ABSTRACT
We assessed the ability of the Etest performed directly on positive blood cultures (ETDIR) to detect fluconazole susceptibility in 6 fluconazole-resistant and 12 fluconazole-susceptible Candida albicans isolates, according to CLSI M27-A3 and EUCAST EDef 7.2 procedures. Categorical agreement between ETDIR and broth microdilution was 100% when the trays were incubated at 25°C and trailing effect was ruled out. ETDIR is a reliable procedure when screening for the presence of fluconazole resistance in C. albicans.
KEYWORDS: Candida albicans, fluconazole, resistance, Etest
TEXT
Fluconazole and echinocandins are the backbone of antifungal treatment for candidemia and invasive candidiasis (1, 2). Different rates of fluconazole resistance have been reported in population-based studies (3–5), and prior exposure to azoles seems to be a risk factor for the development of resistance (6, 7). In Spain, the overall fluconazole resistance rate is below 10% in Candida spp. and below 2% in Candida albicans in particular (5, 8). Although resistance to fluconazole is infrequent in Candida albicans strains isolated from blood, it may complicate the management of patients. A correlation has been detected between mortality and delayed initiation of effective antifungal therapy in patients with candidemia, including cases in which the dose of fluconazole used is suboptimal (5, 6, 9–13).
The mechanisms responsible for azole resistance in C. albicans involve mutations in the ERG11 and ERG3 genes, overexpression of ERG11, overexpression of genes encoding efflux pumps, or a combination of the three (14, 15). Fluconazole-resistant C. albicans isolates can be detected in the clinical microbiology laboratory using gold standard broth microdilution methods (CLSI and EUCAST), commercially available broth microdilution microtiter systems (Sensititre YeastOne), and agar-based methods (Etest) (16, 17).
Conventional methods for detecting fluconazole-resistant isolates delay results for at least 48 h after the detection of Candida spp. in blood cultures. Antifungal susceptibility based on agar diffusion tests performed directly on positive bottles has reduced the time from positivity of blood culture, making it possible to obtain preliminary fluconazole susceptibility values (18–20); a similar approach using marketed microdilution systems (e.g., Vitek, Sensititre YeastOne, and flow cytometry) has proven unsuccessful (21–23).
We previously showed that when performed directly on positive blood culture bottles, the Etest reliably detected fluconazole resistance in non-albicans Candida isolates approximately 24 h after the diagnosis of candidemia is confirmed (19). However, the role of this procedure for the detection of fluconazole resistance in C. albicans has not been properly assessed, because no resistant isolates have been tested to date. In this study, we assessed the ability of the Etest performed directly on positive blood culture bottles to detect fluconazole-resistant C. albicans isolates.
(Data from this study were presented at the 26th European Congress of Clinical Microbiology and Infectious Diseases in Amsterdam, The Netherlands [abstr P1613] [24]).
Isolates and fluconazole susceptibility testing.
We studied 6 fluconazole-resistant C. albicans isolates from patients admitted to our hospital (Hospital Gregorio Marañón, Madrid, Spain). As controls, we used 12 fluconazole-susceptible C. albicans isolates showing different degrees of trailing (25) and 24 fluconazole-resistant non-albicans Candida isolates (C. glabrata, n = 4; C. parapsilosis, n = 1; C. lusitaniae, n = 2; C. krusei, n = 13; C. guilliermondii, n = 3; and C. inconspicua, n = 1) from blood samples. All isolates were identified by sequencing the internal transcribed spacer (ITS1-5.8S-ITS2) regions (26).
The in vitro susceptibility to fluconazole was assessed for the 42 isolates, according to the CLSI M27-A3 and EUCAST EDef 7.2 microdilution procedures (16, 17). The MIC value was defined as the lowest concentration of drug that inhibited ≥50% of growth compared with the growth in the control well. Isolates were considered fluconazole resistant according to the current EUCAST and CLSI breakpoints (fluconazole MIC, >4 mg/liter).
Susceptibility was also assessed using the Etest according to the standard manufacturer's instructions (ETSD), with yeast suspensions adjusted to a 0.5 McFarland standard streaked across the surface of the agar plates. The Etest was performed directly from positive blood culture bottles (ETDIR), as previously described (19). Briefly, a 0.5-ml suspension (0.5 McFarland standard) of each isolate was inoculated into Bactec FX bottles (Becton Dickinson, Cockeysville, MD, USA) and reincubated in the automatic system. When growth of yeast was detected in Gram stains performed in bottles flagged as positive, 10 to 20 drops of broth were poured and streaked onto RPMI 1640 agar plates supplemented with 2% glucose (bioMérieux, Marcy l'Etoile, France). All plates were incubated at 35°C for 24 h before the fluconazole MIC was determined.
Sequencing and gene expression.
The presence of previously reported fluconazole resistance mechanisms was studied in the 18 C. albicans isolates. ERG11 and ERG3 were amplified and sequenced as previously reported (15). The relative expression levels of ERG11, CDR1, CDR2, and MDR1 were also studied after total RNA extraction, reverse transcription, and reverse transcription-quantitative PCR (RT-qPCR). For each isolate, the expression level of the gene was evaluated using the 2−ΔΔCT method, where the CT was the average threshold cycle obtained in 3 independent experiments for the above-mentioned genes. The normalized CT (based on the CT of a housekeeping gene, ACT1) was further compared with that obtained after calculating the mean CT values measured in 3 residual trailing isolates. Relative gene expression between fluconazole-resistant isolates (CA-1 to CA-6) and fluconazole-susceptible isolates (CA-7 to CA-18) was compared using the Mann-Whitney test.
Data analysis.
Categorical agreement between the 4 antifungal susceptibility testing methods was calculated, using CLSI M27-A3 and EUCAST EDef 7.2 as the gold standards. Errors were categorized as very major errors (VMEs) or false susceptible when the ETSD or ETDIR classified an isolate as susceptible and the gold standard classified it as resistant, and as major errors (MEs) or false resistance when an isolate was classified as resistant by ETSD or ETDIR and susceptible by the gold standard (19).
Ethical considerations.
This study (protocol no. 157/16) was approved by the ethics committee of Hospital Gregorio Marañón (CEIC-A1). The need for informed consent was waived, owing to the retrospective design of the study.
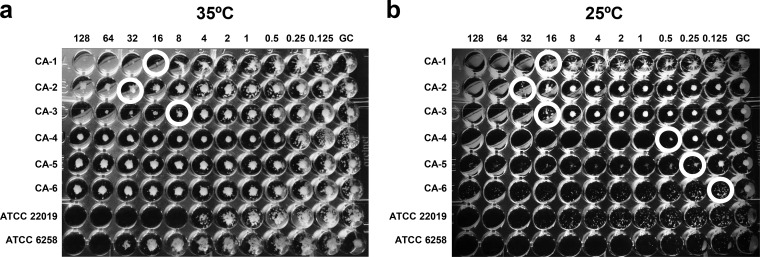
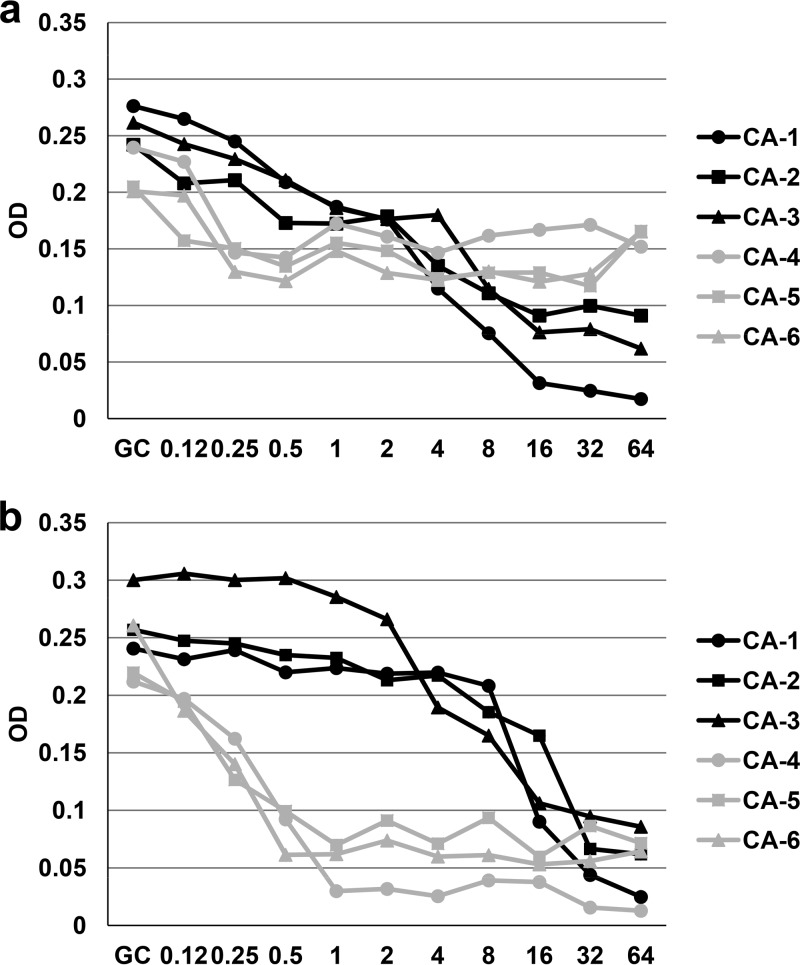
Table 1 shows the fluconazole susceptibilities of the 18 C. albicans isolates obtained by the 4 procedures studied, the mutations found in ERG11 and ERG3, and the relative expression levels of these genes. According to both the EUCAST and the CLSI procedures, 6 isolates (CA-1 to CA-6) were fluconazole resistant (MIC, >4 mg/liter) and showed 2 different growth patterns in the microdilution trays. When the CLSI procedure was used, setting the fluconazole MIC endpoint was easy against isolates CA-1, CA-2, and CA-3, whereas the very prominent growth at all fluconazole concentrations found in isolates CA-4, CA-5, and CA-6 led to an MIC above the highest fluconazole concentration tested (Fig. 1a). Agitation of the plates according to the CLSI procedure is optional and may facilitate the MIC setting in isolates showing heavy trailing (27). However, we retested the isolates (CA-1 to CA-6) and set the MIC after agitating the plates, but it did not have a significant impact on the MICs. According to the EUCAST procedure, the growth inhibition curve kinetics were also different (persistent growth slightly above 50% or sharp inhibition of growth at fluconazole concentrations of ≥8 mg/liter), and both patterns matched those observed with the CLSI procedure (Fig. 2a).
TABLE 1.
Fluconazole MICs, gene mutations, and gene expression levels
| Isolate | Classificationa | Fluconazole MIC (mg/liter) |
Gene mutation(s) |
Relative gene expressionb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EUCAST guideline | CLSI guideline | ETSD | ETDIR | ERG11 | ERG3 | CDR1 | CDR2 | ERG | MDR | ||
| CA-1 | Resistant | 8 | 16 | 12 | 16 | E266D, V488I | V351A, A353T | 1.61 | 2.20 | 1.32 | 1.05 |
| CA-2 | Resistant | 8 | 32 | 32 | 32 | A114Sc | V351A | 1.53 | 46.91 | 1.81 | 2.16 |
| CA-3 | Resistant | 8 | 8 | 32 | 32 | A114Sc, G464Sc | Wild type | 2.06 | 43.25 | 0.71 | 0.59 |
| CA-4 | Resistant | 128 | 256 | 0.125 | 0.125 | D116E, E266D, V488I | H28Y, D219N, S265F, V351A | 1.54 | 10.60 | 2.48 | 4.44 |
| CA-5 | Resistant | 128 | 256 | 0.094 | 0.125 | D116E, V481I | H28Y, D219N, S265Y | 0.42 | 4.52 | 0.96 | 0.67 |
| CA-6 | Resistant | 128 | 256 | 0.38 | 0.75 | Wild type | Wild type | 0.22 | 3.09 | 0.57 | 0.60 |
| CA-7 | Trailer (heavy) | 0.25 | 0.125 | 0.38 | 0.5 | D153E | Wild type | 0.62 | 11.19 | 1.14 | 1.19 |
| CA-8 | Trailer (heavy) | 0.125 | 0.125 | 0.25 | 0.5 | D116E, K128T | V351A | 0.42 | 12.59 | 1.37 | 5.57 |
| CA-9 | Trailer (heavy) | 0.125 | 0.062 | 0.125 | 0.19 | R246C | V351A | 0.72 | 6.19 | 1.22 | 1.45 |
| CA-10 | Trailer (moderate) | 0.25 | 0.25 | 0.38 | 0.5 | D116E, V437I | Wild type | 0.53 | 27.60 | 0.94 | 0.30 |
| CA-11 | Trailer (moderate) | 0.25 | 0.25 | 0.75 | 0.75 | Wild type | V351A | 0.53 | 13.40 | 1.51 | 2.54 |
| CA-12 | Trailer (moderate) | 0.25 | 0.5 | 0.5 | 0.38 | Wild type | V351A | 0.45 | 2.14 | 1.21 | 4.29 |
| CA-13 | Trailer (slightly) | 0.125 | 0.25 | 0.25 | 0.38 | D116E, K128T | N62S | 1.65 | 1.31 | 0.68 | 0.81 |
| CA-14 | Trailer (slightly) | 0.125 | 0.062 | 0.125 | 0.19 | D116E, V437I | H28Y | 0.84 | 1.47 | 0.61 | 0.43 |
| CA-15 | Trailer (slightly) | 0.25 | 0.25 | 0.75 | 0.75 | Wild type | Wild type | 0.71 | 10.10 | 1.22 | 1.13 |
| CA-16 | Trailer (residual) | 0.125 | 0.062 | 0.25 | 0.25 | D116E, V488I, E266D | Wild type | NA | NA | NA | NA |
| CA-17 | Trailer (residual) | 0.125 | 0.125 | 0.5 | 0.5 | E266D | V351A | NA | NA | NA | NA |
| CA-18 | Trailer (residual) | 0.25 | 0.5 | 1 | 0.5 | E266D, V488I | V351A | NA | NA | NA | NA |
Fluconazole-susceptible isolates (CA-7 to CA-18) were classified according to trailing using a previously reported score: residual trailers, 0.1 to 5%; slight trailers, 6 to 10%; moderate trailers, 11 to 15%; and heavy trailers, >15% (25).
NA, not applicable. Residual trailing isolates were used as controls to determine gene expression.
Mutations previously reported as conferring fluconazole resistance (28).
FIG 1.
Tray showing the fluconazole MICs (top, in milligrams per liter) against the 6 fluconazole-resistant C. albicans isolates (CA-1 to CA-6) by CLSI procedure after 24 h of incubation at 35°C (a) and at 25°C (b). Wells in circles indicate the MIC. GC, growth control well.
FIG 2.
Growth inhibition curves of the 6 fluconazole-resistant C. albicans isolates (CA-1 to CA-6) by EUCAST procedure after 24 h of incubation at 35°C (a) or 25°C (b). x axis, concentration in milligrams per liter. y axis, optical density (OD).
Point mutations in ERG11 and ERG3 were found in most isolates, although only 2 isolates had mutations in ERG11, which has been reported to confer resistance (15, 28, 29). The remaining mutations were also previously described in fluconazole-susceptible isolates and do not seem to play a major role in resistance (15, 30–32). No differences in gene expression were observed between the fluconazole-resistant isolates and the fluconazole-susceptible isolates (P > 0.05) (Table 1). However, CA-2 and CA-3 had higher expression levels of CDR2 and were also resistant to voriconazole and posaconazole (data not shown). The lack of a clear correlation between phenotypic and molecular resistance was not surprising, as the molecular explanation for fluconazole resistance in Candida is based on single mechanisms or simultaneous multiple mechanisms, and there might be other unknown underlying mechanisms that play a role in the resistance of these isolates (33–36). Furthermore, there may be an association between the specific resistance mechanisms and the anatomical site at which the isolate has become resistant (37).
Categorical agreement between ETDIR, ETSD, and broth microdilution was 100% for fluconazole-susceptible C. albicans isolates (no MEs) and for fluconazole-resistant non-albicans Candida isolates (no VMEs), thus confirming our previous observations (19). However, ETDIR and ETSD classified only 3 out of the 6 fluconazole-resistant isolates as resistant (isolates CA-1, CA-2, and CA-3; Tables 1 and 2), which yielded 50% of VMEs. Interestingly, inhibition of growth was sharply reduced in the EUCAST curves, and the MIC was easily interpreted using CLSI, whereas the remaining 3 isolates, which showed apparently “false” susceptibility, displayed persistent growth, even at high fluconazole concentrations in both microdilution methods (isolates CA-4, CA-5, and CA-6; Fig. 1a and 2a).
TABLE 2.
Categorical agreement between the recommended conditions of incubation trays as per EUCAST and CLSI, with results obtained after modification of the incubation temperature of 25°Ca
| Isolate | Fluconazole susceptibilitya |
|||
|---|---|---|---|---|
| EUCAST (35°C/25°C) | CLSI (35°C/25°C) | ETSD | ETDIR | |
| CA-1 | R/R | R/R | R | R |
| CA-2 | R/R | R/R | R | R |
| CA-3 | R/R | R/R | R | R |
| CA-4 | R/S | R/S | S | S |
| CA-5 | R/S | R/S | S | S |
| CA-6 | R/S | R/S | S | S |
R, resistant; S, susceptible.
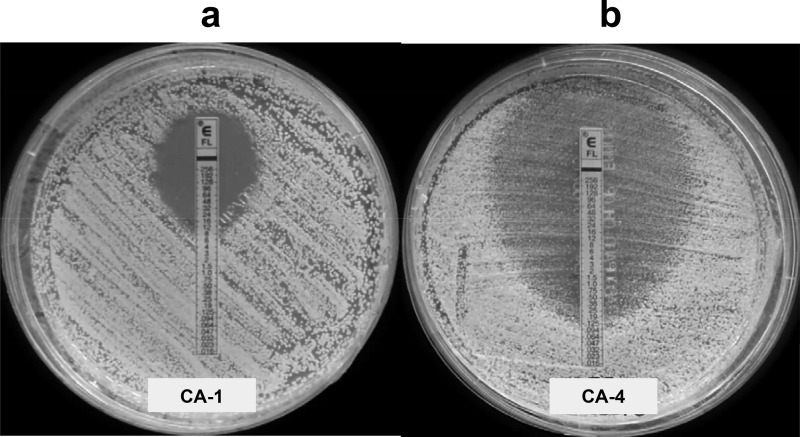
Observation of the ETDIR plate in isolates CA-4, CA-5, and CA-6 revealed prominent growth of slime within the elliptic inhibition zone, thus suggesting that the disagreement between the methods could be due to heavy trailing in the microdilution trays rather than true fluconazole resistance (Fig. 3). Given the fungistatic nature of fluconazole, the trailing effect is frequently observed in C. albicans isolates and may complicate assessment of the MIC. This phenomenon is a consequence of the activation of calcineurin and altered regulation genes, although this effect cannot be explained accurately (38). Trailing can be misinterpreted as resistance in broth microdilution methods, particularly in isolates showing heavy trailing. However, animal models and clinical experience reveal these isolates to be truly fluconazole susceptible (39–41). In a previous report, we found that most of the C. albicans bloodstream isolates displayed fluconazole trailing to some extent: 26% were classified as heavy trailers, with the consequent potential to misclassify isolates as resistant (25).
FIG 3.
Fluconazole MICs obtained by ETDIR against C. albicans isolates CA-1 and CA-4 after 24 h of incubation at 35°C. (a) True resistant isolate (CA-1), in which both ETDIR and microdilution were in agreement. (b) Isolate (CA-4), in which ETDIR classified the isolate as susceptible and microdilution as resistant, albeit with heavy trailing.
In order to unravel whether the persistent growth pattern resembled true resistance or trailing, we modified the CLSI and EUCAST procedures by lowering the incubation temperature of the trays to 25°C and lowering the pH of RPMI broth medium to 4.5. These modifications were previously reported to minimize the interference of trailing when assessing the MIC using CLSI (39, 42). The modification of the broth medium to pH 4.5 did not enable growth of the isolates after 24 h of incubation or clear reduction of trailing (data not shown). However, when both microdilution trays were incubated at 25°C, the trailing effect was considerably reduced, and the 3 isolates classified as resistant by ETDIR (CA-4, CA-5, and CA-6) switched from resistant to susceptible by both EUCAST and CLSI (Fig. 1b and 2b), thus leading to 100% categorical agreement between the 4 methods (Table 2). Previous reports have compared broth microdilution methods and the Etest for fluconazole susceptibility testing. Consistent with our data, the findings were limited by the low number of resistant isolates, thus precluding an evaluation of the role of the Etest for detecting fluconazole resistance (20, 43–45). The small samples of fluconazole-resistant isolates in these studies are partially a consequence of the low frequency of isolation in the clinical microbiology laboratory (46).
We conclude that ETDIR is a reliable procedure when screening for the presence of fluconazole resistance in C. albicans isolates causing candidemia. When using microdilution procedures, true fluconazole resistance should be proven after incubation of the microtiter trays at 25°C if the EUCAST shows a growth inhibition pattern consisting of a persistent growth slightly above 50% or heavy trailing, using CLSI guidelines.
ACKNOWLEDGMENTS
We thank Thomas O'Boyle for editing the article.
This study was supported by grants PI14/00740 and MSI15/00115 from the Fondo de Investigación Sanitaria (FIS; Instituto de Salud Carlos III; Plan Nacional de I+D+I 2013-2016) and cofinanced by the European Regional Development Fund (ERDF) “A way of making Europe.” P.E. (CPI15/00115) and J.G. (CPII15/00006) are recipients of a Miguel Servet contract; L.J.M.-Z. (PI14/00740) is supported by FIS. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
J.G. has received funds for speaking at symposia organized on behalf of Astellas, Gilead, MSD, Scynexis, and United Medical; he has also received funds for research from the Fondo de Investigación Sanitaria, Gilead, and Scynexis. The other authors declare no conflicts of interest.
REFERENCES
- 1.Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 3.Pemán J, Canton E, Minana JJ, Florez JA, Echeverria J, Ortega DN, Alarcon JM, Fontanals D, Sard BG, Moreno BB, Torroba L, Ayats J, Perez MA, Fernandez MA, Reus FS, Natal IF, Garcia GR, Ezpeleta G, Martin-Mazuelos E, Iglesias I, Rezusta A, de Ocariz IR, Nieto AG, el Grupo de Estudio FUNGEMYCA. 2011. Changes in the epidemiology of fungaemia and fluconazole susceptibility of blood isolates during the last 10 years in Spain: results from the FUNGEMYCA study. Rev Iberoam Micol 28:91–99. (In Spanish.) doi: 10.1016/j.riam.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. 2011. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J Clin Microbiol 49:396–399. doi: 10.1128/JCM.01398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guinea J, Zaragoza O, Escribano P, Martin-Mazuelos E, Peman J, Sanchez-Reus F, Cuenca-Estrella M, CANDIPOP Project, GEIH-GEMICOMED (SEIMC), REIPI. 2014. Molecular identification and antifungal susceptibility of yeast isolates causing fungemia collected in a population-based study in Spain from 2010 to 2011. Antimicrob Agents Chemother 58:1529–1537. doi: 10.1128/AAC.02155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puig-Asensio M, Padilla B, Garnacho-Montero J, Zaragoza O, Aguado JM, Zaragoza R, Montejo M, Munoz P, Ruiz-Camps I, Cuenca-Estrella M, Almirante B, CANDIPOP Project, GEIH-GEMICOMED (SEIMC), REIPI. 2014. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect 20:O245–O254. doi: 10.1111/1469-0691.12380. [DOI] [PubMed] [Google Scholar]
- 7.Myoken Y, Kyo T, Kohara T, Fujihara M, Sugata T, Mikami Y. 2003. Breakthrough fungemia caused by azole-resistant Candida albicans in neutropenic patients with acute leukemia. Clin Infect Dis 36:1496–1497. doi: 10.1086/374894. [DOI] [PubMed] [Google Scholar]
- 8.Pemán J, Canton E, Quindos G, Eraso E, Alcoba J, Guinea J, Merino P, Ruiz-Perez-de-Pipaon MT, Perez-del-Molino L, Linares-Sicilia MJ, Marco F, Garcia J, Rosello EM, Gómez-G-de-la-Pedrosa E, Borrell N, Porras A, Yague G, FUNGEMYCA Study Group. 2012. Epidemiology, species distribution and in vitro antifungal susceptibility of fungaemia in a Spanish multicentre prospective survey. J Antimicrob Chemother 67:1181–1187. doi: 10.1093/jac/dks019. [DOI] [PubMed] [Google Scholar]
- 9.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 10.Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pai MP, Turpin RS, Garey KW. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob Agents Chemother 51:35–39. doi: 10.1128/AAC.00474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Xu YC, Hsueh PR. 2016. Epidemiology of candidemia and antifungal susceptibility in invasive Candida species in the Asia-Pacific region. Future Microbiol 11:1461–1477. doi: 10.2217/fmb-2016-0099. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran R, Sherry L, Nile CJ, Sherriff A, Johnson EM, Hanson MF, Williams C, Munro CA, Jones BJ, Ramage G. 2016. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection-Scotland, 2012–2013. Clin Microbiol Infect 22:87–93. doi: 10.1016/j.cmi.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen LM, Xu YH, Zhou CL, Zhao J, Li CY, Wang R. 2010. Overexpression of CDR1 and CDR2 genes plays an important role in fluconazole resistance in Candida albicans with G487T and T916C mutations. J Int Med Res 38:536–545. doi: 10.1177/147323001003800216. [DOI] [PubMed] [Google Scholar]
- 15.Liu JY, Shi C, Wang Y, Li WJ, Zhao Y, Xiang MJ. 2015. Mechanisms of azole resistance in Candida albicans clinical isolates from Shanghai, China. Res Microbiol 166:153–161. doi: 10.1016/j.resmic.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope W, EUCAST-AFST . 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 18.Chang HC, Chang JJ, Chan SH, Huang AH, Wu TL, Lin MC, Chang TC. 2001. Evaluation of Etest for direct antifungal susceptibility testing of yeasts in positive blood cultures. J Clin Microbiol 39:1328–1333. doi: 10.1128/JCM.39.4.1328-1333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinea J, Recio S, Escribano P, Torres-Narbona M, Pelaez T, Sanchez-Carrillo C, Rodriguez-Creixems M, Bouza E. 2010. Rapid antifungal susceptibility determination for yeast isolates by use of Etest performed directly on blood samples from patients with fungemia. J Clin Microbiol 48:2205–2212. doi: 10.1128/JCM.02321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabeen K, Kumar H, Farooqi J, Mehboob R, Brandt ME, Zafar A. 2016. Agreement of direct antifungal susceptibility testing from positive blood culture bottles with the conventional method for Candida species. J Clin Microbiol 54:343–348. doi: 10.1128/JCM.02432-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudensky B, Broide E, Berko N, Wiener-Well Y, Yinnon AM, Raveh D. 2008. Direct fluconazole susceptibility testing of positive Candida blood cultures by flow cytometry. Mycoses 51:200–204. doi: 10.1111/j.1439-0507.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 22.Avolio M, Grosso S, Bruschetta G, De Rosa R, Camporese A. 2009. Direct antifungal susceptibility testing of positive Candida blood cultures by Sensititre YeastOne. New Microbiol 32:179–184. [PubMed] [Google Scholar]
- 23.Idelevich EA, Grunewald CM, Wullenweber J, Becker K. 2014. Rapid identification and susceptibility testing of Candida spp. from positive blood cultures by combination of direct MALDI-TOF mass spectrometry and direct inoculation of Vitek 2. PLoS One 9:e114834. doi: 10.1371/journal.pone.0114834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escribano P, Marcos-Zambrano LJ, Sánchez-Carrillo C, Bouza E, Guinea J. 2016. Disagreement between the Etest performed directly on blood culture bottles and the standard microdilution procedures to detect fluconazole resistance in C. albicans, abstr P1613 26th Eur Cong Clin Microbiol Infect Dis, 9 to 12 April 2016, Amsterdam, The Netherlands. [Google Scholar]
- 25.Marcos-Zambrano LJ, Escribano P, Sanchez-Carrillo C, Bouza E, Guinea J. 2016. Scope and frequency of fluconazole trailing assessed using EUCAST in invasive Candida spp. isolates. Med Mycol 54:733–739. doi: 10.1093/mmy/myw033. [DOI] [PubMed] [Google Scholar]
- 26.White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gefland DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 27.Alp S, Sancak B, Hascelik G, Arikan S. 2010. Influence of different susceptibility testing methods and media on determination of the relevant fluconazole minimum inhibitory concentrations for heavy trailing Candida isolates with low-high phenotype. Mycoses 53:475–480. doi: 10.1111/j.1439-0507.2009.01739.x. [DOI] [PubMed] [Google Scholar]
- 28.Flowers SA, Colon B, Whaley SG, Schuler MA, Rogers PD. 2015. Contribution of clinically derived mutations in ERG11 to azole resistance in Candida albicans. Antimicrob Agents Chemother 59:450–460. doi: 10.1128/AAC.03470-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Sheng F, Zhao J, Chen L, Li C. 2015. ERG11 mutations and expression of resistance genes in fluconazole-resistant Candida albicans isolates. Arch Microbiol 197:1087–1093. doi: 10.1007/s00203-015-1146-8. [DOI] [PubMed] [Google Scholar]
- 30.Morio F, Pagniez F, Lacroix C, Miegeville M, Le Pape P. 2012. Amino acid substitutions in the Candida albicans sterol Δ5,6-desaturase (Erg3p) confer azole resistance: characterization of two novel mutants with impaired virulence. J Antimicrob Chemother 67:2131–2138. doi: 10.1093/jac/dks186. [DOI] [PubMed] [Google Scholar]
- 31.Ying Y, Zhao Y, Hu X, Cai Z, Liu X, Jin G, Zhang J, Liu J, Huang X. 2013. In vitro fluconazole susceptibility of 1,903 clinical isolates of Candida albicans and the identification of ERG11 mutations. Microb Drug Resist 19:266–273. doi: 10.1089/mdr.2012.0204. [DOI] [PubMed] [Google Scholar]
- 32.Xiang MJ, Liu JY, Ni PH, Wang S, Shi C, Wei B, Ni YX, Ge HL. 2013. ERG11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res 13:386–393. doi: 10.1111/1567-1364.12042. [DOI] [PubMed] [Google Scholar]
- 33.Franz R, Kelly SL, Lamb DC, Kelly DE, Ruhnke M, Morschhauser J. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother 42:3065–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Perlin DS. 2005. Establishing surrogate markers for fluconazole resistance in Candida albicans. Microb Drug Resist 11:232–238. doi: 10.1089/mdr.2005.11.232. [DOI] [PubMed] [Google Scholar]
- 35.Lohberger A, Coste A, Sanglard D. 2013. Distinct roles of the drug resistance transcription factors TAC1, MRR1 and UPC2 from Candida albicans in virulence. Eukaryot Cell 13:127–142. doi: 10.1128/EC.00245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman GH, da Silva Ferreira ME, dos Reis Marques E, Savoldi M, Perlin D, Park S, Godoy Martinez PC, Goldman MH, Colombo AL. 2004. Evaluation of fluconazole resistance mechanisms in Candida albicans clinical isolates from HIV-infected patients in Brazil. Diagn Microbiol Infect Dis 50:25–32. doi: 10.1016/j.diagmicrobio.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharya S, Sobel JD, White TC. 2016. A combination fluorescence assay demonstrates increased efflux pump activity as a resistance mechanism in azole-resistant vaginal Candida albicans isolates. Antimicrob Agents Chemother 60:5858–5866. doi: 10.1128/AAC.01252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MK, Williams LE, Warnock DW, Arthington-Skaggs BA. 2004. Drug resistance genes and trailing growth in Candida albicans isolates. J Antimicrob Chemother 53:217–224. doi: 10.1093/jac/dkh040. [DOI] [PubMed] [Google Scholar]
- 39.Marr KA, Rustad TR, Rex JH, White TC. 1999. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother 43:1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Revankar SG, Kirkpatrick WR, McAtee RK, Fothergill AW, Redding SW, Rinaldi MG, Patterson TF. 1998. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J Clin Microbiol 36:153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rex JH, Nelson PW, Paetznick VL, Lozano-Chiu M, Espinel-Ingroff A, Anaissie EJ. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother 42:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal D, Patterson TF, Rinaldi MG, Revankar SG. 2007. Trailing end-point phenotype of Candida spp. in antifungal susceptibility testing to fluconazole is eliminated by altering incubation temperature. J Med Microbiol 56:1003–1004. doi: 10.1099/jmm.0.47168-0. [DOI] [PubMed] [Google Scholar]
- 43.Dannaoui E, Paugam A, Develoux M, Chochillon C, Matheron J, Datry A, Bouges-Michel C, Bonnal C, Dromer F, Bretagne S. 2009. Comparison of antifungal MICs for yeasts obtained using the EUCAST method in a reference laboratory and the Etest in nine different hospital laboratories. Clin Microbiol Infect 16:863–869. doi: 10.1111/j.1469-0691.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 44.Bourgeois N, Dehandschoewercker L, Bertout S, Bousquet PJ, Rispail P, Lachaud L. 2010. Antifungal susceptibility of 205 Candida spp. isolated primarily during invasive candidiasis and comparison of the Vitek 2 system with the CLSI broth microdilution and Etest methods. J Clin Microbiol 48:154–161. doi: 10.1128/JCM.01096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metin DY, Hilmioglu-Polat S, Samlioglu P, Doganay-Oflazoglu B, Inci R, Tumbay E. 2011. Evaluation of antifungal susceptibility testing with microdilution and Etest methods of Candida blood isolates. Mycopathologia 172:187–199. doi: 10.1007/s11046-011-9413-y. [DOI] [PubMed] [Google Scholar]
- 46.Marcos-Zambrano LJ, Escribano P, Sanchez C, Munoz P, Bouza E, Guinea J. 2014. Antifungal resistance to fluconazole and echinocandins is not emerging in yeast isolates causing fungemia in a Spanish tertiary care center. Antimicrob Agents Chemother 58:4565–4572. doi: 10.1128/AAC.02670-14. [DOI] [PMC free article] [PubMed] [Google Scholar]