ABSTRACT
Controversy still surrounds both the etiology and pathophysiology of vestibular neuritis (VN). Especially uncertain is why the superior vestibular nerve (SVN) is more frequently affected than the inferior vestibular nerve (IVN), which is partially or totally spared. To address this question, we developed an improved method for preparing human vestibular ganglia (VG) and nerve. Subsequently, macro- and microanatomical as well as PCR studies were performed on 38 human ganglia from 38 individuals. The SVN was 2.4 mm longer than the IVN, and in 65% of the cases, the IVN ran in two separate bony canals, which was not the case for the SVN. Anastomoses between the facial and cochlear nerves were more common for the SVN (14/38 and 9/38, respectively) than for the IVN (7/38 and 2/38, respectively). Using reverse transcription-quantitative PCR (RT-qPCR), we found only a few latently herpes simplex virus 1 (HSV-1)-infected VG (18.4%). In cases of two separate neuronal fields, infected neurons were located in the superior part only. In summary, these PCR and micro- and macroanatomical studies provide possible explanations for the high frequency of SVN infection in vestibular neuritis.
IMPORTANCE Vestibular neuritis is known to affect the superior part of the vestibular nerve more frequently than the inferior part. The reason for this clinical phenomenon remains unclear. Anatomical differences may play a role, or if latent HSV-1 infection is assumed, the etiology may be due to the different distribution of the infection. To shed further light on this subject, we conducted different macro- and microanatomical studies. We also assessed the presence of HSV-1 in VG and in different sections of the VG. Our findings add new information on the macro- and microanatomy of the VG as well as the pathophysiology of vestibular neuritis. We also show that latent HSV-1 infection of VG neurons is less frequent than previously reported.
KEYWORDS: HSV-1 latency, human, vestibular ganglia, vestibular neuritis
INTRODUCTION
Vestibular neuritis (VN) is a common cause of an “acute unilateral vestibulopathy.” The main clinical symptoms of VN are acute onset of prolonged severe spinning vertigo with spontaneous horizontal torsional nystagmus toward the unaffected ear, postural imbalance, and nausea (1). Its annual incidence is reported to be 3.5 to 15.5 per population of 100,000 (2, 3). It is the third most common cause of peripheral vestibular dysfunction (4, 5). A viral etiology of VN is supported by studies showing inflammatory degeneration of the vestibular nerves and the presence of herpes simplex virus 1 (HSV-1) DNA as well as latency-associated transcript (LAT) in the vestibular ganglia (VG) (6–9). After primary infection, usually occurring via the oral mucosa of the mouth, HSV-1 infects the trigeminal ganglion (TG) and can reach the geniculate ganglion (GG) via the lingual nerve. From there, smaller numbers of cells in the superior VG can be infected via faciovestibular anastomoses. Reactivation can occur during different diseases such as herpes labialis (TG) and, in rarer cases, Bell's palsy (GG), sudden hearing loss, or vestibular neuritis (VG). Such a reactivation of HSV-1 from the VG has been proposed to cause vestibular neuritis, although final confirmation is still lacking (6–8, 10).
The superior vestibular nerve (SVN) supplies the horizontal and anterior canals as well as the utricle and parts of the saccule. The inferior vestibular nerve (IVN) innervates the main portion of the macula sacculi and the posterior ampulla. Vestibular neuritis of the IVN has not been as well characterized as VN of the SVN, but it is generally agreed that the SVN is much more frequently affected than the IVN. The frequency of VN reported in the available literature ranges from 40 to 48% of all cases affecting only the SVN (11–13), 34 to 56% of cases affecting both the SVN and IVN (11–13), and 1.3 to 18% of cases affecting only the IVN (11–14). Possible explanations proposed previously are the longer and narrower bony canal, through which the SVN passes (15), and the double supply of the IVN in two separate bony canals (6).
In the present study, we performed macro- and microanatomical studies as well as PCR experiments on 38 human VG from 38 individuals to determine why the SVN is more frequently affected in vestibular neuritis. Theoretically, this could be due to (i) the different lengths of the SVN and IVN; (ii) the presence of two bony canals for the IVN; (iii) differences in anastomoses, which would facilitate viral transmission between the SVN and IVN; or (iv) differences in the distributions of LAT among neurons projecting to the SVN or IVN.
RESULTS
Macroscopic anatomical evaluation of VG.
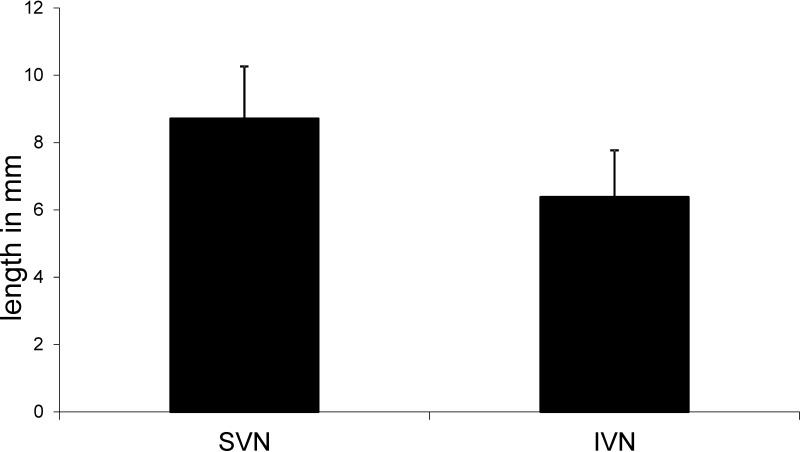
The lengths of the SVN and IVN were microscopically measured in 21/38 VG. The SVN (8.7 ± 2.2 mm [mean ± standard deviation {SD}]) was found to be significantly longer than the IVN (6.3 ± 2 mm [mean ± SD]) (P = 0.000 by a Wilcoxon test). Overall, the SVN was on average 2.4 mm longer than the IVN (Fig. 1).
FIG 1.
Graph showing the lengths (in millimeters) of the superior and inferior vestibular nerves.
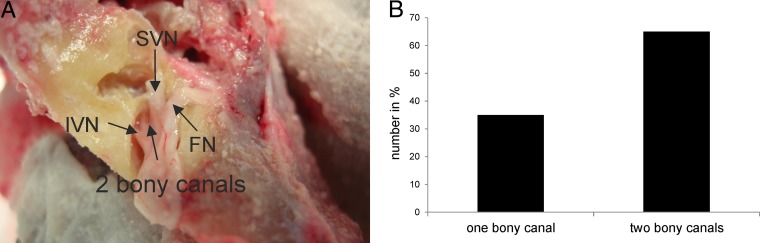
In 65% (25/38) of the analyzed temporal bones, the posterior canal of the vestibular system was innervated by two separate portions of the inferior nerve running in two separate bony canals (Fig. 2). There was no difference in the numbers of bony canals between LAT-positive and LAT-negative ganglia (P = 0.374 by a chi-square test).
FIG 2.
(A) Photographic image of a drilled right temporal bone showing the facial nerve, the superior vestibular nerve, and the inferior nerve running in two separate bony canals. (B) Percentage of the inferior vestibular nerve running in one or two bony canals. FN, facial nerve.
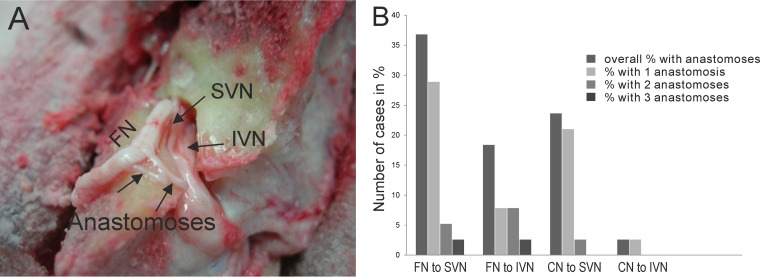
Transnerval anastomoses, which could facilitate viral transmission to the SVN or IVN, occurred more frequently in the SVN than in the IVN (P = 0.008 by a chi-square test) (Fig. 3). When anastomoses from the facial and cochlear nerves were analyzed separately, anastomoses from the facial nerve to the SVN (14/38; 36.8%) were found more frequently than were anastomoses from the facial nerve to the IVN (7/38; 18.4%); however, these differences were not statistically significant (P = 0.073 by a chi-square test). Anastomoses from the cochlear nerve to the SVN (9/38; 23.7%) were found more frequently than were anastomoses from the cochlear nerve to the IVN (2/38; 5.3%) (P = 0.007 by a chi-square test).
FIG 3.
(A) Photographic image of a drilled left temporal bone showing the facial nerve and superior and inferior vestibular nerves. Furthermore, anastomoses between the facial nerve and the superior vestibular nerve are depicted. (B) Graph showing the percentage of cases with anastomoses between the respective nerves. FN, facial nerve; CN, cochlear nerve.
Microscopic anatomical evaluation of the VG.
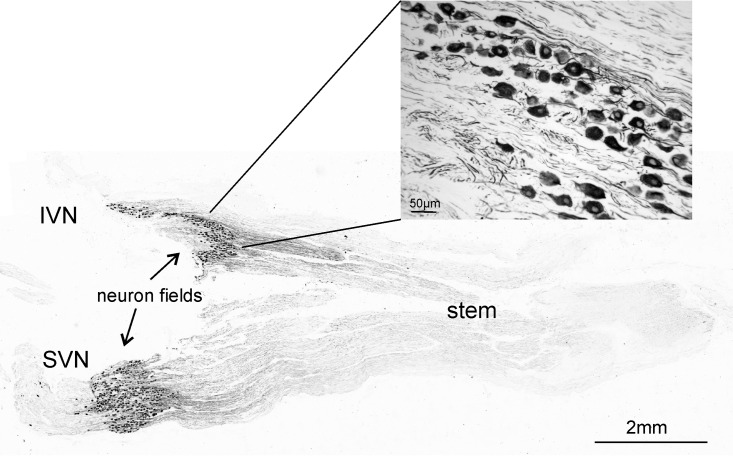
The distributions of the neuronal somata within the VG and the projecting SVN and IVN branches were determined. Neuronal somata were found either only in the stem of the VG (9/38; 23.7%), in the two branches (7/38; 18.4%), in one branch (16/38; 42.1%), or in both the stem and the branches (6/38; 15.8%). An example of the distribution of neurons immunostained for nonphosphorylated neurofilaments is shown in Fig. 4.
FIG 4.
Vestibular nerve stained with antibodies against nonphosphorylated neurofilaments. In this example, the neuronal somata are localized in both branches of the VG. The small image shows an enlarged section of one neuron field.
Detection of HSV-1 LAT in the VG.
Three different experiments were performed to assess the distribution of LAT in the VG. When sections from VG were analyzed, only 7/38 VG were positive for LAT via reverse transcription-quantitative PCR (RT-qPCR) (18.4%). The individual neuronal fields were then analyzed by using laser capture microdissection (LCM) and single-cell RT-qPCR. In 31/38 ganglia, the neuronal somata were located in the stem as well as in the branches, so a clear separation of those projecting to the SVN or IVN was not possible. In these VG, LAT was detected in 4/31 (12.9%) of the ganglia. These results were additionally confirmed by nested PCR for LAT and subsequent sequencing.
The 7 VG in which the neuronal somata were located within the SVN and IVN were then investigated. They could thus be assessed separately for the presence of LAT: 42.8% (3/7) of these VG were positive for LAT, and the positive neurons were located within the SVN in all cases. No positive neurons were detected in the IVN. In the remaining VG, the neurons in the SVN and IVN were negative. Overall, the neurons of the SVN were more frequently LAT positive than were those of the IVN (P = 0.051 by a chi-square test).
DISCUSSION
The major findings of the study are as follows: (i) the SVN was significantly longer than the IVN; (ii) in about 65% of the temporal bones, the posterior canal was innervated by two separate portions of the inferior nerve running in two separate bony canals; (iii) anastomoses from the facial and cochlear nerves to the SVN occurred more frequently than did anastomoses from the facial and cochlear nerves to the IVN; (iv) contrary to previous reports, ganglion cells of the VG were located within different areas (only in the stem, in two branches, in the stem and the branch, or in one branch); and (v) there were many fewer LAT-positive VG than previously reported, and in those ganglia where differential assessment was possible, all positive neurons were found within the SVN.
In the present study, microscopic measurement of the lengths of the SVN and of the IVN revealed that the SVN is on average 2.4 mm longer than the IVN. Gianoli et al. (15) showed that the bony canal of the SVN was seven times longer than the IVN canal, and a higher percentage of bone spicules was present in the superior canal. Similarly, more interspersed reticulated bone was found in the superior vestibular canal (13). These anatomical differences could make this portion of the superior division more susceptible to entrapment and ischemia caused by swelling from an initial viral infection (15).
In addition, we found two separate bony canals for the IVN in 65% of the analyzed temporal bones. Arbusow et al. (6) previously suggested that the frequent double innervation of the posterior ampulla was caused by two nerves running in two separate canals. This could be another explanation for the frequent sparing of IVN-innervated structures. Vestibular neuritis is not functionally equivalent to a total vestibular deficit. This was suspected since vestibular neuritis and benign paroxysmal positional vertigo (BPPV) can occur in the same ear (16). A later three-dimensional analysis of canal function made confirmation possible (17).
Primary infection with HSV-1, usually occurring via the mucosa of the mouth, results in lytic infection, with lysis of epithelial or mucosal cells and the subsequent release of infectious virus. HSV-1 can then enter sensory nerve fibers, innervating the site of inoculation. The virus then travels via retrograde transport to the sensory neurons, which are located in the TG. Here, HSV-1 establishes latency (18, 19). Viral latency is characterized by the retention of a functional viral genome and very limited gene expression without the production of infectious virus particles (20). Due to certain triggers, such as stress, UV light, or immune suppression, the virus can reactivate from the latent state and cause recurrent infections at the original site of infection (herpes labialis) (21, 22). From the TG, the virus is thought to travel via the lingual nerve to the ganglion geniculi and from there to the vestibular ganglion via faciovestibular anastomoses (23, 24). A schematic neuroanatomical drawing showing the geniculate ganglion, the vestibular ganglion, as well as the presumed pathway of virus migration was reported previously (6). In the present study, we showed that anastomoses occur more often in the superior part of the VG and possibly lead to higher rates of infection, which would then result in more frequent reactivation, i.e., the typical clinical picture of neuritis. HSV-1 infection of the VG without an involvement of the associated GG does not necessarily exclude the possibility of viral spread from the GG to the VG via faciovestibular anastomosis. The absence of viral DNA in the GG could be due to the reactivation and subsequent clearance of the virus. Clearance of HSV-1 from the TG (25–27) and the cornea (28) was shown previously in mice. However, it is also possible that HSV-1 may first infect the VG via infected T lymphocytes or retrogradely from the vestibular labyrinth (7).
We found neurons only in the stem of the VG (23.7%), in the two branches (18.4%), in one branch (42.1%), and in both the stem and branches (15.8%). This finding contradicts data from previous studies which found neuronal somata in the stem and the beginning of the two branches (6). Previous studies showed that ganglion cells are scattered in the isthmus and in the nerve branches. They are closely packed and form a rather distinct ganglion, although a few scattered cells are seen at some distance from this cell cluster (24, 29, 30).
The hypothesis that vestibular neuritis was caused by a reactivation of HSV-1 in the VG was based on data from temporal bone studies, which showed that changes in the vestibular nerve were similar to those normally seen in viral infections (31, 32), and studies that detected HSV-1 DNA in the VG (6, 33, 34). Animal studies also reported that HSV-1 antigen was found in the VG and the vestibular nerve of mice experimentally infected with HSV-1 (35, 36). Viral DNA was detected via PCR in 60 to 70% of the VG from human temporal bone (6, 33, 37–39). Using a more sensitive detection method (RT-qPCR), LAT was detected in 63 to 70% of VG (8, 39). Additionally, a previous report from our group showed that LAT transcript levels ranged between 1 and 70 copies (10) in the VG, compared to 1,149 to 2,406 copies in the TG (10).
While DNA has been shown to be present in many parts of the human body, it is now evident that the presence of LAT mRNA indicates a virus capable of reactivation and causing disease, as it is the only prominent transcript during viral latency. In contrast to data from previous studies, we found LAT in only 18.4% of the VG via RT-qPCR. In 42.8% (3/7) of the VG, where the neuronal somata were located in both branches, the SVN was positive and the IVN was negative, while the remaining 4 VG tested were negative. Overall, the SVN of the VG was LAT positive more frequently than was the IVN. Only one other study determined the distribution of HSV-1 among the different branches (6) but evaluated DNA and not mRNA (LAT). HSV-1 was found only in the stem in 24% of VG, in the superior portion in 29%, and in the inferior portion in 14%. The differences from our study could stem from our use of laser microdissection rather than “scratching” (4) and measurement of mRNA instead of DNA levels. These findings correlate with previously reported clinical observations that the involvement of the IVN is much less common than the involvement of the SVN (11–14). The IVN accounts for only 1.3 to 18% of cases of vestibular neuritis (11–14). The rareness of the IVN may be attributed in part to diagnostic difficulties, but temporal bone pathology may also play a role (32, 40). Small sample sizes and restrictive inclusion criteria could explain the different numbers reported in previous studies. Using better examination methods instead of only abnormal caloric irrigation values or an abnormal bedside head impulse test (HIT) as inclusion criteria, those researchers recruited patients on the basis of their symptoms and clinical performance.
Nowadays, the functions of all three semicircular canals, the utricle, and the saccule can be separately tested to evaluate inferior and superior vestibular nerve function: one group specifically investigated the otolith, and the other investigated the ampullary afferents (41). Therefore, both nerve divisions could be affected together but also independently (11, 42, 43). Abnormalities in the tests of inferior nerve function were significantly fewer and less extensive than those in the tests of superior nerve function (11). These contrasting results are thought to be due to anatomical differences in the path of each nerve within the temporal bone (15, 40).
Also, clinical cases in which both the SVN and IVN are affected could have a different explanation. Namely, after infection of SVN neurons, the virus could migrate within the ganglion in those cases in which neurons are located in the stem close to each other. This would explain the finding that patients who initially had normal IVN function later developed a saccular deficit (11, 14).
Differences in HSV-1 infection frequencies (especially the much lower frequency of detection of HSV-1 LAT in the present study than in previous ones) could be explained by several factors. As the temporal bone is the strongest bone in the human body (44), problems with VG preparation, such as a longer preparation time with subsequent heating of the tissue, can lead to disintegration. There may also be damage to the ganglion due to decalcification or freezing and thawing of the specimens (37). Discrepancies could also stem from differences in RNA isolation protocols. Consecutive PCR mixtures, each with about 35 cycles (nested PCR), may be contaminated by neighboring samples, and foreign RNA can lead to false-positive results. The creation of dimers can also give incorrect results, and the use of an unsuitable housekeeping gene can also lead to false-positive results.
In the present study, we found several possible virus-related as well as macro- and microanatomy aspects that could explain the clinically observed higher frequency of SVN involvement in vestibular neuritis than of IVN involvement. These factors probably interact in clinical practice: the lengths of the two nerves are different, two separate portions of the inferior nerve run in two separate bony canals, anastomoses to the SVN occur more frequently, and LAT mRNA can be detected in only SVN neurons. Our improved, rapid method of VG preparation and RNA analysis detected a much lower frequency of LAT in the VG (18.4%) than those reported in previous studies (63 to 70%). While clinical studies evaluating the frequency and pathogenesis of the IVN are still scarce, here, we provide scientifically well-founded possible explanations for this phenomenon.
MATERIALS AND METHODS
The Ethics Committee of the Medical Faculty of the Ludwig Maximilian University of Munich approved the use of autopsy samples for this study. At the time of postmortem examination, none of the individuals had lesions suggestive of an active orolabial herpes infection or a history of cranial nerve disorders. Thirty-eight VG were collected from individuals (aged 19 to 88 years; mean age ± SD, 61.2 ± 20.2 years; postmortem delay [PMD] of 4 to 24 h; mean PMD ± SD, 18.7 ± 3.9 h) and used in most analyses. Some evaluations, e.g., measurement of the lengths of the SVN and IVN, were done on only a subset of these ganglia. Where applicable, this is indicated in Results. The VG from 38 totally unrelated subjects of comparable ages and with similar PMD characteristics were used to study the number of anastomoses.
Preparation of temporal bone to obtain VG.
It is very difficult to prepare VG because the temporal bone is the strongest bone in the human body (44), a fact that has greatly hindered research on human VG. Methods like decalcification do not result in good preservation of the ganglion. Different drilling strategies have been tried, but they have often led to prolonged preparation times, with subsequent heating and disintegration of the VG.
Since it is extremely challenging to dissect the temporal bone in situ, a section of the temporal bone containing the cochlea, the bony labyrinth, and the porus meatus acusticus internus was first removed. The bone in the region of the porus meatus acusticus internus and the area arcuata was gradually removed by using a precision drill until the semicircular canals were exposed. Subsequently, the osseous canal, in which the nervus vestibulocochlearis, nervus facialis, and nervus intermedius run, was exposed. The nervus vestibulocochlearis, nervus facialis, and nervus intermedius were examined for anastomoses, and their number was recorded. Likewise, the lengths of the SVN and IVN were measured. Nerve length was measured from the initial point where the nerve separates from the main vestibular nerve trunk to the exit into the inner ear.
In addition, it was determined whether the inferior nerve separated into one or two bony canals. The nervus facialis and the nervus intermedius were then pushed to one side, and the vestibular nerve was removed. The dissection in our study took approximately 3 min for a trained preparator, in contrast to previous groups, which needed far-longer preparation times (about 2 h), with subsequent heating of the tissue and its disintegration. After dissection had been completed, the temporal bone was returned to the corpse. All ganglia were immediately embedded in Jung tissue freezing medium (Leica Microsystems, Nussloch, Germany) and subsequently stored at −80°C. Frozen sections (10 μm) were cut and mounted onto either positively charged slides (Superfrost Plus; Menzel, Braunschweig, Germany) for immunohistochemistry or PEN (polyethylene naphthalate) slides (Leica Microsystems, Wetzlar, Germany) for laser capture microdissection. Slides were subsequently stored at −20°C or −80°C, respectively. Furthermore, 10 30-μm tissue sections were collected for RNA extraction. Tissue sections from different levels within each ganglion were stained with hematoxylin and eosin for morphological examination. To be certain that no neuron fields were missed in the branches, the entire VG were sectioned. Sections from different levels were used in all experiments.
Immunocytochemical staining.
One VG (obtained at 24 h postmortem) was fixed in 4% paraformaldehyde, equilibrated in increasing concentrations of sucrose (10 to 30%), and cut at 20 μm by using a cryostat. Sections were thaw mounted onto glass slides (Superfrost Plus; Menzel, Braunschweig, Germany) and allowed to dry for at least 2 days. A series of sections was incubated with monoclonal mouse antibodies against nonphosphorylated neurofilaments (SMI32, 1:5,000; BioLegend, Aachen, Germany) for 48 h at 4°C after blocking endogenous peroxidase with incubation in 1% H2O2 in 0.1 M Tris-buffered saline (TBS). The antigen binding site was visualized by subsequent incubation in biotinylated horse anti-mouse antibody (1:200; Vector Lab, Eching, Germany) and Extravidin peroxidase (1:1,000; Sigma, Darmstadt, Germany) and a Ni-intensified diaminobenzidine reaction.
In situ hybridization.
In brief, for in situ hybridization, defrosted tissue sections were paraformaldehyde fixed, endogenous peroxidase blocked by using 1.5% H2O2 in methanol, acetylated with 0.25% acetic anhydride in triethanolamine, and then prehybridized for 1 h in PHO buffer (1× Denhardt's solution, 0.1 μg/ml herring sperm DNA, 5 mg/ml sodium pyrophosphate, and 5 mM Tris-HCl, in 4× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]). Samples were incubated overnight at 37°C with 4 ng/μl of an oligonucleotide probe against HSV-1 LAT, labeled with a 3′ digoxigenin (DIG) tag (5′-CAT AGA GAG CCA GGC ACA AAA ACA C-3′) (Eurofins MWG Operon, Ebersberg, Germany). After further washing and blocking, samples were incubated for 2 h with an anti-DIG-alkaline phosphatase (AP)-conjugated antibody (Roche Diagnostics, Mannheim, Germany), and reactivity was visualized by using nitroblue tetrazolium (NBT)–5-bromo-4-chloro-3-indolylphosphate (BCIP) (Roche Diagnostics).
RNA extraction, reverse transcription, and RT-qPCR.
RNA was extracted from pooled 30-μm sections from each ganglion by using Qiazol (Qiagen, Hilden, Germany) and the miRNeasy minikit (Qiagen). The quality of the isolated RNA was then analyzed with an Agilent 2100 bioanalyzer (Agilent Technologies, Waldbronn, Germany) combined with the Agilent RNA 6000p kit (Agilent Technologies). The RNA concentration was measured by using a NanoDrop 2000c instrument (Thermo Scientific, USA), RNA was then reverse transcribed, and the HSV-1 infection state of the VG was assessed by RT-qPCR for LAT. The protocol as well as the primers and probes are described below.
Laser capture microdissection, reverse transcription of RNA, and RT-qPCR of single cells.
Tissue was treated with 70% isopropanol to dry the sections, thereby making them more susceptible to laser capture microdissection (LCM). The regions of interest (neuronal somata in different branches or in the stem of the VG) were laser microdissected. Neurons were marked electronically, microdissected, and laser captured into single reaction tubes, which were immediately stored on dry ice or at −80°C until further use. Single-cell RT-qPCR was performed by using an Ambion kit (Thermo Scientific, Darmstadt, Germany) according to the manufacturer's instructions. The expression levels of various target genes as well as the expression level of the endogenous control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were evaluated by using commercially available TaqMan gene expression assay mixtures (Thermo Scientific, Darmstadt, Germany) containing optimized primer and probe concentrations. Primer-probe sets consisted of two unlabeled PCR primers and the 6-carboxyfluorescein (FAM)-VIC dye-labeled TaqMan MGB probe formulated into a single mixture.
For GAPDH, assay Hs02758991_g1 (catalog number 4448489) was used. The custom-made primer-and-probe set for LAT used in this study were as follows: forward primer CCCACGTACTCCAAGAAGGC, reverse primer AGACCCAAGCATAGAGAGCCAG, and probe CCCACCCCGCCTGTGTTTTTGTGT.
Statistical analysis.
Data are given as percentages, absolute numbers, and means ± SD. Nominal data were analyzed by using chi-square test metric data with nonparametric tests. A P value of <0.05 was considered significant in all analyses. Analyses were performed with SPSS.
ACKNOWLEDGMENTS
This study was supported by grant IFB-01EO0901 from the BMBF (German Ministry for Education and Research).
We thank Ahmed Messoudi and Christine Glombik for excellent technical assistance. We thank Katie Göttlinger and Judy Benson for copyediting the manuscript.
We declare that we have no conflict of interest.
REFERENCES
- 1.Strupp M, Brandt T. 1999. Vestibular neuritis. Adv Otorhinolaryngol 55:111–136. [DOI] [PubMed] [Google Scholar]
- 2.Sekitani T, Imate Y, Noguchi T, Inokuma T. 1993. Vestibular neuronitis: epidemiological survey by questionnaire in Japan. Acta Otolaryngol Suppl 503:9–12. doi: 10.3109/00016489309128061. [DOI] [PubMed] [Google Scholar]
- 3.Adamec I, Krbot Skoric M, Handzic J, Habek M. 2015. Incidence, seasonality and comorbidity in vestibular neuritis. Neurol Sci 36:91–95. doi: 10.1007/s10072-014-1912-4. [DOI] [PubMed] [Google Scholar]
- 4.Strupp M, Brandt T. 2009. Vestibular neuritis. Semin Neurol 29:509–519. doi: 10.1055/s-0029-1241040. [DOI] [PubMed] [Google Scholar]
- 5.Strupp M, Magnusson M. 2015. Acute unilateral vestibulopathy. Neurol Clin 33:669–685. doi: 10.1016/j.ncl.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Arbusow V, Schulz P, Strupp M, Dieterich M, von Reinhardstoettner A, Rauch E, Brandt T. 1999. Distribution of herpes simplex virus type 1 in human geniculate and vestibular ganglia: implications for vestibular neuritis. Ann Neurol 46:416–419. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Arbusow V, Strupp M, Wasicky R, Horn AK, Schulz P, Brandt T. 2000. Detection of herpes simplex virus type 1 in human vestibular nuclei. Neurology 55:880–882. doi: 10.1212/WNL.55.6.880. [DOI] [PubMed] [Google Scholar]
- 8.Theil D, Arbusow V, Derfuss T, Strupp M, Pfeiffer M, Mascolo A, Brandt T. 2001. Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Brain Pathol 11:408–413. doi: 10.1111/j.1750-3639.2001.tb00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol 163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbusow V, Derfuss T, Held K, Himmelein S, Strupp M, Gurkov R, Brandt T, Theil D. 2010. Latency of herpes simplex virus type-1 in human geniculate and vestibular ganglia is associated with infiltration of CD8+ T cells. J Med Virol 82:1917–1920. doi: 10.1002/jmv.21904. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RL, McGarvie LA, Reid N, Young AS, Halmagyi GM, Welgampola MS. 2016. Vestibular neuritis affects both superior and inferior vestibular nerves. Neurology 87:1704–1712. doi: 10.1212/WNL.0000000000003223. [DOI] [PubMed] [Google Scholar]
- 12.Magliulo G, Gagliardi S, Ciniglio Appiani M, Iannella G, Re M. 2014. Vestibular neurolabyrinthitis: a follow-up study with cervical and ocular vestibular evoked myogenic potentials and the video head impulse test. Ann Otol Rhinol Laryngol 123:162–173. doi: 10.1177/0003489414522974. [DOI] [PubMed] [Google Scholar]
- 13.Chihara Y, Iwasaki S, Murofushi T, Yagi M, Inoue A, Fujimoto C, Egami N, Ushio M, Karino S, Sugasawa K, Yamasoba T. 2012. Clinical characteristics of inferior vestibular neuritis. Acta Otolaryngol 132:1288–1294. doi: 10.3109/00016489.2012.701326. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Kim HJ. 2012. Inferior vestibular neuritis. J Neurol 259:1553–1560. doi: 10.1007/s00415-011-6375-4. [DOI] [PubMed] [Google Scholar]
- 15.Gianoli G, Goebel J, Mowry S, Poomipannit P. 2005. Anatomic differences in the lateral vestibular nerve channels and their implications in vestibular neuritis. Otol Neurotol 26:489–494. doi: 10.1097/01.mao.0000169787.99835.9f. [DOI] [PubMed] [Google Scholar]
- 16.Buchele W, Brandt T. 1988. Vestibular neuritis—a horizontal semicircular canal paresis? Adv Otorhinolaryngol 42:157–161. [DOI] [PubMed] [Google Scholar]
- 17.Fetter M, Dichgans J. 1996. Vestibular neuritis spares the inferior division of the vestibular nerve. Brain 119(Part 3):755–763. [DOI] [PubMed] [Google Scholar]
- 18.Baringer JR, Swoveland P. 1973. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med 288:648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- 19.Stevens JG, Cook ML. 1971. Latent herpes simplex virus in spinal ganglia of mice. Science 173:843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 20.Croen KD, Ostrove JM, Dragovic LJ, Smialek JE, Straus SE. 1987. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene “anti-sense” transcript by in situ hybridization. N Engl J Med 317:1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- 21.Steiner I, Kennedy PG, Pachner AR. 2007. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol 6:1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- 22.Steiner I, Kennedy PG. 1995. Herpes simplex virus latent infection in the nervous system. J Neurovirol 1:19–29. doi: 10.3109/13550289509111007. [DOI] [PubMed] [Google Scholar]
- 23.Whitley RJ. 1988. Herpes simplex virus infections of the central nervous system. A review. Am J Med 85:61–67. doi: 10.1016/0002-9343(88)90389-0. [DOI] [PubMed] [Google Scholar]
- 24.Bergstrom B. 1973. Morphology of the vestibular nerve. I. Anatomical studies of the vestibular nerve in man. Acta Otolaryngol 76:162–172. doi: 10.3109/00016487309121495. [DOI] [PubMed] [Google Scholar]
- 25.Kodukula P, Liu T, Rooijen NV, Jager MJ, Hendricks RL. 1999. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol 162:2895–2905. [PubMed] [Google Scholar]
- 26.Lima GK, Zolini GP, Mansur DS, Freire Lima BH, Wischhoff U, Astigarraga RG, Dias MF, das Gracas Almeida Silva M, Bela SR, do Valle Antonelli LR, Arantes RM, Gazzinelli RT, Bafica A, Kroon EG, Campos MA. 2010. Toll-like receptor (TLR) 2 and TLR9 expressed in trigeminal ganglia are critical to viral control during herpes simplex virus 1 infection. Am J Pathol 177:2433–2445. doi: 10.2353/ajpath.2010.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuest TR, Thapa M, Zheng M, Carr DJ. 2011. CXCL10 expressing hematopoietic-derived cells are requisite in defense against HSV-1 infection in the nervous system of CXCL10 deficient mice. J Neuroimmunol 234:103–108. doi: 10.1016/j.jneuroim.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mott K, Brick DJ, van Rooijen N, Ghiasi H. 2007. Macrophages are important determinants of acute ocular HSV-1 infection in immunized mice. Invest Ophthalmol Vis Sci 48:5605–5615. doi: 10.1167/iovs.07-0894. [DOI] [PubMed] [Google Scholar]
- 29.Wersall J. 1956. Studies on the structure and innervation of the sensory epithelium of the cristae ampulares in the guinea pig; a light and electron microscopic investigation. Acta Otolaryngol Suppl 126:1–85. [PubMed] [Google Scholar]
- 30.Ballantyne J, Engstrom H. 1969. Morphology of the vestibular ganglion cells. J Laryngol Otol 83:19–42. doi: 10.1017/S002221510007002X. [DOI] [PubMed] [Google Scholar]
- 31.Morgenstein KM, Seung HI. 1971. Vestibular neuronitis. Laryngoscope 81:131–139. doi: 10.1288/00005537-197101000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Schuknecht HF, Kitamura K. 1981. Second Louis H. Clerf lecture. Vestibular neuritis. Ann Otol Rhinol Laryngol Suppl 90:1–19. [PubMed] [Google Scholar]
- 33.Furuta Y, Takasu T, Fukuda S, Inuyama Y, Sato KC, Nagashima K. 1993. Latent herpes simplex virus type 1 in human vestibular ganglia. Acta Otolaryngol Suppl 503:85–89. doi: 10.3109/00016489309128081. [DOI] [PubMed] [Google Scholar]
- 34.Furuta Y, Takasu T, Sato KC, Fukuda S, Inuyama Y, Nagashima K. 1992. Latent herpes simplex virus type 1 in human geniculate ganglia. Acta Neuropathol 84:39–44. doi: 10.1007/BF00427213. [DOI] [PubMed] [Google Scholar]
- 35.Davis LE. 1993. Viruses and vestibular neuritis: review of human and animal studies. Acta Otolaryngol Suppl 503:70–73. doi: 10.3109/00016489309128077. [DOI] [PubMed] [Google Scholar]
- 36.Hirata Y, Gyo K, Yanagihara N. 1995. Herpetic vestibular neuritis: an experimental study. Acta Otolaryngol Suppl 519:93–96. doi: 10.3109/00016489509121878. [DOI] [PubMed] [Google Scholar]
- 37.Arbusow V, Theil D, Strupp M, Mascolo A, Brandt T. 2001. HSV-1 not only in human vestibular ganglia but also in the vestibular labyrinth. Audiol Neurootol 6:259–262. doi: 10.1159/000046131. [DOI] [PubMed] [Google Scholar]
- 38.Schulz P, Arbusow V, Strupp M, Dieterich M, Rauch E, Brandt T. 1998. Highly variable distribution of HSV-1-specific DNA in human geniculate, vestibular and spiral ganglia. Neurosci Lett 252:139–142. doi: 10.1016/S0304-3940(98)00573-4. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki S. 1996. Detection of latent herpes simplex virus in human vestibular ganglia. Hokkaido Igaku Zasshi 71:561–571. (In Japanese.) [PubMed] [Google Scholar]
- 40.Goebel JA, O'Mara W, Gianoli G. 2001. Anatomic considerations in vestibular neuritis. Otol Neurotol 22:512–518. doi: 10.1097/00129492-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Curthoys IS. 2012. The interpretation of clinical tests of peripheral vestibular function. Laryngoscope 122:1342–1352. doi: 10.1002/lary.23258. [DOI] [PubMed] [Google Scholar]
- 42.Halmagyi GM, Aw ST, Karlberg M, Curthoys IS, Todd MJ. 2002. Inferior vestibular neuritis. Ann N Y Acad Sci 956:306–313. doi: 10.1111/j.1749-6632.2002.tb02829.x. [DOI] [PubMed] [Google Scholar]
- 43.Murofushi T, Halmagyi GM, Yavor RA, Colebatch JG. 1996. Absent vestibular evoked myogenic potentials in vestibular neurolabyrinthitis. An indicator of inferior vestibular nerve involvement? Arch Otolaryngol Head Neck Surg 122:845–848. doi: 10.1001/archotol.1996.01890200035008. [DOI] [PubMed] [Google Scholar]
- 44.Forge A, Wright T. 2002. The molecular architecture of the inner ear. Br Med Bull 63:5–24. doi: 10.1093/bmb/63.1.5. [DOI] [PubMed] [Google Scholar]