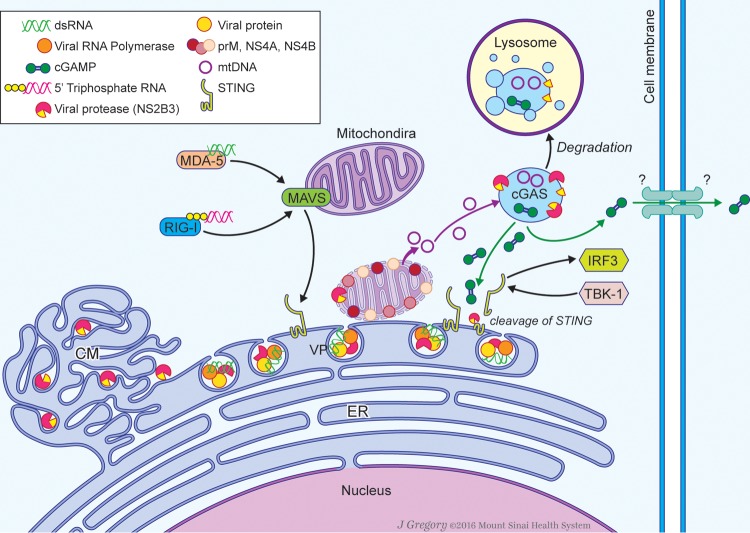
To effectively infect and replicate in the target cells, viruses through coevolution with their hosts have acquired diverse strategies to delay their early detection and control by the innate immune system. Dengue virus (DENV), in particular, is known to counteract the type I interferon (IFN) system by targeting specific host factors involved in the production and signaling of type I IFNs (2). The initial studies on DENV-encoded IFN antagonists were focused on the signaling of IFN. In this regard, different groups have shown that DENV uses at least four viral proteins (namely, NS2A, NS4A, NS4B, and NS5) to target the signal transducer and activator of transcription proteins 1 and 2 (STATs 1 and 2) (2). This inhibition halts the ability of the cell to acquire the antiviral state induced by type I IFN signaling and to propagate it in a paracrine fashion by the expression of hundreds of gene products with antiviral properties (e.g., the products of interferon-stimulated genes [ISGs]). Besides using these specific inhibitory mechanisms, which are mediated by viral proteins, DENV and other viruses with positive-sense single-stranded RNA [(+)ssRNA]genomes use nonstructural viral proteins to substantially modify endoplasmic reticulum (ER)-derived membranes and build semi-isolated nanocompartments that are necessary for viral replication (Fig. 1) (3). These intracellular modifications create a physical barrier that minimizes the availability of viral replication subproducts—5′-phosphorylated RNA and/or double-stranded RNA (dsRNA)—in the cytoplasm. These pathogen-associated molecular patterns (PAMPs) are thus shielded from subsequent sensing by RIG-I-like receptors (RIG-I and MDA-5), the cytoplasmic pattern recognition receptors (PRRs) for RNA species (3). Additionally, the consequences of this pronounced virus infection-induced rearrangement of internal membranes of the cell have not been totally elucidated. Conversely, countermechanisms employed by viruses to prevent sensing triggered by the manipulation of cellular structures are not well understood either.
FIG 1.
Sensing of DENV infection by host cells. DENV replicates in ER membrane-derived vesicles, where it hides its replication products from the cytosolic RIG-I-like receptors (RIG-I and MDA-5), which can recognize viral RNA and signal type I IFN production through MAVS and STING. The DENV NS2B3 protease complex cleaves STING in the ER membrane to inhibit viral-RNA and self-DNA detection. Some viral proteins reach the mitochondrial membrane, resulting in mitochondrial stress and subsequent mtDNA leakage. The DNA sensor cGAS can detect mtDNA in the cytoplasm to engage the type I IFN production through the synthesis of cGAMPs, which can activate STING in the infected cell or translocate to neighboring cells via gap junctions. DENV NS2B, NS3, and NS2B3 proteins can interact with cGAS and inhibit its function. DENV NS2B interacts with cGAS and induces its degradation by an autophagy–lysosome-dependent mechanism, resulting in an inability to produce type I IFN by the host. CM, convoluted membrane; VP, vesicle packets. (Courtesy of Mount Sinai Health System, reproduced with permission.)
ROLE OF THE DNA-SENSING MACHINERY DURING RNA VIRUS INFECTION
Our group recently described evidence that DENV infection triggers mislocalization of mitochondrial DNA (mtDNA) into the cytoplasm of infected cells (4). The leaked mtDNA is then detected by the DNA sensor cyclic GMP-AMP synthase (cGAS, also known as C6ORF160 and MB21D1) and serves as a trigger of the cGAS/stimulator of interferon genes (cGAS/STING) pathway, resulting in type I IFN production. This finding provides an explanation for why DENV, an RNA virus, blocks the cGAS DNA-sensing pathway in infected cells (5). However, it still needs to be determined whether this is a common feature observed among flaviviruses as well as other (+)ssRNA viruses.
DENV specifically blocks the cGAS/STING pathway by directly targeting both STING and cGAS. The adaptor STING, a protein that resides in the ER membrane, is cleaved by the DENV-encoded protease complex NS2B3 in DENV-infected cells, inhibiting the production of type I IFNs (6). STING is known to have a promiscuous role in viral detection, since it can participate in viral RNA sensing through its interaction with the mitochondrial antiviral signaling protein (MAVS) and further transduce the signaling induced by RNA-derived PAMPs through RIG-I-like sensors (RIG-I and MDA-5) (7) (Fig. 1). STING is also known to mediate the sensing of DNA by an exclusive relationship with cGAS through the detection of the second messenger cyclic GMP-AMP (cGAMP), which is synthetized by cGAS upon recognition of double-stranded DNA (dsDNA) or RNA-DNA hybrids (8). The ability of STING to detect cyclic dinucleotides (CDNs) was first described for bacterial CDNs (9); later, the Chen laboratory reported that cGAS can synthetize noncanonical CDNs with high affinity for STING dimers (10). This discovery unveiled a novel role of second messengers in the signaling of the IFN pathway. Furthermore, Ablasser and collaborators described a paracrine effect of cGAMPs in the activation of STING by their intercellular trafficking via gap junctions (11). Our group recently reported that DENV targets cGAS for degradation through the protease cofactor NS2B, which strongly interacts with cGAS and promotes its degradation in an autophagy–lysosome-dependent mechanism (4). We showed that inhibition of lysosome acidification by chloroquine or ammonium chloride, as well as inhibition of its formation by 3-methyladenine (3-MA) treatment, prevented cGAS degradation during DENV infection (4). Of note, DENV NS2B is the first viral protein that directly targets cGAS for degradation (Fig. 1). These findings highlight the relevance of the DNA-sensing pathway for the control of DENV replication and possibly of other (+)ssRNA viruses and open new questions about the reach of cGAMP-dependent STING activation in uninfected cells.
THANK YOUR MOTHER FOR THE IMMUNITY: ROLE OF mtDNA DURING DENV INFECTION
The restricting role of cGAS for (+)ssRNA viruses was first reported using seven different viral genera (5). However, the substrate that is recognized by this DNA sensor during infections with RNA viruses remained a mystery. By isolating cGAS from DENV-infected cells, we were able to determine the identity of the cGAS ligand (4). Analysis of the nucleic acids bound to cGAS during DENV infection revealed a significant enrichment of mtDNA compared to that in purified cGAS from mock-infected cells. Furthermore, the ability of the purified mtDNA to activate the cGAS/cGAMP/STING pathway was confirmed by reporter assays, consistent with reports showing that DNA sensing by cGAS is not sequence specific (12). Wild-type cGAS or two different inactive mutants (DNA binding mutant or nucleotidyltransferase [NTase] domain mutant) were used to confirm that cGAS physically interacts with mtDNA and that cGAMP synthesis is required for IFN-β induction (4). Immunofluorescence data demonstrated the localization of DENV proteins in the mitochondrial membrane; this observation was concomitant with the perturbation of mitochondrial morphology and the presence of mislocalized cellular DNA in the cytoplasm of DENV-infected cells (4). The ability of mtDNA to engage type I IFN production in a cGAS-dependent manner has been described previously for DNA viruses and bacterial pathogens (13, 14). Our work shows a link between an RNA virus infection, perturbation of mitochondria, and cGAS/cGAMP/STING pathway activation through mtDNA recognition (4). Interestingly, several DENV proteins have been reported to be localized into the mitochondrial membrane, which might affect mitochondrion homeostasis and function. For instance, it has been shown that the DENV virus membrane (M) protein can form pores in the mitochondrial membrane, inducing permeabilization, matrix swelling, and loss of mitochondrial membrane potential (15). DENV NS4A protein has also been found in mitochondrion-associated membranes, where it interferes with MAVS-mediated signaling (16). The DENV NS2B3 protease complex was also shown to be present in the mitochondrial membrane, where it cleaves mitofusins 1 and 2 (17). Interestingly, mitofusin 2 mediates the interaction between mitochondria and the ER membrane, which allows communication and molecule interchanges. More recently, the DENV NS4B protein was shown to also localize in the mitochondrial membrane and to induce the elongation of the mitochondria, promoting virus replication and mitigation of RIG-I pathway-dependent type I IFN induction (18). These reports suggest a mechanism by which DENV may disrupt the mitochondrion-ER interaction to avoid the accumulation of DENV proteins in that organelle, which can trigger mtDNA leakage to the cytoplasm. The role of mtDNA in immune defense against pathogens has been described to occur not only in the cell cytoplasm but also extracellularly by forming extracellular neutrophil traps for bacteria (19). Our recent findings add an extra layer of information about the role of mitochondria in innate immunity and RNA virus infection as a “maternal” protection that triggers intracellular pathways of immune defense.
PERSPECTIVES FOR THE RATIONAL EXPLOITATION OF THE cGAS/cGAMP/STING PATHWAY
Since its discovery as a stimulator of type I IFNs, STING has been reported to be a crucial adaptor mediating the signaling cascades induced by a plethora of pathogens (viruses, bacteria, and parasites) that contain RNA and/or DNA in their genomes. Dimers of STING can directly sense CDNs, which are produced by bacteria and by cGAS in host cells. Furthermore, STING can promote immunity against different tumors. Lastly, it is responsible for signaling unwanted chronic activation of the IFN pathway due to accumulation of cytoplasmic self-DNA, which is observed in several autoimmune pathologies. All this information highlights the need for molecular tools that can either activate or silence the cGAS/cGAMP/STING pathway. As an example, preliminary data from the use of CDNs as adjuvants for vaccines and antitumoral therapy show promising results in animal models (20). On the other hand, the compiled information about inhibition of the cGAS/cGAMP/STING pathway by different viruses can be used as a scaffold for the rational design of compounds that mimic the inhibitory effect observed in nature (i.e., by viral antagonists targeting this pathway).
Our group was one of the first to show that the viral protein (DENV NS2B3) can cleave and degrade STING to disrupt its function (6). More recently, we described the first viral protein (DENV NS2B), paradoxically from an RNA virus, that degrades cGAS in order to avoid DNA sensing and cGAMP synthesis. It remains unclear why two different proteins involved in the same signaling pathway are degraded during DENV infection, but it might be related to the paracrine effect that cGAMPs may exert in uninfected tissue. The information described here opens new questions about the promiscuity and redundancy of the innate immune system during the detection of infectious organisms. We have demonstrated that cells can recognize signs of “collateral” damage (in the form of cytosolic DNA) during infection with an RNA virus that efficiently hides its replication products (PAMPs). Future studies will investigate whether host cells have mechanisms to trigger RNA sensor activation by immunogenic cellular RNAs during infection of microbes with DNA genomes.
ACKNOWLEDGMENTS
We thank Viviana Simon and Adolfo Garcia-Sastre for their invaluable discussions and input for this article.
The work from the Fernandez-Sesma laboratory described in this review was funded by NIH/NIAID grants R01AI073450, R21AI116022, and 1U19AI118610 and the DARPA (Prophecy) grant HR0011-11-C-0094 (A.F.-S.).
REFERENCES
- 1.Mayer SV, Tesh RB, Vasilakis N. 2017. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop 166:155–163. doi: 10.1016/j.actatropica.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison J, Aguirre S, Fernandez-Sesma A. 2012. Innate immunity evasion by Dengue virus. Viruses 4:397–413. doi: 10.3390/v4030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida L, Espada-Murao LA, Takamatsu Y, Okamoto K, Hayasaka D, Yu F, Nabeshima T, Buerano CC, Morita K. 2014. The dengue virus conceals double-stranded RNA in the intracellular membrane to escape from an interferon response. Sci Rep 4:7395. doi: 10.1038/srep07395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguirre S, Luthra P, Sanchez-Aparicio MT, Maestre AM, Patel J, Lamothe F, Fredericks AC, Tripathi S, Zhu T, Pintado-Silva J, Webb LG, Bernal-Rubio D, Solovyov A, Greenbaum B, Simon V, Basler CF, Mulder LC, Garcia-Sastre A, Fernandez-Sesma A. 2017. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat Microbiol 2:17037. doi: 10.1038/nmicrobiol.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V, Dabelic R, Manicassamy B, Aitchison JD, Aderem A, Elliott RM, García-Sastre A, Racaniello V, Snijder EJ, Yokoyama WM, Diamond MS, Virgin HW, Rice CM. 2014. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, Maringer K, Bernal-Rubio D, Shabman RS, Simon V, Rodriguez-Madoz JR, Mulder LC, Barber GN, Fernandez-Sesma A. 2012. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog 8:e1002934. doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa H, Barber GN. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mankan AK, Schmidt T, Chauhan D, Goldeck M, Honing K, Gaidt M, Kubarenko AV, Andreeva L, Hopfner KP, Hornung V. 2014. Cytosolic RNA:DNA hybrids activate the cGAS-STING axis. EMBO J 33:2937–2946. doi: 10.15252/embj.201488726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdette D. 2011. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. 2013. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, Hornung V. 2013. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503:530–534. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, Hornung V, Hopfner KP. 2013. Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498:332–337. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. 2015. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiens KE, Ernst JD. 2016. The mechanism for type I interferon induction by Mycobacterium tuberculosis is bacterial strain-dependent. PLoS Pathog 12:e1005809. doi: 10.1371/journal.ppat.1005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catteau A, Roue G, Yuste VJ, Susin SA, Despres P. 2003. Expression of dengue ApoptoM sequence results in disruption of mitochondrial potential and caspase activation. Biochimie 85:789–793. doi: 10.1016/S0300-9084(03)00139-1. [DOI] [PubMed] [Google Scholar]
- 16.He Z, Zhu X, Wen W, Yuan J, Hu Y, Chen J, An S, Dong X, Lin C, Yu J, Wu J, Yang Y, Cai J, Li J, Li M. 2016. Dengue virus subverts host innate immunity by targeting adaptor protein MAVS. J Virol 90:7219–7230. doi: 10.1128/JVI.00221-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu CY, Liang JJ, Li J K, Lee YL, Chang BL, Su CI, Huang WJ, Lai MM, Lin YL. 2015. Dengue virus impairs mitochondrial fusion by cleaving mitofusins. PLoS Pathog 11:e1005350. doi: 10.1371/journal.ppat.1005350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatel-Chaix L, Cortese M, Romero-Brey I, Bender S, Neufeldt CJ, Fischl W, Scaturro P, Schieber N, Schwab Y, Fischer B, Ruggieri A, Bartenschlager R. 2016. Dengue virus perturbs mitochondrial morphodynamics to dampen innate immune responses. Cell Host Microbe 20:342–356. doi: 10.1016/j.chom.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 20.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, Sivick KE, Zeng Q, Soares KC, Zheng L, Portnoy DA, Woodward JJ, Pardoll DM, Dubensky TW Jr, Kim Y. 2015. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 7:283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]