ABSTRACT
The broadly neutralizing antibody (bNAb) VRC01, capable of neutralizing 91% of known human immunodeficiency virus type 1 (HIV-1) isolates in vitro, is a promising candidate microbicide for preventing sexual HIV infection when administered topically to the vagina; however, accessibility to antibody-based prophylactic treatment by target populations in sub-Saharan Africa and other underdeveloped regions may be limited by the high cost of conventionally produced antibodies and the limited capacity to manufacture such antibodies. Intravaginal rings of the pod design (pod-IVRs) delivering Nicotiana-manufactured VRC01 (VRC01-N) over a range of release rates have been developed. The pharmacokinetics and preliminary safety of VRC01-N pod-IVRs were evaluated in a rhesus macaque model. The devices sustained VRC01-N release for up to 21 days at controlled rates, with mean steady-state VRC01-N levels in vaginal fluids in the range of 102 to 103 μg g−1 being correlated with in vitro release rates. No adverse safety indications were observed. These findings indicate that pod-IVRs are promising devices for the delivery of the candidate topical microbicide VRC01-N against HIV-1 infection and merit further preclinical evaluation.
KEYWORDS: monoclonal antibody, broadly neutralizing antibody, VRC01, intravaginal ring, HIV prevention, drug delivery, rhesus macaque model, pharmacokinetics, human immunodeficiency virus
INTRODUCTION
The global human immunodeficiency virus (HIV)/AIDS epidemic continues into its fourth decade, with sub-Saharan Africa being the region most impacted by the epidemic (1–3). Although prevention efforts have resulted in a 40% decrease in new infections from 2.6 million in 2001 to 1.6 million in 2012, more than 4,300 individuals are still newly infected each day in sub-Saharan Africa (4). Women make up approximately 60% of infected individuals, with women aged 15 to 24 years comprising 85% of those currently living with HIV (3). Recent clinical trials have demonstrated that preexposure prophylaxis (PrEP) using small-molecule antiretroviral (ARV) drugs delivered orally or topically as a vaginal ring or gel may prevent HIV infection in a significant number of individuals (5–9). Several additional trials, however, failed to show its efficacy in preventing HIV infection (10, 11). These disparate trial results, along with the potential for the development of viral resistance to ARV agents if individuals using PrEP become HIV infected, suggest the need for continuing research to develop additional methods that use non-ARV microbicides with improved pharmacologic efficacy and high acceptability across diverse populations.
Human monoclonal antibodies (MAbs) may act as highly specific, potent, and mechanistically diverse microbicides against HIV type 1 (HIV-1). The virus exhibits a heavily glycosylated envelope glycoprotein consisting of a gp120 surface unit and gp41 transmembrane domain, resulting in a large viral diversity and the ability of HIV-1 to evade the host immune response. A recent review (12) of HIV-1 immunology described the characteristics of neutralizing antibodies identified in infected individuals. Although most efforts to identify HIV-1-neutralizing antibodies have focused primarily on vaccine development, a passive immunization approach using human MAbs topically to prevent HIV-1 infection in the vaginal mucosa has shown promise in preclinical and early clinical research. First studied were broadly neutralizing antibodies (bNAbs) that target specific regions of the HIV-1 envelope protein (13), including IgG1b12 (b12), targeting the CD4 binding site on gp120 (14–16); 2G12, targeting a glycan cluster on gp120 (14, 17, 18); and 2F5 and 4E10, targeting the membrane proximal extracellular region (MPER) region of the env gp41 subunit (14, 19, 20). Intravenously delivered 2G12 (21) and 2F5 and 4E10 (21, 22), as well as topically applied b12 (23, 24), protected against simian-human immunodeficiency virus (SHIV) infection in challenge studies using rhesus and cynomolgus macaques. Both vaginal gel formulations of a triple MAb combination of 2F5, 2G12, and 4E10 (Mabgel) (25) and 2G12 manufactured in a Nicotiana-based process (26) have completed phase 1 clinical trials.
While first-generation bNAbs exhibit weak potency and/or a poor breadth of reactivity across different strains (12), neutralizing only ca. 40% of HIV-1 isolates, a number of more potent second-generation bNAbs have been isolated. bNAbs PG9 and PG13 neutralized 73% and 79% of the HIV-1 strains tested, respectively (27, 28), and bNAbs VRC01, VRC02, and VRC03 neutralized more than 90% of the HIV-1 strains tested (29). Acute systemic infection following inoculation with HIV-1 BaL was inhibited in hCD4/R5/cT1 humanized mice treated with VRC01 (30). In a phase 1 dose-escalation study in healthy adults, VRC01 infused at 5 to 50 mg kg−1 of body weight retained HIV-1-neutralizing activity in serum, and no anti-VRC01 responses were detected (31). Systemically delivered VRC01 is currently in a clinical trial to investigate the efficacy of antibody-mediated HIV prevention (32).
Protection from initial mucosal infection by MAbs applied topically has been demonstrated in humanized mouse and primate models. A gel formulation of VRC01 afforded significant protection (7/9 mice) against HIV-1 BaL challenge in a RAG humanized mouse infection model, and a combination of b12, 2F5, 4E10, and 2G12 in the same gel completely protected against infection (33). A gel containing the 2G12, 2F5, and 4E10 MAbs protected 10 of 15 cynomolgus macaques vaginally challenged with a 50% infectious SHIV dose (34). A study comparing VRC01 and three other HIV-1 Env-specific MAbs to the anti-CD4 MAb 2D5 administered intravenously showed that although all five displayed a potent neutralizing ability in vitro, only the HIV-specific bNAbs, including VRC01, afforded high levels of protection in vivo in macaque vaginal and rectal challenge models (35). An in vitro study demonstrating that VRC01 can block the transfer of HIV-1 primary isolates from dendritic cells to CD4+ cells suggested that in the mucosa, MAbs may prevent the formation of systemic infection by inhibiting virus dissemination from the initial mucosal infection site to neighboring lymphocytes (36).
The use of MAbs as microbicides for topical HIV prophylaxis, particularly in resource-poor areas, such as sub-Saharan Africa, could be limited by high manufacturing costs and difficulty in scaling production to the quantities necessary for large-scale clinical trials and subsequent product rollout. The production of MAbs is typically carried out in mammalian cell cultures, but the use of a plant-based manufacturing system is an alternative platform that may lower the cost and increase the production scale. Both transgenic maize (37, 38) and an ΔXF knockout variant of Nicotiana benthamiana (39) have been used for the production of 2G12, and a 4E10 variant has been produced using transformed Nicotiana tabacum rhizosecretions (40). A Nicotiana system using a single vector expressed VRC01 at 80 mg MAb/kg plant material, and in an in vitro neutralization assay, the activity of Nicotiana-manufactured VRC01 (VRC01-N) was retained and was comparable to that of VRC01 produced in an HEK cell culture (41). Another VRC01-N variant made in transgenic Nicotiana plants demonstrated a neutralization capacity identical to that of VRC01 produced from human cells in a multistrain HIV-1 neutralization assay (42).
Here we report the sustained in vivo delivery of a human MAb for preventing vaginal HIV infection. Intravaginal ring (IVR) formulations of Nicotiana-manufactured VRC01 using a pod-based design (pod-IVR) (43–45) were fabricated and evaluated in vitro for release characteristics and MAb stability. Preliminary local safety and pharmacokinetics were investigated in a rhesus macaque primate model, in which it was found that steady-state vaginal fluid levels of VRC01-N were sustained over 21 days and the gp120 binding activity of the VRC01-N in the pod-IVR was retained in vivo.
RESULTS
In vitro studies.
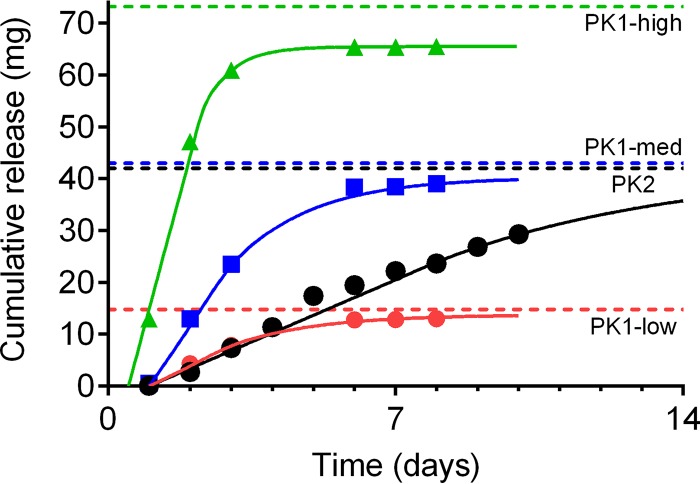
Plots of cumulative release versus time for the four VRC01-N IVR formulations are shown in Fig. 1, and in vitro release rates calculated from the initial linear portions of the release curves (PK1-high, days 1 to 3; PK1-low and PK1-med, days 1 to 6; PK2, days 1 to 10, where PK1-high, PK1-med, and PK1-low represent a 28-day dose-ranging pharmacokinetic [PK] study [PK1] with the three first-generation IVR formulations at high, medium [med], and low dosages, respectively, and PK2 represents a two-phase study [7 days and 28 days] with a second-generation pod-IVR formulation delivering VRC01-N employing more intensive PK sampling and multiple safety measures) are presented in Table S1 in the supplemental material.
FIG 1.
In vitro release of VRC01-N from the four pod-IVR configurations used in the PK1 and PK2 macaque studies. Pod-IVR configuration details are shown in Table S1. For the dose-ranging study (PK1), pod-IVRs releasing VRC01-N at three different initial rates (the zero-order portion of the release curve) were used: 3.8 mg day−1 (low release; red), 12 mg day−1 (medium release; blue), and 30 mg day−1 (high release; green). For PK2, a pod-IVR releasing 3.4 mg day−1 VRC01-N was used (black). Solid lines, the fit of in vitro data to the diffusion-based release model (see reference 45); dashed lines, total drug load for each pod-IVR configuration.
Pharmacokinetic measurements.
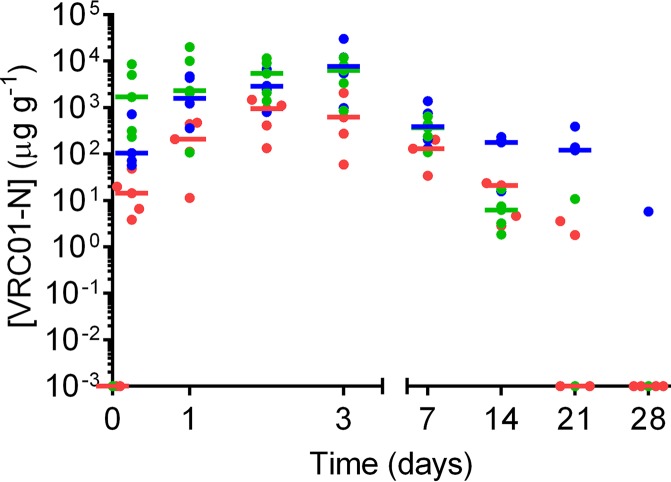
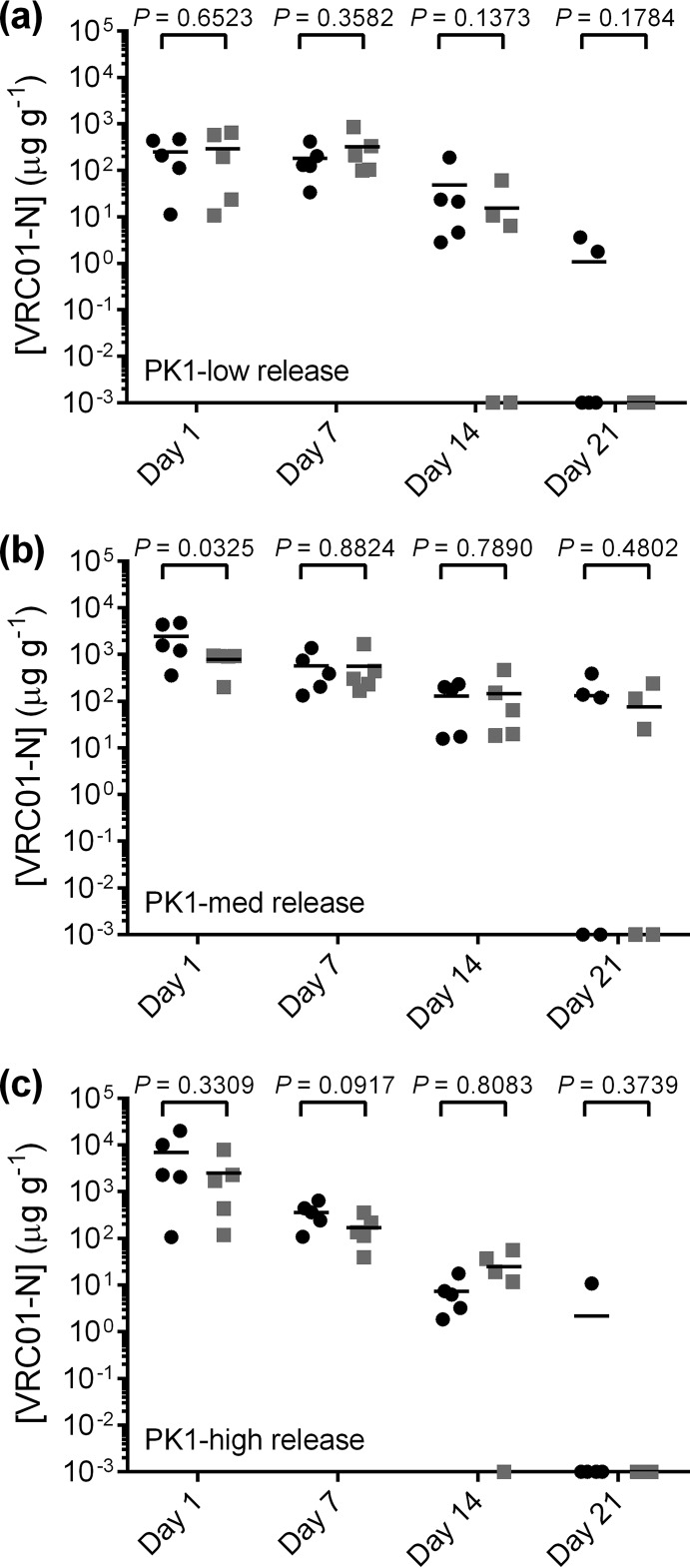
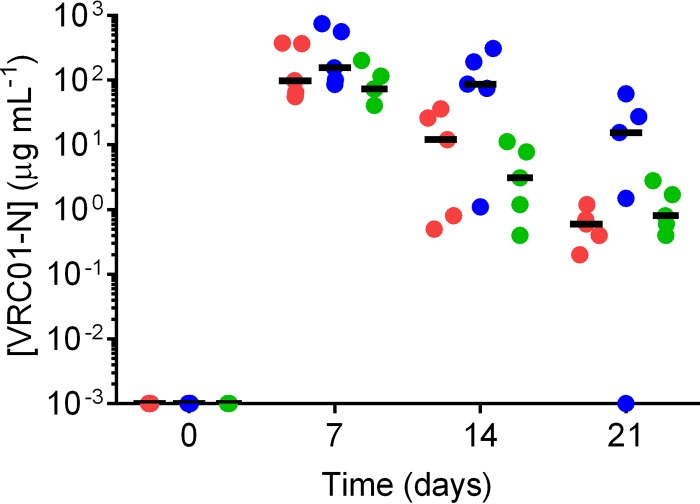
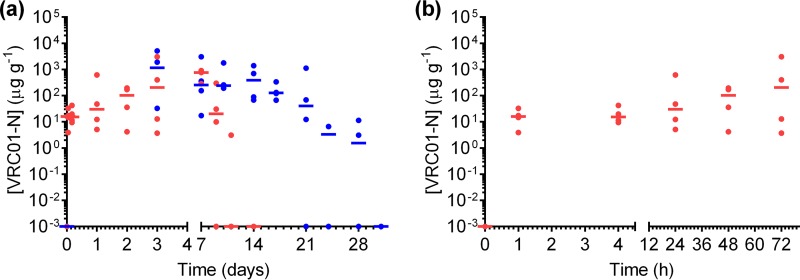
The design and sampling schedule of the PK1 and PK2 sheep studies is given in Fig. 2. The levels of VRC01-N in vaginal fluid collected by a Tear-Flo strip at locations proximal to the IVR (deep vagina) are shown in Fig. 3 for each dose group in PK1. The VRC01-N levels in vaginal fluids sampled in PK1 at locations proximal (deep vagina) and distal (midvagina) to the pod-IVRs are shown in Fig. 4. At each time point, the measurements at the two locations were compared using a ratio paired t test (95% confidence interval [CI]), and a statistically significant difference (P < 0.05) between data sets (i.e., those for the proximal versus distal locations) was observed only for the medium-releasing group on day 1. The levels of VRC01-N in cervicovaginal lavage (CVL) fluid are shown in Fig. 5 for PK1 and were maintained above the lower limit of quantitation (LLOQ) in all but one sample for 21 days. At day 28, all CVL fluid VRC01-N levels were below the limit of quantitation (BLQ). Vaginal fluid levels measured during PK2 are shown in Fig. 6 for both phase A (PK2A; 7-day study) and phase B (PK2B; 28-day study). The VRC01-N levels in CVL fluid for PK2 are shown in Fig. 7. The levels were above the LLOQ in all but two samples (both obtained on day 28), with concentrations of >1 μg ml−1 being obtained at day 21 for all animals.
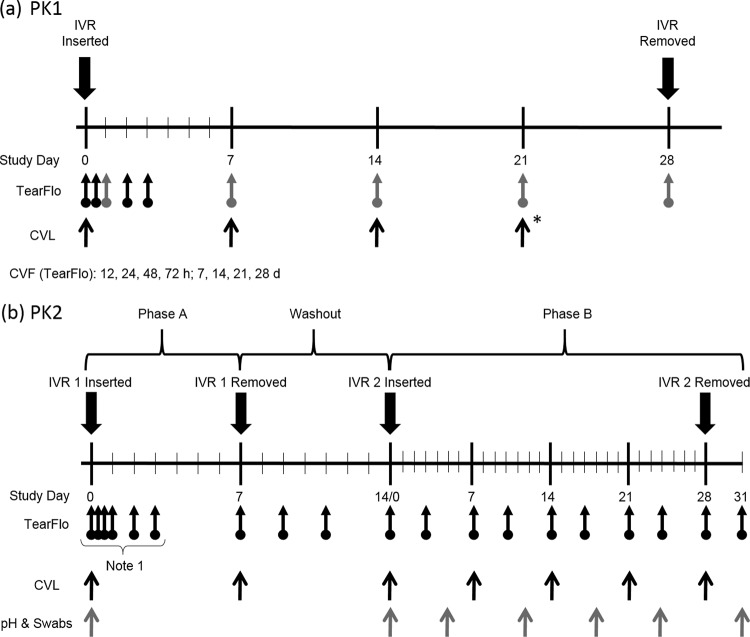
FIG 2.
Nonhuman primate study timelines and biological sample collection points for the PK1 dose-ranging study (n = 5 per dosage group, 3 groups) (a) and the PK2 study (n = 4) (b). Arrows with circles, times of cervicovaginal fluid (CVF) collection using Tear-Flo strips (proximal to the IVR [black], proximal and distal to the IVR [gray]); black arrows, CVL fluid and serum collection (*, only CVL fluid collection); gray arrows, collection of pH and swab samples for determination of the Nugent score. Note 1, vaginal fluid samples in PK2 phase A were collected at 1 h, 4 h, 24 h, 48 h, 72 h, and 168 h (7 days). d, days.
FIG 3.
Plot of vaginal fluid concentrations of VRC01-N obtained at each sampling interval in study PK1. Each data point represents the VRC01-N concentration measured for each animal (n = 5), and horizontal bars represent the median values for each group: high release (green), medium release (blue), and low release (red).
FIG 4.
Comparison of PK1 VRC01-N levels in vaginal fluid as a function of sampling location proximal (circles) and distal (squares) to the IVR on days 1, 7, 14, and 21 for each study group: low release (a), medium release (b), and high release (c). For each day, values for the proximal and distal locations within study groups were compared using a ratio paired t test (95% CI). The data set means are considered to be significantly different for P values of <0.05.
FIG 5.
Plot of cervicovaginal lavage (CVL) fluid concentrations of VRC01-N obtained at each sampling interval in study PK1. Each data point represents the data for each animal (n = 5), and horizontal bars represent the median values for each group: high release (green), medium release (blue), and low release (red).
FIG 6.
(a) Plot of vaginal fluid concentrations of VRC01-N obtained at each sampling interval in the PK2 study. Each data point represents the concentration in each animal in phase A (7 days and a 7-day washout; red) and in phase B (28 days and a 3-day washout; blue). Horizontal bars represent the median values (n = 4). Data are plotted overlaid, with the time of IVR insertion for each phase occurring on day 0. (b) Plot of phase A VRC01-N vaginal fluid concentrations during the first 72 h to show the rapid rise to steady state.
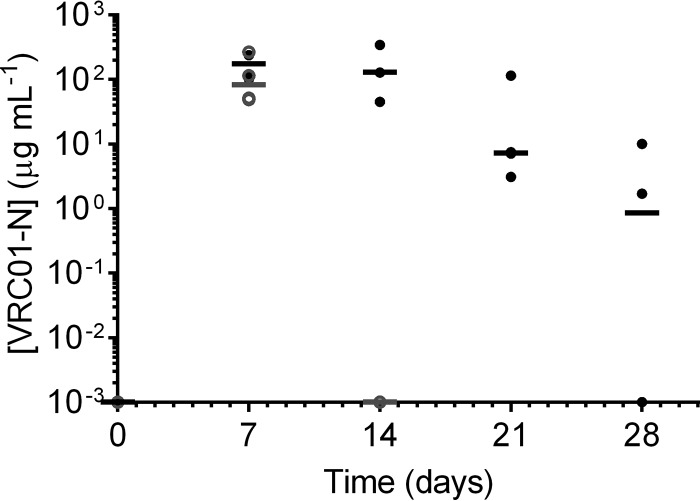
FIG 7.
Plot of CVL fluid concentrations of VRC01-N obtained at each sampling interval in study PK2. Each data point represents the data for each animal (n = 4), and horizontal bars represent the median values for phase A (day 7; gray open circles), the washout period (day 14; gray open circle), and phase B (days 7, 14, 21, and 28; black closed circles).
The mean VRC01-N blood serum levels from measurements for all animals at all time points, as determined by the enzyme-linked immunosorbent assay (ELISA) response, were 27 ± 13 μg ml−1 for PK1 and 13 ± 3 μg ml−1 for PK2. No statistically significant (Bonferroni's multiple-comparison test, P < 0.05) difference in the levels compared to the baseline levels was observed at any time point, suggesting that the ELISA response is due to an interfering IgG or other component of serum and not the accumulation of VRC01-N.
In vivo release and VRC01 stability.
Residual VRC01-N in the pod-IVRs after removal (7 or 28 days) was assayed to determine the in vivo release and stability of the MAb in the presence of vaginal fluids. For PK1, visual inspection of the IVRs after removal indicated that only a trace of pod material remained in the IVRs of all three groups. Table 1 shows the total amount of residual protein, as determined by measurement of the absorbance at 280 nm (optical density at 280 nm [OD280]), and the amount of residual VRC01-N exhibiting gp120 binding activity, as determined by ELISA, for each group and for a pod extracted from an unused IVR. Most pod cavities contained a clear or slightly cloudy gelatinous material that was responsible for the large OD280 signal relative to the ELISA response for the residual pod material. The material remaining in the pods in the IVRs from both phases was extracted and analyzed using ELISA and sodium dodecyl sulfate (SDS)-PAGE to determine if VRC01-N was degraded within the pod-IVR prior to release into vaginal fluids. Table 2 shows the mass of VRC01-N retaining gp120 binding activity by ELISA, and Fig. S3 shows the results of SDS-PAGE analysis of the residual VRC01-N from PK2A on a per pod basis. All pods except for one from macaque 3 showed the expected gel pattern for VRC01-N, as indicated by the VRC01-N standard control lane.
TABLE 1.
Analysis of residual VRC01-N MAb in pod-IVRs from study PK1 by ELISA and OD280 determination
| Study group | Amt of residual VRC01-N MAb (μg) by: |
|
|---|---|---|
| ELISA | OD280 determination | |
| PK1-low | NDb | 0.76 ± 0.12 |
| PK1-med | 0.016 ± 0.032 | 2.11 ± 0.74 |
| PK1-high | 0.001 ± 0.003 | 2.95 ± 0.19 |
| Poda | 9,100 | 11,900 |
A single pod containing 12 mg VRC01-N extracted from an unused pod-IVR.
ND, not detected.
TABLE 2.
Analysis of residual VRC01-N MAb in pod-IVRs from study PK2 by ELISA
| Study | Animal identifier | Residual amt of VRC01-N (mg) |
|---|---|---|
| PK2A | 1A | 52.9 |
| 2A | 48.1 | |
| 3A | 31.6 | |
| 4A | 48.8 | |
| PK2B | 1B | 0.24 |
| 2B | NDa | |
| 3B | ND | |
| 4B | 0.04 |
ND, not detected.
The in vivo rate of VRC01-N release was estimated from the vaginal fluid PK data and a diffusion-based model for MAb release from pod-IVRs described previously (45). Figure S2 shows the release curves generated from the model. Table S1 shows the in vivo release rates, calculated as the slope of the linear (zero-order) portion of this model's in vivo release curve.
Local safety measures.
No ring expulsions or adverse events were noted by colposcopy during the course of the study. In PK1, the cytokine levels at day 0, prior to IVR insertion (the baseline), were compared to the cytokine levels in all groups at all time points with the IVR in place. No statistically significant differences (P < 0.05) in cytokine levels were observed, except for those of interleukin-18 (IL-18), where the levels in the low-releasing IVR group were lower on day 14 (mean, 65 pg/ml) than day 0 (mean, 1,221 pg/ml) (adjusted P = 0.0042). For PK2, no statistically significant differences (P < 0.05) in cytokine levels were observed between time points in any of the animals with the IVR in place and with the device removed. The IL-1 receptor antagonist (IL-1ra) level was above the upper quantitation limit (17.5 ng/ml) in all samples from animals in PK2, including those obtained at the baseline, suggesting that these levels were intrinsic and not induced by the IVR. Nugent scores in the range of 2 to 10 and a pH of vaginal fluids in the range 5.0 to 6.8 were measured in PK2 (Fig. S4).
DISCUSSION
IVR delivery of MAbs.
The high water solubility of MAbs precludes their sustained mucosal delivery using traditional matrix and reservoir IVR designs because MAbs are not able to diffuse through the hydrophobic elastomers, e.g., silicone or ethylene-co-vinyl acetate (EVA), typically used in these devices. A limited number of approaches for mucosal delivery of MAbs have been described previously. Radomsky et al. delivered fluorescently labeled mouse and rabbit human chorionic gonadotropin-binding IgGs from intravaginal devices consisting of particles of IgG mixed with polysaccharides and salts loaded at 30 to 50 wt% into an EVA matrix (46). At this high loading, the particles form a series of channels in the EVA matrix through which the IgG diffuses. Vaginal fluid concentrations of 0.3 to 10 μg ml−1 IgG were sustained over 30 days, with IgG being distributed through the vaginal lumen without penetration into epithelial tissues or migration into the uterine horns (46). Morrow et al. developed an insert IVR that released the HIV monoclonal antibody 2F5 from lyophilized inserts formed from the antibody mixed with hydroxypropyl methylcellulose (47). The in vitro release of 2F5 from these devices was sustained for up to 170 h, with 75 to 85% of the 2F5 activity being maintained following lyophilization and formulation, as measured by ELISA (47). The pod-IVR described here utilizes the diffusion of MAb from a solid core across a rate-controlling polymer membrane and through a macroscale physical channel in the elastomer ring. The release rate from pod-IVRs is determined by the additive effect of the MAb solubility and three independently variable parameters: the composition and thickness of the pod's polymer membrane coating, the size and number of delivery channels through the ring exposing each individual pod to the vaginal fluid, and the number of pods in the ring. The pod-IVR design has been used for the delivery of relatively hydrophilic small-molecule antiretroviral drugs singly and in combination (43, 44, 48) and of ovine IgG as a model for human MAb (45).
Formulation and in vitro release of VRC01-N.
The in vitro release of VRC01-N into vaginal fluid simulant (VFS) from four different pod-IVR formulations was evaluated in preparation for the two in vivo PK studies. The release from all four formulations was initially linear (zero order) and spanned a range of nearly 1 order of magnitude of from 3.8 to 30 mg day−1. The in vitro release kinetics shifted to first order at longer times when a significant fraction of the total VRC01-N load in each pod was depleted. The in vitro release kinetics were similar to those observed for sheep IgG release from a pod-IVR and were consistent with a previously developed, diffusion-based release model (45). The number of pods per IVR and the pod's delivery channel diameter determined the rate of VRC01-N release. Because they release VRC01-N simultaneously, addition of pods increases the total release rate but not the length of time that release may be sustained. For a given delivery channel configuration (i.e., release rate), increasing the mass of VRC01-N in a pod increases the total time that release is maintained at the target rate.
Studies of HIV or simian-human immunodeficiency virus (SHIV) challenge infection in humanized mouse (33) or macaque (34) models have demonstrated that the mucosal delivery of a single MAb does not fully protect from infection and that MAb combinations are required for effective HIV prophylaxis. Because each pod is a separate delivery device, pod-IVRs can deliver multiple MAbs simultaneously with the independent control of release rates. Pod-IVRs for macaques (48, 49) are significantly smaller than those for humans (44, 45) and limit the total drug loading and capacity to deliver multiple MAbs at efficacious rates. The macaque pod-IVRs used here accommodate a maximum of six 25-mg pods, providing a maximum MAb loading of 75 mg from a formulation that contains 50% MAb. Human pod-IVRs accommodate up to 10 pods as large as 4.8 mm in diameter with a load of 200 mg (45) and have the capacity for the sustained delivery of three or more MAbs simultaneously for 30 days at rates of >2 mg day−1 from each pod. The smaller size of macaque IVRs limits the duration that release may be sustained at realistic target levels compared to that for human IVRs. On the basis of the results of the PK1 study, pod-IVRs for the PK2 study were formulated to maintain steady-state VRC01-N levels in vaginal fluids for at least 14 days. Sustained VRC01-N levels above 100 μg g−1 were maintained in PK2 for over 14 days, suggesting the suitability of these pod-IVRs for a future efficacy study in a macaque infection model using a weekly vaginal SHIV challenge and biweekly IVR exchange. This approach was used to evaluate the efficacy of pod-IVRs delivering the antiretroviral combination of tenofovir disoproxil fumarate and emtricitabine (70).
In vivo release.
Initial in vitro release measurements prior to PK1 were carried out with a VRC01-N formulation prepared by lyophilizing an aqueous solution of MAb and buffer components, including stabilizing sugars. The in vitro studies suggested that more than 28 days of release in vivo would be obtained in the low- and medium-releasing groups and approximately 18 days of release would be obtained in the high-releasing group. For PK1, a spray-dried VRC01-N powder optimized for stabilizing the MAb with respect to the loss of binding activity when it was stored as a solid was utilized, and in vitro release studies with the spray-dried formulation were conducted concurrently with the in vivo evaluation. The pod-IVRs fabricated using spray-dried VRC01-N exhibited significantly (∼5-fold) faster release in vitro than those fabricated using the lyophilized formulation and, concomitantly, a faster in vivo release that resulted in depletion of the VRC01-N load in all three dose groups prior to 28 days.
Phase A of PK2 (the 7-day study) was designed so that, on the basis of the measured in vitro release rates, only 20 to 30% of the VRC01-N in each IVR would be released at the time of removal. This would allow determination of the in vivo VRC01-N release rate from the difference between the initial VRC01-N mass in the IVR and the residual mass of VRC01-N in the pod material extracted from the IVR following removal. The VRC01-N ELISA method, however, did not provide the precision required to accurately quantify the mass of VRC01-N released, as residual mass values above and below the initial pod masses were measured. Qualitatively, comparison of ELISA measurements from phase A to phase B (the 28-day study) of PK2 showed that a significant amount of active VRC01-N was present after 7 days when IVRs contained visible solid material, whereas little to no VRC01-N was observed after 28 days in vivo when all of the visible pod mass has been depleted. Consequently, in vivo release rates for both PK1 and PK2 were estimated using the PK data and a diffusion-based model for pod-IVR release (45). The vaginal fluid concentrations for the animals in the three PK1 groups and PK2 phase B were used to determine the time at which release switched from zero order to first order (when steady-state VRC01-N levels in vaginal fluid begin to decrease) and the time at which the entire VRC01-N load had been released (when vaginal fluid VRC01-N levels fall to zero). These time values were input into the diffusion release model, and in vivo release curves were generated (see Fig. S2 in the supplemental material).
Pharmacokinetics of MAb delivery.
Pod-IVRs delivered VRC01-N, with sustained MAb levels in vaginal fluids being maintained for up to 21 days. Following pod-IVR insertion, a rapid increase in the VRC01-N concentration in vaginal fluids was observed, indicating a fast initial diffusion of fluid into the delivery channel to wet the pod and initiate release. Median vaginal fluid levels in study PK1 were >10 μg g−1 within 6 h following IVR insertion and reached steady state by 24 h for the medium- and high-releasing groups and by 48 to 72 h in the low-releasing group. Initially, the median vaginal fluid concentrations of VRC01-N followed the expected trend by VRC01-N dose group, as follows: low dose release (942 ± 539 μg g−1) < medium dose release (2,870 ± 2,170 μg g−1) < high dose release (5,430 ± 4,370 μg g−1) on day 2. After day 3, the steady-state VRC01-N levels began to decrease because the in vivo release rate decreased as the pod mass was depleted, and the VRC01-N vaginal fluid levels in all but one macaque were BLQ by day 28.
A two-phase study design was used for PK2: IVR insertion for 7 days (phase A), followed by a 7-day washout period after IVR removal prior to insertion of identical IVRs for 28 days (phase B). Intensive vaginal fluid sampling immediately following IVR insertion and during the washout period allowed both an initial buildup of the VRC01-N concentration to steady state and a VRC01-N concentration decrease following IVR removal to be observed. Removal of the IVR at day 7 during steady-state release also allowed assessment of VRC01-N degradation or a loss of binding activity within the IVR while it was present in vivo. As shown in Fig. 6b, the median VRC01-N level was >10 μg g−1 1 h after IVR insertion, and a median steady-state concentration above 100 μg g−1 was reached by day 2. Following IVR removal on day 7, VRC01-N levels fell below the limit of detection (LOD) within the washout period. No significant difference between the phase A and phase B VRC01-N levels was observed on either day 3 or day 7, the only time points common to the two phases of PK2, indicating that the in vivo VRC01-N release rate and PK are similar in both study phases. In phase B, VRC01-N steady-state levels were sustained for 14 days in all animals, and fluid levels of >100 μg g−1 VRC01-N were maintained for 17 days in 4 of 4 animals and 21 days in 3 of 4 animals. Maintaining steady-state vaginal fluid levels is critical to avoid zones of vulnerability to HIV infection that could result from significant concentration variability or troughs (50).
The levels of VRC01-N in CVL fluid showed a trend similar to that seen in vaginal fluid, with peak levels on day 7 that decreased through day 21. The CVL fluid measurements may be more representative of the total vaginal fluid VRC01-N levels than the measurements obtained with fluid sampled by the use of site-specific Tear-Flo strips. The CVL rinse collects vaginal fluids from the entire vaginal vault, while Tear-Flo strips sample a small amount of fluid from a single location along the vaginal wall. The median CVL fluid levels showed less variability within groups than fluid levels from Tear-Flo strips. At day 21, median CVL fluid levels remained above 1 μg ml−1, with the value for only one macaque being BLQ. The dilution of the vaginal fluid in phosphate-buffered saline (PBS) containing CVL fluid was not quantified, and the concentrations of VRC01-N in CVL fluid underestimate the actual vaginal fluid concentrations present in the vagina. Lavage sampling has been shown to dilute vaginal fluids by a factor of 10 to 50 (51–53).
Sampling with Tear-Flo strips provides a measure of MAb dissemination and distribution within the vaginal vault. There was minimal spatial variability of vaginal fluid VRC01-N levels across the vaginal mucosal surface in PK1 Tear-Flo samples. In all groups, no statistically significant difference in the VRC01-N concentration was observed at locations proximal and distal to the IVR on days 1, 7, 14, and 21 (Fig. 4), except for the medium-releasing group on day 1, where VRC01-N concentrations were higher proximal than distal to the IVR. This indicates a uniform MAb distribution with rapid spreading of VRC01-N in vaginal fluids from the upper (proximal to the IVR) to the lower (distal to the IVR) vagina.
VRC01 stability in vivo.
The ELISA and SDS-PAGE analyses of residual MAb in the pods for PK2A suggested that the VRC01-N in the IVR does not significantly degrade over 7 days in vivo. The ELISA response for PK2A, where VRC01-N release was still at steady state, showed a high level of binding activity. For PK2B, where vaginal fluid levels and visual IVR inspection indicated the nearly complete release of VRC01-N, the ELISA response was near or below the LOD, suggesting that there is no interfering component from vaginal fluid that artificially enhances the ELISA response for the residual pod material. The SDS-PAGE results also confirmed that VRC01-N does not degrade in the IVR in vivo. As shown in Fig. S3, only one pod from PK2A (macaque 3) did not exhibit the gel pattern for VRC01-N, and ELISA showed that the pod contained less than 1 mg active VRC01-N. Pod-IVRs stabilize ovine IgG against a loss of binding activity during in vitro release studies and long-term IVR storage (45). The stability of VRC01-N and other MAbs during storage and use is particularly important for sustained-delivery devices, such as IVRs, where the MAb is exposed to vaginal fluids and body temperatures continuously for periods of 1 month or more.
Local safety of VRC01 pod-IVRs.
Increased susceptibility to HIV infection may result from compromised mucosal barrier integrity as a result of vaginal epithelial trauma or recruitment of immune cells and the resulting associated inflammation (50, 54). The effect of VRC01-N pod-IVRs in PK2 on the vaginal pH, the microbiome (as indicated by the Nugent score), and the mucosal levels of proinflammatory cytokines was evaluated as a preliminary measure of IVR safety. Variations in vaginal fluid pH (range, 5.0 to 6.8) and Nugent scores (range, 2 to 10) were observed throughout the study (Fig. S4), with changes occurring as a function of time and sex cycle period for a single animal and between animals. The variations were not correlated with IVR insertion or removal and appeared to be natural cycling unrelated to IVR use. Proinflammatory cytokine levels in the CVL fluid samples as a function of time during PK1 and PK2 did not show statistically significant changes as a result of IVR use except for IL-18 levels in the PK1 low-releasing group, which decreased from those at the baseline upon ring insertion. These results indicate that neither the pod-IVR nor the VRC01-N itself leads to an increased inflammatory response, as has been observed previously for pod-IVRs delivering antiretrovirals (ARVs) in pig-tailed macaques (48, 55).
Implications for topical HIV PrEP.
The use of pod-IVRs delivering VRC01-N, a second-generation bNAb shown to neutralize over 90% of HIV-1 strains in vitro, is an alternative or complementary approach to the use of small-molecule ARVs that currently dominate nonvaccine HIV prevention efforts. Because each pod's release characteristics are independent of those of the other pods in the IVR, combinations of multiple MAbs or of MAbs with small-molecule ARV drugs may be delivered simultaneously to the vaginal mucosa to neutralize HIV at different stages of the viral life cycle, decreasing the potential for the development of viral resistance to prophylactic therapy (56). The investigation of topically applied MAbs to prevent sexual HIV infection has thus far been limited primarily to preclinical studies, but this approach has shown promise. The efficacy of a VRC01 gel compared to that of a gel with a combination of first-generation bNAbs b12, 2F5, 4E10, and 2G12 at preventing infection by R5-tropic HIV-1 BaL was evaluated in a RAG humanized mouse vaginal challenge model (33), where the VRC01 gel protected 7 of 9 mice from infection and the bNAb combination protected 5 of 5 mice from infection. The bNAb b12 applied vaginally conferred significant (9 of 12 mice) protection against SHIV 162P4 mucosal challenge in rhesus macaques (24). A subset of bNAbs, including VRC01, has been shown to block HIV-1 transmission by cell-associated virus (57). Two vaginal gel formulations have both completed phase 1 clinical trials: the Mabgel triple combination of 2F5, 2G12, and 4E10 (25) and 2G12 manufactured in a Nicotiana-based process (26). A phase 1 clinical trial to assess the safety of a vaginal film containing VRC01-N is ongoing (58).
These studies have, however, been limited to the topical application of MAbs in on-demand formulations (gels or films) just prior to viral challenge. Devices such as IVRs that provide continuous MAb delivery for over 1 month or more should provide the sustained mucosal levels required for protection against HIV infection (59). The sustained vaginal fluid concentrations of VRC01-N required to prevent infection are not currently known. In studies of passive administration of broadly neutralizing antibodies in SHIV challenge macaque models, it is estimated that MAb serum concentrations of 100 or more times the 50% inhibitory concentration (IC50) can achieve significant protection (28). Wu et al. used a panel of 190 Env-pseudotyped virus strains that included all major clades and determined that 72% of the primary isolates were neutralized with an IC50 of <1 μg ml−1 (29). On the basis of these IC50 values, the dosing levels obtained in PK1A and PK1B have the potential to provide significant protection from a majority of viral strains. Combinations of complementary MAbs or of MAbs and small-molecule ARV agents may lead to a larger breadth of coverage and an increase in potency (28). Because the release of an MAb or some other drug from each pod in the pod-IVR is independent, formulating combinations is a simple matter of combining multiple MAb- or ARV agent-containing pods in a single ring.
MAb manufacturing cost and scale.
Two of the primary limiting factors in the successful implementation of PrEP in the developing world, where HIV infection rates remain the highest, are the manufacturing cost and the availability of production capacity. As a biologic, MAbs cost more to manufacture than small-molecule drugs, with FDA-licensed MAbs currently being among the most expensive drugs. Many factors contribute to the cost of a particular MAb (60), but the primary determinant of price appears to be market driven, and the U.S. and European therapeutic markets currently support a high cost for MAbs (61). As the ability of MAb-based drugs to make significant contributions to global health and disease prevention increases, there is significant growing pressure to dramatically lower the costs of MAb-based products. The expense of antibody manufacturing in mammalian cells has decreased considerably over the last 3 decades, with a cost of <$300 per g being possible using existing and conventional unit operations for large-scale antibody manufacture and purification (62). The sheer size of the unmet need for antibody-based products in global health, however, may be beyond the current worldwide manufacturing capability of animal cell-based production (63). The manufacture of whole antibodies in Nicotiana benthamiana (64, 65) or a fungus (66) may meet the demands of large, cost-sensitive markets. A cost per dose (10 mg) of less than $1 has been estimated for very large scale MAb production in recombinant manufacturing platforms (62).
Conclusion.
The use of pod-IVRs for the topical administration of VRC01-N, a second-generation, Nicotiana-manufactured bNAb with a high neutralizing potency, showed that they exhibited sustained, controlled release over 21 days in rhesus macaques with no adverse safety indications. The high steady-state levels of VRC01-N observed in vaginal fluids suggest that these pod-IVRs may successfully prevent HIV-1 infection following vaginal exposure. Consequently, these devices merit further evaluation both in a preclinical macaque efficacy model and in a clinical setting.
MATERIALS AND METHODS
Materials.
The Nicotiana-manufactured VRC01 MAb (VRC01-N) was obtained from Mapp Biopharmaceuticals, Inc. (San Diego, CA). Poly-d,l-lactide (PLA; 10 to 18 kDa; ester terminated; Resomer R 202 S) was obtained from Evonik Industries AG (Essen, Germany). The polydimethylsiloxane resin (liquid silicone rubber) used for injection molding of macaque-sized ring scaffolds was MED-4940, obtained from Nusil, Inc. (Carpinteria, CA, USA). Dithiothreitol (DTT) and sodium dodecyl sulfate (SDS) were obtained from Thermo Fisher Scientific (Pittsburgh, PA, USA). All other chemicals and solvents were reagent grade or better and were used as received.
Preparation of solid antibody formulation.
A solution of excipients (trehalose, Tween 20, and histidine) and VRC01-N was prepared with 50% total solids, with VRC01-N being approximately 25% (wt/wt) of the solution. Spray-drying of this solution to obtain a solid containing VRC01-N, trehalose, Tween 20, and histidine was performed with a Buchi mini-spray dryer (model B190; Buchi, New Castle, DE) under controlled conditions of 28°C and <10% relative humidity. The liquid feed rate was 0.5 ml min−1. The solution was atomized through the sonic nozzle at a 10-lb/in2 atomization gas pressure. The outlet temperature was 60°C. After spray-drying, 1-g aliquots of VRC01-N powder were transferred to glass vials, and the vials were sealed in foil packets and stored at 4°C. The solid formulation was 51.3% VRC01-N.
Manufacture of silicone pod-intravaginal rings.
Intravaginal rings of the pod-IVR design containing VRC01 were prepared using methods previously reported (44). The IVR dimensions were identical to those used in previous macaque studies (48, 49). Cylindrical cores of ∼25 mg VRC01-N spray-dried formulation (3.2 mm in diameter by ca. 2.5 mm in height) were formed using compaction on a manual pellet press and a 1,470-N force (model MTCM-I; GlobePharma, North Brunswick, NJ). The compressed VRC01-N cores were coated with two layers of PLA from a 5% PLA solution in 2:1 dichloromethane-ethyl acetate. Pods were dipped in the PLA solution for approximately 3 s, removed, placed on a Teflon plate, and allowed to dry for ∼4 h, before a second PLA layer was applied using the same technique. The PLA-coated VRC01-N pods were embedded in silicone ring scaffolds as described previously (44) with one 1.0-mm- or 1.5-mm-diameter delivery channel per pod. Four different VRC01-N formulations were prepared by varying the number of pods, the delivery channel diameter, and the total VRC01-N mass per ring (see Fig. S1 and Table S1 in the supplemental material).
In vitro studies.
The in vitro release of VRC01-N was carried out by placing individual pod-IVRs into glass vials containing 10 ml vaginal fluid simulant (VFS) maintained at 25 ± 2°C on an incubating orbital shaker at 60 rpm. The VFS was adapted from that of Owen and Katz (67) and consisted of 25 mM acetate buffer (pH 4.2) with NaCl added to yield a 200-mosM solution. Aliquots of the release medium were removed at specified time intervals and analyzed by UV absorption spectroscopy at 280 nm (OD280) using a SpectraMax Plus384 96-well plate reader (Molecular Devices, Sunnyvale, CA, USA). The concentration of VRC01-N in the release solution was calculated using the Beer-Lambert law and the absorption coefficient for VRC01-N at 280 nm (1.565 mg−1 · cm−1 · ml). Daily release rates were calculated as the slope of the linear portion of the release curve determined by linear regression using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
Nonhuman primate studies.
Pharmacokinetic (PK) and safety evaluations were carried out at the Yerkes National Primate Research Center (YNPRC). Adult female rhesus macaques (Macaca mulatta) of Indian origin were used for all studies. The animals were born and housed at the YNPRC of Emory University (Atlanta, GA) and were maintained according to the guidelines in the Guide for the Care and Use of Laboratory Animals (68) of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, and policies of the U.S. Department of Health and Human Services. The animals were fed monkey diet (Purina) supplemented daily with fresh fruit or vegetables and water ad libitum. The studies reported herein were performed under IACUC protocol YER200, which was reviewed and approved by the Emory University IACUC and Biosafety Review Committee. The YNPRC has been fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International since 1985.
Two separate studies were conducted: a 28-day dose-ranging PK study with the three first-generation IVR formulations (the PK1 study) and a two-phase (7-day and 28-day) study with a second-generation pod-IVR formulation delivering VRC01-N employing more intensive PK sampling and multiple safety measures (the PK2 study). The study timelines and biological sample collection points are shown in Fig. 2. For both studies, IVRs were inserted into the posterior vagina on day 0 (and on day 14 for PK2 phase B) following baseline sample collection. Vaginal colposcopy was used to confirm the placement and retention of the IVRs and to examine the integrity of the cervicovaginal epithelium. The animals were placed in ventral recumbency while under general anesthesia. A pediatric speculum was used to open the vaginal vault, and a Zeiss colposcope was used to visualize and photograph the IVR placement (Fig. S1).
For PK1, animals in three dosage groups (low, medium, high) with five macaques per group each received a pod-IVR delivering VRC01-N at the doses shown in Table S1. Samples of vaginal fluid, cervicovaginal lavage (CVL) fluid, and blood serum were collected as shown in Fig. 2a over 28 days of pod-IVR insertion. Pod-IVRs were removed on day 28, and a final set of vaginal fluid samples was collected on day 31 to observe VRC01-N washout following IVR removal. For PK2, the study was divided into phases: phase A (IVR 1, 7 days), washout (no IVR, 7 days), and phase B (IVR 2, 28 days plus 3 days after IVR removal). On day 0, four macaques each received a pod-IVR releasing 3.0 mg day−1 in vitro (Table 1). Sampling of vaginal fluid, CVL fluid, and blood serum was carried out as described in Fig. 2b. The IVRs were removed on day 7, and additional vaginal fluid samples were collected to observe VRC01-N washout over a 7-day period with no IVR in place. A second set of baseline vaginal fluid, CVL fluid, and serum samples was collected on day 14 prior to insertion of a pod-IVR identical to that used in phase A. The second IVR remained in place for 28 days (phase B).
Vaginal fluid samples were collected at locations proximal (ectocervix) and distal (introitus) to the ring placement using Tear-Flo strips (Beaver-Visitec International, Waltham, MA, USA). The strips were weighed prior to use, placed against the vaginal wall either proximally or distally to the IVR for 1 min each, and weighed again to determine the fluid volume collected. The strips were placed in microcentrifuge tubes, and 108 μl 1× PBS was added. Strips in PBS buffer were stored at −80°C until analysis by ELISA. For CVL fluid collection, 1× PBS (1 ml) was gently infused into the vaginal vault via a sterile transfer pipette (catalog number Samco 336-1S; Thermo Fisher Scientific). The CVL fluid was collected after 10 aspirations and reinfusions to ensure mixing of the saline with the cervicovaginal fluids. The CVL fluid was observed for the presence of blood or any other discoloration, the amount of mucus was scored, and the volume of fluid collected was noted. The levels of VRC01-N in vaginal fluids, CVL fluid, and serum were measured by ELISA using the method described below. Vaginal pH was measured using MColor pHast test strips (pH 0 to 6.0 and 5 to 10.0; EMD Millipore, Billerica, MA). Samples for Nugent score analysis were collected by use of a BactiSwab (Thermo Fisher-Remel Products, Lenexa, KS, USA) following the manufacturer's protocol.
The VRC01-N remaining in the IVRs following removal was recovered by cutting open the surrounding silicone with a scalpel to expose the remaining pod contents and dissolving the residue in 1.0 ml of 1× PBS ELISA buffer. Extracts were analyzed by determination of the absorbance at 280 nm (OD280) to quantify the total protein content, ELISA measurement of VRC01-N binding to gp120 to quantify the residual active MAb, and SDS-PAGE for qualitative assessment of VRC01-N stability.
Cytokine measurement and histopathology.
Induction of mucosal inflammation was monitored by measuring the vaginal cytokine levels in CVL fluid samples as previously described (49, 69) using fluorescent multiplexed bead-based assays (Milliplex PRCYTMAG40K-PX25; EMD Millipore) in accordance with the manufacturer's instructions. The following cytokines were quantified: soluble CD40L, IL-1β, IL-1ra, IL-6, IL-8, IL-12/23, IL-17A, IL-18, monocyte chemotactic protein 1, transforming growth factor α, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and gamma interferon. The lowest concentration of the standard curve was defined as the LLOQ, and LLOQs ranged from 0.68 pg ml−1 (GM-CSF) to 6.08 pg ml−1 (MIP-1α).
ELISA measurements.
Quantification of VRC01-N exhibiting gp120 binding activity was performed by enzyme-linked immunosorbent assay (ELISA). The HIV-1 gp120 protein coating antigen (catalog number 11233-V08H) was obtained from Sino Biological (Beijing, China), and goat anti-human kappa-horseradish peroxidase (detection antibody; catalog no. 2060-05) was obtained from SouthernBiotech (Birmingham, AL, USA). Bovine serum albumin (BSA) was obtained from Sigma (St. Louis, MO, USA). SureBlue tetramethylbenzidine (TMB) peroxidase substrate was obtained from KPL, Inc. (Gaithersburg, MD, USA). ELISA plates were prepared by sequential incubation in 96-well plates with 100 μl per well of coating antigen (1 ng μl−1 in 1× PBS), 200 μl per well of blocking buffer (2% BSA in 1× PBS), 100 μl of standard or sample, and finally, 100 μl of detection antibody diluted 1:5,000 in wash buffer. All blocking and incubation steps were at room temperature for 1 h with gentle agitation of the plate. The plates were washed 3 to 4 times with wash buffer between each incubation step. The plates were developed by adding 100 μl TMB substrate solution to each well, followed by 50 μl 4 N H2SO4, once color development was observed in the low-concentration standards. The absorption at 450 nm (OD450) was measured using a SpectraMax Plus 384 microplate reader. For each ELISA experiment, a single plate was used for all VRC01-N standards and samples, and all measurements were in triplicate. Standards at eight concentrations ranging from 1.5 to 1,000 ng/ml (as determined by measurement of the OD280) were prepared from spray-dried VRC01-N stored continuously at −80°C. The values for samples with concentrations below 1.5 ng ml−1 were considered to be below the LLOQ. Samples were diluted as necessary to remain within the range of the VRC01-N standards.
SDS-PAGE analysis.
Aliquots of the residual pod material extracted from used IVRs and dissolved in 1× PBS were analyzed by electrophoresis (SDS-PAGE). Reduced samples were denatured by heating at 75°C for 10 min with 7 to 13% (wt/vol) lithium dodecyl sulfate (4× NuPage loading buffer; Thermo Fisher Scientific) and 1.0 mM DTT. Samples were diluted with PBS to obtain 100 μg total protein per sample, as determined by measurement of the OD280. Electrophoresis was carried out using a NuPage Novex 4 to 12% bis-Tris polyacrylamide gel (Thermo Fisher Scientific) with a sample volume corresponding to 20.0 μg of protein/well. Protein bands were visualized with Coomassie blue dye (Simply Blue SafeStain; Thermo Fisher Scientific) according to the manufacturer's instructions. Gel images were obtained using a Bio-Rad Gel Doc EZ imaging system.
Statistical analysis.
Data were analyzed using GraphPad Prism software (version 6.02; GraphPad Software, Inc., La Jolla, CA). The vaginal fluid and CVL fluid concentrations of VRC01-N and cytokines below the limit of detection of the assay were assigned concentrations of 0.001 ng ml−1 to allow plotting on a log scale. The cytokine levels measured after insertion of the IVR and after IVR removal were compared to the baseline levels and to each other in a one-way analysis of variance (ANOVA). Cytokine values were log transformed to normalize the data. A Bonferroni correction was used to avoid inference errors due to multiple comparisons. Statistical significance was defined as a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number U19AI096398 and by the National Institutes of Health under award number P51OD11132 for support of the Yerkes National Primate Research Center. We gratefully acknowledge their support.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02465-16.
REFERENCES
- 1.Hecht R, Stover J, Bollinger L, Muhib F, Case K, de Ferranti D. 2010. Financing of HIV/AIDS programme scale-up in low-income and middle-income countries, 2009-31. Lancet 376:1254–1260. doi: 10.1016/S0140-6736(10)61255-X. [DOI] [PubMed] [Google Scholar]
- 2.Shattock RJ, Warren M, McCormack S, Hankins CA. 2011. Turning the tide against HIV. Science 333:42–43. doi: 10.1126/science.1206399. [DOI] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS. 2013. Report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland: http://www.unaids.org/en/resources/publications/2013 Accessed 3 November 2016. [Google Scholar]
- 4.Joint United Nations Programme on HIV/AIDS. 2016. AIDS by the numbers. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland: http://www.unaids.org/en/resources/documents/2016/AIDS-by-the-numbers Accessed 3 November 2016. [Google Scholar]
- 5.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker L-G, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, et al. 2016. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 375:2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang FR, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdool Karim Q, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 10.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Mâsse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM. 2015. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascola JR, Haynes BF. 2013. HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol Rev 254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stiegler G, Katinger H. 2003. Therapeutic potential of neutralizing antibodies in the treatment of HIV-1 infection. J Antimicrob Chemother 51:757–759. doi: 10.1093/jac/dkg172. [DOI] [PubMed] [Google Scholar]
- 14.Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol 78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PWHI, Sawyer LSW, Hendry RM, Dunlop N, Nara PL, Lamacchia M, Garratty E, Stiehm ER, Bryson YJ, Cao Y, Moore JP, Ho DD, Barbas CF III. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 16.Zhou T, Xu L, Dey B, Hessell AJ, Van Ryk D, Xiang S-H, Yang X, Zhang M-Y, Zwick MB, Arthos J, Burton DR, Dimitrov DS, Sodroski J, Wyatt R, Nabel GJ, Kwong PD. 2007. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445:732–737. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 18.Trkola A, Pomales AB, Yuan H, Korber B, Maddon PJ, Allaway GP, Katinger H, Barbas CF, Burton DR, Ho DD. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol 69:6609–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker F, Katinger H. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol 67:6642–6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PWHI. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol 75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou T-C, Ruprecht RM. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 22.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. 2010. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol 84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A 108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu QX, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med 9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 25.Morris GC, Wiggins RC, Woodhall SC, Bland JM, Taylor CR, Jespers V, Vcelar BA, Lacey CJ. 2014. MABGEL 1: first phase 1 trial of the anti-HIV-1 monoclonal antibodies 2F5, 4E10 and 2G12 as a vaginal microbicide. PLoS One 9:e116153. doi: 10.1371/journal.pone.0116153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiatt A, Zeitlin L, Whaley KJ. 2013. Multiantibody strategies for HIV. Clin Dev Immunol 2013:632893. doi: 10.1155/2013/632893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corti D, Langedijk JPM, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, Silacci C, Pinna D, Jarrossay D, Balla-Jhagjhoorsingh S, Willems B, Zekveld MJ, Dreja H, O'Sullivan E, Pade C, Orkin C, Jeffs SA, Montefiori DC, Davis D, Weissenhorn W, McKnight A, Heeney JL, Sallusto F, Sattentau QJ, Weiss RA, Lanzavecchia A. 2010. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One 5:e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu XL, Yang ZY, Li YX, Hogerkorp CM, Schief WR, Seaman MS, Zhou TQ, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seay K, Qi X, Zheng JH, Zhang C, Chen K, Dutta M, Deneroff K, Ochsenbauer C, Kappes JC, Littman DR, Goldstein H. 2013. Mice transgenic for CD4-specific human CD4, CCR5 and cyclin T1 expression: a new model for investigating HIV-1 transmission and treatment efficacy. PLoS One 8:e63537. doi: 10.1371/journal.pone.0063537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledgerwood JE, Coates EE, Yamshchikov G, Saunders JG, Holman L, Enama ME, DeZure A, Lynch RM, Gordon I, Plummer S, Hendel CS, Pegu A, Conan-Cibotti M, Sitar S, Bailer RT, Narpala S, McDermott A, Louder M, O'Dell S, Mohan S, Pandey JP, Schwartz RM, Hu Z, Koup RA, Capparelli E, Mascola JR, Graham BS, VRC 602 Study Team . 2015. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol 182:289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute of Allergy and Infectious Diseases. 2015. Evaluating the safety and efficacy of the VRC01 antibody in reducing acquisition of HIV-1 infection in women. National Library of Medicine, Bethesda, MD: https://clinicaltrials.gov/ct2/show/NCT02568215. [Google Scholar]
- 33.Veselinovic M, Preston Neff C, Mulder LR, Akkina R. 2012. Topical gel formulation of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 confers protection against HIV-1 vaginal challenge in a humanized mouse model. Virology 432:505–510. doi: 10.1016/j.virol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moog C, Dereuddre-Bosquet N, Teillaud JL, Biedma ME, Holl V, Van Ham G, Heyndrickx L, Van Dorsselaer A, Katinger D, Vcelar B, Zolla-Pazner S, Mangeot I, Kelly C, Shattock RJ, Le Grand R. 2014. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol 7:46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 35.Pegu A, Yang Z-Y, Boyington JC, Wu L, Ko S-Y, Schmidt SD, McKee K, Kong W-P, Shi W, Chen X, Todd J-P, Letvin NL, Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M, Rao SS, Mascola JR, Nabel GJ. 2014. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med 6:243ra288. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su B, Lederle A, Laumond G, Ducloy C, Schmidt S, Decoville T, Moog C. 2014. Broadly neutralizing antibody VRC01 prevents HIV-1 transmission from plasmacytoid dendritic cells to CD4 T lymphocytes. J Virol 88:10975–10981. doi: 10.1128/JVI.01748-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rademacher T, Sack M, Arcalis E, Stadlmann J, Balzer S, Altmann F, Quendler H, Stiegler G, Kunert R, Fischer R, Stoger E. 2008. Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly single-GlcNAc N-glycans. Plant Biotechnol J 6:189–201. doi: 10.1111/j.1467-7652.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 38.Ramessar K, Rademacher T, Sack M, Stadlmann J, Platis D, Stiegler G, Labrou N, Altmann F, Ma J, Stöger E, Capell T, Christou P. 2008. Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc Natl Acad Sci U S A 105:3727–3732. doi: 10.1073/pnas.0708841104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, Glössl J, Weterings K, Pabst M, Steinkellner H. 2008. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J 6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 40.Drake PMW, Barbi T, Sexton A, McGowan E, Stadlmann J, Navarre C, Paul MJ, Ma JK-C. 2009. Development of rhizosecretion as a production system for recombinant proteins from hydroponic cultivated tobacco. FASEB J 23:3581–3589. doi: 10.1096/fj.09-131771. [DOI] [PubMed] [Google Scholar]
- 41.Teh AYH, Maresch D, Klein K, Ma JKC. 2014. Characterization of VRC01, a potent and broadly neutralizing anti-HIV mAb, produced in transiently and stably transformed tobacco. Plant Biotechnol J 12:300–311. doi: 10.1111/pbi.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamorsky KT, Grooms-Williams TW, Husk AS, Bennett LJ, Palmer KE, Matoba N. 2013. Efficient single tobamoviral vector-based bioproduction of broadly neutralizing anti-HIV-1 monoclonal antibody VRC01 in Nicotiana benthamiana plants and utility of VRC01 in combination microbicides. Antimicrob Agents Chemother 57:2076–2086. doi: 10.1128/AAC.02588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baum MM, Butkyavichene I, Churchman SA, Lopez G, Miller CS, Smith TJ, Moss JA. 2015. An intravaginal ring for the sustained delivery of tenofovir disoproxil fumarate. Int J Pharm 495:579–587. doi: 10.1016/j.ijpharm.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baum MM, Butkyavichene I, Gilman J, Kennedy S, Kopin E, Malone AM, Nguyen C, Smith TJ, Friend DR, Clark MR, Moss JA. 2012. An intravaginal ring for the simultaneous delivery of multiple drugs. J Pharm Sci 101:2833–2843. doi: 10.1002/jps.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunawardana M, Baum MM, Smith TJ, Moss JA. 2014. An intravaginal ring for the sustained delivery of antibodies. J Pharm Sci 103:3611–3620. doi: 10.1002/jps.24154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radomsky ML, Whaley KJ, Cone RA, Saltzman WM. 1992. Controlled vaginal delivery of antibodies in the mouse. Biol Reprod 47:133–140. doi: 10.1095/biolreprod47.1.133. [DOI] [PubMed] [Google Scholar]
- 47.Morrow RJ, Woolfson AD, Donnelly L, Curran R, Andrews G, Katinger D, Malcolm RK. 2011. Sustained release of proteins from a modified vaginal ring device. Eur J Pharm Biopharm 77:3–10. doi: 10.1016/j.ejpb.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss JA, Srinivasan P, Smith TJ, Butkyavichene I, Lopez G, Brooks AA, Martin A, Dinh CT, Smith JM, Baum MM. 2014. Pharmacokinetics and preliminary safety study of pod-intravaginal rings delivering antiretroviral combinations for HIV prophylaxis in a macaque model. Antimicrob Agents Chemother 58:5125–5135. doi: 10.1128/AAC.02871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Promadej-Lanier N, Smith JM, Srinivasan P, McCoy CF, Butera S, Woolfson AD, Malcolm RK, Otten RA. 2009. Development and evaluation of a vaginal ring device for sustained delivery of HIV microbicides to non-human primates. J Med Primatol 38:263–271. doi: 10.1111/j.1600-0684.2009.00354.x. [DOI] [PubMed] [Google Scholar]
- 50.Haase AT. 2010. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 51.Dezzutti CS, Hendrix CW, Marrazzo JM, Pan Z, Wang L, Louissaint N, Kalyoussef S, Torres NM, Hladik F, Parikh U, Mellors J, Hillier SL, Herold BC. 2011. Performance of swabs, lavage, and diluents to quantify biomarkers of female genital tract soluble mucosal mediators. PLoS One 6:e23136. doi: 10.1371/journal.pone.0023136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, Salata R, Soto-Torres L, Patterson K, Minnis AM, Gandham S, Gomez K, Richardson BA, Bumpus NN. 2013. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One 8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, Hitti J. 2011. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J Clin Microbiol 49:735–736. doi: 10.1128/JCM.00991-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller CJ, Shattock RJ. 2003. Target cells in vaginal HIV transmission. Microbes Infect 5:59–67. doi: 10.1016/S1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 55.Moss JA, Malone AM, Smith TJ, Butkyavichene I, Cortez C, Gilman J, Kennedy S, Kopin E, Nguyen C, Sinha P, Hendry M, Guenthner P, Holder A, Martin A, McNicholl J, Mitchell J, Pau C-P, Srinivasan P, Smith JM, Baum MM. 2012. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. Antimicrob Agents Chemother 56:5952–5960. doi: 10.1128/AAC.01198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pirrone V, Thakkar N, Jacobson JM, Wigdahl B, Krebs FC. 2011. Combinatorial approaches to the prevention and treatment of HIV-1 infection. Antimicrob Agents Chemother 55:1831–1842. doi: 10.1128/AAC.00976-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, Scheid JF, Eden C, Mouquet H, Nussenzweig MC, Schwartz O. 2013. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med 210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeafBio, Inc. 2015. Vaginal Antibody Safety Trial: safety study of monoclonal antibodies to reduce the vaginal transmission of herpes simplex virus (HSV) and human immunodeficiency virus (HIV) (VAST). National Library of Medicine, Bethesda, MD: https://clinicaltrials.gov/ct2/show/NCT02579083. [Google Scholar]
- 59.Malcolm R, Edwards K, Kiser P, Romano J, Smith T. 2010. Advances in microbicide vaginal rings. Antiviral Res 88(Suppl 1):S30–S39. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Whaley KJ, Zeitlin L. 2013. Antibody-based concepts for multipurpose prevention technologies. Antiviral Res 100:S48–S53. doi: 10.1016/j.antiviral.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Center for Biosecurity of UPMC. 2013. Next-generation monoclonal antibodies: challenges and opportunities. Final report. Center for Biosecurity of UPMC, Washington, DC. [Google Scholar]
- 62.Kelley B. 2007. Very large scale monoclonal antibody purification: the case for conventional unit operations. Biotechnol Prog 23:995–1008. [DOI] [PubMed] [Google Scholar]
- 63.Farid SS. 2007. Process economics of industrial monoclonal antibody manufacture. J Chromatogr B Analyt Technol Biomed Life Sci 848:8–18. doi: 10.1016/j.jchromb.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 64.Whaley K, Morton J, Hume S, Hiatt E, Bratcher B, Klimyuk V, Hiatt A, Pauly M, Zeitlin L. 2014. Emerging antibody-based products. Curr Top Microbiol Immunol 375:107–126. doi: 10.1007/82_2012_240. [DOI] [PubMed] [Google Scholar]
- 65.Whaley KJ, Hiatt A, Zeitlin L. 2011. Emerging antibody products and Nicotiana manufacturing. Hum Vaccin 7:349–356. doi: 10.4161/hv.7.3.14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Estelle D. 2006. Adapting industry practices for the rapid, large-scale manufacture of pharmaceutical proteins, p 39–44. In The bridge. National Academy of Engineering, Washington, DC. [Google Scholar]
- 67.Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95. doi: 10.1016/S0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 68.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf. [Google Scholar]
- 69.Promadej-Lanier N, Srinivasan P, Curtis K, Adams D, Kim C, Luo W, Jia H, Subbarao S, Otten R, Butera S. 2008. Systemic and mucosal immunological responses during repeated mucosal SHIV(162P3) challenges prior to and following infection in pigtailed macaques. Virology 375:492–503. doi: 10.1016/j.virol.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 70.Srinivasan P, Moss JA, Gunawardana M, Churchman SA, Yang F, Dinh CT, Mitchell JM, Zhang J, Fanter R, Miller CS, Butkyavichene I, McNicholl JM, Smith TJ, Baum MM, Smith JM. 2016. Topical delivery of tenofovir disoproxil fumarate and emtricitabine from pod-intravaginal rings protects macaques from multiple SHIV exposures. PLoS One 11:e0157061. doi: 10.1371/journal.pone.0157061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.