ABSTRACT
New and improved treatments for tuberculosis (TB) are urgently needed. Recently, it has been demonstrated that verapamil, an efflux inhibitor, can reduce bacterial drug tolerance caused by efflux pump activity when administered in combination with available antituberculosis agents. The aim of this study was to evaluate the effectiveness of verapamil in combination with the antituberculosis drug candidate Q203, which has recently been developed and is currently under clinical trials as a potential antituberculosis agent. We evaluated changes in Q203 activity in the presence and absence of verapamil in vitro using the resazurin microplate assay and ex vivo using a microscopy-based phenotypic assay for the quantification of intracellular replicating mycobacteria. Verapamil increased the potency of Q203 against Mycobacterium tuberculosis both in vitro and ex vivo, indicating that efflux pumps are associated with the activity of Q203. Other efflux pump inhibitors also displayed an increase in Q203 potency, strengthening this hypothesis. Therefore, the combination of verapamil and Q203 may be a promising combinatorial strategy for anti-TB treatment to accelerate the elimination of M. tuberculosis.
KEYWORDS: Mycobacterium tuberculosis, Q203, verapamil, efflux pump inhibitors, drug efflux, drug resistance
INTRODUCTION
Tuberculosis (TB) is one of the oldest known human diseases and is a major cause of morbidity and mortality worldwide. Indeed, there were 10.4 million newly diagnosed cases of TB and TB accounted for nearly 1.8 million deaths in 2015 (1). TB is an infectious bacterial disease that is primarily caused by Mycobacterium tuberculosis. TB is transmitted when aerosolized droplets, generated by persons with active TB, are inhaled through the large and small airways and onto lung alveolar surfaces (2). In healthy individuals, infection with M. tuberculosis is often asymptomatic, as their immune systems are a sufficient barrier to the growth and division of the bacterium. However, it is estimated that 5% to 10% of people infected with TB will develop clinically active disease at some point in their lives (3).
Recently, the ability to control TB infection has become a global challenge owing to the emergence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB). MDR-TB is present in virtually all countries surveyed, and XDR-TB has now been reported in 117 countries (1). Drug-resistant TB is a greater challenge because it requires a longer treatment course and because there are fewer effective drugs, which are associated with more significant side effects. In addition, the higher cost of second-line drugs implies that management of MDR-TB is a significant financial burden (4).
Intrinsic drug resistance in M. tuberculosis has been attributed to a combination of highly lipid-rich cell walls, which are relatively impermeable, and an active drug efflux mechanism. Efflux is a ubiquitous mechanism of drug resistance in prokaryotic and eukaryotic cells, and M. tuberculosis has numerous transport proteins that pump out toxic antimicrobial compounds from the bacterium. Indeed, M. tuberculosis has one of the largest numbers of putative drug efflux pumps relative to the size of its genome (5). The induction of efflux in response to anti-TB drug treatment has been proposed as the first step in the development of drug resistance, subsequently leading to chromosomal mutation-related resistance (6, 7, 8). While several efflux pumps are known in M. tuberculosis, more studies are required because anti-TB drug discovery has predominantly focused on identifying new active and potent protein target inhibitors. These efforts, however, assume that the identified drugs will be able to reach their target in the bacteria, which may not always be the case, due to the activity of efflux pumps or permeability. Interestingly, the activity of these pumps raises the possibility of using efflux pump inhibitors (EPIs) to increase the activity of existing bactericidal or bacteriostatic agents in the form of combination therapy.
Verapamil is a calcium channel antagonist and an EPI. Verapamil has been shown to reduce tolerance to rifampin in both M. tuberculosis and Mycobacterium marinum. Incubation of M. marinum-infected macrophages with a combination of rifampin and verapamil more successfully cleared bacterial infection compared to rifampin alone (9). Furthermore, verapamil adjunctive therapy in the rodent model was shown to accelerate bactericidal activity and achieve durable sterilization (10). Moreover, Gupta et al. showed that verapamil was able to decrease the MIC of bedaquiline and clofazimine by 8- to 16-fold, which was further confirmed in vivo using a mouse model (11, 12).
We recently discovered Q203, a promising clinical candidate for the treatment of MDR-TB and XDR-TB. Q203 belongs to the imidazopyridine amide (IPA) class of antibiotics and acts by blocking the respiratory cytochrome bc1 complex, leading to reduced intracellular ATP, the energy source of most cellular enzymes, and thereby inhibiting the growth of M. tuberculosis (13). In this study, we have investigated whether efflux can modulate the antituberculosis activity of Q203 in both extracellularly and intracellularly replicating M. tuberculosis.
RESULTS AND DISCUSSION
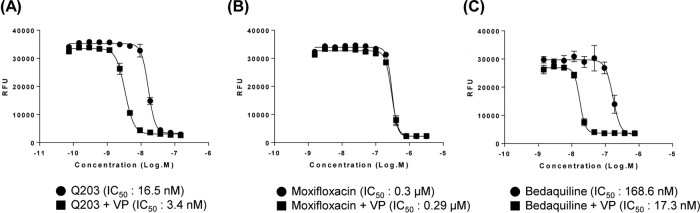
To measure the effects of verapamil on the antimycobacterial activity of Q203, we compared the minimum concentrations required to inhibit the growth of 50% of bacteria (IC50s) of Q203 in the presence and absence of verapamil. For this, verapamil was used to test whether the laboratory strain M. tuberculosis H37Rv displayed efflux-mediated resistance to Q203. We hypothesized that if H37Rv utilizes an efflux resistance mechanism, then the combination of verapamil and Q203 should be able to potentiate the antimycobacterial activity of Q203. In this context, we used two different controls, namely, moxifloxacin and bedaquiline, in these experiments. Moxifloxacin was used as a negative control because its activity, as measured in vitro, was previously unaffected by verapamil. Bedaquiline was included as a positive control because the combination of bedaquiline and verapamil showed greater activity against M. tuberculosis in vitro compared with that of bedaquiline alone due to efflux pump inhibition (11). As shown in Fig. 1, we found that supplementation with verapamil at a concentration of 50 μg/ml decreased the IC50 of Q203 for M. tuberculosis H37Rv by about 4.8-fold (Fig. 1A). As expected, verapamil did not change the IC50 of moxifloxacin (Fig. 1B), while the activity of the positive control bedaquiline against M. tuberculosis was increased 10.3-fold in the presence of 50 μg/ml verapamil (Fig. 1C) as anticipated. Taken together, these results indicate that efflux may influence the activity of Q203 against M. tuberculosis.
FIG 1.
Effect of verapamil on antimicrobial activity. M. tuberculosis H37Rv was grown to mid-log phase in the presence (closed square) or absence (closed circle) of verapamil (VP) (50 μg/ml) and treated with Q203 (A), moxifloxacin (B), and bedaquiline (C). IC50s were calculated based on the concentrations required to inhibit bacterial growth by 50% by fitting the curves with a sigmoidal dose response using GraphPad Prism software (version 6.05).
Next, we confirmed the above finding on clinical isolates of MDR M. tuberculosis. The isolation and drug resistance profiles of the MDR strains against isoniazid, rifampin, streptomycin, and ethambutol are summarized in Table 1. As shown in Fig. S2 in the supplemental material, the combination of Q203 and verapamil showed lower IC50s against all MDR clinical isolates compared to those of Q203 alone. The ratio of IC50 in the presence and absence of verapamil for Q203 ranged from 3.5 to 5.7, which provided further information regarding the role of the efflux mechanism in Q203 resistance (Table 1). We observed that Q203 exhibited similar activities against H37Rv and MDR clinical isolates compared with the previously reported values of 2.5 nM. This indicated that our modified resazurin microplate assay (REMA) (initial inoculum of 5 × 104 CFU/ml and incubation time of 14 days) generated results comparable to those obtained in earlier studies using a green fluorescent protein (GFP) strain of M. tuberculosis (13). The activity of moxifloxacin was not changed in the presence of verapamil as expected.
TABLE 1.
IC50s of Q203 and moxifloxacin against M. tuberculosis in presence or absence of verapamil as determined using REMA
| Strain | Sample origin | Resistant profilea |
IC50b |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STR | EMB | RFM | INH | Q203 (nM [ng/ml]) |
Moxifloxacin (μM [μg/ml]) |
||||||
| VP (+) | VP (−) | Ratio | VP (+) | VP (−) | Ratio | ||||||
| H37Rv | Laboratory strain | S | S | S | S | 3.4 (1.9) | 16.5 (9.2) | 4.8 | 0.29 (0.12) | 0.30 (0.12) | 1.0 |
| MDR 1 | Pleural effusion | S | S | R | R | 9.7 (5.4) | 34.0 (18.9) | 3.5 | 0.34 (0.14) | 0.34 (0.14) | 1.0 |
| MDR 2 | Sputum | S | S | R | R | 3.0 (1.7) | 14.2 (7.9) | 4.7 | 0.23 (0.092) | 0.26 (0.10) | 1.1 |
| MDR 3 | Sputum | R | R | R | R | 0.56 (0.31) | 3.2 (1.8) | 5.7 | 0.12 (0.048) | 0.12 (0.048) | 1.0 |
| MDR 4 | Sputum | R | R | R | R | 6.9 (3.8) | 33.8 (18.8) | 4.9 | 0.30 (0.12) | 0.35 (0.14) | 1.2 |
| MDR 5 | Sputum | R | R | R | R | 7.3 (4.1) | 31.4 (17.5) | 4.3 | 0.30 (0.12) | 0.36 (0.14) | 1.2 |
| MDR 6 | Sputum | R | R | R | R | 1.7 (0.95) | 6.8 (3.8) | 4.0 | 0.16 (0.064) | 0.21 (0.084) | 1.3 |
S, susceptible; R, resistant; STR, streptomycin; EMB, ethambutol; RFM, rifampin; INH, isoniazid.
VP, verapamil; +, present; −, absent.
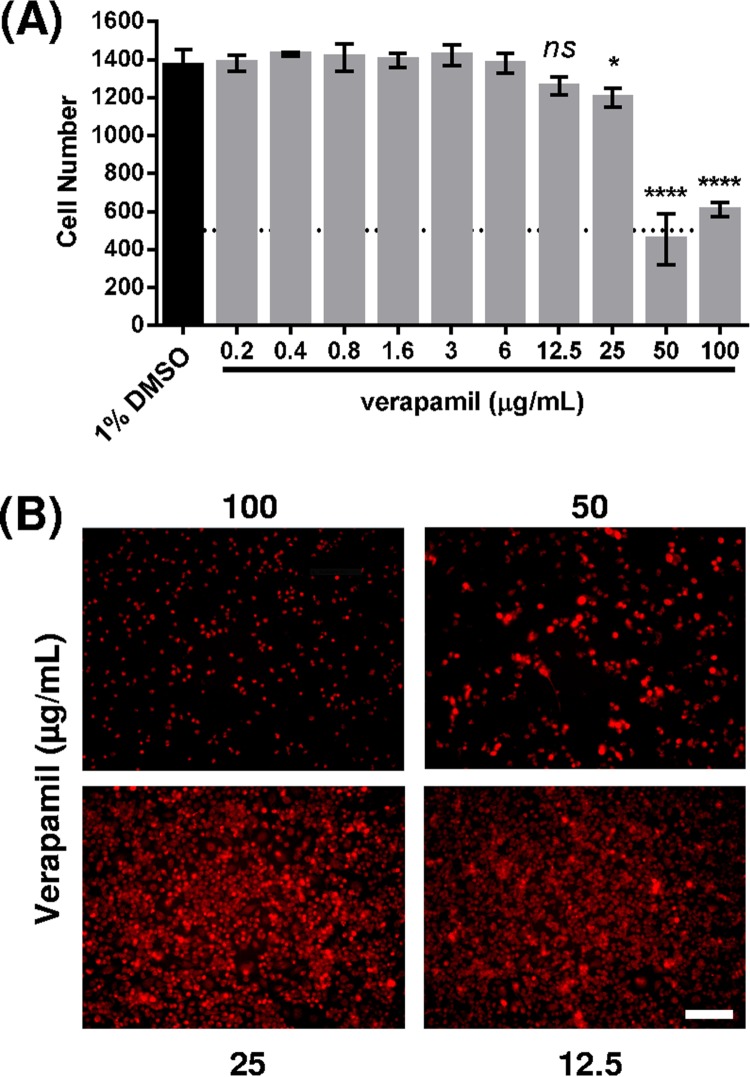
In order to further validate our findings, we tested the potency of Q203 in the presence and absence of verapamil using a rapid phenotypic assay based on the use of automated fluorescence microscopy to monitor intracellular growth of GFP-expressing M. tuberculosis H37Rv in RAW 264.7 macrophages (14). Prior to this assay, we evaluated the toxicity of verapamil in RAW 264.7 macrophages to select the appropriate doses for cell-based activity measurements. We incubated macrophages with various concentrations of verapamil (0.2 to 100 μg/ml) and observed the cytotoxicity under a fluorescence microscope. As shown in Fig. 2A, verapamil was cytotoxic at 50 μg/ml. Indeed, only a fraction of the macrophage cells survived at this concentration in our cell-based analysis, although 50 μg/ml did not inhibit bacterial growth in the resazurin microplate assay. While Adams et al. showed that high verapamil concentrations, up to 80 μg/ml, enhanced isoniazid activity in a dose-dependent manner in THP-1 macrophages (15), we noted that verapamil significantly influenced cell viability even at 25 μg/ml based on the Syto 60 signal in our assay (Fig. 2B). Therefore, we decided to use a fixed verapamil concentration of 12.5 μg/ml in subsequent assays, as this concentration was not cytotoxic in our setting.
FIG 2.
(A) Quantification of the cytotoxic effect of verapamil on RAW 264.7 cells. The number of cells was determined by averaging three different fields per well (n = 4). DMSO (1%) was used as a control. Statistical difference with the control was determined using an unpaired t test. ns, non-statistically significant; *, P value of <0.05; ****, P value of <0.0001. (B) Cytotoxic effect of verapamil on RAW 264.7 cells. Images were acquired using fluorescence microscopy (Operetta; PerkinElmer) at a magnification of ×20. Cells were stained with Syto 60 dye (5 μM) for 20 min prior to imaging at Ex. 630 nm/Em. 690 nm. Scale bar, 100 μm.
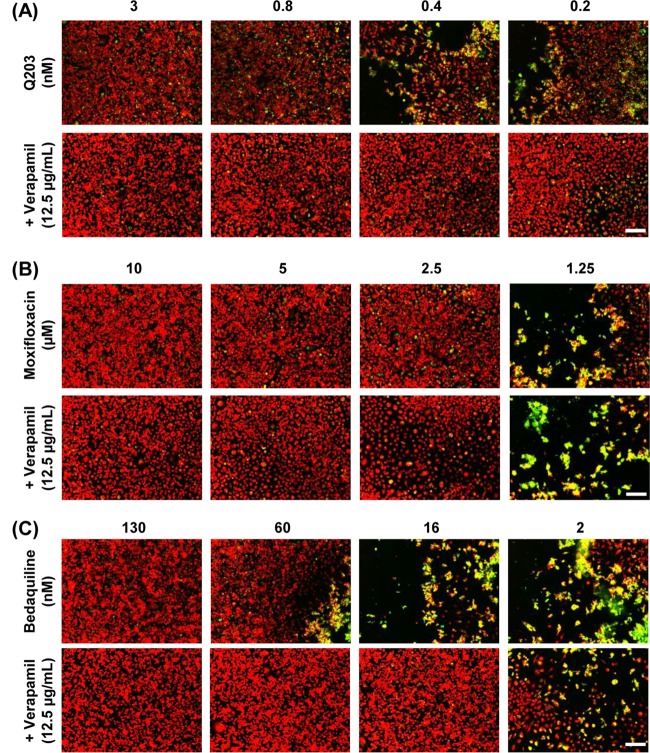
We next tested the combinatorial effect of verapamil and Q203 in the cell-based assay system using bedaquiline and moxifloxacin as positive and negative controls, respectively. After adding each compound in the presence of 12.5 μg/ml verapamil to M. tuberculosis-GFP-infected RAW 264.7 cells, the culture plates were incubated for 5 days at 37°C after which images were acquired and analyzed. As shown in Fig. 3A, addition of verapamil increased the activity of Q203 against M. tuberculosis replication in macrophages. More precisely, Q203 was active against M. tuberculosis H37Rv with an IC50 of 3 nM inside host RAW 264.7 cells, and this value decreased to less than 0.2 nM in the presence of verapamil. As expected, verapamil had no effect on the activity of moxifloxacin (Fig. 3B) while enhancing the activity of bedaquiline against intracellular M. tuberculosis H37Rv (Fig. 3C), producing a similar growth inhibitory profile as observed in a previous report (11). The IC50 of bedaquiline was lowered from 130 nM to 16 nM in the presence of verapamil, corresponding to an 8-fold potentiation.
FIG 3.
Intracellular cellular survival of M. tuberculosis-GFP after treatment with anti-TB drugs in combination with verapamil (VP). M. tuberculosis-GFP-infected RAW 264.7 cells were incubated with Q203 (A), moxifloxacin (B), and bedaquiline (C) in the presence or absence of VP. Scale bar, 100 μm.
Based on these in vitro and ex vivo results showing the effectiveness of the EPI verapamil in potentiating the activity of Q203, we hypothesized that efflux may play a significant role in decreasing the activity of Q203. To add more strength to this hypothesis, additional efflux pump inhibitors (EPIs) were tested for their ability to decrease the IC50 of Q203 in both assays. Reserpine and piperine were first tested using REMA, displaying a similar effect compared to that of verapamil against M. tuberculosis (see Fig. S3 in the supplemental material). In addition, piperine, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), and 2,4-dinitrophenol (DNP) were tested for their ability to increase the activity of Q203 in the macrophage assay. The cytotoxic effect of these compounds was first measured using noninfected cells to determine the highest, noncytotoxic dose that could be used (see Fig. S4 in the supplemental material). For piperine, a concentration of 12.5 μg/ml was found to be suitable, but the compound could be used at a concentration of up to 25 μg/ml with only limited cytotoxicity. However, CCCP could only be used at a concentration of less than 1.6 μg/ml, and DNP started to show significant toxicity from 6 μg/ml. Treatment with nontoxic doses of EPIs in combination with Q203 resulted in enhanced activity in the cell-based assay system. Furthermore, verapamil showed the most significant improvement of Q203 activity, displaying 5- to 10-fold reductions in the IC50 of Q203 on average when used at 12.5 μg/ml. Piperine and CCCP showed more modest reductions (5- and 2.5-fold, respectively) when used at 25 and 0.8 μg/ml, respectively (Fig. S4). Only DNP failed to improve the activity of Q203 at all concentrations tested (up to 12.5 μg/ml). As expected, none of the EPIs significantly modified the activity of moxifloxacin, used here as a negative control (Fig. S4). Altogether, these results were in agreement with our hypothesis that inhibiting efflux by EPIs could increase the activity of Q203.
In a previous study, Gupta et al. reported that supplementation with verapamil considerably potentiated the MIC of bedaquiline against drug-susceptible and drug-resistant clinical isolates (12). For example, an 8- to 16-fold decrease in the MIC of bedaquiline was reported for a number of strains when used in combination with verapamil (11). Furthermore, they showed that coadministration of verapamil and bedaquiline had a bactericidal effect in rodents and also resulted in a lower number of resistant mutants in vivo (12). Bedaquiline targets the atpE gene encoding subunit c of the ATP synthase of M. tuberculosis, thereby affecting the proton pump needed for ATP synthesis in the electron transport chain (ETC) and consequently making bacteria vulnerable to further ATP depletion (16). Likewise, Q203 targets the qcrB subunit of the cytochrome bc1 complex in the ETC and triggers a rapid depletion of ATP, thereby blocking the growth of M. tuberculosis (13). Since both compounds share the same ETC-related mechanism of action, they may be affected by the same efflux pumps although the verapamil-potentiated activity of Q203 was not as significant as that of bedaquiline.
Several efflux pumps have been reported in M. tuberculosis. For example, Rodrigues et al. reported that Mmr (Rv3065), a member of the small multidrug resistance (SMR) family of transporters, is involved in the extrusion of quaternary compounds, such as ethidium bromide, through a genetic study using Mmr-deficient and -overexpressing M. tuberculosis (17). In addition, Andries et al. (18) reported a novel nontarget-based efflux pump mechanism for bedaquiline resistance that was identified by sequencing non-atpE bedaquiline-resistant mutants isolated from patients treated with bedaquiline as well as from mice. These nontarget-based mutants primarily harbored single nucleotide polymorphisms (SNPs) in the Rv0687 gene, which is a transcriptional repressor of mmpS5-mmpL5 (18). The involvement of MmpL5 in the resistance to bedaquiline was further evidenced in vitro (19) and supported by recent clinical data (20, 21). We were unable to identify and confirm which molecular targets were involved in this efflux pump-mediated tolerance to Q203 in the present study because of the low rate of spontaneous Q203-resistant mutants (∼10−8), with SNPs appearing exclusively in the qcrB gene (A937G leading to T313A) (13). In addition, very little is currently known about the potential sources of Q203 resistance, and there are currently no reports of “nontarget” Q203 mutants that do not harbor qcrB mutations. To further characterize this phenomenon, we are currently investigating the role played by MmpL5 and other known efflux pumps in Q203 resistance, using deletion and overexpression mutants generated in vitro as well as resistant strains obtained from clinical isolates.
In conclusion, this is the first report demonstrating the involvement of efflux pumps in the modulation of Q203 activity against M. tuberculosis, which was established using verapamil and other well-characterized EPIs. The combination of verapamil and Q203 potentiated the antibacterial activity of Q203 both in vitro and in an advanced cell-based assay system. Based on these results, we believe that the possibility of using verapamil to sensitize M. tuberculosis to Q203 may be an interesting avenue to explore for in vivo trials. Because Q203 also inhibits the growth of MDR and XDR-TB clinical isolates in culture broth medium in the low nanomolar range, the combination of Q203 and the efflux pump inhibitor verapamil may have significant clinical implication for the treatment of the most resilient forms of drug-resistant TB.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The MDR-TB isolates and H37Rv strain of M. tuberculosis were grown at 37°C in Middlebrook 7H9 liquid medium (Difco) supplemented with albumin dextrose catalase (ADC), glycerol (0.2%, vol/vol), and Tween 80 (0.05%, vol/vol). The M. tuberculosis H37Rv-GFP strain, constitutively expressing a green fluorescent protein (GFP), was grown in the same medium supplemented with 50 μg/ml hygromycin (Invitrogen). RAW 264.7 cells (ATCC TIB-71) were grown in RPMI 1640 medium (Welgene) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen).
REMA.
Drug susceptibility testing (DST) using resazurin was performed under aerobic conditions. Resazurin solution was prepared as a 0.02% (wt/vol) solution in sterile distilled water using resazurin sodium salt powder (Sigma), filter sterilized, and stored at 4°C for not more than 1 week. Bacteria from exponential-phase cultures were harvested and diluted to a final inoculum of 5 × 104 CFU/ml in wells of a 96-well microtiter plate. Twofold serial dilutions of compounds were prepared from 150 to 0.07 nM Q203, from 3.2 μM to 1.5 nM moxifloxacin (Sigma), and from 1,500 to 6 nM bedaquiline (AdooQ BioScience) with or without 50 μg/ml of verapamil (Sigma). Piperine and reserpine were purchased from Sigma-Aldrich and used at a concentration of 32 μg/ml (final concentration in the well) as described previously (22). Plates were then incubated at 37°C for 14 days. Thereafter, 30 μl of 0.02% resazurin was added to the wells. A change in color from blue to pink was used as an indicator of bacterial growth, and the fluorescence was measured using a Victor3 reader (PerkinElmer, Life Sciences). Concentrations required to inhibit bacterial growth by 50% (IC50s) were determined by fitting the curves with a sigmoidal dose-response using GraphPad Prism software (version 6.05).
Intracellular bacterial replication assay.
Efflux pump inhibitors, namely, verapamil, piperine, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), and 2,4-dinitrophenol (DNP), were obtained from Sigma-Aldrich. Stock solutions were prepared at 10 mg/ml in dimethyl sulfoxide (DMSO) and stored at −20°C. For the assessment of the cytotoxicity of the EPI, 2-fold serial dilutions of EPI, starting from 100 μg/ml (final concentration in the well), were tested against noninfected RAW 264.7 cells (ATCC TIB-71). To determine the effect of the EPI on Q203 and moxifloxacin, RAW 264.7 cells were infected with M. tuberculosis H37Rv-GFP at a multiplicity of infection (MOI) of 2:1. Twofold serial dilutions of anti-TB compounds were prepared as described above, with or without EPI at fixed concentrations. For all experiments, the amount of DMSO was kept constant, equal to 1% final in all wells. Cells were dispensed into 384-well plates (25,000 cells/well). After 5 days of infection at 37°C and 5% CO2, macrophages were stained for 20 min with 5 μM Syto 60 dye (Invitrogen) and images were acquired using confocal microscopy (Opera and Operetta; PerkinElmer). Bacterial load and macrophage number were quantified as described previously (14).
Supplementary Material
ACKNOWLEDGMENTS
Institut Pasteur Korea is a member of the Institut Pasteur International Network (https://www.pasteur.fr/en/international-en).
English language editing of the article was carried out by Editage, a division of Cactus Communications.
This work was supported by the National Research Foundation of Korea (grants 2014R1A1A1007464.1, 2016R1D1A1A02937214, and 2014K1A4A7A01074643), funded by the Korean Ministry of Science, ICT and Future Planning (MSIP). Vincent Delorme was supported by the French Ministry of Foreign Affairs. Da Eun Park and Jinsun Jeong were supported by the BK21plus program through the National Research Foundation (NRF), funded by the Ministry of Education of Korea.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02637-16.
REFERENCES
- 1.World Health Organization. 2016. Global tuberculosis report 2016. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Lin PL, Flynn JL. 2010. Understanding latent tuberculosis: a moving target. J Immunol 185:15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young DB, Gideon HP, Wilkinson RJ. 2009. Eliminating latent tuberculosis. Trends Microbiol 17:183–188. doi: 10.1016/j.tim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Blöndal K. 2007. Barriers to reaching the targets for tuberculosis control: multidrug-resistant tuberculosis. Bull World Health Organ 85:387–390. doi: 10.2471/BLT.06.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Silva PEA, Von Groll A, Martin A, Palomino JC. 2011. Efflux as a mechanism for drug resistance in Mycobacterium tuberculosis. FEMS Immunol Med Microbiol 63:1–9. doi: 10.1111/j.1574-695X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 6.Pasipanodya JG, Gumbo T. 2011. A new evolutionary and pharmacokinetic-pharmacodynamic scenario for rapid emergence of resistance to single and multiple anti-tuberculosis drugs. Curr Opin Pharmacol 11:457–463. doi: 10.1016/j.coph.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. 2010. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J Infect Dis 201:1225–1231. doi: 10.1086/651377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado D, Couto I, Perdigão J, Rodrigues L, Portugal I, Baptista P, Veigas B, Amaral L, Viveiros M. 2012. Contribution of efflux to the emergence of isoniazid and multidrug resistance in Mycobacterium tuberculosis. PLoS One 7(4):e34538. doi: 10.1371/journal.pone.0034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. 2011. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Tyagi S, Almeida DV, Maiga MC, Ammerman NC, Bishai WR. 2013. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am J Respir Crit Care Med 188:600–607. doi: 10.1164/rccm.201304-0650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Cohen KA, Winglee K, Maiga M, Diarra B, Bishai WR. 2014. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:574–576. doi: 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Tyagi S, Bishaia WR. 2015. Verapamil increases the bactericidal activity of bedaquiline against Mycobacterium tuberculosis in a mouse model. Antimicrob Agents Chemother 59:673–676. doi: 10.1128/AAC.04019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim S-A, Nam J, Kang H, Kwon H, Oh C-T, Cho Y, Jang Y, Kim J, Chua A, Tan BH, Nanjundappa MB, Rao SPS, Barnes WS, Wintjens R, Walker JR, Alonso S, Lee S, Kim J, Oh S, Oh T, Nehrbass U, Han S-J, No Z, Lee J, Brodin P, Cho S-N, Nam K, Kim J. 2013. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 19:1157–1160. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 14.Christophe T, Jackson M, Hee KJ, Fenistein D, Contreras-Dominguez M, Kim J, Genovesio A, Carralot JP, Ewann F, Kim EH, Lee SY, Kang S, Seo MJ, Eun JP, Škovierová H, Pham H, Riccardi G, Youn NJ, Marsollier L, Kempf M, Joly-Guillou ML, Oh T, Won KS, No Z, Nehrbass U, Brosch R, Cole ST, Brodin P. 2009. High content screening identifies decaprenyl-phosphoribose 2′ epimerase as a target for intracellular antimycobacterial inhibitors. PLoS Pathog 5:e1000645. doi: 10.1371/journal.ppat.1000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams KN, Szumowski JD, Ramakrishnan L. 2014. Verapamil, and its metabolite norverapamil, inhibit macrophage-induced, bacterial efflux pump-mediated tolerance to multiple anti-tubercular drugs. J Infect Dis 210:456–466. doi: 10.1093/infdis/jiu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koul A, Dendouga N, Vergauwen K, Molenberghs B, Vranckx L, Willebrords R, Ristic Z, Lill H, Dorange I, Guillemont J, Bald D, Andries K. 2007. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat Chem Biol 3:323–324. doi: 10.1038/nchembio884. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues L, Villellas C, Bailo R, Viveiros M, Aínsa JA. 2013. Role of the Mmr efflux pump in drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 57:751–757. doi: 10.1128/AAC.01482-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9(7):e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartkoorn RC, Uplekar S, Cole ST. 2014. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander DC, Vasireddy R, Vasireddy S, Philley JV, Brown-Elliott BA, Perry BJ, Griffith DE, Benwill JL, Cameron ADS, Wallace RJ. 2017. Emergence of mmpT5 variants during bedaquiline treatment of Mycobacterium intracellulare lung disease. J Clin Microbiol 55:574–584. doi: 10.1128/JCM.02087-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villellas C, Coeck N, Meehan CJ, Lounis N, de Jong B, Rigouts L, Andries K. 2017. Unexpected high prevalence of resistance-associated Rv0678 variants in MDR-TB patients without documented prior use of clofazimine or bedaquiline. J Antimicrob Chemother 72:684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin J, Zhang J, Guo N, Feng H, Li L, Liang J, Sun K, Wu X, Wang X, Liu M, Deng X, Yu L. 2011. The plant alkaloid piperine as a potential inhibitor of ethidium bromide efflux in Mycobacterium smegmatis. J Med Microbiol 60:223–229. doi: 10.1099/jmm.0.025734-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.