ABSTRACT
The ceftazidime-avibactam antibiotic combination was recently shown to be at risk for the emergence of resistance under treatment. To gain insight into the underlying mechanism, we have analyzed the catalytic properties of a Klebsiella pneumoniae carbapenemase type 2 (KPC-2) β-lactamase harboring the D179Y substitution. We show that impaired inhibition by avibactam combined with significant residual activity for ceftazidime hydrolysis accounts for the resistance. In contrast, the D179Y substitution abolished the hydrolysis of aztreonam and imipenem, indicating that these drugs might provide therapeutic alternatives.
KEYWORDS: β-lactamase inhibitor, avibactam, KPC-2, carbapenemase, ceftazidime
TEXT
Resistance to carbapenems in Enterobacteriaceae is often due to the production of class A β-lactamases belonging to the Klebsiella pneumoniae carbapenemase (KPC) type (1). The most commonly encountered variants worldwide are KPC-2 and KPC-3, which confer high-level resistance to most available β-lactams. The production of KPC β-lactamases is often associated with resistance to other classes of antibiotics, including aminoglycosides, fluoroquinolones, and colistin, leaving few or no therapeutic alternatives (2, 3). Inhibition of KPC enzymes by classical β-lactamase inhibitors (clavulanate, sulbactam, and tazobactam) is not sufficient to restore the activity of β-lactams (4). In this context, a new β-lactam–β-lactamase inhibitor combination, ceftazidime-avibactam (5), has recently obtained regulatory approval in the United States and Europe. The combination has a broad spectrum of activity against multidrug-resistant Enterobacteriaceae and Pseudomonas aeruginosa strains producing class A, C, and some class D β-lactamases (6, 7).
The emergence of resistance to the ceftazidime-avibactam combination has been recently reported in three out of 37 patients infected with carbapenem-resistant Enterobacteriaceae and treated with these drugs (8). The emergence of resistance, which was associated with microbiological failure, was due to amino acid substitutions in the KPC-3 enzymes produced by the K. pneumoniae isolates recovered from these three patients (D179Y, V240G, and D179Y associated with T243M) (9). The impact of these substitutions on the kinetic parameters for hydrolysis of ceftazidime and inhibition by avibactam has not been previously reported. In another study (10), site-directed mutagenesis identified rare substitutions resulting in resistance to the ceftazidime-avibactam combination. A comparison of KPC-2 and KPC-2 D179N did not reveal any modification of the steady-state rate of ceftazidime hydrolysis or inhibition by avibactam (10). However, an initial burst of ceftazidime hydrolysis was detected for KPC-2 D179N but not for the parental enzyme (10).
To gain insight into the modifications of the catalytic properties of KPC enzymes that lead to resistance to the ceftazidime-avibactam combination, we have isolated in vitro a mutant derived from a KPC-2-producing strain of Escherichia coli. The resulting KPC-2 enzyme, which was found to harbor the previously described D179Y substitution, was kinetically characterized with respect to the hydrolysis of various β-lactams and the inhibition by avibactam.
In vitro selection for resistance to the ceftazidime-avibactam combination.
Selection was performed with E. coli TOP10 harboring plasmid pTRC-99kΩblaKPC-2, which enables expression of the blaKPC-2 gene encoding KPC-2 under the control of the Ptrc promoter (11). Approximately 6 × 109 CFU were plated on Mueller-Hinton (MH) agar (Difco) containing isopropyl-β-d-1-thiogalactopyranoside (IPTG) for the induction of Ptrc, a fixed concentration of avibactam (4 μg/ml), and 2-fold-increasing concentrations of ceftazidime (1 to 32 μg/ml). CFU grew after 48 h of incubation at frequencies of 2 × 10−8, 5 × 10−9, and 1× 10−9 on agar plates containing 4, 8, and 16 μg/ml ceftazidime, respectively. Sequencing of the blaKPC-2 gene from 10 putative mutants obtained at the highest concentrations of ceftazidime (8 or 16 μg/ml) revealed the same point mutation leading to the D179Y substitution in 5 resistant clones and the wild-type sequence in the remaining clones. The plasmid encoding KPC-2 D179Y was extracted and introduced by transformation into E. coli TOP10, with selection for resistance to kanamycin conveyed by the vector pTRC-99k. The resulting transformants grew in the presence of ceftazidime (8 μg/ml) and avibactam (4 μg/ml), indicating that the D179Y substitution was sufficient for resistance to the drug combination, as these concentrations correspond to the susceptible breakpoints by FDA and EUCAST criteria.
Modification of the resistance phenotype resulting from the D179Y substitution in KPC-2.
The MICs of β-lactams were determined by the microdilution method in MH broth, according to Clinical and Laboratory Standards Institute (CLSI) recommendations (12). Avibactam and clavulanate were used at a fixed concentration of 4 μg/ml in combination with β-lactams. IPTG (500 μM) was added to the microdilution plates to induce production of the β-lactamase. The precultures were grown in MH broth containing IPTG (500 μM) and kanamycin (50 μg/ml) for plasmid maintenance.
E. coli TOP10 producing wild-type KPC-2 was resistant to all tested β-lactams (MIC, ≧128 μg/ml), whereas the ceftazidime-avibactam combination was active (MIC, 1 μg/ml), according to FDA and EUCAST interpretive criteria (Table 1). In contrast, avibactam did not restore susceptibility to ceftazidime in the strain producing the KPC-2 D179Y variant (MIC, 32 μg/ml), as expected from the selection procedure. Resistance to ceftazidime alone was not abolished by D179Y (MIC, >128 μg/ml), indicating that the β-lactamase remained active in the hydrolysis of this cephalosporin. The D179Y substitution decreased the MICs of ampicillin, ceftriaxone, aztreonam, and meropenem, resulting in susceptibility for aztreonam and meropenem (MICs, 0.5 and <0.12 μg/ml, respectively). Interestingly, clavulanate significantly reduced the MIC of ceftazidime against E. coli TOP10 producing KPC-2 D179Y (from >128 μg/ml to 8 μg/ml). In conclusion, the D179Y substitution led to the acquisition of resistance to the ceftazidime-avibactam combination and of susceptibility to aztreonam and meropenem. These results are in full agreement with previous analyses of the consequences of the D179Y substitution in various genetic backgrounds (9).
TABLE 1.
Impact of D179Y substitution on MICs of β-lactams against E. coli TOP10 producing a KPC-2 β-lactamase in the absence or presence of a β-lactamase inhibitor
| β-Lactamase | MIC (μg/ml) of indicated β-lactams with avibactam (+Avi), with clavulanate (+Clav), or in the absence of inhibitor (none)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin |
Ceftazidime |
Ceftriaxone |
Aztreonam |
Meropenem |
Imipenem |
|||||
| None | +Avi | +Clav | None | +Avi | +Clav | None | None | None | None | |
| None | 2 | 2 | 2 | 0.25 | 0.25 | 0.25 | <0.12 | 0.25 | <0.12 | 0.25 |
| KPC-2 | >128 | 128 | >128 | >128 | 1 | 64 | >128 | >128 | >128 | 128 |
| KPC-2 D179Y | 16 | 8 | 8 | >128 | 32 | 8 | 32 | 0.5 | <0.12 | 0.25 |
Inhibitors were used at a fixed concentration of 4 μg/ml. Data are the median of the results from five determinations.
Impact of the D179Y substitution on the hydrolysis of β-lactams by KPC-2.
Wild-type KPC-2 and KPC-2 D179Y were produced in E. coli TOP10 cells harboring pTRC-99kΩblaKPC-2 or its derivative encoding KPC-2 D179Y, as previously described (11). The β-lactamases were purified by anion-exchange and size-exclusion chromatography (11). All kinetic experiments were performed at 20°C in 2-(N-morpholino)ethanesulfonic acid (MES; 100 mM [pH 6.4]) in a Cary 300 spectrophotometer (Agilent), as previously described (11). Steady-state kinetic parameters (kcat, Km, and kcat/Km) were determined at a fixed concentration of the β-lactamase [E] and various concentrations of the substrate [S]. Initial rate (vi) values were plotted as a function of [S], according to the equation vi = kcat [E][S]/(Km + [S]). Km values that could not be evaluated by this method were determined by analyzing competitive hydrolysis of the β-lactam and the chromogenic cephalosporin CENTA (13), as previously described (11, 14). Variations in the molar extinction coefficients (Δε) resulting from hydrolysis of the β-lactam ring were 7,380 M−1 · cm−1 (λ415 nm) for CENTA, −500 M−1 · cm−1 (λ235 nm) for ampicillin, −9,800 M−1 · cm−1 (λ256 nm) for ceftazidime, −400 M−1 · cm−1 (λ318 nm) for aztreonam, −14,500 M−1 · cm−1 (λ270 nm) for ceftriaxone, −2,500 M−1 · cm−1 (λ227 nm) for clavulanate, and −7,280 M−1 · cm−1 (λ298 nm) for meropenem.
The D179Y substitution severely impaired the hydrolysis of ampicillin, aztreonam, and meropenem, since hydrolysis of these β-lactams by KPC-2 D179Y was not detectable at the highest enzyme concentration tested (10 μM) (Table 2). Accordingly, KPC-2 D179Y did not confer resistance to aztreonam and meropenem (Table 1). For ampicillin, the production of KPC-2 D179Y led to a 4-fold increase in the MIC, suggesting that the enzyme retained residual activity which could not be detected due to the low sensitivity of the in vitro assay for this antibiotic (Δε = −500 M−1 · cm−1 at λ235 nm).
TABLE 2.
Impact of D179Y substitution on kinetic parameters of KPC-2 for hydrolysis of β-lactams
| Kinetic parameter by β-lactam | Resultsb |
|
|---|---|---|
| KPC-2 | KPC-2 D179Y | |
| Ampicillin | ||
| kcat (s−1) | 200 ± 10 | NAa |
| Km (μM) | 200 ± 40 | NA |
| kcat/Km (M−1 · s−1) | (1.0 ± 0.2) × 106 | NA |
| CENTA | ||
| kcat (s−1) | 100 ± 10 | 0.11 ± 0.01 |
| Km (μM) | 32 ± 8 | 23 ± 3 |
| kcat/Km (M−1 · s−1) | (3.1 ± 0.8) × 106 | (4.8 ± 0.6) × 103 |
| Ceftazidime | ||
| kcat (s−1) | >1.4 | (1.3 ± 0.2) × 10−3 |
| Km (μM) | >600 | 19 ± 4 |
| kcat/Km (M−1 · s−1) | (3.7 ± 0.1) × 103 | 70 ± 20 |
| Ceftriaxone | ||
| kcat (s−1) | >25 | (7.0 ± 0.1) × 10−4 |
| Km (μM) | >100 | 0.20 ± 0.03 |
| kcat/Km (M−1 · s−1) | (2.5 ± 0.1) × 105 | (3.5 ± 0.5) × 103 |
| Aztreonam | ||
| kcat (s−1) | >350 | NA |
| Km (μM) | >5 × 103 | NA |
| kcat/Km (M−1 · s−1) | (6.9 ± 0.3) × 104 | NA |
| Meropenem | ||
| kcat (s−1) | 1.8 ± 0.2 | NA |
| Km (μM) | 27 ± 9 | NA |
| kcat/Km (M−1 · s−1) | (6.7 ± 2.3) × 104 | NA |
| Imipenem | ||
| kcat (s−1) | 48 ± 5 | NA |
| Km (μM) | 66 ± 21 | NA |
| kcat/Km (M−1 · s−1) | (7.3 ± 2.4) × 105 | NA |
| Clavulanate | ||
| kcat (s−1) | 5.2 ± 0.7 | NA |
| Km (μM) | 36 ± 4 | NA |
| kcat/Km (M−1 · s−1) | (1.4 ± 0.3) × 105 | NA |
NA, not applicable, as hydrolysis of β-lactams (100 μM, except for aztreonam at 1,000 µM) was not detected at the highest β-lactamase concentration tested (10 μM). Under these conditions, the lower limits of detection correspond to a turnover of <2 × 10−3 · s−1, <2.6 × 10−3 · s−1, <1.5 × 10−4 · s−1, <1.5 × 10−4 · s−1, and <4 × 10−4 · s−1 for ampicillin, aztreonam, meropenem, imipenem, and clavulanate, respectively.
Data are means ± standard errors of the mean (SEM).
The D179Y substitution led to a >900-fold reduction in the kinetic constant kcat for hydrolysis of ceftazidime, ceftriaxone, and the chromogenic cephalosporin CENTA. Binding of these drugs to the β-lactamase did not appear to be impaired, since the D179Y substitution also led to a large reduction in the kinetic constant Km for ceftazidime (>30-fold) and ceftriaxone (>500-fold), whereas that of CENTA was not significantly altered. Overall, the D179Y substitution had a moderate impact on the catalytic efficacy (kcat/Km) of ceftazidime hydrolysis (a 50-fold decrease) due to a partially compensatory effect of the large decrease in kcat (>1,000-fold) by a decrease in Km (>30-fold). Of note, this modification of the kinetic parameters did not prevent the expression of ceftazidime resistance (MIC, >128 μg/ml). The results obtained with ceftriaxone were qualitatively similar, although for this drug, the partial compensation of the decreases in kcat and Km was associated with a lower level of resistance (MIC, 32 μg/ml).
Impact of the D179Y substitution on inhibition of KPC-2 by avibactam.
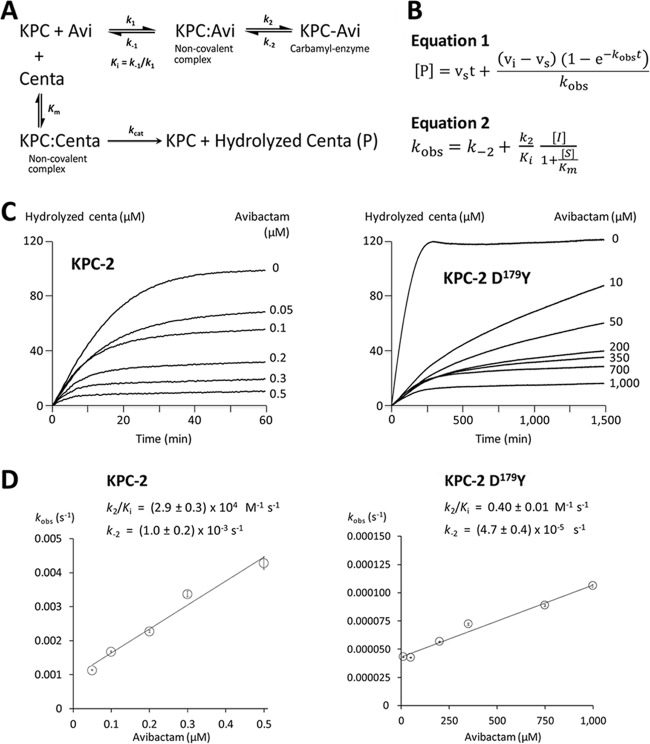
The rate constant for the carbamylation reaction (k2/Ki) was evaluated based on the inhibition of CENTA hydrolysis (100 μM) by avibactam at 37°C in MES (100 mM [pH 6.4]) for a two-step reaction (Fig. 1), as previously described (11, 15). The D179Y substitution led to an approximately 70,000-fold reduction in the carbamylation rate constant k2/Ki. Thus, resistance to the ceftazidime-avibactam combination conveyed by the D179Y substitution is accounted for by a drastic reduction in the efficacy of avibactam coupled to substantial residual activity for ceftazidime hydrolysis.
FIG 1.
Kinetics of KPC-2 and KPC-2 D179Y inhibition by avibactam (Avi). (A) Reaction schemes. (B) Equations for rate constants. vi and vs are the initial and steady-state (inhibited) velocities of the reactions, respectively. [I], [P], and [S] are the concentrations of avibactam, hydrolyzed CENTA, and CENTA, respectively. Km is the kinetic parameter for hydrolysis of CENTA in the absence of avibactam. t, time. (C) Inhibition of CENTA hydrolysis by increasing concentrations of avibactam. Values of kobs were obtained by fitting equation 1 to progress curves. (D) Parameters k2/Ki and k−2 were determined by fitting equation 2 to the data. Error bars shown are ± standard error of the mean (SEM) from the fit to kobs.
Selection of mutants coresistant to imipenem and the ceftazidime-avibactam combination.
E. coli TOP10 harboring pTRC-99kΩblaKPC-2 (ca. 6 × 109 CFU) was plated on MH agar containing avibactam (4 μg/ml), ceftazidime (8 μg/ml), and imipenem (0.12 to 32 μg/ml). Mutants resistant to the triple combination were not obtained (frequency, <2 × 10−10).
The D179Y substitution leads to decreased hydrolysis of meropenem and imipenem by the enzyme and consequently does not confer resistance to those substrates once produced in E. coli (see above, Table 1). To assess whether secondary mutations could restore resistance to carbapenems, derivatives of E. coli TOP10 producing KPC-2 D179Y were selected on MH agar containing imipenem (8 μg/ml). Mutants were obtained at a frequency of 2 × 10−9. Sequencing of the blaKPC-2 gene from 8 out of 8 mutants revealed a reversion to the wild-type sequence. These results indicate that the alteration of KPC-2 conferring coresistance to the ceftazidime-avibactam combination and to imipenem are not readily selected in vitro.
Conclusions.
Here, we show that a single amino acid substitution in KPC-2 (D179Y) is sufficient to prevent inhibition of the β-lactamase by avibactam, leading to resistance to the ceftazidime-avibactam combination. In contrast, previous analyses based on site-directed mutagenesis have shown that reduced inhibition by avibactam due to a modification of position 132 of KPC-2 (N132G) does not confer resistance to the ceftazidime-avibactam combination, since the hydrolysis of ceftazidime is drastically reduced (11). Extensive analysis of the Ω-loop of KPC-2 by site-directed mutagenesis (positions 164, 167, 169, and 179) has identified only a few substitutions that result in resistance to the ceftazidime-avibactam combination (R164 replaced by A or P, and D179 replaced by A, Q, or N) (10). The underlying mechanism was proposed to involve stabilizing interactions (e.g., hydrogen bonds) of ceftazidime within the KPC-2 active site that prevent avibactam from binding to and inhibiting the β-lactamase (10). The mechanism described in reference 10 is clearly distinct from the impaired carbamylation by avibactam reported for the D179Y substitution in the current study.
The D179Y substitution was recently shown to be responsible for the acquisition of ceftazidime-avibactam resistance under treatment in K. pneumoniae isolates producing the closely related enzyme KPC-3 (8, 16). The fact that a single mutation is sufficient for impaired inhibition of KPC β-lactamases by avibactam indicates that the emergence of resistance under treatment may jeopardize the efficacy of the ceftazidime-avibactam combination in the future (17). To date, the emergence of resistance was documented in a single study in ca. 8% of the patients infected with carbapenem-resistant Enterobacteriaceae and treated with the ceftazidime-avibactam combination (8).
The D179Y substitution abolished the hydrolysis of meropenem and aztreonam by KPC-2 and improved inhibition by clavulanate (Table 2). Thus, these β-lactams and classical β-lactamase inhibitors might provide therapeutic alternatives in case of the emergence of ceftazidime-avibactam resistance. It remains to be determined whether such alternatives could also exist for other modifications of KPC enzymes leading to ceftazidime-avibactam resistance. This might be the case since the mutations selected under treatment (8) also led to large decreases in the MICs of carbapenems (18). Similarly, in vitro selection for resistance to the ceftazidime-avibactam combination in KPC-3-producing K. pneumoniae and Enterobacter cloacae strains led to large decreases in the MICs of carbapenems in the majority of the mutants (19). Our attempts to select mutants resistant to the triple combination of ceftazidime, imipenem, and avibactam were unsuccessful. Our selection for imipenem-resistant derivatives of the mutant producing KPC-2 D179Y led to reversion to the wild-type sequence at position 179 and to susceptibility to the ceftazidime-avibactam combination. Recently, Shields et al. also reported such reversions, but resistance to carbapenems and ceftazidime-avibactam has been obtained by multistep exposure (≥4 weeks) of ceftazidime-avibactam-resistant K. pneumoniae strains to meropenem, mainly due to modifications of the porin OmpK36 (20).
Carbapenems and classical β-lactamase inhibitors may provide alternatives for use against variants with impaired inhibition by avibactam, as previously observed for substitutions in the β-lactamases from mycobacteria (21). These observations indicate, as previously discussed (21), that the formulation of β-lactamase inhibitors independently from a β-lactam partner would provide clinicians with access to potentially useful therapeutic regimens based on combining β-lactams with approved inhibitors.
ACKNOWLEDGMENT
Avibactam was provided by AstraZeneca.
We declare no conflicts of interest.
REFERENCES
- 1.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasevic AT, Canton R, Carmeli Y, Friedrich AW, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Nordmann P, Poirel L, Rossolini GM, Seifert H, Vatopoulos A, Walsh T, Woodford N, Monnet DL, European Survey of Carbapenemase-Producing Enterobacteriaceae Working Group. 2017. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 17:153–163. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 3.Monaco M, Giani T, Raffone M, Arena F, Garcia-Fernandez A, Pollini S, Network Eu S-I, Grundmann H, Pantosti A, Rossolini GM. 2014. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill 19:pii=20939 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20939. [DOI] [PubMed] [Google Scholar]
- 4.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev 23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papp-Wallace KM, Bonomo RA. 2016. New beta-lactamase inhibitors in the clinic. Infect Dis Clin North Am 30:441–464. doi: 10.1016/j.idc.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sader HS, Castanheira M, Flamm RK. 2017. Antimicrobial activity of ceftazidime-avibactam against Gram-negative bacteria isolated from patients hospitalized with pneumonia in U.S. medical centers, 2011 to 2015. Antimicrob Agents Chemother 61:e02083-16. doi: 10.1128/AAC.02083-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahiri SD, Bradford PA, Nichols WW, Alm RA. 2016. Structural and sequence analysis of class A beta-lactamases with respect to avibactam inhibition: impact of Ω-loop variations. J Antimicrob Chemother 71:2848–2855. doi: 10.1093/jac/dkw248. [DOI] [PubMed] [Google Scholar]
- 8.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkler ML, Papp-Wallace KM, Bonomo RA. 2015. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV beta-lactamases with single amino acid substitutions in the Ω-loop. J Antimicrob Chemother 70:2279–2286. doi: 10.1093/jac/dkv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ourghanlian C, Soroka D, Arthur M. 2017. Inhibition by avibactam and clavulanate of the β-lactamases KPC-2 and CTX-M-15 harboring the substitution N132G in the conserved SDN motif. Antimicrob Agents Chemother 61:e02510-16. doi: 10.1128/AAC.02510-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2015. Methods for dilution susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed CLSI document M7-A8. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Bebrone C, Moali C, Mahy F, Rival S, Docquier JD, Rossolini GM, Fastrez J, Pratt RF, Frere JM, Galleni M. 2001. CENTA as a chromogenic substrate for studying beta-lactamases. Antimicrob Agents Chemother 45:1868–1871. doi: 10.1128/AAC.45.6.1868-1871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frère JM, Dormans C, Duyckaerts C, De Graeve J. 1982. Interaction of beta-iodopenicillanate with the beta-lactamases of Streptomyces albus G and Actinomadura R39. Biochem J 207:437–444. doi: 10.1042/bj2070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-beta-lactam beta-lactamase inhibitor. Proc Natl Acad Sci U S A 109:11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shields RK, Clancy CJ, Hao B, Chen L, Press EG, Iovine NM, Kreiswirth BN, Nguyen MH. 2015. Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum beta-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against carbapenem-resistant K. pneumoniae. Antimicrob Agents Chemother 59:5793–5797. doi: 10.1128/AAC.00548-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spellberg B, Bonomo RA. 2016. Ceftazidime-avibactam and carbapenem-resistant Enterobacteriaceae: “we're gonna need a bigger boat.” Clin Infect Dis 63:1619–1621. doi: 10.1093/cid/ciw639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Hong Nguyen M. 2017. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum β-lactamases. Antimicrob Agent Chemother, 61:e02534-16. doi: 10.1128/AAC.02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livermore DM, Warner M, Jamrozy D, Mushtaq S, Nichols WW, Mustafa N, Woodford N. 2015. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother 59:5324–5330. doi: 10.1128/AAC.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. In vitro selection of meropenem resistance among ceftazidime-avibactam resistant, meropenem susceptible Klebsiella pneumoniae isolates with variant KPC-3 carbapenemases. Antimicrob Agents Chemother, 61:e00079-17. doi: 10.1128/AAC.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soroka D, Ourghanlian C, Compain F, Fichini M, Dubée V, Mainardi J, Hugonnet J, Arthur M. 2017. Inhibition of β-lactamases of mycobacteria by avibactam and clavulanate. J Antimicrob Chemother 72:1081–1088. doi: 10.1093/jac/dkw546. [DOI] [PubMed] [Google Scholar]