ABSTRACT
Staphylococcus aureus possessing either the smr gene or the qacA/B genes is associated with decreased susceptibility to chlorhexidine gluconate (CHG) and other antiseptics. Previous studies of antiseptic-tolerant staphylococci have focused largely on high-risk populations, and the exact role of health care exposure in the acquisition of these organisms is unclear. We sought to describe the risk factors and features of infection caused by antiseptic-tolerant S. aureus in a general pediatric population. Isolates were selected from an ongoing S. aureus surveillance study. Every third sequential isolate in the year 2014 was selected for inclusion. All isolates underwent PCR for the genes qacA/B and smr. Medical records were reviewed. Five hundred six isolates were included in the study, with 377 (74.3%) being community acquired. One hundred (19.8%) isolates were smr positive and 79 (15.6%) qacA/B positive. In univariable analyses, the presence of either gene was associated with underlying medical conditions, nosocomial acquisition, recent hospitalization, central venous lines, and CHG exposure. In multivariable analyses, only differences between patients with chronic medical conditions (odds ratio [OR] = 1.72; 95% confidence interval [CI], 1.22 to 2.64) and nosocomial acquisition (OR = 2.48; 95% CI, 1.16 to 8.17) remained statistically significant. Among patients without risk factors, 27.9% had infection with an antiseptic-tolerant isolate. smr- or qacA/B-positive S. aureus isolates are common in children and are independently associated with nosocomial acquisition and underlying medical conditions. These findings imply a role for the health care environment in acquisition of these organisms. However, genotypic antiseptic tolerance was seen in >25% of healthy children with an S. aureus infection, indicating that these organism are prevalent in the community as well.
KEYWORDS: chlorhexidine, smr, qacA/B, Staphylococcus aureus, children
INTRODUCTION
Health care-associated infections (HAIs) are associated with substantial morbidity and mortality for individual patients and also place increased resource burdens on the health care system (1, 2). A study published in 2013 revealed that the estimated costs of the five most common HAIs in the United States totaled $9.8 billion (3). One of the most commonly implemented strategies to minimize the incidence of HAIs includes the use of topical antimicrobials and antiseptics, among the most prevalent of which is chlorhexidine gluconate (CHG). CHG-based body washes, oral solutions, and central-line care bundles have been shown to decrease the incidence of HAIs in both adults and children (4–7).
Staphylococcus aureus remains one of the principal causes of HAI in children (8–11). In S. aureus, the smr gene and the qacA/B gene complex have been associated with elevated MICs and minimum bactericidal concentrations (MBCs) of CHG. A number of investigators have discovered an increase in the incidence of organisms bearing these genes following widespread use of CHG in hospital units (12–14) and, more rarely, following exposure to CHG outside the hospital setting (15, 16). Importantly, the presence of these genes has been associated with resistance to other systemic antimicrobial agents, including clindamycin and ciprofloxacin (smr), as well as higher MICs of vancomycin (qacA/B) (17, 18).
The exact influence of health care exposure and, specifically, antiseptic exposure on the acquisition of these organisms is controversial. The impact of the use of these agents on the development of genotypic or in vitro CHG resistance has been minimal in large clinical trials of CHG-based decolonization regimens (15, 19). Previous studies of children have shown an association between antiseptic tolerance genes in S. aureus and the presence of central venous lines (CVLs), as well as a higher rate of invasive infection (18). Many previous pediatric studies, however, have been biased by including high-risk populations, such as neonates/infants and oncology and cardiac surgery patients (12, 18, 20, 21).
The goals of the present study were (i) to define the relative prevalence of the smr and qacA/B genes among a random sample of clinical S. aureus isolates from a general pediatric population and (ii) to determine the clinical features and outcomes associated with S. aureus isolates positive for these genes compared to those of negative controls in children.
RESULTS
During the study period, 1,530 unique S. aureus isolates were catalogued in the Texas Children's Hospital (TCH) S. aureus surveillance database, with 506 viable nonduplicate isolates included in the present study and undergoing screening. The median age of patients was 2.4 years (interquartile range [IQR], 1.1 to 8.2 years), and the racial/ethnic makeup of patients was very similar to that of the greater Houston area (Table 1). The vast majority of infections were community acquired (377/506 [74.3%]), and the most common infectious disease diagnosis was skin and soft tissue infection (SSTI) (386/506 [76.3%]), followed by bacteremia/endocarditis (26/506 [5.1%]) and musculoskeletal infections (24/506 [4.7%]). Invasive infections occurred in 22.1% of patients. An underlying medical illness was present in 177 (35%) patients, of which the most common illnesses were eczema (36/506 [7.1%]), immunocompromising conditions (35/506 [6.9%]), allergic rhinitis (21/506 [4.2%]), and asthma (19/506 [3.8%]).
TABLE 1.
Characteristics of the study group and univariable comparison of isolates with and without antiseptic tolerance genesa
| Parameter | Values for all patients (n = 506)b | Values for patients infected with isolates that were |
P value (smr-positive isolates vs smr-negative isolates) | Values for patients infected with isolates that were |
P value (qacA/B-positive isolates vs qacA/B-negative isolates) | ||
|---|---|---|---|---|---|---|---|
| smr positive (n = 100) | smr negative (n = 406) | qacA/B-positive (n = 79) | qacA/B negative (n = 427) | ||||
| Median age (yr) | 2.4 (1.1–8.2) | 2.1 (0.8–5.5) | 2.5 (1.2–8.3) | 0.2 | 3.1 (0.8–10.3) | 2.2 (1.1–8.2) | 0.6 |
| Female gender | 259 (51.2) | 48 (48) | 211 (52) | 0.5 | 34 (43) | 225 (52.3) | 0.1 |
| African American race | 130 (25.5) | 25 (25) | 105 (25.9) | 0.9 | 22 (27.9) | 108 (25.9) | 0.6 |
| Hispanic ethnicity | 236 (46.6) | 43 (43) | 213 (52.4) | 0.4 | 33 (41.8) | 203 (47.5) | 0.4 |
| Site of acquisition of infection | 0.03 | 0.02 | |||||
| Community acquired | 377 (74.5) | 64 (64) | 313 (77.1) | 53 (67) | 324 (75.9) | ||
| Community-onset health care associated | 104 (20.6) | 29 (29) | 75 (18.5) | 17 (21.5) | 87 (20.4) | ||
| Nosocomial | 25 (4.9) | 7 (7) | 18 (4.4) | 9 (11.3) | 16 (3.7) | ||
| Any underlying medical condition | 177 (35) | 43 (43) | 134 (33) | 0.06 | 39 (49.4) | 138 (32.3) | 0.005 |
| Immunocompromising conditions | 35 (6.9) | 11 (11) | 24 (5.9) | 0.08 | 7 (8.9) | 28 (6.6) | 0.46 |
| Cardiac disease | 18 (3.6) | 5 (5) | 13 (3.2) | 0.37 | 8 (10.1) | 10 (2.3) | 0.003 |
| Hospitalization in the prior 3 mo | 90 (17.8) | 23 (23) | 67 (16.5) | 0.14 | 20 (25.3) | 70 (16.4) | 0.07 |
| Surgery in the prior 3 mo | 56 (11.1) | 16 (16) | 40 (9.9) | 0.37 | 10 (12.7) | 46 (10.7) | 0.55 |
| Receipt of antibiotics in the prior 3 mo | 241 (47.8) | 57 (57) | 184 (45.3) | 0.045 | 35 (44.3) | 206 (48.2) | 0.54 |
| Central venous catheter in situ | 32 (6.3) | 9 (9) | 23 (5.7) | 0.12 | 9 (11.4) | 23 (5.4) | 0.07 |
| Any receipt of CHG in the prior 3 mo | 49 (9.7) | 15 (15) | 34 (8.3) | 0.04 | 11 (13.9) | 38 (8.9) | 0.21 |
| Infection leading to hospital admission | 270 (53.4) | 59 (59) | 211 (51.9) | 0.2 | 37 (46.8) | 233 (54.6) | 0.7 |
| Admission for ≥24 h | 190 (37.5) | 55 (55) | 145 (35.7) | 0.001 | 30 (37.9) | 160 (37.4) | 1 |
| Infection leading to ICU admission | 28 (5.5) | 6 (6) | 22 (5.4) | 0.8 | 11 (13.9) | 17 (3.9) | 0.002 |
| Median length of hospital stay (days) | 5 (2–10) | 3 (2–9) | 3 (2–7) | 0.3 | 8 (4–20) | 3 (2–6) | <0.001 |
| 30-day readmission | 18 (3.6) | 8 (8) | 10 (2.4) | 0.01 | 3 (3.8) | 15 (3.3) | 1 |
| All-cause mortality | 3 (0.6) | 1 (1) | 2 (0.5) | 0.48 | 1 (1.3) | 2 (0.4) | 0.4 |
| Invasive infectionsc | 112 (22.1) | 29 (29) | 83 (20.4) | 0.08 | 25 (31.6) | 87 (20.3) | 0.04 |
| Methicillin resistance | 264 (52.2) | 59 (59) | 205 (50.4) | 0.1 | 15 (18.9) | 249 (58.3) | <0.001 |
| Clindamycin resistance | 70 (13.8) | 21 (21) | 49 (12.1) | 0.02 | 16 (20.3) | 54 (12.6) | 0.07 |
| Vancomycin MIC of ≥2 μg/ml | 12 (2.4) | 5 (5) | 7 (1.7) | 0.06 | 4 (5.1) | 8 (1.9) | 0.09 |
Continuous variables are expressed as medians with interquartile ranges (IQRs). Categorical variables expressed as numbers (percentages) of patients.
The most common diagnoses overall were skin and soft tissue infections (386 [76.3%]), bacteremia/endocarditis (26 [5.1%]), and musculoskeletal infections (24 [4.7%]).
Invasive infections included bacteremia, central-line-associated bloodstream infections, endocarditis, musculoskeletal infection, pneumonia/empyema, peritonitis, central nervous system infection, deep abscesses (such as deep neck abscesses), and deep surgical-site infections.
Antiseptic tolerance genes.
Overall, 100 isolates (19.8%) were positive for the smr gene by PCR, 79 (15.6%) were positive for qacA/B, and 13 (2.5%) were positive for all three genes. In univariable analyses, S. aureus isolates positive for smr were less likely to be community acquired (P = 0.03) and more likely to be clindamycin resistant (21% versus 12.1%; P = 0.02) (Table 1) than isolates negative for smr. In addition, smr-positive isolates were more likely to be associated with prior antibiotic exposure (57% versus 45.3%; P = 0.04) and prior CHG use (15% versus 8.4%; P = 0.04). Furthermore, smr-positive organisms were more often associated with 30-day readmission (8% versus 2.9%; P = 0.01).
In contrast, qacA/B-positive infections were far less likely to be methicillin resistant (18.9% versus 58.3%; P < 0.001) and were associated with higher vancomycin MICs (P = 0.09) (Table 1) in univariable analyses. As with smr-positive organisms, qacA/B-positive organisms were less likely to be community acquired (P = 0.02) and were more likely to be seen in patients with underlying conditions, especially cardiac disease (10.1% versus 2.3%; P = 0.003). In addition, qacA/B-positive organisms were more often associated with invasive infections (31.6% versus 20.3%; P = 0.04), CVLs (11.4% versus 5.4%; P = 0.05), and intensive care unit (ICU) admission than qacA/B-negative organisms (13.9% versus 3.9%; P = 0.002).
Comparisons were made between patients infected with isolates bearing smr and/or qacA/B (see Table S1 in the supplemental material). Infections due to isolates bearing both qacA/B and smr were far less likely to be methicillin resistant (P < 0.001), more likely to be clindamycin resistant (P = 0.02), and more likely to be associated with CHG exposure (P = 0.05) and underlying medical conditions (P < 0.001) than isolates with only one tolerance gene or no tolerance genes. In addition, isolates bearing both genes were more likely to be invasive in nature (P = 0.03) and associated with ICU admission (P = 0.007).
Chlorhexidine exposure.
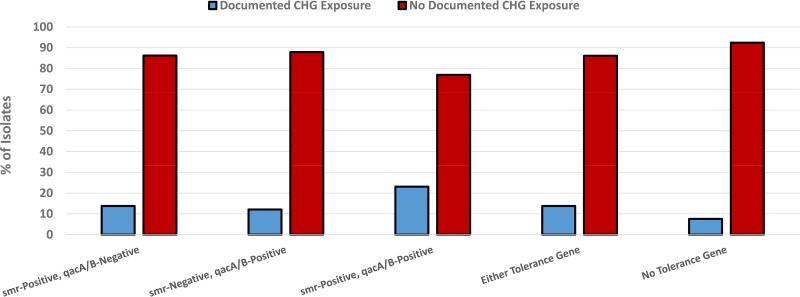
Only 49 patients (9.7%) had documented receipt of at least one application of CHG in the 3 months prior to presentation. Among these, 26 (53.1%) had CHG exposure associated with the presence of a CVL and 33 (67.3%) had CHG exposure associated with surgical procedures; 30 (61.2%) cases were community-onset health care associated (CO-HCA), and 17 (34.7%) were nosocomial. Among isolates obtained from those patients with documented CHG exposure, the presence of either tolerance gene (46.9% versus 31.3%; P = 0.04), specifically smr (30.6% versus 18.6%; P = 0.06), was more common than among those isolates obtained from patients without a history of CHG exposure (Fig. 1). Six patients (1.2%) had CHG exposure without a history of CVLs or surgery. Among these, four had been prescribed topical CHG preparations for recurrent skin infections, and two received CHG-based mouthwashes for gingivitis; four out of these six patients (66.6%) had infection with an smr-positive organism (P = 0.009).
FIG 1.
Impact of CHG exposure on antiseptic tolerance in S. aureus isolates with and without smr and qacA/B. Comparisons of genotypes were done in terms of documented CHG exposure. P was equal to 0.08 in comparisons across all categories; P was equal to 0.03 in a comparison of isolates with either tolerance gene and no tolerance gene.
Risk factors for any antiseptic tolerance gene.
Comparisons were made between infections due to organisms harboring at least one of the antiseptic tolerance genes (n = 166) and isolates without these genes (n = 340) (Table 2). Underlying chronic medical conditions, nosocomial acquisition of infection, previous hospital admission, the presence of central venous lines, and previous exposure to CHG were associated with the presence of either antiseptic tolerance gene in univariable analyses. When these clinical features were included in a multivariable logistic regression model for antiseptic tolerance, only underlying conditions (P = 0.004; odds ratio [OR], 1.72; 95% confidence interval [CI], 1.22 to 2.64) and nosocomial acquisition (P = 0.04; OR, 2.48; 95% CI, 1.16 to 8.17) remained statistically significant. The presence of a combination of these two clinical factors in any given patient with an S. aureus infection was examined in terms of the likelihood of an antiseptic-tolerant isolate. There was an additive effect of the number of risk factors present on the proportion of isolates with genotypic antiseptic tolerance (P < 0.001), ranging from 27.2% (89/328) with neither of these factors present to 40.9% (63/154) with one and 58.3% (14/24) with 2 risk factors present. Among nosocomial S. aureus isolates, 15/25 (60%) were positive for either smr or qacA/B; among isolates obtained from patients with underlying conditions, 76/177 (42.9%) carried at least one of these genes.
TABLE 2.
Characteristics of infections secondary to antiseptic-tolerant versus -susceptible organismsa
| Parameter | Values for patients infected with isolates with: |
Univariable P value | Adjusted multivariable P value | Odds ratio | 95% CI | |
|---|---|---|---|---|---|---|
| Either tolerance gene (n = 166) | No tolerance gene (n = 340) | |||||
| Age (yr) | 2.4 (0.8–8.2) | 2.4 (1.2–8.2) | 0.8 | |||
| Female gender | 75 (45.2) | 184 (54.1) | 0.08 | |||
| African American race | 45 (27.1) | 85 (25) | 0.6 | |||
| Hispanic ethnicity | 71 (42.8) | 165 (48.5) | 0.3 | |||
| Underlying conditions | 76 (45.8) | 101 (29.7) | 0.001 | 0.004 | 1.72 | 1.22–2.64 |
| Immunocompromised | 17 (10.2) | 19 (5.6) | 0.05 | |||
| Cardiac disease | 11 (6.6) | 7 (2.1) | 0.02 | |||
| Nosocomial acquisition | 15 (9) | 10 (2.9) | 0.004 | 0.04 | 2.48 | 1.16–8.17 |
| Hospitalization in prior 3 mo | 40 (24.1) | 50 (14.7) | 0.013 | 0.35 | 1.33 | 0.74–2.44 |
| Antibiotic use in prior 3 mo | 87 (52.4) | 154 (45.3) | 0.2 | |||
| Surgery in prior 3 mo | 24 (14.5) | 32 (9.4) | 0.11 | |||
| CVL in situ | 16 (9.6) | 16 (4.7) | 0.05 | 0.58 | 1.43 | 0.54–2.88 |
| Any CHG use in prior 3 mo | 23 (13.9) | 26 (7.6) | 0.04 | 0.68 | 1.1 | 0.44–2.36 |
| Clinical outcomes | ||||||
| Methicillin-resistant isolate | 72 (43.6) | 192 (56.5) | 0.006 | |||
| Clindamycin-resistant isolate | 32 (19.3) | 38 (11.2) | 0.02 | |||
| Vancomycin MIC of ≥2 μg/ml | 7 (6.6) | 5 (1.5) | 0.01 | |||
| Invasive infection | 49 (29.5) | 63 (18.6) | 0.006 | |||
| Infection leading to hospital admission | 87 (52.4) | 183 (53.8) | 0.7 | |||
| ICU admission | 15 (9) | 13 (3.8) | 0.02 | |||
| Length of stay (days) | 5 (2–10) | 2 (1–6) | 0.001 | |||
| 30-day readmission | 7 (4.2) | 6 (1.8) | 0.1 | |||
| All-cause mortality | 2 (1.2) | 1 (0.3) | 0.2 | |||
Continuous variables are expressed as medians with interquartile ranges. Categorical variables are expressed as numbers (percentages) of patients.
Overall, the presence of either gene was associated with a higher rate of invasive infection (29.5% versus 18.6%; P = 0.006), intensive care unit admission (9% versus 3.8%; P = 0.03), and a longer median length of stay (5 days [IQR, 2 to 10 days] versus 2 days [IQR, 1 to 6 days]; P = 0.001) than found with isolates lacking these genes.
Genotypic antiseptic tolerance in the absence of risk factors.
Two hundred ninety-five patients did not have any of the above-identified clinical features associated with smr- and/or qacA/B-positive S. aureus isolates in univariable analyses (nosocomial acquisition of infection, admission in the prior 3 months, CVL in situ, CHG use, or underlying chronic medical conditions); of these 295 patients, 79 (26.7%) had infection with an isolate with genotypic antiseptic tolerance. Among these healthy children, 50 patients had isolates that were smr positive (16.9%), and 36 had isolates that were qacA/B positive (12.2%). In patients without risk factors, S. aureus isolates with genotypic antiseptic tolerance were more often associated with a longer length of hospital stay (4 days versus 2 days; P = 0.04) and readmission within 30 days (5.2% versus 0.2%; P = 0.04) than susceptible isolates.
Invasive infections.
One hundred twelve patients in the study had invasive infections. Comparisons were made between invasive and noninvasive infections (Table S2). Several clinical variables were associated with invasive infections in univariable analyses; however, in multivariable analyses, only nosocomial acquisition (OR = 10.8; 95% CI, 1.78 to 66.2), underlying medical conditions (OR = 2.02; 95% CI, 1.15 to 3.53), and previous admission (OR = 2.6; 95% CI, 1.2 to 5.63) remained significantly associated with invasive infection.
DISCUSSION
Numerous studies have illustrated the clear benefit of the use of CHG and other antiseptic preparations in the prevention of HAIs. There is concern, however, regarding the potential development of decreased susceptibility to these agents over time. We have performed a cross-sectional study with a large random sample of pediatric S. aureus isolates at a tertiary care children's hospital and found that 32.8% of isolates harbored smr and/or qacA/B. This is higher than the rate of genotypic antiseptic tolerance of 18.5% among a random sample of pediatric S. aureus isolates described in a study performed at Vanderbilt from 2004 to 2009 (22). This discrepancy may reflect both geographic variation and a temporal trend for an increased prevalence of these genes among staphylococci.
There are numerous reports of a temporal relationship between CHG use and the detection of genotypically antiseptic-tolerant staphylococci in hospital units (13, 14). The presence of smr- and/or qacA/B in S. aureus is associated with health care exposure, as illustrated by an association in multivariable analyses with nosocomial acquisition and underlying medical conditions. Notably, the presence of these risk factors in a given patient had an additive effect, such that in patients with both of these factors, the proportion of S. aureus isolates positive for smr and/or qacA/B was 58.3%. Interestingly, while any CHG exposure was associated with qacA/B or smr in univariable analyses, it lost statistical significance in our multivariable analysis; this may potentially be a consequence of the high degree of collinearity between any CHG exposure (as defined in this study) and CO-HCA/nosocomial acquisition and CVLs.
Only a small proportion of our study population (9.7%) had documented CHG exposure in the 3 months preceding their sampling. However, among isolates taken from patients with previous CHG exposure, the proportion of cases bearing either a tolerance gene or specifically smr was higher than among those without CHG exposure. This is consistent with reports of increasing prevalence of qacA/B among staphylococci in hospital settings following the initiation of daily CHG bathing (14). While this finding was statistically significant, the overall effect size is small, with an absolute difference of only 15.6%. This small effect size, which was detectable with our overall large data set, in part may explain the lack of emergence of CHG nonsusceptibility in some clinical trials of this agent for decolonization, particularly with declines in HAI rates after CHG use (19, 23). Fritz et al., in a trial of a mupirocin- and CHG-based regimen to prevent recurrent skin and soft tissue infection in the community, found the emergence of qacA/B-positive S. aureus in only 2/215 (0.9%) patients receiving CHG (15). Interestingly, among six patients with a history of CHG exposure independent of prior surgery or CVL placement, 4/6 (66.7%) had infection secondary to infection with an smr-positive staphylococcus, suggesting that CHG regimens used by practitioners in the community may select for genotypic antiseptic tolerance.
It is notable that 26.7% of patients without any of the above-described risk factors had infection caused by an isolate with either smr or qacA/B. While it is apparent from our data that health care exposure is associated with infection with staphylococci bearing genotypic antiseptic tolerance, it also appears that these organisms exist at a high baseline level in our community. Given the retrospective nature of this study, it is likely that the prevalence of true community-acquired antiseptic-tolerant staphylococci may have been overrepresented; any CHG use and/or other health care exposures not documented in the medical record would not have been captured by our study design. However, the classification of any patient receiving surgery or CVL at our center being considered as having CHG exposure by our study definition helps to minimize this limitation.
The actual impact that the smr and qacA/B genes in S. aureus have on the efficacy of CHG-based antisepsis is controversial. Most studies show that the CHG MICs for these organisms are in the range of 2 to 4 μg/ml, and while this range is higher than for staphylococci lacking these genes (0.5 to 1 μg/ml) (18), it is much lower than the concentration of most CHG preparations in clinical use. What is clear, however, is that these organisms are associated with a more severe clinical phenotype, as manifested by more frequent invasive infections, more ICU admissions, and longer lengths of stay. Importantly, the finding of a longer length of stay was consistently shown in the group of healthy children analyzed. The reasons for the increased severity of illness are unclear but may be related to an unrecognized S. aureus virulence factor that should be further investigated. These findings must be interpreted with caution, as the study lacked a control group with CHG exposure but no infection. Furthermore, the study design precludes determining a causal relationship between genotypic antiseptic tolerance and severity of illness.
The findings in this study have several limitations. The restriction of a 3-month period in our definition of antibiotic and antiseptic exposure may have underrepresented the impact of these particular factors on genotypic antiseptic tolerance. In addition, given the prevalence of smr- and qacA/B-positive staphylococci in otherwise-healthy children, it is possible that this is a consequence of the spread of an advantageous S. aureus clone or clones in our community. Previous work has illustrated that antiseptic-tolerant S. aureus isolates are of highly diverse genetic backgrounds (18), which minimizes, however, the impact of this limitation. In addition, isolates did not undergo CHG MIC determinations for this study, and the presence of smr and/or qacA/B might not directly equate to CHG resistance/tolerance. The large number of isolates in our study makes traditional broth dilution studies very labor-intensive and impractical. In addition, previous work in our center has demonstrated that the presence of these genes is associated with elevated MICs/MBCs of CHG even for community-acquired isolates (24). Finally, given that patients are not routinely screened for S. aureus colonization at our center, we are unable to evaluate the impact that qacA/B or smr may have on the efficacy of CHG-based decolonization regimens in hospitalized patients.
In conclusion, health care exposure, specifically nosocomial acquisition of infection and underlying medical conditions, is associated with genotypic antiseptic tolerance in S. aureus. Furthermore, these infections are associated with a more severe clinical phenotype, including more invasive infections, more ICU admissions, and reduced susceptibility to other systemic antibiotics. smr- and qacA/B-positive S. aureus strains are also common in our community even in the apparent absence of health care exposure. Further study and surveillance are necessary to better understand the consequences of these organisms.
MATERIALS AND METHODS
Patient and isolate selection.
Isolates were selected from an S. aureus surveillance study at TCH ongoing since 2001. All S. aureus isolates identified by the clinical microbiology laboratory at TCH are subcultured and stored in horse blood at −80°C in the Infectious Diseases Research Laboratory. The surveillance study captures only isolates from infectious sources and does not capture colonization cultures. Every third sequential isolate in the calendar year 2014 was selected for inclusion in this study. TCH and the affiliated Texas Children's Pediatric Associates clinics (a network of 52 primary care clinics in the greater Houston area) have an integrated electronic medical record system. For all isolates, a retrospective review of the corresponding inpatient and outpatient electronic medical and pharmacy records was undertaken during a 3-month window prior to their presentation with the infection under study. This study was approved by the institutional review board of Baylor College of Medicine.
Infection control practices.
The routine use of CHG for infection control purposes at our institution has been described elsewhere (18). Briefly, at our institution, CHG is the skin cleanser of choice prior to insertion of central venous lines (CVLs) and their maintenance. Daily CHG bathing is employed at TCH for all hospitalized patients with a CVL in situ. All patients undergoing elective surgery at TCH are encouraged to take a CHG bath the night prior to the operation, and this agent is the skin cleanser of choice in our operating rooms immediately prior to surgery. Daily CHG mouthwashes are routinely prescribed at TCH for hematopoietic stem cell transplant (HSCT) recipients and those with acute myeloid leukemia (AML). Colonization screening for methicillin-resistant S. aureus (MRSA), or for S. aureus in general, is not performed routinely at TCH.
Definitions.
The following types of acquisition of infection were considered: community acquired, community-onset health care associated (CO-HCA), and nosocomial. Community-acquired infections were those occurring in otherwise-healthy children who exhibited the onset of signs and symptoms of infection as outpatients. CO-HCA infections were considered those in which signs and symptoms of infection developed in the outpatient setting in children with underlying medical conditions (25), excluding well-controlled asthma, eczema, and allergic rhinitis. Nosocomial infections were those in which the patient developed signs/symptoms of infection ≥72 h after hospital admission (26). For purposes of this study, patients with underlying medical conditions included all patients with a documented chronic medical illness (including even mild conditions, such as well-controlled asthma). Patients were considered to have CHG exposure in the prior 3 months if they had documented use of any CHG preparation, surgery, or CVL placement at TCH or diagnosis of AML or HSCT; all patients with surgery or CVL placement at TCH were assumed to have CHG exposure even if not clearly documented in the medical record. Invasive S. aureus infections included bacteremia/endocarditis, musculoskeletal infection (osteomyelitis, septic arthritis, and pyomyositis), pneumonia/empyema, peritonitis, central nervous system infection, deep abscesses (such as deep neck abscesses), and deep surgical-site infections (27). Noninvasive infections included skin and soft tissue infections (SSTIs) (such as cellulitis, impetigo, cutaneous abscesses, pustulosis, folliculitis, paronychia, etc.), otitis media, sinusitis, lymphadenitis, and superficial surgical-site infections. Immunocompromising conditions were considered to be malignancy/HSCT, HIV infection, primary immunodeficiency, end-stage renal disease, solid organ transplant, and rheumatologic conditions if the patient was receiving corticosteroids or immunomodulatory agents. For purposes of this study, patients with underlying cardiac disease included patients with congenital heart disease, cardiomyopathy, and medically/surgically managed arrhythmia. Mortality, for purposes of this study, refers to all-cause mortality during hospital admission.
Microbiology and molecular studies.
Testing for susceptibility to oxacillin and clindamycin was performed by the clinical microbiology laboratory in the routine course of care. Vancomycin MICs were determined with the Etest micromethod in the Infectious Diseases Research Laboratory. Whole DNA was prepared from all isolates with the assistance of QIAcube (Qiagen, Valencia, CA). All isolates underwent PCR to detect the presence of smr and qacA/B using previously published primers (18).
Statistical analyses and sample size considerations.
Based on previous studies of health care-associated as well as community-acquired infections at our institution, the proportion of isolates carrying qacA/B or smr was estimated to be between 15 and 25% (18, 20, 24). Given that 1,530 isolates were known to be catalogued in the S. aureus database in 2014, selecting every third sequential isolate allowed for >500 isolates to be included in the study. In the event that the sequential selection of isolates resulted in any given patient having >1 isolate, only the first isolate was included in the study. A 20% prevalence of any given antiseptic tolerance gene was estimated a priori, with allowance for 4:1 matching of susceptible and tolerant isolates. Such a design allowed >80% power to detect a 15% absolute difference in the presence of any given risk factor with an α of 0.05 using a continuity-corrected χ2 value.
Continuous variables were analyzed with either the Wilcoxon rank sum or Kruskal-Wallis test. Categorical variables were compared using Fisher's exact test. In analyses of length of hospital stay, patients admitted for 23-h observation were excluded from these calculations but not excluded from the study as a whole. In analyses of clinical features associated with the presence of either or both antiseptic tolerance genes, variables with a P value of ≤0.1 were included in a multivariable logistic regression model; a priori, it was decided to not include gender or race/ethnicity in the multivariable model. In specific analyses regarding the impact of the mode of infection acquisition (i.e., community-acquired, CO-HCA, or nosocomial), a comparison of nosocomial versus nonnosocomial infections (a combined group of community-acquired and CO-HCA infections) was used in the logistic regression model due to collinearity between CO-HCA acquisition and the presence of underlying medical conditions. The presence of any underlying chronic medical condition rather than individual conditions was included in the regression model. Variables found to be statistically significant in a multivariable analysis were included in a scheme to determine the additive effect of multiple risk factors on the likelihood of antiseptic tolerance. Additional analyses included comparisons of invasive and noninvasive infections (as defined above). All analyses were performed with the assistance of Stata v.13 (StataCorp, College Station, TX).
Supplementary Material
ACKNOWLEDGMENT
This study was funded by NIAID grant K23AI099159 to J. Chase McNeil.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00223-17.
REFERENCES
- 1.Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, Lapetina E, Gemmen E. 2005. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med 165:1756–1761. doi: 10.1001/archinte.165.15.1756. [DOI] [PubMed] [Google Scholar]
- 2.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. 2013. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 173:2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 4.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. 2007. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 167:2073–2079. doi: 10.1001/archinte.167.19.2073. [DOI] [PubMed] [Google Scholar]
- 5.DeRiso AJ II, Ladowski JS, Dillon TA, Justice JW, Peterson AC. 1996. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest 109:1556–1561. doi: 10.1378/chest.109.6.1556. [DOI] [PubMed] [Google Scholar]
- 6.Milstone AM, Elward A, Song X, Zerr DM, Orscheln R, Speck K, Obeng D, Reich NG, Coffin SE, Perl TM, Pediatric SCRUB Trial Study Group. 2013. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 381:1099–1106. doi: 10.1016/S0140-6736(12)61687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darouiche RO, Wall MJ Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, Miller HJ, Awad SS, Crosby CT, Mosier MC, Alsharif A, Berger DH. 2010. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 362:18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 8.Dramowski A, Madide A, Bekker A. 2015. Neonatal nosocomial bloodstream infections at a referral hospital in a middle-income country: burden, pathogens, antimicrobial resistance and mortality. Paediatr Int Child Health 35:265–272. doi: 10.1179/2046905515Y.0000000029. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn RM, Henderson KL, Minaji M, Muller-Pebody B, Johnson AP, Sharland M. 2012. Exploring the epidemiology of hospital-acquired bloodstream infections in children in England (January 2009–March 2010) by linkage of national hospital admissions and microbiological databases. J Pediatr Infect Dis Soc 1:284–292. doi: 10.1093/jpids/pis084. [DOI] [PubMed] [Google Scholar]
- 10.Murray MT, Krishnamurthy G, Corda R, Turcotte RF, Jia H, Bacha E, Saiman L. 2014. Surgical site infections and bloodstream infections in infants after cardiac surgery. J Thorac Cardiovasc Surg 148:259–265. doi: 10.1016/j.jtcvs.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 11.Zingg W, Hopkins S, Gayet-Ageron A, Holmes A, Sharland M, Suetens C, ECDC PPS Study Group. 2017. Health-care-associated infections in neonates, children, and adolescents: an analysis of paediatric data from the European Centre for Disease Prevention and Control point-prevalence survey. Lancet Infect Dis 17:381–389. doi: 10.1016/S1473-3099(16)30517-5. [DOI] [PubMed] [Google Scholar]
- 12.McNeil JC, Hulten KG, Kaplan SL, Mahoney DH, Mason EO. 2013. Staphylococcus aureus infections in pediatric oncology patients: high rates of antimicrobial resistance, antiseptic tolerance and complications. Pediatr Infect Dis J 32:124–128. doi: 10.1097/INF.0b013e318271c4e0. [DOI] [PubMed] [Google Scholar]
- 13.Suwantarat N, Carroll KC, Tekle T, Ross T, Maragakis LL, Cosgrove SE, Milstone AM. 2014. High prevalence of reduced chlorhexidine susceptibility in organisms causing central line-associated bloodstream infections. Infect Control Hosp Epidemiol 35:1183–1186. doi: 10.1086/677628. [DOI] [PubMed] [Google Scholar]
- 14.Warren DK, Prager M, Munigala S, Wallace MA, Kennedy CR, Bommarito KM, Mazuski JE, Burnham CA. 2016. Prevalence of qacA/B genes and mupirocin resistance among methicillin-resistant Staphylococcus aureus (MRSA) isolates in the setting of chlorhexidine bathing without mupirocin. Infect Control Hosp Epidemiol 37:590–597. doi: 10.1017/ice.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fritz SA, Hogan PG, Camins BC, Ainsworth AJ, Patrick C, Martin MS, Krauss MJ, Rodriguez M, Burnham CA. 2013. Mupirocin and chlorhexidine resistance in Staphylococcus aureus in patients with community-onset skin and soft tissue infections. Antimicrob Agents Chemother 57:559–568. doi: 10.1128/AAC.01633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RC, Schlett CD, Crawford K, Lanier JB, Merrell DS, Ellis MW. 2015. Recurrent methicillin-resistant Staphylococcus aureus cutaneous abscesses and selection of reduced chlorhexidine susceptibility during chlorhexidine use. J Clin Microbiol 53:3677–3682. doi: 10.1128/JCM.01771-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi N, Hase M, Kitta M, Sasatsu M, Deguchi K, Kono M. 1999. Antiseptic susceptibility and distribution of antiseptic-resistance genes in methicillin-resistant Staphylococcus aureus. FEMS Microbiol Lett 172:247–253. doi: 10.1111/j.1574-6968.1999.tb13475.x. [DOI] [PubMed] [Google Scholar]
- 18.McNeil JC, Kok EY, Vallejo JG, Campbell JR, Hulten KG, Mason EO, Kaplan SL. 2015. Clinical and molecular features of decreased chlorhexidine susceptibility among nosocomial staphylococcus aureus isolates at Texas Children's Hospital. Antimicrob Agents Chemother 60:1121–1128. doi: 10.1128/AAC.02011-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayden MK, Lolans K, Haffenreffer K, Avery TR, Kleinman K, Li H, Kaganov RE, Lankiewicz J, Moody J, Septimus E, Weinstein RA, Hickok J, Jernigan J, Perlin JB, Platt R, Huang SS, for the Agency for Healthcare Research and Quality (AHRQ) DEcIDE Network and Healthcare-Associated Infections Program and the Centers for Disease Control and Prevention's (CDC) Prevention Epicenters Program. 24 August 2016. Chlorhexidine and mupirocin susceptibility of methicillin-resistant Staphylococcus aureus isolates in the REDUCE-MRSA Trial. J Clin Microbiol. doi: 10.1128/JCM.01444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeil JC, Ligon JA, Hulten KG, Dreyer WJ, Heinle JS, Mason EO, Kaplan SL. 2013. Staphylococcus aureus infections in children with congenital heart disease. J Pediatr Infect Dis Soc 2:337–344. doi: 10.1093/jpids/pit037. [DOI] [PubMed] [Google Scholar]
- 21.Reich PJ, Boyle MG, Hogan PG, Johnson AJ, Wallace MA, Elward AM, Warner BB, Burnham CA, Fritz SA. 2016. Emergence of community-associated methicillin-resistant Staphylococcus aureus strains in the neonatal intensive care unit: an infection prevention and patient safety challenge. Clin Microbiol Infect 22:645.e1–645.e8. doi: 10.1016/j.cmi.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JG, Saye EJ, Jimenez-Truque N, Soper N, Thomsen I, Talbot TR, Creech CB. 2013. Frequency of disinfectant resistance genes in pediatric strains of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 34:1326–1327. doi: 10.1086/673983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlett CD, Millar EV, Crawford KB, Cui T, Lanier JB, Tribble DR, Ellis MW. 2014. Prevalence of chlorhexidine-resistant methicillin-resistant Staphylococcus aureus following prolonged exposure. Antimicrob Agents Chemother 58:4404–4410. doi: 10.1128/AAC.02419-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeil JC, Hulten KG, Kaplan SL, Mason EO. 2014. Decreased susceptibilities to retapamulin, mupirocin, and chlorhexidine among Staphylococcus aureus isolates causing skin and soft tissue infections in otherwise healthy children. Antimicrob Agents Chemother 58:2878–2883. doi: 10.1128/AAC.02707-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulten KG, Kaplan SL, Gonzalez BE, Hammerman WA, Lamberth LB, Versalovic J, Mason EO Jr. 2006. Three-year surveillance of community onset health care-associated Staphylococcus aureus infections in children. Pediatr Infect Dis J 25:349–353. doi: 10.1097/01.inf.0000207404.50143.1e. [DOI] [PubMed] [Google Scholar]
- 26.Hulten KG, Kaplan SL, Lamberth LB, Slimp K, Hammerman WA, Carrillo-Marquez M, Starke JR, Versalovic J, Mason EO Jr. 2010. Hospital-acquired Staphylococcus aureus infections at Texas Children's Hospital, 2001–2007. Infect Control Hosp Epidemiol 31:183–190. doi: 10.1086/649793. [DOI] [PubMed] [Google Scholar]
- 27.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.