ABSTRACT
Leishmaniasis is a neglected tropical disease that affects millions of people worldwide and represents a major public health problem. Information on protein expression patterns and functional roles within the context of Leishmania-infected human monocyte-derived macrophages (MDMs) under drug treatment conditions is essential for understanding the role of these cells in leishmaniasis treatment. We analyzed functional changes in the expression of human MDM genes and proteins during in vitro infection by Leishmania braziliensis and treatment with Glucantime (SbV), using quantitative PCR (qPCR) arrays, Western blotting, confocal microscopy, and small interfering RNA (siRNA) human gene inhibition assays. Comparison of the results from gene transcription and protein expression analyses revealed that glutathione S-transferase π1 (GSTP1), glutamate-cysteine ligase modifier subunit (GCLM), glutathione reductase (GSR), glutathione synthetase (GSS), thioredoxin (TRX), and ATP-binding cassette, subfamily B, member 5 (ABCB5), were strongly upregulated at both the mRNA and protein levels in human MDMs that were infected and treated, compared to the control group. Subcellular localization studies showed a primarily phagolysosomal location for the ABCB5 transporter, indicating that this protein may be involved in the transport of SbV. By inducing a decrease in L. braziliensis intracellular survival in THP-1 macrophages, siRNA silencing of GSTP1, GSS, and ABCB5 resulted in an increased leishmanicidal effect of SbV exposure in vitro. Our results suggest that human MDMs infected with L. braziliensis and treated with SbV express increased levels of genes participating in antioxidant defense, whereas our functional analyses provide evidence for the involvement of human MDMs in drug detoxification. Therefore, we conclude that GSS, GSTP1, and ABCB5 proteins represent potential targets for enhancing the leishmanicidal activity of Glucantime.
KEYWORDS: human leishmaniasis, host-pathogen interaction, Leishmania braziliensis, GSTP1, GSS, ABCB5, Glucantime
INTRODUCTION
Leishmania spp., which are intracellular protozoan parasites, are the causative agents of leishmaniasis, a neglected infectious disease that is found worldwide. Depending on the species of infecting parasite and the host immune status, leishmaniasis can manifest in a variety of clinical conditions with cutaneous, mucocutaneous, or visceral involvement (1, 2). Leishmania (Viannia) braziliensis, which causes cutaneous and mucocutaneous leishmaniasis, is the most prevalent species infecting humans in Central America and South America (3). Currently, the control of leishmaniasis depends on avoidance of exposure to the insect vector through individual or collective protection with the use of insecticides and chemotherapy (4).
Over the past 60 years, pentavalent antimony compounds have been the first-line drugs for treatment of all forms of human leishmaniasis in Central America, South America, North Africa, Turkey, Bangladesh, Nepal, and India (except Bihar) (5). Despite renewed attention regarding the biochemistry and pharmacological effects of these drugs, their metabolism and mechanism of action are not yet fully understood (6). Pentavalent antimony (SbV) is generally considered a prodrug, requiring biological reduction to trivalent antimony (SbIII), within either the parasite or the host macrophages (MΦ), to become active against Leishmania spp. (7, 8).
SbV induces the production of reactive oxygen species (ROS) (mainly H2O2) in Leishmania-infected macrophages through an oxidative burst, via phosphorylation of phosphoinositide 3-kinase (PI3K), protein kinase C (PKC) Ras, and extracellular signal-regulated kinase (ERK) pathways. In addition, nitric oxide (NO) is produced through the PI3K and p38 mitogen-activated protein kinase (MAPK) pathways (9, 10). Consequently, to maintain redox homeostasis and to eliminate ROS, aerobic organisms are equipped with enzymatic and nonenzymatic antioxidants and metal-sequestering proteins that either prevent the generation of prooxidants or intercept or degrade the molecules once they are produced (11). Recent studies have reported at least four different thiol molecules (glutathione, cysteine, cysteinyl-glycine, and trypanothione [only in trypanosomatids]) that are involved in cellular redox control and protect against potentially toxic agents that cause chemical or oxidative stress (12–14). For instance, the reduced form (SbIII) acts directly on the Leishmania parasite and the host cell by perturbing the redox balance and generating cytotoxic effects in both (15, 16). Thiols exhibit a dual role in pentavalent antimonial pharmacology, first promoting drug activation through nonenzymatic reduction of SbV to SbIII and then inducing drug detoxification by forming conjugates with SbIII that are effluxed from and/or sequestrated within the host cell (8).
Different approaches have been applied for understanding the metabolism and mechanism of action of antimonial compounds. Nevertheless, the role of macrophages in human leishmaniasis treatment has not been completely elucidated. Hence, we sought to identify, via functional analysis of antioxidant defense genes, human macrophage proteins that are potentially involved in the detoxification of pentavalent antimony compounds. The results suggest that host factors can be modulated to enhance leishmanicidal drug activity and to promote new strategies to prevent the harmful effects of Leishmania parasites.
RESULTS
Effects of SbV (Glucantime) treatment and L. braziliensis infection on oxidative stress and drug transporter gene expression in human MDMs.
To assess gene expression patterns in human monocyte-derived macrophages (MDMs) in response to late L. braziliensis infection (96 h of parasite infection) and SbV treatment (72 h of drug interaction), we used a commercially available PCR array for oxidative stress and drug transporter genes. After establishing an arbitrary cutoff value of 3-fold differential gene expression in the study groups, compared to the control group (human MDMs without infection and without treatment), we found that 19.6% of the genes (33/168) were upregulated after infection with L. braziliensis and treatment with SbV (Fig. 1A). Of the 33 upregulated genes, 54.5% and 45.5% belong to the oxidative stress and drug transporter pathways, respectively (Fig. 1B and C).
FIG 1.
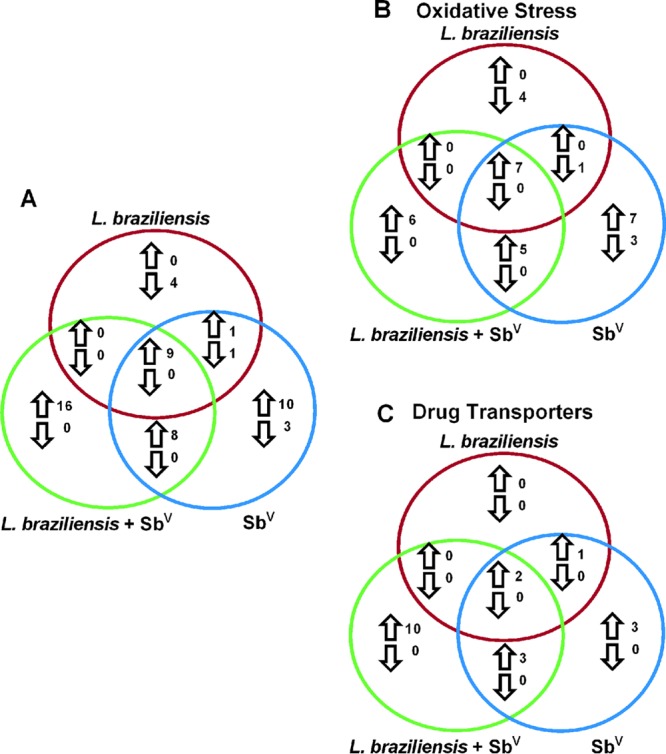
Venn diagram showing the number of transcripts upregulated and downregulated in human MDMs. (A) Total number of genes shared or differentially expressed in human MDMs. (B) Number of genes of the oxidative stress pathway. (C) Number of genes of the drug transporter pathway. MDMs infected with Leishmania braziliensis (red circles), treated with Glucantime (blue circles), and both infected and treated with Glucantime (green circles) are indicated. Upregulated genes are indicated by upward arrows and downregulated genes by downward arrows.
Among the genes that were differentially expressed were GSTP1 (glutathione S-transferase π1), GSS (glutathione synthetase), GSR (glutathione reductase), GPX2 (glutathione peroxidase 2, gastrointestinal), GPX3 (glutathione peroxidase 3, from plasma), CAT (catalase), HMOX1 (heme oxygenase [decycling] 1), TRX (thioredoxin), ABCB5 (ATP-binding cassette, subfamily B [multidrug resistance [MDR]/transporter associated with antigen processing [TAP]], member 5), ABCB6 (ATP-binding cassette, subfamily B [MDR/TAP], member 6), ABCB11 (ATP-binding cassette, subfamily B [MDR/TAP], member 11), SLC7A11 (solute carrier family 7 [anionic amino acid transporter light chain, Xc− system], member 11), and SLC22A1 (solute carrier family 22 [organic cation transporter [OCT]], member 1). The gene expression levels were between 3.06 and 15.98 times higher than those in the control group; GSTP1 showed the greatest upregulation and GPX3 the least (see Tables S2 and S3 in the supplemental material). These results indicate that both L. braziliensis infection and SbV treatment significantly modulate gene expression in the human host cells, particularly for the genes encoding proteins that may be involved in SbV detoxification (i.e., biosynthesis of glutathione, antioxidant defense, and drug transporters). Notably, we did not observe downregulation of genes in human MDMs infected and treated with SbV (Tables S2 and S3).
Furthermore, in the MDM group treated with SbV, 16.7% of the evaluated genes (28/168 genes) exhibited upregulation, with 10 of the genes being exclusive to this group (Fig. 1A; also see Tables S2 and S3). In the group infected with L. braziliensis, 10 upregulated genes were found but none was exclusive to this group (Fig. 1A). In the MDM group infected with L. braziliensis, 3.0% of the genes (5/168 genes) were downregulated; among them, the SOD3 (superoxide dismutase 3, extracellular) gene showed the greatest downregulation, being decreased 5.62-fold (Table S2). In the human MDMs treated with SbV, 2.4% of the genes (4/168 genes) were downregulated (Fig. 1B), with TXNRD1 (thioredoxin reductase 1) showing the most negative regulation, at −11.09-fold (Table S2).
Validation of antioxidant defense and drug transporter gene expression levels in human MDMs infected with L. braziliensis and treated with SbV (Glucantime).
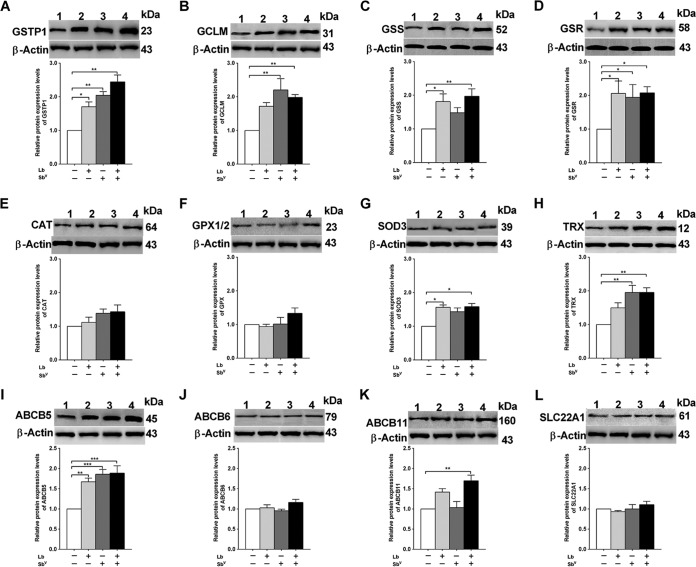
To validate the gene expression levels observed in human MDMs infected with L. braziliensis and treated with SbV, we assessed the levels of GSTP1, glutamate-cysteine ligase modifier subunit (GCLM), GSR, GSS, CAT, GPX2, SOD3, TRX, ABCB5, ABCB6, ABCB11/bile salt export pump (BSEP), and SLC22A1/OCT1 protein expression by Western blotting, using total protein extracts from human MDMs.
The results showed the highest levels of expression for proteins in the glutathione biosynthesis pathway (P < 0.05) in infected and treated human MDMs, as also observed in the gene expression assays. Western blot analyses revealed increased protein expression of GSTP1, GCLM, GSS, and GSR, with levels 2.44, 1.98, 2.08, and 1.97 times higher (P < 0.01), respectively, than those in the control group (Fig. 2). Similarly, the expression of proteins involved in antioxidant defense, such as SOD and TRX, was significantly increased, with levels 1.58-fold (P < 0.05) and 1.95-fold (P < 0.01) higher, respectively, in the infected and treated human MDMs than in the control group. However, no significant changes in the levels of CAT and GPX1/2 protein expression were detected (Fig. 2). In contrast, increased expression of the ABCB5 and ABCB11 drug transporters, with levels 1.89-fold (P < 0.001) and 1.69-fold (P < 0.01) higher, respectively, was observed in MDMs infected with L. braziliensis and treated with SbV, compared to the control group (Fig. 2).
FIG 2.
Modulation of oxidative stress and drug transporter protein expression levels in human MDMs. Protein expression analysis of GSTP1 (A), GCLM (B), GSS (C), GSR (D), CAT (E), GPX1/2 (F), SOD3 (G), TRX (H), ABCB5 (I), ABCB6 (J), ABCB11 (K), and SCL22A1 (L) was performed, with Western blot analyses of soluble protein extracts obtained from human MDMs untreated and uninfected (as a control group) (lanes 1), infected with L. braziliensis (Lb) (lanes 2), treated with 32 μg/ml SbV (lanes 3), and both infected and treated (lanes 4). Equivalent protein loading was assessed by immunodetection of β-actin. Densitometric analysis of the expression of oxidative stress proteins in human MDM groups, as described above, was performed. The results represent the average of values for five healthy donors in duplicate + standard error of the mean (SEM). Significant differences were determined by one-way ANOVA, followed by Bonferroni's multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Molecular sizes (in kilodaltons) are shown to the right of all gel images.
Correlation between gene and protein expression levels in human MDMs.
We attempted to correlate the observed changes in gene expression with the levels of 12 proteins, of which 8 are involved in antioxidant defense and 4 are involved in drug transport pathways in human macrophages. Positive correlations were observed for 6 genes, namely, GSTP1 (r = 0.55, P = 0.006), GCLM (r = 0.50, P = 0.01), GSS (r = 0.43, P = 0.03), GSR (r = 0.63, P = 0.001), TRX (r = 0.74, P = 0.0001), and ABCB5 (r = 0.59, P = 0.003), and a negative correlation was observed for SOD3 (r = −0.39, P = 0.04). No correlations were observed for the remaining proteins.
Subcellular localization of the drug transporter ABCB5.
To explore the possible role of ABCB5 in SbV (Glucantime) transport and because of the positive correlation between gene and protein expression levels, the subcellular localization of this protein was assessed (Fig. S1B and E, top and bottom). The overlay image from confocal microscopy, which was obtained using an anti-lysosome-associated membrane protein 1 (LAMP-1) monoclonal antibody and Hoechst dye staining, showed that parasites were located in phagolysosomes (Fig. S1E). Confocal microscopic analyses of infected macrophages versus infected macrophages treated with SbV allowed the determination of subcellular localization differences for ABCB5, showing the greatest association with phagolysosome membranes where the parasites resided (Fig. S1E, upper).
Silencing of the human GSTP1 gene in THP-1 MΦ cells increasing the intracellular sensitivity of L. braziliensis amastigotes to SbV (Glucantime) treatment.
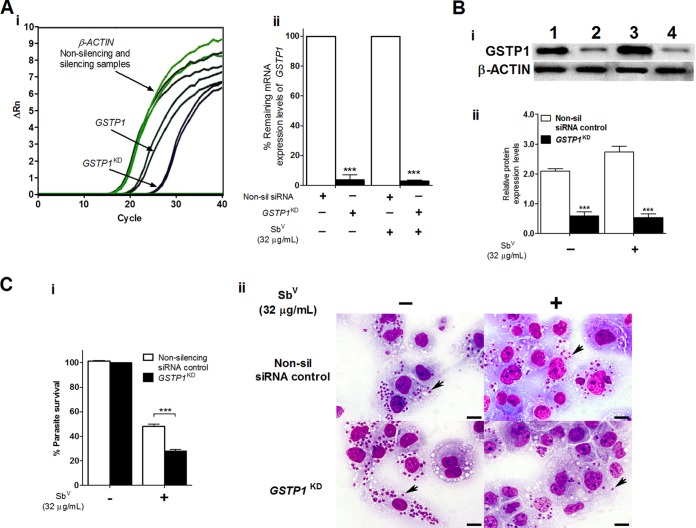
To determine the effects of GSTP1 silencing on intracellular L. braziliensis amastigote survival in THP-1 MΦ cells infected and not treated or infected and treated with SbV, we first confirmed successful inhibition of the GSTP1 gene (GSTP1KD samples) using raw data from reverse transcription (RT)-quantitative PCR (qPCR) analyses (Fig. 3Ai). The change in the fluorescence-normalized reporter signal (ΔRn) versus the number of amplification cycles showed an increase in the threshold cycle (CT) value of approximately 5 cycles for the GSTP1KD target-gene-silenced samples, producing a knockdown effect in expression levels of 96% to 97%. No significant differences in gene expression were observed for silenced MΦ cells in the infected and untreated and infected and Glucantime-treated (32 μg/ml) groups (Fig. 3Aii). The CT values for the β-actin (ACTB) reference gene remained unchanged in both the GSTP1KD target-gene-silenced samples and the nonsilencing small interfering RNA (siRNA)-transfected negative-control samples (nonsilencing siRNA control) (Fig. 3Ai). Interestingly, Western blot analyses of the groups with silenced GSTP1 showed reductions in GSTP1 protein expression levels of 0.59 and 0.52 (P < 0.001), which were consistent with the knockdown effects of 71.8% and 81% in THP-1 MΦ cells infected and untreated and infected and treated with SbV, respectively (Fig. 3Bi and Bii).
FIG 3.
Silencing of the human GSTP1 gene enhancing the intracellular susceptibility of L. braziliensis amastigotes to SbV treatment in THP-1 human MΦ cells. (A) Efficacy of GSTP1 gene silencing determined at the mRNA expression level by real-time PCR analyses. (i) Representative amplification plot for GSTP1 and ACTB genes, showing ΔRn versus the cycles observed for THP-1 MΦ cells infected with L. braziliensis and treated with SbV. (ii) Percentage of the remaining mRNA expression in the infected and infected and treated THP-1 MΦ groups (nonsilencing siRNA negative-control and silenced samples). (Bi) Protein expression analyses by Western blotting. Equivalent protein loading was assessed by immunodetection of β-actin. Lane 1, THP-1 MΦ cells transfected with nonsilencing siRNA negative control and infected; lane 2, THP-1 MΦ cells transfected with GSTP1 siRNA (GSTP1KD) and infected; lane 3, THP-1 MΦ cells transfected with nonsilencing siRNA, infected, and treated with SbV (32 μg/ml); lane 4, THP-1 MΦ cells transfected with GSTP1 siRNA (GSTP1KD), infected, and treated with SbV. (Bii) Densitometric analysis of the signals shown in panel Bi, carried out with ImageJ software. (Ci) Percentage of intracellular survival of L. braziliensis amastigotes infecting THP-1 MΦ cells transfected with nonsilencing siRNA or GSTP1 siRNA and treated with SbV (32 μg/ml). Infected and untreated THP-1 MΦ cells were used as controls. (Cii) Photomicrographs of human THP-1 MΦ cells containing amastigotes (arrows) transfected with nonsilencing siRNA or GSTP1 siRNA and treated or not with 32 μg/ml SbV (Giemsa stain; scale bars = 20 μm). The results represent the average of two or three independent experiments + SEM. Significant differences were determined by two-way ANOVA, followed by Bonferroni's multiple-comparison test. ***, P < 0.01.
Once successful inhibition of the GSTP1 gene was confirmed, we determined the effect of GSTP1 silencing on intracellular L. braziliensis amastigote survival in THP-1 MΦ cells infected and untreated or infected and SbV treated. Our results showed a direct association between GSTP1 gene inhibition and a significant reduction in intracellular survival of 20.4% (P < 0.001) in THP-1 MΦ cells silenced for the target gene (GSTP1KD samples), infected with L. braziliensis, and treated with a dose of 32 μg/ml SbV (Fig. 3C). This effect was also illustrated by a decrease in the number of intracellular amastigotes in this group, compared to the THP-1 MΦ group that was infected and treated but not silenced for this specific target gene. No significant differences in the numbers of amastigotes between the infected groups were observed (Fig. 3Cii; also see Fig. S2A).
Effects of GSS, ABCB5, and TRX gene silencing on the intracellular sensitivity of L. braziliensis amastigotes to SbV (Glucantime) treatment in human THP-1 MΦ cells.
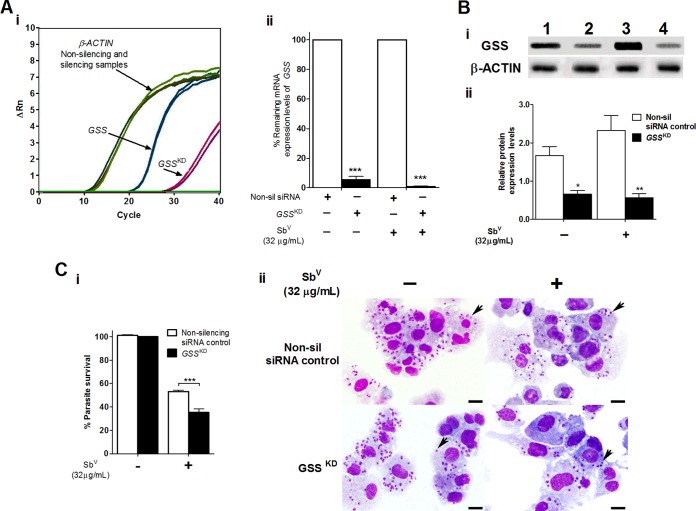
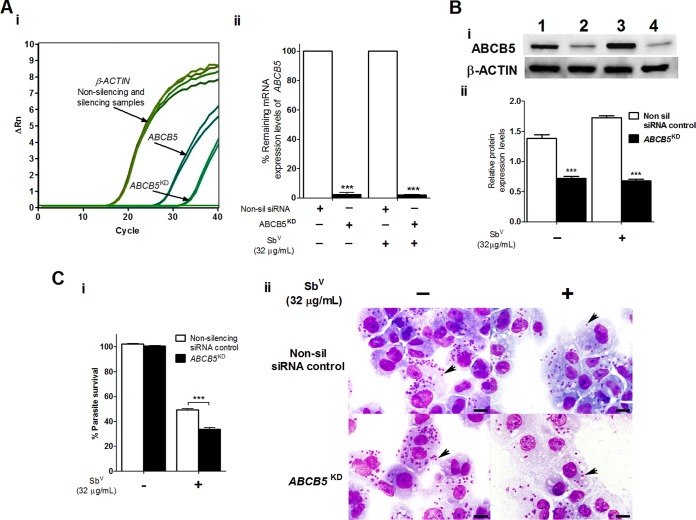
Successful inhibition of GSS, ABCB5, and TRX target gene expression was also obtained; this was confirmed by mRNA and protein expression levels in THP-1 MΦ cells infected and treated or not treated with SbV, as evaluated by RT-qPCR and Western blotting, respectively (Fig. 4 and 5; also see Fig. S3). In these groups, the levels of gene knockdown were 94.5% to 99.1% for GSS, 98.0% for ABCB5, and 83.0% to 87.4% for TRX (Fig. 4Aii and 5Aii; also see Fig. S3Aii).
FIG 4.
Silencing of the human GSS gene enhancing the intracellular susceptibility of L. braziliensis amastigotes to SbV treatment in THP-1 human MΦ cells. (A) Efficacy of GSS gene silencing determined at the mRNA expression level by real-time PCR analyses. (i) Representative amplification plot for the GSS and ACTB genes, showing ΔRn versus the cycles observed for THP-1 MΦ cells infected with L. braziliensis and treated with SbV. (ii) Percentage of the remaining mRNA expression in the infected and infected and treated THP-1 MΦ groups (nonsilencing siRNA negative-control and silenced samples). (Bi) Protein expression analyses by Western blotting. Equivalent protein loading was assessed by immunodetection of β-actin. Lane 1, THP-1 MΦ cells transfected with nonsilencing siRNA negative control and infected; lane 2, THP-1 MΦ cells transfected with GSS siRNA (GSSKD) and infected; lane 3, THP-1 MΦ cells transfected with nonsilencing siRNA, infected, and treated with SbV (32 μg/ml); lane 4, THP-1 MΦ cells transfected with GSS siRNA, infected, and treated with SbV. (Bii) Densitometric analysis of the signals shown in panel Bi, carried out with ImageJ software. (Ci) Percentage of intracellular survival of L. braziliensis amastigotes infecting THP-1 MΦ cells transfected with nonsilencing siRNA or GSS siRNA and treated with SbV (32 μg/ml). Infected and untreated THP-1 MΦ cells were used as controls. (Cii) Photomicrographs of human THP-1 MΦ cells containing amastigotes (arrows) transfected with nonsilencing siRNA or GSS siRNA and treated or not with 32 μg/ml SbV (Giemsa stain; scale bars = 20 μm). The results represent the average of two or three independent experiments + SEM. Significant differences were determined by two-way ANOVA, followed by Bonferroni's multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 5.
Silencing of the human ABCB5 gene enhancing the intracellular susceptibility of L. braziliensis amastigotes to SbV treatment in THP-1 human MΦ cells. (A) Efficacy of ABCB5 gene silencing determined at the mRNA expression level by real-time PCR analyses. (i) Representative amplification plot for ABCB5 and ACTB genes, showing ΔRn versus the cycles observed for THP-1 MΦ cells infected with L. braziliensis and treated with SbV. (ii) Percentage of the remaining mRNA expression for the infected and infected and treated THP-1 MΦ groups (nonsilencing siRNA negative-control and silenced samples). (Bi) Protein expression analyses by Western blotting. Equivalent protein loading was assessed by immunodetection of β-actin. Lane 1, THP-1 MΦ cells transfected with nonsilencing siRNA negative control and infected; lane 2, THP-1 MΦ cells transfected with ABCB5 siRNA (ABCB5KD) and infected; lane 3, THP-1 MΦ cells transfected with nonsilencing siRNA, infected, and treated with SbV (32 μg/ml); lane 4, THP-1 MΦ cells transfected with ABCB5 siRNA, infected, and treated with SbV. (Bii) Densitometric analysis of the signals shown in panel Bi, carried out with ImageJ software. (Ci) Percentage of intracellular survival of L. braziliensis amastigotes infecting THP-1 MΦ cells transfected with nonsilencing siRNA or ABCB5 siRNA and treated with SbV (32 μg/ml). Infected and untreated THP-1 MΦ cells were used as controls. (Cii) Photomicrographs of human THP-1 MΦ cells containing amastigotes (arrows) transfected with nonsilencing siRNA or ABCB5 siRNA and treated or not with 32 μg/ml SbV (Giemsa stain; scale bars = 20 μm). The results represent the average of two or three independent experiments + SEM. Significant differences were determined by two-way ANOVA, followed by Bonferroni's multiple-comparison test. ***, P < 0.001.
Sequential knockdown of the GSSKD, ABCB5KD, and TRXKD genes in infected THP-1 MΦ cells with and without SbV treatment induced significant reductions in the levels of protein expression (P < 0.05). The protein expression levels of the GSSKD samples were 0.66-fold those of control samples (P < 0.05), corresponding to 60.2% protein knockdown, in the infected THP-1 MΦ group and 0.57-fold those of control samples (P < 0.01), corresponding to 75.6% protein knockdown, in the THP-1 MΦ group infected and treated with SbV (Fig. 4Bi and Bii). For the ABCB5KD gene, the protein expression levels were 0.72-fold those of control samples (P < 0.05), corresponding to 47.8% protein knockdown, in the infected THP-1 MΦ group and 0.68-fold those of control samples (P < 0.01), corresponding to 61.0% protein knockdown, in the THP-1 MΦ group infected and treated with SbV (Fig. 5Bi and Bii). Finally, in TRXKD-silenced samples, protein expression levels were 0.74-fold those of control samples (P < 0.001), corresponding to 54.9% protein knockdown, in the infected THP-1 MΦ group and 0.87-fold those of control samples (P < 0.01), corresponding to 59.5% knockdown, in the THP-1 MΦ group infected and treated with SbV (Fig. S3Bi and Bii).
The intracellular survival of L. braziliensis with Glucantime treatment in GSSKD- and ABCB5KD-silenced THP-1 MΦ cells revealed significant reductions in intracellular parasite survival (P < 0.001), as demonstrated by parasite load reductions of 17.8% for GSSKD and 15.5% for ABCB5KD (Fig. 4C and 5C). These effects on intracellular L. braziliensis survival were also illustrated by reductions in the numbers of amastigotes observed in GSSKD and ABCB5KD THP-1 MΦ cells infected and treated with SbV, compared to nonsilenced THP-1 MΦ cells infected and treated but not silenced for the specific target genes (Fig. 4Cii and 5Cii; also see Fig. S2B and C). In contrast, no significant decrease in intracellular survival of L. braziliensis in TRXKD THP-1 MΦ cells after treatment with 32 μg/ml SbV was observed (Fig. S4Ci and Cii).
Validation of the inhibitory effects of antioxidant defense and drug transporter genes on intracellular L. braziliensis survival in THP-1 MΦ cells treated with SbV (Glucantime).
The enhanced leishmanicidal effects of SbV observed in THP-1 MΦ cells that had knocked-down GSTP1, GSS, and ABCB5 genes and were infected with L. braziliensis were validated by assessing intracellular amastigote survival in THP-1 MΦ cells silenced for the constitutively expressed ACTB gene. The efficacy of knockdown after transfection with a specific siRNA ACTB gene was determined by RT-qPCR and Western blot analysis. The remaining relative mRNA expression results showed a significant reduction (P < 0.001) in β-actin mRNA levels, with values from 85.33% to 88.26%, and demonstrated successful inhibition of the target gene in THP-1 MΦ groups infected and untreated and infected and treated with SbV (Fig. S4A). Reductions of between 41.9% (P < 0.01) and 48.3% (P < 0.01) in the levels of β-actin protein expression were confirmed in all experimental groups (Fig. S4B). Intracellular survival of L. braziliensis in ACTBKD THP-1 MΦ cells treated or not treated with 32 μg/ml SbV was not affected by the cell-silencing procedure (Fig. S2E and S4C). Taken together, our results suggest that the GSTP1, GSS, and ABCB5 genes are potentially involved in the detoxification of antimony compounds and that inhibition of these genes significantly increases the in vitro leishmanicidal effect on intracellular L. braziliensis susceptibility to SbV in THP-1 MΦ cells.
Reestablishment of the phenotype of L. braziliensis intracellular sensitivity to SbV (Glucantime) by selective inhibition of GSTP1 in THP-1 MΦ cells.
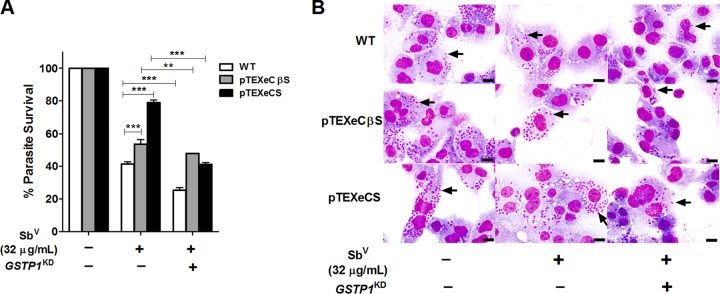
To determine whether GSTP1 gene silencing also enhances the effect of SbV on L. braziliensis mutant survival, as observed for wild-type parasites, we used two L. braziliensis strains generated in our laboratory, which overexpress cystathionine β-synthase (pTEXCβSeGFP) and cysteine synthase (pTEXCSeGFP). Both of these strains showed a loss of sensitivity to SbV in previous studies (17). Our results showed partial restoration of the SbV-sensitive phenotype in these mutant parasites, with reductions in parasite survival of 37.7% and 5.5%, respectively, compared to the 16.2% reduction observed for wild-type parasites (Fig. 6A and B). Survival in THP-1 MΦ cells treated with 32 μg/ml SbV was determined in comparison with cells infected with the mutant and wild-type strains but not treated with SbV (control group), in which parasite survival was considered to be 100%.
FIG 6.
Effects of GSTP1 knockdown and SbV treatment on the viability of L. braziliensis mutants overexpressing cysteine synthase and cystathionine β-synthase. (A) Intracellular survival of L. braziliensis amastigotes infecting THP-1 MΦ cells transfected with nonsilencing siRNA or GSTP1 siRNA and treated with SbV (32 μg/ml). (B) Photomicrographs of THP-1 MΦ cells containing amastigotes (arrows) transfected with nonsilencing siRNA or GSTP1 siRNA and treated with 32 μg/ml SbV (Giemsa stain; scale bars = 20 μm). WT, wild-type. The results represent the average of two independent experiments + SEM. Significant differences were determined by two-way ANOVA, followed by Bonferroni's multiple-comparison test. **, P < 0.01; ***, P < 0.001.
DISCUSSION
Rapid scientific advances in our knowledge of the biology of pathogenic parasites and their transmitting vectors, as achieved by genomic sequencing, has not yet translated into advances in control measures that can interrupt parasite transmission, in vaccine development, or in new and more effective and more accessible therapeutic drugs. In the present study, we report that analysis of mRNA gene expression levels revealed modulation of oxidative stress and drug transporter genes under different experimental conditions. In human MDMs infected with L. braziliensis, downregulation of MBL2, NQO1, PTGS2, and SOD3 was observed; this is in agreement with the ability of macrophages to control parasite infection, as these genes have been reported to be involved in the host cell antioxidant defense system. For instance, a recent study showed that inhibition of SOD1, an isoform of the SOD3 gene, induced a decrease in the parasite load in human macrophages (18). In addition, negative regulation of these genes, together with the observed upregulation of CAT, GSTP1, and GPX2, suggests that the parasite has the ability to modulate the host response, favoring intracellular survival (19).
In human MDMs not infected but treated with SbV (Glucantime), we observed modulation of the CAT, GSR, GSTP1, GCLM, GPX2, HMOX1, and TRX genes, which are related to antioxidant defense and oxidative stress. Similar results have been reported for the THP-1 human macrophage cell line and confirmed in human MDMs, suggesting that SbV induces oxidative stress in human macrophages (20). In our study, overexpression of the CAT, GPX2, HMOX1, and TRX genes confirms the effect of SbV and possibly SbIII in inducing oxidative stress. Additionally, our results revealed that the glutathione biosynthesis pathway was regulated in response to macrophage treatment with Sb, via overexpression of the gene encoding the protein GCLM, an enzyme involved in the first step of reduced glutathione (GSH) biosynthesis. Overexpression of this particular gene has been associated with increases in the intracellular levels of GSH (21), which is widely known as the major antioxidant molecule in mammals and plays a central role in maintaining cell homeostasis.
Furthermore, in human MDMs infected and treated with SbV, our analysis of gene expression confirmed results reported by other authors who used similar methodological approaches. In those studies, antimony compounds, as well as Leishmania infection, were demonstrated to modulate the expression of the GSR gene, which is involved in the reduction of glutathione, and that of the ABCB6 and SLC7A11 genes, which encode drug transporters involved in drug distribution or drug reduction in human macrophages (20, 22). We also found upregulation of genes involved in the glutathione biosynthesis pathway, antioxidant defense, and drug transporters of the ABCB family, highlighting the expression levels observed for GSTP1, GSS, TRX, and ABCB5.
The observed regulation of both gene and protein expression in human macrophages not only confirmed previous reports of host-pathogen-drug interactions (23, 24) but also allowed the identification of new genes that encode enzymes involved in the glutathione biosynthesis pathway, such as GSS. In addition, the role of GSTP1 in catalyzing the conjugation of xenobiotic compounds with GSH products and the involvement of the ABCB5 drug transporter in the efflux of drugs were identified. Regulation of these human genes in response to L. braziliensis infection and treatment with SbV (Glucantime) had not yet been described in human macrophages.
GSTP1 belongs to the glutathione S-transferase superfamily, the members of which play important roles in the cellular defense system, particularly in the detoxification of xenobiotic compounds. These enzymes catalyze the conjugation of GSH with electrophilic compounds, thereby facilitating their efflux from the cell (25). Notably, drug conjugation with GSH can occur either spontaneously or by catalytic action via the GST enzyme subclass P1 (26, 27). In models using arsenic (As), a metal closely related to Sb, an increase in the expression of GST-P in mouse cell lines has been reported, resulting in an increase in cellular tolerance to arsenic through the formation of drug conjugates with GSH and ABC drug transporter family efflux of As (28, 29). The increased protein expression of GSTP1 observed in the present study suggests that this enzyme may play a role similar to that described for As detoxification, by forming Sb conjugates with GSH to efflux Sb from human macrophages. Interestingly, selective inhibition of antioxidant defense factor GSTP1, GSS, and TRX and ABCB5 drug transporter expression had a direct effect on the reduction of intracellular parasite survival in THP-1 MΦ cells upon treatment with SbV (Glucantime). This effect was also evident with short exposure times or low doses of SbV (data not shown). These findings, in addition to protein expression analysis in time course and dose dependence assays, reinforce the hypothesis that the GSTP1 protein may be involved in detoxification of antimony compounds in human macrophages via formation of a Sb(GS)3 complex (data not shown). The description herein of the selective inhibition of GSTP1 due to interference with gene expression is the first report, to our knowledge, of the specific inhibition of this molecule and establishes the possible role of GSTP1 in the mechanism of antimony compound detoxification in the THP-1 human cell line.
Our results suggest that selective inhibition of the human GSTP1 gene significantly increases the intracellular leishmanicidal activity of SbV (Glucantime) in human THP-1 macrophages in vitro. This effect was also demonstrated by restoration of the SbV (Glucantime)-sensitive phenotype of intracellular L. braziliensis using the mutant strains pTEXCβSeGFP and pTEXCSeGFP, both of which exhibit a loss of sensitivity to SbV (17). These findings suggest that a strategy combining the specific inhibitors of human GSTP1 with antimonial compounds may improve the efficacy of first-line drugs for leishmaniasis treatment and could lead to reverse-resistance phenotypes in clinical trials, which are our current interests. Additional studies are required to better understand the molecular mechanisms and effects of inhibiting human genes in increasing the sensitivity of parasites that are naturally resistant to antimony compounds.
GSS is an important enzyme catalyzing the second step of GSH thiol biosynthesis (30, 31). GSH has been reported to have a dual function, by participating in the detoxification of xenobiotic products through the formation of conjugates that can be exported out of the cell and by participating in antimony compound reduction (i.e., nonenzymatic conversion of SbV to the active trivalent form SbIII) (12, 30). Our functional analyses indicate that GSS also may be involved in L. braziliensis intracellular survival in human macrophages treated with SbV (Glucantime), as indicated by a significant decrease of 17.8% for the GSSKD knockdown phenotype of THP-1 macrophages. Decreased GSS expression in the host cell may result in reduced intracellular GSH levels and may induce an intracellular oxidative environment, promoting a low level of drug complexation and subsequent lower drug efflux and promoting the death of intracellular parasites.
Members of the ABCB drug transporter subfamily have been characterized as molecules located in the plasma membrane, as well as in different cell organelles, such as mitochondria and the endoplasmic reticulum, that act as main drug efflux transporters (32, 33). ABCB5 has been described as a drug transporter of chemotherapeutic agents, such as doxorubicin, camptothecin, 10-hydroxycamptothecin, and 5-fluorouracil, which are widely used in cancer treatment (34). This drug transporter belongs to MDR gene family and exhibits 70% amino acid sequence homology to the ABCB1 (P-glycoprotein [P-gp], MDR1) drug transporter, which is largely associated with drug efflux, enhancing resistance in melanocytes and melanoma cells (35). Several studies reported that members of the ABCB1, ABCB6, and ABCB11 protein subfamilies are also located in lysosomes, suggesting an influx function for xenobiotic products, enhancing sequestration or leading to a reduction in the intracellular distribution of such compounds (22, 36, 37). In our study, localization of the ABCB5 drug transporter mainly in phagolysosomal membranes, where the parasite resides, in THP-1 macrophages treated with SbV (Glucantime) suggests a possible role for this transporter in the susceptibility of L. braziliensis to SbV (Glucantime). Functional analysis using siRNA for specific inhibition of the ABCB5 gene had a direct effect on intracellular survival of L. braziliensis, enhancing the leishmanicidal activity of SbV in THP-1 MΦ cells.
Functional selective inhibition of ABCB5 suggests that this drug transporter may be involved in the detoxification of antimony compounds through efflux of the active form of the drug, SbIII, from the host cell. The involvement of ABCB family members, such as ABCB1 (P-gp, MDR1), in Sb efflux has been described by other authors; positive modulation of this gene results in a significant reduction in the intracellular content of Sb (38, 39). Our findings for the subcellular localization of ABCB5 in phagolysosome membranes and the possible role of this protein in the detoxification of antimony compounds constitute the first report of this drug transporter in human THP-1 macrophages.
The association of GSTP, GSS, and ABCB5 with antimony compound detoxification is in agreement with existing knowledge regarding Sb detoxification mechanisms and the involvement of the host cell in these processes, with increased activity of γ-glutamate-cysteine ligase (γ-GCL) raising intracellular thiol levels (21, 40). Increased intracellular thiol levels have also been reported to be involved in SbV activation or in complex formation with SbIII (41, 42). Furthermore, antimony compounds may enter host cells through channels, such as aquaporin 7 (AQP7) and AQP9, and the influx of antimony into specialized organelles, such as phagolysosomes, might be mediated by the ABCB6 drug transporter (22, 43, 44).
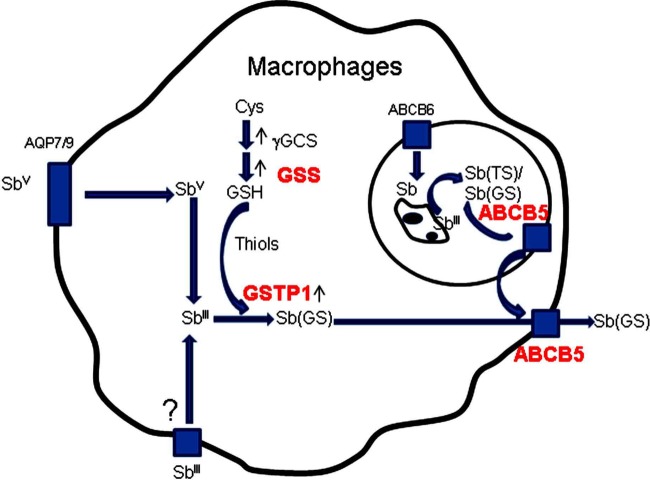
In Fig. 7, we propose a model of interaction between the GSTP, GSS, and ABCB5 enzymes, suggesting that these enzymes may be involved in the mechanism of Sb detoxification, with enhanced intracellular parasite survival in human macrophages treated with SbV (Glucantime) occurring through three major mechanisms. In the first mechanism, the amount of GSH available for potentiating the reduction of SbV (Glucantime) into the active form of antimony, SbIII, in macrophages may be affected because GSS might be one of the enzymes responsible for maintaining adequate levels of GSH. In the second mechanism, regarding SbV reduction, GSH availability also facilitates GSH conjugation with SbIII compounds present in the host cell via the catalytic activity of GSTP1. The third possible mechanism is related to SbIII drug efflux through involvement of the ABCB5 drug transporter, decreasing intracellular drug contact with the parasite and enhancing survival of the parasite in the host cell.
FIG 7.
Proposed model for Glucantime detoxification in human macrophages infected with L. braziliensis. The detoxification pathway includes three main mechanisms. The first mechanism involves the amount of GSS available relative to the macrophage GSH level. In the second mechanism, GSTP1 catalyzes the conjugation of thiol molecules with SbIII compounds. In the third mechanism, ABCB5 participates in the efflux of SbIII. Sb(TS), SbIII conjugated with trypanothione; Sb(GS), SbIII conjugated with glutathione; Cys, cysteine. The proteins highlighted in red were characterized in this study.
Our study expands the current knowledge regarding the involvement of host cells in the possible mechanism of SbV (Glucantime) detoxification, and the GSTP1 gene was proven to be an interesting factor for enhancing the leishmanicidal activity of the drug. These results, together with recent studies on improving Glucantime bioavailability through nanotechnology, may be useful for the development of innovative topical formulations of antimony compounds for leishmaniasis treatment.
MATERIALS AND METHODS
Ethics statement.
The study protocol, informed consent material, and sampling procedures were approved by the Federal University of Santa Catarina (UFSC) institutional review board for studies involving human subjects (process 2190 FR 453659). The study was conducted in compliance with national and international guidelines for the protection of human subjects from research risks. Written informed consent was obtained from all participants.
Parasite cultures.
L. braziliensis mutant strains overexpressing cystathionine β-synthase (pTEXCβSeGFP) and cysteine synthase (pTEXCSeGFP) promastigotes were generated. L. braziliensis (MHOM/BR/75/M2904) and other strains are stored in the protozoan culture collection of the Protozoology Laboratory of UFSC (mutant parasites are available upon request) (17). Parasites were propagated at 26°C in Schneider's Drosophila medium (Sigma-Aldrich) supplemented with 5% heat-inactivated fetal bovine serum (FBS) (45). Infective stationary-phase promastigotes were obtained as described previously, with some modifications (46). Briefly, promastigotes cultured at 26°C in biphasic human blood agar-based medium were harvested after 6 days of passage and opsonized by treatment for 1 h at 34°C with RPMI 1640 medium containing 10% human-type AB-positive serum.
Isolation and culture of human MDMs.
Peripheral blood (150 ml) was obtained from five healthy adult volunteers. Donors originating from leishmaniasis-free areas were tested for Leishmania infection by PCR (J. Téllez, I. Romero, M. Soares, M. Steindel, and A. Romanha, unpublished data). Peripheral blood mononuclear cells (PBMCs) were obtained from the peripheral blood by Ficoll-Hypaque 1077 density gradient centrifugation. Monocytes were allowed to differentiate into macrophages as described previously (47). Briefly, 1 × 107 PBMCs were allowed to adhere for 2 h at 37°C in 6-well plates. After being washed, the adhering cells were differentiated to macrophages for 7 days in culture at 37°C, with 5% CO2, in RPMI 1640 medium supplemented with 20% human autologous plasma.
Human THP-1-derived macrophages.
Cells of the human acute monocytic leukemia cell line THP-1 (ATCC TIB202) were cultured and differentiated into macrophages as described previously (17). THP-1-derived macrophages (THP-1 MΦ) were used to evaluate the functional involvement of GSTP1, GSS, ABCB5, and TRX in this cell line infected with L. braziliensis and treated with SbV, as described below.
L. braziliensis infection and Glucantime treatment.
Human MDMs and THP-1 MΦ cells were infected with opsonized L. braziliensis promastigotes at a parasite/cell ratio of 10:1. Infection was allowed to proceed for 1 h at 34°C, with 5% CO2, in FBS-free RPMI medium. Free parasites were removed with 2 or 3 washes with serum-free RPMI medium. Following an additional 24 h of incubation at 34°C with 5% CO2, to allow the complete development of amastigotes, infected human MDMs and THP-1 MΦ cells were treated for 48 h with experimentally determined optimum Glucantime (meglumine antimoniate SbV) at a concentration of 32 μg/ml, with replenishment and incubation continuing for 24 h at 34°C with 5% CO2 (48). Infection-free and drug-free macrophage controls were included in all assays. Samples from human MDMs were collected and stored directly in TRIzol (Invitrogen) for evaluation of the gene expression profile by PCR array or were pelleted for determination of protein expression by Western blot analysis. In parallel, identical infected and drug-treated cells seeded in a chamber slide were evaluated to assess the levels of infection using conventional microscopy and Giemsa staining. Percentages of parasite survival were determined as described previously by Sereno et al. (49).
RNA extraction and first-strand cDNA synthesis.
Total RNA was extracted using an RNeasy minikit (Qiagen), following the manufacturer's instructions, eluted in 40 μl of water, and quantified using a PicoDrop P200 spectrophotometer (PicoDrop Technologies). First-strand cDNA synthesis was achieved using a RT2 first strand kit (SABiosciences). Total RNA (70 ng) was reverse transcribed in a final volume of 20 μl, following the manufacturer's recommendations, with an additional step for elimination of genomic DNA. Reverse transcriptase was inactivated by heating at 95°C for 5 min. cDNA was diluted to 111 μl with RNase-free water and stored at −20°C until use.
RT2 Profiler PCR arrays of human MDMs.
cDNA was mixed with RT2 SYBR green/ROX qPCR Mastermix (SABiosciences), following the manufacturer's instructions. Thereafter, 25 μl/well was loaded in 96-well plates with predispensed specific primer sets to evaluate the expression levels of 168 genes, which included 84 genes of the human oxidative stress RT2 Profiler PCR array and 84 genes of the human drug transporters RT2 Profiler PCR array (catalog numbers PAHS-065Z and PAHS-070Z, respectively; SABiosciences). The arrays contain a panel of proprietary controls to monitor genomic DNA contamination as well as first-strand synthesis and real-time PCR efficiency. All PCR array experiments were performed using an ABI 7900HT FAST instrument (Applied Biosystems) under the following conditions: 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C.
PCR array data analysis.
We employed the ΔΔCT method using the online analysis tool provided by the supplier of the PCR arrays (http://www.sabiosciences.com/pcrarraydataanalysis.php). Genes with CT values greater than 35 cycles were considered nondetectable. The average for five housekeeping genes (β2-microglobulin [B2M], hypoxanthine phosphoribosyltransferase 1 [HPRT1], ribosomal protein L13A [RPL13A], glyceraldehyde-3-phosphate dehydrogenase [GAPDH], and β-actin [ACTB]) was used to obtain the ΔCT value for each gene of interest. Fold changes were calculated as 2−ΔΔCT, which represents the expression levels for each gene in MDMs infected with L. braziliensis, in MDMs treated with SbV (Glucantime), and in MDMs infected with L. braziliensis and treated with SbV (Glucantime) versus the expression level in the control sample (human MDMs from healthy donors without in vitro infection and not treated with SbV [Glucantime]).
Western blot analysis.
Whole-cell protein extracts were obtained using 6-well plate cell cultures of primary human MDMs from five healthy donors of the aforementioned groups. Cells were washed in 1× Dulbecco's phosphate-buffered saline (PBS) and lysed by repeated aspiration in ice-cold lysis buffer (0.25 M sucrose, 0.25% Triton X-100, 10 mM EDTA) containing a protease inhibitor mixture (Sigma-Aldrich). Cell debris was removed by centrifugation at 12,000 × g for 20 min at 4°C (50). All proteins were stored at −20°C, and their concentrations were determined with a Bio-Rad protein assay kit (Bradford), using bovine serum albumin (BSA) as a standard. Western blotting was performed as described previously (50). Briefly, soluble protein extracts (22 μg) from MDMs under different treatments were fractionated by 12% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the predicted protein size and then were electroblotted onto nitrocellulose membranes (GE Healthcare) (51). The membranes were incubated with mouse monoclonal antibodies against CAT, GPX1/2, GSR, GSS, GSTP1, SOD3, TRX, and ABCB11, goat polyclonal anti-ABCB5, and rabbit polyclonal anti-ABCB6 and SLC22A1. Goat antiactin (Santa Cruz Biotechnology) was used as the loading control. All primary antibodies were used at 1:200. Detection of positive reactions was carried out using appropriate horseradish peroxidase-conjugated IgG secondary antibodies (1:10,000) and an enhanced chemiluminescence kit (Pierce), according to the manufacturer's recommendations. The blots were digitally analyzed using ImageJ v.1.463r (http://imagej.nih.gov/ij/index.html), as described previously (50).
Subcellular localization of the ABCB5 drug transporter.
Cultures on a chamber slide of human MDMs under the different treatments were washed twice with 1× PBS, fixed for 10 min with 4% paraformaldehyde at room temperature, and permeabilized for 5 min in 0.05% Triton X-100 in 1× PBS. After blocking with 1% BSA in 1× PBS, macrophages on the chamber slide were incubated with a specific antibody against ABCB5. Colocalization of ABCB5 within parasitophorous vacuoles containing L. braziliensis parasites was carried out by double labeling with a mouse monoclonal antibody to the phagosome marker LAMP-1 and secondary antibodies conjugated to Alexa 488 (anti-mouse IgG) and Alexa 594 (anti-goat IgG). Hoechst dye H6024 (Sigma-Aldrich) was used as a nuclear marker. Slides were observed and analyzed with a Leica SP5 confocal laser microscope (Leica Microsystems).
RNA interference with GSTP1, GSS, ABCB5, and TRX in L. braziliensis-infected and SbV (Glucantime)-treated THP-1 MΦ cells.
To study the functional involvement of GSTP1, GSS, ABCB5, and TRX genes in THP-1 MΦ cells infected with L. braziliensis and treated with SbV, specific inhibition of each gene using three validated siRNA sequences was performed using a FlexiTube siRNA kit (Qiagen), following the manufacturer's instructions with some modifications. The target genes are listed in Table S1 in the supplemental material. Briefly, 2.5 × 105 THP-1 MΦ cells in 24-well plates and 1 × 105 THP-1 MΦ cells on chamber slides were transfected using HiPerFect (Qiagen). Each siRNA sequence (25 nM) for the targets (GSTP1, GSS, ABCB5, and TRX), nonsilencing scrambled control siRNA (AllStars AF488 negative control; Qiagen), or siRNA targeting ACTB (used as the positive control) was added to 100 μl of serum-free RPMI and 9 μl of transfection reagent. The mixture was incubated for 10 min at room temperature to allow complex transfection formation of the siRNA target gene-HiPerFect reagents. The complex formed was left in contact with cells for 6 h, in a final volume of 600 μl of serum-free RPMI medium, under controlled-atmosphere conditions. The medium was then changed, and the cells were incubated again for 1 h and subsequently infected with L. braziliensis and treated with SbV, as described above. The use of THP-1 MΦ cells for gene silencing assays was described previously (52, 53).
Successful knockdown of target genes in wild-type and mutant cells was confirmed by RT-qPCR and Western blotting using specific primers for genes and antibodies for proteins, as described above. The effects of GSTP1, GSS, ABCB5, TRX, and ACTB gene silencing on the intracellular sensitivity of L. braziliensis to SbV in THP-1 MΦ cells were determined by assessing the percentage of survival of the parasite, as described above.
Statistical analyses.
Statistical differences were analyzed by one-way or two-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests, as indicated in the figure legends. Analyses were performed with GraphPad Prism 5 software (GraphPad Inc., San Diego, CA), and P values of <0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
J.T. and I.R. were recipients of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) scholarships, and M.S. was supported by Financiadora de Estudos e Projetos (FINEP). This work was supported by CAPES and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) - Brazilian Government Agencies (grant 475474/2011-2). The funders had no role in the study design, data generation and analysis, decision to publish, or preparation of the manuscript.
We thank Melissa J. Caimano (Department of Medicine, University of Connecticut Health Center) for critical reading and suggestions for the manuscript. We thank the Program for Technological Development in Tools for Health/Fiocruz for use of their facilities (platform RPT07C). We extend thanks to the healthy adult volunteers who participated in this study as PBMC donors and the staff at the UFSC Hospital Polydoro Ernani de São Thiago for assistance with donor screening.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02099-16.
REFERENCES
- 1.Herwaldt BL. 1999. Leishmaniasis. Lancet 354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 2.Antinori S, Schifanella L, Corbellino M. 2012. Leishmaniasis: new insights from an old and neglected disease. Eur J Clin Microbiol Infect Dis 31:109–118. doi: 10.1007/s10096-011-1276-0. [DOI] [PubMed] [Google Scholar]
- 3.Goto H, Lindoso JA. 2010. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther 8:419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 4.Croft SL, Sundar S, Fairlamb AH. 2006. Drug resistance in leishmaniasis. Clin Microbiol Rev 19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray HW, Berman JD, Davies CR, Saravia NG. 2005. Advances in leishmaniasis. Lancet 366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 6.Frézard F, Demicheli C. 2010. New delivery strategies for the old pentavalent antimonial drugs. Expert Opin Drug Deliv 7:1343–1358. doi: 10.1517/17425247.2010.529897. [DOI] [PubMed] [Google Scholar]
- 7.Hansen C, Hansen EW, Hansen HR, Gammelgaard B, Stürup S. 2011. Reduction of Sb(V) in a human macrophage cell line measured by HPLC-ICP-MS. Biol Trace Elem Res 144:234–243. doi: 10.1007/s12011-011-9079-9. [DOI] [PubMed] [Google Scholar]
- 8.Frézard F, Demicheli C, Kato KC, Reis PG, Lizarazo-Jaimes EH. 2013. Chemistry of antimony-based drugs in biological systems and studies of their mechanism of action. Rev Inorg Chem 33:1–12. doi: 10.1016/j.inoche.2013.03.027. [DOI] [Google Scholar]
- 9.Mookerjee Basu J, Moorkerjee A, Sen P, Bhaumik S, Sen P, Banerjee S, Naskar K, Choudhuri SK, Saha B, Raha S, Roy S. 2006. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob Agents Chemother 50:1788–1797. doi: 10.1128/AAC.50.5.1788-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 11.Wrenger C, Schettert I, Liebau E. 2013. Oxidative stress in human infectious diseases—present and current knowledge about its druggability, p 227–250. In El-Shemy HA. (ed), Drug discovery. InTech, Rijeka, Croatia. [Google Scholar]
- 12.Ferreira CS, Martins PS, Demicheli C, Brochu C, Ouellette M, Frézart F. 2003. Thiol-induced reduction of antimony(V) into antimony(III): a comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathione. Biometals 16:441–446. doi: 10.1023/A:1022823605068. [DOI] [PubMed] [Google Scholar]
- 13.Fairlamb AH, Cerami A. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol 46:695–729. doi: 10.1146/annurev.micro.46.1.695. [DOI] [PubMed] [Google Scholar]
- 14.Haddad JJ. 2002. Oxygen-sensing mechanisms and the regulation of redox-responsive transcription factors in development and pathophysiology. Respir Res 3:26. doi: 10.1186/rr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyllie S, Cunningham ML, Fairlamb AH. 2004. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J Biol Chem 279:39925–39932. doi: 10.1074/jbc.M405635200. [DOI] [PubMed] [Google Scholar]
- 16.Mehta A, Shaha C. 2006. Mechanism of metalloid-induced death in Leishmania spp.: role of iron, reactive oxygen species, Ca2+, and glutathione. Free Radic Biol Med 40:1857–1868. doi: 10.1016/j.freeradbiomed.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Romero I, Téllez J, Romanha AJ, Steindel M, Grisard EC. 2015. Upregulation of cysteine synthase and cystathionine β-synthase contributes to Leishmania braziliensis survival under oxidative stress. Antimicrob Agents Chemother 59:4770–4781. doi: 10.1128/AAC.04880-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khouri R, Bafica A, Silva MP, Noronha A, Kolb JP, Wietzerbin J, Barral A, Barral-Neto M, Van Weyenbergh J. 2009. IFN-β impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J Immunol 182:2525–2531. doi: 10.4049/jimmunol.0802860. [DOI] [PubMed] [Google Scholar]
- 19.Bhardwaj S, Srivastava N, Sudan R, Saha B. 2010. Leishmania interferes with host cell signaling to devise a survival strategy. J Biomed Biotechnol 2010:109189. doi: 10.1155/2010/109189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Fadili K, Imbeault M, Messier N, Roy G, Gourbal B, Bergeron M, Tremblay MJ, Légaré D, Ouellette M. 2008. Modulation of gene expression in human macrophages treated with the anti-Leishmania pentavalent antimonial drug sodium stibogluconate. Antimicrob Agents Chemother 52:526–533. doi: 10.1128/AAC.01183-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. 2005. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem 280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- 22.Gómez MA, Navas A, Márquez R, Rojas LJ, Vargas DA, Blanco VM, Koren R, Zilberstein D, Saravia NG. 2014. Leishmania panamensis infection and antimonial drugs modulate expression of macrophage drug transporters and metabolizing enzymes: impact on intracellular parasite survival. J Antimicrob Chemother 69:139–149. doi: 10.1093/jac/dkt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeddi F, Piarroux R, Mary C. 2011. Antimony resistance in Leishmania, focusing on experimental research. J Trop Med 2011:695382. doi: 10.1155/2011/695382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashutosh, Sundar S, Goyal N. 2007. Molecular mechanisms of antimony resistance in Leishmania. J Med Microbiol 56:143–153. doi: 10.1099/jmm.0.46841-0. [DOI] [PubMed] [Google Scholar]
- 25.Hayes JD, Flanagan JU, Jowsey IR. 2005. Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 26.Ploemen JH, Van Schanke A, Van Ommen B, Van Bladeren PJ. 1994. Reversible conjugation of ethacrynic acid with glutathione and human glutathione S-transferase P1-1. Cancer Res 54:915–919. [PubMed] [Google Scholar]
- 27.Ruzza P, Rosato A, Rossi CR, Floreani M, Quintieri L. 2009. Glutathione transferases as targets for cancer therapy. Anticancer Agents Med Chem 9:763–777. doi: 10.2174/187152009789056895. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z. 2010. Roles of vertebrate aquaglyceroporins in arsenic transport and detoxification. Adv Exp Med Biol 679:71–81. doi: 10.1007/978-1-4419-6315-4_6. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Chen H, Miller DS, Saavedra JE, Keefer LK, Johnson DR, Klaassen CD, Waalkes MP. 2001. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol Pharmacol 60:302–309. [DOI] [PubMed] [Google Scholar]
- 30.Lu SC. 2013. Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris D, Khurasany M, Nguyen T, Kim J, Guilford F, Mehta R, Gray D, Saviola B, Venketaraman V. 2013. Glutathione and infection. Biochim Biophys Acta 1830:3329–3349. doi: 10.1016/j.bbagen.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Glavinas H, Krajcsi P, Cserepes J, Sarkadi B. 2004. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv 1:27–42. doi: 10.2174/1567201043480036. [DOI] [PubMed] [Google Scholar]
- 33.Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, Sayegh MH, Frank MH. 2003. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem 278:47156–47165. doi: 10.1074/jbc.M308700200. [DOI] [PubMed] [Google Scholar]
- 34.Gillet J, Efferth T, Remacle J. 2007. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim Biophys Acta 1775:237–262. [DOI] [PubMed] [Google Scholar]
- 35.Chen KG, Szakács G, Annereau JP, Rouzaud F, Liang XJ, Valencia JC, Nagineni CN, Hooks JJ, Hearing VJ, Gottesman MM. 2005. Principal expression of two mRNA isoforms (ABCB 5α and ABCB 5β) of the ATP-binding cassette transporter gene ABCB 5 in melanoma cells and melanocytes. Pigment Cell Res 18:102–112. doi: 10.1111/j.1600-0749.2005.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiss K, Brozik A, Kucsma N, Toth A, Gera M, Berry L, Vallentin A, Vial H, Vidal M, Szakacs G. 2012. Shifting the paradigm: the putative mitochondrial protein ABCB6 resides in the lysosomes of cells and in the plasma membrane of erythrocytes. PLoS One 7:e37378. doi: 10.1371/journal.pone.0037378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajagopal A, Simon SM. 2003. Subcellular localization and activity of multidrug resistance proteins. Mol Biol Cell 14:3389–3399. doi: 10.1091/mbc.E02-11-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vernhet L, Allain N, Bardiau C, Anger JP, Fardel O. 2000. Differential sensitivities of MRP1-overexpressing lung tumor cells to cytotoxic metals. Toxicology 142:127–134. doi: 10.1016/S0300-483X(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 39.Vernhet L, Courtois A, Allain N, Payen L, Anger JP, Guillouzo A, Fardel O. 1999. Overexpression of the multidrug resistance-associated protein (MRP1) in human heavy metal-selected tumor cells. FEBS Lett 443:321–325. doi: 10.1016/S0014-5793(98)01716-5. [DOI] [PubMed] [Google Scholar]
- 40.Wyllie S, Fairlamb AH. 2006. Differential toxicity of antimonial compounds and their effects on glutathione homeostasis in a human leukaemia monocyte cell line. Biochem Pharmacol 71:257–267. doi: 10.1016/j.bcp.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 41.Frézard F, Demicheli C, Ferreira CS, Costa MA. 2001. Glutathione-induced conversion of pentavalent antimony to trivalent antimony in meglumine antimoniate. Antimicrob Agents Chemother 45:913–916. doi: 10.1128/AAC.45.3.913-916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan S, Li F, Ding K, Sun H. 2003. Reduction of pentavalent antimony by trypanothione and formation of a binary and ternary complex of antimony(III) and trypanothione. J Biol Inorg Chem 8:689–697. doi: 10.1007/s00775-003-0468-1. [DOI] [PubMed] [Google Scholar]
- 43.Rosen BP. 2002. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp Biochem Physiol A Mol Integr Physiol 133:689–693. doi: 10.1016/S1095-6433(02)00201-5. [DOI] [PubMed] [Google Scholar]
- 44.Jahn TP, Bienert GP (ed). 2010. MIPs and their role in the exchange of metalloids. Adv Exp Med Biol 679:1–145. [PubMed] [Google Scholar]
- 45.Duszenko M, Ferguson MA, Lamont GS, Rifkin MR, Cross GA. 1985. Cysteine eliminates the feeder cell requirement for cultivation of Trypanosoma brucei bloodstream forms in vitro. J Exp Med 162:1256–1263. doi: 10.1084/jem.162.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rey JA, Travi BL, Valencia AZ, Saravia NG. 1990. Infectivity of the subspecies of the Leishmania braziliensis complex in vivo and in vitro. Am J Trop Med Hyg 43:623–631. [DOI] [PubMed] [Google Scholar]
- 47.Wahl LM, Wahl SM, Smythies LE, Smith PD. 2006. Isolation of human monocyte populations. Curr Protoc Immunol Chapter 7:Unit 7.6A. doi: 10.1002/0471142735.im0706as70. [DOI] [PubMed] [Google Scholar]
- 48.Romero I, Téllez J, Suárez Y, Cardona M, Figueroa R, Zelazny A, Gore Saravia N. 2010. Viability and burden of Leishmania in extralesional sites during human dermal leishmaniasis. PLoS Negl Trop Dis 4:e819. doi: 10.1371/journal.pntd.0000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sereno D, Holzmuller P, Lemesre JL. 2000. Efficacy of second line drugs on antimonyl-resistant amastigotes of Leishmania infantum. Acta Trop 74:25–31. doi: 10.1016/S0001-706X(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 50.Romero I, Téllez J, Yamanaka LE, Steindel M, Romanha AJ, Grisard EC. 2014. Transsulfuration is an active pathway for cysteine biosynthesis in Trypanosoma rangeli. Parasit Vectors 7:197. doi: 10.1186/1756-3305-7-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallagher S, Winston SE, Fuller SA, Hurrell JG. 2008. Immunoblotting and immunodetection. Curr Protoc Mol Biol Chapter 10:Unit 10.8. doi: 10.1002/0471142727.mb1008s83. [DOI] [PubMed] [Google Scholar]
- 52.Li N, Sun J, Benet ZL, Wang Z, Al-Khodor S, John SP, Lin B, Sung MH, Fraser ID. 2015. Development of a cell system for siRNA screening of pathogen responses in human and mouse macrophages. Sci Rep 5:9559. doi: 10.1038/srep09559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dos Santos JC, Henhuis B, Gomes RS, Damen MS, Real F, Mortara RA, Keating ST, Dinarello CA, Joosten LA, Ribeiro-Dias F. 2017. Cytokines and microbicidal molecules regulated by IL-32 in THP-1-derived human macrophages infected with New World Leishmania species. PLoS Negl Trop Dis 11:e0005413. doi: 10.1371/journal.pntd.0005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.