ABSTRACT
The maturation process of high-affinity antibodies is a result of intricate interactions between B cells and follicular helper T (Tfh) cells occurring in lymphoid germinal centers. HIV infection induces significant chronic immune activation, phenotypic skewing, and inflammation driven by years of continuous viral replication. High levels of viremia as well as immune activation and dysfunction have been demonstrated to have a perturbing impact on the B cell memory compartment and contribute to B cell exhaustion. Counterintuitively, the factors associated with perturbation of the B cell compartment seem to be favorable for the generation of highly affinity-matured Env-specific antibodies in a minority of HIV-infected individuals. Thus, the impact of HIV antigenemia on B cells and Tfh cell interactions warrants further exploration. We therefore studied immunophenotypes of HIV-specific B cells in individuals with differing levels of viral control using HIV Env gp120 probes and characterized the functionality of matched T cells in peripheral blood. While CXCR5+ CD4+ T cells were significantly diminished in HIV progressors, we found that a small subset of gp120-specific interleukin-21 (IL-21)-secreting CXCR5+ CD4+ T cells were significantly associated with gp120-specific B cell frequencies. In contrast, neither bulk CXCR5+ CD4+ T cells nor other HIV antigen specificities were associated with gp120-specific B cell levels. HIV-specific B cells derived from elite controllers displayed greater amounts of gp120-specific B cells in the resting memory subset, whereas HIV-specific B cells in progressors accumulated in tissue-like and activated memory subsets. Furthermore, CXCR5+ CD4+ T cells from elite controllers showed a stronger ex vivo capacity to induce B cell maturation and immunoglobulin class switching than cells from HIV progressors.
IMPORTANCE Dissecting the factors that are involved in B cell maturation and antibody development is important for HIV vaccine design. In this study, we found that HIV Env-specific CXCR5+ CD4+ T cells that secrete interleukin-21 are strongly associated with B cell memory phenotypes and function. Moreover, we found that the immune responses of HIV controllers showed intrinsically better helper activity than those of HIV progressors.
KEYWORDS: B cell memory, CD4 T cells, IL-21, Tfh cells, elite controllers, human immunodeficiency virus
INTRODUCTION
There is general consensus that an effective human immunodeficiency virus (HIV) vaccine will likely necessitate induction of high-affinity, protective antibody responses against a diverse repertoire of viral isolates (termed broadly neutralizing antibodies [bNAbs]) (1). Attempts to induce such bNAbs through vaccination have mostly been ineffective. Interestingly, in natural infection, serologic breadth develops only under conditions that seem counterintuitive to efficient B cell maturation processes, such as years of chronic immune activation, loss of CD4 T cells, high levels of exhaustion marker expression, and other phenotypic lymphocyte abnormalities (2–5). In addition, high viral loads and coevolution of viral quasispecies have been implicated in the development of serologic breadth, suggesting that constant immune activation and antigen recognition may play a key role in HIV antibody maturation processes. However, while immune activation and lack of controlled HIV viremia appear to be favorable for bNAb elicitation, increased frequencies of peripheral CD4 T cells expressing combinations of functional markers present on germinal center (GC) T follicular helper (Tfh) cells have also been associated with the development of bNAbs and B cell memory phenotypes (6–8). It is well known that Tfh cells play a crucial role in the development of long-lived, high-affinity antibody responses during the germinal center reaction (9). Furthermore, the extensive amount of somatic hypermutation (SHM) in monoclonal bNAbs points to a critical involvement of this process in the setting of HIV (10). Thus, dissecting the relative impact of chronic immune activation and dysfunction versus Tfh cell signaling on B cell maturation and antibody development is critical for vaccine development.
While it has been shown that Tfh cells are expanded during chronic HIV infection (8, 11), their ability to help induce maturation and class switching provided to B cells appears to be diminished, in part due to a functional impairment of these cells within the GC (12, 13). Similarly, memory B cells have been demonstrated to be phenotypically skewed during chronic HIV infection by direct and bystander activation mechanisms (14, 15). This perturbation is typified by an increased relative fraction of activated memory cells, plasmablasts, and exhausted B cells and a reduction in long-lived plasma cells. Phenotypic and functional abnormalities therefore are well documented for both T and B cell compartments and are induced by viral replication-driven chronic immune activation. However, the generation of protective humoral immunity likely requires the induction of neutralizing antibodies that are secreted from long-lived plasma cells, a process which is likely hindered by HIV-associated immune dysfunction. Paradoxically, these abnormalities do not seem to interfere with B cell maturation processes in cases where serologic breadth is achieved. Therefore, other immunologic phenomena may be influential in differentiating between individuals that generate bNAbs and those that do not.
A small minority of HIV-infected individuals, known as controllers, spontaneously maintains prolonged control of viremia and normal CD4 T cell counts in the absence of antiretroviral therapy (ART). While there are several factors that contribute to this durable control, a strong adaptive cellular response is a cornerstone of the ability of these individuals to resist the phenotypic and functional abnormalities driven by the immune-activating effects of ongoing viral replication (16). In contrast, progressors do not mount such antiviral immune responses, and uncontrolled viral replication in these individuals leads to chronic immune activation and, if left untreated, the onset of AIDS. Interestingly, despite preserving intact cellular and humoral compartments, HIV controllers seldom develop serologic breadth. Thus, interrogating B and T cell interactions with cells derived from both controllers and progressors may help elucidate important factors in HIV-specific antibody development. Due to a lack of available lymphoid tissues and specimens from individuals with defined serologic breadth, we focused on interactions between lymphocytes isolated from peripheral blood that are functionally related to bona fide Tfh (termed pTfh) cells. We hypothesized that chronic immune activation must affect only certain subsets of antigen-specific B cells and does not necessarily impede T/B cell interactions critical for Env-specific antibody maturation. Correspondingly, we studied the phenotypic and functional differences of HIV-specific pTfh and B cells between controllers and progressors to ascertain whether antigenemia and immune activation may influence Tfh cell functionality and its subsequent impact on B cell differentiation. We observed differences in memory B cell subset distribution, with controllers having an enrichment of Env-specific B cells in the resting memory compartment relative to progressors. CXCR5+ interleukin-21-positive (IL-21+) CD4+ T cells from HIV controllers displayed a greater ability to promote B cell maturation and Ig isotype class switching than did those from progressors as well. Together, these results indicate a critical role for Tfh functionality rather than immune activation in influencing Env-specific B cell maturation in the setting of HIV infection and can serve to inform improved vaccine and therapeutic design.
RESULTS
Bulk B cells are expanded in uncontrolled HIV infection but not T cells.
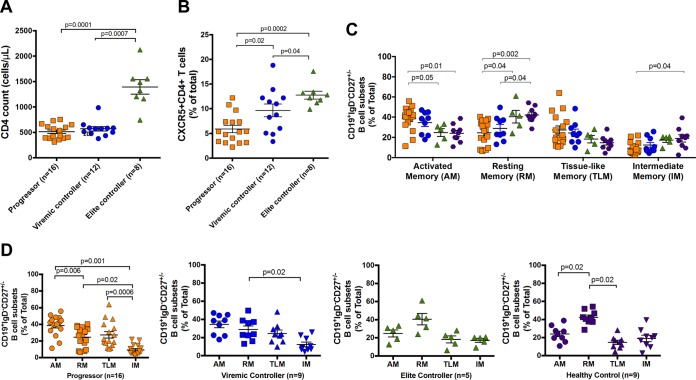
To initially address the impact of antigenemia on the dynamics of T helper cells and B cells, we first quantified the frequencies of CXCR5+ CD4+ T cells and CD19+ B cells in cross-sectional samples from three groups of HIV-infected subjects with high viremia (termed chronic progressors), individuals with controlled viremia (50 to 2,000 HIV RNA copies/ml, termed viremic controllers [VC]), and individuals able to spontaneously control viral loads below the limit of detection in the absence of ART (<50 HIV RNA copies/ml, termed elite controllers [EC]). As expected, we found significantly higher bulk CD4 T cell counts in HIV EC (1,395 ± 399 cells/μl) than in HIV progressors (512 ± 143 cells/μl) and HIV VC (570 ± 152 cells/μl) (P = 0.0001 and P = 0.0007, respectively [Fig. 1A]). Similarly, HIV progressors had a significantly lower frequency of CXCR5+ CD4+ T cells (5.9% ± 3.0%) circulating in peripheral blood than did HIV VC (9.7% ± 4.5% [P = 0.02]) and HIV EC (12.8% ± 2.3% [P = 0.0002]). In addition, CXCR5+ CD4+ T cell levels in HIV VC were lower than in HIV EC (P = 0.04 [Fig. 1B]).
FIG 1.
Cross-sectional analysis of CD4 T and B cells isolated from HIV progressors, HIV viremic controllers, HIV elite controllers, and HIV-uninfected individuals. (A) Comparison of absolute numbers of CD4 T cell counts. (B) Frequency of CXCR5+ CD4+ T cells. (C) Frequencies of B cell subpopulations. Memory B cell subgroups in CD19+ IgD− CD27+/− B cells were identified as CD21− CD27+ activated memory (AM), CD21+ CD27+ resting memory (RM), CD21− CD27− tissue-like memory (TLM), and CD21+ CD27− intermediate memory (IM). (D) Comparison of B cell subpopulation frequencies in each subgroup. Bars represent means ± SEM and groups compared by Mann-Whitney tests. For all graphs, colors of symbols represent HIV progressors (orange), HIV viremic controllers (blue), HIV elite controllers (green), and HIV-uninfected individuals (purple).
As Tfh cell expansion has been associated with a concurrent expansion of B cells and plasma cells in lymphoid germinal centers, we next studied bulk and HIV-specific B cell phenotypes in the peripheral blood of the cohorts to determine how the observed frequencies of CXCR5+ CD4+ T cells in peripheral blood affect B cell phenotypes. Initially, we stratified antigen-experienced bulk B cells by gating out IgD+ CD27− CD19+ B cells to remove IgD+ naive B cells while retaining IgD+ CD27+ memory B cells. To analyze the effects of antigenemia on memory B cell subsets, bulk B cells were separated into the four following memory subsets based on expression of CD21 and CD27 as previously described (14, 15, 17): CD27+ CD21− activated memory (AM), CD27+ CD21+ resting memory (RM), CD27− CD21− tissue-like memory (TLM), and CD27− CD21+ intermediate memory (IM). We found that peripheral blood mononuclear cells (PBMCs) from HIV-infected individuals have altered profiles of B cell memory subtypes compared to those from uninfected individuals. Comparison of the subset distribution in each cohort revealed that both HIV progressors and HIV VC displayed the highest AM and the lowest IM cell frequencies. However, HIV EC showed a pattern similar to that of healthy controls, namely, high RM and low TLM cell frequencies (Fig. 1C and D). Indeed, we found that compared to healthy donors, both HIV progressors and HIV VC contained lower frequencies of bulk RM cells (P = 0.0002 and P = 0.01) and higher frequencies of bulk TLM cells (P = 0.02 and P = 0.04, respectively). Moreover, we found that HIV progressors had higher frequencies of bulk AM cells than did both HIV EC and healthy donors (P = 0.03 and P = 0.003, respectively). Additionally HIV progressors had lower frequencies of both bulk RM and IM cells than did HIV EC (P = 0.04 and P = 0.04, respectively) (Fig. 1C and D). These data show that there is an expansion of circulating bulk memory B cells similar to that in GCs in uncontrolled HIV infection and confirm aberrant B cell phenotypes previously described (18).
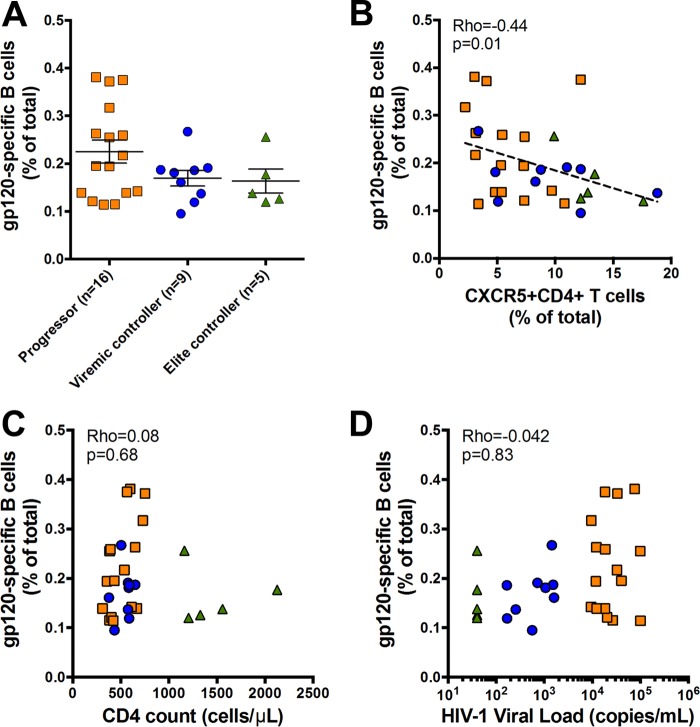
To study the impact of antigenemia on HIV-specific B cells, we next used a His-tagged HIV Env gp120 protein probe study the approximate frequency of gp120-specific B cells between progressors, HIV EC, and HIV VC by flow cytometry, as previously described (2). Healthy donors were assessed as negative controls. We found only a trending difference between frequencies of gp120-specific memory (IgD+ CD27−) B cells in PBMCs from HIV progressors, HIV VC, and HIV EC (0.22 ± 0.09, 0.17 ± 0.05, and 0.16 ± 0.05, respectively) (Fig. 2A). Moreover, there were no differences between gp120-specific B cell frequencies in the three subpopulations (P > 0.05), despite progressors having clearly higher viral loads and fewer CD4 T cells than both controller subgroups. Thus, our data illustrate that high antigenemia may not independently drive HIV-specific B cell activation and antibody production.
FIG 2.
Associations of gp120-specific B cells with clinical parameters in HIV infection. (A) Frequencies of HIV gp120-specific B cells in progressors, viremic controllers, and elite controllers. (B) Significant inverse association between frequencies of CXCR5+ CD4+ T cells and HIV gp120-specific B cells. (C) Frequency of HIV gp120-specific B cells is not associated with absolute CD4 T cell counts. (D) Frequency of HIV gp120-specific B cells is not associated with HIV-1 loads. Symbols represent individual study participants. Horizontal bars represent means ± SEM. Regression was analyzed by the Spearman correlation test.
Given the clear alteration in bulk, but not HIV-specific, B cell frequency between progressors and controllers, we hypothesized that viral replication may indirectly affect the B cell compartment by skewing the T cell compartment. Thus, we next interrogated T cell markers involved in mediating B cell help to further address the role of viral load in helper T cell interactions with B cells. While we and others have established that circulating CXCR5+ CD4+ T cells are heterogeneous (19), there is a large body of evidence showing that IL-21-secreting HIV-specific CD4 T cells comprise a small subset within this T cell subpopulation and provide B cell help (19). Interestingly, however, we found a strong inverse association between the frequency of CXCR5+ CD4+ T cells and gp120-specific B cells (rho = −0.44; P = 0.01) in all groups of HIV-infected individuals (Fig. 2B). These data are irrespective of viral load or bulk CD4 T cell count, as we found no association between gp120-specific B cell frequency with CD4 T cell count (rho = 0.08; P = 0.68 [Fig. 2C]) or HIV load (r = 0.042; P = 0.83 [Fig. 2D]). Thus, our data indicate that CXCR5 expression alone may not be the best indicator for HIV-specific B cell activation/maturation, and analyses of functional cytokines/surface molecules involved in B cell help are likely important.
Functional antigen-specific T cells are associated with gp120-specific B cells.
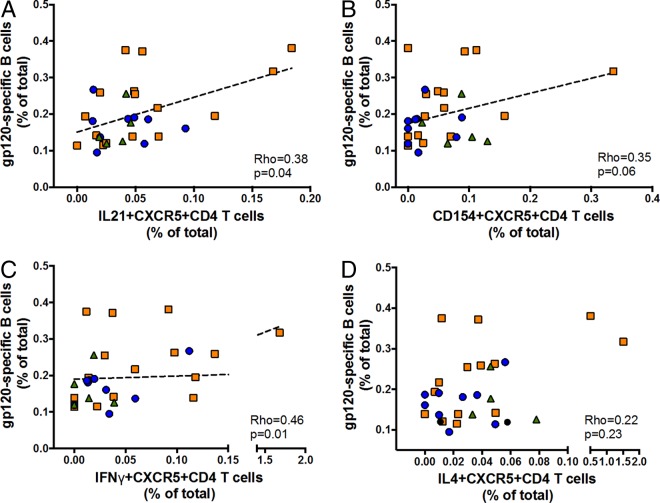
Provision of B cell help by GC Tfh cells is well defined, and studies have shown that CD154 (CD40L) works together with IL-21 to induce activation-induced cytidine deaminase activation, which, in turn, drives class switch recombination (CSR), SHM, and affinity maturation (20); other cytokines, such as IL-4, have been suggested to contribute to humoral responses by inducing Th2-biased immune responses. Thus, to further study gp120-specific B cell interactions with functional antigen-specific T cell responses in HIV+ specimens, we interrogated associations between frequencies of gp120-specific B cells and functional T cell markers in Env- or Gag-stimulated T cells using a standardintracellular cytokine staining (ICS) protocol. While we found no association between Gag-specific responses and gp120-specific B cell frequency (data not shown), our data show that gp120-specific B cell frequency is associated with Env-specific expression of several molecules involved in B cell help by CXCR5+ CD4+ T cells. In particular, the frequency of gp120-specific IL-21+ CXCR5+ CD4+ T cells and that of gp120-specific B cells were significantly associated (rho = 0.38; P = 0.04 [Fig. 3A]). We further found a trending positive association between frequencies of Env gp120-specific CD154+ CXCR5+ CD4+ (rho = 0.35; P > 0.05 [Fig. 3B]) and IFN-γ+ CXCR5+ CD4+ (rho = 0.46; P = 0.01 [Fig. 3C]) T cell responses but not IL-4+ CXCR5+ CD4+ (rho = 0.22; P > 0.05 [Fig. 3D]) or IL-10+ CXCR5+ CD4+ (data not shown) T cell responses with gp120-specific B cells.
FIG 3.
Immunologic associations between gp120-specific B cells and Env-specific CXCR5+ CD4 T cell responses. (A) HIV gp120-specific B cells are significantly associated with Env-specific IL-21+ CXCR5+ CD4+ T cells. (B) A trending association exists between HIV gp120-specific B cells and Env-specific CD154+ CXCR5+ CD4+ T cells. (C) A significant positive association exists between HIV gp120-specific B cells and IFN-γ-specific CXCR5+ CD4+ T cells. (D) No association observed between HIV gp120-specific B cells and Env-specific IL-4+ CXCR5+ CD4+ T cells. Symbols indicate individual study participants. Orange squares represent HIV progressors; blue circles and green triangles represent HIV VC and HIV EC, respectively. Regression was analyzed by the Spearman correlation test.
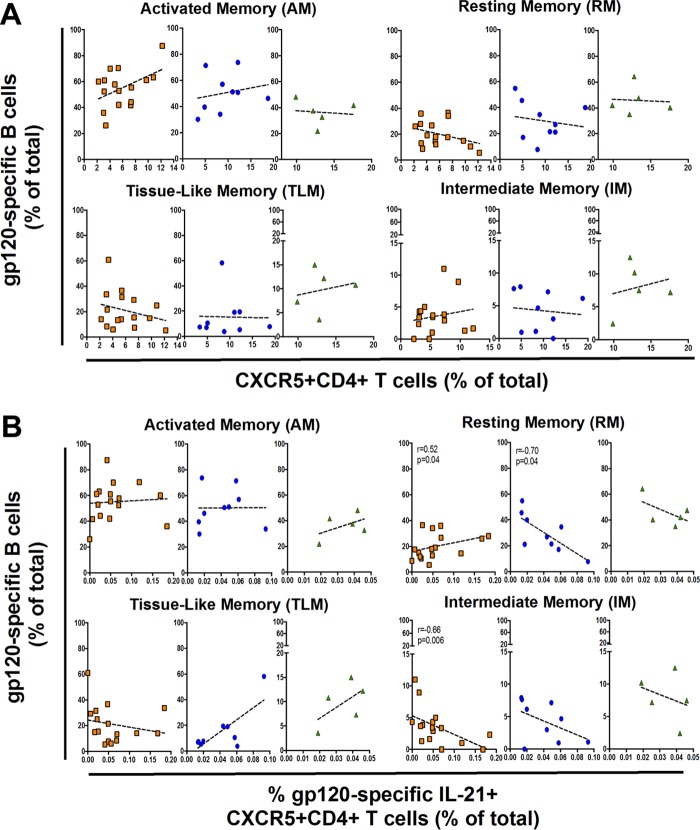
IL-21-expressing Env-specific CXCR5+ CD4+ T cells associated with gp120-specific B cell memory subsets.
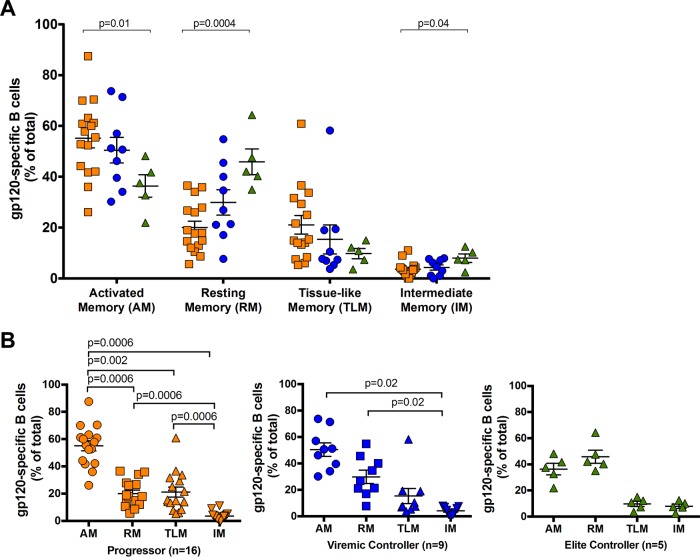
As expression of the CXCR5 chemokine receptor on CD4+ T cells ostensibly defines a subset of pTfh cells in peripheral blood (7, 19, 21), we assessed whether specific gp120-specific B cell memory subsets were associated with CXCR5+ CD4+ T cells. Overall, gp120-specific RM and IM cells were significantly expanded in HIV EC (P = 0.0004 and P = 0.04, respectively), while the AM cell subset was larger in HIV progressors (P = 0.01), as was the TLM subset, albeit to a lower degree (Fig. 4A). We further compared gp120-specific B cell distribution between memory compartments and observed distributions similar to those observed in bulk CD19+ cells. Comparing the distributions of gp120-specific B cells showed that they were highest in the AM compartment in both HIV progressors and HIV VC, whereas they were found in the highest levels in the RM compartment in HIV EC (Fig. 4B). However, we found no correlation of gp120-specific B cell memory subsets with the frequency of peripheral CXCR5+ CD4+ T cells (Fig. 5A). We therefore assessed whether memory gp120-specific B cell subsets were associated with pTfh cells that expressed B helper cytokines upon stimulation with HIV antigens. Again, while we detected high levels of Gag-specific CXCR5+ CD4+ T cell responses, we found no association between Gag-specific CXCR5+ CD4+ T cells that secrete any cytokine and HIV-specific B cell memory subsets (data not shown). Similarly, we found no associations between memory B cell subsets and gp120-specific CD154+, IL-4+, or IFN-γ+ CXCR5+ CD4+ T cells (data not shown). In contrast, however, we found that gp120-specific IL-21+ CXCR5+ CD4+ T cell responses were associated with gp120-specific B cell memory subsets in a diametric fashion in HIV controllers compared to progressors. Indeed, we found that gp120-specific IL-21+ CXCR5+ CD4+ T cells have a weak positive association with gp120-specific RM B cell frequency in HIV progressors (rho = 0.52; P = 0.04), while there is a strong inverse correlation in HIV VC (rho = −0.70; P = 0.04) (Fig. 5B). Interestingly, gp120+ IM B cells in HIV progressors were strongly inversely associated with IL-21+ CXCR5+ CD4+ T cells (rho = −0.66, P = 0.006), while AM and TLM gp120+ B cells showed no association (Fig. 5B). Taken together, our data demonstrate that certain memory B cell subsets are correlated with Env-specific IL-21+ CXCR5+ CD4+ T cells but not bulk CXCR5+ CD4+ T cells, supporting our previous study showing that IL-21 secretion may better identify pTfh cells (19). Moreover, our partially divergent association in HIV progressors versus controllers suggests a functional impairment of HIV-specific pTfh cells in HIV progressors, in accordance with data for GC Tfh cells (12).
FIG 4.
Frequencies of HIV gp120-specific B cells by memory subsets in HIV progressors, HIV VC, and HIV EC. (A) Frequencies of HIV gp120-specific B cells in each B cell subpopulation. (B) Comparison of frequencies of HIV gp120-specific B cells in each B cell subpopulation (AM, RM, TLM, and IM) in each group. Bars represent means ± SEM and groups compared by Mann-Whitney tests.
FIG 5.
Relationship between gp120-specific B cells and T follicular helper cells. (A) No significant correlation between HIV gp120-specific memory B cell subsets and CXCR5+ CD4+ T cells. (B) Several diametric associations between Env-specific IL-21+ CXCR5+ CD4+ T cells and HIV gp120-specific memory B cell subsets. Orange squares represent HIV progressors; blue circles and green triangles represent HIV VC and HIV EC, respectively. Correlation was analyzed by the Spearman correlation test.
Bulk progressor- and controller-derived CXCR5+ T cells have equivalent B helper capabilities.
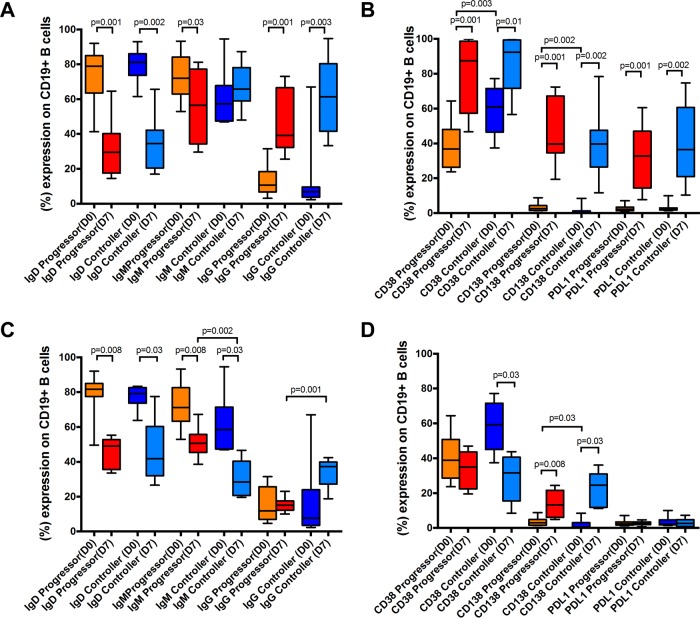
To further explore functional impairment in pTfh cells in HIV progressors compared to controllers, we next performed coculture assays to directly determine whether CD4 helper quality varies between PBMCs derived from these two cohorts in response to both global and antigen-specific stimulation, as previously shown (21, 22). Specimens from both HIV VC and HIV EC were grouped for this analysis to achieve sufficient statistical power. Thus, we assayed 11 HIV progressors and 10 HIV controllers (combined from HIV VC and HIV EC) for staphylococcal enterotoxin B (SEB) stimulation experiments and 8 HIV progressors and 6 HIV controllers for Env stimulation experiments, due to sample availability. We initially used the superantigen SEB as the stimulating agent because it selectively but maximally stimulates CD4+ T cells through the T cell receptor (TCR). Surprisingly, we found that CXCR5+ CD4+ T cells derived from HIV controllers and progressors comparably induced class switching in our coculture assay (Fig. 6A). For HIV progressors and HIV controllers, we found similar reductions of IgD (P = 0.001 and P = 0.002, respectively) and inductions of IgG (P = 0.001 and P = 0.003, respectively) in B cells cocultured with pTfh cells for 7 days in the presence of SEB. Surprisingly, we found reduction of IgM only in HIV progressors (P = 0.03) and not in controllers (P > 0.05), suggesting a functional impairment in B cells in HIV progressors relative to controllers. Moreover, we found that CXCR5+ CD4+ T cells from HIV progressors and HIV controllers were equally capable of activating B cells after 7 days of coculture, indicated by the upregulation of CD38 (P = 0.001 and P = 0.01, respectively) and differentiation of plasma cells (CD138 expression) (P = 0.001 and P = 0.002, respectively). Similarly, CXCR5+ CD4+ T cells from HIV progressors and HIV controllers equally induced PDL1 expression on B cells (P = 0.001 and P = 0.002, respectively) (Fig. 6B).
FIG 6.
) CXCR5+ CD4+ T cells derived from HIV controllers provide B cell help superior to that from cells derived from HIV progressors. Naive B cells isolated from peripheral blood were cocultured with autologous CXCR5-enriched CD4 T cells for 7 days in the presence of CD28/CD49d costimulating antibodies and SEB or HIV-1 PTE ENV peptide pools. (A) Naive B cells cocultured with CXCR5+ CD4+ T cells in the presence of SEB lose expression of IgD and increase expression of IgG similarly in HIV progressors and HIV controllers. (B) No difference in phenotypic changes of B cells in expression of CD38, CD138, or PDL1 expression after stimulation with SEB between HIV controllers and HIV progressors. (C) Significant differences between HIV controllers and HIV progressors in the class switching of naive B cells cocultured with Env-stimulated CXCR5+ CD4+ T cells. (D) No differences in phenotypic changes of B cells in CD38, CD138, or PDL1 expression after stimulation with ENV PTE peptide pools between HIV controllers and HIV progressors. Data are presented as boxes and whiskers and groups compared by Mann-Whitney tests.
Env-specific controller-derived CXCR5+ T cells have enhanced B helper capability.
As using SEB as a stimulating agent assumes a general and not specific superiority of the immune response capability of HIV controllers versus progressors, we next assessed whether gp120-specific CXCR5+ CD4+ T cell responses have an enhanced helper function. We cocultured CXCR5+ CD4+ T cell responses with B cells in the presence of HIV Env gp120 potential T cell epitope (PTE) peptide pools for 7 days and then assessed changes in B cell phenotype by flow cytometry (Fig. 6C and D). Both gp120-specific CXCR5+ CD4+ T cell responses in HIV progressors and controllers similarly reduced IgD expression (P = 0.008 and P = 0.03, respectively) on autologous B cells. In addition, cocultured B cells from HIV progressors and controllers had a reduction of IgM (P = 0.008 and P = 0.03, respectively) and enhancement of IgG. Interestingly, cocultured B cells from HIV controllers had a more significant reduction of IgM (P = 0.002) and enhancement of IgG (P = 0.001) than did those derived from HIV progressors (Fig. 6C). Alternatively, while cells from HIV controllers showed a stronger reduction in CD38 (P = 0.03), cells from both HIV progressors and controllers similarly showed an enhancement of CD138 (P = 0.008 and P = 0.03, respectively) and no change in PDL1 expression (Fig. 6D). Interestingly, cocultured B cells from HIV controllers had a significant enhancement of plasma cells (P = 0.03) compared to those derived from HIV progressors (Fig. 6D). These data suggest that B cell function in HIV controllers is better than B cell function in HIV progressors. Taken together, our data show a stronger B helper capacity of HIV controller-derived CXCR5+ CD4+ T cells as shown by induction of CSR, B cell activation (CD38 expression), and plasma cell differentiation (CD138 expression) than for those derived from HIV progressors.
DISCUSSION
Development of high-affinity class-switched antibodies is dependent on delicate and effective interactions between B cells and helper T cells in the GC regions of lymphoid tissues. Indeed, the dysregulation of such interactions culminates in disease states characterized by overactive or ineffective humoral responses. In HIV, bNAbs represent a class of highly affinity-matured antibodies that develop in spite of, and partly dependent on, significant chronic immune activation leading to immune dysregulation of both the T and B cell compartments. While much has recently been revealed with regard to the biology of HIV-mediated lymphoid tissue dysregulation, the mechanisms underlying B and helper T cell interactions and consequences in chronic infection remain poorly understood. We therefore sought to describe differences in B and helper T cell phenotype and function between two populations of HIV-infected individuals: those that spontaneously control viral replication (controllers) but cannot produce highly affinity-matured antibodies and those who can under certain unknown circumstances but nonetheless progress to disease (progressors). Interestingly, we found superior pTfh helper function in HIV controllers compared to progressors. Our data show an overall strong inverse association between CXCR5+ CD4+ T cells and gp120-specific B cells. We further found a corresponding strong association between CXCR5+ CD4+ T cells that express functional cytokines upon stimulation and Env-specific B cells in an Env-specific, but not Gag-specific, manner. In particular, we found a diametric relationship between Env-specific memory B cell subsets and IL-21+ CXCR5+ helper T cells in controllers and progressors that suggested a functional impairment of HIV-specific Tfh responses in progressors and further directly confirmed this point through in vitro coculture assays.
Recent studies with both humans and monkeys demonstrated that GC Tfh cells expand over the course of HIV or simian immunodeficiency virus (SIV) infection (8, 23). Moreover, while these studies could not establish a direct link between individual viral loads and Tfh cell expansion, the presence of viremia was associated with an increased frequency of Tfh cells and a concurrent reduction of memory B cells in germinal centers, along with peripheral hypergammaglobulinemia (8). In this study, we showed that unlike GC Tfh cells, peripheral CXCR5+ cells are more abundant in the absence of viremia, suggesting that peripheral CXCR5+ T cell dynamics more closely mimic those of bulk CD4+ T cells and not of Tfh cells in the B cell follicles. The role of a peripheral counterpart of GC Tfh cells remains contentious, but it has been shown in mice that pTfh cells may be a memory subset poised to quickly respond and migrate into lymphoid tissues to provide B cell help. The data presented here imply that this may also be the case in HIV-infected individuals, as controllers have more pTfh cells in circulation than progressors. One key limitation of this study is our inability to assay for neutralization activity in our study cohort; thus, we cannot unequivocally conclude whether the higher frequency of pTfh cells in circulation plays a role in the inability of controllers to produce highly affinity-matured antibodies.
We observed stronger IL-21+ cell production in HIV controllers than in progressors, with qualitative differences in the nature of cytokines produced in response to stimulation by Env or Gag. Similar observations have been described in our previous report showing that Env-specific pTfh cells share features of Th2 cells, including higher expression of GATA3 transcription factor and frequency of IL-4, IL-5, and IL-13, as well as lower IFN-γ and tumor necrosis factor alpha (TNF-α) production (19). Our data suggest that the Env-specific IL-21+ CXCR5+ CD4+ T cells, but not Gag-specific responses, have a stronger impact on B cells in the coculture helper assays. Yet how these divergent cellular responses influence HIV-specific B cells in vivo remains unanswered and may be impacted by several factors. The interactions between B cells and helper T cells that result in productive antibody responses are fragile and sensitive to the correct balance of signals. Indeed, abnormal antibody responses and B cell phenotypes have been reported from aberrant gp120 and Nef signaling (24, 25), and IL-10 and CD154 can be either helpful or deleterious in combination with different factors (26). Moreover, HIV exploits these interactions and hijacks the CD40 signaling pathway to induce alterations in B cell phenotype and function (27). Thus, the nature of the helper T cell response would be expected to influence antibody response to particular viral antigens. Interestingly, several groups have posited that Env- and Gag-specific antibodies are differentially regulated and directly affected not by viral loads but rather by different amounts of required T cell help (28).
Antigen-experienced B cells expressing CD27 can be differentiated into several distinct phenotypic and functional subsets based on lineage and differentiation markers, and the prevalence of these subpopulations is profoundly skewed in HIV infection (14, 18). The dysregulation of the B cell compartment in HIV infection is likely caused by both direct immune stimulation and bystander mechanisms of immunologic damage (14); some of which persist in the presence of ART (15). A recent study reported that bulk, non-antigen-specific RM B cells in peripheral blood are depleted during HIV infection, while AM and TLM cell frequencies expand. Unlike the case with AM and TLM cells, the depletion of the RM subset is not corrected by ART treatment. Moreover, using various distinct HIV Env probes, Kardava et al. showed that the majority of Env-specific B cells lie in abnormal memory subsets and that control of viremia results in enrichment of these responses in the RM cell compartment (17). We similarly found that Env-specific B cells in controllers are enriched in the RM B cell subset, whereas in progressors, Env-specific B cells are significantly contained in the TLM and, to a lesser extent, AM subsets. Moreover, we observed a significant, diametric relationship between Env-specific IL-21+ CXCR5+ CD4+ T cells and Env-specific memory B cell subsets present in controllers and progressors, supporting our previous study demonstrating the IL-21-secreting cells more closely represent a peripheral blood counterpoint of GC Tfh cells (19). Furthermore, while assessment of humoral responses in the cohort studies described here was outside the scope of this study due to sample limitations, we showed enhanced antigen-specific B helper activity in specimens derived from controllers compared to progressors. Of note, using a similar technique, Doria-Rose et al. reported that plasmablasts were expanded in peripheral blood of HIV+ PBMCs and accounted for most of the antigen-specific antibody-secreting cells (ASC) in HIV-infected samples. However, they only found a relationship between development of bNAbs and viral load, and not Env-specific ASC, CD4+ T cell count, or years since diagnosis (2). Thus, the present findings builds upon those of Kardava et al., who reported no significant associations between CD4 T cells (frequency or count) and percent RM Env-specific B cells (17).
In summary, the results reported here provide insights into the effects of viral replication-induced chronic activation on bulk and antigen-specific B cell memory subsets, the frequency of circulating helper T cells, and their helper capabilities. We took advantage of recently developed probes to demonstrate that Env-specific B cells are enriched in different memory B cell compartments in controllers versus progressors and are differentially associated with pTfh cells. Importantly, we further show that helper activity was preserved in the absence of chronic immune activation. Further studies will be required to elucidate how bNAbs develop in HIV progressors but not controllers. Thus, our current study provides additional immunologic insight into the interrelatedness of HIV viremia, helper T cell, and B cell dynamics that may inform the process by which B cells receive the necessary signals to produce highly affinity-matured antibodies in spite of tremendous immune dysfunction.
MATERIALS AND METHODS
Study participants.
Cryopreserved PBMCs from treatment-naive chronically HIV type 1 (HIV-1)-infected individuals were used in this study. Frozen PBMCs of 16 chronically HIV-infected subjects were collected under the Walter Reed Army Institute of Research Institutional Review Board (IRB)-approved clinical study RV149. In addition, 20 HIV-infected adults were sampled from the SCOPE cohort, a clinic-based cohort of more than 1,000 chronically HIV-infected individuals at the University of California, San Francisco. We evaluated three distinct groups of HIV-infected individuals based on their respective viral loads: (i) HIV progressors with viral loads of >2000 copies/ml; (ii) HIV viremic controllers (VC), who have maintained plasma HIV RNA levels of between 50 and 2,000 copies/ml; and (iii) HIV elite controllers (EC), defined as HIV-seropositive individuals maintaining plasma HIV RNA levels of <50 copies/ml (Table 1). Nine healthy donors were included as negative controls. All study subjects gave informed consent, and approval was obtained from the IRB at the Walter Reed Army Institute of Research.
TABLE 1.
Clinical characterization of HIV-1-infected participants and healthy controls
| Parameter | Datum for: |
|||
|---|---|---|---|---|
| HIV progressors | Viremic controllers | Elite controllers | Healthy donors | |
| No. of subjects | 16 | 12 | 8 | 9 |
| Antiretroviral therapy | Naive | Naive | Naive | |
| Age (yrs), mean ± SD | 39 ± 9 | |||
| Gender (male:female) | 16:00 | |||
| Duration of infection (yrs), mean ± SD | 17 ± 6.3 | |||
| CD4+ T cells/mm3, mean ± SD | 512 ± 143 | 570 ± 152 | 1,395 ± 399 | |
| CD8+ T cells/mm3, mean ± SD | 1,293 ± 520 | 1,060 ± 429 | ||
| CD4/CD8 ratio, mean ± SD | 0.52 ± 0.25 | 1.45 ± 0.58 | ||
| Median plasma HIV-1 RNA (copies/ml), mean ± SD | 19,414 ± 30,548 | 894 ± 607 | <40 | |
Intracellular cytokine staining and phenotype analysis.
Frozen PBMCs from HIV-infected individuals were thawed and separated into B cell-depleted and -enriched fractions via a CD19+ MultiSort kit according to the manufacturer's instructions (Miltenyi Biotec). The flowthrough, CD19-depleted fraction was stimulated with HIV envelope potential T cell epitope (PTE) peptide pools (1 μg/ml; NIH AIDS Reagent Program) for 6 h in complete RPMI medium in the presence of costimulating antibodies CD28/49d (BD Biosciences) and low endotoxin azide free (LEAF)-purified CD40 monoclonal antibody (MAb; Biolegend). After 1 h of stimulation, a cocktail of Golgi Stop (monensin; BD) and brefeldin A (Sigma) was added to the PBMCs for the remainder of the incubation. Following stimulation, cells were washed with phosphate-buffered saline (PBS) and stained with amine-reactive viability dye (LIVE/DEAD Aqua; Life Technologies) for 20 min at room temperature. The cells were washed with staining buffer (PBS with 2% fetal calf serum [FCS]) and then stained for 20 min at 4°C with the surface markers CXCR5-Alexa Fluor (AF)488 (CD185, J252D4; Biolegend), PD-1-eFluor650NC (J105; eBioscience), CD45RO-Brilliant Violet (BV)711 (UCHL1; Biolegend), and CD8-allophycocyanin (APC; SK1; BD) in the dark. All cells were fixed for 15 min at room temperature with 2% paraformaldehyde–PBS (Sigma-Aldrich) and subsequently permeabilized. Intracellular staining was done with CD3-Qdot605 (UCHT1; Invitrogen), CD4-phycoerythrin (PE)-CF594 (RPA-T4; BD), CD154-PE-Cy5 (CD154, TRAP1; BD), IFN-γ–AF700 (B27; Biolegend), IL-4–BV421 (MP4-25D2; Biolegend), IL-10–PE–Cy7 (JES3-9D7; Biolegend), and IL-21–PE (4BG1; Biolegend) for 30 min at 4°C in the dark. After being washed, all cells were resuspended in fluorescence-activated cell sorter (FACS) buffer and analyzed on a FACS LSRII flow cytometer with FACS Diva software (Becton Dickinson, San Jose, CA). Data were analyzed using FlowJo 9.0.2 (Ashland, OR).
HIV envelope-specific B cell phenotyping.
HIV Env-specific B cells were detected as shown previously (2). Briefly, B cells were detached from CD19-binding beads, blocked with LEAF-purified CD4 for 30 min at 4°C, and incubated with His-tagged HIV-1 CN-54 gp120 recombinant protein (10 μg/ml). Env-specific B cells were detected with His-AF488 (mouse IgG1/clone AD1.1.10). Nonspecific background levels were determined by using both HIV-negative cells incubated with gp120 as described above and HIV-positive cells with His-AF488 only. After incubation, cells were washed in cold PBS and resuspended in FACS buffer. Cells were subsequently washed and stained with viability dye and surface markers CD3-PE-CF594 (UCHT1; BD), CD19-BV785 (HIB19; Biolegend), CD21-peridinin chlorophyll protein (PerCP)-Cy5.5 (Bu32; Biolegend), CD27-BV421 (M-T271; BD), CD38-BV711 (HIT2; BD), CD138-APC (DL-101; Biolegend), CD274-PE-Cy7 (PDL1, M1H1; eBioscience), IgD-AF700 (IA6-2; Biolegend), IgG-APC-Cy7 (HP6017; Biolegend), and IgM-PE-Cy5 (G20 + 127; BD) for 30 min at 4°C in the dark. After being washed, all cells were resuspended in FACS buffer and analyzed on a FACS LSRII flow cytometer with FACS Diva software (Becton Dickinson, San Jose, CA). Data were analyzed using FlowJo 9.0.2 (Ashland, OR).
CD4 T/B cell helper assay.
CD4+ T cells and naive B cells were negatively isolated using an untouched human CD4 T cell kit (Dynabeads; Invitrogen) and naive B cell isolation kit II (Miltenyi), respectively. pTfh cells were further enriched from CD4+ T cells by incubating with biotin-conjugated anti-CXCR5 and subsequently using streptavidin microbeads (Miltenyi Biotec). CXCR5+ CD4+ T cells were then cocultured with autologous naive B cells at a 1:1 ratio in the presence of either staphylococcal enterotoxin B (SEB) or HIV-1 PTE Env pool peptides and costimulation antibodies CD28/CD49d for 7 days in R20 medium (RPMI 1640, 20% [vol/vol] fetal calf serum, 1% sodium pyruvate, 1% nonessential amino acids, apo-transferrin at 40 μg/ml, 0.1% β-mercaptoethanol) at 37°C. Cells were harvested on day 7, washed twice with FACS buffer, stained for viability (LIVE/DEAD Aqua; Life Technologies) and B cell surface markers, T cell surface markers, and intracellular cytokine staining markers (as described above), and then processed for flow cytometry analysis.
Statistics.
Qualitative differences data between samples were examined using a Mann-Whitney test and Spearman correlation coefficients on GraphPad Prism v7 and Stata statistical software (release 13; StataCorp., College Station, TX). Data are presented as means and standard errors of the means (SEM), determined using Prism software. A P value of <0.05 was regarded as significant utilizing nonparametric statistical tests.
ACKNOWLEDGMENTS
We thank Jintanat Ananworanich for her valuable input.
This work was funded by the National Institutes of Health (NIH; R01 AI091450-01 and R01 AI094602-01) and a cooperative agreement (W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD). The study was further supported by NIH grant P30 AI027763.
The following were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 PTE Gag and Env peptides. Human recombinant IL-2 was obtained from Maurice Gately, Hoffmann-La Roche Inc.
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense.
REFERENCES
- 1.Streeck H, D'Souza MP, Littman DR, Crotty S. 2013. Harnessing CD4(+) T cell responses in HIV vaccine development. Nat Med 19:143–149. doi: 10.1038/nm.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doria-Rose NA, Klein RM, Daniels MG, O'Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, Mascola JR, Connors M. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol 84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sather DN, Carbonetti S, Kehayia J, Kraft Z, Mikell I, Scheid JF, Klein F, Stamatatos L. 2012. Broadly neutralizing antibodies developed by an HIV-positive elite neutralizer exact a replication fitness cost on the contemporaneous virus. J Virol 86:12676–12685. doi: 10.1128/JVI.01893-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Euler Z, Bunnik EM, Burger JA, Boeser-Nunnink BD, Grijsen ML, Prins JM, Schuitemaker H. 2011. Activity of broadly neutralizing antibodies, including PG9, PG16, and VRC01, against recently transmitted subtype B HIV-1 variants from early and late in the epidemic. J Virol 85:7236–7245. doi: 10.1128/JVI.00196-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog 7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK, Poignard P, Crotty S. 2013. Human circulating PD-1(+)CXCR3(−)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze zur Wiesch J, Streeck H. 2012. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest 122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. 2016. Follicular helper T cells. Annu Rev Immunol 34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 10.Pissani F, Streeck H. 2014. Emerging concepts on T follicular helper cell dynamics in HIV infection. Trends Immunol 35:278–286. doi: 10.1016/j.it.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrovas C, Koup RA. 2014. T follicular helper cells and HIV/SIV-specific antibody responses. Curr Opin HIV AIDS 9:235–241. doi: 10.1097/COH.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 12.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G Jr, Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. 2013. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cubas R, Perreau M. 2014. The dysfunction of T follicular helper cells. Curr Opin HIV AIDS 9:485–491. doi: 10.1097/COH.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moir S, Fauci AS. 2013. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev 254:207–224. doi: 10.1111/imr.12067. [DOI] [PubMed] [Google Scholar]
- 15.Amu S, Ruffin N, Rethi B, Chiodi F. 2013. Impairment of B-cell functions during HIV-1 infection. AIDS 27:2323–2334. doi: 10.1097/QAD.0b013e328361a427. [DOI] [PubMed] [Google Scholar]
- 16.Walker BD, Yu XG. 2013. Unravelling the mechanisms of durable control of HIV-1. Nat Rev Immunol 13:487–498. doi: 10.1038/nri3478. [DOI] [PubMed] [Google Scholar]
- 17.Kardava L, Moir S, Shah N, Wang W, Wilson R, Buckner CM, Santich BH, Kim LJ, Spurlin EE, Nelson AK, Wheatley AK, Harvey CJ, McDermott AB, Wucherpfennig KW, Chun TW, Tsang JS, Li Y, Fauci AS. 2014. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J Clin Invest 124:3252–3262. doi: 10.1172/JCI74351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moir S, Ogwaro KM, Malaspina A, Vasquez J, Donoghue ET, Hallahan CW, Liu S, Ehler LA, Planta MA, Kottilil S, Chun TW, Fauci AS. 2003. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc Natl Acad Sci U S A 100:6057–6062. doi: 10.1073/pnas.0730819100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz BT, Teigler JE, Pissani F, Oster AF, Kranias G, Alter G, Marovich M, Eller MA, Dittmer U, Robb ML, Kim JH, Michael NL, Bolton D, Streeck H. 2016. Circulating HIV-specific interleukin-21(+)CD4(+) T cells represent peripheral Tfh cells with antigen-dependent helper functions. Immunity 44:167–178. doi: 10.1016/j.immuni.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Pallikkuth S, Pahwa S. 2013. Interleukin-21 and T follicular helper cells in HIV infection: research focus and future perspectives. Immunol Res 57:279–291. doi: 10.1007/s12026-013-8457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. 2011. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, Xu H, Pascual V, Banchereau J, Garcia-Sastre A, Palucka AK, Ramilo O, Ueno H. 2013. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA. 2012. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest 122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. 2006. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J Immunol 176:3931–3941. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- 25.Swingler S, Zhou J, Swingler C, Dauphin A, Greenough T, Jolicoeur P, Stevenson M. 2008. Evidence for a pathogenic determinant in HIV-1 Nef involved in B cell dysfunction in HIV/AIDS. Cell Host Microbe 4:63–76. doi: 10.1016/j.chom.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller F, Aukrust P, Nordoy I, Froland SS. 1998. Possible role of interleukin-10 (IL-10) and CD40 ligand expression in the pathogenesis of hypergammaglobulinemia in human immunodeficiency virus infection: modulation of IL-10 and Ig production after intravenous Ig infusion. Blood 92:3721–3729. [PubMed] [Google Scholar]
- 27.Epeldegui M, Thapa DR, De la Cruz J, Kitchen S, Zack JA, Martinez-Maza O. 2010. CD40 ligand (CD154) incorporated into HIV virions induces activation-induced cytidine deaminase (AID) expression in human B lymphocytes. PLoS One 5:e11448. doi: 10.1371/journal.pone.0011448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binley JM, Klasse PJ, Cao Y, Jones I, Markowitz M, Ho DD, Moore JP. 1997. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol 71:2799–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]