ABSTRACT
Mosquito-borne arboviruses are a major source of human disease. One strategy to reduce arbovirus disease is to reduce the mosquito's ability to transmit virus. Mosquito infection with the bacterial endosymbiont Wolbachia pipientis wMel is a novel strategy to reduce Aedes mosquito competency for flavivirus infection. However, experiments investigating cyclic environmental temperatures have shown a reduction in maternal transmission of wMel, potentially weakening the integration of this strain into a mosquito population relative to that of other Wolbachia strains. Consequently, it is important to investigate additional Wolbachia strains. All Zika virus (ZIKV) suppression studies are limited to the wMel Wolbachia strain. Here we show ZIKV inhibition by two different Wolbachia strains: wAlbB (isolated from Aedes albopictus mosquitoes) and wStri (isolated from the planthopper Laodelphax striatellus) in mosquito cells. Wolbachia strain wStri inhibited ZIKV most effectively. Single-cycle infection experiments showed that ZIKV RNA replication and nonstructural protein 5 translation were reduced below the limits of detection in wStri-containing cells, demonstrating early inhibition of virus replication. ZIKV replication was rescued when Wolbachia was inhibited with a bacteriostatic antibiotic. We observed a partial rescue of ZIKV growth when Wolbachia-infected cells were supplemented with cholesterol-lipid concentrate, suggesting competition for nutrients as one of the possible mechanisms of Wolbachia inhibition of ZIKV. Our data show that wAlbB and wStri infection causes inhibition of ZIKV, making them attractive candidates for further in vitro mechanistic and in vivo studies and future vector-centered approaches to limit ZIKV infection and spread.
IMPORTANCE Zika virus (ZIKV) has swiftly spread throughout most of the Western Hemisphere. This is due in large part to its replication in and spread by a mosquito vector host. There is an urgent need for approaches that limit ZIKV replication in mosquitoes. One exciting approach for this is to use a bacterial endosymbiont called Wolbachia that can populate mosquito cells and inhibit ZIKV replication. Here we show that two different strains of Wolbachia, wAlbB and wStri, are effective at repressing ZIKV in mosquito cell lines. Repression of virus growth is through the inhibition of an early stage of infection and requires actively replicating Wolbachia. Our findings further the understanding of Wolbachia viral inhibition and provide novel tools that can be used in an effort to limit ZIKV replication in the mosquito vector, thereby interrupting the transmission and spread of the virus.
KEYWORDS: Wolbachia, Zika virus, arthropod vectors, vector biology
INTRODUCTION
Arboviruses are zoonotic viruses transmitted by arthropods. A large number of arboviruses are human pathogens, including dengue virus (DENV), West Nile virus (WNV), Chikungunya virus (CHIKV), and yellow fever virus (YFV). Among arboviruses that cause human disease, 90% are transmitted by mosquitoes (1). Mosquitoes are an effective vector for rapidly disseminating arboviral diseases around the globe. This has been evidenced by the spread of DENV, WNV, and CHIKV into the Western Hemisphere. Zika virus (ZIKV) is the fourth arbovirus to cause a Western Hemisphere epidemic in the last 20 years (2). Trying to control the spread of these diseases through control of the mosquito vector has long been attempted, with mixed results. Traditional attempts to suppress arboviruses include mosquito population control through insecticides (1, 3). There have been many successes, but eradication is limited by the mosquito's resistance to insecticides and widespread population dynamics (3). The irregular success of insecticide-based arbovirus eradication strategies has highlighted the need for alternative strategies.
One promising alternative strategy is to limit arbovirus transmission and spread through the use of endosymbiotic bacteria, such as Wolbachia pipientis (4). Wolbachia organisms are obligate intracellular bacterial endosymbionts of arthropods and nematodes. Wolbachia bacteria are maternally transmitted and affect host reproductive phenotypes. This allows efficient integration into a population (5, 6). As a result, it is estimated that up to 40% of all insects are infected with diverse strains of Wolbachia (7). Wolbachia strains which have been investigated for the ability to inhibit arboviruses span two major phylogenetic clades (supergroup A and supergroup B) (8). Wolbachia strains from both clades cultured in Aedes mosquito cells have been shown to inhibit the replication of viral pathogens (9–14).
Wolbachia-infected Aedes mosquitoes have a strong resistance to infection with various arboviruses. wMelPop is a pathogenic strain of Wolbachia native to Drosophila melanogaster which broadly inhibits DENV (11), CHIKV (11, 15), YFV (15), and WNV (12). However, the extreme density of wMelPop has too large of a fitness cost to the mosquito to successfully integrate into the mosquito population (13). This led to the investigation of other Wolbachia strains that do not overgrow in the mosquito host.
Wolbachia strain wMel, also indigenous to D. melanogaster, does not have the fitness costs incurred by wMelPop (6, 16, 17). Nevertheless, introduction of wMel into the Aedes mosquito host (10, 16, 18) also limits DENV (10, 19, 20), ZIKV (21, 22), and CHIKV (23) infections. wMel's lower density causes a limited reduction in fitness of the Aedes host, but the strain is less effective at reducing viral titers than wMelPop (10, 15, 16, 18). Field trials investigating the integration of wMel and suppression of DENV are currently being conducted (13, 20, 24). While some models predict successful control of arboviruses by Wolbachia strain wMel (6), concerns have been raised that over time, ongoing evolutionary adaptations of Wolbachia/vector/virus interactions may undermine the long-term effectiveness of wMel to control arboviruses (25). For example, recent studies suggest that wMel may struggle to integrate into large mosquito populations (26). This is due to a loss of maternal transmission and density and a reduced ability to induce cytoplasmic compatibility at tropical cyclic temperatures simulated in laboratory experiments (26). As a result, strategies employing alternative Wolbachia strains, which effectively reduce viral titers without large host fitness costs, have been suggested to improve the efforts of a Wolbachia-mediated suppression strategy (27).
In this study, we investigated two Wolbachia strains belonging to supergroup B, wAlbB and wStri, to determine if they effectively inhibit ZIKV in vitro. wAlbB has been reported to have opposing phenotypes for different viruses in different mosquito hosts (28–31), making it an interesting Wolbachia strain for study. wStri of the leafhopper Laodelphax striatella has also been established in Aedes albopictus culture (32), making it potentially useful for future vector suppression approaches, yet it has never been studied in the context of arboviruses. Our results show that both Wolbachia strains inhibit ZIKV in A. albopictus cells. Our data for wStri-infected cells demonstrate the early inhibition of virus infection prior to or at transcription and translation of virus. We also provide evidence that competition for cholesterol and/or other lipids plays a partial role in the suppression of viral infection in Aedes cells harboring the endosymbiont Wolbachia. We conclude that wStri and wAlbB are effective inhibitors of ZIKV.
RESULTS
Phylogenetically distinct Wolbachia strains, wStri and wAlbB, successfully established in mosquito cell cultures inhibit ZIKV in vitro.
Previous work showed that Wolbachia strain wMel, belonging to supergroup A (33), inhibits ZIKV in vivo (21, 22). To increase the repertoire of Wolbachia strains available for ZIKV control and to develop an in vitro system amenable to high-throughput approaches, we investigated whether the wAlbB and wStri strains of Wolbachia were capable of restricting ZIKV infection in mosquito cells. These strains are phylogenetically distant from the wMel and wMelPop strains (Fig. 1A). wStri and wAlbB form a clade with wPip, consistent with previous reports defining each of these strains as group B Wolbachia strains (34) (Fig. 1A). Because wAlbB and wStri are adapted to Aedes cell culture, we investigated them further to determine if they are candidates for ZIKV control.
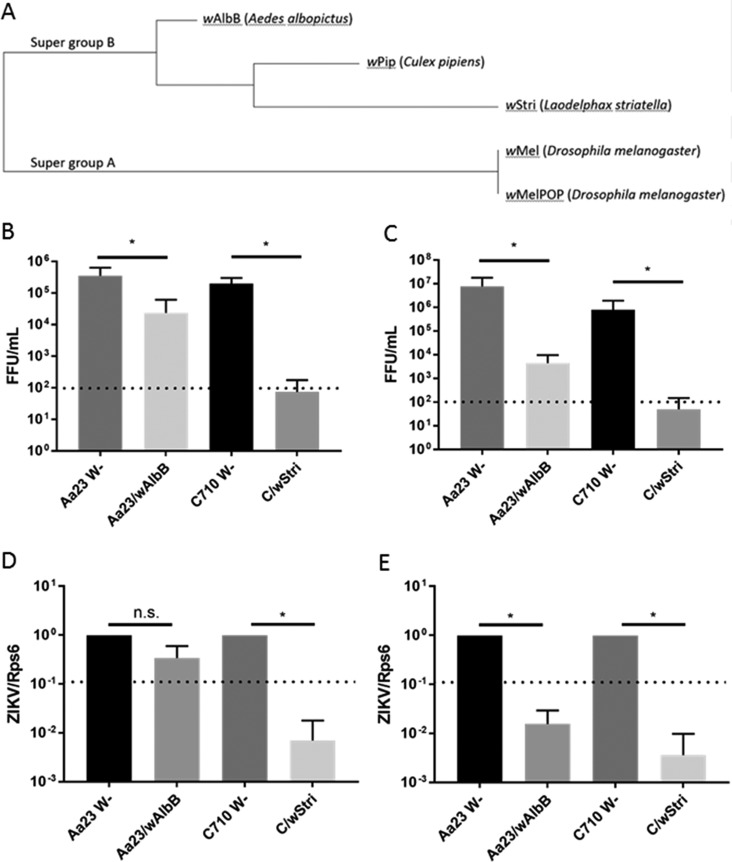
FIG 1.
Phylogenetically distinct Wolbachia strains, wStri and wAlbB, significantly inhibit ZIKV production of infectious virions in A. albopictus cells. (A) Phylogenetic analysis was performed on five concatenated multilocus sequence typing genes (coxA, fbpA, ftsZ, gatB, and hcpA) (33) by MUSCLE alignment and the Tamura-Nei model to produce a maximum likelihood tree in MEGA6 (48). (B) A. albopictus cells (Aa23 W− and C710 W−) produced >105 infectious units/ml after initial infection at an MOI of 0.01. Wolbachia strain wAlbB significantly reduced ZIKV MR766 in each experiment (P < 0.013 for each experiment). Wolbachia strain wStri inhibited ZIKV MR766 in each experiment (P < 0.016 for each experiment). Statistical significance was determined using the Holm-Sidak method, with an alpha value of 0.05. Each experiment was analyzed individually, without assuming a consistent standard deviation. Statistical tests were calculated by GraphPad Prism. Data shown are means and standard deviations of three independent experiments with a minimum of two technical replicates each. (C) Wolbachia strain wAlbB significantly reduced ZIKV PRVABC FFU in each experiment (P < 0.05 for each experiment). Wolbachia strain wStri significantly inhibited ZIKV PRVABC in each experiment (P < 0.01 for each experiment). Statistical significance was determined using the Holm-Sidak method, with an alpha value of 0.05. Each experiment was analyzed individually, without assuming a consistent standard deviation. Statistical tests were calculated by GraphPad Prism. Data shown are means and standard deviations from three independent experiments with a minimum of two technical replicates each. The dotted line represents the limit of detection. (D) After infection at an MOI of 0.01, cells were incubated for 5 days. Cells were assayed for viral genome by qRT-PCR. The limit of detection was determined based on a no-input control. wAlbB did not significantly reduce ZIKV MR766 viral genome copies (P > 0.05). wStri reduces ZIKV MR766 genome copies to undetectable levels (P < 0.05 for C710 compared to C/wStri). Statistical significance was determined by a ratio-paired t test. Statistics were calculated on the collective of three independent experiments. Statistical tests were calculated by GraphPad Prism. Data are means from three independent experiments with no less than two technical replicates each. n.s., not significant. (E) After infection with PRVABC59 at an MOI of 0.01, cells were incubated for 5 days and assayed for viral genome by qRT-PCR. wAlbB and wStri significantly reduced ZIKV PRVABC59 below the limit of detection (dotted line) determined by a no input control (P < 0.05 for both strains compared to their respective Wolbachia-free lines). Statistical significance was determined by a ratio-paired t test. Statistics were calculated on the collective of three independent experiments. Statistical tests were calculated by GraphPad Prism. Data are means from three independent experiments with no less than two technical replicates each. *, P < 0.05.
While no investigations have pursued wStri, wAlbB has been shown to inhibit DENV, a relative of ZIKV, in Aedes mosquitoes (27). Group B Wolbachia strains have been shown to have an inhibitory effect on DENV growth in A. albopictus (14, 24). Thus, we hypothesized wAlbB and wStri may inhibit ZIKV. To investigate this hypothesis, we utilized A. albopictus cells with persistent Wolbachia infection. Aa23 wAlbB-infected cells and their respective Wolbachia-free cell line (Aa23 W−), as well as C/wStri-infected cells and their respective Wolbachia-free line (C710 W−), were infected with ZIKV at a low multiplicity of infection (MOI), i.e., 0.01. After 5 days of incubation, titers were determined by focus forming assay (FFA). Wolbachia strain wAlbB significantly inhibited titers of African strain ZIKV MR766, by approximately 1 log (93%), from a mean of 3.5 × 105 to 2.4 × 104 focus-forming units (FFU) per ml (Fig. 1B). wAlbB-containing mosquito cells were also resistant to infection with a clinical isolate of the Asian lineage of ZIKV. Puerto Rican strain PRVABC59, isolated in 2016, produced fewer infectious virions in wAlbB-infected cells than in wAlbB-free cells, from 7.6 × 107 to 4.4 × 103 FFU/ml (Fig. 1C). Cells infected with Wolbachia strain wStri also showed significantly less replication of ZIKV MR766, with a titer from wStri-free cells of 2.0 × 105, compared to a titer of 7.3 × 101 from cells containing wStri (Fig. 1B). There was a similar decrease in replication of ZIKV PRVABC, from 7.8 × 105 in wStri-free cells to 4.9 × 101 in cells containing wStri (Fig. 1C). Three independent experiments showed ZIKV titers in wStri-infected cells of ∼102, representing repression close to or at the limit of detection in this assay. wStri consistently reduced titers below the limit of detection, while wAlbB cells always grew low levels of virus (Fig. 1B and C).
We confirmed these findings by quantitative reverse transcription-PCR (qRT-PCR) of cells to determine the production of viral genome copies. We first looked at viral genome copies by infecting Wolbachia-free and Wolbachia-infected cells with ZIKV at a low multiplicity of infection (MOI = 0.01). After 5 days of incubation, cell lysates were collected and analyzed by quantitative RT-PCR. ZIKV MR766 was only nonsignificantly reduced, from a cycle threshold (CT) of ∼19 in Wolbachia-free Aa23 cells to a CT of ∼22 in Aa23 cells infected with wAlbB, consistent with the 1-log reduction observed by focus forming assay (Fig. 1B and D). ZIKV MR766 RNA was significantly reduced, from a CT of ∼17 in Wolbachia-free C710 cells to a CT of 27 or equivalent to that of the no-template control (termed undetectable) in C/wStri cells (Fig. 1D). ZIKV MR766 genome copies were significantly restricted in C/wStri cells compared to C710 W− cells, consistent with the reduction observed by focus forming assay (Fig. 1B and D). Wolbachia wAlbB and wStri had a robust inhibitory effect on ZIKV PRVABC59 (Fig. 1E). ZIKV PRVABC59 RNA was significantly reduced, from a CT of ∼17 in Aa23 cells to a CT of ∼25 in wAlbB-infected cells. Likewise, a reduction of CT of ∼16 in C710 cells to CT of 29 or undetectable in C/wStri cells was observed (Fig. 1E). Both wAlbB-infected cells and wStri-infected cells showed significantly reduced ZIKV PRVABC59 viral genome copies, to below the limit of detection, showing a viral-strain-specific effect (Fig. 1C and E). Overall, the data suggest that wStri is more effective at reducing ZIKV MR766 and PRVABC59 titers than wAlbB.
Wolbachia wAlbB and wStri cell lines are infected at similar frequencies yet different densities.
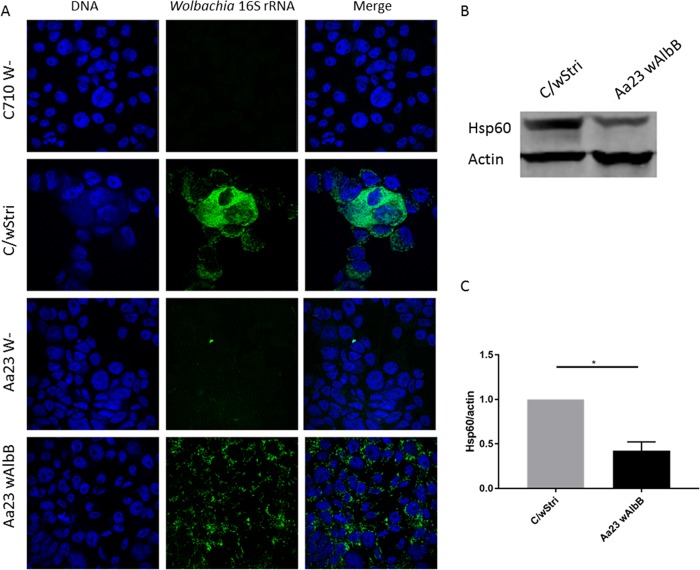
To better characterize the Wolbachia wAlbB- and wStri-infected cells, we determined the frequency of Wolbachia infection in each cell line by imaging. Fluorescent in situ hybridization by two different probes showed that each Wolbachia strain infected 94% or more cells in each cell line (Table 1; Fig. 2A). Wolbachia density has been demonstrated to be a determinant of DENV inhibition (35). Thus, we hypothesized that the stronger inhibition by wStri might be attributed to the higher density of this Wolbachia strain than of wAlbB in cells. We quantitated Wolbachia density by Hsp60 band intensity (Fig. 2B and C). wAlbB-infected cells showed a density 2 to 3 times lower than that of wStri-infected cells (Fig. 2C). Wolbachia density in a host is predetermined by the genetics of the Wolbachia strain (36). We therefore hypothesize that Wolbachia strain density caused by strain-specific interactions with their host confers an antiviral phenotype. Reduction of wStri density may reduce viral protection, while increasing wAlbB density may increase the antiviral phenotype to that observed in wStri-infected cells.
TABLE 1.
Frequency of Wolbachia infections in cell linesa
| Cell line | No. of cells infected/total (%) as determined with: |
|
|---|---|---|
| Probe 1 | Probe 2 | |
| C/wStri | 125/129 (96.9) | 48/51 (94.1) |
| Aa23 wALbB | 112/114 (98.2) | 161/162 (99.4) |
Using the DIC channel to delineate cell membranes, cells were counted and recorded as Wolbachia infected or Wolbachia free to determine the frequency of Wolbachia infection in each cell line. Two different Wolbachia 16S rRNA probes were used to confirm the reliability of this technique across two independent experiments.
FIG 2.
Wolbachia wAlbB and wStri lines are infected at similar frequencies but different densities. (A) Fluorescent in situ hybridization of Aa23 cells with and without wAlbB and C710 cells with and without wStri. Using the differential interference contrast (DIC) channel to delineate cell barriers, cells were counted and recorded as Wolbachia infected or Wolbachia free to determine the frequency of Wolbachia infection in each cell line. Data are recorded in Table 1. (B) Western blot to quantitate Wolbachia density (Hsp60) relative to host (actin) proteins. (C) Hsp60 normalized to actin band intensity was quantified for three independent experiments to compare wStri density to wAlbB density in A. albopictus cells (significance, P < 0.05). wStri density is normalized to 1 for each experiment to compare wAlbB density. Statistical significance was determined by paired t test (one-tailed; alpha = 0.05) on the natural log of the (Hsp60/actin) ratio accounting for nonnormal distribution of fluorescent intensities. Statistical tests were calculated by GraphPad Prism.
Tetracycline treatment reduces Wolbachia titer, rescuing ZIKV growth.
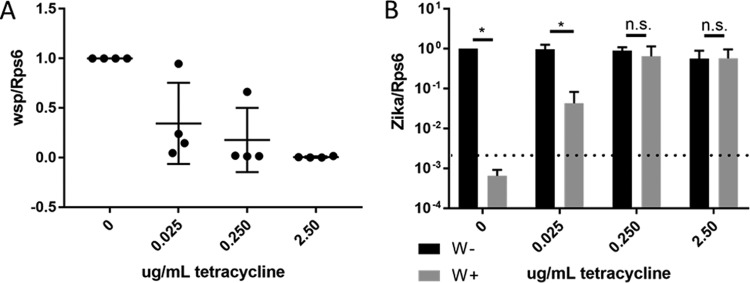
To further investigate the role of Wolbachia, we asked if the rescue of ZIKV growth is dependent on the density of Wolbachia present in ZIKV-challenged cells. Treatment of wStri-containing cells with increasing concentrations of tetracycline demonstrated a reduction in Wolbachia titers from a mean of six copies of Wolbachia surface protein (wsp) per host ribosomal protein (Rps6) to less than one copy of wsp per 20 copies of Rps6 (Fig. 3A). When these cells were infected with ZIKV, there was an inverse dose-dependent increase in ZIKV replication rescue (Fig. 3A and B). ZIKV RNA copies in C/wStri cells treated with tetracycline increased from undetectable concentrations to CTs of 18 to 22, similar to the Wolbachia-free C710 control CT of 20 to 22 (Fig. 3B). Thus, ZIKV inhibition in the C/wStri line is due to the presence of Wolbachia directly, rather than a result of epigenetic or genetic changes caused by Wolbachia that altered the cells' permissiveness.
FIG 3.
Removal of Wolbachia from C/wStri cells rescues ZIKV growth. (A) Tetracycline dose response of Wolbachia as determined by Wolbachia surface protein, quantified by qRT-PCR. Wolbachia concentration was normalized to nontreatment conditions. Data shown are means from three independent experiments with no less than two technical replicates each. (B) Tetracycline dose response of C/wStri cells treated with tetracycline after infection with ZIKV PRVABC. ZIKV infection is significantly reduced in wStri-infected cells at 0 and 0.025 μg/ml of tetracycline (P < 0.001 and P < 0.005, respectively). At tetracycline concentrations of 0.25 μg/ml and 2.5 μg/ml, ZIKV infection is not significantly different from W- comparable treatment (P > 0.05), demonstrating a Wolbachia-specific inhibition in C/wStri cells. Statistical significance was independently calculated by Student's t test to determine if ZIKV was reduced in Wolbachia-infected cells under each dose of tetracycline. Discovery was determined using the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli, with a Q value of 1%. Statistical tests were calculated by GraphPad Prism. Each tetracycline concentration was analyzed individually, without assuming a consistent standard deviation. Data were normalized to C710 W− (no treatment). Data are the means and standard deviations from three independent experiments.
Wolbachia wStri inhibits ZIKV early in viral infection.
We next wanted to investigate the stage of viral infection which is blocked by Wolbachia to gain mechanistic insight. Toward this goal, we chose the strain with the most robust effect on virus growth, wStri. We divided the virus life cycle into two phases to investigate a stage of viral inhibition: (i) viral entry, transcription, and translation and (ii) assembly and release of mature virions (Fig. 4A). The previous results demonstrated Wolbachia inhibition of the production of viral genome and infectious particles at a low multiplicity of infection (MOI = 0.01) (Fig. 1). To determine if C/wStri cells were highly resistant to ZIKV infection, we next infected cells at a high multiplicity of infection (MOI = 10) and assayed the production of infectious units by FFA. We found a consistent significant reduction in FFU in C/wStri cells compared to C710 cells (P < 0.05) (Fig. 4B). We investigated differences in virus production upstream of the production of infectious particles to determine if there is an early block of virus production by infecting C710 and C/wStri cells with ZIKV PRVABC59 at a high multiplicity of infection (MOI =10) and analyzing viral genome copies by qRT-PCR 3 days postinfection. We observed a consistent significant reduction in viral genome copies, from a CT of ∼14 in C710 cells to a CT of ∼26 in C/wStri cells (Fig. 4C).
FIG 4.
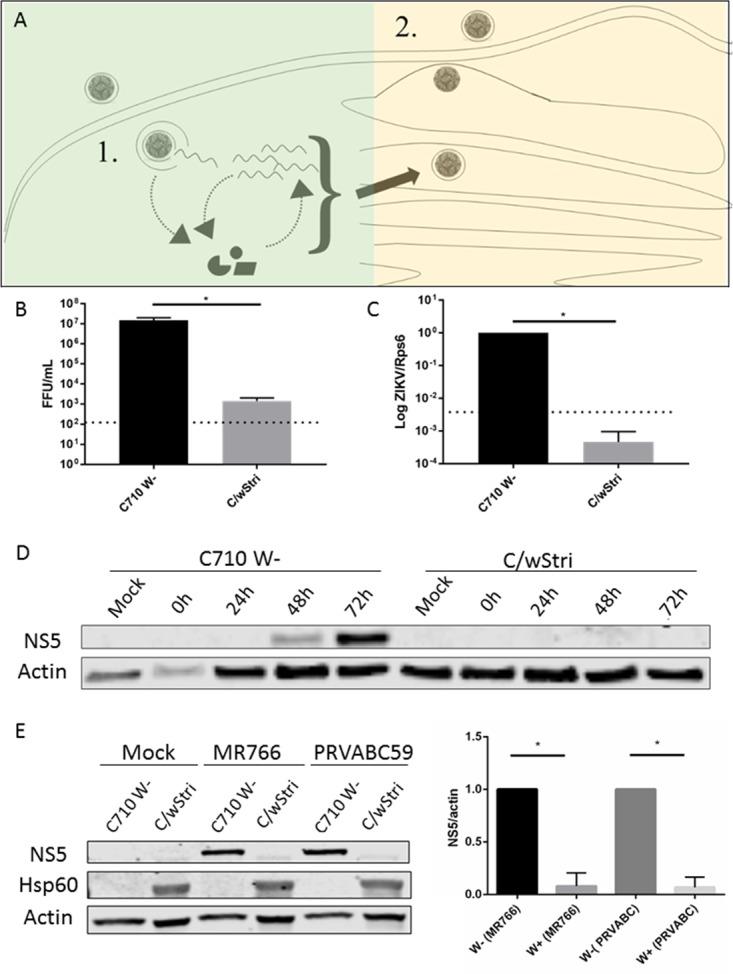
wStri inhibits ZIKV early in virus infection. (A) Isolation of ZIKV life cycle: viral entry, transcription, and translation (1) and assembly and release of mature virions (2). Quantitative RT-PCR and Western blotting were used to assess early infection. Focus forming assay assessed late infection. (B) Infectious virus was assayed by focus forming assay 3 days postinfection at a high multiplicity of infection (MOI = 10) (P < 0.05). Statistical significance was determined by a paired t test. Statistics were calculated on the collective of three independent experiments. Statistical tests were calculated by GraphPad Prism. (C) After infection at an MOI of 10, cells were incubated for 3 days. Cells were assayed for viral genome by qRT-PCR. wStri reduces ZIKV PRVABC59 genome copies to undetectable levels (P < 0.05). Statistical significance was determined by a ratio-paired t test on the collective of three independent experiments calculated by GraphPad Prism. Data are means from three independent experiments with no less than two technical replicates each. (D) C710 and C/wStri cells were assayed for NS5 production by Western blotting 1, 2, and 3 days postinfection at MOI = 10. (E) ZIKV MR766 and PRVABC NS5 expression is significantly reduced following a high multiplicity of infection (MOI = 10) in wStri-infected cells (P < 0.05 for both ZIKV strains). Statistical significance was determined by a one-tailed ratio-paired t test under the assumption that samples are from a population where the log of the ratios follows a Gaussian distribution. Statistics were calculated on the collective of three independent experiments. Statistical tests were calculated by GraphPad Prism. Data are means and standard deviations from three independent experiments.
Viral RNA is replicated by nonstructural protein 5 (NS5), a viral protein not packaged in the infectious virion. We assessed early virus infection by analyzing NS5 translation by Western blotting. Cells were infected with ZIKV PRVABC59 at a high MOI (MOI = 10), restricting our analysis to fewer rounds of virus amplification, and collected after 1, 2, and 3 days. C/wStri cells infected with ZIKV MR766 translated undetectable amounts of NS5 at all time points (Fig. 4D). Wolbachia-free C710 cells produced significantly more NS5, with the most robust signal at 3 days postinfection (Fig. 4D). We repeated this analysis with both ZIKV strains MR766 and PRVABC59 for three independent experiments to quantitate ZIKV NS5 in C710 and C/wStri cells. Consistent with the time course, ZIKV NS5 was significantly reduced in C/wStri cells (Fig. 4E). Hsp60 indicated Wolbachia infection in the C710 cells (Fig. 4E). In conclusion, Wolbachia wStri inhibits ZIKV at or prior to translation and genome replication.
Addition of cholesterol-lipid supplement partially rescues ZIKV growth in C/wStri cells.
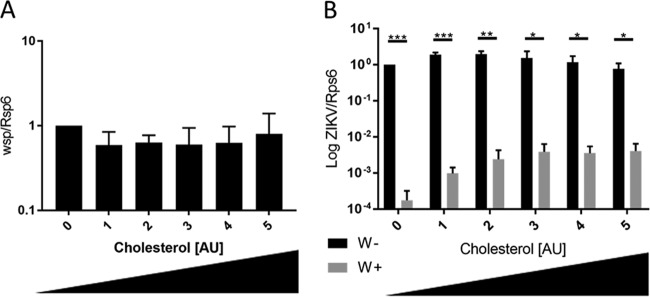
Wolbachia inhibition of Drosophila C virus (DCV) has been shown to be mediated by competition for cholesterol (37). When cholesterol is added to the flies' diet, DCV grows to lethal titers in the presence of Wolbachia (37). Cholesterol is important for several steps in flavivirus infection, including entry early in infection (38). To determine if the Drosophila observed phenotype translates to a human pathogen, we investigated if the addition of cholesterol would rescue ZIKV growth in Wolbachia. We infected cells at an MOI of 0.01 with ZIKV PRVABC59 for 1 h. A cholesterol-lipid supplement designed for cell culture was added in increasing concentrations. After 6 days, we observed no change in Wolbachia density quantitated by qRT-PCR (Fig. 5A). However, ZIKV RNA was increased >1 log with the addition of the cholesterol-lipid solution (Fig. 5B). This result strongly implies a role for the competition for cholesterol and/or other lipids in limiting ZIKV infection in cells as a result of persistent Wolbachia infection.
FIG 5.
Addition of cholesterol-lipid supplement partially rescues ZIKV growth in C/wStri cells. (A) Wolbachia density quantitated by wsp is unchanged by the addition of cholesterol concentrate. Data are normalized to no-treatment control. (B) ZIKV RNA is partially rescued in wStri-infected cells relative to Wolbachia-free (C710) cells. Statistical significance was determined using the Holm-Sidak method, with an alpha value of 0.05. Each experiment was analyzed individually, without assuming a consistent standard deviation. Statistical tests were calculated by GraphPad Prism. Data are the means and standard deviations from three independent experiments with a minimum of two technical replicates each.
DISCUSSION
The data presented here show that ZIKV replication is compromised by two different Wolbachia strains. wAlbB reduced virus growth by 1 to 3 logs. wStri was more effective, consistently ablating growth below detection. This could be due to the different cell types the Wolbachia strains are transinfected into, C710 and Aa23. However, C710 and Aa23 cells are equally permissive to ZIKV growth, suggesting a Wolbachia strain-specific phenotype. This is to our knowledge the first report of wStri inhibition of any flavivirus.
An alternative hypothesis for the weaker inhibition by wAlbB is that wAlbB is native to A. albopictus. Nonnative Wolbachia strains have been reported to be more effective at pathogen inhibition than native Wolbachia strains in the native host. wAlbB restricts DENV dissemination in its native host, A. albopictus (29, 39), albeit to a lesser extent than wAlbB restricts DENV in A. aegypti, a nonnative host (27). Consistent with this hypothesis, transinfection of nonnative wMel into A. albopictus or A. aegypti induces a strong antiviral phenotype (17). Our data show a similar trend such that wStri, a Wolbachia strain nonnative to A. albopictus, is more effective at reducing ZIKV titers than wAlbB, a native Wolbachia endosymbiont of A. albopictus.
Differences in the native versus nonnative virus growth phenotype trends with different Wolbachia densities. wAlbB inhibition of DENV has been shown to be dependent on Wolbachia titer (35). wAlbB grows to a higher per-cell density in nonnative Aedes aegypti hosts and lower densities in its native A. albopictus host (35). We observed reduced titers of wAlbB relative to wStri in A. albopictus, consistent with a density-dependent phenotype. Higher densities, like that demonstrated by wMelPop (24) than for wMel, are more effective at reducing viral titers. Too high a Wolbachia titer comes with a fitness cost (13). It is important to next investigate if wStri reduces ZIKV in vivo and with limited transinfection fitness cost.
Mechanism of action.
The stage of flavivirus life cycle that is inhibited in Wolbachia-infected cells has not been identified. Our studies suggest that Wolbachia restriction of ZIKV occurs at an early step of infection. It is likely that many flaviviruses are restricted at this step based on flavivirus similarities. One study suggested that the alphavirus Semliki Forest virus is inhibited by Wolbachia early in viral inhibition (40), consistent with our data. Thus, Wolbachia may block many positive RNA viruses by the same mechanism.
Previous investigations into a mechanism of Wolbachia-mediated virus suppression have focused on molecular pathways in the immune system (41, 42) and on metabolic competition (37). We chose to look at the impact of cholesterol on virus growth in Wolbachia-infected cells because Wolbachia bacteria have been shown to compete with the host for cholesterol, lipids, and amino acids (43, 44). Wolbachia protection from Drosophila C virus is dependent on cholesterol in Drosophila (37). We found an increase in ZIKV growth in Wolbachia-infected cells but not Wolbachia-free cells when cholesterol-lipid concentrate was added to cell media.
This suggests a partial dependence on cholesterol and/or other lipids in Wolbachia-mediated virus inhibition. This competition may play a role in inhibiting viral entry since increased cholesterol has a strong impact on virus entry (45). However, additional mechanisms must also contribute to viral inhibition.
In vitro studies.
Cell lines offer a valuable tool to dissect molecular aspects of virus-host interactions. While C6/36 mosquito cells are permissive to ZIKV, other mosquito cell lines, such as CCL-125s, do not allow ZIKV growth (46). Often ZIKV is propagated in C6/36 cell lines, because they are defective in the antiviral RNA interference (RNAi) response (47). However, this also renders them limited for vector competence and RNAi screens. Here we demonstrate that two additional insect cell lines, Aa23 and C710, are competent for ZIKV. The availability of a cell line system to investigate Wolbachia ZIKV suppression offers a platform for future RNAi screens and other high-throughput approaches aimed at determining the mechanism and pathways of viral suppression by Wolbachia. This is the first evidence that Wolbachia bacteria block ZIKV in cell culture, showing that blockage occurs in a cell-autonomous manner independent of systemic immunity.
In vivo application.
Our results have implications for using Wolbachia to control arbovirus. The current Wolbachia-based strategy to inhibit arboviruses employs only wMel (4, 21). Multiple models have predicted successful establishment of wMel control (6, 19). Field trials are currently being conducted. However, there are concerns that wMel will adapt to its new Aedes host (25). This may cause loss of virus inhibition (25). Investigation of additional strains will provide additional tools to wMel to improve upon disease control strategies. Our data suggest that wStri and wAlbB to a lesser extent could also be used to inhibit ZIKV replication in mosquitoes.
Our study highlights two important aspects that support improving the utilization of Wolbachia to limit ZIKV transmission. The first is that the robust inhibition of ZIKV by wStri is optimal for the investigation of Wolbachia-arbovirus interaction. Understanding this mechanism of Wolbachia-caused virus suppression might allow direct targeting of arboviruses in vivo. Second, this work provides precedence for the use of wStri in vivo. Studies will need to be conducted to assess if wStri can successfully inhibit ZIKV in A. aegypti. Future studies should include the transinfection of wStri into A. aegypti in vitro to adapt to A. aegypti for in vivo approaches.
MATERIALS AND METHODS
Insect cell culture.
A. albopictus C710 and C/wStri1 cells were a kind gift from Ann Fallon. C/wStri cells were derived from C710 cells transinfected with Wolbachia pipientis wStri from the planthopper Laodelphax striatellus (32). C710 and C/wStri cells were grown at 28°C with 5% CO2 and subcultured weekly at a 1:5 dilution in E-5 medium as previously described (32).
A. albopictus Aa23 cells with and without a stable Wolbachia pipientis wAlbB infection were a kind gift from Zhiyong Xi. Aa23 cells are derived from A. albopictus with a natural Wolbachia pipientis wAlbB infection. Aa23TET cells were treated with tetracycline to remove Wolbachia as previously described (35). wAlbB-infected cells were subcultured weekly at a 1:5 dilution. Cells were grown in Schneider's medium with 10% tetracycline-tested fetal bovine serum (FBS), 50 μg/ml of penicillin, and 50 μg/ml of streptomycin at 28°C with 5% CO2.
A. albopictus C6/36 cells were cultured in minimal essential medium (MEM) with 10% fetal bovine serum, 1× nonessential amino acids, and 2 mM glutamine. C6/36 cells were subcultured weekly at a 1:10 dilution.
Mammalian cell culture.
Macaca mulatta kidney LLC-MK2 cells were cultured in Dulbecco's modified Eagle medium with 10% fetal bovine serum and 2 mM glutamine. LLC-MK2 cells were subcultured weekly at a 1:10 dilution.
Phylogenetic analysis.
Phylogenetic analysis was performed by generating a concatenated sequence using five multilocus sequence typing genes (coxA, fbpA, ftsZ, gatB, and hcpA) (33). wMel sequences identified in the assembled genome are available on the NCBI website (accession number AE017196.1). All other genes were identified by BLAST search of unassembled genome sequence contigs. wStri sequences were identified from GenBank accession numbers LRUH01000003.1 (coxA and gatB), LRUH01000022.1 (ftsZ), LRUH01000049.1 (fbpA), and LRUH01000065.1 (hcpA). wAlbB sequences were identified from GenBank accession numbers CAGB01000095.1 (coxA and gatB), CAGB01000132.1 (ftsZ), CAGB01000163.1 (fbpA), and CAGB01000012.1 (hcpA). wMelPop sequences were identified from GenBank accession numbers AQQE01000026.1 (coxA), AQQE01000016.1 (gatB), AQQE01000047.1 (ftsZ), AQQE01000005.1 (fbpA), and AQQE01000033.1 (hcpA). wPip sequences were identified from GenBank accession numbers CACK01000072.1 (coxA), CACK01000072.1 and CACK01000085.1 (gatB), CACK01000123.1 (ftsZ), CACK01000089.1 (fbpA), and CACK01000050.1 (hcpA). Sequences were aligned by MUSCLE and the Tamura-Nei model to produce a maximum likelihood tree in MEGA6 (48).
Virus stocks.
ZIKV strains MR766 and PRVABC59 were obtained from the Biodefense and Emerging Infections Research Resources Repository. Virus was grown in C6/36 cells infected at an MOI of 0.01 and harvested at 7 days. Virus supernatant was filtered and aliquoted. To concentrate virus for high-MOI experiments, 8% polyethylene glycol 8000 was incubated with virus overnight at 4°C. Virus was pelleted at a relative centrifugal force (RCF) of 30,000 and resuspended in NaCl-Tris-EDTA (NTE) buffer. Infectious virus units were quantified by focus forming assay.
Focus forming assay for infectious virus production.
Cells were infected at an MOI of 0.01 for 1 h at 28°C. The virus inoculum was removed and complete medium was added to each well. Cells were incubated at 28°C with 5% CO2 for 5 days. After incubation, medium was removed for quantitation by focus forming assay. The focus forming assay protocol was adapted from that previously described by Paul et al. (49). Briefly LLC-MK2 cells were inoculated with serial dilutions of supernatant and incubated for 1 h. Virus was removed and cells were rinsed one time before addition of 1% agar in MEM with 10% FBS. Plates were incubated for 72 h, followed by fixation in 10% formalin for 1 h at room temperature and then permeabilization with 70% ethanol for 30 min. Cell were stained with cross-reactive primary human anti-dengue E virus protein antibody (D11C) (50) in phosphate-buffered saline (PBS) with 0.01% Tween 20 and 5% nonfat dry milk (NFDM), followed by goat anti-human horseradish peroxidase (HRP). Foci were developed with 0.5 mg/ml diaminobenzidine in 25 mM Tris-HCl, pH 7.2. Foci were counted and graphed in GraphPad Prism.
Tetracycline treatment.
Cells were infected at an MOI of 0.01 for 1 h at 28°C. The virus inoculum was removed and complete medium with either 0.0235, 0.25, or 2.5 μg/ml of tetracycline was added to each well. Cells were incubated at 28°C with 5% CO2 for 5 days. After incubation, medium was removed and cells were rinsed one time with PBS. Cellular RNA was extracted with a Qiagen RNeasy kit per manufacturer recommendations. Quantitative RT-PCR was carried out with a Roche one-step SYBR green kit as described below.
Fluorescent in situ hybridization.
Fluorescent in situ hybridization was performed with minor modifications to a method previously described (51). Briefly, cells were fixed with 4% paraformaldehyde in serum-free medium with 0.1% Triton X-100 and 0.1% Tween 20 for 1 h. Cells were then incubated in hybridization buffer (50% formamide, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 250 mg/liter of salmon sperm DNA, 0.5× Denhardt's solution, 20 mM Tris HCl, 0.1% SDS) at 37°C for 1 h. Probes were added in in situ buffer at a concentration of 1 ng/μl (probe 1) or 7.5 pg/μl (probe 2). Fluorescent in situ hybridization utilized two Wolbachia 16S rRNA probes: probe 1 (5′ Cy3-ATCTTGCGACCGTAGTCC 3′ and probe 2 (5′ Alexa Fluor 488-ACATGCTCCACCGCTTGTGCGGGTCCCCGTCAATT 3′). Probes were incubated with cells for a minimum of 3 h at 37°C and then washed in buffer 1 (1× SSC, 20 mM Tris-HCl, and 0.1% SDS) followed by wash buffer 2 (0.5× SSC, 20 mM Tris-HCl, and 0.1% SDS) at 37°C for 15 min each. Cells were stained with Hoechst at 0.5 μg/ml in wash buffer 2 for 30 min at room temperature. Finally, cells were rinsed with wash buffer 2 twice and mounted in ProLong gold for imaging on an Olympus FV100 FluoView confocal microscope.
Quantitative RT-PCR of virus genome copies.
Cells were infected at an MOI of 0.01 for 1 h at 28°C in serum-free medium. The virus inoculum was removed, and complete medium was added to each well. Cells were incubated at 28°C with 5% CO2 for 5 days. After incubation, medium was removed and cells were rinsed one time with PBS. Cellular RNA was extracted with a Qiagen RNeasy kit per manufacturer recommendations. Quantitative RT-PCR was carried out with a Roche one-step SYBR green kit. ZIKV primers (ZIKV_For [AARTACACATACCARAACAAAGTGGT] and ZIKV_Rev [TCCRCTCCCYCTYTGGTCTTG]) were previously published (52). wStri primers (wStri_For [TCAAGCAAAAGCTGGTGTTAGC] and wStri_Rev [CAGCATCATCCTTAGCTGCC]) and wAlbB primers (wAlbB_For [AGCATCTTTTATGGCTGGTGG] and wAlbB_Rev [AATGTTGCACCACCAACGTC]) were made against Wolbachia surface protein. All reactions were annealed at 55°C.
Western blots.
Cells were infected with ZIKV at an MOI of 10 for 1 h at 28°C in serum-free medium. The virus inoculum was removed and complete medium was added to each well. Cells were incubated at 28°C with 5% CO2 for 1, 2, or 3 days. After incubation, medium was removed and cells were rinsed one time with PBS. Protein was extracted by NP-40 buffer with protease inhibitor on ice for 5 min. Fifteen micrograms of protein was loaded per well in a 10% SDS TGX minigel and subsequently transferred to low-fluorescence polyvinylidene difluoride (PVDF) paper (Bio-Rad). After blocking for 1 h in Odyssey PBS blocking buffer, proteins were probed for with Genetex polyclonal rabbit anti-Zika virus NS5, Abcam mouse anti-Hsp60 LK-2, and Millipore mouse anti-actin in Odyssey blocking buffer with 0.01% Tween 20 overnight at 4°C. Blots were then incubated with Licor secondary antibodies, IRDye 680LT donkey anti-mouse IgG (H+L), and IRDye 800CW donkey anti-rabbit IgG (H+L) at a dilution of 1:5,000 in Odyssey blocking buffer with 0.01% Tween 20 and 0.01% SDS. Membranes were visualized with the Licor Odyssey Clx. Quantitation of band intensity was performed with Image Studio and graphed in GraphPad Prism. To determine the appropriate statistical test, we consulted a computational biologist/statistician (Tom Kepler, Microbiology, Mathematics & Statistics, Boston University), who advised us to log transform the intensity ratios, which typically produces normally distributed errors and ameliorates heteroscedasticity in data.
Cholesterol supplementation.
Cells were infected at an MOI of 0.01 for 1 h at 28°C in serum-free medium. The virus inoculum was removed and complete medium with or without cholesterol-lipid concentrate (Thermo Fisher; catalog number 12531018). Incubation took place at 28°C with 5% CO2 for 5 days. The supernatant was removed. Cells were rinsed one time with PBS and then lysed with Qiagen RLT buffer. RNA was extracted with an RNeasy kit per the manufacturer's recommendations. Quantitative RT-PCR was carried out as described above.
ACKNOWLEDGMENTS
We thank Ann Fallon and Zhiyong Xi for kindly sharing cell lines infected and not infected with Wolbachia. We thank Tom Kepler (Microbiology, Mathematics & Statistics, BU) for statistical consultation for Western blot quantitation.
Funding was provided by the following: The Directors Fund (BU NEIDL) and the National Institute of Allergy and Infectious Diseases (R21 NS101151, 1R56AI097589, and 1R56AI097589-01A1).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.McGraw EA, O'Neill SL. 2013. Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol 11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Morens DM. 2016. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 3.Liu N. 2015. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol 60:537–559. doi: 10.1146/annurev-ento-010814-020828. [DOI] [PubMed] [Google Scholar]
- 4.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, Bossin HC, Moretti R, Baton LA, Hughes GL, Mavingui P, Gilles JRL. 2014. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop 132(Suppl):S150–S163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O'Neill SL. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson NM, Hue Kien DT, Clapham H, Aguas R, Trung VT, Bich Chau TN, Popovici J, Ryan PA, O'Neill SL, McGraw EA, Long VT, Dui LT, Nguyen HL, Vinh Chau NV, Wills B, Simmons CP. 2015. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med 7:279ra237. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zug R, Hammerstein P. 2012. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kittayapong P, Baisley KJ, Baimai V, O'Neill SL. 2000. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J Med Entomol 37:340–345. doi: 10.1603/0022-2585(2000)037[0340:DADOWI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Frentiu FD, Robinson J, Young PR, McGraw EA, O'Neill SL. 2010. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One 5:e13398. doi: 10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O'Neill SL, Hoffmann AA. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 11.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O'Neill SL. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Hussain M, Lu G, Torres S, Edmonds JH, Kay BH, Khromykh AA, Asgari S. 2013. Effect of Wolbachia on replication of West Nile virus in a mosquito cell line and adult mosquitoes. J Virol 87:851–858. doi: 10.1128/JVI.01837-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen TH, Nguyen HL, Nguyen TY, Vu SN, Tran ND, Le TN, Vien QM, Bui TC, Le HT, Kutcher S, Hurst TP, Duong TTH, Jeffery JAL, Darbro JM, Kay BH, Iturbe-Ormaetxe I, Popovici J, Montgomery BL, Turley AP, Zigterman F, Cook H, Cook PE, Johnson PH, Ryan PA, Paton CJ, Ritchie SA, Simmons CP, O'Neill SL, Hoffmann AA. 2015. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors 8:563. doi: 10.1186/s13071-015-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian G, Xu Y, Lu P, Xie Y, Xi Z. 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, Day A, Higgs S, O'Neill SL. 2012. Impact of Wolbachia on infection with Chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis 6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blagrove MSC, Arias-Goeta C, Di Genua C, Failloux A-B, Sinkins SP. 2013. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits Chikungunya virus. PLoS Negl Trop Dis 7:e2152. doi: 10.1371/journal.pntd.0002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blagrove MSC, Arias-Goeta C, Failloux A-B, Sinkins SP. 2012. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci U S A 109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobson SL, Rattanadechakul W, Marsland EJ. 2004. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity 93:135–142. doi: 10.1038/sj.hdy.6800458. [DOI] [PubMed] [Google Scholar]
- 19.Ye YH, Carrasco AM, Frentiu FD, Chenoweth SF, Beebe NW, van den Hurk AF, Simmons CP, O'Neill SL, McGraw EA. 2015. Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Negl Trop Dis 9:e0003894. doi: 10.1371/journal.pntd.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, McGraw EA, O'Neill SL. 2014. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis 8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutra Heverton Leandro C, Rocha Marcele N, Dias Fernando Braga S, Mansur Simone B, Caragata Eric P, Moreira Luciano A. 2016. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19:771–774. doi: 10.1016/j.chom.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aliota MT, Peinado SA, Velez ID, Osorio JE. 2016. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci Rep 6:28792. doi: 10.1038/srep28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliota MT, Walker EC, Uribe Yepes A, Velez ID, Christensen BM, Osorio JE. 2016. The wMel strain of Wolbachia reduces transmission of Chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis 10:e0004677. doi: 10.1371/journal.pntd.0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caragata EP, Dutra HLC, Moreira LA. 2016. Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol 32:207–218. doi: 10.1016/j.pt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Zug R, Hammerstein P. 2015. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol Rev Camb Philos Soc 90:89–111. doi: 10.1111/brv.12098. [DOI] [PubMed] [Google Scholar]
- 26.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA. 2017. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog 13:e1006006. doi: 10.1371/journal.ppat.1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joubert DA, Walker T, Carrington LB, De Bruyne JT, Kien DHT, Hoang NLT, Chau NVV, Iturbe-Ormaetxe I, Simmons CP, O'Neill SL. 2016. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog 12:e1005434. doi: 10.1371/journal.ppat.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodson BL, Hughes GL, Paul O, Matacchiero AC, Kramer LD, Rasgon JL. 2014. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl Trop Dis 8:e2965. doi: 10.1371/journal.pntd.0002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mousson L, Zouache K, Arias-Goeta C, Raquin V, Mavingui P, Failloux A-B. 2012. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl Trop Dis 6:e1989. doi: 10.1371/journal.pntd.0001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raquin V, Valiente Moro C, Saucereau Y, Tran F-H, Potier P, Mavingui P. 2015. Native Wolbachia from Aedes albopictus blocks Chikungunya virus infection in cellulo. PLoS One 10:e0125066. doi: 10.1371/journal.pone.0125066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joubert DA, O'Neill SL. 2017. Comparison of stable and transient Wolbachia infection models in Aedes aegypti to block dengue and West Nile viruses. PLoS Negl Trop Dis 11:e0005275. doi: 10.1371/journal.pntd.0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallon AM, Baldridge GD, Higgins LA, Witthuhn BA. 2013. Wolbachia from the planthopper Laodelphax striatellus establishes a robust, persistent, streptomycin-resistant infection in clonal mosquito cells. In Vitro Cell Dev Biol Anim 49:66–73. doi: 10.1007/s11626-012-9571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MC, Tettelin H, Werren JH. 2006. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Meer MMM, Witteveldt J, Stouthamer R. 1999. Phylogeny of the arthropod endosymbiont Wolbachia based on the wsp gene. Insect Mol Biol 8:399–408. doi: 10.1046/j.1365-2583.1999.83129.x. [DOI] [PubMed] [Google Scholar]
- 35.Lu P, Bian G, Pan X, Xi Z. 2012. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl Trop Dis 6:e1754. doi: 10.1371/journal.pntd.0001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9:e1003896. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caragata EP, Rancès E, Hedges LM, Gofton AW, Johnson KN, O'Neill SL, McGraw EA. 2013. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog 9:e1003459. doi: 10.1371/journal.ppat.1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smit JM, Moesker B, Rodenhuis-Zybert I, Wilschut J. 2011. Flavivirus cell entry and membrane fusion. Viruses 3:160–171. doi: 10.3390/v3020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bian G, Zhou G, Lu P, Xi Z. 2013. Replacing a native Wolbachia with a novel strain results in an increase in endosymbiont load and resistance to dengue virus in a mosquito vector. PLoS Negl Trop Dis 7:e2250. doi: 10.1371/journal.pntd.0002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rainey SM, Martinez J, McFarlane M, Juneja P, Sarkies P, Lulla A, Schnettler E, Varjak M, Merits A, Miska EA, Jiggins FM, Kohl A. 2016. Wolbachia blocks viral genome replication early in infection without a transcriptional response by the endosymbiont or host small RNA pathways. PLoS Pathog 12:e1005536. doi: 10.1371/journal.ppat.1005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rancès E, Johnson TK, Popovici J, Iturbe-Ormaetxe I, Zakir T, Warr CG, O'Neill SL. 2013. The Toll and Imd pathways are not required for Wolbachia-mediated dengue virus interference. J Virol 87:11945–11949. doi: 10.1128/JVI.01522-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. 2012. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caragata EP, Rances E, O'Neill SL, McGraw EA. 2014. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb Ecol 67:205–218. doi: 10.1007/s00248-013-0339-4. [DOI] [PubMed] [Google Scholar]
- 44.Molloy JC, Sommer U, Viant MR, Sinkins SP. 2016. Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl Environ Microbiol 82:3109–3120. doi: 10.1128/AEM.00275-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stiasny K, Koessl C, Heinz FX. 2003. Involvement of lipids in different steps of the flavivirus fusion mechanism. J Virol 77:7856–7862. doi: 10.1128/JVI.77.14.7856-7862.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Offerdahl DK, Dorward DW, Hansen BT, Bloom ME. 2017. Cytoarchitecture of Zika virus infection in human neuroblastoma and Aedes albopictus cell lines. Virology 501:54–62. doi: 10.1016/j.virol.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brackney DE, Scott JC, Sagawa F, Woodward JE, Miller NA, Schilkey FD, Mudge J, Wilusz J, Olson KE, Blair CD, Ebel GD. 2010. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl Trop Dis 4:e856. doi: 10.1371/journal.pntd.0000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul LM, Carlin ER, Jenkins MM, Tan AL, Barcellona CM, Nicholson CO, Michael SF, Isern S. 2016. Dengue virus antibodies enhance Zika virus infection. Clin Transl Immunol 5:e117. doi: 10.1038/cti.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, Bazzone LE, Hogancamp G, Figueroa Sierra M, Fong RH, Yang ST, Lin L, Robinson JE, Doranz BJ, Chernomordik LV, Michael SF, Schieffelin JS, Isern S. 2013. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. J Virol 87:52–66. doi: 10.1128/JVI.02273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toomey ME, Panaram K, Fast EM, Beatty C, Frydman HM. 2013. Evolutionarily conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc Natl Acad Sci U S A 110:10788–10793. doi: 10.1073/pnas.1301524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faye O, Faye O, Dupressoir A, Weidmann M, Ndiaye M, Alpha Sall A. 2008. One-step RT-PCR for detection of Zika virus. J Clin Virol 43:96–101. doi: 10.1016/j.jcv.2008.05.005. [DOI] [PubMed] [Google Scholar]